Abstract
Rationale
Given evidence for age-related differences in the effects of drugs of abuse, surprisingly few preclinical studies have explored effects of opioids in adolescents (versus adults).
Objectives
This study compared the motor stimulating and ataxic effects of repeatedly-administered morphine in adolescent, late-adolescent, and adult mice.
Methods
Mice were treated with saline or morphine (10–100 mg/kg, i.p.) once per day for 4 days, and morphine (3.2–56 mg/kg)-induced locomotion was assessed 3 days or 5 weeks later. Different mice were treated repeatedly with morphine and ataxia was measured.
Results
Acute administration of morphine increased locomotion more in adolescents than in adults. Repeated morphine enhanced morphine-induced locomotion, assessed 3 days later, to a similar extent in each age group (minimum effective dose: 17.8 mg/kg). This sensitization was still evident 5 weeks later when the adolescents had become adult, but was smaller and occurred at a higher dose (56 mg/kg). In animals treated repeatedly with morphine as adults, sensitization was no longer apparent 5 weeks later. Intermittent morphine was at least 10-fold less potent to produce body weight loss in adolescents than in adults. Repeated morphine did not alter morphine-induced ataxia at any age.
Conclusions
Compared with adults, adolescents were more sensitive to the acute locomotor stimulating effects of morphine and to its long-lasting locomotor sensitizing effects, consistent with overactivity of dopamine systems during adolescence. In contrast, adolescents were less sensitive than adults to body weight loss induced by intermittent morphine, an effect indicative of morphine withdrawal in adult rodents.
Keywords: Morphine, Locomotion, Ataxia, Sensitization, Withdrawal, Adolescent, Adult, Mouse
The misuse and abuse of prescription opioids, particularly in adolescents, has become a major public health concern (Compton and Volkow 2006). Opioid analgesic abuse is particularly problematic for adolescents because of uncertain implications for future addiction. Like other drug-related conditions, opioid analgesic abuse is mostly concentrated in adolescents and young adults (Substance Abuse and Mental Health Services Administration 2003), yet little is known about opioid effects in these age groups. Most of what we know about opioid abuse and addiction has been learned from heroin addiction in 20 to 40-year-old individuals, and from research in adult animals.
Most drugs of abuse, including prescription opioids, interact with the dopamine system. This system changes during development, and appears to be overactive during adolescence (Wahlstrom et al. 2010). This overactivity could be involved in the different balance of rewarding and aversive effects of drugs of abuse in adolescents compared with adults (Schramm-Sapyta et al. 2009): adolescents may be more sensitive to the rewarding effects of drugs and less sensitive to aversive effects. Evidence for this differential sensitivity has been obtained in animal models with nicotine (Shram et al. 2006), ethanol (Philpot et al. 2003), and cocaine (Badanich et al. 2006; Schramm-Sapyta et al. 2006). Unfortunately, only a few studies have compared dopamine-related effects of opioids in adolescent and adult rats (for example, see White and Holtzman 2005; White et al. 2008; Doherty and Frantz 2012, 2013) or in adolescent or adult mice (for example, see Hodgson et al. 2009; Zhang et al. 2009).
The present study is part of an effort to examine effects of morphine in adolescent and adult mice. Previously, acute administration of morphine was found to stimulate locomotion with similar potency in all age groups (Koek et al., 2012). However, maximal stimulation was higher in adolescents than in late adolescents, and higher in late adolescents than in adults. Morphine stimulates dopamine systems indirectly by inhibiting GABAergic interneurons that inhibit dopaminergic neurons (Johnson and North, 1992). Overactivity of this system during adolescence (Wahlstrom et al., 2010) conceivably underlies age differences in the acute effects of morphine on locomotion (Koek et al., 2012). When administered repeatedly, the locomotor-stimulating effects of many drugs of abuse are enhanced. This sensitization is thought to reflect long-lasting brain changes involved in addiction (e.g., Robinson and Berridge, 2003). For a recent review of sensitization processes in drug addiction, see Vanderschuren and Pierce (2010). Whereas repeated administration of morphine is known to produce locomotor sensitization in adult rodents (e.g., Kalivas and Duffy 1987; Kuribara 1996; Vanderschuren 1997), only a few studies have compared morphine-induced locomotor sensitization in adolescent and adult rats (White and Holtzman 2005; White et al. 2008; Hofford et al. 2012; Doherty and Frantz 2013), and studies in mice are lacking.
The goal of this study was to examine the effects of repeated administration of morphine on morphine-induced locomotion in adolescent and adult mice. To broadly cover the adolescent period, the study examined mice between postnatal days 28–42, described as prototypical adolescence by Spear (2000), and mice between postnatal days 44–58, described as late adolescence (Adriani et al. 2002). Often, effects of repeated drug administration are examined by studying only a single dose. Results of such studies can be difficult to interpret, especially when an initially increasing dose-response function inverts and decreases at higher doses; in such a case, a horizontal shift of the dose-response curve implies increased effects of some doses, but decreased effects of others (see Corfield-Sumner and Stolerman 1978; Carlton 1983). Because morphine stimulates locomotion along an inverted U-shaped dose-response curve (Koek et al. 2012), the present study examined the ability of repeated morphine to shift its dose-response curve for locomotor stimulation. Sensitization was examined 3 days after repeated drug administration (experiment 1) and, to investigate possible long-lasting effects, 5 weeks after repeated drug administration, when adolescents had become adult (experiment 2). Locomotor activity was measured also during repeated drug administration, to examine dose- and age-related effects of morphine on the onset of locomotor sensitization.
Upward shifts of biphasic dose-response curves are often thought to evidence sensitization, but could also be due to tolerance to effects responsible for the descending part of the dose-response curve (Zernig et al. 2004). Because morphine-induced locomotion is likely limited at higher doses by morphine-induced ataxia, the effects of repeated administration on morphine-induced ataxia were studied to examine the involvement of tolerance to morphine-induced ataxia in upward shifts of the dose-response curve for morphine-induced locomotion (experiment 3).
When drugs are administered intermittently, spontaneous withdrawal may occur. To explore the possible occurrence of withdrawal under the conditions of the present experiments, morphine-induced body weight loss, an effect indicative of spontaneous withdrawal in adult male C57BL/6J mice (Papaleo and Contarino, 2006), was measured.
Methods
Subjects
Adolescent (postnatal day 28–42), late adolescent (postnatal day 44–58), and adult (postnatal day 64–78) male C57BL/6J mice were obtained by breeding C57BL/6J mice from the Jackson Laboratory (Bar Harbor, ME). Housing and rearing conditions were identical to those described in Koek et al. (2012). Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996).
Procedures
Locomotor activity
Locomotor activity was assessed using eight acrylic boxes equipped with infrared light beams and enclosed separately in sound-attenuating chambers [for details, see Koek et al. (2012)]. On the first day of the experiment, basal activity was measured for 2 h. During each of the next four days, animals received an i.p. injection of saline or a particular dose of morphine (10, 17.8, 32, 56, 100 mg/kg) and activity was measured for 2 h. Three days (experiment 1) or 5 weeks (experiment 2) later, animals in each repeated treatment condition received an i.p. injection of saline or a particular test dose of morphine (3.2, 5.6, 10, 17.8, 32, 56 mg/kg; n=6 per test dose) and activity was again measured for 2 h.
Ataxia
In the horizontal wire test, conducted as described in Koek et al. (2012), mice grasp a horizontally wire with both forepaws. If they did not grasp the wire with at least one hindpaw they failed the test (scored as 1). During each of four consecutive days, mice were tested before and repeatedly (at 15, 30, 45, 60, 90, and 120 min) after an i.p. injection of saline or of 100 mg/kg morphine (n=20), a dose that produces maximal ataxia in adolescent, late adolescent, and adult mice (Koek et al., 2012).
Data analyses
ANOVAs were conducted using NCSS 2007 for windows (NCSS, Kaysville, Utah, USA). Repeated measures ANOVA used the Geisser-Greenhouse adjustment to correct for possible violations of sphericity. Locomotion on the first day of the experiment was analyzed by means of one-factor ANOVA with age (adolescent, late adolescent, adult) as between-subjects factor. There were significant baseline differences in locomotion among the age groups during the first day (consistent with previous findings reported in Koek et al., 2012), and during the next four days in animals treated repeatedly with saline (see Results). Therefore, drug effects on locomotion were expressed as a percentage of locomotion in saline controls [for additional details of this approach, see results and discussion in Koek et al. (2012)]. Locomotion during the four repeated treatment days was analyzed separately for each repeated treatment condition (i.e., saline, or 10–100 mg/kg morphine) by two-factor ANOVA with age as between-subjects factor and day (2–5) as within-subjects factor. Locomotion during the test day, which was conducted 3 days (experiment 1) or 5 weeks (experiment 2) after the last repeated treatment day, was analyzed separately for each repeated treatment dose and each age group by two-factor ANOVA with repeated treatment dose and test dose as between-subjects factors. Comparisons among age groups were conducted with the Tukey test, and doses were compared with saline by Dunnett’s test.
Morphine-induced locomotion on the test day was analyzed also by plotting mean locomotion as a function of morphine test dose separately for each repeated treatment dose and each age group, and by calculating for each dose-response curve the area under the curve (AUC) using the trapezoid rule (GraphPad Prism 6.01 for Windows; GraphPad Software, La Jolla, CA, USA). Differences between the AUC values obtained after repeated treatment with morphine or saline were plotted as a function of the repeated treatment dose to obtain a dose-response curve for morphine to induce sensitization in each age group. These dose-response curves were analyzed by fitting a straight line to the linear part of each curve, and by using the F ratio test in Prism to compare the slopes of these lines. Dose-response data obtained in adolescent animals were compared with those obtained in adults by simultaneously fitting straight lines with a common slope to the data and expressing the logED50 of the curve in adolescents as the sum of the logED50 of the curve in adults and the log of the potency ratio, which yielded an estimate of the potency ratio and its 95% confidence limits (see EC50 shift equation in Prism). Potency differences were considered statistically significant if the 95% confidence interval of the potency ratio did not include 1. To examine the generality of drug effects on test days expressed as percentage of saline controls, morphine-induced locomotion was analyzed also by expressing drug effects as difference from saline controls.
Morphine-induced ataxia was examined by summing the scores obtained from 0 to 120 min post-injection for each animal (maximum = 6). Summed scores were analyzed separately for each age group by two-factor ANOVA with injection condition as between-subjects factor and day (1–4) as within-subjects factor. Results obtained during days 2–4 with those obtained during day 1 were compared by Tukey’s test. Age effects were examined by two-factor ANOVA, with age and injection condition as between-subjects factors, performed on ataxia scores averaged across days 1–4.
Morphine-induced body weight loss was examined by subtracting the body weight immediately before each daily injection from the body weight measured on day 1 of the experiment, and by expressing the difference as a percentage of the body weight on day 1. These percentages were analyzed separately for each age group by two-factor ANOVA with dose as between-subjects factor and day as within-subjects factor. Dunnett’s test compared results obtained at each dose with saline control data.
Drugs
Morphine sulfate (National Institute on Drug Abuse, Research Technology Branch, Research Triangle Park, NC) was dissolved in physiological saline and injected i.p. in a volume of 10 ml/kg.
Results
Locomotor activity: test 3 days after repeated treatment (experiment 1)
During the initial 2 h exposure to the activity chambers on day 1, basal locomotion was significantly lower in adolescents compared with adults [F(2,756)=272.5, p<0.0001] (data not shown). Adolescents were significantly less active than late adolescents, and late adolescents significantly less than adults (Tukey test): mean beam breaks (95% confidence limits) were 2244 (2193–2348) in adolescents, 3569 (3436–3701) in late adolescents, and 4473 (4318–4627) in adults. Similar age-related activity differences were also apparent during subsequent sessions on days 2–5 in animals that received an injection of saline before each session (Fig. 1, upper left panel). In these animals, activity was significantly affected by age [F(2,119)=15.74, p<0.0001], session [F(3,357)=4.50, p=0.0058], and an age by session interaction [F(6,357)=2.35, p=0.037]: adolescents, but not late adolescents, showed significantly less activity than adults during each of the four sessions. Because of these age- and session-related differences, drug effects on locomotion were not expressed as a percentage of within-subjects basal locomotion assessed on day 1, but as a percentage of locomotion in saline controls at each session, as was done in the previous study of locomotion induced by a single administration of morphine in mice (Koek et al., 2012).
Fig. 1.
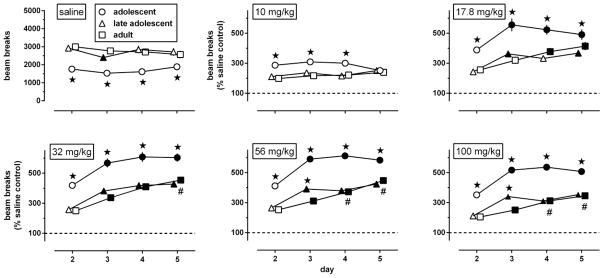
Locomotor activity, measured as number of breaks of horizontal infrared beams (upper left panel) or expressed as a percentage of the data obtained in corresponding age-matched saline controls (all other panels) during 4 daily 2h exposures to the activity chambers immediately after an i.p. injection of saline (upper left panel) or morphine (10–100 mg/kg; all other panels) in adolescent, late adolescent, and adult male C57/BL6J mice (n=42 per group). Results are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Asterisks indicate p<0.05 compared with adults, filled symbols indicate p<0.05 compared with day 2, and pound signs indicate p<0.05 compared with day 3.
Repeated administration of morphine stimulated locomotion in an age- and daily session-related manner (Fig. 1, upper middle, upper right, and lower panels). The effects of morphine depended on age [F(2,124)≥8.02, p<0.001]: all doses stimulated locomotion significantly more in adolescents than in the other age groups; in late adolescents 56 and 100 mg/kg morphine stimulated locomotion more than in adults, but only on day 3. Locomotor sensitization did not occur after repeated administration of 10 mg/kg morphine [main effect of day: F(3,372)=1.66, p=0.18], but became apparent at doses of 17.8 mg/kg and higher [main effect of day: F(3,384)≥25.11, p<0.0001]. These latter doses stimulated locomotion significantly more after the second injection (on day 3) than after the first (on day 2), in all age groups, except in adults treated with 17.8 mg/kg. Age effects interacted significantly with those of repeated administration of 17.8, 56, and 100 mg/kg [F(6,384)≥2.80, p<0.02], but not of 32 mg/kg [F(6,327)=1.17, p=0.32]. In adolescents and late adolescents the effects of repeated administration reached an apparent maximum after the second injection (day 3), but in adults the effects continued to increase after subsequent injections (days 4 and 5).
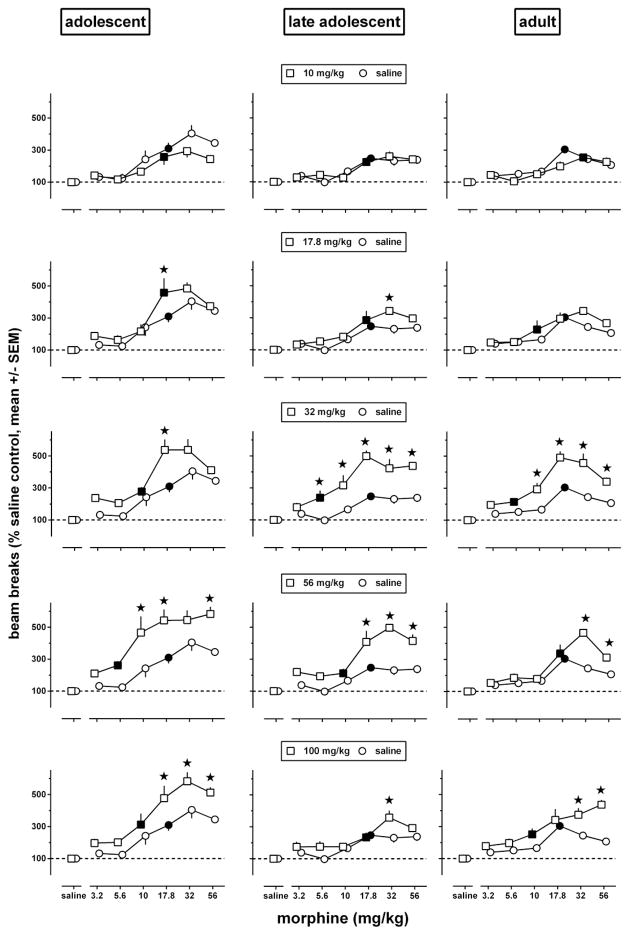
The data in Fig. 1 show sensitization as enhanced effects of particular doses. When the basic dose-response curve is biphasic, as it is for morphine-induced locomotion (e.g., Koek et al. 2012), dose-response data are necessary to characterize sensitization. Dose-response data shown in Fig. 2 were obtained during a test session on day 8, 3 days after repeated treatment with morphine ended. The dose-response curves obtained after repeated administration of saline in each age group (Fig. 2, circles, re-plotted in each row to facilitate comparisons with repeated administration of morphine) had the same minimum significant dose (i.e., 17.8 mg/kg), appeared biphasic, attained significantly higher levels in adolescents than in late adolescents and adults (at 32 and 56 mg/kg), and were generally shifted upward and often to the left in each of the age groups by repeated treatment with morphine at doses of 17.8 mg/kg and higher. Each age group showed significant effects of repeated treatment [F(5,209)≥21.0, p<0.0001], test dose [F(6,209)≥72, p<0.0001], and repeated treatment by test dose interaction [F(30,209)≥1.81, p≤0.02].
Fig. 2.
Locomotor activity in adolescent (left panels), late adolescent (middle panels), and adult (right panels) male C57/BL6J mice during a 2h exposure to the activity chambers immediately after an i.p. injection of morphine or its vehicle (n=6 per dose; each animal received only one injection), 3 days after repeated administration of saline (circles, re-plotted in each row to facilitate comparisons with repeated administration of morphine) or morphine [squares, one repeatedly administered dose (10–100 mg/kg) per row]. Results are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Data obtained in morphine-treated animals were expressed as a percentage of the data obtained in their corresponding age-matched saline controls. Asterisks indicate p<0.05 of differences between animals repeatedly treated with morphine (squares) or saline (circles), and filled symbols indicate the minimum significant dose (p<0.05) of each dose-response curve.
For each of the curves shown in Fig. 2 an AUC value was calculated. These AUC values were used to quantify sensitization in each age group by subtracting the value obtained after repeated administration of saline from the values obtained after each of the repeatedly administered doses of morphine. The AUC value differences were plotted as a function of the repeated treatment dose, yielding in each age group a dose-response curve for morphine to induce locomotor sensitization (Fig. 3). This dose-response relation was biphasic in each of the age groups. The ascending part of the dose-response curves could be fitted by straight lines with slopes that did not differ significantly among the age groups [F(2,4)=0.10, p=0.90]. Straight lines with a common slope (1600, 95% CL 1300–2000) adequately (R2: 0.94–0.98) fitted the data and yielded a potency ratio (95% CL) of 1.1 (0.80–1.4) when comparing adolescents with adults, and a potency ratio of 1.2 (0.76–1.6) when comparing late adolescents with adults. Because each of these 95% CL included 1, the results indicate that the potency with which morphine induced locomotor sensitization did not differ significantly among adolescents, late adolescents, and adults. The ED50 of morphine to produce locomotor sensitization, calculated by using a maximal effect averaged across age groups, was 22 (20–26) mg/kg. The maximal locomotor sensitization produced by morphine attained a value of 710 in adolescents and 880 in late adolescents at a dose of 32 mg/kg, and attained a value of 930 in adolescents at a dose of 56 mg/kg. The descending part of the dose-response curves suggested the possibility that the potency with which morphine produced effects that appeared to limit locomotor sensitization differed among the age groups. The descending part of the dose-response curves could be fitted with straight lines with slopes that were not significantly different [F(2,2)=0.67, p=0.60]. Straight lines with a common slope (−1200, 95% CL −2300, −140) adequately fitted the adolescent and late adolescent data (R2: 0.91–0.99) but not the data in adults (R2: 0.24), thereby precluding statistical comparisons of potency differences with adults. When comparing adolescents with late adolescents, the obtained potency ratio (1.8, 0.4–3.2) did not differ significantly from 1.
Fig. 3.
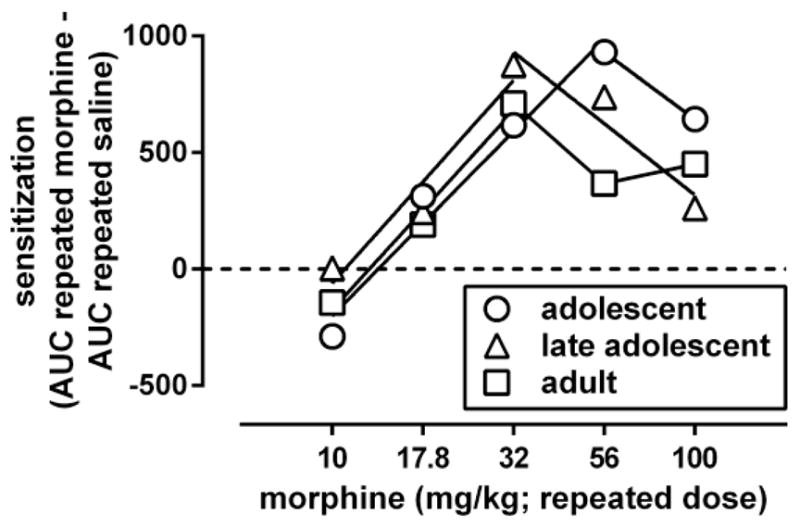
Dose-response curves for morphine to induce locomotor sensitization 3 days after its repeated administration in adolescent, late adolescent, and adult male C57/BL6J mice. Ordinate: sensitization, measured by the difference between the area under the curve (AUC) for the morphine dose-response curve obtained after repeated treatment with morphine and the morphine dose-response curve obtained after repeated treatment with saline (shown in Fig. 2). Abscissa: dose of morphine that was administered repeatedly. Dose-response data were analyzed by fitting straight lines through the ascending and descending parts of the curves.
Locomotor activity: test 5 weeks after repeated treatment with morphine (experiment 2)
The second experiment differed from the first experiment in the length of the interval between the repeated treatment with morphine and the subsequent testing of the sensitivity to the locomotor-enhancing effects of morphine. Also, the second experiment was conducted in adolescents and adults but not in late adolescents, because adolescents differed more markedly from adults than late adolescents with respect to motor effects of morphine (first experiment in the present study; Koek et al., 2012). In addition, the effects of repeated treatment were examined over a smaller dose range (i.e., 32–100 mg/kg) than in the first experiment (10–100 mg/kg), because initial results suggested that repeatedly administered morphine was less potent to produce sensitization 5 weeks later than 3 days later.
During days 1 to 5, the animals in experiment 2 were treated identically to those used in experiment 1, and the results shown in Fig. 4 were therefore obtained under the same conditions as the results shown in Fig. 1. Like in experiment 1, adolescents that received an injection of saline before each session on days 2–5 in experiment 2 (Fig. 4, upper left panel) showed significantly less activity than adult saline controls [age: F(1,64)=28.35, p<0.0001; session: F(3,192)=1.82, p=0.16; age by session: F(3,192)=2.33, p=0.088]. Repeated administration of morphine stimulated locomotion in an age- and daily session-related manner (Fig. 4). All three doses tested produced locomotor sensitization [main effect of day: F(3,189)≥83.01, p<0.0001] and stimulated locomotion significantly more after the second injection (on day 3) than after the first (on day 2) in both age groups. Morphine age-dependently increased locomotion [main effect of age: F(1,68)≥145.7, p<0.0001], and all doses stimulated locomotion significantly more in adolescents than in adults. Age by day interactions were statistically significant at each dose of morphine [F(3,189)≥4.64, p<0.015]. In both age groups, the effects of repeated administration did not reach an apparent maximum after the second injection (day 3), but continued to increase after subsequent injections.
Fig. 4.
Locomotor activity, measured as number of breaks of horizontal infrared beams (upper left panel) or expressed as a percentage of the data obtained in corresponding age-matched saline controls (all other panels) during 4 daily 2h exposures to the activity chambers immediately after an i.p. injection of saline (upper left panel) or morphine (32–100 mg/kg; all other panels) in adolescent and adult male C57/BL6J mice (n=42 per group). Results are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Asterisks indicate p<0.05 compared with adults, filled symbols indicate p<0.05 compared with day 2, and pound signs indicate p<0.05 compared with day 3.
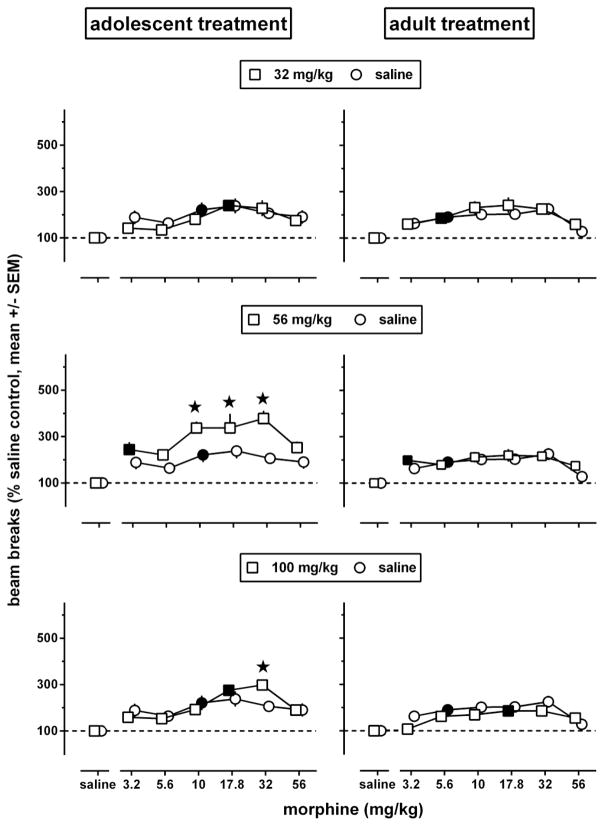
The dose-response data shown in Fig. 5 were obtained during a test session conducted 5 weeks after repeated treatment with morphine ended, i.e., when the animals repeatedly treated with morphine while adolescent (starting at postnatal day 29) had become adult [postnatal day 29 + (5×7) days = 64]. The dose-response curve obtained after repeated administration of saline in each age group (circles, re-plotted in each row to facilitate comparisons with repeated administration of morphine) was biphasic and attained similar levels in adults previously treated with saline during adolescence or during adulthood. Further, this dose-response curve was shifted upward in adults repeatedly treated with 56 or 100 mg/kg morphine during adolescence, but not in adults repeatedly treated with morphine during adulthood. Each age group showed significant effects of repeated treatment [F(3,136)≥5.21, p<0.0025] and of test dose [F(6,136)≥19.50, p<0.0001] and a non-significant repeated treatment by test dose interaction [F(18, 136)≤1.18, p≥0.20].
Fig. 5.
Locomotor activity in adult male C57/BL6J mice during a 2h exposure to the activity chambers immediately after an i.p. injection of morphine or its vehicle (n=6 per dose; each animal received only one injection), 5 weeks after repeated administration of saline (circles, re-plotted in each row to facilitate comparisons with repeated administration of morphine) or morphine [squares, one repeatedly administered dose (32–100 mg/kg) per row] while the animals were adolescent (left panels) or adult (right panels). Results are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Data obtained in morphine-treated animals were expressed as a percentage of the data obtained in their corresponding age-matched saline controls. Asterisks indicate p<0.05 of differences between animals repeatedly treated with morphine (squares) or saline (circles), and filled symbols indicate the minimum significant dose (p<0.05) of each dose-response curve.
An AUC value was calculated for each of the curves shown in Fig. 5, and AUC values obtained after repeated administration of saline were subtracted from the AUC values obtained after each of the repeatedly administered doses of morphine. The results were plotted as a function of the repeated treatment dose to obtain a dose-response curve for morphine to induce locomotor sensitization (Fig. 6, filled symbols). This dose-response curve was biphasic in animals repeatedly treated with morphine during adolescence and tested 5 weeks later as adults. The ascending part of this dose-response curve was compared with that of the dose-response curve of morphine to induce locomotor sensitization in adolescents (Fig. 6, open circles; replotted from Fig. 3). The ascending part of both curves could be fitted by straight lines with slopes that did not differ significantly between the age groups [F(2,4)=0.10, p>0.20]. Straight lines with a common slope (1600, 95% CL 1000–2400) fitted the data adequately (R2: 0.88–0.97) and yielded a potency ratio of 2.3 (95% CL 1.1–3.4). Because the 95% CL did not include 1, these results indicate that repeated morphine during adolescence was significantly less potent to produce locomotor sensitization after 5 weeks than after 3 days. The maximal locomotor sensitization produced by repeated morphine during adolescence, which attained a value of 710 at a dose of 56 mg/kg after 3 days, attained a value of 530 at a dose of 56 mg/kg after 5 weeks. In animals repeatedly treated with morphine as adults, a dose-response relation for morphine to produce locomotor sensitization, which was apparent after 3 days (Fig. 3, squares), was no longer apparent 5 weeks later (Fig. 6, squares).
Fig. 6.
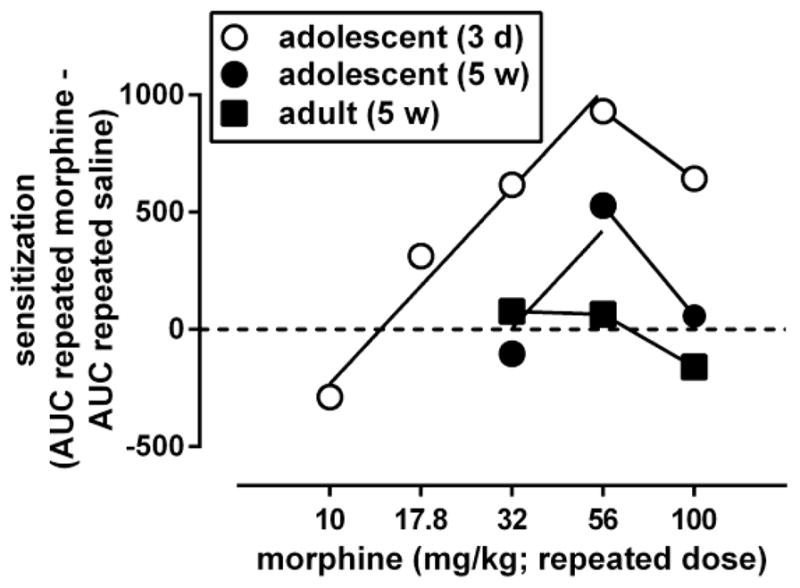
Dose-response curves for morphine to induce locomotor sensitization in adult male C57/BL6J mice, 5 weeks after its repeated administration while the animals were adolescent (filled circles) or adult (filled squares). Ordinate: sensitization, measured by the difference between the area under the curve (AUC) for the morphine dose-response curve obtained after repeated treatment with morphine and the morphine dose-response curve obtained after repeated treatment with saline (shown in Fig. 5). Abscissa: dose of morphine that was administered repeatedly. Dose-response data were analyzed by fitting straight lines through the ascending and descending parts of the curves. For comparison, data obtained in adolescents tested 3 days after repeated treatment with morphine (open circles) are re-plotted from Fig. 3.
Expressing drug effects on test days in experiments 1 and 2 as difference from saline control values, instead of as percentage of saline controls, yielded results similar to those in figures 2 and 5, and yielded curves relating repeated dose to increased AUC similar to those in figures 3 and 6 (data not shown). From these curves, an ED50 (95% CL) of 17 (13–21) mg/kg was obtained for morphine to produce short-term sensitization in all three age groups, and a ratio of 3 (2.6–3.4) for the relative potency with which morphine produced short- and long-term sensitization in adolescents. These values were similar to those obtained from analyses of percentages of saline control values [see above: ED50 = 22 (20–26) mg/kg; potency ratio = 2.3 (1.1–3.4)].
Body weight (experiments 1 and 2)
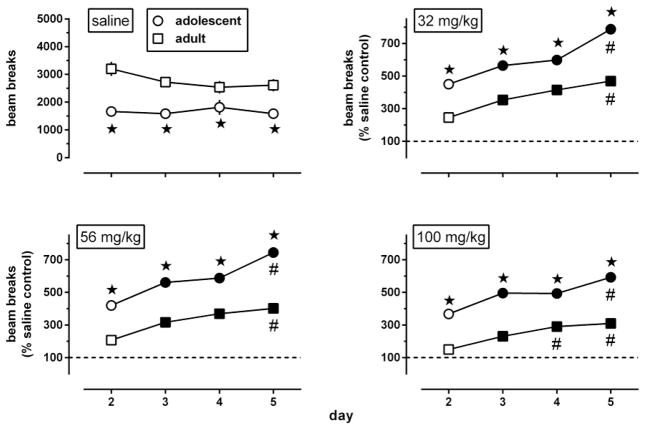
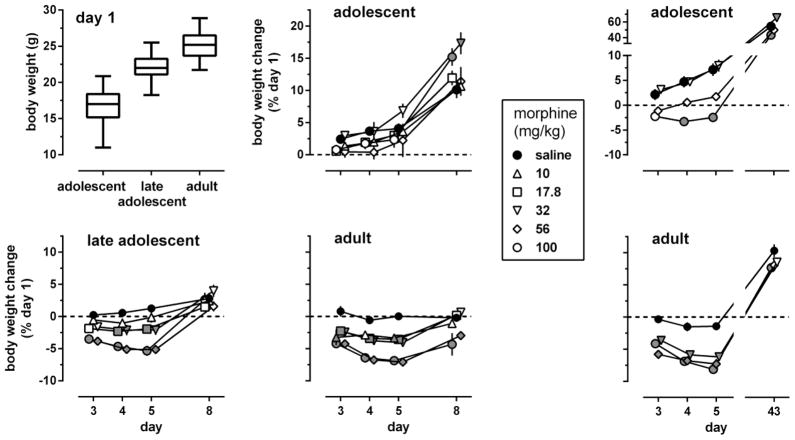
The body weights of adolescent, late adolescent, and adult mice differed at baseline, on day 1 of experiment 1 (Fig. 7, upper left panel) [F(2,750)=985, p<0.0001]; late adolescents weighed significantly more than adolescents and significantly less than adults (Tukey test). During the 8 days of experiment 1, the body weight of saline-treated adolescents and late adolescents significantly increased 10 and 2.6%, respectively, while the body weight of saline-treated adults did not significantly change (Fig. 7, lower left panel and middle panels, black-filled circles). Intermittent administration of morphine affected body weight in a dose-, daily session-, and age-related manner; it produced weight loss in late adolescents and adults, but not in adolescents. In adolescents (Fig. 7, upper middle panel), body weight changes showed a main effect of day [F(3,711)=295, p<0.0001], but not of dose [F(5,237)=1.7, p=0.13]; a dose x day interaction [F(15,711)=3.38, p=0.00016] followed by multiple comparisons with controls repeatedly treated with saline showed that repeated treatment with 32 or 100 mg/kg morphine produced a statistically significant weight gain of 5–7% on day 8, three days after the last injection. In late adolescents and adults (Fig. 7, lower left and lower middle panels), body weight changes showed a main effect of day [F(3,702)=160, F(3,660)=46.9, respectively, p<0.0001], of dose [F(5,234)=8.43, F(5,220)=16.6, p<0.0001], and a dose x day interaction [F(15,702)=6.04, F(15, 660)=3.94, p<0.0001]. On day 3, twenty-four hours after the first injection of morphine, late adolescents and adults showed a 4–5% weight loss compared with saline controls. The minimum significant dose to produce this effect was 56 mg/kg in late adolescents and ≤10 mg/kg in adults. In late adolescents, morphine became more potent to produce weight loss when given repeatedly (minimum significant dose 24 h after two (day 4) and three (day 5) injections: 17.8 mg/kg). On day 8, three days after the last injection, body weight had returned to normal in all drug-treated late adolescents. In contrast, the body weight of adults repeatedly treated with 56 or 100 m g/kg morphine was still below saline control values on day 8.
Fig. 7.
Effects of morphine, administered i.p. once daily during 4 consecutive days (days 2–5 of the experiments), on body weight changes during experiments 1 (lower left and middle panels) and 2 (right panels) (n=42 per group). Baseline values, obtained on day 1 before any injections were given, are shown as box plots (mean, interquartile range, 95% CL) (upper left panel). Body weight changes (expressed as a percentage of baseline values on day 1) were examined in adolescent, late adolescent, and adult male C57/BL6J mice 24 h after 1, 2, and 3 daily injections (i.e., on days 3–5 of the experiments), and 3 (experiment 1) or 38 (experiment 2) days after 4 daily injections. All body weight changes are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Gray-filled symbols represent data points that are significantly different from saline control (Dunnett’s test).
On day 1 of experiment 2, the mean body weight was 16.8 (0.2) g in adolescents and 25.4 (0.2) g in adults (data not shown). On day 43 of the experiment, when adolescents had become adult, the body weight of saline-treated adolescents and adults was significantly increased 50 and 10%, respectively (Fig. 7, right panels, black-filled circles). In both age groups, body weight changes showed effects of day [F(3,363)=1350, F(3,327)=920, p<0.0001] and dose [F(3,121)=13.2, F(3,109)=23.6, p<0.0001], and a day x dose interaction [F(9,363)=4.69, F(9,327)=4.33, p<0.003] (adolescents and adults, respectively). In adults, but not in adolescents, a single injection of morphine significantly decreased body weight by about 5% 24 h later (on day 3). Repeated administration of morphine produced body weight loss in both age groups, but less potently in adolescents (minimum significant dose: 100 mg/kg) than in adults (minimum significant dose: ≤32 mg/kg). On day 43, five weeks after the last injection of morphine, adults treated repeatedly with 100 mg/kg morphine still showed a statistically significant body weight loss of about 2.5%. Similarly, adolescents repeatedly treated with 100 mg/kg morphine showed a statistically significant weight loss of about 6% on day 43, when they had become adult. In contrast, adolescents repeatedly treated with 32 mg/kg morphine showed an about 16% weight gain as adults.
Ataxia (experiment 3)
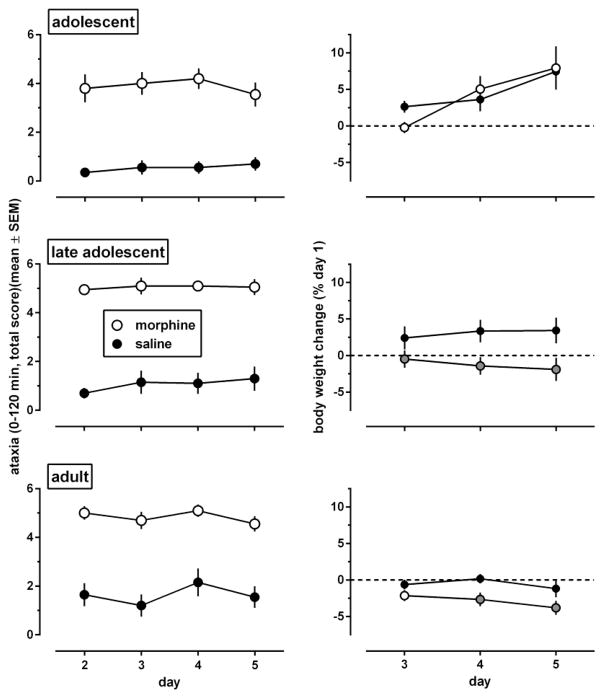
On day 1 of the experiment, before any injections were given, wire test performance did not differ significantly among the age groups [F(2,116)=0.28, p=0.76; overall score: 0.96 (0.13) (data not shown)]. Acute administration of 100 mg/kg morphine produced ataxia in all age groups (Fig. 8, left panels, day 2) [main effect of injection condition (morphine, saline): F(1,38)≥40.8, p<0.0001]. These effects did not change when morphine was administered repeatedly in adolescents and late adolescents [main effect of days: F(3,114)≤0.85, p>0.40; interaction between days and injection condition: F(3,114)≤1.35, p>0.20]. In adults, the main effect of days was statistically significant [F(3,114)=3.15, p=0.033], but not the days by injection condition interaction [F(3,114)=0.62, p=0.59]. In each injection condition in each age group, results obtained during days 3–5 did not differ significantly from those obtained during day 2. Adolescents, but not late adolescents, showed significantly less ataxia than adults, and these age effects [F(2,114)=5.36, p=0.006] did not interact significantly with those of the injection conditions [F(2,114)=0.78, p=0.46].
Fig. 8.
Effects of morphine, administered i.p. once daily during 4 consecutive days (days 2–5 of the experiment) on motor coordination (left panels) and body weight (right panels). Morphine-induced ataxia [measured by summing horizontal wire test scores obtained from 0 to 120 min post-injection of 100 mg/kg morphine or saline (n=20 per dose)] was assessed during 4 consecutive daily sessions in adolescent, late adolescent, and adult male C57/BL6J mice. Body weight changes (expressed as a percentage of baseline values on day 1) were examined 24 h after 1, 2, and 3 daily injections (i.e., on days 3–5 of the experiment). Results are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Data points in the right panels that are significantly different from saline control (Dunnett’s test) are represented by gray-filled symbols.
During the 5 days of experiment 3, morphine induced body weight loss in late adolescents and adults, but not in adolescents (Fig. 8, right panels). Body weight increased in saline-treated adolescents, and remained the same in the other saline-treated age groups (Fig. 8, right panels, black-filled circles). On day 3, 24 h after the first injection of 100 mg/kg morphine, body weights were significantly decreased only in late adolescents. On days 4 and 5 of the experiment, 24 h after 2 and 3 injections of morphine, respectively, body weights were significantly decreased in late adolescents and adults. In adolescents, ANOVA showed a significant main effect of days [F(2,74)=14, p=0.0002], a non-significant main effect of dose [F(1,37)=0.02, p=0.89], and a non-significant dose x days interaction [F(2,74)=1.70, p=0.20]. Late adolescents showed a significant effect of dose [F(1,26)=4.48, p=0.044], but not of days [F(2,52)=0.12, p=0.80], and the dose x days interaction did not reach statistical significance [F(2,52)=3.43, p=0.059]. In adults, both main effects were significant [dose: F(1,38)=4.42, P=0.042; day: F(2,76)=3.54, p=0.043], but not their interaction [F(2,76)=0.95, p=0.38].
Discussion
Adolescent and late adolescent male mice showed significantly less activity than adults upon initial exposure to the locomotor activity chambers, and similar age-related activity differences were also apparent during subsequent sessions in saline-treated controls in experiments 1 and 2. These observations confirm and extend our previous findings (Koek et al., 2012), but contrast with the notion that adolescent animals are typically hyperactive in novel situations compared with adults (Spear 2000). However, there are other reports that adolescent male rodents are less active when first exposed to a test environment, and the novelty of the test situation has been proposed to be a factor that affects differences in locomotion between adolescents and adults (Laviola et al. 2003). Thus, differences in the relative novelty of the test environment may account for the apparent discrepancies among studies.
Morphine is well-known to stimulate motor activity in several strains of mice [(e.g., C57BL/6J (Shuster et al. 1963); Swiss-Webster, C3H/HeJ (Goldstein and Sheehan 1969)]. Consistent with this, in the present study acute administration of morphine dose-dependently increased locomotion in male C57BL/6J mice, and did so along an inverted dose-response curve. Its potency to stimulate locomotion was similar in all age groups. However, morphine increased locomotion to higher levels in adolescents than in late adolescents and adults, after its first administration on day 2, and on the last day of the experiments in animals repeatedly treated with saline. These observations confirm and extend previous findings in mice (Koek et al., 2012) and rats (Spear et al. 1982; White et al. 2008) that adolescents are more sensitive to the acute locomotor-stimulating effects of morphine than adults. Morphine stimulates dopamine systems indirectly by inhibiting GABAergic interneurons in the ventral tegmental area that inhibit dopaminergic neurons (Johnson and North 1992). The adolescent brain undergoes extensive changes, including changes in the mesolimbic dopamine system (Wahlstrom et al. 2010). The activity of ventral tegmental dopamine neurons peaks during adolescence, potentially because GABA tone increases as adulthood is reached (McCutcheon et al., 2012). This increased activity of ventral tegmental dopamine neurons during adolescence relative to adulthood conceivably underlies the age-related effects of morphine on locomotion.
If morphine stimulates locomotor activity in adolescents by indirectly stimulating an overactive dopamine system, adolescents would be expected to be more sensitive also to other effects of morphine that are mediated indirectly through the dopamine system, such as its rewarding effects. Surprisingly, adolescent rats have been reported not to exhibit morphine-induced place preference (Bolanos et al. 1996), or to show morphine-induced place preference similar in magnitude to that shown by adults (Campbell et al. 2000). Also, compared with adults, adolescents self-administered less morphine (Doherty et al. 2009) and less oxycodone (Zhang et al. 2009). In the latter study, which was conducted in mice, oxycodone increased striatal dopamine levels more in adolescents than in adults. This enhanced response is consistent with the interpretation that low rates of self-administration could reflect a leftward shift of the inverted U-shaped dose-response function, i.e., a higher sensitivity to the reinforcing effects of oxycodone in adolescents. However, a more recent study (Doherty and Frantz 2012) failed to find evidence for robust age differences in the acute reinforcing effects of heroin, and reported less extinction responding and reinstatement in adolescent than in adult rats. Clearly, further studies are needed to delineate the conditions under which the rewarding effects of opioids depend on age.
The locomotor-stimulating effects of morphine increased during its repeated administration. A single administration of morphine at a dose of 17.8–32 mg/kg or higher was sufficient to enhance its effects 24 h later, in all age groups, and in both experiments. These results are consistent with other findings of locomotor sensitization after a single administration of morphine in rats (Vanderschuren et al. 2001) and mice (Valjent et al. 2010; Luo et al. 2011). In the study by Luo et al. (2011), sensitization was observed 4–21 days after exposure to morphine, but not before. In the present study, sensitization was already apparent 24 h after the first injection. Procedural differences, including enhanced power of the present study because of a large group size (n=42 per repeated treatment dose), could explain this discrepancy. In the first experiment reported here, the locomotor-stimulating effects of morphine continued to increase after subsequent injections in adults, but appeared to have reached an apparent maximum after the first injection in adolescents and late adolescents. In the second experiment, however, the locomotor-stimulating effects of morphine continued to increase after subsequent injections both in adults and adolescents. Taken together, these results show sensitization to be evident after a single injection of morphine in adolescents, late adolescents, and adults, and generally to increase further after subsequent injections in all age groups.
Because morphine stimulated locomotion in all age groups along an inverted U-shaped dose-response curve (Koek et al. 2012; present results), the present study examined the ability of repeated morphine to shift this curve, and used data relating the magnitude of the shift of this dose-response curve to the repeatedly administered dose to characterize and compare morphine-induced sensitization in adolescents and adults. Three days after repeated drug administration, morphine induced sensitization to its locomotor stimulating effects along biphasic dose-response curves in all age groups, with similar potency [overall ED50 (95% CL): 22 (20–26) mg/kg] and to a similar maximum (attained at 32–56 mg/kg). These results suggest that repeated administration of morphine has similar short-term effects on locomotion in adolescents and adults.
The long-term effect of repeated drug administration, however, were different in animals treated repeatedly with morphine during adolescence compared with animals repeatedly treated with morphine during adulthood. Five weeks after repeated drug administration, when animals treated during adolescence had become adult, locomotor sensitization was still apparent in animals exposed to morphine as adolescents, but was no longer apparent in animals exposed to morphine as adults. These results are consistent with previous findings in rats that treatment with morphine during adolescence produces more locomotor sensitization during adulthood than treatment with morphine during adulthood (White and Holtzman, 2005; White et al., 2008), and extend these findings to mice. Thus, repeated exposure to morphine in adolescents and adults appears to have similar short-term effects on its ability to stimulate locomotion, but different long-term effects.
In addition to its ability to stimulate locomotion, morphine can also interfere with motor coordination. Using the horizontal wire test, we observed that morphine induced ataxia in all age groups, consistent with previous findings (Koek et al., 2012). These effects did not change when morphine was administered repeatedly, on four consecutive days. Because tolerance to morphine-induced ataxia did not develop under conditions similar to those used in the locomotor experiments, the enhanced locomotion observed in these experiments likely involves sensitization of locomotor stimulation, not tolerance to ataxia.
Intermittent administration of morphine produced body weight loss in an age- and dose-related manner. Late adolescents and adults showed body weight loss in all three experiments, and these effects were already apparent 24 h after morphine had been administered only once or twice, at doses as low as 10 mg/kg. In contrast, adolescents showed body weight loss only in one of the three experiments, and only after repeated administration of the highest dose examined. Body weight loss is a prominent somatic sign of opioid withdrawal, apparent from one to at most eight days after discontinuation of repeated intermittent opioid administration, in adult rats (e.g., Langerman et al. 2001; Becker et al. 2010) and mice (e.g., Pappaleo and Contarino 2006). The present findings therefore suggest the possibility that adolescents are less sensitive to opioid withdrawal than adults, consistent with reports that adolescent mice show less affective withdrawal from morphine than do adults, as measured in the forced swim test (Hodgson et al. 2009), and that adolescent rats show less physical withdrawal (somatic signs and body weight loss) from heroin than do adults (Doherty and Frantz 2013). Although it is tempting to interpret the differential effects on body weight observed in the present experiments in terms of differential withdrawal, such an interpretation awaits a comparison of additional opioid withdrawal-related effects in adolescents and adult mice.
Taken together, the results of the present experiments show that, compared with adults, adolescent mice were more sensitive to some effects of morphine (acute locomotor stimulation, long-term enhancement of locomotor stimulation) and less sensitive to others (body weight loss). Previously, morphine was found to have similar pharmacokinetic characteristics in adolescent and adult mice (Koek et al. 2012). Together, these observations suggest that the age-related effects of morphine reported here are not due to developmental differences in the pharmacokinetic properties of morphine, but involve overactivity of nigrostriatal/mesolimbic dopamine systems during adolescence relative to adulthood.
Acknowledgments
The authors thank Jason Persyn, Chris Limas, Bindumahi Sudaabattula, and Sonia Cano for technical assistance. The work was supported by US Public Health Service Grant DA23261
References
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Becker GL, Gerak LR, Li J-X, Koek W, France CP. Precipitated and conditioned withdrawal in morphine-treated rats. Psychopharmacology. 2010;209:85–94. doi: 10.1007/s00213-009-1773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Carlton PL. A primer of behavioral pharmacology. New York: Freeman; 1983. [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Corfield-Sumner PK, Stolerman IP. Behavioral tolerance. In: Blackman DE, Sanger DJ, editors. Contemporary research in behavioral pharmacology. New York: Plenum Press; 1978. [Google Scholar]
- Doherty JM, Frantz KJ. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology. 2012;219:763–773. doi: 10.1007/s00213-011-2398-x. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Attenuated effects of experimenter-administered heroin in adolescent vs. adult male rats: physical withdrawal and locomotor sensitization. Psychopharmacology. 2013;225:595–604. doi: 10.1007/s00213-012-2847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Sheehan P. Tolerance to opioid narcotics. I. Tolerance to the “running fit” caused by levorphanol in the mouse. J Pharmacol Exp Ther. 1969;169:175–184. [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Schul DL, Wellman PJ, Eitan S. Social influences on morphine sensitization in adolescent rats. Addict Biol. 2012;17:547–56. doi: 10.1111/j.1369-1600.2011.00315.x. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons. J Pharmacol Exp Ther. 1987;241:204–12. [PubMed] [Google Scholar]
- Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology. 2012;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H. Effects of interdose interval on ambulatory sensitization to methamphetamine, cocaine and morphine in mice. Eur J Pharmacol. 1996;316:1–5. doi: 10.1016/s0014-2999(96)00635-8. [DOI] [PubMed] [Google Scholar]
- Langerman L, Piscoun B, Bansinath M, Shemesh Y, Turndorf H, Grant GJ. Quantifiable dose-dependent withdrawal after morphine discontinuation in a rat model. Pharmacol Biochem Behav. 2001;68:1–6. doi: 10.1016/s0091-3057(00)00442-1. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Luo J, Jing L, Qin W-J, Zhang M, Lawrence AJ, Chen F, Liang J-H. Transcription and protein synthesis inhibitors reduce the induction of behavioural sensitization to a single morphine exposure and regulate Hsp70 expression in the mouse nucleus accumbens. Int J Neuropsychopharmacol. 2011;14:107–121. doi: 10.1017/S146114571000057X. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–30. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Contarino A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res. 2006;170:110–118. doi: 10.1016/j.bbr.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–9. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–52. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster L, Hannam RV, Boyle WE., Jr A simple method for producing tolerance to dihydromorphinone in mice. J Pharmacol Exp Ther. 1963;140:149–154. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Overview of Findings from the 2002 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville MD: 2003. [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D, Girault J-A. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacol. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, Schoffelmeer AN. A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. Eur J Neurosci. 2001;14:1533–1538. doi: 10.1046/j.0953-816x.2001.01775.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–95. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Tjon GH, Nestby P, Mulder AH, Schoffelmeer AN, De Vries TJ. Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regimen. Psychopharmacology. 1997;131:115–22. doi: 10.1007/s002130050273. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”? Psychopharmacology. 2004;171:349–351. doi: 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]