Abstract
Purpose
To determine whether hard exudates (HE) within one disc diameter of the foveola is an acceptable criterion for the referral of diabetic patients suspected of clinically significant macular edema (CSME) in a screening setting.
Methods
143 adults diagnosed with diabetes mellitus were imaged using a non-mydriatic digital fundus camera at the Alameda County Medical Center in Oakland, CA. Non-stereo fundus images were graded independently for the presence of HE near the center of the macula by two graders according to the EyePACS grading protocol. The patients also received a dilated fundus exam on a separate visit. CSME was determined during the dilated fundus examination using the criteria set forth by the Early Treatment Diabetic Retinopathy Study. Subsequently, the sensitivity and specificity of hard exudates within one disc diameter of the foveola in non-stereo digital images used as a surrogate for the detection of CSME diagnosed by live fundus examination were calculated.
Results
The mean age of 103 patients included in the analysis was 56±17years. CSME was diagnosed in 15.5% of eyes during the dilated exam. For the right eyes, the sensitivity of hard exudates within one disc diameter from the foveola as a surrogate for detecting CSME was 93.8% for each of the graders; the specificity was 88.5% and 85.1%. For the left eyes, the sensitivity was 93.8% and 75% for each of the two graders, respectively; the specificity was 87.4% for both graders.
Conclusions
This study supports the use of HE within a disk diameter of the center of the macula in non-stereo digital images for CSME detection in a screening setting.
Keywords: clinically significant macular edema, hard exudates, diabetic retinopathy, teleophthalmology
The International Diabetes Federation recommends that patients with type 2 diabetes mellitus receive a dilated fundus exam by a qualified provider at the time of diagnosis of diabetes and annually thereafter if no retinopathy is present.1 More frequent retinal examinations are indicated if any retinopathy is present. For patients with type 1 diabetes mellitus, a dilated fundus exam for retinopathy screening is recommended from age 11 years and after 2 years after the diagnosis and annually thereafter.2 The current data indicate that, on average, only 60% of patients with diagnosed diabetes comply with these recommendations.3 Even poorer compliance is reported among patients of lower socioeconomic status.4 This underscores the need for a simple and effective screening tool for the detection of sight threatening retinopathy, since early detection and prompt treatment of retinal disease among diabetic patients can prevent vision loss.5,6
Tele-ophthalmology screening for diabetic retinopathy has been shown to be effective in detecting diabetic retinopathy in a primary care setting7. Stereoscopic digital retinal photography with pupil dilation has been validated as an acceptable method for detecting and grading the severity of diabetic retinopathy (DR) and diabetic macular edema (DME).8,9 The international classification of diabetic retinopathy (ICDR) developed by the International Council of Ophthalmology and adopted by the American Academy of Ophthalmology uses the presence and severity of retinal lesion types to stratify the risk of progression to sight-threatening complications from DR.10,11 Several organizations throughout the world, including the Canadian Teleophthalmology Network in Alberta and Inoveon diabetic retinopathy screening program in Oklahoma, have implemented diabetic retinopathy detection programs using stereoscopic retinal photography and the ICDR.12,13
Non-mydriatic retinal cameras have been developed to reduce the discomfort and potential hazards of pupil dilation. However, stereoscopic photography without pharmacological pupil dilation is difficult to perform, and it results in images ungradable for retinal thickening in up to 20% of eyes.14,15 On the other hand, determining retinal thickening in non-stereoscopic images is not possible. Therefore, a number of diabetic retinopathy detection programs, such as the Scottish Diabetic Retinopathy Screening Program16 and the Veterans Administration Diabetic Retinopathy Screening Program17 use the presence and location of hard exudates close to the center of the macula, as a surrogate to detect and stage DME. There has been only limited validation of hard exudates as a surrogate for DME. Bresnick et al.18 performed a retrospective analysis of the photographic database of the Early Treatment of Diabetic Retinopathy Study (ETDRS) using the criterion of hard exudates within one disc diameter of the foveola, and identified CSME with a sensitivity of 94% and a specificity of 54%. Rudnisky et al.19 reported the sensitivity of HE within two disc diameters of the foveola to be 93.9% in detecting ophthalmoscopically confirmed CSME; the specificity was reported to be 81.6%. Retinal images, in both of these studies, were obtained after pupillary dilation.
The purpose of the present study was to test, using a prospective clinical design, the validity of using hard exudates located within one disc diameter of the foveola in non-mydriatic, non-stereo digital retinal images as a criterion for referring diabetic patients suspected of having CSME, compared with the standard clinical technique of stereo biomicroscopy using a condensing lens or a contact fundus lens.
METHODS
This study was conducted at the Alameda County Medical Center in Oakland, CA, a diabetic retinopathy screening site within the EyePACS telemedicine network20. This clinic serves uninsured local patients, and the limited clinic budget did not provide Optical Coherence Tomography (OCT). Adult patients with known type 2 diabetes were recruited for the study. The recruitment process was purposely enriched by patients who were deemed likely to have diabetic retinopathy based on a greater than 5 year history of diabetes, elevated HbA1c >9.0, age >40, and self-reported comorbidities such as angina or stroke. Written informed consent for the use and disclosure of protected health information was obtained from all subjects before being enrolled in the study. Institutional Review Board approval was granted by the Alameda County Medical Center, the University of California, Berkeley, and Indiana University.
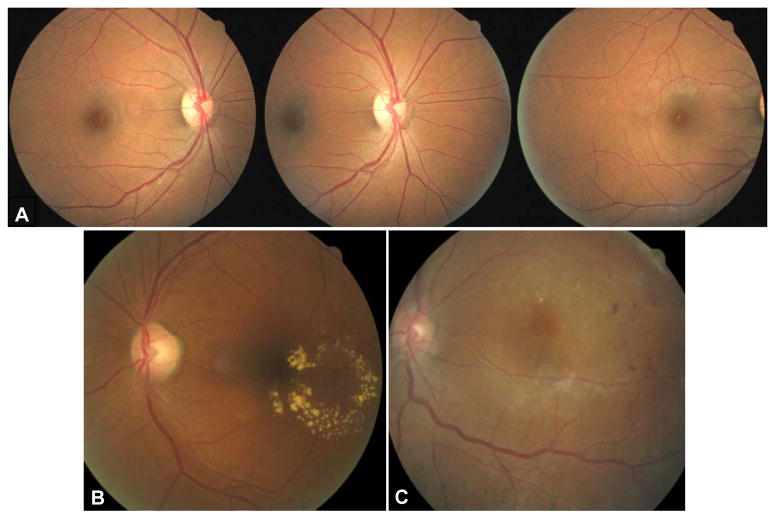
The study protocol required two patient visits to the clinic, one for retinal photography alone and the other for a dilated retinal exam. This procedure mimics the actual screening process in the county system where a patient undergoes fundus imaging during the first visit to the clinic, images are reviewed and the follow up appointment with clinician is scheduled based on the outcomes of the image review. The time between the two visits varies according to a number of factors. Such factors include physician and photographer availability issues coupled with reliability and scheduling issues of this indigent population. It was important to mimic that process in order to assess the utility of hard exudates as a referral criterion for CSME in a practical setting. If due to logistical issues, the interval between the first and the second clinic visit exceeded 100 days, the patient was excluded from the study. The actual mean interval was 33 ± 31 days. Retinal imaging was performed during the first clinic visit using a non-mydriatic fundus camera, the CR6-45NM (Canon Inc, Tokyo, Japan) without pupillary dilation. Non-stereoscopic, 45 degree images of three retinal fields per eye were obtained in accordance with the EyePACS imaging protocol21. The primary field included the macula and the optic nerve, the centers of which were located equidistant from the center of the image (default position of the camera, Figure 1a left). The second field was obtained with the optic nerve at the center of the image (Figure 1a center). The third field was captured with the optic nerve to the far nasal side of the field with the macula below and nasal to the center of the picture (Figure 1A right). An EyePACS image in the primary position is shown with large HE within one disc diameter of the foveola (Figure 1B) and an image with smaller HE in (Figure 1C).
Figure 1.
Images obtained according to the EyePACS imaging protocol. (A) normal retina: left primary field OD-temporal retina and optic nerve, center) field centered on optic nerve OD, right) optic nerve and nasal retina OD. (B) and (C) would each yield referrals for retinal edema because of hard exudates within 1 disc diameter of foveola. (B) circinate ring of hard exudates involving macula OS,(C) small hard exudates within1 disc diameter of foveola.w
The captured images were uploaded to the EyePACS website20 and graded independently for macular edema by two graders (TJG and TVL) according to the EyePACS grading protocol.22 A presumptive diagnosis of clinically significant macular edema (CSME) was made when hard exudates were noted at or within one disc diameter of the foveola. During the second clinic visit, patients received a dilated fundus exam of the macular region by TVL using a non-contact 90D condensing lens and a biomicroscope. The examiner was masked to the retinal imaging findings. As stated above, there was typically a delay between imaging and the exam, and the patients were not necessarily seen sequentially, lessening the potential bias of results from grading the images to exam results. The presence, extent, and location of retinal thickening were noted, as well as the presence and location of hard exudates. In cases of uncertainty about the presence of macular edema, Goldmann macular contact lens was also used. The diagnosis of CSME on the dilated fundus exam was made according to the criteria set forth by the Early Treatment Diabetic Retinopathy Study23: 1) retinal thickening within 500 microns of the center of the macula; or 2) hard exudates within 500 microns of the center of the macula with adjacent retinal thickening; or 3) retinal thickening of one disc area in size or greater, any part of which is located at or within one disc diameter from the center of the macula.
The sensitivity and specificity values for CSME detection using hard exudates at or within one disc diameter of the foveola graded in the retinal images were calculated and compared to CSME identified during the dilated fundus exam as the standard. The statistical analysis was performed separately for the right and the left eyes.
RESULTS
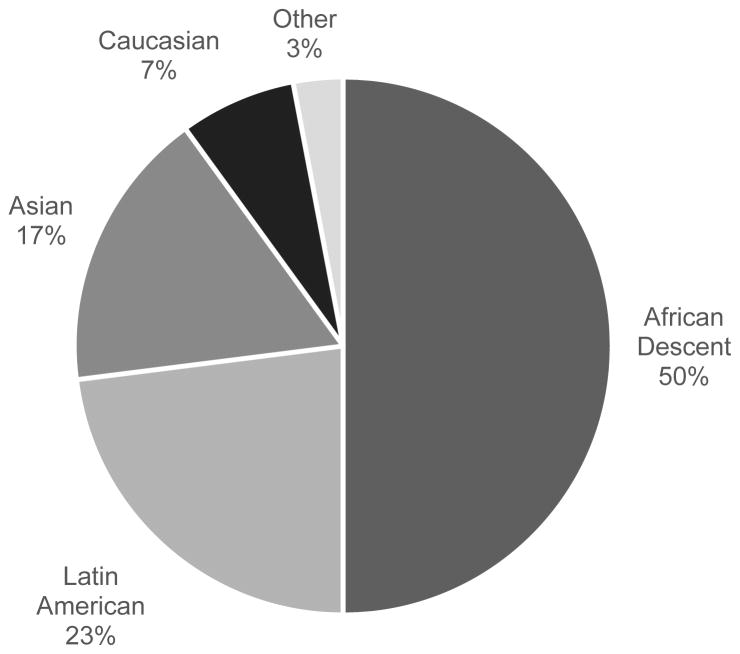
One hundred forty three adult diabetic patients were recruited for the study. Forty of these patients were excluded from the study because the time between the first and the second clinic visits exceeded 100 days, reinforcing the scheduling difficulties in this patient population. The mean time interval between the first and the second clinic visits of the remaining 103 patients was 33±31 days. Forty nine percent were females. The mean age of the included patients was 56±17 years. Ethnic composition of the study population is presented in Figure 2.
Figure 2.
Ethnicity of patient group.
For the right eyes, CSME was diagnosed in 16 (15.5%) eyes by biomicroscopy during the dilated exam. Based on retinal images, a presumptive diagnosis of CSME was made independently by the two graders in 28 (26.4%) and in 25 (24.2%) cases, respectively (Table 1). In the right eyes, the sensitivity of hard exudates located within one disc diameter from the center of the macula as a surrogate for detecting CSME was 93.8% for each of the graders; the specificity was 88.5% and 85.1%; positive predictive value was 60% and 53.57%; and negative predictive value was 98.7% and 98.7% (Table 2).
Table 1.
CSME: Dilated biomicroscopic exam vs. image grading.
| CSME On Fundus Exam |
CSME Grader 1 On Image Grading |
CSME Grader 2 On Image Grading |
||||
|---|---|---|---|---|---|---|
| OD | OS | OD | OS | OD | OS | |
| Number of eyes | 16 | 16 | 28 | 26 | 25 | 23 |
| Percent Value | 15.5% | 15.5% | 26.4% | 25.2% | 24.2% | 22.3% |
Table 2.
Predictive power of the surrogate method of CSME detection.
| Grader 1 | Grader 2 | |||
|---|---|---|---|---|
| OD | OS | OD | OS | |
| Sensitivity | 93.75% | 93.75% | 93.75% | 75% |
| Specificity | 88.51% | 87.36% | 85.05% | 87.36% |
| PPV | 60% | 57.69% | 53.57% | 52.17% |
| NPV | 98.72% | 98.70% | 98.67% | 95% |
For the left eyes, CSME was diagnosed in 16 (15.5%) cases by biomicroscopy during the dilated exam. Based on retinal images, CSME was diagnosed by Grader 1 in 26 (25.2%) cases, while Grader 2 diagnosed CSME in 23 (22.3%) (Table 1). Based on the results of the left eye analysis, the sensitivity of hard exudates located within one disc diameter from the center of the macula as a surrogate for detecting CSME was 93.8% and 75% for each of the two graders, respectively; the specificity was 87.4% for both graders; positive predictive value was 57.7% and 52.17%; negative predictive value was 98.7% and 95% respectively (Table 2).
CONCLUSIONS
When hard exudates were present at or within one disc diameter from the foveola in undilated non-stereoscopic fundus photos, CSME was detected (determined by a dilated biomicroscopic fundus examination) with good sensitivity with an acceptable level of over diagnosis for a screening situation. These results indicate that HE are a valid surrogate marker for the detection of CSME when stereophotography and OCT are inadequate or simply unavailable for financial reasons.
Having a valid, inexpensive alternative for the detection of CSME is important for several reasons. It assures that diabetic retinopathy screenings can successfully detect sight-threatening macular edema without dilation, stereoscopic photos of the macula, or OCT. In addition, diabetic retinopathy screenings that rely on non-mydriatic stereo-imaging can result in a high proportion of ungradable images for the detection of retinal thickening.14,15 When one image of a macular stereo-pair is unusable, a surrogate marker for CSME is very useful. The results of this study also suggest that HE near the center of the macula can be used by primary care providers to screen for CSME using direct ophthalmoscopy, with some consequent over referral to retinal specialists. We do not suggest that direct ophthalmoscopy is a substitute for a dilated retinal examination.
Our sensitivities are comparable to results published by Bresnick18 comparing hard exudates within one disc diameter of the center of the macula versus the then current “gold standard for CSME” graded in ETDRS stereoscopic photographs. Our specificities are somewhat higher than those reported by Bresnick, although both sensitivities and specificities are similar to data published by Rudnisky in 200619 reporting the ability of hard exudates within two disc diameters of the fovea to detect CSME that was confirmed by a dilated fundus examination using a retinal contact lens. However, our study is different from these other two studies because we obtained our retinal images undilated, more closely matching the common screening condition of non-mydriatic, non-stereo retinal imaging.
With regard to the quality of the grading, the lower sensitivity demonstrated by Grader 2 in detection of CSME in the left eyes prompted further investigation. Of the three CSME cases that Grader 2 missed, one case showed unmistakable exudates well within one disc diameter of the foveola, and therefore may have been a case of data-entry error. The other two cases showed a single small exudate at the border of one disc diameter from the foveola. The difference in grading in those cases may be attributed to variability in judgment between graders for the threshold of hard exudates detection by each grader. This highlights the importance of testing grading systems for intra- and inter-grader repeatability, and of performing quality control of image grading in DR screening programs.
Low positive predictive values point to a relatively high rate of “over-referral,” although it may be acceptable in a screening setting especially in a patient population with generally poor access to health care. This study shows the utility of the use of the hard exudates surrogate for detection of diabetic macular edema by screening programs utilizing non-stereoscopic images. In our opinion, this is important for rapid screening of large diabetic populations with relatively low equipment expense, low false negative rates, and reasonable false positive rates. It is better to err on the positive side and send the patient to a retina specialist who does not find serious pathology than miss an actually active disease and lead to blindness on the part of the patient.
This study has limitations. Firstly, the time interval between imaging and dilated eye exams was 33±31 days whereas ideally imaging and examination would have been done at the same visit. In the framework of population screening, it is common to have a screening visit and then a follow-up examination of those subjects showing clinically significant retinopathy so some interval is largely unavoidable. For the specific situation of diabetic macular edema, the ETDRS reported that macular edema tended to be chronic and that spontaneous visual recovery was unusual23. Additionally a recent study by Kwon24 followed two groups of patients with mild and moderate diabetic macular edema measured by OCT over a period of 6 months without treatment and found no significant changes in the 250–300 micron central macular thickness group. There was significant progressive decrease in retinal thickness in the 300–500 micron group, but the eyes still were at an average of 318 microns at 1 month and 284 microns at 6 months. Thus, though it is possible that there were some changes in degree of edema over this one month period between imaging and examination, it is unlikely there were significant changes having a major impact on our results. Additionally, our findings are similar to those reported by Rudnisky19. In their study, fundus photos were obtained on the same day as a dilated fundus exam and the presence of HE within 2DD of the fovea was used as a surrogate for CSME detection. They report that HE within 2DD of the foveola has a sensitivity of 93.9% and a specificity of 81.6% for CSME detection. Thus, it may be inferred that any significant changes in retinal thickening occur slowly, and that the elapsed time in our study likely did not have a major impact on our results.
Secondly the historically accepted gold standard for detecting CSME is the use of 30 degree -film-based stereo macular photos performed and graded according to the ETDRS protocol25. However, our use of the dilated biomicroscopic exam as the standard in the present study is supported by the high correlation reported between CSME detected by contact lens biomicroscopy compared with CSME detection by the ETDRS protocol.26
Additionally, further validation studies to compare the hard exudates surrogate for macular thickening with more objective means such as OCT are planned. While OCT is widely accepted as an objective method for detecting diabetic maculopathy, it is currently too costly and technically challenging to integrate into existing retinopathy detection programs in primary care settings. Low-cost and reliable methods of detecting CSME, such as the use of a hard exudate surrogate marker described here, are needed to meet the challenge of widespread screening for this vision threatening condition.
Acknowledgments
This study was supported by grant EY020017 from the National Eye Institute, National Institute of Health. Involved in design and conduct of study (TVL, MSM, AEE,GYO, GHB); data collection and management of data (TVL, TJG, JAC, MSM); analysis and interpretation of data (TVL, GHB, GYO) and preparation, review, or approval of the manuscript (TVL, GHB, GYO, JAC, AEE, MSM, TJG). We would also like to thank our photographers Lai Pang, Nathan Louie, and Jessica Archambault. This study was presented in the form of a Scientific Poster (115858) at the annual meeting of the American Academy of Optometry in 2011.
References
- 1.International Diabetes Federation. Clinical Guidelines Task Force. [Accessed January 3, 2014];Global Guideline for Type 2 diabetes. 2005 Available at: http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf.
- 2.International Society for Pediatric and Adolescent Diabetes (ISPAD), International Diabetes Federation (IDF) Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence. International Diabetes Federation; 2011. [Accessed January 3, 2014]. Available at: http://www.idf.org/sites/default/files/Diabetes-in-Childhood-and-Adolescence-Guidelines.pdf. [Google Scholar]
- 3.Hazin R, Barazi MK, Summerfield M. Challenges to establishing nationwide diabetic retinopathy screening programs. Curr Opin Ophthalmol. 2011;22:174–9. doi: 10.1097/ICU.0b013e32834595e8. [DOI] [PubMed] [Google Scholar]
- 4.Brechner RJ, Cowie CC, Howie LJ, Herman WH, Will JC, Harris MI. Ophthalmic examination among adults with diagnosed diabetes mellitus. JAMA. 1993;270:1714–8. [PubMed] [Google Scholar]
- 5.Han Y, Schneck ME, Bearse MA, Jr, Barez S, Jacobsen CH, Jewell NP, Adams AJ. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:4106–12. doi: 10.1167/iovs.04-0405. [DOI] [PubMed] [Google Scholar]
- 6.Garg S, Davis R. Diabetic retinopathy screening update. Clin Diabetes. 2009;27(4):140–5. [Google Scholar]
- 7.Andonegui J, Serrano L, Eguzkiza A, Berastegui L, Jimenez-Lasanta L, Aliseda D, Gaminde I. Diabetic retinopathy screening using tele-ophthalmology in a primary care setting. J Telemed Telecare. 2010;16:429–32. doi: 10.1258/jtt.2010.091204. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard LD, Sun W, Cleary PA, Danis RP, Hainsworth DP, Peng Q, Susman RA, Aiello LP, Davis MD, Diabetes C Complications Trial/Epidemiology of Diabetes I, Complications Study Research G. Comparison of digital and film grading of diabetic retinopathy severity in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Arch Ophthalmol. 2011;129:718–26. doi: 10.1001/archophthalmol.2011.136. [DOI] [PubMed] [Google Scholar]
- 9.Li HK, Danis RP, Hubbard LD, Florez-Arango JF, Esquivel A, Krupinski EA. Comparability of digital photography with the ETDRS film protocol for evaluation of diabetic retinopathy severity. Invest Ophthalmol Vis Sci. 2011;52:4717–25. doi: 10.1167/iovs.10-6303. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 11.Preferred Practice Pattern Guidelines. American Academy of Ophthalmology Retina/Vitreous Panel. American Academy of Ophthalmology; 2008. [Accessed January 3, 2014]. Diabetic Retinopathy. Available at: http://one.aao.org/Assets/99d84214-4366-4acd-97ad-2a073c1a6be8/634965436190300000/diabetic-retinopathy-pdf. [Google Scholar]
- 12.Ng M, Nathoo N, Rudnisky CJ, Tennant MT. Improving access to eye care: teleophthalmology in Alberta, Canada. J Diabetes Sci Technol. 2009;3:289–96. doi: 10.1177/193229680900300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fransen SR, Leonard-Martin TC, Feuer WJ, Hildebrand PL The Inoveon Health Research Group. Clinical evaluation of patients with diabetic retinopathy: accuracy of the Inoveon diabetic retinopathy-3DT system. Ophthalmology. 2002;109:595–601. doi: 10.1016/s0161-6420(01)00990-3. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed J, Ward TP, Bursell SE, Aiello LM, Cavallerano JD, Vigersky RA. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205–9. doi: 10.2337/dc06-0295. [DOI] [PubMed] [Google Scholar]
- 15.Bursell SE, Cavallerano JD, Cavallerano AA, Clermont AC, Birkmire-Peters D, Aiello LP, Aiello LM Joslin Vision Network Research Team. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology. 2001;108:572–85. doi: 10.1016/s0161-6420(00)00604-7. [DOI] [PubMed] [Google Scholar]
- 16.Scotland National Health Service. [Accessed January 3, 2014];Scottish Diabetic Retinopathy Grading Scheme. 2007 1.1:1–2. Available at: http://www.ndrs.scot.nhs.uk/ClinGrp/Docs/Grading%20Scheme%202007%20v1.1.pdf. [Google Scholar]
- 17.Cavallerano AA, Conlin PR. Teleretinal imaging to screen for diabetic retinopathy in the Veterans Health Administration. J Diabetes Sci Technol. 2008;2:33–9. doi: 10.1177/193229680800200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bresnick GH, Mukamel DB, Dickinson JC, Cole DR. A screening approach to the surveillance of patients with diabetes for the presence of vision-threatening retinopathy. Ophthalmology. 2000;107:19–24. doi: 10.1016/s0161-6420(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 19.Rudnisky CJ, Tennant MT, de Leon AR, Hinz BJ, Greve MD. Benefits of stereopsis when identifying clinically significant macular edema via teleophthalmology. Can J Ophthalmol. 2006;41:727–32. doi: 10.3129/i06-066. [DOI] [PubMed] [Google Scholar]
- 20.EyePACS, LLC. [Accessed January 3, 2014];Eye Picture Archive Communication System. 2010 Available at: www.eyepacs.com.
- 21.EyePACS, LLC. [Accessed January 3, 2014];Photographer Manual. 2013 Mar; Available at: http://eyepacs.com/modules/ckeditor-6.x-1.2/ckeditor/ckfinder/core/connector/php/sites/default/files/files/EyePACS%20Handbook_For%20Photographers_March%202013.pdf.
- 22.Cuadros J, Bresnick G. EyePACS: an adaptable telemedicine system for diabetic retinopathy screening. J Diabetes Sci Technol. 2009;3:509–16. doi: 10.1177/193229680900300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 24.Kwon SI, Baek SU, Park IW. Comparison of natural course, intravitreal triamcinolone and macular laser photocoagulation for treatment of mild diabetic macular edema. Int J Med Sci. 2013;10:243–9. doi: 10.7150/ijms.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs- -an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 26.Kinyoun J, Barton F, Fisher M, Hubbard L, Aiello L, Ferris F, 3rd The ETDRS Research Group. Detection of diabetic macular edema. Ophthalmoscopy versus photography—Early Treatment Diabetic Retinopathy Study Report Number 5. Ophthalmology. 1989;96:746–50. doi: 10.1016/s0161-6420(89)32814-4. [DOI] [PubMed] [Google Scholar]