Abstract
Rationale
Risperidone use in children and adolescents for the treatment of various neuropsychiatric disorders (e.g. schizophrenia, autism, disruptive behavior, etc.) has increased substantially in recent decades. However, its long-term effect on the brain and behavioral functions is not well understood.
Objective
The present study investigated how a short-term risperidone treatment in adolescence impacts antipsychotic response in adulthood in the conditioned avoidance response and PCP-induced hyperlocomotion tests.
Methods
Male adolescent Sprague-Dawley rats (postnatal days [P] 40-44 or 43-48) were first treated with risperidone (0.3, 0.5 or 1.0 mg/kg, sc) and tested in the conditioned avoidance or PCP (3.2 mg/kg, sc)-induced hyperlocomotion model daily for 5 consecutive days. After they became adults (~P 76-80), they were challenged with risperidone (0.3 mg/kg, sc) to assess their sensitivity to risperidone re-exposure. A quinpirole (a D2/3 receptor agonist, 1.0 mg/kg, sc)-induced hyperlocomotion test was later conducted to assess the risperidone-induced functional changes in D2 receptor.
Results
In the risperidone challenge test in adulthood, adult rats previously treated with risperidone in adolescence made significantly fewer avoidance responses and exhibited significantly lower PCP-induced hyperlocomotion than those previously treated with vehicle. They also appeared to be more hyperactive than the vehicle-pretreated ones in the quinpirole-induced hyperlocomotion test. Prepulse inhibition of acoustic startle or fear-induced 22 kHz ultrasonic vocalizations in adulthood was not altered by adolescence risperidone treatment.
Conclusions
Adolescent risperidone exposure induces a long-term increase in behavioral sensitivity to risperidone that persists into adulthood. This long-lasting change might be due to functional upregulation of D2-mediated neurotransmission.
Keywords: Risperidone; Conditioned avoidance response; Quinpirole, 22 kHz ultrasonic vocalization; Phencyclidine; Motor activity; Prepulse inhibition; Adolescence; Sensitization
INTRODUCTION
Antipsychotic treatment in children and adolescents has seen a dramatic increase in recent decades (Kalverdijk et al. 2008; Rani et al. 2008). Among those who are treated with antipsychotic drugs, more than 90% are on atypical drugs (e.g. risperidone, clozapine, olanzapine) (Olfson et al. 2006) which causes a concern for potential adverse long-term impacts on brain and behavioral development (Almandil and Wong 2011). Preclinical studies strongly suggest that antipsychotic exposure in adolescence could alter basic brain and behavioral functions. For example, animal receptor binding studies show that antipsychotic exposure during adolescence increases or decreases various neuroreceptors, including dopamine D1, D2 and D4 receptors (Moran-Gates et al. 2006; Vinish et al. 2013), serotonin 5-HT1A and 5-HT2A receptors (Choi et al. 2010), and ionotropic NMDA and AMPA glutamatergic receptors (Choi et al. 2009). Behavioral studies also suggest that early adolescent antipsychotic exposure enhances animals’ sensitivity to reward stimuli (Vinish et al. 2013), impairs their working memory, delays the extinction process of fear memory in adulthood (Milstein et al. 2013), and prevents the development of various psychosis-like behaviors (e.g. prepulse inhibition deficit, latent inhibition deficit, etc.) induced by maternal immune activation (Poly I:C) (Meyer et al. 2010; Piontkewitz et al. 2011; Piontkewitz et al. 2009; Piontkewitz et al. 2012).
In addition to the effects on the basic brain and behavioral functions, adolescent antipsychotic exposure could also alter later antipsychotic response in adulthood. In adult rats, two behavioral patterns (sensitization and tolerance) are often associated with repeated drug treatment (Sun et al. 2009; Swalve and Li 2012; Zhang and Li 2012). Using two distinct tests of antipsychotic activity - the conditioned avoidance response (CAR) and phencyclidine (PCP)-induced hyperlocomotion, we showed that prior treatment of haloperidol, olanzapine and risperidone causes an increased antipsychotic sensitivity (i.e. sensitization), whereas prior clozapine treatment causes a decreased sensitivity (i.e. tolerance) (Li et al. 2010). Recently, we extended these findings into adolescent rats and found that olanzapine treatment during the adolescence period [postnatal day 43-48] makes animals more sensitive to olanzapine re-exposure when they become adults, whereas clozapine treatment in adolescence makes animals less sensitive to clozapine in adulthood (Qiao et al. 2013; Shu et al. 2013).
Building on these important findings, the present study examined risperidone, another atypical antipsychotic drug, in both the CAR and the PCP-induced hyperlocomotion models to determine the generality of the altered antipsychotic sensitivity patterns (sensitization versus tolerance) from the adolescence to adulthood. Unlike olanzapine and clozapine, risperidone is a Food and Drug Administration (FDA)-approved antipsychotic drug for pediatric use. It has indications for the treatment of schizophrenia in adolescents aged 13-17 years, for the short-term treatment of acute manic or mixed episodes associated with Bipolar I Disorder in children and adolescents aged 10-17 years, and irritability associated with autism in children and adolescents 5–16 years of age. In one large study sample of children and adolescents (Patel et al. 2005), risperidone was the most prescribed antipsychotic agent from 1996 to 2000. Therefore, testing risperidone in adolescent animals also has significantly clinical relevance. Mechanistically, because of its similar receptor binding profile on dopamine D2 and serotonin 5-HT2A/2C receptors with olanzapine and clozapine, risperidone could induce either a sensitization (like olanzapine) or tolerance (like clozapine) effect. No prior study has examined the potential long-lasting effect of adolescent risperidone treatment. To investigate the possible involvement of dopamine D2 receptor as a receptor mechanism underlying adolescent risperidone effect, we compared the quinpirole (a selective D2/3 receptor agonist)-induced hyperlocomotion in adult rats that had been treated with risperidone or vehicle in adolescence. This approach was chosen because the quinpirole-induced hyperlomotion is a widely used method assessing drug or non-drug induced changes in D2/3 functions (Tenk et al. 2007; Vorhees et al. 2009). Finally, we also assessed whether adolescent risperidone treatment caused any instrumental learning deficit in a modified avoidance conditioning task in adulthood and attention deficit in a prepulse inhibition test (Swerdlow et al. 2000).
MATERIALS AND METHODS
Animals
Male Sprague-Dawley adolescent rats from Charles River Inc. (Portage, MI) were used. In Experiments 1 and 2, on the delivery date, they were approximately 22-26 days old and weighed 51-75 g (postnatal days, P 22-26, assumed averaged age = ~P 24). In Experiments 3 and 4, the adolescent rats were approximately 33-37 days old (averaged age = ~ P 35) and weighed 101-125 g. After arrival, they were housed two per cage, in 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages under 12-h light/dark conditions (light on between 6:30 am and 6:30 pm). Room temperature was maintained at 22 ± 1°C with a relative humidity of 45-60%. Food and water was available ad libitum. Animals were allowed at least 5 days of habituation to the animal facility before being used in experiments. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln.
Drugs and choice of doses
Risperidone (RIS) (gift from the NIMH drug supply program) was dissolved in distilled sterile water with 0.5-1.0% glacial acetic acid and was administrated subcutaneously (sc) at 1.0 ml/kg. In the conditioned avoidance response test (Experiment 1), we tested RIS at 0.5 and 1.0 mg/kg based on our and others’ work showing that RIS at these doses disrupts avoidance response in a clear dose-dependent fashion (Aguilar et al. 1997; Li et al. 2007; Li et al. 2009; Mead and Li 2010; Zhang et al. 2011). In the PCP-induced hyperlocomotion test (Experiment 3), we tested RIS at 0.3 and 1.0 mg/kg and PCP at 3.2 mg/kg based on our previous work showing that RIS at these doses suppressed PCP-induced hyperlocomotion dose-dependently (Sun et al. 2010b). In this dose range, RIS gives rise to clinically comparable striatal D2 occupancy (65–80%) (Kapur et al. 2003; Wadenberg et al. 2001). Quinpirole was tested at 1.0 mg/kg which presumably targets postsynaptic D2 receptors and causes an increase in motor activity (Koller et al. 1987; Luque-Rojas et al. 2013; Nakamura et al. 1994; Prosser et al. 1989).
Apparatus
The two-way shuttle box custom designed and manufactured by Med Associates (St. Albans, VT) and the prepulse inhibition of acoustic startle reflex apparatus (Kinder Scientific, Julian, CA) have been described in detail previously (Qiao et al. 2013). A detailed description of the motor activity monitoring apparatus (Aero Apparatus Sixbeam Locomotor System v1.4, Toronto, Canada) can be found in Qin et al. (2013).
Experiment 1: Long-term effect of adolescent risperidone treatment on adulthood antipsychotic response in the conditioned avoidance response model
The experimental procedure is described in details in Table 1.
Table 1.
Timeline of events occurred in Experiment 1
| Days of Study | Approximate age | Manipulation |
|---|---|---|
| 1-2 | PND 31-PND 32 | CAR box habituation 30 min |
| 3-9 | PND 33-PND 39 | 7 days of CAR training (CS-US) |
| 10-14 | PND 40-PND 44 | 5 days of drug test (CS-only) |
| 15 | PND 45 | 1st PPI test |
| 16-36 | PND 46-PND 66 | rest |
| 37 | PND 67 | 2nd PPI test |
| 38 | PND 68 | CAR box re-habituation 30 min |
| 39-45 | PND 69-PND 75 | 7 days of CAR training (CS1+CS2) |
| 46 | PND 76 | rest |
| 47 | PND 77-PND 79 | 3 days of CAR retraining (CS-US) |
| 48 | PND 80 | RIS 0.3 mg/kg challenge test |
Avoidance training and repeated risperidone testing in adolescence
Forty eight adolescent rats (~P 31) were first habituated to the shuttle boxes for 2 days (30 min/day) and then trained for conditioned avoidance responding for 7 consecutive days/sessions. Each session consisted of 30 trials with a white noise (maximum duration = 10 s) serving as the conditioned stimulus (CS) and a continuous scrambled foot shock (0.8 mA, maximum duration = 5 s) serving as the unconditioned stimulus (US). An avoidance response was recorded if a rat moved from one compartment into the other within the 10 s of CS presentation. If the rat did not respond during the entire 5 s presentation of the shock, the trial was terminated and the intertrial intervals started (30 - 60 s). The total number of avoidance responses and intertrial crossings were recorded for each session.
At the end of the training session (~P 40), rats were first matched on avoidance performance on the last training day (i.e. predrug) and then randomly assigned to one of three groups: vehicle (sterile water, n = 16), risperidone 0.5 mg/kg (RIS 0.5, n = 16), and risperidone 1.0 mg/kg (RIS 1.0, n = 16), and tested daily for avoidance response for 5 consecutive days. During each drug test, rats were first injected with RIS (0.5 or 1.0 mg/kg, sc) or sterile water. One hour later, they were placed in the CAR boxes and tested under the CS-only (no shock, 30 trials/daily session) condition. Number of avoidance and intertrial crossings in each session was recorded. In addition, ultrasonic vocalizations at the 22 kHz range (20 - 32 kHz, as measure of conditioned fear/anxiety) (Mead et al. 2008; Sun et al. 2010a) were also recorded for the first 10 min of the testing using Avisoft Recorder software (Version 3.4). This measure was taken to assess the behavioral specificity of the RIS effect, as has been done previously (Qiao et al. 2013).
Avoidance retraining/testing in adulthood
Rats remained in their home cages until ~ P 68 when all rats were returned to the CAR boxes for one habituation session, followed by 7 days of CAR training in a modified training procedure. This novel procedure has been used before (Chen et al. 2011; Li et al. 2009) and was used in the present study to assess the extent to which early adolescent risperidone treatment might have altered animals’ learning and memory ability. A less salient and less informative signal (pure tone as CS2 which was paired with the US in only half of the trials) was added in the training for two reasons. The first one is that it would provide an additional measure of learning and memory. The second one is that a weaker CS2 avoidance might be more sensitive to any subtle effect of adolescent RIS treatment than CS1 avoidance. Each training session consisted of 30 trials. Ten trials used a 10 s 76 dB white noise as the CS (CS1) with its termination immediately followed by a shock if the rats did not make an avoidance response. The remaining 20 trials used a pure tone (10 s, 2800 kHz, 85 dB) as the CS (CS2). In 15 CS2 trials, the CS2 was followed by the shock if the rat failed to respond to the CS2; whereas, in the remaining 5 trials no shock was used. The 10 CS1 trials were randomly intermixed with the 20 CS2 trials. Two days after the last CS1-CS2 training session, all rats were trained again for 3 days using the same procedure as used in the adolescence period (white noise as the only CS) to ensure that their avoidance responding was at a high level (> 50% avoidance trials) before the challenge test.
Risperidone assessment in adulthood (~P 80)
The long-term effect of adolescent RIS treatment was assessed 1 day after the 3rd retraining session (~P 80) with all rats being injected with a challenge dose of RIS 0.3 mg/kg and tested for avoidance performance, 22 kHz USV and intertrial crossings in the CS (white noise)-only condition (30 trials) 1 h later.
PPI assessment
Two separate PPI tests were conducted to assess the possible effect of adolescent RIS treatment on the sensorimotor gating. One test was done at the late adolescence period (~P 45, 1 day after the 5 drug test days) and another one at the early adulthood period (~P 67, 2 days before the adulthood CAR training procedure). The PPI test procedure was adapted from (Culm and Hammer 2004) and has been described in details previously (Qiao et al. 2013).
Experiment 2: Long-term effect of adolescent risperidone treatment in the avoidance response model on adulthood quinpirole-induced hyperlocomotion test
This experiment examined whether the D2 receptor system is involved in RIS sensitization as assessed in the conditioned avoidance response model. The basic procedure was similar to that in Experiment 1 with the exception that PPI tests were omitted. Twenty four rats were assigned to 2 groups (n = 12/group) and treated with VEH (sterile water with 1.0% glacial acetic acid) and RIS 1.0 for 5 days in adolescence. After they became adults and were retrained in the CAR task for 10 days (7 days of CS1+CS2-US sessions and 3 CS1-US sessions), as done in Experiment 1, they were habituated to the locomotor boxes for 1 day on PND 80 (30 min/day). One day later (PND 81), all rats were challenged with quinpirole (1.0 mg/kg, sc) and motor activity was recorded for 120 min in 12 10-min blocks. Finally, the RIS sensitization in the avoidance task was assessed on PND 84 after 1 day of avoidance retraining (CS-US condition) under the RIS 0.3 mg/kg challenge.
Experiment 3: Long-term effect of adolescent risperidone treatment on adulthood antipsychotic response in the PCP-induced hyperlocomotion model
Table 2 details the timeline of major events.
Table 2.
Timeline of events occurred in Experiment 2
| Days of study | Approximate age | Manipulation |
|---|---|---|
| 1-2 | PND 42-43 | Habituation to the motor activity boxes |
| 3-7 | PND 44-48 | 5 days of drug testing |
| 8 | PND 49 | 1st PPI test |
| 9-26 | PND 50-66 | Rest |
| 27 | PND 67 | 2nd PPI test |
| 28-35 | PND 68-74 | Rest |
| 36 | PND 75 | Habituation to the motor activity boxes |
| 37 | PND 76 |
Drug challenge test: RIS 0.3 mg/kg, PCP 3.2
mg/kg |
| 38 | PND 77 | 3rd PPI test |
Repeated risperidone treatment in adolescence
Twenty four adolescent rats (~P 42-43) were first handled and habituated to the locomotor activity apparatus for 2 days (30 min/day). On each of the next 5 consecutive days, adolescent rats (~P 44-48) were first injected with vehicle (sterile water), RIS 0.3 mg/kg, or RIS 1.0 mg/kg, and then immediately placed in the boxes for 30 min. At the end of the 30-min period, they were taken out and injected with PCP (3.2 mg/kg, sc) or saline and placed back in the boxes for another 60 min. Motor activity was measured throughout the entire 90-min test session. There were a total of four groups (n = 6/group): VEH+VEH (sterile water + saline), VEH+PCP, RIS 0.3+PCP and RIS 1.0+PCP.
Risperidone assessment in adulthood (~P 76)
Twenty nine days after the last PCP test, after the rats became adults (~P 75), they were returned to the locomotor activity boxes for 1 re-habituation session (30 min), followed by the first RIS sensitization challenge test 1 day later (~P 76). On the challenge test day, all rats were first injected with a small dose of RIS 0.3 mg/kg and then immediately placed in the motor activity boxes for 30 minutes. At the end of the 30-min period, rats were taken out and injected with PCP (3.2 mg/kg) and placed back in the boxes for another 60 min. Motor activity was recorded for the entire 90-min test session.
PPI assessment
A total of three PPI tests were conducted throughout the developmental period. The first one was done during the late adolescent period (~P 49, 1 day after the 5 drug test days), the second one during the early adulthood period (~P 67). The third one (~P 77) was conducted 1 day after the 1st sensitization test.
Experiment 4: Long-term effect of adolescent risperidone treatment in the PCP model on adulthood quinpirole-induced hyperlocomotion test
This experiment examined whether postsynaptic D2 receptor is involved in the long-lasting RIS sensitization from adolescence to adulthood in the PCP-induced hyperlocomotion model. The procedure was similar to that used in Experiment 3 with the exception that PPI tests were omitted. Sixteen adolescent rats were assigned to 2 groups (n = 8/group): VEH (sterile water with 1.0% glacial acetic acid)+PCP, and RIS 1.0+PCP, and tested for PCP-induced hyperlocomotion for 5 days in adolescence (P 43-48). After they became adults (PND 76), they were challenged with quinpirole (1.0 mg/kg, sc) and motor activity was recorded for 120 min.
Statistical Analysis
All data were expressed as mean + SEM. Data from the repeated drug test period or retraining period were analyzed using a factorial repeated measures analysis of variance (ANOVA) with the between-subjects factor of drug group and the within-subjects factors of test session, type of CS trial or time block, followed by post hoc Tukey tests. Percent PPI data were analyzed using repeated measures ANOVAs with drug treatment group as a between-subjects factor and prepulse level as a within-subjects factor. For all analyses, p < 0.05 was considered statistically significant and all data were analyzed using SPSS version 21.
RESULTS
Experiment 1: Long-term effect of adolescent risperidone treatment on adulthood antipsychotic response in the conditioned avoidance response model
Avoidance training and repeated risperidone treatment in adolescence
Avoidance response
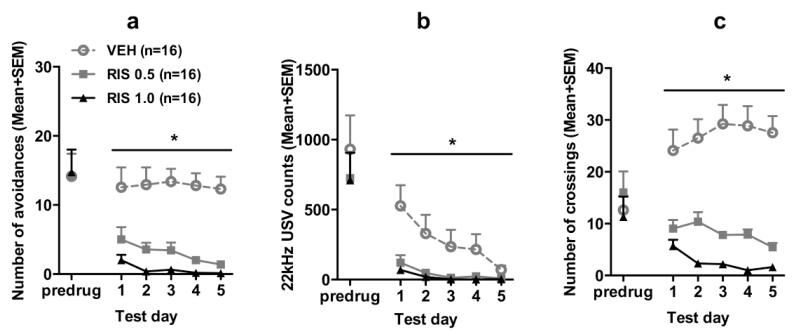
Fig. 1a shows the number of avoidance responses on the last training (predrug) day and 5 drug test days. There was no group difference on the last training day. Throughout the 5 drug test days, RIS treatment disrupted avoidance response persistently. Repeated measures ANOVA revealed a main effect of group, F(2, 45) = 26.400, p < 0.001; day, F(4, 180) = 2.666, p = 0.034, but no significant group × day interaction, F(8, 180) = 0.912, p = 0.508. Post hoc Tukey tests revealed that the two RIS groups had significantly lower avoidance than the VEH group, all ps < 0.001, although they did not differ significantly from each other.
Fig. 1.
Effects of repeated risperidone treatment (0.5 or 1.0 mg/kg, sc) on the avoidance responses (a), 22 kHz USV counts (b) and intertrial crossings (c) in adolescence. Data on the last training (predrug) day and throughout the 5 drug test days are expressed as mean + SEM. * p < 0.05 relative to the VEH group.
22 kHz USV
RIS also decreased the number of 22 kHz USV (Fig. 1b). On the predrug day, there was no significant group difference, p = 0.707. During the drug test phase, the two RIS groups had fewer 22 kHz USVs in comparison to the VEH group. Repeated measures ANOVA revealed a main effect of group, F(2, 45) = 6.100, p = 0.005, day, F(4, 180) = 10.123, p < 0.001 and a significant group × day interaction, F(8, 180) = 3.187, p = 0.002.
Intertrial crossing
No significant group difference was found on the last training day (Fig. 1c). During the drug test phase, RIS dose-dependently decreased intertrial crossings. Repeated measures ANOVA revealed a main effect of group, F(2, 45) = 47.091, p < 0.001, group × day, F(8, 180) = 2.308, p < 0.023. Post hoc tests showed that the two RIS groups made significantly fewer intertrial crossings than the VEH group, all ps < 0.001.
Avoidance retraining/testing in adulthood: Effect of adolescence risperidone treatment on the acquisition of CS2 avoidance and re-acquisition of CS1 avoidance
Throughout the 7 avoidance test sessions to the two CS trials, avoidance response to the CS1 was higher than avoidance response to the CS2 (data not shown). The main effect of group was not significant, and neither were its interactions with session and CS type, all ps > 0.175, suggesting that prior RIS treatment did not cause a significant impairment of acquisition of a new avoidance response (i.e. CS2 avoidance) and expression of a learned one (i.e. CS1 avoidance).
RIS assessment in adulthood on ~P 80
Avoidance response
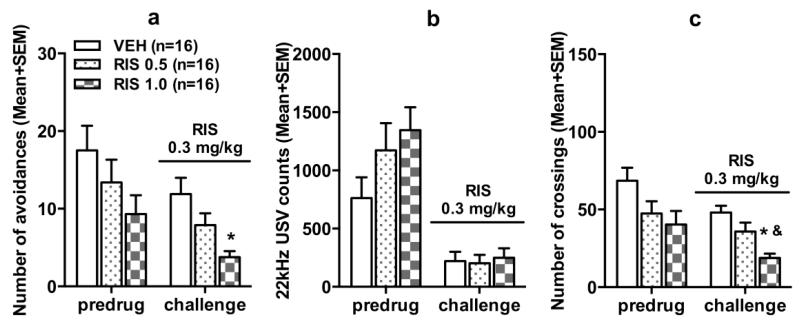
Fig. 2a shows the number of avoidance responses on the predrug day and the RIS sensitization test day (~P 80). Before the RIS challenge, there was no significant group difference. On the challenge day when all rats were injected with RIS 0.3 mg/kg, the two RIS groups made fewer avoidance responses than the VEH group. One-way ANOVA confirmed this observation as there was a significant effect of group, F(2, 45) = 6.735, p = 0.003. Post hoc Tukey tests showed that the RIS 1.0 but not RIS 0.5 group was significantly different from the VEH group, p = 0.002. Exclusion of rats with less than 50% avoidance on the predrug day yielded the same result (data not shown).
Fig. 2.
Number of avoidance responses (a), 22 kHz USV counts (b) and intertrial crossings (c) made by the rats in the risperidone (0.5 mg/kg), risperidone (1.0 mg/kg) and vehicle groups on the last retraining (predrug) day and on the risperidone challenge test (sensitization assessment). * p < 0.05 relative to the VEH group, & p < 0.05 relative to the RIS 0.5 group.
22 kHz USV and intertrial crossing
No significant group difference on the 22 kHz USV was detected on the predrug day and on the challenge day (Fig. 2b). The number of intertrial crossing differed among groups on the challenge day (Fig. 2c). One-way ANOVA showed a main effect of group, F(2, 45) = 10.943, p < 0.001. Post hoc tests showed that the RIS 1.0 group made fewer crossings than the other two groups, ps < 0.026.
These findings indicate that repeated RIS treatment in adolescence induced a long-lasting sensitization effect that persisted into adulthood. This effect was dose-dependent and behaviorally specific, as it was only shown in avoidance, but not in 22 kHz USV.
PPI assessment
PPI data from the 2 time points of testing (~P 45 and 67) did not reveal any significant group difference, p = 0.931 and 0.541, respectively (data not shown). The group × prepulse level interactions were also not significant p = 0.119 and 0.762, respectively. These findings suggest that repeated RIS treatment did not significantly impair the sensorimotor gating ability.
Experiment 2: Long-term effect of adolescent risperidone treatment in the avoidance response model on adulthood quinpirole-induced hyperlocomotion test
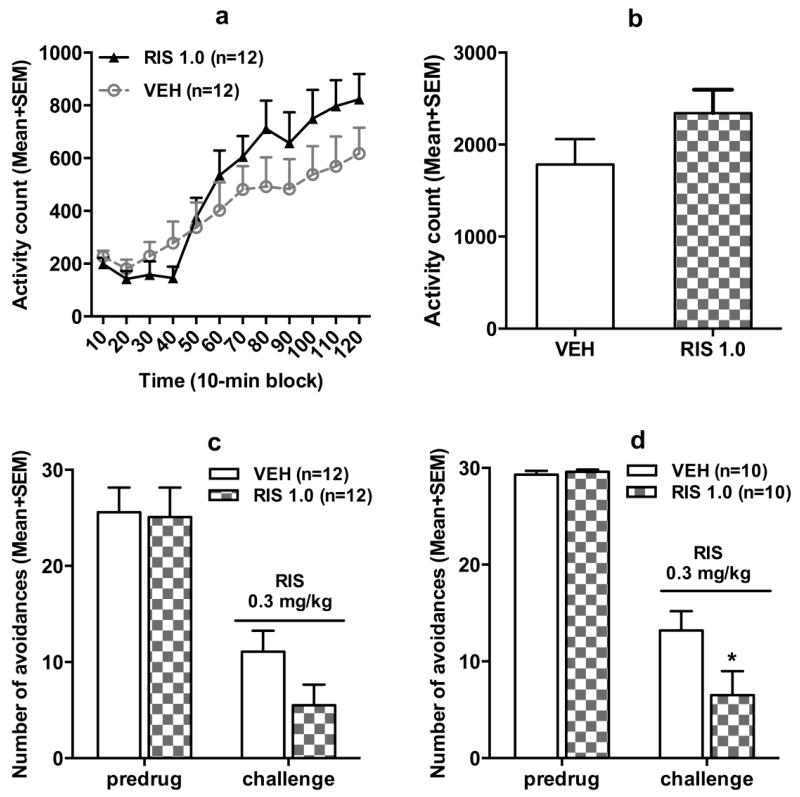
Avoidance data during the adolescent treatment period and adulthood retraining period were similar to those reported in Experiment 1 (data not shown). In the quinpirole-induced hyperlocomotion test, rats previously treated with RIS in adolescence appeared to make more motor activity than those previously treated with vehicle (Fig. 3a and 3b). Repeated measures ANOVA revealed a significant main effect of time block, F(11,242) = 46.825, p < 0.001, a significant block × group interaction, F(11,242) = 2.942, p = 0.001, but no main effect of group, F(1,22) = 0.743, p = 0.398. The group difference on the total motor activity in 120 min was also not significant, t(22) = −1.482, p = 0.152. These data suggest that adolescent RIS treatment slightly increased drug sensitivity to quinpirole in adulthood, as manifested in the increased motor activity over time.
Fig. 3.
Quinpirole-induced locomotor activity in 12 10-min blocks (a) or in 120 min (b) in the quinpirole-induced hyperlocomotion test. All rats were injected with quinpirole (1.0 mg/kg, sc) and then measured for motor activity for 120 min. After 1 retraining (predrug) session, all rats were injected with risperidone (0.3 mg/kg, sc) and avoidance responses (c, d) were measured 60 min later. c shows data of all rats, while d shows only of those with avoidance > 50% on the predrug day. All data are expressed as mean + SEM. * p < 0.05 relative to the VEH group.
After the quinpirole test, all rats were retrained (CS-US session) 1 day and then challenged with risperidone (0.3 mg/kg) in the CAR to verify the RIS sensitization in adulthood. As showed in Fig. 3c, on the challenge day, the RIS 1.0 group made fewer avoidance responses than the VEH group, this difference was marginally significant, t(22) = 1.822, p = 0.082. When 4 rats with less than 50% avoidance on the predrug day were excluded from the analysis, the group difference was significant, t(18) = 2.107, p = 0.049 (Fig. 3d), confirming the RIS sensitization effect. Overall, results from this experiment indicate that adolescent RIS treatment might have induced a long-lasting supersensitivity of D2/3 receptors that persisted into adulthood, which may serve as a mechanism underlying RIS sensitization.
Experiment 3: Long-term effect of adolescent risperidone treatment on adulthood antipsychotic response in the PCP-induced hyperlocomotion model
Repeated risperidone treatment in adolescence
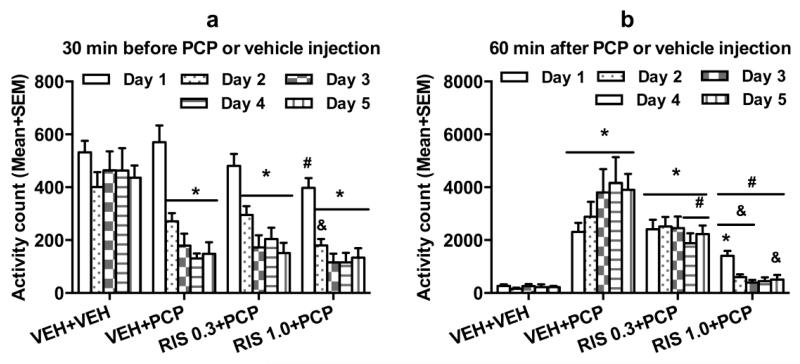
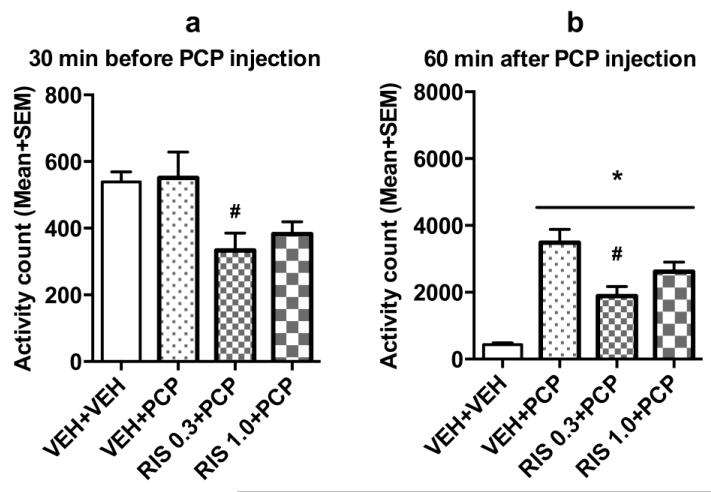
Fig. 4a shows the mean motor activity of the 4 groups of rats during the 30-min period before PCP or vehicle injection throughout the 5 days of drug testing. Repeated measures ANOVA revealed a main effect of group, F(3, 20) = 16.166, p < 0.001, a main effect of test day, F(4, 80) = 32.719, p < 0.001, and significant effect on group × day interaction, F(12, 80) = 2.719, p = 0.004. Post hoc Tukey tests revealed that the 3 PCP treated groups had significantly lower motor activity than the VEH+VEH group, all ps < 0.001, mainly on Days 2-5.
Fig. 4.
Effect of repeated risperidone (0.3 and 1.0 mg/kg) treatment on PCP-induced hyperlocomotion across the 5 test days (n = 6/group) during adolescence. Locomotor activity in the 30-min before vehicle or PCP injection (a) and 60-min after PCP injection (b) are expressed as mean + SEM for each group. * p < 0.05 relative to the VEH+VEH group; # p < 0.05 relative to the VEH+PCP group; & p < 0.05 relative to the RIS 0.3+PCP group.
Fig. 4b shows the mean motor activity of the 4 groups of rats during the 60-min period after PCP or vehicle injection throughout the 5 days of drug testing. Two-way repeated measures ANOVA revealed a significant main effect of group, F(3, 20) = 33.520, p < 0.001, and a significant group × day interaction, F(12, 80) = 2.413, p = 0.023, but no main effect of test day, F(4, 80) = 0.202, p = 0.937. Post hoc Tukey tests revealed that the two RIS (0.3 and 1.0 mg/kg) groups showed significantly lower motor activity compared to the VEH+PCP group, all ps < 0.027. The VEH+VEH group had significantly lower motor activity than the VEH+PCP group, p < 0.001, the RIS 0.3+PCP group, p < 0.001.
Sensitization assessment during adulthood on ~P 76
On the RIS sensitization challenge test, in the first 30 min (Fig. 5a), the 2 RIS groups had lower motor activity than the vehicle group. One-way ANOVA revealed a main effect of group, F(3, 20) = 4.424, p = 0.015. Post hoc Tukey tests showed that the RIS 1.0+PCP group had significantly lower motor activity than the VEH+PCP group, p = 0.037. In the 60 min test period after PCP injection (Fig. 5b), 1 rat in the RIS 0.3+PCP was identified as an outlier because it had a motor activity value on the challenge test more than 3 box lengths from the 75 percentile value of the group, thus was excluded from the data analysis. One-way ANOVA revealed a main effect of group, F(3, 19) = 21.037, p < 0.001. Post hoc two group comparisons revealed that the RIS 0.3+PCP group had significantly lower motor activity than the VEH+PCP group, p = 0.006, indicating a RIS sensitization effect. The 3 PCP-treated groups also had significantly higher motor activity than the VEH+VEH group that received vehicle injection in adolescence, all ps < 0.013, indicating a PCP-induced psychomotor sensitization that was not prevented by adolescent RIS treatment.
Fig. 5.
Locomotor activity during the 30-min test period before PCP injection (a) and the 60-min test period after PCP injection (b) on the risperidone challenge test day (~P 76). * p < 0.05 relative to the VEH+VEH group; # p < 0.05 relative to the VEH+PCP group.
Collectively, results from this experiment confirmed RIS sensitization. Like RIS sensitization observed in the avoidance conditioning test (Experiment 1), this effect was dose-dependently and long-lasting. It was manifested as an enhanced inhibition of PCP-induced hyperlocomotion (an index of antipsychotic activity) in the RIS treated animals.
PPI assessment
PPI data from the 3 time points of testing (~P 49, 67 and 77) did not reveal any significant group difference, p = 0.817, 0.7403, and 0.535, respectively (data not shown). The group × prepulse level interactions were also not significant p = 0.983, 0.149, and 0.275, respectively. These findings suggest that repeated RIS treatment and PCP treatment did not significantly impair the sensorimotor gating ability.
Experiment 4: Long-term effect of adolescent risperidone treatment in the PCP model on adulthood quinpirole-induced hyperlocomotion test
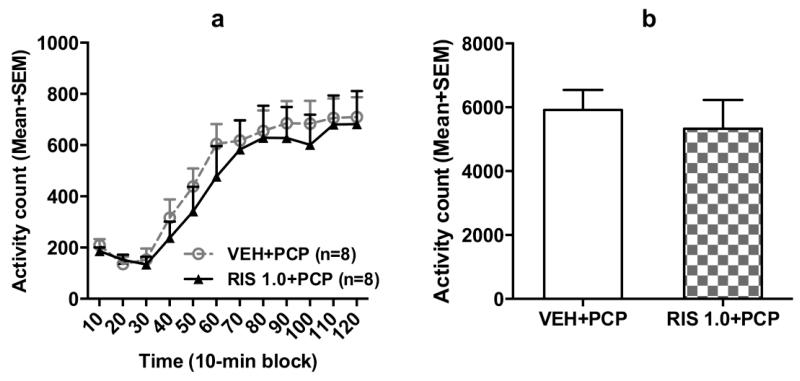
In the adolescent RIS treatment phase, repeated RIS treatment (1.0 mg/kg, sc) progressively enhanced its inhibition of PCP-induced hyperlocomotion (data not shown). In the quinpirole-induced hyperlocomotion test in adulthood, rats previously treated with RIS 1.0+PCP in adolescence did not seem to make more motor activity than those previously treated with vehicle (Fig. 6a). Repeated measures ANOVA revealed a significant main effect of time-block, F(11,154) = 29.296, p < 0.000, but no main effect of group, F(1,14) = 0.285, p = 0.602, nor block × group interaction, F(11,154) = 0.241, p = 0.994. The group difference of the total motor activity in 120 min was also not significant (Fig. 6b), t(14) = 0.533, p = 0.602. These data suggest that adolescent RIS treatment in the PCP model may not have induced a long-lasting change in D2/3 receptor sensitivity.
Fig. 6.
Quinpirole-induced locomotor activity in 12 10-min blocks (a) and in the 120 min test period (b) of rats pretreated with risperidone (1.0 mg/kg) or VEH followed by PCP (3.2 mg/kg, sc) for 5 days in adolescence. All rats were injected with quinpirole (1.0 mg/kg, sc) and then measured for motor activity for 120 min. Data are expressed as mean + SEM.
DISCUSSION
In this study, we addressed the issue of how early adolescence RIS treatment influences antipsychotic efficacy of RIS in adulthood. We demonstrated that 5 daily administration of RIS in adolescence dose-dependently increased behavioral sensitivity to RIS in both the CAR and PCP-induced hyperlocomotion models. This potentiated antipsychotic effect as indexed by the enhanced inhibition of avoidance response and enhanced suppression of PCP-induced hyperlocomotion was observed during the repeated drug test period (the induction phase) in adolescence and during the challenge test (the expression phase) in adulthood. This effect was long-lasting and behavioral specific, as it manifested in avoidance, intertrial crossing and PCP-induced increase in motor activity, but not in 22 kHz USV. The fact that it was observed in two independent preclinical tests of antipsychotic activity strongly suggests that this antipsychotic sensitization across development is a general phenomenon associated with repeated drug administration. In this regard, this study reinforces the notion that antipsychotic drug treatment history or experience can permanently alter drug sensitivity.
Much of adolescent antipsychotic work has been focusing on how early antipsychotic treatment alters the development of brain and behavioral functions. One research area is to identify possible adverse effects on basic psychological functions such as attention, emotion, and learning and memory (Milstein et al. 2013; Vinish et al. 2013). Another important area is to determine the potential preventive or treatment effects of adolescence antipsychotic exposure on behavioral and brain abnormalities in animal models of schizophrenia (Piontkewitz et al. 2011; Piontkewitz et al. 2009). The present study differed from most in the literature in that it focused on how antipsychotic treatment during adolescence alters adult antipsychotic response. In adult rats, we have shown that repeated RIS treatment (0.2-1.0 mg/kg) for 7 days caused an increased sensitivity to RIS (0.33 mg/kg) challenge 3 weeks later (Mead and Li 2010). The present study further showed that this RIS sensitization effect could persist from adolescence into adulthood against the background of brain maturation and induced in the PCP-induced hyperlocomotion model. Because the inhibition of PCP-induced hyperlocomotion is commonly used as a screening tool to determine the potential antipsychotic activity (Arnt 1995; Gleason and Shannon 1997; Sun et al. 2010b), this finding implies that repeated RIS treatment might lead to an increase in its antipsychotic efficacy. Because repeated RIS treatment caused a similar sensitization profile as olanzapine does in both models, but dissimilar effect to that of clozapine (e.g. tolerance) (Qiao et al. 2013; Shu et al. 2013), RIS may share a similar therapeutic and side effect profile with olanzapine, rather than with clozapine. In this regard, our paradigm may be useful in identifying clozapine-like compounds.
The neurobiological mechanisms that mediate persistent RIS sensitization are not known. Based on the finding that adolescent RIS exposure (0.3, 1.0 and 3.0 mg/kg) for 3 weeks selectively and dose-dependently increased levels of D2 receptors in the medial prefrontal cortex and hippocampus (Moran-Gates et al. 2007), we used the quinpirole-induced hyperlocomotion test to assess whether 5 days of RIS treatment in adolescence would give rise to a long-lasting functional upregulation of dopamine D2 receptors that may underlie adolescent RIS sensitization. Results from Experiment 2 provide a support for this hypothesis, although the evidence was weak due to a lack of significant group difference. Results from Experiment 4 did not support this hypothesis: adult rats that were treated with RIS in adolescence did not exhibit higher motor activity than those treated with vehicle, possibly due to the masking effect from PCP. This result was in conflict with our recent demonstration that the same 5 days of RIS treatment in adult rats (not in adolescents) induced a long-last functional upregulation of dopamine D2 receptors as assessed 50 days later in the quinpirole-induced hyperlocomotion test, an effect parallel to the long-lasting RIS sensitization (Gao and Li 2013). One possible reason for the discrepancy is the high degree of brain plasticity of adolescent brain (versus the adult brain). It is possible that the brain maturation process from adolescence to adulthood might have dampened the impact of antipsychotic treatment on D2 receptors in the limbic regions. Another possibility is that our motor activity assessment system is not sensitive enough to pick up subtle changes induced by RIS. Therefore, whether RIS-induced functional upregulation of dopamine D2 receptors serves as a mechanism of across development RIS sensitization could not be determined based on the current evidence. More work directly measuring D2 receptor density or function is required.
In addition to impact on D2 receptors, the same RIS treatment (0.3, 1.0 and 3.0 mg/kg for 3 weeks) was found to increase D1 receptors in the striatum, D4 receptors in the striatum and hippocampus of adolescent rats (Moran-Gates et al. 2007). It also increased 5-HT1A receptors in the medial-prefrontal and dorsolateral-frontal cortices, and also in the hippocampus (at the high dose), but reduced 5-HT2A levels in the medial-prefrontal and dorsolateral-frontal cortices (Choi et al. 2010). RIS (1.0 and 3.0 mg/kg/day) also had an effect on NMDA and AMPA receptors (Choi et al. 2009). These receptor binding findings suggest that the long-term changes of specific dopamine, serotonin and glutamate receptors in various limbic regions implicated in action of antipsychotic drugs (Swerdlow et al. 1994) could contribute to RIS sensitization. These changes could alter the trajectory of the brain and behavioral development in adolescent animals, which in turn may change their later response to drug treatment as adults. Future research should test the involvement of these receptors.
RIS remains to be one of widely prescribed second generation antipsychotic drugs, especially for a variety of disorders prevalent in childhood and adolescence, including schizophrenia, autism, attention deficit and hyperactivity disorder (ADHD), Tourette’s disorder, disruptive behaviors, bipolar disorder, and mood disorders (Correll et al. 2011). Because adolescence is such an important period in which the brain undergoes dramatic transitions and re-organization (Brenhouse and Andersen 2011), any drug exposure during this period could potentially derail the normal brain developmental process. Antipsychotic drugs are no exception. Currently in psychopharmacology, much attention has been focused on the harmful consequences of adolescent exposures to drugs of abuse (e.g. nicotine, methamphetamine). With increased number of children and adolescents being treated with antipsychotic drugs, it is critical to pay more attention to the long-term consequences of early antipsychotic exposure. Our finding that as little as 5 days of RIS exposure in adolescence could induce a long-lasting effect that persists into adulthood suggests that there is a real possibility of brain and behavioral functional changes. Because pediatric patients are likely to receive continued antipsychotic treatment into adulthood, the present work has significant clinical implications for clinical practice involving adolescence antipsychotic treatments. For example, when psychiatrists determine the drug dose and treatment schedule for younger individuals, they need to consider the patients’ early drug treatment histories.
Acknowledgments
Professor Ming Li was supported by a visiting professorship grant from the Key Laboratory of Cognition and Personality and Institute of Psychology at Southwest University, China and by National Institute of Mental Health grant R01MH085635.
Footnotes
Disclosures
All authors declare no conflict of interest.
REFERENCES
- Aguilar MA, Rodriguez-Arias M, Mari-Sanmillan MI, Minarro J. Effects of risperidone on conditioned avoidance responding in male mice. Behav Pharmacol. 1997;8:669–76. doi: 10.1097/00008877-199712000-00001. [DOI] [PubMed] [Google Scholar]
- Almandil NB, Wong IC. Review on the current use of antipsychotic drugs in children and adolescents. Archives of disease in childhood Education and practice edition. 2011;96:192–6. doi: 10.1136/archdischild-2011-300054. [DOI] [PubMed] [Google Scholar]
- Arnt J. Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of D-amphetamine. Eur J Pharmacol. 1995;283:55–62. doi: 10.1016/0014-2999(95)00292-s. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang Z, Li M. Multiple ‘hits’ during postnatal and early adulthood periods disrupt the normal development of sensorimotor gating ability in rats. J Psychopharmacol. 2011;25:379–92. doi: 10.1177/0269881109354929. [DOI] [PubMed] [Google Scholar]
- Choi YK, Gardner MP, Tarazi FI. Effects of risperidone on glutamate receptor subtypes in developing rat brain. Eur Neuropsychopharmacol. 2009;19:77–84. doi: 10.1016/j.euroneuro.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Moran-Gates T, Gardner MP, Tarazi FI. Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. Eur Neuropsychopharmacol. 2010;20:187–94. doi: 10.1016/j.euroneuro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ, March JS. Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry. 2011;72:655–70. doi: 10.4088/JCP.11r07064. [DOI] [PubMed] [Google Scholar]
- Culm KE, Hammer RP., Jr. Recovery of sensorimotor gating without G protein adaptation after repeated D2-like dopamine receptor agonist treatment in rats. J Pharmacol Exp Ther. 2004;308:487–94. doi: 10.1124/jpet.103.057158. [DOI] [PubMed] [Google Scholar]
- Gao J, Li M. Time-dependence of risperidone and asenapine sensitization and associated D receptor mechanism. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- Kalverdijk LJ, Tobi H, van den Berg PB, Buiskool J, Wagenaar L, Minderaa RB, de Jong-van den Berg LT. Use of antipsychotic drugs among Dutch youths between 1997 and 2005. Psychiatr Serv. 2008;59:554–60. doi: 10.1176/ps.2008.59.5.554. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–31. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Koller W, Herbster G, Anderson D, Wack R, Gordon J. Quinpirole hydrochloride, a potential anti-parkinsonism drug. Neuropharmacology. 1987;26:1031–6. doi: 10.1016/0028-3908(87)90245-0. [DOI] [PubMed] [Google Scholar]
- Li M, Fletcher PJ, Kapur S. Time course of the antipsychotic effect and the underlying behavioral mechanisms. Neuropsychopharmacology. 2007;32:263–72. doi: 10.1038/sj.npp.1301110. [DOI] [PubMed] [Google Scholar]
- Li M, He W, Mead A. Olanzapine and risperidone disrupt conditioned avoidance responding in phencyclidine-pretreated or amphetamine-pretreated rats by selectively weakening motivational salience of conditioned stimulus. Behav Pharmacol. 2009;20:84–98. doi: 10.1097/FBP.0b013e3283243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sun T, Zhang C, Hu G. Distinct neural mechanisms underlying acute and repeated administration of antipsychotic drugs in rat avoidance conditioning. Psychopharmacology (Berl) 2010;212:45–57. doi: 10.1007/s00213-010-1925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Rojas MJ, Galeano P, Suarez J, Araos P, Santin LJ, de Fonseca FR, Calvo EB. Hyperactivity induced by the dopamine D2/D3 receptor agonist quinpirole is attenuated by inhibitors of endocannabinoid degradation in mice. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:661–76. doi: 10.1017/S1461145712000569. [DOI] [PubMed] [Google Scholar]
- Mead A, Li M. Avoidance-suppressing effect of antipsychotic drugs is progressively potentiated after repeated administration: an interoceptive drug state mechanism. J Psychopharmacol. 2010;24:1045–53. doi: 10.1177/0269881109102546. [DOI] [PubMed] [Google Scholar]
- Mead A, Li M, Kapur S. Clozapine and olanzapine exhibit an intrinsic anxiolytic property in two conditioned fear paradigms: contrast with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav. 2008;90:551–62. doi: 10.1016/j.pbb.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–23. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein JA, Elnabawi A, Vinish M, Swanson T, Enos JK, Bailey AM, Kolb B, Frost DO. Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS One. 2013;8:e57308. doi: 10.1371/journal.pone.0057308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moran-Gates T, Gan L, Park YS, Zhang K, Baldessarini RJ, Tarazi FI. Repeated antipsychotic drug exposure in developing rats: dopamine receptor effects. Synapse. 2006;59:92–100. doi: 10.1002/syn.20220. [DOI] [PubMed] [Google Scholar]
- Moran-Gates T, Grady C, Shik Park Y, Baldessarini RJ, Tarazi FI. Effects of risperidone on dopamine receptor subtypes in developing rat brain. Eur Neuropsychopharmacol. 2007;17:448–55. doi: 10.1016/j.euroneuro.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Lopez-Moreno JA, Rodriguez de Fonseca F, Navarro M. Behavioural effects of quinpirole following withdrawal of chronic treatment with the CB1 agonist, HU-210, in rats. Behavioural pharmacology. 2005;16:441–6. doi: 10.1097/00008877-200509000-00017. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Yue JL, Goshima Y, Miyamae T, Ueda H, Misu Y. Non-effective dose of exogenously applied L-dopa itself stereoselectively potentiates postsynaptic D2 receptor-mediated locomotor activities of conscious rats. Neuroscience letters. 1994;170:22–6. doi: 10.1016/0304-3940(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–85. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Patel NC, Crismon ML, Hoagwood K, Johnsrud MT, Rascati KL, Wilson JP, Jensen PS. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44:548–56. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2011;37:1257–69. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–46. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Bernstein HG, Dobrowolny H, Bogerts B, Weiner I, Keilhoff G. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun. 2012;26:353–63. doi: 10.1016/j.bbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Prosser ES, Pruthi R, Csernansky JG. Differences in the time course of dopaminergic supersensitivity following chronic administration of haloperidol, molindone, or sulpiride. Psychopharmacology (Berl) 1989;99:109–16. doi: 10.1007/BF00634463. [DOI] [PubMed] [Google Scholar]
- Qiao J, Li H, Li M. Olanzapine sensitization and clozapine tolerance: from adolescence to adulthood in the conditioned avoidance response model. Neuropsychopharmacology. 2013;38:513–24. doi: 10.1038/npp.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin R, Chen Y, Li M. Repeated asenapine treatment produces a sensitization effect in two preclinical tests of antipsychotic activity. Neuropharmacology. 2013;75C:356–364. doi: 10.1016/j.neuropharm.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani F, Murray ML, Byrne PJ, Wong IC. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics. 2008;121:1002–9. doi: 10.1542/peds.2007-2008. [DOI] [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–31. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Shu Q, Hu G, Li M. Adult response to olanzapine or clozapine treatment is altered by adolescent antipsychotic exposure: A preclinical test in the phencyclidine hyperlocomotion model. J Psychopharmacol. 2013 doi: 10.1177/0269881113512039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, He W, Hu G, Li M. Anxiolytic-like property of risperidone and olanzapine as examined in multiple measures of fear in rats. Pharmacol Biochem Behav. 2010a;95:298–307. doi: 10.1016/j.pbb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hu G, Li M. Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: relevance to animal models of antipsychotic drugs. Eur J Pharmacol. 2009;602:334–42. doi: 10.1016/j.ejphar.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhao C, Hu G, Li M. Iptakalim: a potential antipsychotic drug with novel mechanisms? Eur J Pharmacol. 2010b;634:68–76. doi: 10.1016/j.ejphar.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Swalve N, Li M. Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: roles of number of drug exposure, drug dose, and test-retest interval. Behav Pharmacol. 2012;23:380–91. doi: 10.1097/FBP.0b013e32835651ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–54. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Allan M, Talangbayan H, Tracey A, Szechtman H. Locomotor sensitization to quinpirole: environment-modulated increase in efficacy and context-dependent increase in potency. Psychopharmacology. 1997;134:193–200. doi: 10.1007/s002130050442. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Goodwill AM, Szechtman H. Locomotor sensitization to quinpirole in rats: effects of drug abstinence and sex. Psychopharmacology. 2000;152:304–11. doi: 10.1007/s002130000538. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Foley KA, Kavaliers M, Ossenkopp KP. Neonatal immune system activation with lipopolysaccharide enhances behavioural sensitization to the dopamine agonist, quinpirole, in adult female but not male rats. Brain, behavior, and immunity. 2007;21:935–45. doi: 10.1016/j.bbi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Vinish M, Elnabawi A, Milstein JA, Burke JS, Kallevang JK, Turek KC, Lansink CS, Merchenthaler I, Bailey AM, Kolb B, Cheer JF, Frost DO. Olanzapine treatment of adolescent rats alters adult reward behaviour and nucleus accumbens function. Int J Neuropsychopharmacol. 2013:1–11. doi: 10.1017/S1461145712001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Johnson HL, Burns LN, Williams MT. Developmental treatment with the dopamine D2/3 agonist quinpirole selectively impairs spatial learning in the Morris water maze. Neurotoxicol Teratol. 2009;31:1–10. doi: 10.1016/j.ntt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–41. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- Zhang C, Fang Y, Li M. Olanzapine and risperidone disrupt conditioned avoidance responding by selectively weakening motivational salience of conditioned stimulus: further evidence. Pharmacol Biochem Behav. 2011;98:155–60. doi: 10.1016/j.pbb.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li M. Contextual and behavioral control of antipsychotic sensitization induced by haloperidol and olanzapine. Behav Pharmacol. 2012;23:66–79. doi: 10.1097/FBP.0b013e32834ecac4. [DOI] [PMC free article] [PubMed] [Google Scholar]