Abstract
BACKGROUND
The study of novel urinary biomarkers of acute kidney injury has expanded exponentially. Effective interpretation of data and meaningful comparisons between studies require awareness of factors that can adversely affect measurement. We examined how variations in short-term storage and processing might affect measurement of urine biomarkers.
STUDY DESIGN
Cross-sectional, prospective.
SETTING & PARTICIPANTS
Hospitalized patients from two sites: Yale New Haven Hospital (n= 52) and University of California, San Francisco Medical Center (n=36)
PREDICTORS
We tested the impact of 3 urine processing conditions on these biomarkers: a) centrifugation and storage at 4°C for 48 hours before freezing at −80°C, b) centrifugation and storage at 25°C for 48 hours before freezing at −80°C, and c) uncentrifuged samples immediately frozen at −80°C.
OUTCOMES
Urine concentration of five biomarkers: neutrophil gelatinase-associated lipocalin (NGAL), interleukin 18 (IL-18), kidney injury molecule 1 (KIM-1), liver-type fatty acid–binding protein (L-FABP) and cystatin C
MEASUREMENTS
We measured urine biomarkers by an established ELISA method. Biomarker values were log-transformed, and agreement with a reference standard of immediate centrifugation and storage at −80°C was compared using concordance correlation coefficients (CCCs).
RESULTS
Neither storing samples at 4°C for 48 hours nor centrifugation had a significant effect on measured levels, with CCCs above 0.9 for all biomarkers tested. For samples stored at 25°C for 48 hours, excellent CCC values (>0.9) were also noted between the test sample and the reference standard for NGAL, cystatin C, L-FABP and KIM-1. However, the CCC for IL-18 between samples stored at 25°C for 48 hours and the reference standard was 0.81 (95% CI, 0.66–0.96).
Limitations
No comparisons to fresh “unfrozen” samples, no evaluation of the effect of protease inhibitors.
CONCLUSIONS
All candidate markers tested using the specified assays showed high stability with both short-term storage at 4°C and without centrifugation prior to freezing. For optimal fidelity, urine for IL-18 measurement should not be stored at 25°C before long-term storage or analysis.
Keywords: proteins, storage, handling, concordance, urine biomarker, AKI, acute renal failure (ARF), protein stability, biospecimen handling
Development of novel biomarkers for early diagnosis, risk-stratification and prognosis of acute kidney injury (AKI) is a top priority in kidney research.(1) There are more than one hundred published studies examining more than twenty novel urine biomarkers in various settings of AKI and chronic kidney disease (CKD).(2–4) Most studies do not measure novel biomarkers immediately upon collection. Instead, urine samples are often collected, processed and stored under different protocols and subsequently assayed in batch. These protocols are largely based on opinion and convenience without evidence-based consensus about the optimal handling and processing of urine for evaluation.
The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study is a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)–sponsored multi-site research consortium whose goal is to examine the long-term outcomes of AKI.(5) A recent NIDDK workshop (Urine Biospecimen Handling Conference, February 22–23, 2010)(6) identified variations in storage and handling conditions as a major problem in using reposited samples. In response, we systematically examined how different “real-world” processing conditions might affect biomarker measurement, study interpretation, and eventual clinical application. The current standard for processing urine samples for clinical research studies is centrifugation and immediate storage at −80°C. However, in the clinical setting, samples may remain at room temperature or be refrigerated for several hours prior to processing with or without centrifugation. Therefore, we tested the effect of centrifugation and variations in short-term storage conditions on several promising urine biomarkers including neutrophil gelatinase-associated lipocalin (NGAL),(7, 8) interleukin 18 (IL-18),(9, 10) kidney injury molecule 1 (KIM-1),(11, 12) liver-type fatty acid–binding protein (L-FABP),(13) and Cystatin C (14, 15).
Methods
Patient Population
Hospitalized adult patients at the Yale-New Haven Hospital (YNHH) and the University of California, San Francisco Medical Center (UCSF) were screened for eligibility. Each site targeted a different patient population. The YNHH site enrolled fifty-two unique ICU patients admitted after cardiac surgery (coronary artery bypass graft surgery or valve replacement); biospecimens were collected within forty-eight hours of surgery. The UCSF site enrolled thirty-six unique patients admitted from the Emergency Department to the ICU or with a congestive heart failure exacerbation; biospecimens were collected as close as possible to the time of admission (for a description of the parent cohorts, see references (23, 24). Patients who were pregnant or nursing, were treated with long-term dialysis, had received a kidney transplant, or had a urostomy or nephrostomy were excluded from the study. At least 20 ml of urine was collected directly from the proximal reservoirs of a Foley catheter or a freshly voided clean-catch sample. All demographic and baseline information was obtained via medical chart review during the screening process. When ascertaining kidney function, the following definitions were employed: AKI stage 1: a 0.3 mg/dl or 50% increase in serum creatinine concentration during hospitalization; AKI stage 2: a >100% increase in serum creatinine concentration from baseline during hospitalization; CKD: an eGFR <60 ml/min/1.73 m2 using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation (20).
The study was approved by the Institutional Review Boards of Yale University, Kaiser Permanente Northern California and the University of California, San Francisco. All patients or their surrogates provided informed consent, with the exception of (1) patients who died before they or their surrogate could be approached for informed consent and (2) patients whose critical illness precluded them from providing informed consent and for whom a surrogate could not be identified after 28 days at the San Francisco site. For these two categories of patients, a waiver of consent was obtained.
Processing
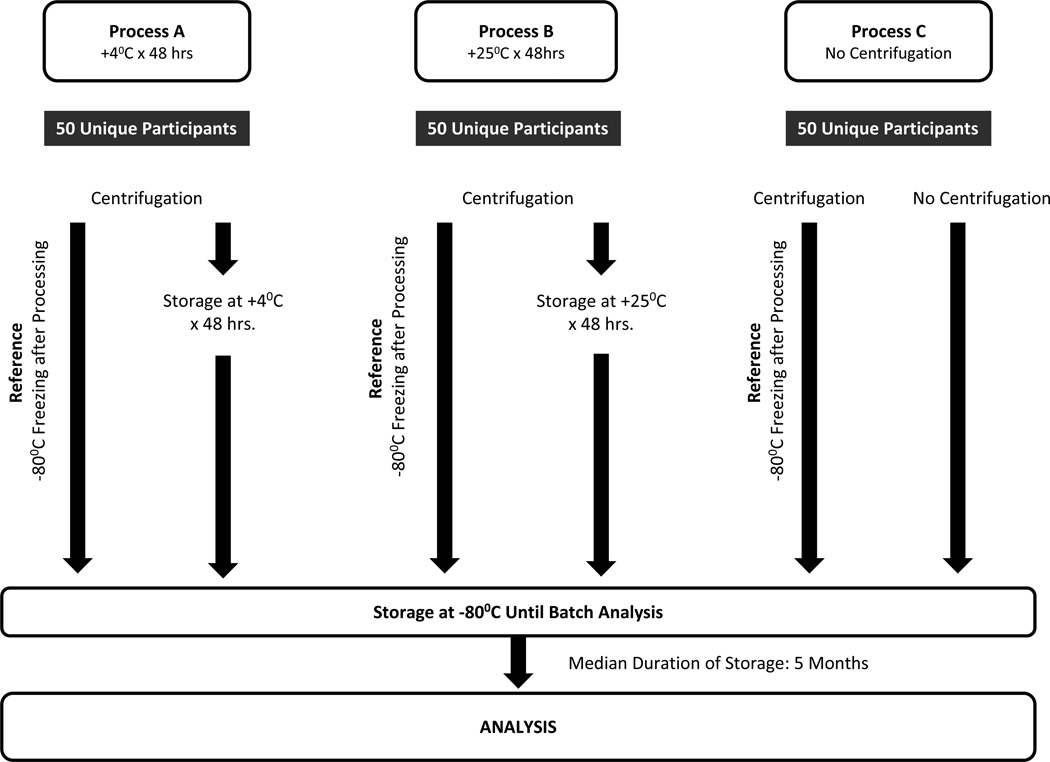
The general approach is depicted in Figure 1. Urine samples from each subject were divided and 10 ml aliquots were processed using the reference protocol (immediate centrifugation at 1000 g at 4°C for 10 minutes, followed by immediate aliquoting and storage at −80°C) and one of three test processes including: process A: Centrifugation followed by immediate aliquoting and temporary storage at 4°C for 48 hours prior to freezing at −80°C; process B: Centrifugation followed by immediate aliquoting and temporary storage at 25°C for 48 hours prior to freezing at −80°C; process C: Samples not centrifuged but immediately aliquoted and stored at −80°C. (Figure 1)
Figure 1.
Schematic representation of the experimental protocol.
Fifty pairs of samples were dedicated to each condition tested. Some patients contributed to multiple conditions if sufficient urine was available.
Biomarker Analysis
The samples for each experimental condition and reference standard were frozen at −80°C for a median of 5 months before biomarker analysis. All the biomarkers were measured in one batch. The laboratory and staff measuring the biomarkers were blinded to the study conditions.
Urine IL-18 was measured using a commercially available ELISA kit (Medical & Biological Laboratories Co., Nagoya, Japan) per manufacturer’s instructions (intra-assay and inter-assay CVs, 7.2% and 7.5%, respectively). The urine KIM-1 ELISA was constructed using commercially available reagents (Duoset DY1750, R & D Systems Inc., Minneapolis, MN) with intra-assay and interassay CVs of 5.6% and 4.9%, respectively. Urinary creatinine was measured by a modified Jaffe method. The analytical sensitivity of the creatinine assay is 0.05 mg/dl (intra-assay and interassay CVs, = 0.6% and 1.1%, respectively). The urine NGAL ELISA was performed using a commercially available assay (NGAL ELISA Kit 036; Bioporto, Grusbakken, Denmark) that specifically detects human NGAL17. The intra-assay and inter-assay CVs for NGAL were 2.1% and 9.1%, respectively. Urine L-FABP was measured using a commercially available ELISA kit (CMIC Co., Tokyo, Japan) per manufacturer’s instructions. Intraassay and inter- assay CVs for L-FABP were 10.9% and 2.7%, respectively. Cystatin C was measured by latex particle-enhanced nephelometric immunoassay on a commercial nephelometer (Siemens, Erlangen, Germany). Intra-assay and inter-assay CVs for urine cystatin C were 5.2% and 4.8%, respectively.
Statistical Methods
The objective of the statistical analysis was to determine the agreement between the measurements of the urinary biomarkers between the reference standard and values obtained in samples prepared as process A, process B, and process C. Therefore, we calculated the concordance correlation coefficient (CCC), introduced by Lin, for each scenario.(18, 19) The CCC has a scale ranging between -1 (perfect negative agreement) and 1 (perfect agreement). Zero reflects no agreement. The CCC is a more appropriate statistic than the Pearson correlation coefficient for assessing the level of agreement between two measurements of the same item. This is because the latter only quantifies the linear relationship whereas the former quantifies the linear relationship under the assumption that the slope equals one and the intercept equals zero. In addition to calculating estimates of the CCC, we also calculated the 95% confidence intervals (CIs). We required that the CCC must exceed 0.90 and 0.80 in order to claim that the paired measurements of a urinary biomarker have excellent and good agreement, respectively, under the two conditions of each process. The former follows the recommendation by Lin (19), whereas the latter is based on our own judgment.
There were two noteworthy features of our collected data: (1) the data for each urinary biomarker were asymmetrically distributed including some very large positive values, and (2) some data points could not be determined because they were below the lower limit of detection (LLD) for the assay. Because the CCC is based on a squared distance function, it can be very sensitive to the effects of large outliers. Also, the typical approach to dealing with a paired data point with at least one value below the LLD is simply to delete the pair. In order to address these two issues appropriately, we analyzed data with both raw values and log-transformed values. We performed analyses by calculating the CCC and its 95% CI under the assumption of bivariate normality and/or deleting any data pair with at least one value below the LLD. We then applied maximum likelihood estimation to calculate the means, variances, and covariance of the bivariate distribution and the standard errors of these estimates.
The common approaches of deleting the values below the LLD or imputing the fixed value (such as one half of the lower limit or the lower limit itself) present statistical issues of concern. The former can yield biased and imprecise results because of the loss of data, whereas the latter overstates the precision by underestimating the standard errors. In order to explore these biases, we also performed a sensitivity analysis by constructing a likelihood function based on the bivariate log-normal distribution in which values below the LLD were included as left-censored values, i.e., their contribution to the likelihood function was the probability of being less than or equal to the LLD. This reduced the number of samples deleted from analysis. All calculations were performed using SAS 9.3 (SAS Institute Inc) statistical software.
Results
Eighty-six unique patients participated in the study with some patients contributing to multiple experimental processes. The mean age of the participants was 70.5 ± 11.9 (standard deviation) years, with the majority being white (81.4%) and male (64%) (Table 1). The distribution of biomarker values allowed us to compare the effect of sample handling at both high and low biomarker levels (Table 2). However, for IL-18, L-FABP and cystatin C, more than 25% of samples had a value below the detection limit of the assay. Time elapsed during each condition was recorded (Table S1, available as online supplementary material) and was comparable between the two sites.
Table 1.
Demographic and Baseline Characteristics
| Value | |
|---|---|
| DEMOGRAPHICS | |
| Age (y) | 70.5 ± 11.9 |
| Female sex | 31 (36%) |
| Race Non-white White |
16 (18%) 70 (81%) |
| Hispanic ethnicity | 3 (4%) |
| CLINICAL CHARACTERISTICS | |
| AKI Network Stage Non-AKI AKI stage 1 ≥AKI stage 2 |
37 (43%) 40 (47%) 9 (11%) |
| Diabetes Mellitus | 28 (33%) |
| CKD | 19 (22%) |
| SCr (mg/dl) Baseline On day sample collected Peak during hospitalization |
1.1 ± 0.4 1.2 ± 0.7 1.6 ± 1.1 |
| DIPSTICK RESULTS | |
| Protein Negative Trace 30 (+) >100 (++) Hematuria Negative Trace Small (+) >Moderate or large (++) |
53 (62%) 15 (17%) 8 (9%) 10 (12%) 21 (24%) 26 (30%) 11 (13%) 28 (33%) |
Note: N=86. Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation. Conversion factor for serum creatinine in mg/dL to µmol/L, ×88.4. AKI stage 1: Defined as 0.3-mg/dL or 50% increase in SCr concentration during hospitalization; AKI stage 2: Defined as >100% increase in SCr concentration from baseline during hospitalization; CKD: defined as estimated glomerular filtration rate <60 ml/min/1.73 m2.
AKI, acute kidney injury; CKD, chronic kidney disease; SCr, serum creatinine
Table 2.
Biomarker levels in study participants
| Median (10th–90th percentile) |
LLD of Assay |
No. (%) samples < LDD |
|
|---|---|---|---|
| IL-18 (pg/ml) | 20.3 (12.5–99.32) | 12.5 | 99 (33.0%) |
| NGAL (ng/ml) | 19.4 (4.0–275) | 4 | 48 (16.0%) |
| KIM-1 (pg/ml) | 731 (79–3260) | 59 | 21 (7.0%) |
| L-FABP (ng/ml) | 6.9 (3–55) | 3 | 87 (29.0%) |
| Urine cystatin C (mg/ml) | 0.02 (0.005–0.20) | 0.005 | 80 (26.7%) |
Note: For descriptive purposes, values below the LLD have been imputed with the LLD.
LLD: lower limit of detection; IL-18 (interleukin 18); NGAL (neutrophil gelatinase-associated lipocalin); KIM-1 (kidney injury molecule 1); L-FABP (liver-type fatty acid–binding protein)
Our primary analysis focused on the CCC for log-transformed biomarker levels above the LLD (Table 3, Figure S1). For the biomarkers tested, neither storing samples at 4°C for 48 hours nor lack of centrifugation was significantly associated with the levels of IL-18, NGAL, KIM-1, L-FABP, or cystatin C. There was excellent agreement between processes for all biomarkers tested with CCC above 0.9. Similarly, NGAL, KIM-1, L-FABP, and cystatin C demonstrated excellent concordance between levels in samples at 25°C for 48 hours and the reference standard. Results were similar for untransformed biomarker levels (data not shown). However, there was only good concordance for IL-18 between samples stored at 25°C for 48 hours and the reference standard for both log-transformed (CCC, 0.81; 95% CI, 0.66–0.96) and untransformed (CCC, 0.79; 95% CI, 0.45–0.85) values. To examine the impact of including pairs with values below the LLD, we also performed a sensitivity analysis by developing a statistical model based on a bivariate lognormal distribution that allowed for left-censoring of low data points. This analysis demonstrated similar results to the aforementioned analyses (Table 3).
Table 3.
Concordance Correlation Coefficients for 3 Processes
| Process | IL-18 | NGAL | KIM-1 | L-FABP | Cystatin C* |
|---|---|---|---|---|---|
| Log-Transformed Results* | |||||
| A: Initial 48 h at 4°C | 0.92 (0.85, 0.98) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) | 0.99 (0.98, 1.00) | 0.94 (0.89, 0.99) |
| B: Initial 48 h at 25°C | 0.81 (0.66, 0.96) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) | 0.95 (0.92, 0.99) | 0.93 (0.87, 0.99) |
| C: No centrifugation | 0.98 (0.96, 1.00) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) |
| Log-Transformed Results & Accounting for Values <LLD | |||||
| A: Initial 48 h at 4°C | 0.83 (0.74, 0.92) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) | 0.99 (0.98, 1.00) | 0.97 (0.95, 0.99) |
| B: Initial 48 h at 25°C | 0.68 (0.51, 0.85) | 0.98 (0.97, 0.99) | 0.99 (0.99, 1.00) | 0.96 (0.94, 0.98) | 0.95 (0.91, 0.98) |
| C: No centrifugation | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) |
Note: Values are given as concordance correlation coefficient (95% confidence interval) versus the reference protocol (immediate centrifugation at 1000g at 4 degrees Celsius followed by immediate aliquoting and storage at −80 degrees Celsius).
Only sample pairs with both values above LLD were included in analysis; for KIM-1 and NGAL, 42–45 pairs; for LFABP and cystatin C, 33–35 pairs; and for IL-18, 25–30 pairs remained in the three processes.
LLD, lower limit of detection; IL-18 (interleukin 18); NGAL (neutrophil gelatinase-associated lipocalin); KIM-1 (kidney injury molecule 1); L-FABP (liver-type fatty acid–binding protein)
The CCC was also determined for pre-specified subgroups including site (New Haven vs. San Francisco), AKI (yes/no), use of a Foley catheter (yes/no), diabetes (yes/no), hematuria (yes/no) and proteinuria (yes/no). Similar to the combined group analysis, IL-18, NGAL, KIM-1, L-FABP, and cystatin C demonstrated good or excellent concordance for these sub-groups.
Discussion
For all biomarkers tested (IL-18, NGAL, KIM-1, L-FABP, cystatin C), short-term storage for 48 hours at 4°C and centrifugation prior to storage had no impact on biomarker levels. High levels of agreement with the reference standard were also observed during storage at 25°C for 48 hours. These observations are consistent with previous biomarker stability studies which showed that NGAL and cystatin C remained stable in short term storage. (21, 22) However, for IL-18, a modest decrease in concordance to 0.81 was observed compared with reference measurements. Measurements were not affected by proteinuria, hematuria, presence of diabetes or Foley catheter use. In an earlier report, urine KIM-1 and NGAL concentrations were decreased by 5%–10% after storage at 25°C for 24 hours and by 1%–2% after storage at 4°C for 24 hours.(16)
Among the biomarkers measured, IL-18 had the highest proportion of values below the detectable range, with nearly half of the sample pairs not included in the primary concordance analysis. In addition, the absolute levels of IL-18 in urine (in picograms/milliliter) were the lowest among all biomarkers tested. Thus, it is possible that even minor degradation would affect IL-18 measurement more than that of the other biomarkers, where concentrations are in the nanogram to microgram range. These two factors could have contributed to the diminished CCC for IL-18 observed in this study.
There are certain limitations to the study that need to be addressed before the clinical applicability of these biomarkers can be entertained. Firstly, the results are only generalizable to the five analytes measured using the specified assays; different assays may produce varying results. However, these analytes and assays were chosen based on the extensive ongoing efforts to validate them for clinical use. Furthermore, we have additional stored aliquots of these samples with which we can test the impact of short-term storage on biomarker levels for other novel biomarkers and biomarker assays. A second limitation is that a comparison of fresh urine with samples stored at −80°C was not incorporated into the study design. In clinical settings, biospecimens are analyzed immediately (forgoing any short term −80°C storage). However, there are currently no assays available to analyze these biomarkers in real time. Even if assays do become accessible, they may not be immediately obtainable in all medical centers. It should be feasible to limit storage at 25°C to no more than 2 days, even if samples have to be transported to specialty laboratories for novel biomarker measurements. As such, we did not examine the impact of longer storage times at 4°C, 25°C or −80°C. However, for clinical research purposes (where long term storage is commonplace), these conditions warrant further investigation. The third limitation is the effect of protease inhibitors that may have an independent effect on biomarker measurement, which we did not evaluate.
In summary, the results of this study should reassure investigators and clinicians that variations in short-term handling using the assays described do not substantially affect the levels of the novel AKI biomarkers tested. However, for optimal fidelity, urine for IL-18 measurement should not be stored at 25°C before long-term storage. These results highlight the importance of prospective and systematic testing of sample storage conditions on biomarker levels prior to the adoption of these tests in clinical practice or research.
Supplementary Material
Acknowledgements
The ASSESS-AKI Study Investigators are as follows: Vernon M. Chinchilli; Alan S. Go; Jonathan Himmelfarb; T. Alp Ikizler; James S. Kaufman; Paul L. Kimmel; Chirag R. Parikh; and John B. Stokes (in memoriam). Additional collaborators are as follows: Yale: Steven Coca; London, Canada: Amit Garg; Kaiser Permanente Northern California: Juan D. Ordonez and Sijie Zheng; Penn State: Nasrollah Ghahramani; Vanderbilt: Julia B. Lewis and Lorraine Ware; Cincinnati: Catherine Krawczeski, Michael Bennett; Montreal: Michael Zappitelli; Seattle: Mark Wurfel.
Support: This study was supported by the supplemental American Recovery and Reinvestment Act funds and research grants U01-DK082223, U01-DK082185, U01DK082192 and U01DK082183 from the NIDDK of the National Institutes of Health (NIH), U.S. Department of Health and Human Services. This publication was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through University of California, San Francisco–Clinical & Translational Science Institute grant UL1 RR024131. Drs Hsu and Siew are additionally supported by grants K24 DK92291 and 5K23DK088964-02, respectively. The opinions expressed in this article are the authors’ own and do not reflect the view of the NIH, the Department of Health and Human Services, or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: CRP is a co-inventor on a patent for the use of IL-18 as a marker of AKI (no financial value). PD is a co-inventor on a patent for the use of NGAL as a marker of AKI. EDS is a consultant for Alere Inc. KDL is a consultant for Astute Biomedical. The other authors declare that they have no other relevant financial interests.
References
- 1.American Society of Nephrology Renal Research Report. Journal of the American Society of Nephrology ; JASN. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 2.Parikh CR, Lu JC, Coca SG, Devarajan P. Tubular proteinuria in acute kidney injury: a critical evaluation of current status and future promise. Annals of clinical biochemistry. 2010;47:301–312. doi: 10.1258/acb.2010.010076. [DOI] [PubMed] [Google Scholar]
- 3.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. Journal of the American Society of Nephrology ; JASN. 2011;22:810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 4.Liu KD, Yang W, Anderson AH, et al. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney international. 2013 doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Parikh CR, Ikizler TA, et al. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC nephrology. 2010;22:11. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Best Practices for Sample Storage: A Report from the Workshop on Urine Biospecimen Handling. 2010 http://www3.niddk.nih.gov/fund/other/Best_Practices_for_Sample_Storage.pdf.
- 7.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology : JASN. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 8.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 9.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 12.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Noiri E, Ono Y, et al. Renal L-type fatty acid--binding protein in acute ischemic injury. Journal of the American Society of Nephrology ; JASN. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 14.Herget-Rosenthal S, Marggraf G, Husing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney international. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahlstrom A, Tallgren M, Peltonen S, Pettila V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;62:344–350. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 16.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clinical journal of the American Society of Nephrology ; CJASN. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 19.King TS, Chinchilli VM. Robust estimators of the concordance correlation coefficient. Journal of biopharmaceutical statistics. 2001;11:83–105. doi: 10.1081/BIP-100107651. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herget-Rosenthal S, Feldkamp T, Volbracht L, Kribben A. Measurement of urinary cystatin C by particle-enhanced nephelometric immunoassay: precision, interferences, stability and reference range. Ann Clin Biochem. 2004 Mar;41(Pt 2):111–118. doi: 10.1258/000456304322879980. [DOI] [PubMed] [Google Scholar]
- 22.Grenier FC, Ali S, Syed H, Workman R, Martens F, Liao M, Wang Y, Wong PY. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem. 2010 Apr;43(6):615–620. doi: 10.1016/j.clinbiochem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013 Apr 1;187(7):736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park M, Vittinghoff E, Liu KD, Shlipak MG, Hsu CY. Urine Biomarkers Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule-1 (KIM-1) Have Different Patterns in Heart Failure Exacerbation. Biomark Insights. 2013;8:15–18. doi: 10.4137/BMI.S11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.