Abstract
We sought to analyze utilization and survival outcomes of cytoreductive nephrectomy in patients with metastatic renal cell carcinoma (RCC) before and after introduction of targeted therapy. We identified patients with metastatic RCC between 1993 and 2010 in the SEER registry and examined temporal trends in utilization. We performed a joinpoint regression to determine when changes in utilization of cytoreductive nephrectomy occurred. We fitted multivariable proportional hazard models in full and propensity score-matched cohorts. We performed a difference-in-difference analysis to compare survival outcomes before and after introduction of targeted therapy. The proportion of patients undergoing cytoreductive nephrectomy increased from 1993 to 2004, from 29% to 39%. We identified a primary joinpoint of 2004, just prior to the introduction of targeted therapy. Beginning in 2005, there was a modest decrease in utilization of cytoreductive nephrectomy. Cytoreductive nephrectomy was associated with a lower adjusted relative hazard (0.41, 95% confidence interval 0.34 to 0.43). Median survival among patients receiving cytoreductive nephrectomy increased in the targeted therapy era (19 versus 13 months), while median survival among patients not receiving cytoreductive nephrectomy increased only slightly (4 versus 3 months). Difference-in-difference analysis showed a significant decrease in hazard of death among patients who received cytoreductive nephrectomy in the targeted therapy era. Despite decreased utilization in the targeted therapy era, cytoreductive nephrectomy remains associated with improved survival. Prospective randomized trials are needed to confirm the benefit of cytoreductive nephrectomy among patients with metastatic RCC treated with novel targeted therapies.
Keywords: Carcinoma, Renal Cell/epidemiology; Carcinoma, Renal Cell/mortality; Carcinoma, Renal Cell/surgery; Kidney Neoplasms/epidemiology; Nephrectomy/trends
Introduction
Nearly one in five patients diagnosed with kidney cancer (renal cell carcinoma, RCC) are found to have distant metastases.1 Metastatic RCC portends a poor prognosis, with only 12% of patients alive five years after diagnosis. In the United States in 2013, 13,780 deaths are predicted from kidney cancers.2
The medical and surgical management of metastatic RCC has evolved significantly in the past 30 years. Prior to effective medical therapies for metastatic RCC, surgical removal of the primary tumor (cytoreductive nephrectomy) was reserved for palliative purposes.3 In 1992 cytokine therapy (interferon-alpha, interleukin-2) was introduced and the role of cytoreductive nephrectomy became controversial. Randomized controlled clinical trials ultimately showed a 6-month survival advantage in patients receiving cytoreductive nephrectomy and cytokine therapy compared with cytokines alone.4–6
Since 2005, the FDA has approved seven novel agents for use in patients with metastatic RCC and these targeted therapeutics have quickly replaced cytokines as the dominant systemic therapy.7 The optimal use of cytoreductive nephrectomy in the targeted therapy era is not yet defined. It is unknown whether cytoreductive nephrectomy continues to afford patients a survival benefit above and beyond that provided by newer systemic therapies. We sought to describe temporal trends in utilization of cytoreductive nephrectomy and to compare the survival of patients treated with cytoreductive nephrectomy before and during the targeted therapy era. We hypothesized that, in the targeted therapy era, cytoreductive nephrectomy would afford a survival benefit equal to, or above that afforded in earlier years.
Materials and Methods
Analytic Cohort
We identified all cases of RCC in the Surveillance Epidemiology and End Results (SEER) database diagnosed from 1993-2010. We restricted our cohort to RCC using ICD-O-3 site code C649 to exclude kidney cancers originating in the renal pelvis. We further limited our cohort to RCC specific histology codes (8032, 8140, 8240, 8270, 8290, 8310, 8312, 8316, 8317, 8319, 8320, 8963). Our analytic cohort included patients 18 years or older with metastatic disease at presentation (“SEER Historic Stage A”=distant or “CS mets at dx”>0).
Patient and Tumor Characteristics and Survival Outcomes
We abstracted demographic data from SEER, including age at diagnosis, year of diagnosis, sex, race/ethnicity (white, Black, and other), SEER registry region, and marital status. Tumor characteristics (stage, tumor size, and histology) were also abstracted from SEER registry data. Tumor size was generated as a composite of two variables. For patients missing “EOD10_SZ” size data, “CS_SIZE” was used. We set all tumor sizes >30 cm as missing to ensure biologic plausibility. Overall survival was defined as the time in months from the date of diagnosis to the date of last contact.
Cytoreductive Nephrectomy Definition
We ascertained the receipt of cytoreducive nephrectomy (CN) if patients had any documented kidney cancer surgery codes for “partial nephrectomy”, “complete” or “radical” nephrectomy, or “nephrectomy NOS” (30, 40, 50, 70, or 80) among the surgery variables (“SS_SURG” [1993 to 1997], “SRPRIM02” [1998 to 2002], “SURGPRIM” [1998 and beyond]).
Temporal Trends in Utilization of Cytoreductive Nephrectomy
We calculated the proportion of patients receiving cytoreductive nephrectomy for each study year. We analyzed the trends in utilization of cytoreductive nephrectomy using Joinpoint version 4.04 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute). We performed a joinpoint regression, also known as spline regression or piecewise linear regression, to identify the intersections of the lines of best fit.8 The ideal number of “joinpoints” and calculation of statistical significance were calculated by the Joinpoint software, using the permutation test. The standard error of the proportion of patients undergoing cytoreductive nephrectomy each year was calculated using the assumption of asymptotic normality of the estimated model parameters.
Factors Associated With Receipt of Cytoreductive Nephrectomy
We examined associations among patient and tumor variables and receipt of cytoreductive nephrectomy using Student's t-test for continuous and the χ2 test for categorical variables. We fit unadjusted and multivariable logistic regression models to estimate the odds of cytoreductive nephrectomy associated with patient and tumor characteristics. We modeled the year of treatment first as a continuous variable and then also as a categorical variable using the Joinpoint value to establish treatment “eras” (before versus after the joinpoint year). For all variables associated with cytoreductive nephrectomy, we tested for effect modification by treatment era. We evaluated model discrimination using the concordance (“c”) statistic, corresponding to the area under the receiver operating characteristic (ROC) curve. We used to the Hosmer-Lemeshow 2 goodness-of-fit test to assess model calibration.
Survival Analysis
Survival curves were generated using the Kaplan-Meier product-limit method stratified by receipt of cytoreductive nephrectomy and by treatment era. We fit unadjusted and multivariable proportional hazards (“Cox”) models to determine the association of patient and tumor characteristics with mortality. We first modeled treatment year as a continuous variable, and then used a dichotomized variable representing the treatment era using the joinpoint year. For all variables associated with mortality, we tested for effect modification by treatment era.
Propensity Score Analysis
Given the differences in patient characteristics between patients treated with and without cytoreductive nephrectomy, we constructed a companion analysis using a propensity score-matched cohort. We included all patient and tumor characteristics, as well as treatment era as matching variables. We then computed the logit of the estimated propensity score and then used a two digit greedy matching algorithm. We calculated Kaplan-Meier product limit estimates using the matched cohort stratified by treatment era and receipt of cytoreductive nephrectomy. We repeated Cox multivariable proportional hazards models to test the significance of cytoreductive nephrectomy in this matched cohort.
Difference-in-Difference Survival Analysis
In order to compare survival outcomes in the cytokine (1993 to 2004) and targeted therapy era (2005 and beyond), we fit a multivariable proportional hazards models to compare overall survival among patients who did and did not receive cytoreductive nephrectomy by treatment era. Next, we performed a difference-in-difference analysis to determine whether cytoreductive nephrectomy was associated with differential survival by treatment era. We modeled overall survival for patients stratified by cytoreductive nephrectomy status (yes/no) and treatment era including an interaction term of CN status * treatment era as seen in the equation below.
Covariates included factors associated with survival in the multivariable Cox models. To minimize the likelihood that patients diagnosed in the targeted therapy era might have received cytokine therapy and vice versa, we compared the treatment era defined as 1993 to 2004 versus 2007 and beyond. In both models, the γ represents the difference-in-difference in survival attributable to the receipt of cytoreductive nephrectomy in the more recent treatment era. We considered inference tests with 2-tailed p-values <0.05 to be statistically significant. We conducted all analyses using SAS 9.3 (Cary, NC) and formatted graphics for publication using JMP Pro 10 (Cary, NC).
Results
Table 1 describes the characteristics of the 20,104 adults with metastatic RCC between 1993 and 2010. Of these, 6915 (34%) underwent cytoreductive nephrectomy. In the full cohort, patients who underwent cytoreductive nephrectomy tended to be younger (60.8 vs. 67.8 years), male, and white. The western SEER region accounted for the majority of cases (56%).
Table 1.
Patient characteristics of the full cohort stratified by receipt of cytoreductive nephrectomy.
| CN+ (n=6915) | CN− (n=13,189) | P value | |
|---|---|---|---|
| Age (mean ± SD) | 60.8 ± 11.30 | 67.8 ± 12.77 | <0.0001 |
| Age Group (%): | |||
| <64 | 4,319 (62.5%) | 5,231 (39.7%) | <0.0001 |
| 65-69 | 981 (14.2%) | 1,738 (13.2%) | |
| 70-74 | 792 (11.4%) | 1,783 (13.5%) | |
| 75-79 | 513 (7.4%) | 1,795 (13.6%) | |
| >80 | 310 (4.5%) | 2,642 (20.0%) | |
| Sex (%): | |||
| Male | 4,786 (69.2%) | 8,465 (64.2%) | <0.0001 |
| Female | 2,129 (30.8%) | 4,724 (35.8%) | |
| Race / Ethnicity (%): | |||
| White | 5,935 (85.8%) | 10,890 (82.6%) | <0.0001 |
| Black | 554 (8.0%) | 1,497 (11.3%) | |
| Other or Unknown Race | 426 (6.2%) | 802 (6.1%) | |
| Marital Status (%): | |||
| Single | 778 (11.3%) | 1,826 (13.8%) | <0.0001 |
| Married | 4,664 (67.4%) | 7,121 (54.0%) | |
| Divorced/Widowed | 1,295 (18.7%) | 3,749 (28.4%) | |
| Unknown | 180 (2.6%) | 493 (3.7%) | |
| Region (%): | |||
| West | 3,979 (57.5%) | 7,327 (55.6%) | 0.0002 |
| Midwest | 852 (12.3%) | 1,874 (14.2%) | |
| Northeast | 919 (13.2%) | 1,823 (13.8%) | |
| South | 1,165 (16.8%) | 2,165 (16.4%) | |
| Vital Status (%) | |||
| Alive | 1,640 (23.7%) | 969 (7.3%) | <0.0001 |
| Dead | 5,275 (76.3%) | 12,220 (92.7%) |
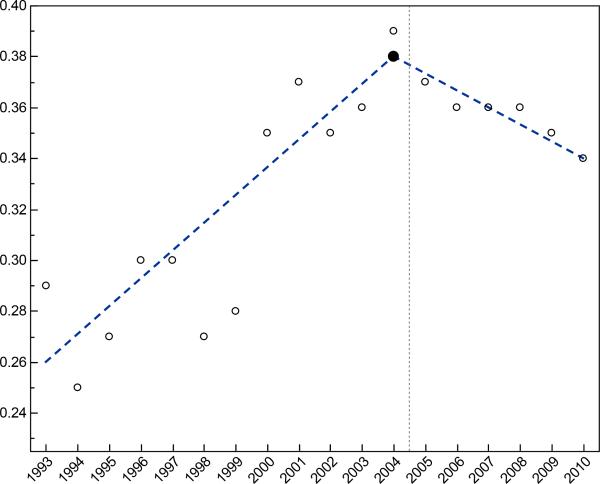
Figure 1 shows the proportion of patients undergoing cytoreductive nephrectomy by study year. The rate of cytoreductive nephrectomy increased from the lowest rate in 1994 (25%) and peaked in 2004 (39%). Joinpoint analysis identified a single joinpoint (2004) as the simplest model with permutation testing confirming a significant difference between 0 and 4 joinpoints (p=0.007) but no difference between 1 and 4 joinpoints (p=0.24). The best-fit linear regression suggested an additional 1.1% of patients per year received cytoreductive nephrectomy from 1993 to 2004 (annual percentage change 3.4%, 95% confidence interval [95% CI] 2.0 to 4.8). Starting in 2005, utilization of cytoreductive nephrectomy declined slightly at a rate of 0.6% per year (annual percentage change −1.8%, 95% CI −4.0 to 0.4).
Figure 1.
Proportion of patients with metastatic RCC receiving cytoreductive nephrectomy by year of diagnosis. We identified 2004 as the year when utilization trends changed (joinpoint). This coincides with the introduction of targeted therapies in 2005, represented by the vertical dotted reference line.
Patients who were younger, male, married, and those with larger tumors had higher odds of receiving CN (Table 2). When analyzed by treatment era using the joinpoint year cutoff (before 2005 versus 2005 and beyond), patients in the targeted therapy era had 11% higher odds of receipt of cytoreductive nephrectomy. However, in multivariable analysis, treatment era was no longer independently associated with receipt of cytoreductive nephrectomy. The association of treatment era with cytoreductive nephrectomy was modified by race / ethnicity (p=0.011) and tumor size (p=0.0008), with fewer Black patients (OR 0.74, 95% CI 0.59 to 0.92), and fewer patients with tumors <4cm (OR.0.63, 95% CI 0.52 to 0.82) receiving cytoreductive nephrectomy in the targeted therapy era.
Table 2.
Characteristics associated with the odds of receipt of cytoreductive nephrectomy including unadjusted (left), and multivariable model including treatment era (right).
| Characteristic | Unadjusted Odds Ratio (95%CI) | Multivariable Odds Ratio (95%CI) |
|---|---|---|
| Age (per 10 year increase) | 0.63 (0.62, 0.65) | 0.63 (0.61, 0.65) |
| Male vs Female | 1.26 (1.18, 1.34) | 1.06 (0.99, 1.15) |
| Year of Diagnosis: | ||
| Continuous | 1.02 (1.02, 1.03) | -- |
| Treatment Era | 1.11 (1.05, 1.18) | 1.02 (0.95, 1.09) |
| Race / Ethnicity (vs. white): | ||
| Black | 0.68 (0.61, 0.75) | 0.68 (0.58, 0.74) |
| Other/ Unknown | 0.98 (0.86, 1.10) | 0.93 (0.81, 1.07) |
| Marital Status (vs. Married): | ||
| Single | 0.65 (0.59, 0.71) | 0.47 (0.42, 0.52) |
| Separated/Divorced/Widowed | 0.53 (0.49, 0.57) | 0.70 (0.64, 0.76) |
| Unknown | 0.56 (0.47, 0.66) | 0.59 (0.48, 0.73) |
| Tumor Size (cm): | ||
| 4-<7 vs <4 | 1.42 (1.25, 1.61) | 1.39 (1.21, 1.58) |
| 7-<10 vs <4 | 1.98 (1.75, 2.25) | 1.79 (1.57, 2.04) |
| >=10 vs <4 | 2.27 (2.01, 2.56) | 1.83 (1.61, 2.08) |
| Region: | ||
| Midwest vs West | 0.84 (0.77, 0.92) | 0.84 (0.76, 0.93) |
| Northeast vs West | 0.93 (0.85, 1.01) | 1.09 (0.98, 1.21) |
| South vs West | 0.99 (0.91, 1.07) | 0.97 (0.88, 1.07) |
Differences that meet p<0.05 shown in bold.
Patients who received cytoreductive nephrectomy differed from those who did not in nearly every category (Table 1). Propensity score matching resulted in a cohort of 3191 patients who received CN (46%) matched with controls in a 1:1 ratio. Because we forced matching on patient (age, sex, race/ethnicity, region) and tumor characteristics (tumor size) as well as propensity scores, the baseline characteristics were well balanced between the two groups.
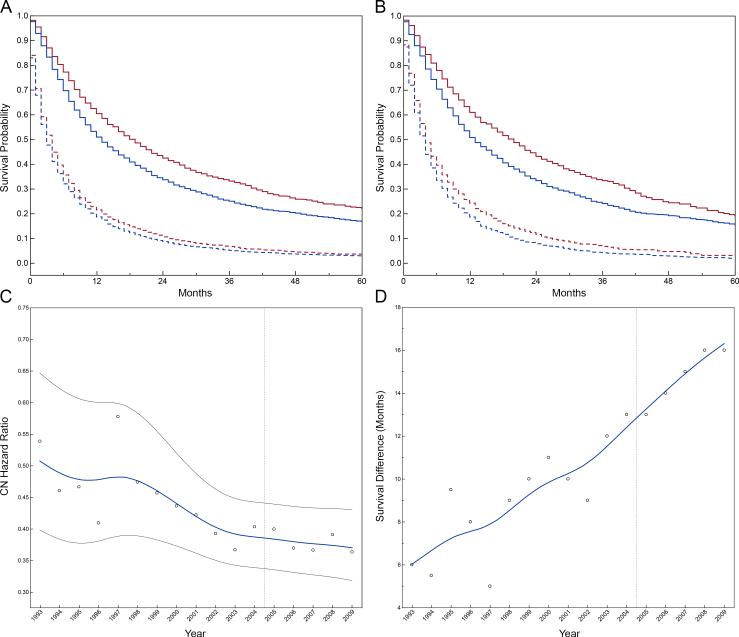
Median follow-up was 12 months (interquartile range 5 to 30, mean 23.6) for patients that received CN and 3 months (interquartile range 1 to 9, mean 8.1) for those that did not. Overall survival of the full and propensity score-matched cohorts stratified by treatment era and receipt of cytoreductive nephrectomy is shown in Figure 2. Median survival was significantly longer for patients treated with cytoreductive nephrectomy (15 versus 4 months, p<0.001); the survival difference increased over the study period. (Figure 2c) The risk of death was higher among older patients and patients with larger tumors, as expected. (Table 3) While Black race was associated with a 33% reduction in odds of receipt of cytoreductive nephrectomy, Black race was not independently associated with survival after adjusting for tumor and treatment characteristics. In multivariable models, cytoreductive nephrectomy was associated with a lower relative hazard of mortality (0.41, 95% CI 0.34 to 0.43) in the full cohort. Propensity score matched results were virtually identical (Supplemental Table 1).
Figure 2.
Product-limit survival estimates stratified by treatment era in the full (A) and propensity score-matched (B) cohorts. Patients treated in the targeted therapy era (≥2005) are shown in red. Patients treated before 2005 are shown in blue. Survival curves of patients treated with cytoreductive nephrectomy are represented as a solid line, while those not receiving cytoreductive nephrectomy are represented as dotted lines. Median overall survival of patients treated with CN increased to 19 months from 13 months in the targeted therapy era. Median overall survival of patients not receiving CN, increased to 4 from 3 months. C) The temporal trend of the adjusted hazard of mortality associated with cytoreductive nephrectomy by diagnosis year with 95% CI. D) The increasing difference in median survival for patients receiving cytoreductive nephrectomy. The beginning of the targeted therapy era is represented by the vertical dotted reference line.
Table 3.
Multivariable Cox proportional hazards model demonstrating the relative mortality hazard.
| Characteristic | Hazard Ratio (95% CI) |
|---|---|
| Cytoreductive nephrectomy | 0.41 (0.39, 0.43) |
| Age (per 10 year increase) | 1.09 (1.08, 1.11) |
| Sex (Male vs. Female) | 0.97 (0.94, 1.01) |
| Year of diagnosis: | |
| Treatment Era (≥2005 vs. <2005) | 0.87 (0.84, 0.90) |
| Race / Ethnicity: | |
| Black vs. white | 1.03 (0.98, 1.10) |
| Other/Unknown vs. white | 0.84 (0.78, 0.90) |
| Marital Status (vs. Married): | |
| Single (never married) | 1.10 (1.05, 1.16) |
| Separated/Divorced/Widowed | 1.08 (1.03, 1.13) |
| Unknown | 1.03 (0.93, 1.15) |
| Tumor size (cm): | |
| 4-7 vs. <4 | 1.21 (1.05, 1.20) |
| 7-10 vs. <4 | 1.29 (1.21, 1.38) |
| ≥10 vs.. <4 | 1.45 (1.36, 1.54) |
| Region: | |
| Midwest vs. West | 1.00 (0.95, 1.05) |
| Northeast vs. West | 0.93 (0.88, 0.99) |
| South vs. West | 1.00 (0.96, 1.05) |
Differences that meet p<0.05 shown in bold.
Overall survival improved over time, with a 1.3% per year reduction in the relative hazard in multivariable analysis of the full cohort (data not shown). Median survival in the targeted therapy era was 19 and 4 months among those treated with and without cytoreductive nephrectomy, respectively. The targeted therapy era was associated with a lower relative hazard of mortality in the full (0.87, 95% CI 0.84 to 0.90) and the propensity score-matched (0.84, 95% CI 0.80 to 0.88) cohorts. Among patients diagnosed in 2005 and beyond, those treated with cytoreductive nephrectomy had a 20% (95% CI 15 to 24%) reduction while those not receiving cytoreductive nephrectomy had a 9% reduction (95% CI 5 to 13%) in the mortality hazard. (Supplemental Table 2)
There was a trend in decline in adjusted relative hazard of death associated with receipt of cytoreductive nephrectomy over the study period (Figure 2c). We noted significant interactions among treatment era and patient age (p=0.009), with older patients experiencing increased mortality hazard in the targeted therapy era (HR 1.04, 95% CI 1.01 to 1.07 per 10 year increase in age), and geographic region (p=0.003), with improving survival in the Northeast region in the targeted therapy era. The relative reduction in mortality associated with CN was more pronounced in the targeted therapy era in our difference-in-difference analysis (p<0.0001, Table 4). Results were similar in a sensitivity analysis comparing the association of cytoreductive nephrectomy with mortality before 2005 and after 2007 (p<0.0001).
Table 4.
Difference-In-Difference Cox proportional hazard model using cytoreductive nephrectomy status and the treatment era interaction term.
| Characteristic | <2005 vs. ≥2005 Hazard Ratio (95% CI) |
|---|---|
| Cytoreductive nephrectomy (yes vs. no) | 0.43 (0.42, 0.46) |
| Age (per 10 year increase) | 1.09 (1.07, 1.11) |
| Sex (Male vs. Female) | 0.97 (0.94, 1.01) |
| Year of diagnosis: | |
| Treatment Era (≥2005 vs. <2005) | 0.93 (0.89, 0.97) |
| Race / Ethnicity: | |
| Black vs. white | 1.03 (0.98, 1.09) |
| Other/Unknown vs. white | 0.84 (0.78, 0.91) |
| Marital Status: | |
| Single (never married) vs. Married | 1.10 (1.04, 1.16) |
| Separated/Divorced/Widowed vs. Married | 1.08 (1.03, 1.13) |
| Unknown vs. Married | 1.03 (0.93, 1.14) |
| Tumor size (cm): | |
| 4-7 vs. <4 | 1.13 (1.06, 1.20) |
| 7-10 vs. <4 | 1.30 (1.22, 1.39) |
| ≥10 vs. <4 | 1.46 (1.37, 1.55) |
| Difference in Difference | |
| CN * Era | 0.84 (0.78, 0.91) |
Differences that meet p<0.05 shown in bold.
Discussion
The utilization of cytoreductive nephrectomy increased over the initial study period until peaking in 2004. Beginning in 2005, the proportion of patients receiving cytoreductive nephrectomy steadily declined. Joinpoint analysis confirmed that 2004 represented a statistical point of change in utilization rates. This year coincides with the introduction of targeted therapies for metastatic RCC and is consistent with previous studies.9–11 There are several possible explanations of the decrease in cytoreductive nephrectomy since 2004. First, the randomized clinical trials supporting the survival benefit of CN were performed prior to the introduction of targeted therapies in patients treated with interferon-alpha.4,5 Subgroup analyses from these trials, and subsequent studies, suggest that the survival benefit for patients receiving cytoreductive nephrectomy was primarily seen among patients with good performance status.12–15 With the introduction of targeted therapies in 2005, it is likely that providers regarded treatment with the oral targeted therapies as less morbid compared to cytoreductive nephrectomy and recommended surgery for fewer patients. In addition, the introduction of these new therapies has led to uncertainty as to whether cytoreductive nephrectomy provides survival benefits above and beyond those observed from the targeted therapies alone.
The odds of receipt of cytoreductive nephrectomy did vary by patient and tumor characteristics, as well as by region. More than likely, the lack of level I evidence establishing the role of cytoreductive nephrectomy in patients receiving targeted therapies contributes to significant practitioner, institutional, and regional variation in referral for CN. Older patients were also less likely to receive CN. While age and comorbidity are appropriate considerations for surgical candidacy, it is unclear whether these fully account for the more than 30% lower odds of receipt of cytoreductive nephrectomy for every 10 year increase in age. It is important to note that cytoreductive nephrectomy utilization differed by race. Black patients were significantly less likely to undergo cytoreductive nephrectomy compared with whites, and this difference persisted after adjustment for age at diagnosis, sex, marital status, and tumor size. Reasons for differential application of cytoreductive nephrectomy by race are unknown, but warrant more attention.
The survival of patients with metastatic RCC has increased following the introduction of targeted therapies, as has been reported previously.11,16 While survival did improve among patients not receiving cytoreductive nephrectomy, the poor overall survival of this group reflects the severity of metastatic RCC and the challenge to alter outcomes for these patients with medical interventions. Overall improvements in survival were most impressive among patients treated with cytoreductive nephrectomy. Cytoreductive nephrectomy is an independent predictor of survival, even after adjusting for available patient and tumor factors. Importantly, cytoreductive nephrectomy remained associated with survival in the targeted therapy era (after 2005) in our multivariable models in the full and propensity score-matched analyses, offering support for its continued role in the contemporary management of patients with metastatic RCC. Additionally, a difference-in-difference analysis demonstrated that cytoreductive nephrectomy was more protective in the targeted therapy era compared with the cytokine era. The observation of increased benefit of cytoreductive nephrectomy in the targeted therapy era remained significant when excluding patients treated during the initial years following introduction of targeted therapy.
Strengths of our analysis include the use of SEER data inclusive of 2010 to document the current temporal trends in survival. SEER data is broadly representative of treatment trends in the United States, includes a large sample size, multiple races and ethnicities, and detailed tumor data. These data have greater external validity than single institution experience or randomized trials. In the phase III trials testing these novel therapies, receipt of cytoreductive nephrectomy was often an inclusion criteria, thus prohibiting any extrapolation on the effect of combined surgical and medical therapy.12 Currently, the Clinical Trial to Assess the Importance of Nephrectomy (CARMENA, NCT00930033) is recruiting patients to compare sunitinib alone versus sunitinib following cytoreductive nephrectomy, but results are not expected until after 2015. The number of newly approved targeted therapeutics for metastatic RCC also raises concerns that a trial using a single agent may not be generalizable to other agents.
In the absence of randomized controlled data, we employed several approaches to minimize the potential bias of confounding by indication. First, our logistic regression models demonstrate that the treatment era was not an independent predictor of receipt of cytoreductive nephrectomy. While we did not directly account for performance status or comorbidity, we do show that measured factors were similar among patients receiving cytoreductive nephrectomy in the cytokine and targeted therapy eras. We also demonstrated that overall survival among patients not treated with cytoreductive nephrectomy (which serve as the reference group in our analysis) changed very little in the targeted therapy era. We fitted multivariable adjusted survival models in the full cohort as well as a propensity score-matched cohort. Finally, we applied a difference-in-difference analysis to compare the interaction between receipt of cytoreductive nephrectomy and the treatment era. This econometrics methodology is not commonly seen in survival analysis of cancer outcomes, but is well suited to compare outcomes after a significant change in practice, as was observed for RCC around 2005.
This study has noteworthy limitations. First, the potential of selection bias remains a concern when using retrospective registry data. Younger and healthier patients are more likely to be considered appropriate surgical candidates and undergo cytoreductive nephrectomy, and are also more likely to experience longer survival times. SEER data do not include individual patient performance status or comorbidities, which might enable us to better account for selection effects. Additionally, we used the diagnosis year and treatment period as a surrogate for whether a patient received cytokine or targeted systemic therapy. It is possible that some patients initially treated with systemic therapy went on to receive a cytoreductive nephrectomy that was not captured in the SEER registry. More recent reports have described initial targeted therapy with subsequent cytoreductive nephrectomy for select patients.17,18 It is unlikely that this practice had significant effects on outcomes for patients treated prior to 2010. Moreover, the inclusion of patients that tolerated targeted therapy and underwent a delayed cytoreductive nephrectomy would likely improve survival outcomes for the group of patients categorized as not having a cytoreductive nephrectomy in this study. While our outcome was overall survival, we do not attempt to quantify the morbidity associated with cytoreductive nephrectomy (e.g., pain, postoperative complications, etc.). Finally, we were not able to ascertain potential period effects that affect the quality of end of life care for patients with metastatic disease.
Approximately one in three patients with metastatic RCC undergo cytoreductive nephrectomy in the targeted therapy era. While the proportion of patients receiving cytoreductive nephrectomy decreased modestly beginning in 2005, we found that cytoreductive nephrectomy remains associated with a survival benefit, and this apparent benefit has increased in the targeted therapy era. Randomized trials of targeted chemotherapeutic agents with and without cytoreductive nephrectomy will be required to definitively determine the optimal management strategies in patients with metastatic RCC.
Supplementary Material
Novelty and Impact Statements.
Cytoreductive nephrectomy has been shown to have a survival advantage in randomized trials of patients receiving immunotherapy. It is not known if this survival advantage has persisted in the targeted therapy era. In this study, we report that despite decreased utilization in the targeted therapy era, cytoreductive nephrectomy remains associated with improved survival in multivariable and propensity-score matched cohorts. Moreover, cytoreductive nephrectomy is associated with a greater survival benefit in the targeted therapy era. Prospective randomized trials are needed to confirm the benefit of cytoreductive nephrectomy among patients with metastatic RCC treated with novel targeted therapies.
Acknowledgments
Funding:
This work was supported by the National Institute of Health (grant numbers K23 DK089086 to JL, K24 DK085446 to GC).
Footnotes
Financial Disclosures:
none
References
- 1.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Dekernion JB, Ramming KP, Smith RB. The natural history of metastatic renal cell carcinoma: a computer analysis. J Urol. 1978;120:148–152. doi: 10.1016/s0022-5347(17)57082-2. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 5.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 6.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 7.Coppin C, Le L, Porzsolt F, Wilt T. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev. 2008;(2):CD006017. doi: 10.1002/14651858.CD006017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Tsao CK, Small AC, Kates M, Moshier EL, Wisnivesky JP, Gartrell BA, Sonpavde G, Godbold JH, Palese MA, Hall SJ, Oh WK, Galsky MD. Cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy in the United States: a SEER analysis. World J Urol. 2012 doi: 10.1007/s00345-012-1001-3. In Press. doi:10.1007/s00345-012-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeldres C, Baillargeon-Gagne S, Liberman D, Isbarn H, Capitanio U, Shariat SF, Sun M, Lughezzani G, Perrotte P, Montorsi F, Graefen M, Karakiewicz PI. A population-based analysis of the rate of cytoreductive nephrectomy for metastatic renal cell carcinoma in the United States. Urology. 2009;74:837–841. doi: 10.1016/j.urology.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Pal SK, Nelson RA, Vogelzang N. Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PLoS ONE. 2013;8:e63341. doi: 10.1371/journal.pone.0063341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, McDermott DF, Rini BI, Heng DY. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–66. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Aben KK, Heskamp S, Janssen-Heijnen ML, Koldewijn EL, van Herpen CM, Kiemeney LA, Oosterwijk E, van Spronsen DJ. Better survival in patients with metastasised kidney cancer after nephrectomy: a population-based study in the Netherlands. Eur J Cancer. 2011;47:2023–2032. doi: 10.1016/j.ejca.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Shuch B, La Rochelle JC, Wu J, Klatte T, Riggs SB, Kabbinavar F, Belldegrun AS, Pantuck AJ. Performance status and cytoreductive nephrectomy: redefining management in patients with poor performance. Cancer. 2008;113:1324–1331. doi: 10.1002/cncr.23708. [DOI] [PubMed] [Google Scholar]
- 15.Belldegrun AS, Klatte T, Shuch B, LaRochelle JC, Miller DC, Said JW, Riggs SB, Zomorodian N, Kabbinavar FF, Dekernion JB, Pantuck AJ. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989-2005): a benchmark for emerging targeted cancer therapies. Cancer. 2008;113:2457–2463. doi: 10.1002/cncr.23851. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powles T, Blank C, Chowdhury S, Horenblas S, Peters J, Shamash J, Sarwar N, Boleti E, Sahdev A, O'Brien T, Berney D, Beltran L, et al. The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. Eur Urol. 2011;60:448–454. doi: 10.1016/j.eururo.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Kim SP, Thompson RH. Sunitinib prior to planned cytoreductive nephrectomy: is this the new litmus test for metastatic renal cell carcinoma? Eur Urol. 2011;60:455–457. doi: 10.1016/j.eururo.2011.05.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.