Abstract
Rationale
Consumption of alcohol begins during late adolescence in a majority of humans, and the greatest drinking occurs at 18–25 years then decreases with age.
Objectives
The present study measured differences in ethanol intake in relation to age at the onset of ethanol access among non-human primates to control for self-selection in humans and isolate age effects on heavy drinking.
Methods
Male rhesus macaques were assigned first access to ethanol during late adolescence (n = 8), young adulthood (n = 8) or early middle age (n = 11). The monkeys were induced to drink ethanol (4% w/v in water) in increasing doses (water, 0.5 g/kg, 1.0 g/kg, 1.5 g/kg) using a fixed-time (FT) 300-s schedule of food delivery, followed by 22 hours/day concurrent access to ethanol and water for 12 months. Age-matched controls consumed isocaloric maltose-dextrin solution yoked to the late adolescents, expected to be rapidly maturing (n = 4).
Results
Young adult monkeys had the greatest daily ethanol intake and blood-ethanol concentration (BEC). Only late adolescents escalated their intake (ethanol, not water) during the second compared to the first 6 months of access. On average, testosterone was consistent with age differences in maturation, and tended to increase throughout the experiment more for control than ethanol-drinking adolescent monkeys.
Conclusions
Young adulthood in non-human primates strongly disposes toward heavy drinking independently of sociocultural factors present in humans. Drinking ethanol to intoxication during the critical period of late adolescence is associated with escalation to heavy drinking.
Keywords: ethanol self-administration, rhesus monkeys, age at onset, testosterone, blood-ethanol concentration, schedule-induced polydipsia
Introduction
Across cultures, ethanol use most often begins during late adolescence (Degenhardt et al. 2008). Worldwide, harmful use of ethanol results in 2.5 million deaths/year, and accounts for 9% of deaths (320,000 deaths/year) among 15–29 year olds according to the World Health Organization (2012). Heavy drinking is most common among young adults, 18–25 years (Chen and Kandel 1995; Dawson et al. 2008). Individuals who begin drinking during late adolescence (high school) have 3-fold increased likelihood of heavy drinking as middle-aged adults (Merline et al. 2004). Early age at the onset of ethanol drinking is associated with increased likelihood of future dependence (Dawson et al. 2008; Grant and Dawson 1997; Grant et al. 2001, 2006) and predicts ethanol-related problems (Schuckit and Russell 1983; Hingson et al. 2006). Age at first ethanol-induced intoxication also predicts subsequent harmful drinking (Warner and White 2003; Morean et al. 2012). Quantity of ethanol consumed during youth may influence the risk of alcohol use disorder (Norberg et al. 2009).
Epidemiological and clinical studies acknowledge that humans are not randomized to drink as adolescents so there is a possibility of self-selection. Covariates of early-onset ethanol use could influence the trajectory to dependence. Early-onset drinkers may have heavy drinking parents, a stressful environment, deviant peers, or weaker social roles that, if present, compete with ethanol drinking (Rossow and Kuntsche 2013; Skidmore and Murphy 2011). As targets for changing adolescent ethanol use, social factors are difficult to manipulate. Biological variables proposed to account for alcoholism risk from early-onset drinking include hypothalamic pituitary-gonadal (HPG) axis activity. Pubertal maturation, orchestrated by the HPG axis, is positively correlated with ethanol use after controlling for age (Biehl et al. 2007). The likelihood of ever having consumed ≥ 1 glass of ethanol increased as sex hormones testosterone or estradiol increased in a cross-sectional study of 11–16-year old boys, controlling for age, but only estradiol correlated with lifetime ethanol use (de Water et al. 2012).
Studies of humans rely on self-report, which may be biased (Sartor et al. 2011). We developed a model in which non-human primates are induced to drink ethanol and then have 12 months of daily access to ethanol and water 22 hours/day. We previously observed heavy ethanol drinking (> 3.0 g/kg ethanol/day, equivalent of about 12 drinks) and average blood-ethanol concentration (BEC) exceeding the legal definition of intoxication (80 mg/dl), in 40% of young adult cynomolgus monkeys (Grant et al. 2008). Our definition in monkeys matches human alcoholic drinking, which may include > 22 drinks/day for several days (Majchrowicz and Mendelson 1970; Mello and Mendelson 1970) and exceeds binge drinking (≥ 5 drinks/occasion) (Dawson et al. 2005). In monkeys, we documented physical dependence during abstinence (Cuzon-Carlson et al., 2011), tremors the morning after heavy drinking (Welsh et al., 2011), fatty liver after 6 months of ethanol drinking and liver inflammation after 18 months, consistent with the onset of alcoholic liver disease in humans (Ivester et al. 2007). The three studies reference above used the same ethanol self-administration protocol as the present study, and therefore can capture trajectories to harmful ethanol use.
Non-human primates have similar ethanol pharmacokinetics, genetics, social organization and endocrine characteristics to humans (Green et al. 1999; Grant and Bennett 2003). A key advantage of the monkey model is the ability to study the onset of chronic drinking to intoxication and future heavy drinking. The present study is the first in which primates were randomly assigned to age at first ethanol access. This experimental design isolates the contribution of age to escalation of ethanol intake, spanning late adolescence, young adulthood and middle age. This study does not explore factors that lead to early onset of drinking ethanol. 6 These are circumvented by having age as an independent variable. The data address the very basic question of whether age at the onset of ethanol drinking or first intoxication is related to future heavy ethanol consumption in primates.
Methods and Materials
The subjects were male rhesus macaques (n = 31, Macaca mulatta, 5.3–10.6 kg) differing in age at the onset of ethanol access: late adolescents with access to ethanol (n = 8, 4.0–4.6 years), late adolescent controls (n = 4, 4.4–4.5 years), young adults (n = 8, 5.0–5.6 years) and middle-aged adults (n = 11, 6.9–9.7 years). The experiment investigated a time of rapid growth and physiological changes, so yoked controls were included for late adolescents to track maturation via testosterone. As young adults and middle-aged adults were mature, controls were not included. All the monkeys were born and reared at the Oregon National Primate Research Center with their mothers until 2–3 years of age. In nature, puberty begins during the breeding season at 4 years in male rhesus monkeys (Mann et al. 1993). In lab studies, the onset of puberty in male rhesus macaques occurs between 2–4 years based on descent of testes, with change in perinea color coinciding with peak growth at 4.5 years on average (Watts and Gavan 1982). Monkeys in the current study were individually housed in quadrant cages (0.8 m × 0.8 m × 0.9 m) in a temperature- (20–22°C) and humidity- (65%) controlled room with a 11-h light cycle (lights on, 6:00–8:00 am). Individually housed monkeys had continuous visual, auditory, and olfactory contact with the other monkeys in the housing room. Each cage had an operant panel on one wall that delivered food and liquids. All monkeys were weighed weekly. The monkeys were trained and given three meals per day as previously described (Grant et al. 2008). Daily meals were modified to allow steady body weight increase throughout the experiment. Due to age differences, the percentage weight gain from after acclimation to the end of the study was greatest among late adolescents (late adolescent drinkers, 30 ± 10%; late adolescent controls, 33 ± 5%; young adults, 18 ± 17%; middle-aged adults, 11 ± 8%). All animal procedures were approved by the Oregon National Primate Research Center IACUC and were performed in accordance with the NIH and the Guide for the Care and Use of Laboratory Animals.
Apparatus
The panel within each animal’s cage is updated from a previously described apparatus (Vivian et al. 2001). A computerized system (Dell Computer Corporation, Round Rock, TX, with interface and programming environment from National Instruments Corporation, Austin, TX) controlled each panel, which had two drinking spouts, a row of three lights (red, white, and green) above each spout, a central recess containing a dowel pull and light, and a magazine for delivery of banana-flavored pellets (Bio-Serv, Frenchtown, NJ) via tubing connected to a pellet dispenser (Med Associates, St. Albans, VT) secured to the cage. The panel also contained a hole with a poke-sensitive infrared switch (OTBVR81L, Banner Engineering, Minneapolis, MN). Tubing connected the spouts through computer-controlled valves (Parker Fluidics, Cleveland, OH) to a fluid reservoir on a digital scale (AV4101C, Ohaus Corporation, Pine Brook, NJ), which interfaced with the computer.
Ethanol Access
Ethanol self-administration was induced using schedule-induced polydipsia (Grant et al. 2008; Vivian et al. 2001). One banana-flavored pellet was delivered every 5 min [fixed time (FT) 300-s schedule] until a volume of water equivalent to 1.5 g/kg ethanol (4% w/v, in water) or a programmed dose was consumed, after which pellets were delivered [fixed-ratio (FR)-1] after a 3-hour time out. The FR-1 schedule occurred until the daily ration of pellets was consumed or the end of the session (22 hours/day). The programmed dose of ethanol increased every 30 days beginning at 0 g/kg/day (a volume of water equivalent to 1.5 g/kg ethanol), then 0.5 g/kg/day (mean ± SEM, 86 ± 3 ml), 1.0 g/kg/day (157 ± 6 ml), and finally 1.5 g/kg/day (228 ± 10 ml). After induction of 1.5 g/kg/day of ethanol, the monkeys had concurrent access to ethanol (4% w/v) and water 22 hours/day, or to maltose-dextrin (10% w/v, in water) and water (control monkeys). Isocaloric controls were yoked to late adolescent monkeys with ethanol access and provided maltose-dextrin to match the calories in the average dose of ethanol consumed the previous week by their experimental counterpart. Maltose-dextrin was provided daily to control monkeys during induction and access to ethanol 22 hours/day, but drinking of maltose-dextrin was not induced using the FT schedule.
Assays
The monkeys were trained to participate in awake venipuncture using food treats (Porcu et al. 2006). Circadian variation in testosterone approaches a nadir during the morning in adult male rhesus macaques (Urbanski 2011). Morning (6:00 am–8:00 am) blood draws (3 ml) prior to the first meal provided plasma for assay. Samples were set on ice until centrifuged (3000 rpm, 15 min, 4°C) and stored at −80°C. Total testosterone was assayed by Endocrine Services Technology Laboratory at ONPRC using a Roche Cobas e411 (sensitivity, 0.025–15 ng/ml; inter-assay variation, 5.7%). Blood-ethanol concentration (BEC) was measured by gas chromatography from samples obtained 30, 60 or 90 min after the onset of the 0.5 g/kg, 1.0 g/kg and 1.5 g/kg induction sessions, respectively, and approximately every 5 days, 7 hours after onset of 22 hours/day-access sessions (Grant et al. 2008).
Data Analysis
The dependent variables included ethanol intake (g/kg), drinking bout measures (continuous fluid displacement with < 5 min between records), BEC, percentage of heavy drinking days (daily intake > 3.0 g/kg, about 12 drinks) and testosterone. Bout measures reveal drinking patterns suggestive of gulping, e.g., consuming the daily dose in fewer bouts or in bouts of greater volume and duration. The independent variables were age (three levels: late adolescent, young adult, middle-aged adult), age at first intoxication as indicated by BEC ≥ 80 mg/dl (two levels: before 5 years, after 5 years), and time (two levels: first 6 months of 22 hours/day access, 181–201 sessions/monkey; second 6 months of 22 hours/day access, 159–227 sessions/monkey). Dependent measures were compared between the first and second 6 months of the experiment because of rapid growth and physiological changes during late adolescence. Control monkeys were included in the analysis of testosterone. The data were analyzed using linear mixed models in which monkey was the subject variable, and the covariance structure was the best fit according to Schwarz’s Bayesian Information Criteria. This statistical approach based on the likelihood function instead of analysis of variance and is appropriate for longitudinal data sets in which individuals differ in the trajectory of behavior, implying unequal covariance among measures within subjects (Littell et al. 1996; Krueger and Tian 2004). Post-hoc testing for significant main effects and interactions were conducted using Bonferroni-corrected planned comparisons (t-tests). All analyses were conducted using SAS 9.2 (Cary, NC), α < 0.05.
Results
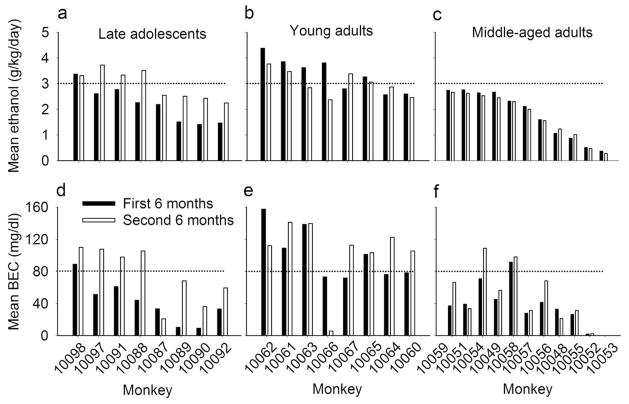
Young adult rhesus monkeys self-administered greater doses of ethanol (mean ± SEM, 3.2 ± 0.02 g/kg/day, (352–354 days/monkey) compared to late adolescent (2.5 ± 0.02 g/kg/day, 360–361 days/monkey) and middle-aged monkeys (1.7 ± 0.02 g/kg/day, 407–408 days/monkey), F(2, 24) = 10.0, p = 0.0007. Most (6/8) young adults were heavy drinkers, with daily intake > 3.0 g/kg throughout 22-hour/day sessions. In contrast, few monkeys in the other age groups were heavy drinkers (3/8 late adolescents, 0/11 middle-aged adults). Among young adults, BEC measured every fifth day was significantly greater (102 ± 2.4 mg/dl, 62–64 samples/monkey) than middle-aged adults (43 ± 2.1 mg/dl, 45–47 samples/monkey) or late adolescents (54.2 ± 2.3 mg/dl, 65–66 samples/monkey), F(2, 23) = 8.1, p = 0.002. Mean BEC for most (7/8) young adults was > 80 mg/dl, exceeding the U.S. legal limit for intoxication (Figure 1).
Fig. 1.
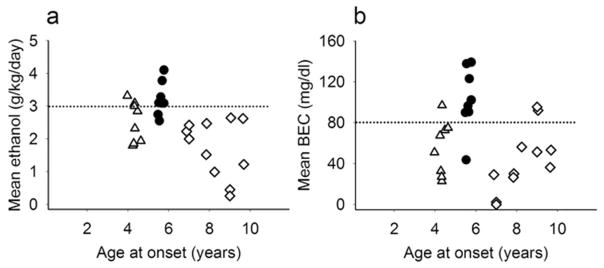
a) Young adult rhesus monkeys (filled circles) self-administered greater doses of ethanol compared to late adolescent (triangles) or middle-aged adult monkeys (diamonds) as measured from 393–408 sessions/monkey, and b) young adults had greater blood-ethanol concentrations 7 hours after the onset of daily access (47–66 samples/monkey) during more than 12 months of daily 22 hours/day continuous access to ethanol. Heavy drinking was defined as > 3.0 g/kg/day (equivalent of 12 drinks; Vivian et al., 2001; Grant et al., 2008) and > 80 mg/dl (horizontal lines)
Age at the onset of ethanol access among late adolescents ranged 4.0–4.6 years and did not correlate with average ethanol intake. However, age at which drinking to BEC ≥ 80 mg/dl was first measured (first intoxication) during 22 hours/day access to ethanol varied from 4.4–5.6 years and inversely correlated with average daily ethanol intake over 12 months of access, r = −0.75, p = 0.03. Late adolescents that first became intoxicated before 5 years of age self-administered greater doses of ethanol during 12 months of access (n = 4, 3.0 ± 0.02 g/kg/day) compared to monkeys with intoxication delayed to > 5 years of age (n = 4, 2.1 ± 0.03 g/kg/day), F(1, 6) = 6.6, p = 0.04 (Figure 2). Monkeys that drank to intoxication before 5 years of age had~2-fold greater BEC (69.3 ± 3.2 mg/dl) compared to monkeys that first drank to intoxication after 5 years of age (39.1 ± 2.9 mg/dl), but this was not statistically significant, F(1, 6) = 1.2. Of those first intoxicated before 5 years, 3/4 had mean BEC > 70 mg/dl, and 3/4 first intoxicated after 5 years had mean BEC < 40 mg/dl. Age at first intoxication did not correlate with average daily intake among the other age groups.
Fig. 2.
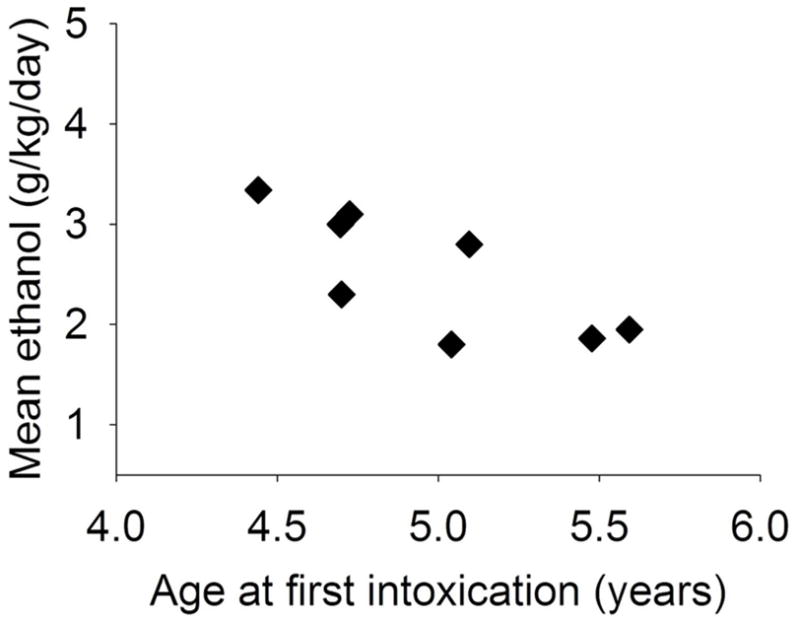
The age at which late adolescent male rhesus monkeys first became intoxicated (BEC ≥ 80 mg/dl) correlated significantly (r = −0.75, p = 0.03) with the mean daily dose of ethanol that they subsequently consumed during more than 12 months of 22 hours/day continuous access to ethanol
Monkeys with first access to ethanol as late adolescents had increased ethanol intake over months [F(2, 24) = 415.1, p < 0.0001] according to post-hoc tests [t(22) = −26.9, p < 0.001: first 6 months, 2.2 ± 0.03 g/kg/day; second 6 months, 3.0 ± 0.02 g/kg/day]. In contrast, ethanol intake over the same intervals decreased among young adults by the equivalent of almost 2 drinks/day [t(22) = 12.8, p < 0.001: 3.4 ± 0.03 g/kg/day to 3.0 ± 0.02 g/kg/day] and decreased slightly among middle-aged adults [t(22) = −5.8, p < 0.001: 1.8 ± 0.02 g/kg/day to 1.7 ± 0.02 g/kg/day; Figure 3]. The average percentage of total calories from ethanol was 25 ± 6% among late adolescents (first 6 months, 20.3 ± 5.8%; second 6 months, 28.9 ± 7.0%), 37 ± 5% among young adults (first 6 months, 37.5 ± 6.5%; second 6 months, 37.4 ± 6.0%) and 23 ± 11% (first 6 months, 21.2 ± 10.4%; second 6 months, 22.8 ± 11.8%) among middle-aged adults. The percentage of total calories from ethanol differed significantly between the first and second 6 months of access only for late adolescents, F(5, 22) = 8.7, p = 0.0001, t(22) = −5.0, p = 0.0009. Ethanol self-administration increased in most (7/8) late adolescents and decreased in most (6/8) young adults (Figure 3). Among late adolescents, the heaviest drinkers showing a steep and steady increase in the daily dose of self-administered ethanol throughout the first 6 months of access (Supplemental Figure 1a). The slope to asymptotic drinking could not be calculated due to multiple or no plateaus of intake. Overall, the Supplemental figures show that consistent trends of increasing or decreasing drinking were absent among middle-aged monkeys, in contrast to the intake of late adolescents or young adults.
Fig. 3.
Mean daily ethanol self-administration (a–c) and blood-ethanol concentration (BEC, d–f) measured every five days, 7 hours after onset of the 22-hour/day session during approximately the first (filled bars; 181–202 sessions/monkey) and second (open bars; 152–227 sessions/monkey) 6 months of access to ethanol among monkeys differing in age at the onset of ethanol access (a and d, late adolescents; b and e, young adults; c and f, middle-aged adults)
Among late adolescents, BEC 7 hours after session onset was significantly greater, F(2, 22) = 6.2, p = 0.007, during the second (75.6 ± 3.7 mg/dl) compared to the first (39.6 ± 2.6 mg/dl) 6 months of 22-hours/day access. Greater intoxication during the second 6 months among late adolescents was not determined by food intake (see Methods), and the percentage change in meal size from the first to the second 6 months of ethanol access did not correlate with the percentage change in ethanol intake (r = 0.34, p = 0.41) or BEC (r = 0.16, p = 0.71). In contrast, BEC did not differ between the first and second 6 months among young adult (101.0 ± 3.3 mg/dl to 103.0 ± 3.6 mg/dl) or middle-aged (37.7 ± 2.8 mg/dl to 47.4 ± 3.0 mg/dl) monkeys. Regardless of age at first intoxication, late adolescents had greater BEC during the second (intoxication < 5 years, 84.2 ± 5.5 mg/dl); intoxication > 5 years, 67.1 ± 4.8 mg/dl) compared to the first 6 months of access (intoxication < 5 years, 59.4 ± 3.9 mg/dl; intoxication > 5 years, 19.8 ± 2.7 mg/dl), F(1, 6) = 32.6, p = 0.001. Age at first intoxication among late adolescents was significantly correlated with mean BEC during the first 6 months (r = −0.79, p = 0.02) but not the second 6 months (r = −0.34, p = 0.41), when BEC was greater.
From the first to the second 6 months of access to ethanol, the percentage of days with intake > 3.0 g/kg increased ~2-fold (23 ± 8% to 48 ± 12%) among late adolescents. All late adolescents had more heavy drinking days during the second compared to the first 6 months. In contrast, the percentage of heavy drinking days was greater in young adults, but decreased from 63 ± 10% (Figure 4b, where the vertical line bisects the frequency distribution) during the first 6 months to 49 ± 10% during the second 6 months (6/8 monkeys). The percentage of heavy drinking days was lower among middle-aged adults, with a slight increase from the first to the second 6 months of access (7 ± 2% to 11± 4%; Figure 4), observed in only 4/11 monkeys using a criteria of 1% change. Abstinent days (defined as < 0.1 g/kg) were rare (late adolescents, 2.2 ± 1.7%; young adults, 2.1 ± 0.3%), but among middle-aged adults intake was < 1.0 g/kg on one third of days, and 6.7 ± 11% of days were abstinent.
Fig. 4.
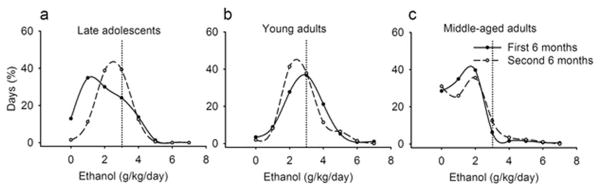
Frequency distributions of daily ethanol intake across 1-g/kg intervals among rhesus macaques differing in age at onset (a, late adolescents; b, young adults; c, middle-aged adults). The vertical line at 3.0 g/kg indicates the operational definition of heavy drinking (Grant et al., 2008)
The ages did not differ in water intake, F(2, 25) = 3.3, p = 0.06. Water intake decreased between the first and second 6 months of access among late adolescents (985 ± 11 ml/day to 901 ± 9 ml/day) and young adults (1639 ± 19 ml/day to 1531 ± 21 ml/day) and increased among middle-aged adults (1199 ± 13 ml/day to 1306 ± 11 ml/day), F(2, 24) = 93.8, p < 0.0001. The escalation of fluid intake among late adolescents was therefore specific to ethanol.
With regard to drinking pattern, maximum duration of ethanol bouts, F(2, 24) = 44.3, p < 0.0001, and maximum bout volume, F(2, 24) = 42.6, p < 0.0001, increased significantly among late adolescents from the first to the second 6 months of access and were stable among young and middle-aged adults (Table 1). Increased bout volume and duration only among late adolescents is consistent with changes in ethanol intake and BEC. Among monkeys that first became intoxicated before or after 5 years of age, maximum bout duration and volume increased from the first to second 6 months of access. Within these groups, age at first intoxication correlated with ethanol bouts and maximum bout duration, but only during the first (r = −0.80, p = 0.02 and r = −0.72, p = 0.04, respectively) and not the second (r = −0.69, p = 0.06 and r = −0.57, p = 0.14, respectively) 6 months. In contrast to changes in ethanol bout duration and volume, the number of bouts in which the daily ethanol dose was consumed was similar across 6-month intervals for all age groups. During 12 months of access, number of ethanol bouts (r = 0.18, p < 0.0001), maximum bout duration (r = 0.27, p < 0.0001) and maximum bout volume (r = 0.40, p < 0.0001) correlated with BEC, during both the first and second 6 months of access, except bout number (Table 1).
Table 1.
Characteristics of ethanol self-administration during the first and second 6 months of 22 hours/day continuous access as indicated by bouts (continuous fluid displacement with < 5 min between records) among rhesus monkeys of different ages at the onset of ethanol access. Data are median ± SEM. For each measure, the total number of observations including all monkeys are listed in parentheses.
| Bouts | Max bout duration (s) | Max bout volume (ml) | Correlation with BEC (r, p) | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Bouts | Max bout duration | Max bout volume | ||||
|
|
||||||
| Late adolescents | ||||||
| First | 19 ± 0.2 (1600) | 389 ± 14 | 102 ± 1 | 0.17, 0.01 | 0.40, < 0.0001 | 0.44, < 0.0001 |
| Second | 20 ± 0.2 (1215) | 541 ± 24** | 131 ± 2** | 0.34, < 0.0001 | 0.43, < 0.0001 | 0.50, < 0.0001 |
| Young adults | ||||||
| First | 20 ± 0.2 (967) | 425 ± 15 | 127 ± 2 | 0.38, < 0.0001 | 0.39, < 0.0001 | 0.31, < 0.0001 |
| Second | 19 ± 0.2 (1337) | 472 ± 11 | 129 ± 2 | −0.002, < NS | 0.22, 0.003 | 0.19, 0.01 |
| Middle-aged adults | ||||||
| First | 17 ± 0.2 (2025) | 436 ± 41 | 87 ± 2 | 0.07, NS | 0.24, 0.003 | 0.39, < 0.0001 |
| Second | 18 ± 0.2 (2461) | 348 ± 18 | 90 ± 1 | 0.19, 0.01 | 0.29, < 0.0001 | 0.33, < 0.0001 |
p < 0.001, compared to first 6 months
Consistent with group differences in maturation, late adolescents (mean ± SEM: drinkers, 1.3 ± 0.2 ng/ml; controls, 1.7 ± 0.2 ng/ml) had significantly lower testosterone at the onset of the experiment compared to young adults (8.9 ± 4.0 ng/ml) and middle-aged adults (10.3 ± 1.2 ng/ml), but similar testosterone to age-matched controls, F(3, 27) = 22.1, p < 0.0001. Testosterone did not change significantly between the first and second 6 months of self-administration (late adolescent drinkers, 2.3 ± 0.3 ng/ml to 3.6 ± 0.6 ng/ml; late adolescent controls, 3.0 ± 0.5 ng/ml to 5.9 ± 1.5 ng/ml; young adults, 8.3 ± 1.0 ng/ml to 6.9 ± 1.0 ng/ml; middle-aged adults, 10.0 ± 0.8 ng/ml to 11.5 ± 0.9 ng/ml. Although not significant, testosterone increased ~ 2-fold among late adolescent controls, for which the percentage increase during the second compared to the first 6 months of access was, on average, greater among controls (231 ± 70%) compared to late adolescents with access to ethanol (159 ± 21%). Testosterone among adults was consistent between the first to the second 6 months of ethanol access (young, 81 ± 10%; middle-aged, 114 ± 12%). Across and within groups, testosterone at baseline was not correlated with mean daily ethanol intake during the first (r = 0.17, p = 0.33) or second (r = −0.16, p = 0.42) 6 months of access.
Age differences in response to induction and experience with ethanol intoxication during schedule-induced polydipsia did not contribute to age differences in ethanol intake during 22 hours/day access. During induction, BEC increased significantly with dose for each group [F(2, 45) = 138, p < 0.0001; Table 2]. However, across 0.5 g/kg, 1.0 g/kg and 1.5 g/kg induction, there were no group differences in BEC, including late adolescents that first experienced intoxication before or after 5 years of age. There were no consistent and significant differences between the groups in duration and volume of the largest bouts during each phase of induction (Table 2). Maximum bout duration was weakly, but positively, correlated with BEC, r = 0.15, p = 0.003, but as shown in Table 2, this widely varied across the age groups and phases. Maximum bout volume, however, positively correlated with BEC, r = 0.63, p < 0.0001, and also among each age group in most induction phases.
Table 2.
Characteristics of ethanol self-administration during the phases of induction as indicated by bouts (continuous fluid displacement with < 5 min between records) and blood-ethanol concentration (BEC) among rhesus monkeys of different ages at the onset of ethanol access. Data are median (± SEM) unless otherwise noted. For each measure, the total number of observations are listed in parentheses.
| 0.5 g/kg | 1.0 g/kg | 1.5 g/kg | |
|---|---|---|---|
| Late adolescents | |||
| Mean BEC (mg/dl) | 17.0 ± 2.3 (48) | 43.2 ± 4.7 (56) | 59.0 ± 8.2 (55) |
| Max bout duration (min) | 28.4 ± 2.6 (240) | 49.9 ± 4.3 (256) | 100.3 ± 5.6 (496) |
| r, p | −0.58, 0.001 | −0.71, < 0.0001 | −0.26, NS |
| Max bout volume (ml) | 71.5 ± 1.3 (240) | 140.4 ±2.5 (256) | 199.1 ± 3.1 (496) |
| r, p | 0.45, 0.02 | 0.79, < 0.0001 | 0.60, 0.0002 |
| Young adults | |||
| Mean BEC (mg/dl) | 17.6 ± 2.0 (48) | 45.9 ± 4.0 (48) | 65.9 ±6.0 (48) |
| Max bout duration (min) | 15.0 ± 1.4 (239) | 24.2 ± 1.3 (239) | 29.9 ± 1.2 (488) |
| r, p | 0.16, NS | −0.14, NS | 0.09, NS |
| Max bout volume (ml) | 91.9 ± 1.4 (239) | 146.0 ± 4.1 (239) | 191.4 ± 4.7 (488) |
| r, p | −0.08, NS | 0.32, 0.04 | 0.55, 0.0001 |
| Middle-aged adults | |||
| Mean BEC (mg/dl) | 24.4 ± 1.6 (66) | 44.9 ± 2.7 (66) | 60.8 ± 3.9 (66) |
| Max bout duration (min) | 17.1 ± 1.1 (360) | 31.0 ± 1.8 (359) | 57.1 ± 2.8 (941) |
| r, p | −0.39, 0.003 | −0.18, NS | −0.06, NS |
| Max bout volume (ml) | 99.3 ± 1.6 (360) | 194.8 ± 4.3 (359) | 277.0 ± 5.0 (941) |
| r, p | 0.38, 0.003 | 0.63, < 0.0001 | 0.58, < 0.0001 |
Discussion
These data show two main influences of age on ethanol self-administration in macaques. First, young adults were heavier drinkers compared to late adolescents or middle-aged adults, similar to humans (Dawson et al. 2008; Grant et al. 2001). Second, late adolescents escalated their ethanol intake as they matured into young adulthood (Figures 3 and 4, Supplemental Figure 1). The age at which monkeys first became intoxicated ( BEC > 80 mg/dl) strongly and inversely correlated with the average dose of ethanol consumed during 22-hours/day access across the experiment (Figure 2). This finding resembles data in humans that self-reported age at first intoxication predicted the frequency and amount of drinking (Morean et al. 2012) and problem drinking (Warner and White 2003) during adulthood. In humans, the percentage of individuals using ethanol increases steeply between 14–18 years, a time of transition from adolescence to young adulthood (Chen and Kandel 1995). In addition, the similarity of the current data to cross-sectional or longitudinal data in humans depends on the common assumption that self-report provides an estimate of ethanol intake, and that age differences in cross-sectional analyses are similar to longitudinal patterns within individuals. Although it appears that age differences in ethanol intake in monkeys are consistent with the patterns suggested by human self-report, the longitudinal design of the present study includes accurate and frequent measures of both ethanol intake and BEC not feasible in humans. The importance of these data is to demonstrate the capacity of the model to address potential mechanisms involved in ethanol use that are likely to be important in humans, and could be targets for intervention.
Plasma testosterone was lower among late adolescent compared to young adult and middle-aged monkeys, indicating that late adolescents were relatively immature at the onset of the study. Compared to testosterone in late adolescent controls that on average increased nearly two-fold between the first and second 6 months, testosterone increased only slightly among ethanol drinkers in this age group. Ethanol intake therefore appeared to suppress gonadal maturation in macaques, consistent with reports that testosterone and luteinizing hormone concentrations among adolescent males that abused drugs and alcohol were about two-fold lower compared to controls (Diamond et al. 1986). The current study did not find a correlation between plasma testosterone and ethanol intake, in agreement with a cross-sectional study of Dutch boys showing that plasma testosterone did not correlate with recent or lifetime ethanol consumption (de Water et al. 2012).
The current data show statistically similar ethanol intake among late adolescent and young adult monkeys, although the frequency of ethanol use, the percentage of daily drinkers (Chen and Kandel 1995), and monthly binge drinkers (Substance Abuse and Mental Health Services Administration 2012) differs greatly between late adolescent and young adult humans. This difference could be related to access. The percentage of 8th, 10th and 12th grade students reporting that ethanol is easy to obtain is about 58%, 79% and 90%, respectively (Johnston et al. 2013). It is unknown whether human adolescents would drink similar amounts as young adults given equal access. Access to ethanol was matched between age groups in the current study, and intake among late adolescents was nearly as great as young adults. Limited access may therefore be an important factor controlling ethanol use in human adolescents, because when access was equivalent among the age groups in the current study, late adolescents consumed nearly as much ethanol as young adults.
In contrast to agreement between the current data and data in humans, other animal models collectively find greater ethanol intake during adolescence compared to young adulthood (Barr et al. 2004; Brunell and Spear 2005; Melendez 2011). Previous studies comparing heavy drinking in adolescent and adult rodents did not report longitudinal measures of BEC and session durations varied from 1 hour/day (Broadwater et al. 2011) to 20–24 hours/day (Brunell and Spear 2005; Vetter et al. 2007). The only previous study of rhesus monkeys found that adolescent females < 4 years of age, who were reared with peers instead of their mothers, drank more flavored ethanol in 1-hour sessions over 3 weeks compared to young adults (Barr et al. 2004). Comparison of the results of the present study with Barr et al. (2004) is not possible because the access conditions and duration of study were very different.
Age differences in average daily ethanol intake in the current study were not related to differences in learning to drink ethanol, or ethanol pharmacokinetics, as BEC and drinking bout parameters did not differ between the age groups during schedule-induced polydipsia. With each phase of induction, BEC increased in association with increased maximum bout volume, whereas increased maximum bout duration was not correlated, or was negatively correlated, with BEC. These relationships were similar between the age groups. The data suggest that adjunctive drinking in response to intermittent reinforcement schedules is similar between late adolescence and middle age in primates, although comparison across FT schedules would be needed to confirm this. The similarity of ethanol intake during induction suggests an absence of age differences in ethanol pharmacokinetics, and thus differences in intake during 22 hours/day access are likewise not due to pharmacokinetics.
Age differences in ethanol intake and intoxication emerged during 22-hours/day access, when the FT schedule of pellet delivery was absent. In the current study, monkeys that began drinking during late adolescence escalated their ethanol intake and BEC only during 22 hours/day access, eventually matching the intake of young adults. This escalation appears to be unique to primates. Adolescent rats do not escalate their intake compared to adults (Vetter et al. 2007). Escalated ethanol intake is expected to reflect homeostatic adaptation to repeated challenge (i.e., tolerance). Greater escalation of ethanol intake among late adolescents in this study suggests that the endogenous processes modulated by ethanol, e.g., GABAergic and glutamatergic neurotransmission (Cuzon Carlson et al. 2011), adapted more quickly to repeated exposure to ethanol.
Patterns of intake during 22 hours/day access differed between the age groups, suggesting age differences in regulatory consumption and homeostatic adaptation to chronic ethanol. Overall, ethanol intake among young adults upon initial access was high, whereas late adolescents’ initial intake was lower, similar to middle-aged monkeys. Age at onset of access, therefore, not initial intake, was associated with future escalation of ethanol self-administration. Escalation is a criterion for ethanol dependence (American Psychiatric Association 2000), and appears to characterize the drinking of humans with late adolescent onset (Clark et al. 1998). These data in non-human primates show that escalation of ethanol intake after the onset of drinking during adolescence occurs when sociocultural factors that are covariates of adolescent onset of ethanol drinking in humans are held constant (e.g., family, peers). Escalation of intake was observed in nearly all monkeys that began drinking during late adolescence, but in few monkeys that began drinking as young or middle-aged adults, suggesting a critical period for escalation of ethanol intake.
Supplementary Material
Daily ethanol intake among late adolescent male rhesus monkeys ordered from the heaviest to the lightest drinker. The data in panels a, b, c and e are from monkeys who first became intoxicated prior to 5 years of age. The first and second 6 months of access to ethanol are split (break)
Daily ethanol intake among young adult male rhesus monkeys ordered from the heaviest to the lightest drinker. The first and second 6 months of access to ethanol are split (break)
Daily ethanol intake among middle-aged rhesus monkeys ordered from the heaviest to the lightest drinker. The first and second 6 months of access to ethanol are split (break)
Acknowledgments
This research was supported by NIH grants OD011092, AA109431, AA010760, AA013510.
Footnotes
Conflict of interest: The authors do not have a financial relationship with the organization that sponsored this research. The authors have full control of all primary data and agree to allow the journal to review their data if requested.
Some of the data in this manuscript were presented at the meeting of the Research Society on Alcoholism, San Antonio, TX, June 26–30, 2010 and at the meeting of the International Society for Biomedical Research on Alcoholism, Sapporo, Japan, September 8–12, 2012.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 2000. text rev. [Google Scholar]
- Barr CS, Schwandt ML, Newman TK, Higley JD. The use of adolescent nonhuman primates to model human alcohol intake. Ann NY Acad Sci. 2004;1021:221–233. 34. doi: 10.1196/annals.1308.027. [DOI] [PubMed] [Google Scholar]
- Biehl MC, Natsuaki MN, Ge X. The influence of pubertal timing on alcohol use and heavy drinking trajectories. J Youth Adol. 2007;36:153–167. [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects of tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Dep. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism, clinical and experimental research. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li T-K. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol Clin Exp Res. 2005;29:902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li T-K. Impact of age at first drink on stress-reactive drinking. Alcohol Clin Exp Res. 2007;31:69–77. doi: 10.1111/j.1530-0277.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu W-T, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, de Girolamo G, Gureje O, Huang Y, Karam A, Kostyuchenko S, Lepine JP, Mora MEM, Neumark Y, Ormel JH, Pinto-Meza A, Posada-Villa J, Stein DJ, Takeshima T, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Braams BR, Crone EA, Peper JS. Pubertal maturation and sex steroids are related to alcohol use in adolescents. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Diamond F, Jr, Ringenberg L, MacDonald D, Barnes J, Hu CS, Duckett G, Sweetland M, Root A. Effects of drug and alcohol abuse upon pituitary-testicular function in adolescent males. J Adolesc Health Care. 1986;7:28–33. doi: 10.1016/s0197-0070(86)80091-2. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Sub Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Johanson CE. The nature of the scheduled reinforcer and adjunctive drinking in nondeprived rhesus monkeys. Pharmacol Biochem Behav. 1988;29:295–301. doi: 10.1016/0091-3057(88)90159-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of alcohol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis) Alcohol Clin Exp Res. 1999;23:611–616. [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2006;36:109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Helms CM, Gonzales S, Green HL, Szeliga KT, Rogers LSM, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 2013 doi: 10.1007/s00213-013-3052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McClintick M, Grant KA. Social rank, chronic ethanol self-administration and hypothalamic-pituitary-adrenal axis response in monkeys. Psychopharmacology. 2012;224:133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Arch Ped Adol Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Ivester P, Roberts LJ, 2nd, Young T, Stafforini D, Vivian J, Lees C, Young J, Daunais J, Friedman D, Rippe RA, Parsons CJ, Grant KA, Cunningham C. Ethanol self-administration and alterations in the livers of the cynomolgus monkey, Macaca fascicularis. Alcohol Clin Exp Res. 2007;31:144–155. doi: 10.1111/j.1530-0277.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on drug use: 2012 overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res for Nurs. 2004;6:151–157. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Majchrowicz E, Mendelson JH. Blood concentrations of acetaldehyde and ethanol in chronic alcoholics. Science. 1970;168:1100–1102. doi: 10.1126/science.168.3935.1100. [DOI] [PubMed] [Google Scholar]
- Mann DR, Akinbami MA, Gould KG, Tanner JM, Wallen K. Neonatal treatment of male monkeys with a gonadotropin-releasing hormone agonist alters differentiation of central nervous system centers that regulate sexual and skeletal development. J Clin Endocrinol Metab. 1993;76:1319–1324. doi: 10.1210/jcem.76.5.8496324. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programmed and spontaneous drinking. J Pharmacol Exp Ther. 1970;173:101–116. [PubMed] [Google Scholar]
- Merline AC, O’Malley PM, Schulenberg JE, Bachman JG, Johnston LD. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am J Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Fromme K. Age of first use and delay to first intoxication in relation to trajectories of heavy drinking and alcohol-related problems during emerging adulthood. Alcohol Clin Exp Res. 2012;36:1991–1999. doi: 10.1111/j.1530-0277.2012.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg KE, Bierut LJ, Grucza RA. Long-term effects of minimum drinking age laws on past-year alcohol use and drug use disorders. Alcohol Clin Exp Res. 2009;33:2180–2190. doi: 10.1111/j.1530-0277.2009.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Rogers LS, Morrow AL, Grant KA. Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav. 2006;84:618–27. doi: 10.1016/j.pbb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Rossow I, Kuntsche E. Early onset of drinking and risk of heavy drinking in young adulthood – a 13-year prospective study. Alcohol Clin Exp Res. 2013;37:E297–E304. doi: 10.1111/j.1530-0277.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Bucholz KK, Nelson EC, Madden PAF, Lynskey MT, Heath AC. Reporting bias in the association between age at first alcohol use and heavy episodic drinking. Alcohol Clin Exp Res. 2011;35:1418–1425. doi: 10.1111/j.1530-0277.2011.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Russell JW. Clinical importance of age at first drink in a group of young men. Am J Psychiatry. 1983;140:1221–1223. doi: 10.1176/ajp.140.9.1221. [DOI] [PubMed] [Google Scholar]
- Skidmore JR, Murphy JG. The effect of drink price and next-day responsibilities on college student drinking: a behavioral economic analysis. Psychol Addict Behav. 2011;25:57–68. doi: 10.1037/a0021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of alcohol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Watts ES, Gavan JA. Postnatal growth of nonhuman primates: the problem of the adolescent spurt. Human Biol. 1982;54:53–70. [PubMed] [Google Scholar]
- Warner LA, White HR. Longitudinal effects of age at onset and first drinking situations on problem drinking. Substance Use Misuse. 2003;38:1983–2016. doi: 10.1081/ja-120025123. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Daily ethanol intake among late adolescent male rhesus monkeys ordered from the heaviest to the lightest drinker. The data in panels a, b, c and e are from monkeys who first became intoxicated prior to 5 years of age. The first and second 6 months of access to ethanol are split (break)
Daily ethanol intake among young adult male rhesus monkeys ordered from the heaviest to the lightest drinker. The first and second 6 months of access to ethanol are split (break)
Daily ethanol intake among middle-aged rhesus monkeys ordered from the heaviest to the lightest drinker. The first and second 6 months of access to ethanol are split (break)