Abstract
Low sensitivity to the acute effects of alcohol is a risk factor for heavy drinking and related problems. However, little research has tested process explanations for such effects. The current study tested the hypothesis that low sensitivity is associated with automatic approach biases for alcohol cues, coupled with deficits inhibiting responses in the presence of such cues. Eighty-five participants varying in alcohol sensitivity completed an Alcohol-Approach Avoidance Task and a Cued Go/No-Go Task while event-related potentials were recorded. Low sensitivity (LS) individuals showed evidence of automatic approach tendencies toward alcohol cues in both tasks, and experienced deficits inhibiting prepotent responses cued by alcohol images. Additionally, the event-related potential data indicated that LS individuals experienced more conflict when attempting to inhibit alcohol-cued responses, but not nonalcohol-cued responses, compared with their high-sensitivity counterparts. Together, these data indicate that alcohol cues elicit an approach bias among LS individuals, translating into greater difficulty inhibiting behavioral responses in the presence of such cues, a pattern generally supportive of dual process models of substance use.
Keywords: alcohol, dual process models, addiction, event-related potentials
Researchers have long observed that alcohol’s acute effects vary widely between individuals (e.g., Li, 2000; Sher & Wood, 2005). This variability is often characterized in terms of differential alcohol sensitivity, sometimes referred to as level of response to alcohol (see Schuckit, 1994; Schuckit & Smith, 2000; Schuckit, Smith, Anderson, & Brown, 2004; Schuckit, Tipp, Smith, Wiesbeck, & Kalmijn, 1997). The etiologic relevance of alcohol sensitivity has been demonstrated by studies showing that a low level of response (i.e., low sensitivity [334]LS[335]) significantly increases the risk of developing alcohol use disorder (Schuckit, 1994; Schuckit & Smith, 2000) and that this risk is dissociable from that conveyed by other relevant factors, including behavioral undercontrol, coping, positive expectancies, comorbid psychiatric disorders, and personality (e.g., Schuckit, Kelsoe, Braff, & Wilhelmsen, 2003; Trim, Schuckit, & Smith, 2009; Viken, Rose, Morzorati, Christian, & Li, 2003).
It is estimated that 50% of the variance in risk for alcohol use disorder is accounted for by genetic factors (e.g., Knopik et al., 2004; Schuckit et al., 2001) and that alcohol sensitivity accounts for 40%–60% of that genetic risk (e.g., Schuckit, 1999). Research also suggests that environmental factors, such as affiliation with heavy-drinking peers, can contribute to development of LS (Schuckit et al., 2011). Still, the psychological processes by which genetic and other factors contributing to alcohol sensitivity confer risk for alcohol abuse are not well understood. The purpose of the current research was to test for potential differences in basic motivational propensities and behavioral control abilities between LS and high-sensitivity (HS) drinkers that theoretically contribute to heavier patterns of alcohol use among LS individuals, within the framework of recent dual process models of substance use.
Dual Processes in Alcohol Use and Abuse
Recent theorizing (e.g., see Wiers et al., 2007; Wiers & Stacy, 2006) posits that drug use decisions are governed by the strength of two functionally and neuroanatomically distinct but interactive systems: a fast impulsive system, governed by affective reactivity reflecting associations in long-term memory that automatically trigger a motivational orientation (e.g., to approach) and a slower reflective system, associated with conscious deliberation and emotion regulation and governed by cognitive control processes rooted in the prefrontal cortex. According to this model, alcohol use disorders develop when there is an imbalance between these two systems such that the impulsive system becomes sensitized—for example, by repeated exposure to alcohol and accompanying reward—while the reflective regulatory system is compromised (e.g., by alcohol exposure), leading to dysregulated approach and consummatory responses in the presence of alcohol-related cues.
This model is particularly useful in making predictions concerning the propensity for alcohol-related problems in young drinkers. Developmental neuroscience has established that neural systems involved in evaluating the affective significance of stimuli develop much more quickly—relevant structures are generally well-formed by early adolescence (e.g., Adolphs, 2001; Nelson, Leibenluft, McClure, & Pine, 2005)—than the prefrontal cortical structures involved in self-regulatory control, which typically are not fully developed in humans until the early- to mid-twenties (e.g., Casey, Geidd, & Thomas, 2000; Gogtay et al., 2004). This imbalance means that the impulsive system often has greater influence over behavioral decisions during adolescence than does the reflective system.
In the dual process model literature, there is evidence that the impulsive system is bidirectional (e.g., Deutsch, Gawronski, & Strack, 2006; Strack & Deutsch, 2004), meaning that motivational orientation toward a cue automatically triggers approach tendencies, whereas approach and consummatory behaviors produce motivational orientations to relevant cues, creating a mutually reinforcing behavioral system. A recent meta-analysis of neuroimaging studies (Chein & Schneider, 2005) indicated that behavioral responses rooted in such bidirectional associations become largely automated and do not recruit neural regions implicated in cognitive control. To the extent that this process operates differentially for LS and HS individuals in relation to alcohol, it could be that for LS drinkers stronger and more automatic bidirectional associations are created between the motivation to drink and approach tendencies associated with achieving this goal, while at the same time the influence of top-down cognitive control is reduced. This process is likely to be exacerbated with increasing consumption, given considerable evidence that acute intoxication impairs self-regulatory cognitive control (e.g., Bartholow, Dickter, & Sestir, 2006; Casbon, Curtin, Lang, & Patrick, 2003; Curtin & Fairchild, 2003; Giancola, 2000, 2004; Pihl, Paylan, Gentes-Hawn, & Hoaken, 2003).
Another, related model of addiction processes, known as the Incentive Sensitization Hypothesis (e.g., Robinson & Berridge, 1993), similarly posits that the strength of automatic approach tendencies evoked by the presence of drug-related cues is an important predictor of drug use. According to this hypothesis, hypersensitivity to the incentive-motivational effects of drugs fosters an attentional processing bias toward drug-related cues (Robinson & Berridge, 1993, 2000, 2003, 2008), such that the cues begin to take on the motivational properties of the drug itself (e.g., Berridge, 2001). Through a series of persistent neuroadaptations, drug-related cues transform from mere visual percepts to enticing incentives (Robinson & Berridge, 2001; Berridge & Robinson, 2003). Berridge (2001) called these incentive stimuli “motivational magnets” because of the way they elicit approach actions and even consummatory behaviors. Importantly, the neuroadaptations reflecting incentive sensitization occur only in circuits that mediate “wanting” (i.e., craving, approach motivation), not circuits that mediate “liking” (i.e., hedonic pleasure) (see Smith, Berridge, & Aldridge, 2011), and thus increases in approach and consumption are driven not by actual pleasure derived from drug effects but from anticipated pleasure triggered by drug-related cues. Testing predictions derived from this model has the potential to explain why LS individuals, despite not feeling the same amount of subjective pleasure from a given dose of alcohol, show distinct patterns of enhanced reactivity to alcohol cues (Bartholow, Henry, & Lust, 2007; Bartholow, Lust, & Tragesser, 2010) and repeated heavy drinking that separate them from their HS peers.
Considering the tenets of these models suggests that individuals who are especially susceptible to the sensitizing properties of alcohol cues, together with relatively weak regulatory control abilities, should be especially prone to heavy drinking and related problems. As Robinson and Berridge (2003) nicely summarized it, “loss of inhibitory control over behavior and poor judgment, combined with sensitization of addicts’ motivational impulses to obtain and take drugs, makes for a potentially disastrous combination” (p. 46). One of the key hypotheses tested in the current research is that, relative to their HS counterparts, LS drinkers will display this “potentially disastrous combination” of heightened incentive salience for alcohol cues and poor inhibitory control in the presence of such cues.
Laboratory Analogues of Incentive Sensitization and Inhibitory Control
Aspects of this hypothesis have been tested in previous studies. For example, Shin, Hopfinger, Lust, Henry, and Bartholow (2010) reasoned that, to the extent that alcohol cues are motivationally salient to LS individuals, they should display an attention bias for such cues. In that study, HS and LS individuals performed a modified dot-probe task (see Townsend & Duka, 2001) in which alcoholic and nonalcoholic beverage cues were presented bilaterally followed by a target that required categorization by color. Consistent with their hypothesis, Shin et al. found that response times were faster for targets appearing in alcohol-cued than nonalcohol-cued locations for LS but not for HS participants. Additionally, Bartholow and colleagues have found that alcohol cues elicit enhanced P300 (P3) event-related brain potential (ERP) amplitudes among LS relative to HS individuals (Bartholow et al., 2007), that this enhanced neural reactivity is specific to alcohol cues and does not generalize to other arousing, appetitive stimuli (Bartholow et al., 2010), and that alcohol cue-elicited P3 amplitude predicts heavy drinking prospectively (Bartholow et al., 2007). Given considerable evidence linking P3 amplitude with the motivational salience or significance of eliciting stimuli (e.g., Delplanque, Silvert, Hot, Rigoulot, & Sequeira, 2006; Schupp et al., 2000; see also Nieuwenhuis, Aston-Jones, & Cohen, 2005), these data also point to the conclusion that alcohol cues are imbued with incentive salience for LS drinkers.
In part, the current study seeks to build on this prior evidence by testing whether LS individuals also display an approach motivational bias for alcohol cues, as would be predicted by the incentive sensitization hypothesis. To investigate this possibility, the current study involved measurement of automatically triggered action tendencies (i.e., approach/avoid) using the Alcohol Approach Avoidance Task (Alcohol-AAT; Wiers, Rinck, Dictus, & Van den Wildenberg, 2009). In this task, participants are instructed to respond to various images (e.g., alcohol and nonalcohol) by either pulling or pushing a joystick as quickly as possible. The use of a joystick task to measure action tendencies is based on the premise that arm flexion (pulling) is associated with more positive evaluations and approach motivational tendencies, whereas arm extension (pushing) is associated with more negative evaluations and avoidance motivational tendencies (Cacioppo, Priester, & Bernston, 1993; Palfai, 2006). In such tasks, any approach bias is indicated by faster “pull” versus “push” responses to a given class of stimuli. Unlike other tasks used to assess implicit biases for alcohol (e.g., Alcohol Implicit Association Task; see Wiers, Van Woerden, Smulders, & De Jong, 2002), which require participants to associate alcohol with valence judgments (e.g., good vs. bad), the Alcohol-AAT requires participants to respond on the basis of the physical orientation of each image (tilt to the left or right), thereby rendering the actual contents of the image irrelevant to their task. In this way, any biases in responding are implicit in nature (see De Houwer et al., 2009). Evidence of external validity has been established in recent studies using the Alcohol-AAT among heavy drinkers (Peeters et al., 2012; Wiers, Rinck, Kordts, Houben, & Strack, 2010), alcoholic inpatients (Wiers, Eberl, Rinck, Becker, & Lindenmeyer, 2011), and carriers of a gene variation implicated in stronger alcohol effects and cue induced craving (Wiers et al., 2009), which all show approach biases toward alcohol-related cues among these high-risk groups. Here, it was predicted that LS (but not HS) individuals would show an approach bias toward alcohol images, represented in faster reaction times (RTs) on trials in which alcohol cues are pulled relative to pushed.
The implementation of behavioral control in the presence of alcohol cues is the second causal factor theorized in dual process models as important to regulating drug use. Behavioral control is often represented in the laboratory by performance on tasks that require participants to rapidly respond to some types of stimuli but to withhold responses to other stimuli (i.e., Go/No-Go Tasks; see Logan & Cowan, 1984). Here, our interest was in whether behavioral control ability would vary according to whether alcohol cues were present. That is, according to the current model, LS individuals should experience more difficulty inhibiting responses in the presence of alcohol compared with nonalcohol cues, particularly if alcohol cues elicit an automatic approach tendency. To test this idea, a modified Go/No-Go Task was used, in which go and no-go targets were preceded by alcohol and nonalcohol cues (see Fillmore, Ostling, Martin, & Kelly, 2009; Fillmore, Marczinski, & Bowman, 2005). In addition, the relationship between cue type (alcohol or nonalcohol) and target type (go or no-go) was manipulated within trial blocks, such that the cues differentially predicted response execution and inhibition. It was predicted that, relative to their HS peers, LS individuals would make more inhibition errors (i.e., executing a response to a no-go target) on alcohol-cued trials, especially when alcohol-cued no-go targets were a low-probability event, requiring unexpected implementation of inhibitory control.
Neurophysiological Markers of Behavioral Control
Neural support of prepotent response inhibition has been associated with activity in at least two specific components of the ERP. In particular, the N200 or N2 component is a negative-going deflection of the stimulus-locked ERP waveform peaking around 200 –350 ms following stimulus onset (see Folstein & Van Petten, 2008). Initially associated with inhibitory processes per se (e.g., Bokura, Yamaguchi, & Kobayashi, 2001; Falkenstein, Hoormann, & Hohnsbein, 1999), the N2 is now thought to index conflict monitoring (e.g., van Veen & Carter, 2002), including the conflict between activation of a prepotent response and the need to inhibit that response (e.g., Heil, Osman, Wiegelmann, Rolke, & Henninghausen, 2000; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). Thus, stemming from the idea that LS participants would experience greater conflict than their HS counterparts inhibiting responses in the presence of alcohol cues, we predicted that LS participants would show larger N2 amplitudes on alcohol-cued no-go trials.
In addition to the N2, the P3, a positive-going deflection of the stimulus-locked ERP waveform peaking around 300 – 600 ms following stimulus onset (see Fabiani, Gratton, & Federmeier, 2007), has been implicated in response inhibition. In tasks requiring the inhibition of a prepotent response, the P3 component tends to be larger in magnitude when a planned action must be cancelled (e.g., Enriquez-Geppert, Konrad, Pantev, & Huster, 2012; Kok, Ramautar, De Rutter, Band, & Ridderinkhof, 2004; Smith, 2011). Thus, as with our prediction regarding N2 amplitude, we also predicted that LS participants would show larger P3 amplitudes on alcohol-cued no-go trials than their HS peers.
Method
Participants
An initial group of more than 2,000 undergraduates enrolled in introductory psychology completed a set of questionnaire measures, including the Alcohol Sensitivity Questionnaire (ASQ; O’Neill, Sher, & Bartholow, 2002) and current/recent alcohol use measures, as part of a mass testing protocol at the beginning of the semester. ASQ scores were calculated for the approximately 60% of these individuals who self-identified as current drinkers. From this group, a random selection of 200 individuals whose ASQ responses fell within the upper and lower quartiles of the distribution (stratified by gender) was invited to participate. Those who expressed interest were further screened using a semistructured telephone interview to determine their eligibility for the study. Individuals who reported color blindness, history of head injury, visual impairment, major medical problems, psychological disorders, or a history of substance abuse were excluded from participation. The final sample included 85 undergraduates (51% LS; 89% Caucasian; 54% women; M age = 19.5) who participated in exchange for course credit in their introductory psychology course.
Self-Report Measures
Alcohol-related demographic information as assessed by the measures described in this section is reported in Table 1.
Table 1.
Means (and SDs) for Alcohol Use and Problems as a Function of Alcohol Sensitivity Group
| Alcohol variables | Group
|
Between-groups differences | |
|---|---|---|---|
| HS | LS | ||
| Q/F past 3 months | 6.98 (7.07) | 13.45 (11.26) | t(84) = 3.86, p < .01 |
| Q/F past 30 days | 10.60 (12.75) | 13.32 (16.63) | t(84) = 3.39, p < .01 |
| Negative consequences | 3.71 (3.08) | 8.11 (7.92) | t(84) = 9.38, p < .001 |
| Dependence features | 1.86 (1.76) | 3.28 (4.47) | t(84) = 3.24, p = .03 |
| Lifetime max. drinks | 11.95 (9.16) | 15.86 (6.19) | t(84) = 9.27, p = .01 |
Note. Q/F = alcohol quantity/frequency, calculated as the number of drinking occasions per week multiplied by the typical number of drinks consumed on one occasion; HS = high sensitivity; LS = low sensitivity.
ASQ
Self-reported sensitivity to the acute effects of alcohol was measured using the 15-item ASQ developed by O’Neill et al. (2002). The first nine items ask about the effects of alcohol associated the ascending limb of intoxication, such as feeling relaxed and more flirtatious. For each of these items, respondents are asked to indicate whether they have ever experienced the effect in question as a result of drinking alcohol and, if so, to estimate the minimum number of drinks they would need to consume in order to feel the effect. The remaining six items assess effects of alcohol associated with the descending limb of intoxication, such as vomiting and blacking out. These items are structured similarly, except that respondents are asked to estimate the maximum number of drinks they can consume without experiencing the effect. A composite alcohol sensitivity score is calculated for each respondent by averaging the number of drinks reported for each item, excluding items representing effects the respondent has never experienced. Internal consistency for the ASQ in the current study was excellent (α = .91) and similar to that reported in previous studies (α = .94 –.97; see Bartholow et al., 2010; O’Neill et al., 2002; Shin et al., 2010). Sensitivity groups did not differ in terms of age, race, or gender, and also did not differ in terms of the average number of alcohol effect items they endorsed (Ms = 12.4 and 11.6 for LS and HS groups, respectively), t(83) = 0.16.
Family history of alcoholism
Participants were asked to complete the Family Tree Questionnaire (Mann, Sobell, Sobell, & Pavan, 1985), which measures the extent to which respondents’ family members have experienced alcohol-related problems. Participants were asked to list each of their first- and second-degree relatives and to categorize each as an abstainer, a nonproblem drinker, or a problem drinker. For the purposes of the current study, participants were considered to be at increased familial risk if any first- or second-degree relatives were identified as having an alcohol problem (n = 52) and at low familial risk if no relatives were identified as such (n = 33). As in previous work (Bartholow et al., 2007, 2010), familial risk and alcohol sensitivity levels were uncorrelated (r = .1).
Alcohol-related negative consequences
Negative consequences of alcohol consumption were measured using a 24-item self-report questionnaire (see Hurlbut & Sher, 1992). Items inquired about consequences in several domains: legal (e.g., DWI), social/interpersonal (e.g., losing friends), physical (e.g., fighting), and occupational/educational (e.g., missing school or work). The measure also included nine items specifically asking about features of dependence (e.g., withdrawal, tolerance, continued use despite problems). Each item asked whether or not a given consequence was incurred by the participant. Response options included, “Never,” “Yes, but not in the past year,” “In the past year but not the past 3 months,” “Yes, in the past 3 months: once; twice; three times, or four times,” (scored 0, .3, .5, 1, 2, 3, and 5, respectively). For each participant, an overall “negative consequences” score was calculated as the sum of their responses to the full 24-item scale (α = .80), and a separate “dependence” score was calculated as the sum of their responses to the nine dependence-related items (α = .74).
Typical alcohol use
Participants were asked to report the average number of drinking occasions experienced per week and average number of drinks consumed per occasion in both the past 3 months and the past 30 days (scored on a per week basis), using items adapted from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Task Force recommendations (NIAAA, 2003). An alcohol quantity/frequency variable (AlcQF) was created by multiplying the number of typical weekly drinking occasions by estimated number of drinks typically consumed per occasion (see Table 1).
Laboratory Tasks
Alcohol-AAT Task
The Alcohol-AAT was developed by Wiers and colleagues (2009) to measure the strength of implicit/automatic approach-motivational biases for alcohol cues. In this task, participants are asked to respond to a series of images tilted 3° to either the right or left by pushing or pulling a joystick. Format movement assignments were counterbalanced (i.e., 50% of participants pulled right-tilted images and pushed left-tilted images, and the others received the opposite instructions). When the joystick is pulled or pushed, images gradually become larger or smaller, respectively, producing a sensation of pulling the image toward oneself or pushing it away.
Although an irrelevant feature of participants’ task, one third of the images (n = 15) are of alcoholic beverages (e.g., Four Loko, Burnett’s Raspberry Vodka), one third are of nonalcoholic beverages (e.g., McAllister’s Sweet Tea, Simply Orange), and one third are other, nonconsummable liquids (e.g., Brut cologne, Noxema). The strength of motivational biases (i.e., to approach or avoid) for the three image types is calculated by subtracting the average “pull” RT from the average “push” RT for each image category, with positive difference score values indicating an approach bias (i.e., faster to pull than to push). Each of the 45 images was presented four times in the pull condition and four times in the push condition. The resulting 360 trials were presented in quasi-random order (maximally three images of one category and three images of the same tilt in a row). To orient them to the task prior to the experimental trials, participants completed 10 practice trials consisting of tilted gray rectangles.
Cued Go/No-Go Task
The strength of behavioral inhibition and its neural correlates in the presence of alcohol cues was assessed using a version of the Cued Go/No-Go Task (e.g., Fillmore et al., 2009). A typical go/no-go task requires participants to press a button when a “go” target is presented and to withhold a button press when a “no-go” target is presented. In a Cued Go/No-Go Task, an additional stimulus—a cue—is presented prior to the target on every trial, to provide information concerning the probability that the upcoming target will require a response (e.g., Low & Miller, 1999; Miller, Schäffer, & Hackley, 1991). In the current study, participants were presented with one of two cues—a beer mug or a water bottle—which changed color to either green or blue (i.e., the target) following a randomly varying stimulus onset asynchrony of 100, 300, or 500 ms to indicate whether or not a response was required. A central fixation cross was presented for 800 ms preceded the cue on each trial. Participants were told to respond as quickly as possible (via a button press) at the presentation of a green target (i.e., go) and to withhold a response at the presentation of a blue target (i.e., no-go). Go-targets remained on the screen until a response was made (or for 1000 ms). Trials were separated by a 700-ms intertrial interval.
The task was divided into two blocks of 225 trials each. During the first block, alcohol cues were followed by go targets 80% of the time (i.e., High-Probability Alcohol Go) and nonalcohol cues were followed by no-go targets 80% of the time (i.e., High Probability Nonalcohol No-Go). The cue-target probability was reversed during the second block such that alcohol cues were followed by no-go targets on 80% of the time (i.e., High Probability Alcohol No-Go) and nonalcohol cues were followed by go targets on 80% of the time (i.e., High Probability Nonalcohol Go). This cue-target manipulation effectively created eight types of trials such that every possible cue-target combination was presented in both a low- and high-probability context (see Table 2), allowing effects of probability to be separated from effects of cue type. All participants completed the blocks in the same order to ensure that the influence of the individual difference variable of interest (alcohol sensitivity) was expressed in the same way in every participant and was not differentially influenced by block order.
Table 2.
Cue-Target Probabilities as a Function of Block
| Cue type | Block 1
|
Block 2
|
||
|---|---|---|---|---|
| Go targets | No-go targets | Go targets | No-go targets | |
| Alcohol | .80 | .20 | .20 | .80 |
| Nonalcohol | .20 | .80 | .80 | .20 |
Note. Total trials = 450.
Electrophysiological Recording and ERP Measurement
The electroencephalogram (EEG) was recorded during the Cued Go/No-Go Task from 64 silver/silver chloride electrodes fixed in a stretch-lycra cap and placed according to the standard 10 –10 system (American Electroencephalographic Society, 1994). All electrodes were referenced online to the right mastoid, and an average mastoid reference was calculated offline. Vertical and horizontal electrooculographic activity were recorded with additional electrodes placed above and below the left eye and approximately 2 cm outside the outer canthus of each eye. A ground electrode was placed along the frontal midline (FPz). All signals were amplified using a Neuroscan Synamps2 amplifier (Compumedics, Charlotte, NC) and filtered online at .01 to 40 Hz at a sampling rate of 1000 Hz. Impedance was kept below 5 KΩ at all channels. Ocular artifacts were corrected from the EEG signal offline using a regression-based procedure (Semlitsch, Anderer, Schuster, & Presslich, 1986). Trials containing voltage deflections of ±75 μV were discarded. After artifact elimination, EEG data were averaged according to participant, electrode, and stimulus conditions and low-pass filtered at 18 Hz. For each participant, the N2 was quantified as the mean amplitude between 200- and 335-ms posttarget presentation. Because of considerable P3 latency differences across participants, the P3 was quantified for each participant individually by identifying each participant’s peak amplitude value within an epoch from 350 to 600 ms posttarget and deriving the average amplitude from 140 ms surrounding the latency of that peak (i.e., peak ± 70 ms).
Procedure
Upon arriving at the lab, participants were told that the purpose of the study was to test RT abilities on two different tasks; all gave informed consent for participation. Participants were then led to a private electrophysiological recording room where the experimenters attached the recording electrodes. Participants then completed a battery of questionnaires related to their alcohol use, experiences, and beliefs, after which they completed the Alcohol-AAT and then the Cued Go/No-Go Task (a 5-min break was inserted between the tasks). Upon task completion, the electrode cap was removed and participants were shown to a private washroom to clean up. Participants were debriefed on the nature of the experiment, thanked for their time, given contact information for the primary investigator, and excused.
Results
Analytic Approach
Eight participants (4 LS) were dropped from ERP analyses because of problems with their EEG recording (these individuals’ behavioral data were retained, however), and four participants’ data (2 LS) were dropped from Cued Go/No-Go behavioral analyses due to data recording error (their ERP data were retained, however). As in prior work (Bartholow et al., 2007, 2010), alcohol sensitivity level and AlcQF were significantly correlated (r = .30, p < .05); therefore, ancillary analyses included AlcQF as a covariate.1 To produce a more normal distribution, RT data from both tasks were trimmed globally by excluding all RTs faster than 200 ms and slower than 2000 ms. RTs were further trimmed within subjects such that all RTs ± 2 SD from an individual’s mean RT were excluded from analysis. Accuracy data (proportion correct) for the Cued Go/No-Go Task were examined as a function of group and trial type, such that any individual accuracy score >2.5 SD below the group mean for a given trial type was modified to the value of the next-closest, nonoutlying value in the distribution (Tabachnick & Fidell, 1989; Tukey, 1990; Wilcox, 1995). Less than 2% of all individual data points were modified in this way and did not differ as a function of sensitivity group. These modified proportion data were transformed using the arcsine of the square root to produce a distribution more suitable for analysis of variance (see McDonald, 2009). Initial analyses indicated that none of the effects of interest were moderated by gender, so this factor was collapsed in all analyses reported here. A correlation matrix of the main variables is presented in Table 3.
Table 3.
Correlations Among Primary Measures
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. AAT Bias | — | |||||
| 2. GNG RT | 0.17 | — | ||||
| 3. N200 | 0.13 | −0.03 | — | |||
| 4. P300 | 0.11 | −0.09 | 0.21 | — | ||
| 5. GNG Accuracy | 0.25* | −0.12 | 0.27* | 0.05 | — | |
| 6. Typical Q/F | 0.05 | 0.21 | −0.14 | 0.05 | −0.24* | — |
| 7. Sensitivity | 0.27* | 0.22 | −0.19 | 0.31* | −0.14 | 0.33* |
Note. AAT = approach/avoidance task; GNG = go/no-go task; The GNG RT variable is a difference score (nonalcohol-cued “go” trial RTs – alcohol-cued go trial RTs), such that more negative values indicate faster RTs to alcohol cues relative to nonalcohol cues. P300 = amplitude of the P300 elicited by low probability, alcohol-cued no-go trials during the Cued Go/No-Go Task; N200 = amplitude of the N200 elicited by low-probability, alcohol-cued no-go trials during the Cued Go/No-Go Task. GNG Accuracy = proportion of low probability, alcohol-cued no-go trials during the Cued Go/No-Go Task (arcsine transformed) where no response was made; larger (more positive) values indicate better performance; Q/F = alcohol quantity-frequency, calculated as the number of drinking occasions per week multiplied by the typical number of drinks consumed on one occasion.. Sensitivity is scored such that greater (more positive) values represent lower sensitivity (i.e., needing more drinks to feel a given effect).
p < .05.
Alcohol Approach Bias: Alcohol-AAT
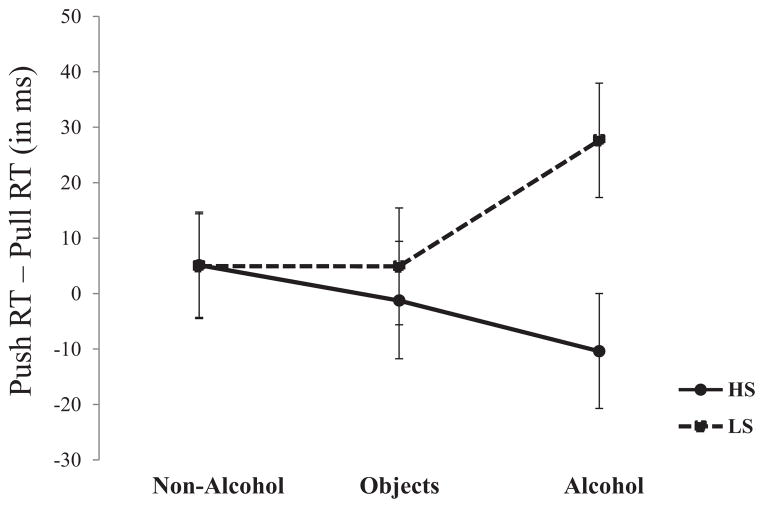
Trials in which errors were made (e.g., initiating a push action on a pull trial) were excluded from analyses (<3% of total trials). Bias scores for each cue type were calculated by subtracting each participant’s mean pull trial RT from their mean push trial RT, such that positive values indicate faster RTs to pull relative to push trials (i.e., an approach bias). These bias scores, illustrated in Figure 1, were submitted to a 2 (Group; LS, HS) × 3 (Cue; alcohol, nonalcohol, other liquids) mixed factorial analysis of variance (ANOVA) with repeated measures on the second factor. As hypothesized, this analysis showed a Group × Cue interaction, F(2, 166) = 3.78, p = .025. Follow-up ANOVAs testing the effect of Group separately for each cue type indicated that, although LS and HS individuals did not differ in their approach/avoidance responses to nonalcohol cues or other liquids, Fs(1, 83) < 1, ps > 0.6, the groups’ responses differed significantly for alcohol cues, F(1, 83) = 6.74, p < .01. One-sample t test comparisons showed that the LS group’s mean bias score for alcohol cues was significantly positive (M = 27.65 ms, SD = 9.91), indicating a significant approach bias, t(42) = 2.79, p < .01. The HS group’s mean bias score was marginally negative (M = −10.39 ms, SD = 10.43), though did not differ reliably from zero, t(41) = −0.96, p = .34.
Figure 1.
Push RT–Pull RT in the Alcohol-AAT as a function of cue type and sensitivity group. Positive values indicate faster average RTs to pull than to push (i.e., an approach bias); zero indicates the absence of bias. Vertical bars indicate ±1 SE.
Cued Go/No-Go Task
RT
Go trial RTs were submitted to a 2 (Group; LS, HS) × 2 (Cue; alcohol, nonalcohol) × 2 (Cue-target probability; high, low) mixed factorial ANOVA with repeated measures on the latter factors. This analysis showed a Group × Cue interaction, F(1, 79) = 3.97, p < .05. LS participants were faster to respond to alcohol-cued targets (M = 341 ms) relative to nonalcohol-cued targets (M = 347 ms), t(39) = 2.8, p < .01, but HS participants showed no RT bias as a function of cue type (Ms = 348 ms for both cues). No other effects were significant in this analysis.
Inhibition accuracy
To test whether LS individuals differentially fail to inhibit prepotent responses to alcohol-cued relative to nonalcohol-cued targets, transformed accuracy rates for no-go trials were submitted to a 2 (Group; LS, HS) × 2 (Cue; alcohol, nonalcohol) × 2 (Cue-target probability; high, low) mixed factorial ANOVA with repeated measures on the latter factors. This analysis showed a significant Group × Cue × Probability interaction, F(1, 79) = 4.33, p = .04 (see Figure 2). To determine the source of this interaction, follow-up Cue × Probability ANOVAs were conducted separately on the data from the HS and LS groups. The ANOVA on the LS group’s data showed a significant Cue × Probability interaction, F(1, 40) = 13.67, p < .001. Follow-up tests indicated that more errors occurred on low-probability, alcohol-cued no-go trials (M = 1.39) than on low-probability nonalcohol-cued no-go trials (M = 1.50), t(40) = −3.80, p < .001, but there was no effect of cue type on high-probability trials t(40) = −1.12, p > .20. The ANOVA on the HS group’s data showed a main effect of probability, F(1, 39) = 9.52, p < .01, such that more errors were made on low-probability trials, but these errors did not differ as a function of cue type, F(1, 39) = 0.43, p = .5.
Figure 2.
Inhibition accuracy on no-go trials in the Cued Go/No-Go Task as a function of cue type and congruency. (A) LS group; (B) HS group. Vertical bars indicate ±1 SE.
N2 amplitude
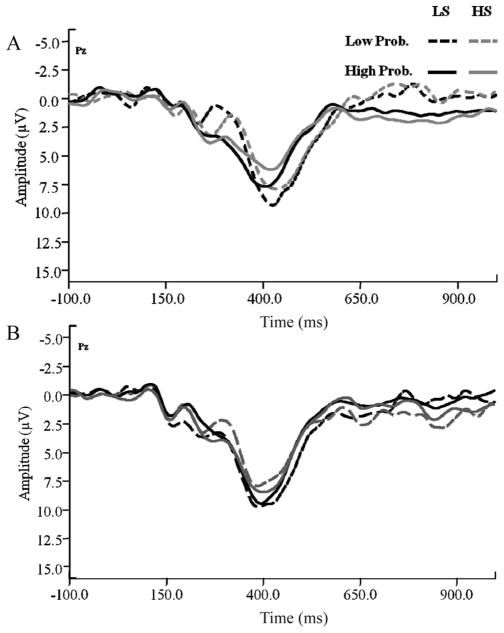
Initial analyses focusing on data recorded from a core set of 15 electrode sites (F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, CP4, P3, PZ, and P4, representing locations from frontal to parietal regions and across both hemispheres) using a 5 (Coronal location; frontal, fronto-central, central, centro-parietal, parietal) × 3 (Lateral location; left, midline, right) repeated-measures ANOVA showed a Coronal × Lateral interaction, indicating that N2 amplitude was most prominent at frontal and fronto-central electrode locations, F(4, 308) = 49.11, p < .001, consistent with numerous previous reports (e.g., Folstein & Van Petten, 2008). Thus, our primary analyses focused on data recorded at six frontal and fronto-central electrodes (F3, Fz, F4, FC3, FCz, and FC4). ERP waveforms recorded at FCz are presented in Figure 3.
Figure 3.
ERP waveforms measured at the FCz electrode elicited by alcohol-cued (A) and nonalcohol-cued (B) no-go trials as a function of sensitivity group and cue-target probability. Target onset occurred at 0 ms.
The primary prediction for the N2 data involved a three-way interaction, such that, relative to their HS peers, LS individuals would show increased N2 amplitude on low-probability no-go trials cued by alcohol (i.e., where it was necessary to withhold a prepotent behavioral response following an alcohol cue), but that no such group difference would occur on nonalcohol-cued trials or when inhibition trials were highly probable. To test this prediction, mean N2 amplitudes elicited during successfully inhibited no-go trials were submitted to a 2 (Group; LS, HS) × 2 (Cue; alcohol, nonalcohol) × 2 (Probability; high, low) × 2 (Coronal location) × 3 (Lateral location) mixed factorial ANOVA with repeated measures on all but the first factor. Consistent with predictions, the analysis showed a significant Group × Cue × Probability interaction, F(1, 76) = 5.78, p < .05, the essence of which is displayed in Figure 4. No other effects of interest were significant in this analysis.
Figure 4.
N2 amplitude at electrode FCz on no-go trials in the Cued Go/No-Go Task as a function of cue type and congruency for the LS group (A) and the HS group (B). Vertical bars indicate ±1 SE.
To understand this complex interaction, follow-up Cue × Probability ANOVAs were performed separately on the data from the two sensitivity groups. The analysis of the LS group’s data indicated a significant Cue × Probability interaction, F(1, 41) = 14.07, p < .001. Follow-up contrasts showed that alcohol-cued trials elicited significantly larger (more negative) N2 amplitude when no-go probability was low (M = −0.80 μV) relative to when no-go probability was high (M = 1.79 μV), F(1, 41) = 26.10, p < .0001, but that N2 amplitude elicited by nonalcohol-cued trials was nearly identical regardless of whether no-go probability was low or high (Ms = 1.94 and 1.97 μV, respectively; F < .01). Moreover, whereas there was no difference in N2 amplitude elicited by alcohol-cued and nonalcohol-cued targets when no-go probability was high (F < 1), alcohol-cued targets elicited much larger N2 amplitude than nonalcohol-cued targets when no-go probability was low, F(1, 41) = 19.9, p < .001. The ANOVA on the HS group’s data showed only a main effect of probability, F(1, 35) = 15.45, p < .001, indicating a predictable increase in N2 amplitude when no-go probability was low relative to high (see also Nieuwenhuis et al., 2003). However, contrary to the LS group, this effect did not differ as a function of cue type (F < 1). Stated another way, LS individuals showed larger N2 amplitudes when inhibiting a prepotent behavioral response cued by alcohol, but not when cued by a nonalcohol beverage. In contrast, the N2 response among HS participants did not differentiate alcohol-cued and nonalcohol-cued trials.
P3 amplitude
An initial analysis from the same core set of 15 electrode sites used in the N2 analysis showed that P3 amplitude on no-go trials was most prominent at central, centro-parietal, and parietal locations, F(4, 308) = 83.34, p < .001. Thus, primary analyses were carried out using data recorded at nine central to parietally located electrodes (C3, Cz, C4, CP3, CPz, CP4, P3, Pz, and P4). ERP waveforms recorded at Pz are presented in Figure 5.
Figure 5.
ERP waveforms measured at the Pz electrode elicited by alcohol-cued (A) and nonalcohol-cued (B) no-go trials as a function of sensitivity group and cue-target congruency. The dashed vertical line on the x-axis indicates stimulus onset.
Similarly to the N2 data, the primary prediction for the P3 data involved a three-way interaction, such that LS individuals, relative to their HS counterparts, would show increased P3 amplitude in response to low-probability inhibition trials cued by alcohol but not those cued by nonalcohol and not when inhibition was a high-probability event. This prediction was tested by submitting P3 amplitude elicited during successfully inhibited no-go trials to a 2 (Group; LS, HS) × 2 (Cue; alcohol, nonalcohol) × 2 (Probability; high, low) × 3 (Coronal location) × 3 (Lateral location) mixed factorial ANOVA with repeated measures on all but the first factor. Although the predicted Group × Cue × Probability interaction was not significant, F(1, 76) = 2.83, p = .09, it was qualified by a significant Group × Cue × Probability × Coronal location interaction, F(2, 152) = 3.55, p = .03. Visual inspection of the means indicated that the predicted three-way interaction was generally evident across the locations we analyzed but was most prominent at centro-parietal and parietal locations. Means associated with this interaction are depicted in Figure 6.
Figure 6.
P3 amplitude at electrode Pz on “no-go” trials in the Cued Go/No-Go Task as a function of cue type and congruency for the LS group (A) and the HS group (B). Vertical bars indicate ±1 SE.
Follow-up ANOVAs testing the Cue × Probability effect separately by sensitivity group showed that the predicted interaction was significant for those in the LS group, F(1, 41) = 8.72, p = .005. As indicated in Figure 6, whereas P3 amplitude elicited by nonalcohol-cued trials did not differ as a function of cue-target probability (t < 1), the P3 elicited on alcohol-cued trials that unexpectedly required inhibition of a response elicited a much larger P3 (M = 9.65 μV) relative to alcohol-cued trials on which inhibition was highly probable (M = 6.57 μV), t(41) = 4.53, p < .001. In contrast, the ANOVA on the HS group’s P3 data indicated only a significant main effect of Cue, F(1, 35) = 9.36, p < .001; the Cue × Probability interaction was not significant (F < 1).
Discussion
LS to the acute effects of alcohol has long been associated with increased alcohol use and risk for alcohol-related problems (e.g., Schuckit, 1994; Schuckit & Smith, 2000). However, relatively little research has investigated mechanisms that might help to explain this association. By adopting a dual-process framework (see Wiers & Stacy, 2006), the current research sought to test the extent to which LS is associated with differences in motivational, neurocognitive, and behavioral responses in the presence of alcohol-related cues that previous research has suggested could lead to enhanced risk for alcohol use and abuse.
The current findings provide support for the notion that LS to alcohol is characterized by automatic approach tendencies for alcohol cues, coupled with difficulty regulating responses in the presence of such cues. This pattern represents the “potentially disastrous combination” Robinson and Berridge (2003, p. 46) described in outlining how incentive salience can produce problematic levels of drug use. As such, these findings have important implications for understanding how low alcohol sensitivity serves as a risk mechanism for alcohol misuse and the development of alcohol use disorders. Previous work (Bartholow et al., 2007, 2010; Shin et al., 2010) has shown that alcohol cues differentially capture attention and engage neural responses indicative of enhanced motivational relevance in LS relative to HS individuals. The current data are the first to show that these markers of motivated attention have implications for behavioral approach responses as well as regulation of such responses, in a way consistent with the tenets of the incentive salience hypothesis (Everitt & Robbins, 2005; Robinson & Berridge, 2003).
Specifically, the Alcohol-AAT showed that task-irrelevant alcohol cues elicited an automatic approach bias among LS individuals, as evidenced by faster RTs on trials where alcoholic images were pulled toward the body compared with trials where these images were pushed away. This bias was absent among the HS individuals, who if anything tended to show an avoidance bias for alcohol cues. Further evidence for an automatic approach bias was found in the Cued Go/No-Go Task, in which LS individuals showed faster RTs to alcohol-cued go targets in comparison to nonalcohol-cued go targets. This apparent approach bias might not be of concern from an etiological standpoint if it were not for the additional evidence indicating that LS participants had considerable difficulty regulating that bias. Specifically, LS individuals showed a marked failure to implement the behavioral control necessary to successfully inhibit responses in the presence of alcohol cues, as indicated by their increased rate of inhibition errors on low-probability, alcohol-cued (relative to nonalcohol-cued) no-go trials.
Moreover, on these same, critical alcohol-cued no-go trials, LS individuals showed an enhancement of the N2 and P3 components of the ERP waveform, indicating that LS individuals were allotting an unusual amount of motivated attention to alcohol cues that required an unexpected inhibitory response (P3 amplitude) and that such trials elicited marked conflict between the prepotent tendency to respond and the need to inhibit that response (N2 amplitude). Stated another way, relative to nonalcohol-cued trials, on alcohol-cued no-go trials LS individuals had to recruit considerably more cognitive control resources in order to successfully inhibit a response (see Bekker, Kenemans, & Verbaten, 2005; Heil et al., 2000; Nieuwenhuis et al., 2003). Still, despite these efforts at control, the fact that LS individuals experienced more inhibition failures on unexpected alcohol-cued no-go trials suggests that they were unable to regulate the prepotent approach tendency elicited by alcohol cues.
To the extent that laboratory analogues of action tendencies and behavioral regulation reflect drinkers’ responses to alcohol cues outside the lab (see Wiers et al., 2011, 2010), the current findings could have implications for understanding how LS individuals develop patterns of use that can lead to problems. Returning to the central premises of the incentive salience hypothesis, it could be that an unusually strong affective response to the pleasurable properties of alcohol (i.e., liking) leads to rapid acceleration of drinking behaviors during adolescence, thereby sensitizing the appetitive system to alcohol cues prior to the maturation of pre-frontal cortical structures that would support regulation of these approach tendencies (e.g., Casey et al., 2000; Gogtay et al., 2004). The current study was not designed to get at the root of these questions, but the results are suggestive that further, longitudinal research will be necessary to investigate the extent to which such a process characterizes the experiences of LS relative to HS drinkers and whether this process is at the root of LS drinkers’ increased susceptibility to alcohol use disorders.
Despite its strengths, the current study had some key limitations. Most importantly, the current study was unable to address a fundamental question concerning the association between alcohol sensitivity and risk status: do some people drink heavily because they are low in sensitivity to alcohol, or are they low in sensitivity because they drink heavily? Although the study’s participants were likely early in their drinking careers and none met criteria for alcohol use disorder, the sampling frame was limited to college students, some of whom had already initiated fairly heavy patterns of use. Future work should endeavor to study whether biases in motivation and attention for alcohol cues are present in some individuals prior to the onset of heavy drinking and whether the magnitude of such biases predicts which individuals will develop low alcohol sensitivity and/or drinking problems. In addition, because the current sample consisted of primarily first-year students at a Midwestern university, they are relatively homogenous in ethnic, racial, and socioeconomic background, making future work with more diverse populations important to generalizing the results of this study.
In conclusion, the results of this study are supportive of a dual process model of addiction for individuals low in sensitivity to the acute effects of alcohol. In accordance with such models (e.g., Wiers et al., 2007) and with the tenets of the incentive salience hypothesis (Robinson & Berridge, 2003), LS individuals exhibited heightened approach-motivational tendencies when presented with a motivationally salient alcohol cue, which translated into greater difficulty inhibiting such approach tendencies when contextually necessary, as evidenced by both behavioral and neurophysiological measures. Continued investigation into this phenomenon and how it fits with other prominent theories of alcohol addiction could lead to important theoretical and practical advances in the study of hazardous alcohol use, risk assessment, and intervention.
Acknowledgments
Preparation of this article was supported by Grants P60 AA011998 and T32 AA013526 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Inclusion of the AlcQF covariate in all main analyses did not increase the p-value.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/S0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society. Guideline thirteen: Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. doi: 10.1097/00004691-199401000-00014. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: Cognitive control of inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90:272–287. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychology of Addictive Behaviors. 2007;21:555–563. doi: 10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Lust SA, Tragesser SL. Specificity of P3 event-related potential reactivity to alcohol cues in individuals low in alcohol sensitivity. Psychology of Addictive Behaviors. 2010;24:220–228. doi: 10.1037/a0017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Source analysis of the N2 in a cued Go/NoGo task. Cognitive Brain Research. 2005;22:221–231. doi: 10.1016/j.cogbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives and expectations. In: Medin DL, editor. Psychology of learning and motivation. Vol. 40. New York, NY: Academic Press; 2001. pp. 223–278. [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neuroscience. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/S1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Priester JR, Bernston GG. Rudimentary determinants of attitudes: II. Arm flexion and extension have differential effects on attitudes. Journal of Personality and Social Psychology. 1993;65:5–17. doi: 10.1037/0022-3514.65.1.5. [DOI] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, Patrick CJ. Deleterious effects of alcohol intoxication: Diminished cognitive control and its behavioral consequences. Journal of Abnormal Psychology. 2003;112:476–487. doi: 10.1037/0021-843X.112.3.476. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Geidd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/S0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: FMRI and meta-analytic evidence of a domain-general control network for learning. Cognitive Brain Research. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology. 2003;112:424– 436. doi: 10.1037/0021-843X.112.3.424. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Teige-Mocigemba S, Spruyt A, Moors A. Implicit measures: A normative analysis and review. Psychological Bulletin. 2009;135:347–368. doi: 10.1037/a0014211. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. International Journal of Psychophysiology. 2006;60:315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Deutsch R, Gawronski B, Strack F. At the boundaries of automaticity: Negation as reflective operation. Journal of Personality and Social Psychology. 2006;91:385–405. doi: 10.1037/0022-3514.91.3.385. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Konrad C, Panteva C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage. 2010;51:877–887. doi: 10.1016/j.neuroimage.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Federmeier KD. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. New York, NY: Cambridge University Press; 2007. pp. 85–119. [DOI] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/S0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug and Alcohol Dependence. 2009;100:91–99. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning: A conceptual framework for alcohol-related aggression. Experimental and Clinical Psychopharmacology. 2000;8:576–597. doi: 10.1037/1064-1297.8.4.576. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning and alcohol-related aggression. Journal of Abnormal Psychology. 2004;113:541–555. doi: 10.1037/0021-843X.113.4.541. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Osman A, Wiegelmann J, Rolke B, Henninghausen E. N200 in the Eriksen-task: Inhibitory executive process? Journal of Psychophysiology. 2000;14:218–225. doi: 10.1027//0269-8803.14.4.218. [DOI] [Google Scholar]
- Hurlbut SC, Sher KJ. Assessing alcohol problems in college students. Journal of American College Health. 1992;41:49–58. doi: 10.1080/07448481.1992.10392818. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PAF, Bucholz KK, Slutske WS, Nelson EC, Martin NG. Genetic effects on alcohol dependence risk: Re-evaluating the importance of psychiatric and heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/S0033291704002922. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GPH, Ridderinkhof RK. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Li TK. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. Journal of Studies on Alcohol. 2000;61:5–12. doi: 10.15288/jsa.2000.61.5. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/0033-295X.91.3.295. [DOI] [PubMed] [Google Scholar]
- Low KA, Miller J. The usefulness of partial information: Effects of go probability in the choice/nogo task. Psychophysiology. 1999;36:288–297. doi: 10.1017/S0048577299980332. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug and Alcohol Dependence. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- McDonald JH. Handbook of biological statistics. 2. Baltimore, MD: Sparky House Publishing; 2009. [Google Scholar]
- Miller J, Schäffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychologica. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-R. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Task force on recommended questions of the National Council on Alcohol Abuse and Alcoholism: Recommended sets of alcohol consumption questions; October 15–16, 2003; 2003. Retrieved from http://www.niaaa.nih.gov/research/guidelines-and-resources/recommended-alcohol-questions. [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social reorientations of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision-making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/CABN.3.1.17. [DOI] [PubMed] [Google Scholar]
- O’Neill SE, Sher KJ, Bartholow BD. Alcohol susceptibility and tolerance in young adults. Alcoholism: Clinical and Experimental Research. 2002;26:119A. [Google Scholar]
- Palfai TP. Activating action tendencies: The influence of action priming on alcohol consumption among male hazardous drinkers. Journal of Studies on Alcohol. 2006;67:926–933. doi: 10.15288/jsa.2006.67.926. [DOI] [PubMed] [Google Scholar]
- Peeters M, Wiers RW, Monshouwer K, van de Schoot R, Janssen T, Vollebergh WAM. Automatic processes in at-risk adolescents: The role of alcohol-approach tendencies and response inhibition in drinking behavior. Addiction. 2012;107:1939–1946. doi: 10.1111/j.1360-0443.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Paylan SS, Gentes-Hawn A, Hoaken PNS. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcoholism: Clinical and Experimental Research. 2003;27:773–779. doi: 10.1097/01.ALC.0000065434.92204.A1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95:91–117. doi: 10.1046/j.1360-0443.95.8s2.19.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society: B Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. New findings in the genetics of alcoholism. Journal of the American Medical Association. 1999;281:1875–1876. doi: 10.1001/jama.281.20.1875. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich L, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:323–329. doi: 10.1111/j.1530-0277.2001.tb02217.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Kelsoe JR, Braff DK, Wilhelmsen KC. Some possible genetic parallels across alcoholism, bipolar disorder, and schizophrenia. Journal of Studies on Alcohol. 2003;64:157–159. doi: 10.15288/jsa.2003.64.157. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. Journal of Studies on Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: Social information processing model of alcoholism risk: A 20-year prospective study. Alcoholism: Clinical and Experimental Research. 2004;28:1881–1889. doi: 10.1097/01.ALC.0000148111.43332.A5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn JA. The relationship between self-rating of the effects of alcohol and alcohol challenge results in ninety-eight young men. Journal of Studies on Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito TA, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. doi: 10.1111/1469-8986.3720257. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD. Subjective effects of alcohol II: Individual differences. In: Earleywine M, editor. Mind altering drugs: Scientific evidence for subjective experience. New York, NY: Oxford; 2005. pp. 135–153. [Google Scholar]
- Shin E, Hopfinger JB, Lust SA, Henry EA, Bartholow BD. Electrophysiological evidence of alcohol-related attentional bias in social drinkers low in alcohol sensitivity. Psychology of Addictive Behaviors. 2010;24:508–515. doi: 10.1037/a0019663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL. To Go or not to go, that is the question: Do the N2 and P3 reflect stimulus- or response-related conflict? International Journal of Psychophysiology. 2011;82:143–152. doi: 10.1016/j.ijpsycho.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review. 2004;8:220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. New York, NY: Harper & Row; 1989. [Google Scholar]
- Townsend JM, Duka T. Attentional bias associated with alcohol cues: Differences between heavy and occasional social drinkers. Psychophamlacology. 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationship of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: A discrete-time survival analysis. Alcoholism: Clinical and Experimental Research. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Data-based graphics: Visual display in the decades to come. Statistical Science. 1990;5:327–339. doi: 10.1214/ss/1177012101. [DOI] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective intoxication in response to alcohol challenge: Heritability and covariation with personality, breath alcohol level, and drinking history. Alcoholism: Clinical and Experimental Research. 2003;27:795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RCME, Sher KJ, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacology, Biochemistry and Behavior. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker E, Lindenmeyer J. Re-training automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science. 2011;22:490– 497. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, Van Den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain and Behavior. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Implicit cognition and addiction. Current Directions in Psychological Science. 2006;15:292–296. doi: 10.1111/j.1467-8721.2006.00455.x. [DOI] [Google Scholar]
- Wiers RW, Van Woerden N, Smulders FTY, De Jong PJ. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. Journal of Abnormal Psychology. 2002;111:648– 658. doi: 10.1037/0021-843X.111.4.648. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Statistics for the social sciences. San Diego, CA: Academic; 1995. [Google Scholar]