SUMMARY
Dnmt1 epigenetically propagates symmetrical CG methylation in many eukaryotes. Their genomes are typically depleted of CG dinucleotides, because of imperfect repair of deaminated methylcytosines. Here, we extensively surveyed diverse species lacking Dnmt1 and show that, surprisingly, symmetrical CG methylation is nonetheless frequently present, catalyzed by a novel DNA methyltransferase family, Dnmt5. Numerous Dnmt5-containing organisms that diverged over a billion years ago exhibit clustered methylation specifically in nucleosome linkers. Clustered methylation occurs at unprecedented densities and directly disfavors nucleosomes, contributing to nucleosome positioning between clusters. Dense methylation is enabled by a novel regime of genomic sequence evolution that enriches CG dinucleotides, driving the highest CG frequencies known. Species with linker methylation have small, transcriptionally active nuclei that approach the physical limits of chromatin compaction. These features constitute a previously unappreciated genome architecture, in which dense methylation influences nucleosome positions, likely facilitating nuclear processes under extreme spatial constraints.
INTRODUCTION
Methylation of carbon at the fifth position (C5) of cytosine is a feature of diverse eukaryotic genomes. In most studied species, Dnmt1 propagates epigenetic information stored in symmetrical methylation of double-stranded 5′-CG-3′ sites by copying methylation after DNA replication with the help of Uhrf1 (Law and Jacobsen, 2010). Methylation by chromomethylases in plants and green algae occurs at 5′-CNG-3′ sites (treated as 5′-CHG-3′, where H is A, C or T, to disambiguate sites from overlapping CG, i.e. 5′-CGG-3′) (Feng et al., 2010; Law and Jacobsen, 2010; Zemach et al., 2010). Other cytosine sequence contexts (treated as 5′-CHH-3′ to disambiguate from overlapping CG and CHG sites) can be methylated by Dnmt3 enzymes, as well as other plant and fungal enzymes (Feng et al., 2010; Law and Jacobsen, 2010; Zemach et al., 2010, 2013). There are also other predicted families of C5 cytosine methyltransferases in eukaryotes identified bioinformatically (e.g. Dnmt5) that have not been functionally characterized (Iyer et al., 2011; Ponger and Li, 2005).
DNA methyltransferases (and related proteins) are thought to profoundly affect genome sequence evolution. They drive cytosine hypermutability, because of imperfect repair of spontaneously deaminated C5-methylated cytosines (and other mutational processes), which depletes methylation target sites and causes a substantial fraction of human diseases (Pfeifer, 2006; Walsh and Xu, 2006). Proteins related to DNA methyltransferases (called RID, Masc1 or Dnmt4 (Ponger and Li, 2005)) also mediate repeat-induced point mutation in some fungi, a process that directly induces mutation of cytosines in repeated sequences, such as transposable elements (TEs) (Galagan and Selker, 2004; Zemach and Zilberman, 2010).
Targeting of methyltransferases across a genome relies on numerous factors, including transcriptional activity, histone modification states, production of small RNAs, the action of nucleosome remodelers, local sequence composition and sequences that specifically bind to proteins (Huff and Zilberman, 2012; Jones, 2012; Law and Jacobsen, 2010; Schübeler, 2012; Zemach et al., 2013). In addition, there could be a role for positioned nucleosomes in localizing DNA methylation, which is not resolved in the published literature. Several studies demonstrated that methyltransferase activity is generally reduced by nucleosomes, which apparently results in preferential methylation of DNA outside or at the edges of nucleosome cores (Felle et al., 2011; Gowher et al., 2005; Jiang et al., 2011; Kelly et al., 2012; Okuwaki and Verreault, 2004; Robertson et al., 2004; Takeshima et al., 2006, 2008), whereas another study instead showed that genomic methylation is modestly enriched within nucleosome core DNA (Chodavarapu et al., 2010). On the other hand, several studies have suggested that methylated DNA has effects on the positioning of nucleosomes (Collings et al., 2013; Jimenez-Useche et al., 2013; Pennings et al., 2005; Pérez et al., 2012), though biological functions for such effects have not been clearly demonstrated.
Eukaryotic DNA methylation is perhaps best known for repressing the transcription of TEs in many plants, fungi and vertebrates (Law and Jacobsen, 2010; Zemach and Zilberman, 2010). Generally, the transcription start sites of genes are kept free of DNA methylation, presumably because it would otherwise interfere with transcription (Long et al., 2013; Zemach and Zilberman, 2010), with important exceptions including regulated silencing of some genes during organismal development and inappropriate silencing of genes in cancer cells (Jones, 2012). In many plants and animals, methylation is also located in the bodies of genes, where it can antagonize localization of the histone variant H2A.Z (Coleman-Derr and Zilberman, 2012; Zemach and Zilberman, 2010) and affect alternative splicing (Lyko and Maleszka, 2011; Maunakea et al., 2013; Shukla et al., 2011). However, whether TE or gene body methylation is ancestral in eukaryotes has not been fully resolved, because of the sporadic distribution in extant species of these features. Furthermore, aspects of genomic methylation targeting have likely changed throughout eukaryotic diversification (Suzuki and Bird, 2008; Zemach and Zilberman, 2010). To assess better the mechanisms of methylating DNA in an evolutionary context, we surveyed eukaryotic species from the most diverse lineages available.
RESULTS
Dnmt1-independent CG methylation in many eukaryotic lineages
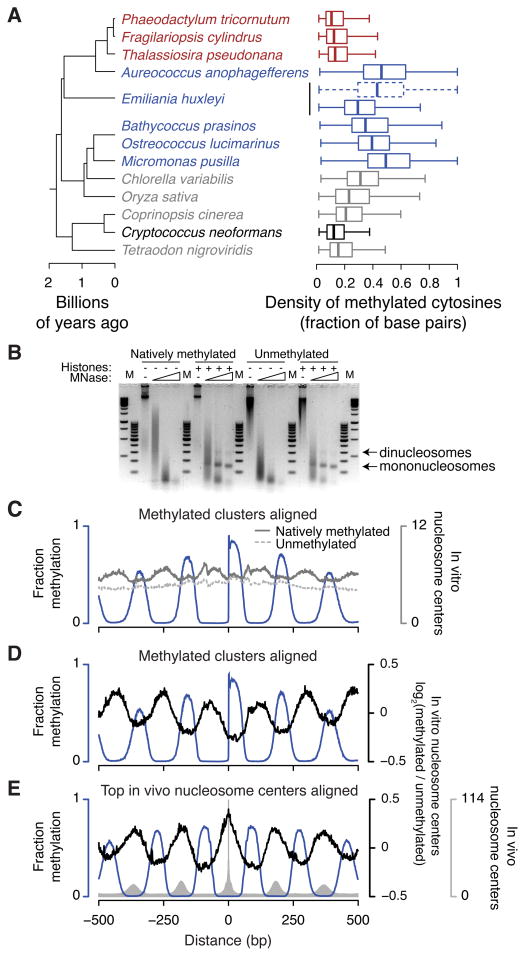
For species with sequenced, assembled genomes that each encode at least one predicted methyltransferase (Figures 1A–C and Table S1), we performed genome-wide bisulfite sequencing to quantify C5-methylated cytosine levels at single-base resolution and high coverage (representing many individual cells). Surprisingly, this uncovered CG methylation in a variety of species that lack Dnmt1; all but one of these species also lack Uhrf1 (Figure 1B–D and Table S1). These divergent marine algae, a photosynthetic assemblage responsible for half of global primary production (Field et al., 1998), span much of the eukaryotic tree and include the diatoms Phaeodactylum tricornutum, Fragilariopsis cylindrus and Thalassiosira pseudonana, the pelagophyte Aureococcus anophagefferens, the coccolithophore Emiliania huxleyi and the prasinophyte green algae Bathycoccus prasinos, Ostreococcus lucimarinus and Micromonas pusilla. This demonstrates that the Dnmt1/Uhrf1 pathway is not strictly required for eukaryotic CG methylation, and that Dnmt1-indpendent CG methylation is widespread and likely ancient. Curiously, we also identified two types of methylation in specific sequence contexts in the mitochondrial genome of A. anophagefferens (Figure S1A), which have not been previously reported in any eukaryote.
Figure 1. Dnmt1-independent CG methylation is associated with Dnmt5 in diverse eukaryotes.
(A) Predicted C5 cytosine methyltransferase domains from eukaryotes and bacteria were aligned and a maximum likelihood tree was inferred. The gray branches are mostly bacterial sequences, but also include several “orphan” sequences from eukaryotes for which homologous sequences from other eukaryotic lineages were not apparent. Eukaryotic families are colored. The families we found are essentially equivalent to those identified using fewer sequences (Ponger and Li, 2005), so we retained the previous naming scheme, which includes the Dnmt4, Dnmt5, and Dnmt6 families. Numbers indicate percent bootstrap support for the nodes uniting each eukaryotic family. Divergent families, such as Dnmt2, Dnmt3, Dnmt4 and Dnmt6, have weak support (49–79% of bootstraps). The family groupings of Dnmt1, chromomethylase (Cmt), Dim-2 and Dnmt5 sequences are each strongly supported (92–100% of bootstraps). (B) Divergent lineages leading to individual species profiled here are single branches. Groups of species are shown as collapsed nodes, indicating earliest divergence times for species previously profiled for genome-wide methylation (Feng et al., 2010; Zemach et al., 2010). B. prasinos and O. lucimarinus are shown on the same branch, because their divergence time has not been estimated. (C) Predicted methyltransferases and Uhrf1 are indicated for each species or group in panel B. Families are colored as in panel A. In groups, presence is denoted for genes found in at least one member species. The dashed box highlights general lack of Dnmt1 and Uhrf1 and the solid box highlights Dnmt5. (D) Mean methylation in each sequence context (darkest to lightest: CG, CHG, CHH) is calculated for each species or group in panel B. For groups with multiple species, each bar represents the mean of member species with the line showing the range of underlying values for each species. *For Fungi non-CG methylation does not generally fall into CHG and CHH categories (Zemach et al., 2010). (E) Domains in a representative Dnmt5 protein; scale bar is 100 amino acids (aa). See also Figure S1 and Table S1.
Dnmt5 is a novel symmetrical CG methyltransferase in eukaryotes
To implicate a specific methyltransferase in catalyzing Dnmt1-independent CG methylation, we compared the complement of genes present in each genome (Figure 1A–D). The species have only one predicted DNA methyltransferase family in common, Dnmt5 (Ponger and Li, 2005) (Dnmt2 is a tRNA methyltransferase (Goll et al., 2006)). The rhodophyte Cyanidioschyzon merolae and the excavate pathogen Leishmania major both lack Dnmt5 (and also Dnmt1) and do not exhibit CG-specific methylation (Table S1). Each Dnmt5 protein contains a methyltransferase domain that is far diverged from other families, but conserved between Dnmt5 homologs (Figure 1A). Dnmt5 has a unique architecture, which includes a RING finger following the methyltransferase domain and a long C-terminal region of SNF2-family homology (Figure 1E). The SNF2 regions belong to a larger family (Figure S1B), which includes known E3 ubiquitin ligase/DNA-dependent ATPase proteins (Unk et al., 2010), suggesting that Dnmt5 proteins are multifunctional enzymes.
To determine experimentally if Dnmt5 mediates CG methylation, we examined the opportunistically pathogenic basidiomycete yeast Cryptococcus neoformans. This organism is ideal, because Dnmt5 is the only DNA methyltransferase homolog present in its genome (Dnmt2 is also absent), and targeted gene deletion is currently feasible, unlike in the algal species. We found that C. neoformans exhibits CG methylation, which is entirely lost in a strain with a deletion of the DNMT5 gene (Figure 2A and Table S1), strongly implicating Dnmt5 as a novel CG-specific DNA methyltransferase. The alternative is that another methyltransferase catalyzes CG methylation in C. neoformans and utilizes Dnmt5 as a required accessory or regulatory protein. This is a vanishingly remote possibility, especially because all known C5 cytosine methyltransferases belong to a single superfamily (Iyer et al., 2011), of which Dnmt5 is a member (Figure 1A). Also of note, loss of Dnmt5 causes decreased C. neoformans infectivity in mice (Liu et al., 2008), making it a potential target for pharmacological inhibition to combat infection.
Figure 2. Dnmt5 mediates symmetrical CG methylation.
(A) Fractional CG methylation is shown with Tukey’s running median smoothing across chromosome 10. The positions of TEs are shown as black vertical lines at the top. Further analyses of TEs are in Figure S2B. Data are shown for wild-type genomic DNA grown and prepared at ATCC (WT) and the DNMT5-deleted strain we grew (dnmt5Δ). We also grew a control “wild-type” strain (WT47, a deletion of the unrelated gene SXI1), constructed with the same procedure as the strain with deletion of DNMT5 (Liu et al., 2008), to ensure that the absence of methylation in dnmt5Δ was not an artifact of the strain construction process or growth conditions. This also allows assessment of the reproducibility of our methylation data, which show strong quantitative similarity (Pearson’s r = 0.90 for the unsmoothed whole-genome data). For comparison, the data for the two WT strains are not correlated with those for the dnmt5Δ strain (Pearson’s r < 0.01 for both comparisons). (B) We analyzed the symmetry of methylation at CGCG sites genome-wide. To remove from the analysis uninformative regions where all of the cytosines are either methylated or unmethylated, we selected the CGCG sites that contain at least one cytosine with high (>85%) and one with low (<15%) methylation in the population of cells and with all cytosines having at least 10-fold sequencing coverage. For these informative sites in each species, the first bar (blue) shows the Pearson’s r of cytosines within CG sites and the second bar (orange) shows the Pearson’s r of the internal cytosines between adjacent CG sites. High-resolution examples of symmetrical methylation at CG sites are in Figure S2A.
Observation of individual CG sites suggests that Dnmt5-associated CG methylation is generally symmetrical (Figure S2A), similar to that catalyzed by Dnmt1 (Law and Jacobsen, 2010). However, genomes are patchworks of methylated and unmethylated regions, so an analysis of all genomic CG sites would yield many fully methylated and fully unmethylated sites, even if methylation were not generally symmetrical. To overcome this issue, we analyzed all pairs of immediately adjacent CG dinucleotides (5′-CGCG-3′) that were neither fully methylated nor fully unmethylated. At these sites, correlations of methylation levels within a given CG site are strongly positive, whereas those between the internal cytosines of neighboring CG sites are strongly negative in all species with Dnmt5-associated CG methylation (Figure 2B). Therefore, across populations of cells, these informative sites are almost exclusively composed of symmetrically methylated CG sites neighboring symmetrically unmethylated CG sites, comparable to the symmetrical CG methylation catalyzed by a Dnmt1 enzyme in Arabidopsis thaliana (Figure 2B).
Methylation of nucleosomal linker DNA in diverse eukaryotic groups
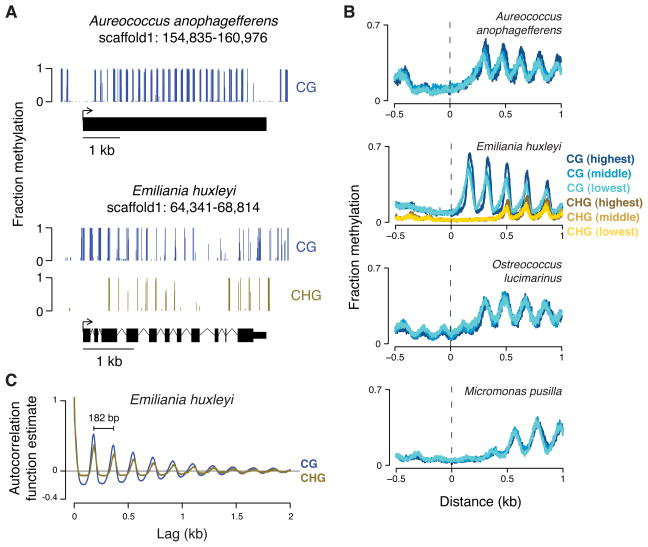
CG methylation in C. neoformans and diatoms is apparently concentrated within TEs (Figures 2A and S2B). These TEs also exhibit little to no RNA production, consistent with silencing by methylation in these species (Figure S2B). However, the genomes of A. anophagefferens, E. huxleyi, B. prasinos, O. lucimarinus and M. pusilla exhibit a methylation pattern unlike that of any eukaryote previously described (Figures 3A,B and S3A). Methylation occurs in clusters that are regularly spaced in the populations of cells with periodicities between 168 and 206 bp, depending on the organism (Figures 3C and S3B). Almost every gene body is methylated, regardless of expression levels (Figures 3B and S3C), suggesting that periodic methylation is a structural component of these genomes.
Figure 3. Genomes of diverse species have periodic methylation in gene bodies, regardless of expression levels.
(A) A snapshot of genomic methylation is shown for A. anophagefferens (top) and E. huxleyi (bottom) with each gene model below. (B) We assessed transcription by RNA-seq of total RNA. For each organism, genes were binned into 5 equally sized groups (quintiles) based on expression measured by FPKM (fragments per kilobase of transcript per million mapped reads). The mean methylation at each base pair position aligned to transcription start sites of genes from different quintiles is shown (CG, blue; CHG, gold). Only data for the first (lowest; lightest color), third (middle; middle color), and fifth (highest; darkest color) quintiles are shown for clarity. The average FPKM differences from the lowest to highest quintiles are approximately 100- to 1000-fold, depending on the organism. A similar plot for B. prasinos is in Figure S3A. (C) The autocorrelation function estimate for CG and CHG methylation is shown for each lag (offset) across the largest scaffold of E. huxleyi. The apparent periodicity for both is 182 base pairs (bp). Autocorrelation function estimates for other species are in Figure S3B.
To discover periodic methylation in species without sequenced genomes, we developed an assay that uses a methylation-dependent endonuclease on purified DNA. We used this assay to identify periodic methylation in Isochrysis galbana and Imantonia rotunda, which belong to the same class (Prymnesiophyceae) as E. huxleyi, as well as to confirm the presence of periodic methylation in most of the species we sequenced (Figure S4).
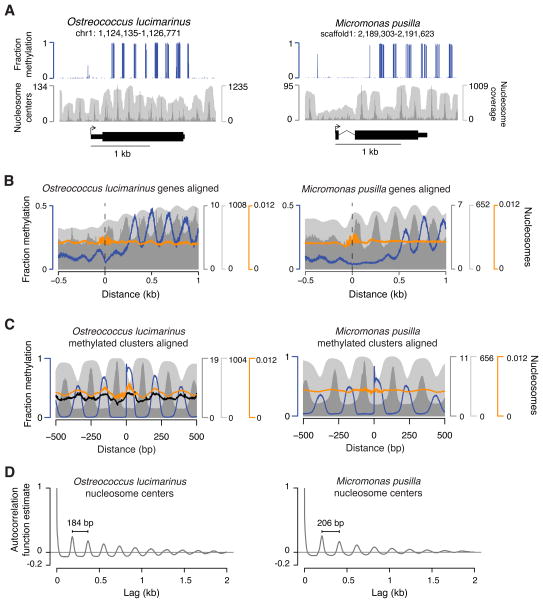
Given the similarity between known nucleosomal repeat lengths and methylation periodicities described here (Figures 3C and S3B), we mapped nucleosomes to high coverage in O. lucimarinus and M. pusilla by micrococcal nuclease (MNase) digestion. The resulting maps show well-positioned nucleosomes across these genomes (Figure 4). Despite the high G+C contents in these algae (Palenik et al., 2007; Worden et al., 2009), nucleosomes are phased downstream of transcription start sites (Figure 4A,B), similar to previously studied A+T-rich genomes (Chang et al., 2012; Struhl and Segal, 2013). Notably, linkers in M. pusilla are typically 22 bp longer than those in O. lucimarinus (Figure 4C,D), consistent with the presence of a gene for linker histone H1 in M. pusilla but not in O. lucimarinus (Worden et al., 2009). A sequence-based algorithm for predicting nucleosomes (Kaplan et al., 2009) performs poorly in O. lucimarinus and M. pusilla (orange trace in Figure 4B,C), suggesting that factors beyond DNA sequence have a strong influence on nucleosome positioning in these species.
Figure 4. Periodic methylation occurs specifically in nucleosome linkers.
(A) A snapshot of genomic CG methylation and nucleosomes is shown for O. lucimarinus (left) and M. pusilla (right) with the gene model below. (B) Means at each position aligned to transcription start sites are shown for methylation and nucleosomes. (C) Means at each position aligned to CG methylation clusters are shown for methylation and nucleosomes. (D) The autocorrelation function estimates of nucleosome center counts per base are shown for each lag (offset) across the largest scaffold/chromosome of O. lucimarinus and M. pusilla. Apparent periodicities are indicated in base pairs (bp). Blue is fractional CG methylation, dark gray is center counts and light gray is fragment fold-coverage for in vivo nucleosomes. Black is fragment centers from naked MNase-digested O. lucimarinus DNA on the same scale as in vivo nucleosome centers. Orange is predicted nucleosome center probabilities from a published algorithm (Kaplan et al., 2009). See also Figure S4.
Comparison of methylation and nucleosome data reveals that periodic methylation in O. lucimarinus and M. pusilla is located in the linkers between nucleosomes and is nearly completely excluded from the DNA within nucleosome cores, except for some base pairs near their edges (Figure 4A–C). MNase digestion of naked O. lucimarinus DNA instead shows preferential depletion of unmethylated sequences (black trace in Figure 4C), demonstrating that our results for in vivo nucleosome positions are not compromised by inherent enzymatic biases. A single amino acid difference in the methyltransferase domain of Dnmt3 can influence its ability to methylate nucleosome cores (Shen et al., 2010), so some of the amino acids found only in Dnmt5 methyltransferase domains of species with linker methylation may analogously be responsible for linker specificity (Figure S5). Methylation apparently either begins in the first linkers downstream of the transcription start sites or is missing from the first one or two linkers, depending on the species (Figures 3A,B, S3A and 4A,B). This indicates that addition (and/or removal) of methylation is affected by factors beyond bulk nucleosome positions, such as the presence of histone variants and modifications.
Linker methylation is extremely dense and directly disfavors nucleosomes
In many of the periodically methylated genomes, local densities of methylated bases are unprecedented for eukaryotic DNA, frequently with more than half of the base pairs containing methylated cytosines (Figure 5A). The CG methylation density is somewhat lower in E. huxleyi, but there appears to be a compensating mechanism: E. huxleyi has CHG methylation (Figure 1B,D and Table S1). CHG methylation is likely mediated by a chromomethylase (Cmt; Figure 1C) and occurs in genes with the same periodicity as CG methylation (Figure 3). Incorporating both CHG and CG sites in E. huxleyi yields densely methylated clusters more closely resembling other species that only have periodic CG methylation (Figure 5A). This suggests that the overall density of linker methylation, rather than methylation in a specific sequence context, is important for function.
Figure 5. Methylation occurs at unprecedented densities and contributes to nucleosome positioning.
(A) Distributions are shown of the density in each methylated region of base pairs containing methylated cytosines on either strand. For each group in Figure 1B we selected the species with the highest densities of genomic CG methylation (gray) to compare with periodically methylated genomes (blue). Diatoms and C. neoformans are colored red and black, respectively. For E. huxleyi, addition of CHG to CG sites is shown as a dashed box and whiskers. Boxes indicate the medians, first and third quartiles with whiskers indicating the most extreme values up to 1.5 times the interquartile ranges away from the boxes. (B) Digestion with increasing amounts of MNase reveals nucleosomes formed with purified recombinant histones and either natively methylated O. lucimarinus genomic DNA or unmethylated equivalent, generated by in vitro replication. M, 100 bp and 1 kb GeneRuler markers (Thermo). (C–E) Nucleosome positioning data from in vitro assemblies. In panel C the number of reads is shown for nucleosome centers assembled from natively methylated DNA (solid dark gray line) or from unmethylated DNA (dashed light gray line). Panels D and E show the base-2 logarithms of the ratios of reads in the methylated versus unmethylated assemblies (black lines), centered at log2(ratio)=0. In vitro nucleosome centers are aligned to methylation clusters (panels C,D) as in Figure 4C or to the top genomic positions for in vivo nucleosome centers (panel E). The blue lines show fractional CG methylation, and the gray bars in panel E show in vivo nucleosome centers for comparison. See also Figure S5.
The apparent importance of the density of DNA methylation in nucleosome linkers and the reliable positioning of nucleosomes in O. lucimarinus and M. pusilla (Figure 4) together suggest that dense methylation may disfavor nucleosomes, contributing to overall nucleosome positioning in vivo. To test this hypothesis, we mapped the positions of nucleosomes assembled in vitro using purified natively methylated O. lucimarinus genomic DNA and recombinant core histones (Figure 5B). To isolate the effects of DNA methylation, we also mapped the positions of nucleosomes assembled onto fully unmethylated O. lucimarinus genomic DNA (Figure 5B), generated by in vitro replication. Strikingly, nucleosomes assembled onto periodically methylated DNA are preferentially positioned over sequences that are unmethylated in vivo, whereas nucleosomes assembled onto fully unmethylated DNA show no such preference (Figure 5C,D). Nucleosomes assembled onto natively methylated DNA also better recapitulate in vivo nucleosome positions (Figure 5E). These results demonstrate that dense periodic DNA methylation contributes directly to nucleosome positioning throughout the genome.
Dense CG methylation is enabled by a novel regime of genome evolution
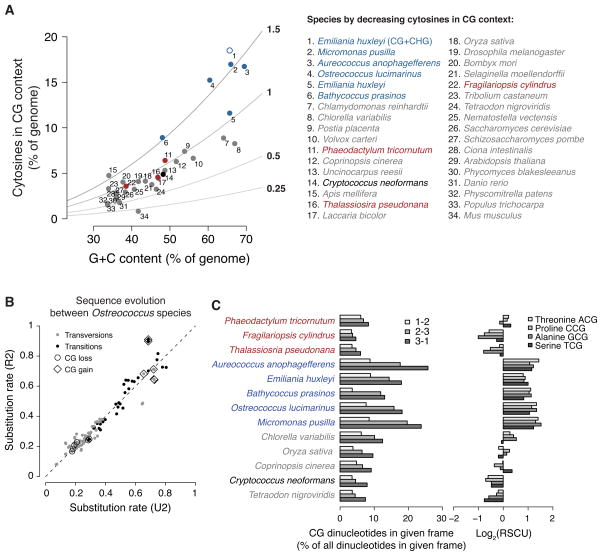
The high density of periodic methylation is made possible by frequent occurrence of CG dinucleotides, which are strongly overrepresented in the genomes of A. anophagefferens, B. prasinos, O. lucimarinus and M. pusilla (Figure 6A). E. huxleyi has modest enrichment of CG sites, but the additional contribution of CHG sites makes the level of cytosines that can be methylated higher than that of other periodically methylated species (Figure 6A). Enrichment of CG dinucleotides is remarkable, because CG dinucleotides are depleted relative to G+C content in the vast majority of previously studied CG-methylated genomes (Figure S6A). For example, transitions away from methylated CG dinucleotides are greatly accelerated relative to other substitutions in primates (Cohen et al., 2011). In striking contrast, analysis of homologous Ostreococcus chromosomes reveals that the rates of transitions away from CG (to TG and CA dinucleotides) are decelerated throughout Ostreococcus genomes, and transversions away from CG may also be slowed (Figure 6B and Table S2). In addition, all substitution rates toward CG are accelerated. This process of CG enrichment, which is unlike any previously described regime of eukaryotic genome evolution, is apparent even outside regions that are methylated (Figure S6B).
Figure 6. Novel regime of CG-enriching genome evolution.
(A) Cytosines in a CG context as percent of genomic bases versus G+C contents of individual genomes are plotted. The colors are as in Figure 5A, but gray includes most species with published methylation profiles. Observed-to-expected ratios for CG content are shown as lines with values immediately right of the plot. The addition of CHG to CG sites for E. huxleyi (#1) is an open blue circle. The complete legend is at the right. (B) Substitution rates between Ostreococcus species were estimated using either a general reversible (R2) or a general unrestricted (U2) dinucleotide model (Siepel and Haussler, 2004) from the non-coding sequences of aligned chromosomes (Table S2). R2 assumes that sequence evolution is time-reversible, but has the advantage of fewer mathematical parameters to estimate. We show the results of both R2 and U2 models to demonstrate that they generally agree (the dashed line shows the expectation if they had produced identical estimates). Transversions and transitions are colored gray and black, respectively. Substitutions that lose and gain CG sites are labeled with circles and diamonds, respectively. (C) For each of the species in Figure 5A, the frequency of CG dinucleotides as a percent of all dinucleotides in each codon frame (phase) is shown (left). “1–2” represents CG in the first and second positions of codons (lightest fill). These are CGN codons specifying arginine and are somewhat overrepresented in A. anophagefferens, E. huxleyi, O. lucimarinus and M. pusilla. “2–3” represents CG in the second and third positions of codons (medium fill). These codons encode amino acids that can be encoded by other codons. The enrichment of CG in this frame causes codon usage bias, shown by the base-2 logarithms of relative synonomous codon usages (RSCU (Sharp et al., 1986)) for each of the four NCG codons at right (lightest to darkest fill: ACG, CCG, GCG, TCG). High log2(RSCU) values for A. anophagefferens, E. huxleyi, B. prasinos, O. lucimarinus and M. pusilla NCG codons indicate that each of these codons is used to encode its respective amino acid more frequently than if codon usage were unbiased. “3–1” represents CG occurring across neighboring codons (center; darkest fill), which is the only frame in which CG enrichment does not necessarily alter encoded proteins or introduce codon usage bias. See also Figure S6.
If the evolutionary process of CG sequence enrichment were also operating in coding regions, where dense CG methylation is located (Figures 3A,B, S3A,C and 4A,B), it would be expected to frequently alter encoded proteins. This would generate deleterious alleles that should generally be removed from populations by negative selection, leaving the sites that least affect encoding to accumulate CG sequences. Indeed, CG dinucleotides in coding sequences are primarily in degenerate codon positions, often across neighboring codons (Figure 6C). However, CG enrichment causes pronounced codon usage bias and is still extreme enough to alter protein sequences, in particular through high levels of CGN codons specifying arginine (Figure 6C). Thus, the regime of sequence evolution that allows dense CG methylation to occur has had considerable impact on coding sequences.
Periodically methylated genomes are highly compacted
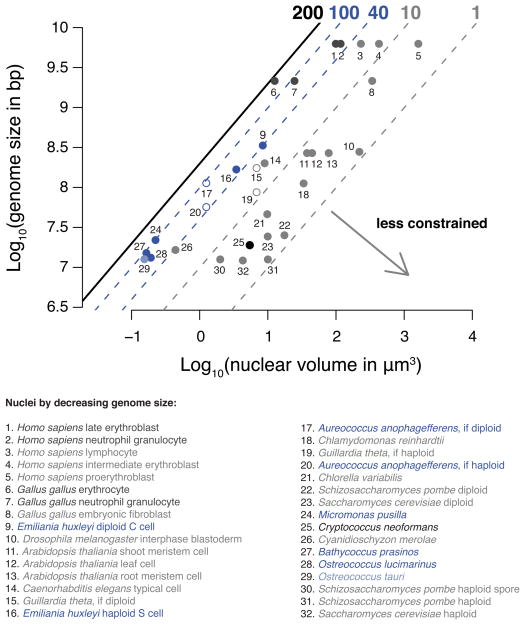
A. anophagefferens, E. huxleyi, B. prasinos, O. lucimarinus and M. pusilla share a genome architecture of CG-enriched chromosomes with dense methylation of nucleosome linkers within gene bodies, implying a function with strong evolutionary benefit. A potential clue to this benefit is another shared feature, small nuclei that contain among the highest average densities of DNA known (Figure 7 and Table S3). This density is comparable to that in vertebrate nuclei composed mostly of transcriptionally quiescent heterochromatin and approaches the physical limit of nucleosomal DNA compaction (Leforestier and Livolant, 1997). However, unlike vertebrate cells with compacted nuclei, these algal cells are growing and dividing, and accordingly are transcriptionally active (Figure S7). Our results suggest that dense, genome-wide linker methylation is a component of chromosome architecture that could facilitate DNA-templated processes (e.g. nucleosome positioning) under tight spatial constraints.
Figure 7. The nuclei of periodically methylated organisms contain genomes under extreme spatial constraints.
The base-10 logarithms of genome sizes in base pairs versus nuclear volumes in μm3 are shown for individual species/cell types. The black line at left shows the approximate upper limit for DNA concentration (200 mg ml−1) in nucleosomes (Leforestier and Livolant, 1997). The dashed blue lines show the highest and lowest average DNA concentrations among periodically methylated nuclei, approximately 100 and 40 mg ml−1, respectively. For reference the dashed gray lines at right show average DNA concentrations of 1 and 10 mg ml−1. Periodically methylated genomes are shown in dark blue (#9, 16, 17, 20, 24, 27 and 28). Both haploid (1N; #16) and diploid (2N; #9) E. huxleyi cell types are shown. Open circles are for species in which ploidy is unresolved. For example A. anophagefferens will be more constrained if diploid (if 2N; #17) than if haploid (if 1N; #20). The light blue point in the lower left is a relative of O. lucimarinus, O. tauri (#28), for which we did not profile DNA methylation, but for which highly accurate nuclear volume measurements are available (Henderson et al., 2007). The black point at lower center is C. neoformans (#25). We did not find published data for the nuclear volumes of diatoms. Gray points are species/cell types with a wide range of spatial constraints. The dark gray points at the upper center are vertebrate nuclei that contain mostly highly compacted heterochromatin (#1, 2, 6 and 7). References are in Table S3. See also Figure S7.
DISCUSSION
Evolutionary implications of a novel eukaryotic DNA methyltransferase
We unexpectedly found that a novel family of DNA methyltransferases, Dnmt5, catalyzes symmetrical CG methylation in diverse species (Figures 1 and 2). This discovery demonstrates that such DNA modification, considered a key mechanism for cellular inheritance of epigenetic information, is more common than previously thought and performed by at least two different methyltransferase families in eukaryotes. The presence of both Dnmt5 and Dnmt1 in some extant fungi and the generally widespread distribution of Dnmt5 (Figure 1B,C) suggest that Dnmt5 is ancient and may have coexisted with Dnmt1 in the last eukaryotic common ancestor.
Many lineages have independently lost Dnmt1 (Zemach and Zilberman, 2010) and Uhrf1 (Figure 1B,C). Our results show that CG methylation was not necessarily lost concomitantly. In such instances, the presence of Dnmt5 could have compensated for Dnmt1/Uhrf1 loss. For example, many basidiomycete fungi that methylate silenced TEs (Zemach et al., 2010) have both Dnmt1 and Dnmt5 (Iyer et al., 2011), but the lineage leading to C. neoformans apparently lost Dnmt1, yet still has CG methylation of silenced TEs (Figures 2A and S2B). Conversely, the presence of Dnmt1/Uhrf1 may have compensated for the loss of Dnmt5 in other lineages. Thus, the history of eukaryotic methylation is more complex than previously thought (Zemach and Zilberman, 2010), because there has been evolutionary turnover of the most basic methylation mechanisms.
We found genomic methylation in diatom TEs, corroborating a previous report examining P. tricornutum (Veluchamy et al., 2013). The lineage leading to diatoms diverged from plants, animals and fungi during the initial radiation of eukaryotes (Parfrey et al., 2011). Thus, our results lend further support to the idea that TE silencing was a root function of methylation in eukaryotes. It is even possible that the TE methylation in basidiomycete fungi (Zemach et al., 2010) that have both Dnmt1 and Dnmt5 represents an ancestral eukaryotic state. The presence of Dnmt5 may also explain the observation that the ascomycete fungus Uncinocarpus reesii has an apparently CG-specific repeat-induced point mutation process (Zemach et al., 2010).
CG-enriching sequence evolution
The genomes studied here harbor the highest CG dinucleotide frequencies known (Figure 6A). We found that accelerated gain and decelerated loss of CG dinucleotides (Figures 6B and S6B) combine to drive such distinctively enriched CG content. This process is not consistent with previously described mechanisms of sequence evolution (Charlesworth and Charlesworth, 2010; Hodgkinson and Eyre-Walker, 2011) and compromises the general assumption that genomic cytosine hypermutability is an inevitable consequence of C5 methylation (Pfeifer, 2006; Walsh and Xu, 2006).
Accelerated generation of CG sites may be driven by an overly efficient repair system that erroneously creates its repair product, as observed for very short patch repair in Escherichia coli (Gläsner et al., 1995; Walsh and Xu, 2006). Efficient CG repair would also explain decelerated loss of CG sites. The SNF2 regions of Dnmt5 proteins are related to enzymes (e.g. Rad5, Rad16 and human HLTF and SHPRH; Figure S1B) involved in various aspects of DNA repair (Unk et al., 2010), so it is tempting to speculate that multifunctional Dnmt5 enzymes could directly participate in a highly efficient CG repair system. However, any repair mechanism envisioned likely operates in both regions that are methylated and those that remain unmethylated (Figure S6B). One possible scenario is that the SNF2 region of Dnmt5 acquired sequence specificity by fusion to a CG-specific methyltransferase domain, which already could scan a genome in search of the appropriate sequences to methylate.
Interplay of DNA methylation and nucleosomes
Data from O. lucimarinus and M. pusilla show exquisite genomic exclusion of DNA methylation from the central regions of nucleosome cores (Figure 4A,C), consistent with previous reports that methyltransferase activities are reduced by positioned nucleosomes and that methylation tends to be in linkers (Felle et al., 2011; Gowher et al., 2005; Jiang et al., 2011; Kelly et al., 2012; Okuwaki and Verreault, 2004; Robertson et al., 2004; Takeshima et al., 2006, 2008). Therefore, the default for DNA methyltransferases appears to be that their activities are reduced by nucleosomes. In the case of Dnmt3, a single amino acid change that occurred in the evolution of mammalian Dnmt3b is sufficient to enhance enzymatic activity on nucleosome cores (Shen et al., 2010). Along similar lines, we highlighted candidate amino acids in the methyltransferase domains of Dnmt5 proteins that could function in the opposite direction, preventing Dnmt5 proteins from accessing nucleosome cores in those species with linker methylation (Figure S5). However, it is also possible that the SNF2 regions of Dnmt5 or additional accessory proteins are responsible for such exquisite sensitivity to nucleosomes.
We identified for the first time a chromomethylase outside of plants and green algae. Chromomethylase is targeted by histone H3 lysine 9 methylation in plants (Du et al., 2012). This modification is removed from plant genes, thus excluding chromomethylase (Huff and Zilberman, 2012; Inagaki et al., 2010), so abundant CHG methylation in E. huxleyi genes was not anticipated (Figure 3A,B). Furthermore, CHG methylation has a periodicity similar to CG methylation (Figure 3C), suggesting that in this organism chromomethylase is blocked by nucleosome cores, similar to Dnmt5.
Densely methylated DNA regions are relatively rigid, which may disfavor optimal curvature of DNA within nucleosomes (Pennings et al., 2005; Pérez et al., 2012). In addition, the methyl groups may locally alter the major and minor grooves, which appears especially detrimental to nucleosome stability where CG major grooves face toward the histones (Jimenez-Useche et al., 2013). Either or both of these effects would disfavor nucleosomes positioned over densely methylated regions, because such nucleosomes would either be unstable or fail to form in the first place. We tested this prediction genome-wide and demonstrated the ability of purified, natively methylated DNA to provide positioning information for nucleosomes formed from purified recombinant histones (Figure 5C–E). These results should be applicable to any system with similar biochemical properties, such as densely methylated regions in the human genome. More generally, we envision that methylation affects in vivo nucleosome positioning in parallel with other mechanisms, such as DNA sequence features and ATP-dependent nucleosome remodelers (Struhl and Segal, 2013).
We propose a model wherein densely methylated DNA clusters disfavor nucleosomes, on the one hand, and act in concert with nucleosomes that block methyltransferases, on the other, to constitute a mutually antagonistic loop contributing to the reliable nucleosome positioning observed across populations of cells (Figure 4). The heritable nature of symmetrical CG methylation and its effects on nucleosomes provide a plausible mechanism for epigenetic inheritance of nucleosome positions in eukaryotic species.
A previously unappreciated genome architecture
Periodically methylated algae share extremely compact nuclei (Figure 7). Extreme constraint results in special cellular requirements, such as a particularly compact mitotic spindle in the smallest known free-living eukaryote, Ostreococcus tauri (Gan et al., 2011). Analogously, dense periodic methylation could be a key component facilitating nuclear processes at extremely high DNA densities. For example, space may be saved by relying less on bulky ATP-dependent chromatin remodelers and more on dense DNA methylation for positioning nucleosomes. However, both an unconventional mitotic spindle and dense linker methylation are likely adaptations to a compact nucleus, rather than the cause of compaction. A compact nucleus is one component of small cell size, which generally results in other cellular properties, such as better light absorption, faster growth rate and lower sinking velocity (Finkel et al., 2010), which may contribute to the evolutionary success of these algae.
We identified periodic methylation in multiple species from the Mamiellophyceae (includes the prasinophytes B. prasinos, O. lucimarinus and M. pusilla) and Prymnesiophyceae classes. The simplest explanation is that periodic methylation goes back hundreds of millions of years to the respective evolutionary radiations of these groups (Figure S4). With periodic methylation in a third group (Figures 3A,B and S3B; A. anophagefferens is a member of the class Pelagophyceae), our data indicate either remarkably convergent genomic evolution or retention from common ancestors of periodic methylation in at least three classes that diverged from each other more than a billion years ago, a timescale comparable to the separation of plants and animals (Figure 1B). Thus, periodic methylation and associated chromosomal and nuclear features constitute an ancient genome architecture that may date back to the initial eukaryotic radiation.
EXPERIMENTAL PROCEDURES
Trees
Divergence time trees were adapted largely from previous estimates (Parfrey et al., 2011). For phylogenetic trees, amino acid sequences were aligned and trimmed to include only the alignable portions, and long-branch wandering sequences and the longest-branch paralogs from the same species were removed. Maximum likelihood phylogenetic trees were inferred using RAxML v7 (Stamatakis, 2006). Additional information on tree construction is available in the Extended Experimental Procedures.
Nucleic acids and cells
Marine algal genomic DNA, total RNA, and frozen cells were obtained directly from the Provasoli-Guillard National Center for Marine Algae and Microbiota: A. anophagefferens, CCMP1984; B. prasinos, CCMP1898; E. huxleyi, CCMP1516; F. cylindrus, CCMP1102; I. rotunda, CCMP456; I. galbana, CCMP1323; M. pusilla, CCMP1545; O. lucimarinus, CCMP2972; P. tricornutum, CCMP632; P. parkeae, CCMP725; T. pseudonana, CCMP1335. C. neoformans var. grubii H99 WT DNA (208821D) and L. major strain Seidman DNA (PRA-309D) were obtained from ATCC. Published (Liu et al., 2008) C. neoformans var. grubii H99 control (WT47; sxi1Δ) and dnmt5Δ (D632) strains were obtained from the Fungal Genetics Stock Center. A live culture of C. merolae (NIES-1332) was obtained from the Microbial Culture Collection at the National Institute for Environmental Studies, Japan.
Sequencing experiments
Assaying cytosine methylation from genomic DNA by bisulfite sequencing was performed as described (Ibarra et al., 2012). Total RNA was used to prepare strand-specific sequencing libraries with the Encore Complete RNA-Seq Library System I (NuGEN). Assaying nucleosome positions in vivo by MNase sequencing was performed by modifiying an existing protocol (Teves and Henikoff, 2012). For in vitro nucleosome position analyses, we first generated an unmethylated equivalent of O. lucimarinus genomic DNA by in vitro replication with unmethylated deoxynucleotides, such that approximately 95% of the resulting DNA molecules contain entirely unmethylated cytosines. Natively methylated O. lucimarinus genomic DNA or the unmethylated equivalent was assembled with purified recombinant histones into nucleosomes by salt dilution using an EpiMark Nucleosome Assembly Kit (New England Biolabs). Assembled nucleosome positions were mapped by MNase sequencing, similar to the procedure for analyzing in vivo positions. All sequencing was performed with the Illumina HiSeq 2000 or 2500 platforms. Details are available in the Extended Experimental Procedures.
Data access
Protein accessions and phylogenetic trees are available from TreeBASE (Study 15142). Sequencing data are available from GEO (GSE46692).
Supplementary Material
HIGHLIGHTS.
Dnmt5 is a novel eukaryotic family of symmetrical CG methyltransferases
Numerous Dnmt5-containing species have dense methylation of nucleosomal linkers
Dense DNA methylation directly disfavors nucleosomes
Dense methylation is enabled by a novel evolutionary regime of genomic CG enrichment
Acknowledgments
We thank C. Schuman at the Provasoli-Guillard National Center for Marine Algae and Microbiota for biological materials; the Fungal Genetics Stock Center for C. neoformans strains; the Vincent J. Coates Genomics Sequencing Laboratory for sequencing; A. Zemach, T.-F. Hsieh and S. Branco for experimental advice; T. Nishimura for computational help; and R. Fischer, A. Plocik and S. Roy for critical reading of the manuscript. This work was supported by a Young Investigator award from the Arnold and Mabel Beckman Foundation to D.Z. and a genomics training grant from the National Institutes of Health to J.T.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang GS, Noegel AA, Mavrich TN, Müller R, Tomsho L, Ward E, Felder M, Jiang C, Eichinger L, Glöckner G, et al. Unusual combinatorial involvement of poly-A/T tracts in organizing genes and chromatin in Dictyostelium. Genome Res. 2012;22:1098–1106. doi: 10.1101/gr.131649.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. Elements of Evolutionary Genetics. Roberts and Company Publishers; 2010. [Google Scholar]
- Chodavarapu RK, Feng S, Bernatavichute YV, Chen PY, Stroud H, Yu Y, Hetzel JA, Kuo F, Kim J, Cokus SJ, et al. Relationship between nucleosome positioning and DNA methylation. Nature. 2010;466:388–392. doi: 10.1038/nature09147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NM, Kenigsberg E, Tanay A. Primate CpG islands are maintained by heterogeneous evolutionary regimes involving minimal selection. Cell. 2011;145:773–786. doi: 10.1016/j.cell.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Coleman-Derr D, Zilberman D. DNA methylation, H2A.Z, and the regulation of constitutive expression. Cold Spring Harb Symp Quant Biol. 2012 doi: 10.1101/sqb.2012.77.014944. [DOI] [PubMed] [Google Scholar]
- Collings CK, Waddell PJ, Anderson JN. Effects of DNA methylation on nucleosome stability. Nucleic Acids Res. 2013;41:2918–2931. doi: 10.1093/nar/gks893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhong X, Bernatavichute YV, Stroud H, Feng S, Caro E, Vashisht AA, Terragni J, Chin HG, Tu A, et al. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell. 2012;151:167–180. doi: 10.1016/j.cell.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle M, Hoffmeister H, Rothammer J, Fuchs A, Exler JH, Längst G. Nucleosomes protect DNA from DNA methylation in vivo and in vitro. Nucleic Acids Res. 2011;39:6956–6969. doi: 10.1093/nar/gkr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Cokus SJ, Zhang X, Chen PY, Bostick M, Goll MG, Hetzel J, Jain J, Strauss SH, Halpern ME, et al. Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A. 2010;107:8689–8694. doi: 10.1073/pnas.1002720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, Behrenfeld, Randerson, Falkowski Primary production of the biosphere: integrating terrestrial and oceanic components. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- Finkel ZV, Beardall J, Flynn KJ, Quigg A, Rees TAV, Raven JA. Phytoplankton in a changing world: cell size and elemental stoichiometry. J Plankton Res. 2010;32:119–137. [Google Scholar]
- Galagan JE, Selker EU. RIP: the evolutionary cost of genome defense. Trends Genet TIG. 2004;20:417–423. doi: 10.1016/j.tig.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Gan L, Ladinsky MS, Jensen GJ. Organization of the smallest eukaryotic spindle. Curr Biol CB. 2011;21:1578–1583. doi: 10.1016/j.cub.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläsner W, Merkl R, Schellenberger V, Fritz HJ. Substrate preferences of Vsr DNA mismatch endonuclease and their consequences for the evolution of the Escherichia coli K-12 genome. J Mol Biol. 1995;245:1–7. doi: 10.1016/s0022-2836(95)80033-6. [DOI] [PubMed] [Google Scholar]
- Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- Gowher H, Stockdale CJ, Goyal R, Ferreira H, Owen-Hughes T, Jeltsch A. De novo methylation of nucleosomal DNA by the mammalian Dnmt1 and Dnmt3A DNA methyltransferases. Biochemistry (Mosc) 2005;44:9899–9904. doi: 10.1021/bi047634t. [DOI] [PubMed] [Google Scholar]
- Henderson GP, Gan L, Jensen GJ. 3-D ultrastructure of O. tauri: electron cryotomography of an entire eukaryotic cell. PloS One. 2007;2:e749. doi: 10.1371/journal.pone.0000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson A, Eyre-Walker A. Variation in the mutation rate across mammalian genomes. Nat Rev Genet. 2011;12:756–766. doi: 10.1038/nrg3098. [DOI] [PubMed] [Google Scholar]
- Huff JT, Zilberman D. Regulation of biological accuracy, precision, and memory by plant chromatin organization. Curr Opin Genet Dev. 2012;22:132–138. doi: 10.1016/j.gde.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Ibarra CA, Feng X, Schoft VK, Hsieh TF, Uzawa R, Rodrigues JA, Zemach A, Chumak N, Machlicova A, Nishimura T, et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science. 2012;337:1360–1364. doi: 10.1126/science.1224839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki S, Miura-Kamio A, Nakamura Y, Lu F, Cui X, Cao X, Kimura H, Saze H, Kakutani T. Autocatalytic differentiation of epigenetic modifications within the Arabidopsis genome. EMBO J. 2010;29:3496–3506. doi: 10.1038/emboj.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Abhiman S, Aravind L. Natural history of eukaryotic DNA methylation systems. Prog Mol Biol Transl Sci. 2011;101:25–104. doi: 10.1016/B978-0-12-387685-0.00002-0. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Schneck JL, Grimes M, Taylor AN, Hou W, Thrall SH, Sweitzer SM. Methyltransferases prefer monomer over core-trimmed nucleosomes as in vitro substrates. Anal Biochem. 2011;415:84–86. doi: 10.1016/j.ab.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Jimenez-Useche I, Ke J, Tian Y, Shim D, Howell SC, Qiu X, Yuan C. DNA methylation regulated nucleosome dynamics. Sci Rep. 2013;3:2121. doi: 10.1038/srep02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Kaplan N, Moore IK, Fondufe-Mittendorf Y, Gossett AJ, Tillo D, Field Y, LeProust EM, Hughes TR, Lieb JD, Widom J, et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature. 2009;458:362–366. doi: 10.1038/nature07667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TK, Liu Y, Lay FD, Liang G, Berman BP, Jones PA. Genome-wide mapping of nucleosome positioning and DNA methylation within individual DNA molecules. Genome Res. 2012;22:2497–2506. doi: 10.1101/gr.143008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leforestier A, Livolant F. Liquid crystalline ordering of nucleosome core particles under macromolecular crowding conditions: evidence for a discotic columnar hexagonal phase. Biophys J. 1997;73:1771–1776. doi: 10.1016/S0006-3495(97)78207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135:174–188. doi: 10.1016/j.cell.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HK, Sims D, Heger A, Blackledge NP, Kutter C, Wright ML, Grützner F, Odom DT, Patient R, Ponting CP, et al. Epigenetic conservation at gene regulatory elements revealed by non-methylated DNA profiling in seven vertebrates. eLife. 2013;2013:e00348. doi: 10.7554/eLife.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko F, Maleszka R. Insects as innovative models for functional studies of DNA methylation. Trends Genet. 2011;27:127–131. doi: 10.1016/j.tig.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Chepelev I, Cui K, Zhao K. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013 doi: 10.1038/cr.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuwaki M, Verreault A. Maintenance DNA methylation of nucleosome core particles. J Biol Chem. 2004;279:2904–2912. doi: 10.1074/jbc.M310111200. [DOI] [PubMed] [Google Scholar]
- Palenik B, Grimwood J, Aerts A, Rouzé P, Salamov A, Putnam N, Dupont C, Jorgensen R, Derelle E, Rombauts S, et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci U S A. 2007;104:7705–7710. doi: 10.1073/pnas.0611046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfrey LW, Lahr DJG, Knoll AH, Katz LA. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci U S A. 2011;108:13624–13629. doi: 10.1073/pnas.1110633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings S, Allan J, Davey CS. DNA methylation, nucleosome formation and positioning. Brief Funct Genomic Proteomic. 2005;3:351–361. doi: 10.1093/bfgp/3.4.351. [DOI] [PubMed] [Google Scholar]
- Pérez A, Castellazzi CL, Battistini F, Collinet K, Flores O, Deniz O, Ruiz ML, Torrents D, Eritja R, Soler-López M, et al. Impact of methylation on the physical properties of DNA. Biophys J. 2012;102:2140–2148. doi: 10.1016/j.bpj.2012.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer GP. Mutagenesis at methylated CpG sequences. Curr Top Microbiol Immunol. 2006;301:259–281. doi: 10.1007/3-540-31390-7_10. [DOI] [PubMed] [Google Scholar]
- Ponger L, Li WH. Evolutionary diversification of DNA methyltransferases in eukaryotic genomes. Mol Biol Evol. 2005;22:1119–1128. doi: 10.1093/molbev/msi098. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Geiman TM, Sankpal UT, Hager GL, Robertson KD. Effects of chromatin structure on the enzymatic and DNA binding functions of DNA methyltransferases DNMT1 and Dnmt3a in vitro. Biochem Biophys Res Commun. 2004;322:110–118. doi: 10.1016/j.bbrc.2004.07.083. [DOI] [PubMed] [Google Scholar]
- Schübeler D. Epigenetic islands in a genetic ocean. Science. 2012;338:756–757. doi: 10.1126/science.1227243. [DOI] [PubMed] [Google Scholar]
- Sharp PM, Tuohy TM, Mosurski KR. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986;14:5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Gao G, Zhang Y, Zhang H, Ye Z, Huang S, Huang J, Kang J. A single amino acid substitution confers enhanced methylation activity of mammalian Dnmt3b on chromatin DNA. Nucleic Acids Res. 2010;38:6054–6064. doi: 10.1093/nar/gkq456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature. 2011;479:74–79. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Haussler D. Phylogenetic estimation of context-dependent substitution rates by maximum likelihood. Mol Biol Evol. 2004;21:468–488. doi: 10.1093/molbev/msh039. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinforma Oxf Engl. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Struhl K, Segal E. Determinants of nucleosome positioning. Nat Struct Mol Biol. 2013;20:267–273. doi: 10.1038/nsmb.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Suetake I, Shimahara H, Ura K, Tate S, Tajima S. Distinct DNA methylation activity of Dnmt3a and Dnmt3b towards naked and nucleosomal DNA. J Biochem (Tokyo) 2006;139:503–515. doi: 10.1093/jb/mvj044. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Suetake I, Tajima S. Mouse Dnmt3a preferentially methylates linker DNA and is inhibited by histone H1. J Mol Biol. 2008;383:810–821. doi: 10.1016/j.jmb.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Teves SS, Henikoff S. Salt fractionation of nucleosomes for genome-wide profiling. Methods Mol Biol Clifton NJ. 2012;833:421–432. doi: 10.1007/978-1-61779-477-3_25. [DOI] [PubMed] [Google Scholar]
- Unk I, Hajdú I, Blastyák A, Haracska L. Role of yeast Rad5 and its human orthologs, HLTF and SHPRH in DNA damage tolerance. DNA Repair. 2010;9:257–267. doi: 10.1016/j.dnarep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Veluchamy A, Lin X, Maumus F, Rivarola M, Bhavsar J, Creasy T, O’Brien K, Sengamalay NA, Tallon LJ, Smith AD, et al. Insights into the role of DNA methylation in diatoms by genome-wide profiling in Phaeodactylum tricornutum. Nat Commun. 2013;4:2091. doi: 10.1038/ncomms3091. [DOI] [PubMed] [Google Scholar]
- Walsh CP, Xu GL. Cytosine methylation and DNA repair. Curr Top Microbiol Immunol. 2006;301:283–315. doi: 10.1007/3-540-31390-7_11. [DOI] [PubMed] [Google Scholar]
- Worden AZ, Lee JH, Mock T, Rouzé P, Simmons MP, Aerts AL, Allen AE, Cuvelier ML, Derelle E, Everett MV, et al. Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science. 2009;324:268–272. doi: 10.1126/science.1167222. [DOI] [PubMed] [Google Scholar]
- Zemach A, Zilberman D. Evolution of eukaryotic DNA methylation and the pursuit of safer sex. Curr Biol CB. 2010;20:R780–785. doi: 10.1016/j.cub.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328:916–919. doi: 10.1126/science.1186366. [DOI] [PubMed] [Google Scholar]
- Zemach A, Kim MY, Hsieh PH, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer SL, Zilberman D. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153:193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.