Abstract
Observations from a wide range of organisms show the centromeres form associations of pairs or small groups at different stages of meiotic prophase. Little is known about the functions or mechanisms of these associations, but in many cases synaptonemal complex elements seem to play a fundamental role. Two main associations are observed: homology-independent associations very early in the meiotic program – sometimes referred to as centromere coupling, and a later association of homologous centromeres, referred to as centromere pairing or tethering. The later centromere pairing initiates during synaptonemal complex assembly, then persists after the dissolution of the synaptonemal complex. While the function of the homology-independent centromere coupling remains a mystery, centromere pairing appears to have a direct impact on the chromosome segregation fidelity of achiasmatic chromosomes. Recent work in yeast, Drosophila, and mice suggest centromere pairing is a previously unappreciated, general meiotic feature that may promote meiotic segregation fidelity of the exchange and non-exchange chromosomes.
Keywords: meiosis, chromosome, centromere pairing, centromere coupling, ZIP1, SYCP1
Prophase of meiosis I is characterized by the establishment of associations between the homologous chromosomes; (reviewed in (Bhalla and Dernburg, 2008; Zickler and Kleckner, 1998) (Fig. 1). In most organisms the homologous partners become tethered by crossovers, the sites of genetic exchange, which are viewed cytologically as chiasmata. These crossovers are formed as the homologous chromosomes are tightly aligned by the synaptonemal complex, a tripartite structure with two outer lateral elements and a connecting central element, that runs along their length (Fig. 1 and Fig. 2). The synaptonemal complex disassembles in late prophase, leaving the homologs connected by their chiasmata (Fig. 1). The homologs remain associated until anaphase I, when they segregate away from each other as new chromosomes comprised of a mixture of maternal and paternal sequence blocks, due to the exchange of genetic material through recombination. Centromeres are fundamental for this segregation, since their specific chromatin structure promotes the assembly of the kinetochore, a protein apparatus able to associate with microtubule fibers and to couple microtubule de-polymerization with chromosomal movement; reviewed in (Westermann et al., 2007).
Figure 1. Centromere behavior in budding yeast and mouse spermatocytes in meiotic prophase.
Two homologous pairs of chromosomes (blue and red) each one comprised of two sister chromatids are represented at different stages of meiosis I in budding yeast and mouse spermatocytes. Budding yeast begins meiosis with the centromeres clustered next to the spindle pole body (the Rabl conformation) which is common in many other organisms, while in mouse spermatocytes the centromeres appear more dispersed with some evidence for limited associations at meiotic entry. In yeast, the centromeres disperse from the Rabl cluster and form non-homologous couples (CEN coupling), in mice, however, no evidence of coupling has been seen. In most organisms, including yeast and mice, at the pachytene stage, the homologous chromosomes are aligned along their length forming a proteinaceous structure between them called the synaptonemal complex (synapsis). At the end of prophase I the synaptonemal complex along the arms disassembles, but persists at the centromeres. In metaphase I the centromeres of the homologous chromosomes are pulled towards opposite poles, in preparation for chromosome segregation that will take place in anaphase (not shown).
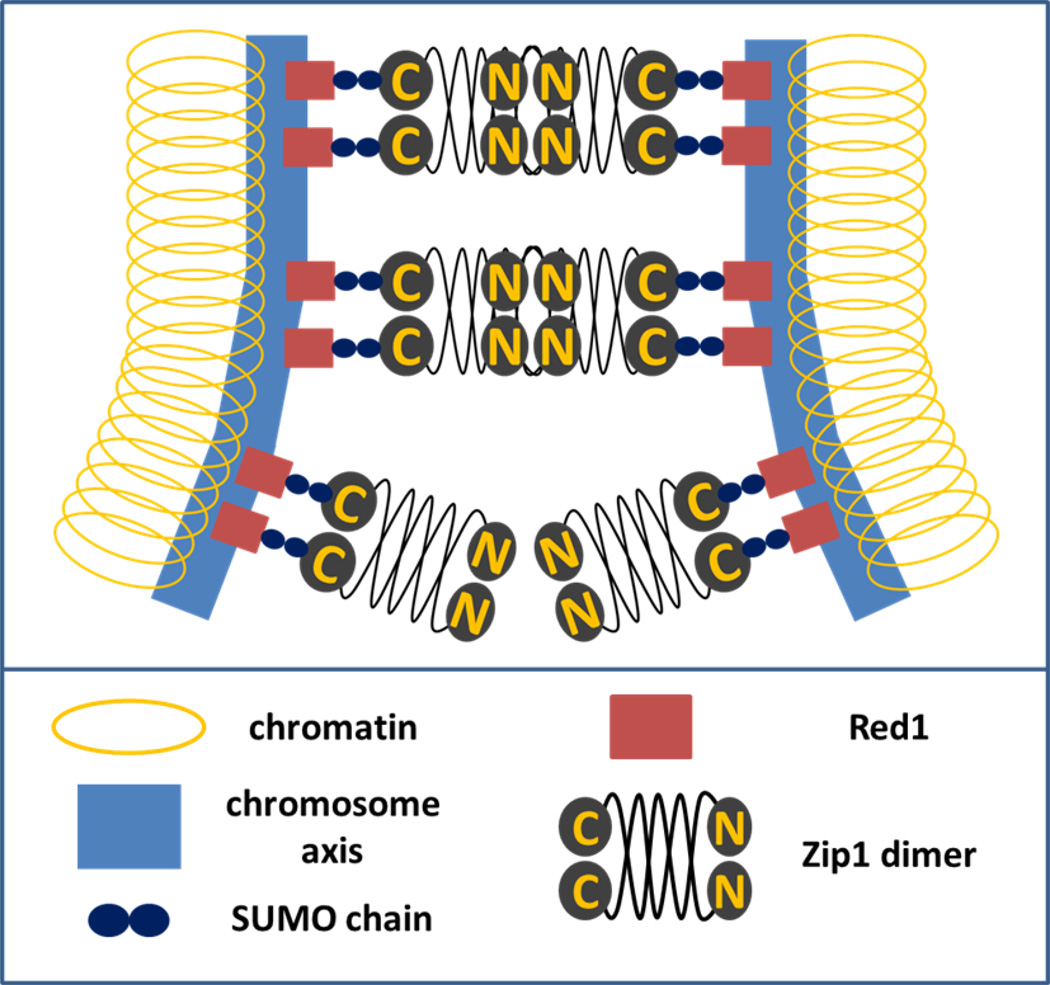
Figure 2. Model of budding yeast synaptonemal complex.
The synaptonemal complex is responsible for tight chromosome synapsis during the pachytene stage of meiosis. It appears “ladder like”; the chromosome axes of the two homologous chromosomes (colored blue) are called lateral elements (LEs) at this stage. The chromatin loops out (colored yellow) from the chromosome axes. The LEs become connected by transverse filaments (the steps of the ladder), which in budding yeast are constituted by the protein Zip1. Recent data suggest that polymeric SUMO chains (blue ovals) play a role on synaptonemal complex formation by binding to both Zip1 and the chromosome axis protein Red1 (red square). Zip1 binds SUMO noncovalently, Red1 can either form covalent SUMO conjugates or bind it non-covalently (not represented). SUMO interactions with other synaptonemal complex elements, like Ecm11, probably play an important role in synaptonemal complex formation and dynamics (not represented). Figure 3. Model of centromere associations and synaptonemal complex formation
In addition to promoting interactions with microtubules, meiotic centromeres have also been observed to form associations with other centromeres in ways that are still not well understood. Observations of centromere associations have been made in a wide range of organisms including mice (Bisig et al., 2012; Brinkley et al., 1986; Qiao et al., 2012; Scherthan et al., 1996), wheat (Bennett, 1979) (Corredor et al., 2007), onion (Church and Moens, 1976), rice (Prieto et al., 2004), fruit fly (Dernburg et al., 1996; Takeo et al., 2011; Tanneti et al., 2011), fission yeast (Ding et al., 2004), budding yeast (Kemp et al., 2004; Tsubouchi and Roeder, 2005) and Arabidopsis (Ronceret et al., 2009). These associations fall into two broad categories based on when they occur in the meiotic program. First, early in meiosis, before homologous pairing occurs, centromere clusters or pairs are observed in many organisms. These associations are often between non-homologous chromosomes. In at least two circumstances, mouse spermatocytes (Bisig et al., 2012; Qiao et al., 2012) and maize (Carlton and Cande, 2002), these types of centromere behaviors could not be observed. Second, in yeast, flies, and mouse spermatocytes, associations between homologous centromeres are observed in late prophase (Fig. 1). It is important to note that these many observations of centromere associations have been made in widely divergent cell types, each with their own peculiar meiotic features, and the observations have been made with a range of methodologies. Given this, though there are clear parallels in the observations, it is not known whether the centromeric associations observed in different organisms are performing identical functions, nor is it known if the structures that promote the non-homologous and homologous centromeric associations are the same. In this review we will refer to the initial non-homologous centromere associations as “centromere coupling” and the later associations between the homologous centromeres as “centromere pairing”. The term “centromere coupling” has been used to describe pairwise association of non-homologous centromeres observed in early meiotic prophase in budding yeast (Tsubouchi and Roeder, 2005), to differentiate it from the close association of homologous centromeres that occurs later when homologous chromosomes synapse, and we will keep that nomenclature.
Early meiotic centromeric associations in plants
Much of what we know about centromere associations in meiosis comes from studies of plants (reviewed in (Naranjo and Corredor, 2008)). A pioneering study of meiotic chromosome behavior in the onion Allium fistulosum was the first to describe centromeric associations in early meiotic prophase (Church and Moens, 1976). In this study, electron microscopy was used to characterize the distribution of centromeres at different stages of meiosis. In premeiotic S-phase the authors observed single centromeres and associations that contained two to approximately seven centromeres. Although they could not test the model directly, the fact that in early prophase there were no specific patterns to the associations and the fact that groups of three or more centromeres must contain at least one that is not homologous to the others, led to the suggestion that the associations were homology-independent. In contrast, at the later stage of pachytene, the centromeres were organized into homologous pairs, which the authors inferred by the observation of the synaptonemal complex passing through the centromere pairs, uninterrupted.
A subsequent study in the wheat Triticum aestivum showed that in early meiosis, well before the formation of any recognizable synaptonemal complex, most of the centromeres were arranged in pairs. As in the previous studies with Allium, this study did not demonstrate directly whether the pairs were between homologous or non-homologous centromeres (Bennett, 1979). Immunofluorescence staining of centromeres during male meiosis in Lilium longiflorum (Suzuki et al., 1997) also revealed clustered centromeres during pre-meiotic interphase. After entry into the leptotene stage these centromeric associations were dissolved, as indicated by an increase in the number of foci observed when staining with an anti-centromere antibody, reaching 24 in late leptotene (the diploid number of chromosomes in this organism). At zygotene the number of fluorescent spots then gradually decreased, resulting in 12 fluorescence spots in pachytene, corresponding to the synapsis of homologous pairs.
The first clear demonstration that early meiotic centromere associations are homology independent was accomplished using fluorescence in situ hybridization in wheat cells carrying an additional pair of rye or barley chromosomes (Martinez-Perez et al., 1999). The wheat genome is polyploid, which makes it possible to substitute or add single chromosome pairs from other related species. Very early in the meiotic program the authors observed non-homologous centromere associations that ultimately gave way to homologous associations. At this early stage centromeres were clustered near one pole as in many other organisms (Rabl configuration), however in wheat they can be visualized as individual structures rather than one tight cluster of centromeres such as is seen, for example, in yeast nuclei (Hayashi et al., 1998; Jin et al., 1998). Using centromeric and telomeric probes they observed that while telomeres were present in a diploid number, centromere foci were present in a haploid number consistent with the formation of non-homologous couples. While the chromosomes were still in the Rabl configuration they changed from non-homologous to homologous pairs (Martinez-Perez et al., 1999). Additional studies of wheat and barley have reinforced these initial findings showing that centromeres become organized in homology-independent couples in early meiosis then disperse from these pairs concomitant with grouping of the telomeres in what is called the bouquet stage (Martinez-Perez et al., 2001; Phillips et al., 2012).
Centromere coupling
Budding yeast centromeres form pairwise associations, mostly between non-homologous chromosomes, early in meiosis. The synaptonemal complex central element component Zip1 localizes to the coupled centromeres and is necessary for this association to occur, illustrating a connection between the synaptonemal complex functions and centromere interactions (Obeso and Dawson, 2010; Tsubouchi and Roeder, 2005). Most of what we know about Zip1 is related to its function as an element of the synaptonemal complex. Given the emerging importance of Zip1, and related synaptonemal complex components in other organisms, in mediating centromere associations (see below), we provide here an overview of synaptonemal complex assembly in budding yeast, and the role of Zip1 in that process.
Zip1 is a meiosis-specific protein that it is required for homolog synapsis (Sym et al., 1993) and also has a critical function in recombination (Storlazzi et al., 1996). It is a component of the central element of the synaptonemal complex, zipping together the two lateral elements (Fig. 2) (Dong and Roeder, 2000; Sym and Roeder, 1995). In zip1 deletion mutants the lateral elements of the synaptonemal complex are present and the homologs are able to align but they never become intimately synapsed.
Zip1 is predicted to form an alpha-helical coiled coil, flanked by globular domains at the N and C termini (Figure 2). Electron microscopy using domain-specific anti-Zip1 antibodies revealed that the N-terminus of Zip1 is in the middle of the central element and the C-terminus is embedded in the lateral elements of the complex (Dong and Roeder, 2000). Consistent with this organization, mutant studies have shown that the C terminus, but not the N terminus, is necessary for the localization of Zip1 to the lateral elements (Tung and Roeder, 1998).
All these studies suggest a model in which Zip1 localizes to the chromosomes interacting with the lateral elements by its C-terminal domain. The building block of the central element of the synaptonemal complex has been proposed to be pairs of Zip1 dimers, positioned in an anti-parallel orientation, partially overlapping via their N-termini (Fig. 2), and spanning from the lateral element of one homolog to the lateral element of the other (Dong and Roeder, 2000).
In budding yeast there is a critical relationship between Zip1’s role in chromosome synapsis and SUMOylation, a post-translational modification consisting of the covalent transfer of a small SUMO polypeptide (Smt3 in budding yeast) to a target protein (reviewed in (Watts and Hoffmann, 2011). Zip1 has a SUMO binding motif on the C-terminal domain (Cheng et al., 2006). This motif appears important for Zip1 polymerization along the chromosome, as Zip1 protein that is lacking the SUMO binding motif is defective in synaptonemal complex assembly (Cheng et al., 2006). Red1, a member of the synaptonemal complex lateral element becomes conjugated by Smt3 at the time of synaptonemal complex formation (Eichinger and Jentsch, 2010), and can also associate non-covalently with Smt3 (Lin et al., 2010). The central (Zip1) and lateral elements (Red1) of the synaptonemal complex are then probably associating through Smt3. The formation of Red1-Smt3 conjugates seems to depend on Zip3, which is essential for synaptonemal complex assembly and has a SUMO E3 ligase activity (Cheng et al., 2006) (Eichinger and Jentsch, 2010). It was originally proposed that synaptonemal complex assembly may be driven by Zip1 association with Zip3-dependent Red-Smt3 conjugates along meiotic chromosomes (Cheng et al., 2006), however, during meiosis the levels of Red-1-Smt3 conjugates are low and these conjugates seem to trigger the initiation of synaptonemal complex formation rather than participate directly in the assembly of the entire synaptonemal complex (Eichinger and Jentsch, 2010). New models have been proposed in which Red-Smt3 conjugates help to initiate the first Red1-Zip1 associations (Eichinger and Jentsch, 2010), and free Smt3 chains, assembled at the sites of synapsis, promote the extension of the synaptonemal complex through the formation of non-covalent sandwiches of Red1, free polymeric Smt3 chains, and Zip1 (Lin et al., 2010). Additionally, two meiosis-specific proteins that co-localize with Zip1, Ecm11 and Gmc2, are important for synaptonemal complex formation. Sumoylation of Ecm11 promotes Zip1 assembly into the synaptonemal complex (Humphryes et al., 2013). In contrast to the situation in budding yeast, in Caenorhabditis elegans, rice, and mice, Zip3 homologs (ZHP-3, HEI10 and RNF212) are not required for synaptonemal complex assembly. This raises the possibility that the role for sumoylation in synaptonemal assembly in budding yeast may not be strictly conserved. Instead, in C. elegans, rice and mice, the Zip3 homologs localize to the synaptonemal complex and play critical roles in crossover control (Bhalla et al., 2008; Jantsch et al., 2004; Reynolds et al., 2013; Wang et al., 2012).
While the efficient synaptonemal complex assembly requires interactions of multiple proteins, Zip1 also has self-assembly characteristics that might contribute to centromere coupling. Over-expression of Zip1 causes the formation of polycomplexes - Zip1 aggregates that are not associated with chromosomes - indicating an intrinsic ability of Zip1 to polymerize (Sym and Roeder, 1995). These Zip1 aggregates are independent of the lateral element components (Red1 and Hop1).
Zip1 localizes to coupled centromeres and deletion of ZIP1 results in a failure to initiate centromere coupling (Obeso and Dawson, 2010; Tsubouchi and Roeder, 2005). Zip1 localizes to the centromeres early in meiotic prophase (Bardhan et al., 2010). This localization is dependent on the meiosis-specific cohesin component, Rec8 (Bardhan et al., 2010), which preferentially loads at centromeres in S-phase (Klein et al., 1999). Centromere coupling requires this meiosis-specific form of cohesin: there is no preferential centromeric localization of Zip1 and no centromere coupling when REC8 is substituted by its mitotic counterpart MCD1/SCC1 (Bardhan et al., 2010).
How does Zip1 promote the coupling of centromeres in early meiosis? Three studies suggest that coupling is not achieved through the assembly of a conventional synaptonemal complex structure between centromeres. First, while Zip2, Zip3 and Red1 are all required for synaptonemal complex assembly, none of these are required for centromere coupling (Bardhan et al., 2010; Cheng et al., 2006). Second, the zip1- S75E mutation (see below) has negligible effects on synaptonemal complex assembly but results in severe defects in centromere coupling (Falk et al., 2010). Third, while sumoylation of Ecm11 is important for synaptonemal complex Zip1 assembly, neither Ecm11 or its partner Gmc2 are necessary for centromere coupling (Humphryes et al., 2013).
Possible role of centromere coupling
The function of early coupling between non-homologous centromeres remains a mystery, and in some organisms such as mice (Bisig et al., 2012; Qiao et al., 2012) and maize (Carlton and Cande, 2002) systematic evaluations of centromere dynamics have revealed no evidence of centromere coupling. The main goal of meiotic prophase is to bring homologous chromosomes together, so that DNA exchange will occur and the two partners will segregate to opposite poles. Why then does the meiotic process start by associating non-homologous chromosomes at their centromeres? Several hypotheses have been proposed: One notion is that centromeres repeatedly pair and dissociate from other centromeres in early meiosis, providing an opportunity for assessments of homology along the arms (Tsubouchi and Roeder, 2005). While this remains a possibility, the fact that zip1-S75E mutants (with no centromere coupling) are proficient at synapsis and recombination demonstrates that centromere coupling is not essential for this process (Falk et al., 2010). Second, centromere coupling could act to sequester homologous centromeres from one another, thereby contributing to the repression of homologous recombination that occurs around centromeres in many organisms (Obeso and Dawson, 2010). Third, studies in wheat led to the suggestion that centromere coupling could counter-balance telomere-lead chromosome re-organization in meiosis (Corredor et al., 2007). However, studies in yeast suggest the most dramatic chromosome movements and re-organization occur at stages after centromere coupling has been dissolved (Lee et al., 2012). Finally, it has also been proposed that centromere coupling may be a by-product of the inherent tendency of Zip1 to selfassociate (Henderson and Keeney, 2005). By this model, it remains possible that the preferential deposition of Zip1 at centromeres in early prophase plays a significant (and yet unknown) function, but that the coupling itself is without functional consequences.
Switching from non-homologous coupling to homologous centromere pairing
Non-homologous centromeres undergoing coupling must disengage in order for homologous centromere pairing to take place. Studies in budding yeast have shown that the release of centromere coupling is tied to the repair of programmed double strand DNA breaks that are used to initiate meiotic recombination. First, in spo11 mutants, in which no DNA breaks are formed, centromere coupling initiates at its normal time, but then persists through meiotic prophase (Obeso and Dawson, 2010). This coordination of centromere coupling and double strand break repair occurs through modulation of the phosphorylation status of Zip1 by the opposing actions of Mec1 kinase and PP4 phosphatase, which localizes to prophase centromeres (Falk et al., 2010). Zip1 possesses a Mec1 consensus phosphorylation site at serine 75 and Mec1- dependent phosphorylation at this position triggers Zip1 hyper-phosphorylation (Falk et al., 2010). Hyper-phosphorylated Zip1 accumulates in PP4 mutants and coupling is abolished. By substituting Zip1 serine 75 with alanine, the centromere coupling defect of the phosphatase mutants could be suppressed. Together these findings led to the model that Mec1 is activated to phosphorylate Zip1 upon double strand break formation, releasing centromeres from their non-homologous partners and thus facilitating homologous chromosome alignments. Intriguingly, in wild-type strains, prophase cells in which all of the centromeres are unpaired are never observed (Obeso and Dawson, 2010). Thus, there is not a protracted period in which the centromeres linger between the coupled and homologously-paired configurations. Instead it has been suggested that following Mec1 phosphoryation and the release of coupling, centromeric PP4 quickly de-phosphorylates Zip1 permitting the centromere to establish a new pairwise association (Falk et al., 2010).
Studies in bread wheat have also implicated a locus that is important for the switch from non-homologous to homologous centromere interactions. The Ph1 locus, which might encompass several genes, ensures correct homologue pairing and recombination. In this organism, while the chromosomes are still in a Rabl configuration, the chromosomes are able to change from non-homologous to homologous pairs (Martinez-Perez et al., 1999). Ph1 is necessary for this transition, and in its absence, non-homologously-associated centromeres do not separate at the beginning of meiosis. Rather, the deletion of Ph1 leads to the synapsis of non-homologous chromosomes and initiation of non-homologous recombination (Martinez-Perez et al., 1999).
When non-homologous centromeres become uncoupled, how are new homologous centromere interactions selected and stabilized? In budding yeast this switching is probably not driven by centromeric DNA homology. Although double strand DNA breaks are generated near the centromeres, a classic study demonstrated that in budding yeast it is the arms and not the centromeres that promote the association and pairing of the homologous chromosomes (Clarke and Carbon, 1983). Subsequent studies in wheat (Corredor et al., 2007) and mouse spermatocytes (Bisig et al., 2012) have led to similar conclusions. Moreover, in budding yeast, even though centromere pairing in late prophase takes place between homologous chromosomes, centromere pairing has been shown to be homology-independent (Dawson et al., 1986). Therefore, it seems likely that in budding yeast it is the homologous interactions on the chromosome arms, and subsequent synapsis, that are the driving force for locking-in homologous centromere associations (Fig. 3).
Figure 3. Models of centromere associations and synaptonemal complex formation in budding yeast.
Two pairs of homologous chromosomes are represented (red and blue), each one composed of two sister chromatids. A) At leptotene (early prophase), coupling occurs in a homology independent fashion, usually between non-homologous centromeres. Coupling coincides with the beginning of recombination and synaptonemal complex formation. Centromere coupling is unstable due to the interplay of Mec1/PP4 on Zip1 phosphorylation status; phosphorylation of Zip1 blocks coupling. B) Synaptonemal complex formation is thought to start at sites of crossover initiation and spread along the chromosomes. SC initiated in this manner might propagate to centromeres, promoting the pairing of homologous centromeres and forming long stretches of synaptonemal complex with the centromeric region at one end. C) It has also been proposed that synaptonemal complex formation may start from the centromeres, which are rich in the central element component Zip1. D) In the pachytene stage full chromosome synapsis has been achieved. The pairing between the homologous centromeres is likely stabilized by the synaptonemal complex. Zip1 has a fundamental role in both bridging synaptonemal complex lateral elements and pairing the centromeres; however, genetic data show that are differences in the way Zip1 handles those processes (indicated by dark green and black respectively, see text). At the end of prophase I the synaptonemal complex disassembles; however, Zip1 is retained at the centromeres, where it mediates centromere pairing.
In a possible contradiction with this hypothesis, it has been shown in budding yeast that synapsis may initiate, not just from sites of recombination initiation, (Chua and Roeder, 1998), but also from the centromeres (Tsubouchi et al., 2008). Indeed, chromosomes engaged in the early stages of synapsis (zygotene) frequently have abundant Zip1 at their paired centromeres, often extending out from one side or another from the centromeres, consistent with the model that synapsis had initiated at and was moving outward from the paired centromeres (Fig 3 B). These blocks of centromeric Zip1 can form independently of Zip3, implying that they occur independently of recombination initiation (Agarwal and Roeder, 2000; Tsubouchi et al., 2008). An alternate explanation for these tracks of centromeric Zip1 on synapsing chromosomes is that the centromeres pose a barrier to polymerization of synaptonemal complex rather than a site of initiation (Henderson and Keeney, 2005), as it is probably the case in human (Brown et al., 2005) and mouse spermatocytes (Bisig et al., 2012; Qiao et al., 2012).
Recent studies have suggested that Zip3 and the proline isomerase, Fpr3, normally act to block the assembly of synaptonemal complex from the centromeres (Macqueen and Roeder, 2009) raising the possibility that the Zip1 at paired centromeres may be carried with them as they transition from non-homologous coupling to homologous pairing. In this capacity, the Zip1 may help promote homologous chromosome synapsis by locking together homologous centromeres that have been brought into juxtaposition by homologous interactions on the arms (Fig. 3) (Macqueen and Roeder, 2009), but not necessarily through the assembly of canonical synaptonemal complex.
Recent studies in Drosophila females have revealed centromere behaviors that have several parallels to what has been seen in yeast and mice, though further work will be required to determine whether these are truly related phenomena. In Drosophila oocytes, centromeres are clustered in early prophase (see below) and the synaptonemal complex protein C(3)G, which may be the functional homolog of Zip1, accumulates at the clustered centromeres as well as at other sites along the euchromatic chromosome arms (Takeo et al., 2011; Tanneti et al., 2011). Some of the euchromatic C(3)G foci co-localize with sites of meiotic double strand breaks (Tanneti et al., 2011). The analysis of synaptonemal complex from staged oocytes suggests that the synaptonemal complex is formed by assembly at multiple points on the arms rather than processive growth from one or a few initiation sites (Takeo et al., 2011; Tanneti et al., 2011). Additionally, mutants in the ORD protein, which are defective in centromere clustering and centromeric deposition of C(3)G, are still proficient at later stages of synaptonemal complex assembly along the chromosome arms, although it is not clear this late forming synaptonemal complex is structurally equivalent to that formed in wildtype flies. These and other observations (Takeo et al., 2011; Tanneti et al., 2011) suggest that synapsis of the chromosome arms may not be dependent on earlier synapsis at the clustered centromeres. Instead, the accumulation of synaptonemal complex proteins at the clustered centromeres may be important for chromosomal organization in the pairing and synapsis process (Takeo et al., 2011; Tanneti et al., 2011).
If interactions between homologous chromosome arms drive the final partner choice for centromere pairing how then are non-exchange chromosome partners in budding yeast able to pair their centromeres? It has been proposed that they may pair by an “exclusion mechanism”. By this model, as centromeres become paired with their homologous partners, they become eliminated from the pool of “available centromeres” with which non-exchange chromosomes to pair. Centromeres from a final non-exchange pair will end up pairing since they are, by exclusion, the only partners available to one another (Kemp et al., 2004). This model is consistent with the suggestion that centromeres go through cycles of pairing and unpairing in prophase (Falk et al., 2010; Tsubouchi and Roeder, 2005). The fact that the non-exchange partners fail to assemble a continuous synaptonemal complex (Gladstone et al., 2009; Loidl et al., 1994; Newnham et al., 2010) but instead mainly pairing at their centromeres, supports the idea that synaptonemal complex assembly is not efficiently triggered by centromere pairing alone.
Centromere pairing
The correct, bipolar, attachment of chromosomes to the metaphase spindle, in which one chromosome will be pulled to each pole at anaphase I, depends upon the generation of tension at the centromeres (Nicklas and Koch, 1969). When the two partners of a homologous pair that is tethered by chiasmata are tugged towards opposite poles of the spindle through their microtubule attachments, tension at the kinetochores is created which stabilizes the microtubule attachments (Fig. 1, metaphase). Conversely, the microtubule-kinetochore attachments of mono-oriented homologs are unstable, and mono-oriented chromosomes trigger the spindle checkpoint, which delays the transition of the cell out of metaphase until all the chromosomes are properly bi-oriented (Li and Nicklas, 1995). In mitotic cells, the microtubule attachments of bi-oriented chromosomes are stabilized in part, because the kinetochores are pulled away from a cloud of Aurora kinase, which is localized between the kinetochores and acts to release nearby kinetochore-microtubule attachments (Liu et al., 2009). Recent studies in fission yeast, suggest a similar process may be used in meiosis, where a reservoir of Aurora kinase (Ark1) between the homologous kinetochores is thought to release the microtubule attachments of mono-oriented chromosomes. The pulling of the bi-oriented kinetochores that are connected by chiasmata, away from each other and out of this cloud of Aurora kinase is thought to allow the microtubule attachments to remain stable (Sakuno et al., 2011) (reviewed in (Watanabe, 2012)). It is critical that some linkage persists, after the dissolution of the synaptonemal complex, to hold the homologous partners together as they become bi-oriented. The formation of chiasmata between homologous partners typically provides this physical association. However, observations in diverse organisms point to the existence of other types of linkages that that may serve a similar function as chiasmata by linking the partner chromosomes; reviewed in (Wolf, 1994).
Some organisms, such as Drosophila melanogaster males and Bombyx mori (silk moth) females, undergo meiosis in the absence of exchange for all chromosomes. In Bombyx females, the homologs are joined together until metaphase by the retention of a modified synaptonemal complex (Rasmussen, 1977). In male Drosophila melanogaster, meiosis occurs in the absence of both recombination and a recognizable synaptonemal complex. Here, the mechanism of homolog association depends on the maintenance of somatic pairing between the homologs. Tagging the chromosomes with GFP-lac repressor protein has shown complex centromeric association patterns and suggested that, in early spermatocyte development, homologous interactions are restricted to euchromatic regions of the autosomes, while heterochromatic regions interact nonspecifically (Vazquez et al., 2002).
As early as 1936, using genetic studies, it was discovered that in Drosophila females the X chromosomes often failed to form crossovers; however they still exhibited high rates of correct segregation (Sturtevant and Beadle, 1936). Similarly, the small chromosome 4 of the fruit fly was found to never undergo recombination, but to still segregate correctly in the vast majority of meiosis (Carpenter, 1973; Grell, 1964). Elegant genetic studies of the behavior of chromosomes with or without blocks of peri-centric heterochromatin led to the proposal that pairing between heterochromatic regions could promote the proper segregation of non-exchange partners in meiosis I (Hawley et al., 1992). This model was confirmed by direct fluorescence in situ hybridization, which revealed that the heterochromatin regions of homologous nonexchange partners remained associated after the chromosomes de-synapsed in Drosophila females (Dernburg et al., 1996). Together these studies led the authors to propose that multiple segregation mechanisms are in place in the Drosophila oocyte. While chiasmata could provide the most stable type of physical linkage, associations between blocks of peri-centric heterochromatin could provide an alternate mechanism to link and co-orient non-exchange homologs (Dernburg et al., 1996; Hawley et al., 1992). Indeed, a different study on flies using artificial achiasmatic mini-chromosomes demonstrated the role of the heterochromatin for the correct segregation of the artificial pairs (Karpen et al., 1996).
In fission yeast it has been observed, using both genetic and direct microscopy methods, that the segregation of the chromosomes when recombination is artificially abolished (using rec12 deletion mutants, the S. pombe homolog of SPO11) is better than expected by random segregation (Davis and Smith, 2003). Subsequent experiments showed that in rec12 mutants, while the arms of the homologs are not able to establish stable associations, their centromeres are able to pair (Ding et al., 2004).
Studies in budding yeast also demonstrated that artificial (Dawson et al., 1986; Mann and Davis, 1986) or natural (Guacci and Kaback, 1991) partner chromosomes that did not recombine were able to segregate from one another. As in some situations in Drosophila (Hawley et al., 1992) this segregation was independent of their extent of homology (Ross et al., 1996) and was due to the existence of centromeric associations (Kemp et al., 2004). Whereas in wild-type Drosophila females achiasmate partners are common, making the need for a second non-chiasma-based segregation system obvious; this is not true in budding yeast, which experiences high levels of crossingover, and in which achiasmate partners are rare (Kaback et al., 1992). This fact, and the discovery that non-exchange segregation in budding yeast involved a process – centromere pairing – that might be experienced by both exchange and non-exchange partners, suggested that the process was not exclusive to the achiasmatic chromosomes. Instead, centromere pairing was proposed to be a general mechanism that could provide higher fidelity segregation for all of the chromosomes (Stewart and Dawson, 2004).
The mechanism of centromere pairing
In budding yeast, Zip1 was shown to be essential for centromere pairing through the study of achiasmatic homeologous chromosomes that do not experience crossovers in meiosis. Zip1 was found to localize to the paired centromeres of these achiasmatic chromosomes at both the pachytene and diplotene stages, and if ZIP1 was deleted the achiasmatic chromosomes were no longer able to pair at their centromeres and segregated nearly randomly (Gladstone et al., 2009; Newnham et al., 2010). Zip1 was retained at the centromeres of exchange chromosomes after synaptonemal complex disassembly and deletions of Zip1 appeared to disrupt both the pairing and disjunction of exchange chromosomes (Gladstone et al., 2009; Newnham et al., 2010; Tsubouchi et al., 2008). The impact of ZIP1 deletions on the segregation of exchange chromosomes was less dramatic than for non-exchange partners, but this is presumably because the exchange chromosomes could still rely on their crossovers to promote bi-orientation on the meiosis I spindle. While inactivation of either ZIP1 or the spindle checkpoint gene, MAD2, resulted in modest non-disjunction of exchange chromosomes, the double mutants exhibited random meiosis I segregation. This supports the model that centromere pairing and the spindle checkpoint both act to promote bi-orientation and in the absence of either mechanism, the other becomes essential (Gladstone et al., 2009; Stewart and Dawson, 2004).
The fact that Zip1 is required for both centromere coupling, and later in prophase for centromere pairing, suggests that the two phenomena may be occurring through the same mechanism. However, while the synaptonemal complex genes ZIP2, ZIP3, ZIP4 and SPO16 are not required for coupling, deletion of these genes greatly reduces the efficiency of centromere pairing between non-exchange chromosome pairs (Gladstone et al., 2009; Newnham et al., 2010). This may mean that centromere pairing has a requirement for these genes. Alternatively mutations in these genes may affect centromere paring indirectly. For example, these mutations result in the accumulation of Zip1 in aberrant structures in prophase (when centromere pairing is happening but after coupling has occurred), which may deplete the levels of Zip1 available for centromere pairing. Clearly, major questions remain to be answered regarding how coupling and pairing are mediated, whether they operate by the same mechanism, and what proteins beyond Zip1 are participating.
What has become clear is that the mechanism of centromere pairing in diverse organisms exhibits some conserved characteristics. As in yeast, centromeres have also been observed to remain associated after synaptonemal complex disassembly in mouse spermatocytes (Fig. 4) and Drosophila oocytes (Bisig et al., 2012; Qiao et al., 2012; Takeo et al., 2011). In both cases the homolog of Zip1 persists at the centromeres after synaptonemal complex disassembly and is essential for centromere associations. In mouse spermatocytes, all the elements of the mouse synaptonemal complex tested, including the synaptonemal complex central element SYCP1, the functional homolog of Zip1, and the lateral element component SYCP3, are retained at the centromeres until diplotene. Structured illumination microscopy showed that the synaptonemal complex at the centromere was composed of transverse filaments connecting two lateral elements (Qiao et al., 2012). However, at the time when microtubules begin to assemble around the chromosomes (pro-metaphase), only SYCP3 is maintained at the paired centromeres, rand remains there until anaphase I (Bisig et al., 2012). Although SYCP1 is not detectable between the paired centromeres when microtubule attachments take place, it is essential for pairing, since centromere pairing is abolished in SYCP1 knockout mice (Bisig et al., 2012; Qiao et al., 2012). Thus, it seems that while SYCP1 is necessary for establishing the centromere pairing, it is the presence of SYCP3 at the centromeres that maintains their association beyond diplotene. In Drosophila oocytes the centromeres associate in one or a few clusters rather than individual pairs. This clustering requires the Zip1 homolog C(3)G, at least one other synaptonemal complex component (Cona), cohesins (SMC1) and ORD, a meiosis-specific protein required for cohesion. Of these C(3)G, Cona and SMC1 were tested and shown to persist at the centromeres after synaptonemal complex disassembly from the chromosome arms. (Takeo et al., 2011).
Figure 4. Centromere pairing in mouse.
Both exchange and non-exchange chromosomes undergo centromere pairing in mouse spermatocytes. A) Chromosome spreads of mouse spermatocytes in late meiotic prophase I stained with antibodies against the central element of the synaptonemal complex SYCP1 and the lateral element SYCP3. The spread contains several desynapsed chromosome pairs in which the SYCP1 has largely disassembled, but with persisting blocks of SYCP1 at the centromeres (one end of the telocentric chromosome) and also at the chiasmata. B) Large images of an exchange and a non-exchange chromosome pair from the spread shown in panel A, with schematic diagrams. Figure 5. Model for centromere pairing and rotational freedom.
Therefore, at least in budding yeast, Drosophila, and mice the removal of synaptonemal complex components from the centromeres must be mechanistically different from its removal at the arms. The process of synaptonemal complex disassembly is still not well understood. It is driven, at least in part, by the phosphorylation of central element proteins, by the polo-like kinase homologs, Cdc5 in yeast and PLK1 in mice (Jordan et al., 2012; Sourirajan and Lichten, 2008; Sun and Handel, 2008). Whereas the PLK1 target remains to be identified, both SYCP1 and SYCP3 have potential PLK1 phosphorylation sites. Indeed, SYCP1 and SYCP3 can be efficiently phosphorylated in vitro by extracts from isolated pachytene cells but not from extracts obtained from cells that have been driven into metaphase I by okadaic acid treatment (Tarsounas et al., 1999).
The processes of synaptonemal complex and cohesion removal from the chromosome arms, and retention at the centromeres, have strong similarities. In mouse spermatocyte cultures, driven out of prophase by treatment with okadaic acid, both SYCP1 and the cohesins REC8 and RAD21L were removed from the chromosome arms but retained at the centromeres (Ishiguro et al., 2011). Interestingly, in these experiments, addition of a PLK1 inhibitor induced the retention of both the cohesins and SYCP1 at the chromosome arms. Similarly, in budding yeast, Cdc5 removes a subpopulation of cohesin from chromosomes before the onset of anaphase I (Brar et al., 2006; Yu and Koshland, 2005). Given the similarities of cohesin and synaptonemal complex removal it has been proposed that shugoshin and PP2A phosphatase (SGO2/PP2A) may protect centromere pairing by antagonizing the action of PLK1 on synaptonemal complex components, similar to their role in protecting cohesion at centromeres by antagonizing cohesin phosphorylation (Bisig et al., 2012).
Tethering of partner chromosomes by synaptonemal complex at noncentromeric locations
Late centromere pairing seems to be mediated by a persistence of the synaptonemal complex, or a modified form of synaptonemal complex, at the centromeres. Similarly, in several organisms, synaptonemal complex components at other locations link partner chromosomes when chiasmata are not present. In marsupials X and Y chromosomes do not share homologous regions, and during prophase there is no continuous synaptonemal complex between them. The XY chromosome pair is therefore achiasmatic. The meiotic segregation of the XY pair has been studied in spermatocytes of the marsupial Thylamys elegans. In this animal the pair is joined until metaphase by the persistence of a structure rich in SYCP3 and SYCP1 (Page et al., 2006). Studies in another mammal that undergoes achiasmatic segregation of the sex chromosomes, the Mongolian gerbil Meriones unguiculatus, have also implicated the central element of the synaptonemal complex in the formation of a structure that associates the sex chromosomes until their segregation (de la Fuente et al., 2007). Other examples of the synaptonemal complex tethering partner chromosomes have been described in (Wolf, 1994)
Function of centromere pairing
While the role of centromere coupling is still not clear, the later pairing of homologous centromeres appears to play a direct role in chromosome segregation in budding yeast. The conserved features of the process suggest it may serve the same function in other organisms where centromere pairing has been observed. In all of these systems, including yeast, a complication in studying centromere pairing is that the synaptonemal complex proteins that are critical for pairing are essential for other critical meiotic processes as well. Thus the mutations in these genes that have been used to study centromere pairing typically ablate multiple processes, thus making it difficult to ascertain which phenotypes are due to failures in centromere pairing and which are due to other defective processes.
Current models favor the idea that centromere pairing promotes correct chromosome segregation either by facilitating a kinetochore geometry that promotes the bipolar attachment of the microtubules, by providing a tether that will transmit tension when kinetochores become bi-oriented, or by facilitating the connecting of chromatin near the centromeres to tether them together. The first notion was originally suggested by Östergren more than 50 years ago (Östergren, 1951). He reasoned that, in the absence of an interaction between the centromeres, the two chromosomes of a homologous pair should just as often be directed to the same, as to opposite poles (Fig. 5). This is because without some connection the partner centromeres could have rotational freedom, thus having a high probability of pointing towards the same pole at the time of microtubule attachment. In contrast, centromere pairing might position the active faces of the homologous kinetochores away from one another, thereby increasing the chances of a correct bi-polar attachment (Fig. 5). This would lessen the load on the spindle checkpoint, which in meiosis, sometimes offers only brief delays to correct potential errors (Cheslock et al., 2005; LeMaire-Adkins et al., 1997; Shonn et al., 2000). Consistent with this model, in budding yeast disruption of either ZIP1 (centromere pairing) or MAD2 (the spindle checkpoint) alone resulted in modest levels of missegregation, but disrupting both at the same time resulted in random chromosome segregation (Gladstone et al., 2009).
Figure 5. Model of centromere pairing and rotational freedom.
A) Representation of bipolar kinetochore geometry of a homologous pair of chromosomes. Such geometry would promote the initial attachment of the kinetochores to microtubules coming from opposite poles (bipolar attachment). B) In absence of a link between the centromeres, the kinetochores may be able to rotate (represented by a twisted arrow). Kinetochores oriented to the same pole are represented. Such a configuration may increase the chances of attachment of both kinetochores to microtubules coming from the same spindle pole body (monopolar attachments) and consequent chromosome non-disjunction (not represented). C) Centromere pairing, by providing a link between the centromeres (black squares), will favor the formation of bipolar attachments by eliminating or reducing rotational freedom (red cross over twisted arrow) and inducing a bipolar kinetochore geometry. The centromere pairing may also provide or promote connections between the centromeres that provide tension when the centromeres become attached to microtubules from opposite poles, thereby stabilizing correct, bipolar, attachments. The presence of a crossover near the centromere might serve the same purposes (not represented).
Recent observations in Drosophila have led to the intriguing model that interactions at the centromeres might allow the formation of chromatin threads between the centromere regions of homologous chromosomes (Hughes et al., 2009). There are multiple examples in the literature that suggest the existence of thread-like or even undetectable tethers between chromosomes (LaFountain et al 2002; Chan, North Hickson 2007; Wang, Schhwarzbraun, Nigg, 2008). Imaging of the metaphase movements of achiasmate partners in Drosophila oocytes revealed that the partner chromosomes could separate then re-associate, and exhibit apparently co-ordinated movements during the bi-orientation process (Hughes et al., 2009). These separated partners were frequently connected by thin heterochromatic DNA threads. These threads could provide tension between the chromosomes facilitating their proper segregation. How these connections are formed and whether centromere associations in prophase are part of the process are exciting areas of future research.
In mice, the frequency of apparent non-exchange chromosomes (those that by imaging approaches appear to be free of chiasmata) is about 4% (Anderson et al., 1999; Bisig et al., 2012; Jagiello and Fang, 1979; Speed, 1977). Cytological studies in mouse spermatocytes suggest that these achiasmate partners are tethered at their centromeres in diplotene (Bisig et al., 2012; Qiao et al., 2012) (Fig. 4). Qiao and colleagues noted that centromeres could be connected by two types of bridges: those positive for centromere protein-staining CREST antibodies in late diplotene and stretched SYCP3 structures between centromeres of chromosomes in diakinesis/metaphase I nuclei. These SYCP3 bridges arose independently of chiasmata and are reminiscent of the Drosophila heterochromatin bridges, which may be a sign that such bridges promote disjunction in a range of organisms. Could it be that a role for centromere pairing in pachytene is to promote the formation of chromatin connections that in turn help with the bi-orientation process in pro-metaphase?
In humans, aneuploidy caused by meiotic chromosome segregation errors is a leading cause of infertility, birth defects and mental retardation. For some chromosomes, failure to recombine in meiosis I is correlated with their non-disjunction. Remarkably, recent studies have shown that while smaller chromosomes (21 and 22) fail to experience crossovers in about 5% of meioses (Cheng et al., 2009; Fledel-Alon et al., 2009; Oliver et al., 2008) they are estimated to non-disjoin at lower levels (<1%) (Fledel-Alon et al., 2009; Hassold et al., 1993; Oliver et al., 2008; Tease et al., 2002). This has led to the proposal that humans have non-exchange based systems that promote the disjunction of achiasmate partners as well (Cheng et al., 2009; Fledel-Alon et al., 2009). By this model, non-disjunction in humans might occur by dual failures, first a failure to generate a crossover, and second, the failure of a back-up system(s) that promotes proper segregation of achiasmate partners (Fledel-Alon et al., 2009; Lamb et al., 1997). The finding that centromere pairing is conserved among many species, and in mouse spermatocytes can mediate the pairing on non-exchange partners, raises the possibility that in humans it could be important for preventing non-disjunction of certain error-prone chromosome pairs. We are in the early days of understanding the mechanisms and roles of centromere pairing and future work will be required to address this question.
Conclusion
The utilization of synaptonemal complex elements, including the central element, to link and promote the segregation of achiasmatic chromosomes seems to be a recurrent theme in biology. Future experiments will be needed to confirm whether this mechanism is also a general meiotic feature that promotes the segregation fidelity of exchange chromosomes, as recent work in yeast, flies and mice has begun to suggest. Even more so, considerable mystery surrounds the early, homology-independent centromeric associations that have been observed in several organisms. Are these associations simply by-products of the self-assembly properties of synaptonemal complex components or do they carry out an undiscovered meiotic role? Only additional experiments will tell us.
ACKNOWLEDGEMENTS
The authors thank Scott Hawley and Emily Kurdzo for their comments on the manuscript. The authors acknowledge their colleagues in the Program of Cell Cycle and Cancer Biology for providing many thought-provoking discussions of centromere behavior in meiosis. RJP was supported NIH NIGMS award 1P20GM103636 and by OCAST grant HR10-48S. DSD was supported by NIH NIGMS grant GM087377.
REFERENCES
- Agarwal S, Roeder GS. Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell. 2000;102:245–255. doi: 10.1016/s0092-8674(00)00029-5. [DOI] [PubMed] [Google Scholar]
- Anderson LK, Reeves A, Webb LM, Ashley T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics. 1999;151:1569–1579. doi: 10.1093/genetics/151.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan A, Chuong H, Dawson DS. Meiotic cohesin promotes pairing of nonhomologous centromeres in early meiotic prophase. Mol Biol Cell. 2010;21:1799–1809. doi: 10.1091/mbc.E09-05-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MD. Centromere arrangements in. Triticum aestivum and their relationship to synapsis. Heredity. 1979;43:157. [Google Scholar]
- Bhalla N, Dernburg AF. Prelude to a division. Annual Review Of Cell And Developmental Biology. 2008;24:397–424. doi: 10.1146/annurev.cellbio.23.090506.123245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla N, Wynne DJ, Jantsch V, Dernburg AF. ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet. 2008;4:e1000235. doi: 10.1371/journal.pgen.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisig CG, Guiraldelli MF, Kouznetsova A, Scherthan H, Hoog C, Dawson DS, Pezza RJ. Synaptonemal complex components persist at centromeres and are required for homologous centromere pairing in mouse spermatocytes. PLoS genetics. 2012;8:e1002701. doi: 10.1371/journal.pgen.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar GA, Kiburz BM, Zhang Y, Kim JE, White F, Amon A. Rec8 phosphorylation and recombination promote the step-wise loss of cohesins in meiosis. Nature. 2006;441:532–536. doi: 10.1038/nature04794. [DOI] [PubMed] [Google Scholar]
- Brinkley BR, Brenner SL, Hall JM, Tousson A, Balczon RD, Valdivia MM. Arrangements of kinetochores in mouse cells during meiosis and spermiogenesis. Chromosoma. 1986;94:309–317. doi: 10.1007/BF00290861. [DOI] [PubMed] [Google Scholar]
- Brown PW, Judis L, Chan ER, Schwartz S, Seftel A, Thomas A, Hassold TJ. Meiotic synapsis proceeds from a limited number of subtelomeric sites in the human male. American journal of human genetics. 2005;77:556–566. doi: 10.1086/468188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton PM, Cande WZ. Telomeres act autonomously in maize to organize the meiotic bouquet from a semipolarized chromosome orientation. The Journal of cell biology. 2002;157:231–242. doi: 10.1083/jcb.200110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AT. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics. 1973;73:393–428. doi: 10.1093/genetics/73.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Lo YH, Liang SS, Ti SC, Lin FM, Yeh CH, Huang HY, Wang TF. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes & Development. 2006;20:2067–2081. doi: 10.1101/gad.1430406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EY, Hunt PA, Naluai-Cecchini TA, Fligner CL, Fujimoto VY, Pasternack TL, Schwartz JM, Steinauer JE, Woodruff TJ, Cherry SM, et al. Meiotic recombination in human oocytes. PLoS Genet. 2009;5:e1000661. doi: 10.1371/journal.pgen.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslock PS, Kemp BJ, Boumil RM, Dawson DS. The roles of MAD1, MAD2 and MAD3 in meiotic progression and the segregation of nonexchange chromosomes. Nature Genetics. 2005;37:756–760. doi: 10.1038/ng1588. [DOI] [PubMed] [Google Scholar]
- Chua PR, Roeder GS. Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell. 1998;93:349–359. doi: 10.1016/s0092-8674(00)81164-2. [DOI] [PubMed] [Google Scholar]
- Church K, Moens PB. Centromere behavior during interphase and meiotic prophase in Allium fistulosum from 3-D E.M. reconstruction. Chromosoma. 1976;56:249–263. [Google Scholar]
- Clarke L, Carbon J. Genomic substitutions of centromeres in Saccharomyces cerevisiae. Nature. 1983;305:23–28. doi: 10.1038/305023a0. [DOI] [PubMed] [Google Scholar]
- Corredor E, Lukaszewski AJ, Pachon P, Allen DC, Naranjo T. Terminal regions of wheat chromosomes select their pairing partners in meiosis. Genetics. 2007;177:699–706. doi: 10.1534/genetics.107.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Smith GR. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics. 2003;163:857–874. doi: 10.1093/genetics/163.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DS, Murray AW, Szostak JW. An alternative pathway for meiotic chromosome segregation in yeast. Science. 1986;234:713–717. doi: 10.1126/science.3535068. [DOI] [PubMed] [Google Scholar]
- de la Fuente R, Parra MT, Viera A, Calvente A, Gomez R, Suja JA, Rufas JS, Page J. Meiotic pairing and segregation of achiasmate sex chromosomes in eutherian mammals: the role of SYCP3 protein. PLoS Genet. 2007;3:e198. doi: 10.1371/journal.pgen.0030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg AF, Sedat JW, Hawley RS. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell. 1996;86:135–146. doi: 10.1016/s0092-8674(00)80084-7. [DOI] [PubMed] [Google Scholar]
- Ding DQ, Yamamoto A, Haraguchi T, Hiraoka Y. Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev Cell. 2004;6:329–341. doi: 10.1016/s1534-5807(04)00059-0. [DOI] [PubMed] [Google Scholar]
- Dong H, Roeder GS. Organization of the yeast Zip1 protein within the central region of the synaptonemal complex. J Cell Biol. 2000;148:417–426. doi: 10.1083/jcb.148.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger CS, Jentsch S. Synaptonemal complex formation and meiotic checkpoint signaling are linked to the lateral element protein Red1. Proc Natl Acad Sci U S A. 2010;107:11370–11375. doi: 10.1073/pnas.1004248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JE, Chan AC, Hoffmann E, Hochwagen A. A Mec1- and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Developmental Cell. 2010;19:599–611. doi: 10.1016/j.devcel.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Fledel-Alon A, Wilson DJ, Broman K, Wen X, Ober C, Coop G, Przeworski M. Broad-scale recombination patterns underlying proper disjunction in humans. PLoS Genet. 2009;5:e1000658. doi: 10.1371/journal.pgen.1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone MN, Obeso D, Chuong H, Dawson DS. The synaptonemal complex protein Zip1 promotes bi-orientation of centromeres at meiosis I. PLoS genetics. 2009;5:e1000771. doi: 10.1371/journal.pgen.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell RF. Distributive Pairing: The Size-Dependent Mechanism for Regular Segregation of the Fourth Chromosomes in Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1964;52:226–232. doi: 10.1073/pnas.52.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci V, Kaback DB. Distributive disjunction of authentic chromosomes in Saccharomyces cerevisiae. Genetics. 1991;127:475–488. doi: 10.1093/genetics/127.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt PA, Sherman S. Trisomy in humans: incidence, origin and etiology. Curr Opin Genet Dev. 1993;3:398–403. doi: 10.1016/0959-437x(93)90111-2. [DOI] [PubMed] [Google Scholar]
- Hawley RS, Irick H, Zitron AE, Haddox DA, Lohe A, New C, Whitley MD, Arbel T, Jang J, McKim K, et al. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev Genet. 1992;13:440–467. doi: 10.1002/dvg.1020130608. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Ogawa H, Kohno K, Gasser SM, Hiraoka Y. Meiotic behaviours of chromosomes and microtubules in budding yeast: relocalization of centromeres and telomeres during meiotic prophase. Genes Cells. 1998;3:587–601. doi: 10.1046/j.1365-2443.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- Henderson KA, Keeney S. Synaptonemal complex formation: where does it start? Bioessays. 2005;27:995–998. doi: 10.1002/bies.20310. [DOI] [PubMed] [Google Scholar]
- Hughes SE, Gilliland WD, Cotitta JL, Takeo S, Collins KA, Hawley RS. Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet. 2009;5:e1000348. doi: 10.1371/journal.pgen.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphryes N, Leung WK, Argunhan B, Terentyev Y, Dvorackova M, Tsubouchi H. The Ecm11-Gmc2 complex promotes synaptonemal complex formation through assembly of transverse filaments in budding yeast. PLoS Genet. 2013;9:e1003194. doi: 10.1371/journal.pgen.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y. A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO reports. 2011;12:267–275. doi: 10.1038/embor.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagiello G, Fang JS. Analyses of diplotene chiasma frequencies in mouse oocytes and spermatocytes in relation to ageing and sexual dimorphism. Cytogenet Cell Genet. 1979;23:53–60. doi: 10.1159/000131302. [DOI] [PubMed] [Google Scholar]
- Jantsch V, Pasierbek P, Mueller MM, Schweizer D, Jantsch M, Loidl J. Targeted gene knockout reveals a role in meiotic recombination for ZHP-3, a Zip3-related protein in Caenorhabditis elegans. Molecular and cellular biology. 2004;24:7998–8006. doi: 10.1128/MCB.24.18.7998-8006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Trelles-Sticken E, Scherthan H, Loidl J. Yeast nuclei display prominent centromere clustering that is reduced in nondividing cells and in meiotic prophase. J Cell Biol. 1998;141:21–29. doi: 10.1083/jcb.141.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PW, Karppinen J, Handel MA. Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes. J Cell Sci. 2012;125:5061–5072. doi: 10.1242/jcs.105015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback DB, Guacci V, Barber D, Mahon JW. Chromosome size-dependent control of meiotic recombination. Science. 1992;256:228–232. doi: 10.1126/science.1566070. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Le MH, Le H. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science. 1996;273:118–122. doi: 10.1126/science.273.5271.118. [DOI] [PubMed] [Google Scholar]
- Kemp B, Boumil RM, Stewart MN, Dawson DS. A role for centromere pairing in meiotic chromosome segregation. Genes & Development. 2004;18:1946–1951. doi: 10.1101/gad.1227304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, Nasmyth K. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- Lamb NE, Feingold E, Savage A, Avramopoulos D, Freeman S, Gu Y, Hallberg A, Hersey J, Karadima G, Pettay D, et al. Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum Mol Genet. 1997;6:1391–1399. doi: 10.1093/hmg/6.9.1391. [DOI] [PubMed] [Google Scholar]
- Lee CY, Conrad MN, Dresser ME. Meiotic chromosome pairing is promoted by telomere-led chromosome movements independent of bouquet formation. PLoS genetics. 2012;8:e1002730. doi: 10.1371/journal.pgen.1002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. The Journal of Cell Biology. 1997;139:1611–1619. doi: 10.1083/jcb.139.7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Lin FM, Lai YJ, Shen HJ, Cheng YH, Wang TF. Yeast axial-element protein, Red1, binds SUMO chains to promote meiotic interhomologue recombination and chromosome synapsis. The EMBO journal. 2010;29:586–596. doi: 10.1038/emboj.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl J, Scherthan H, Kaback DB. Physical association between nonhomologous chromosomes precedes distributive disjunction in yeast. Proc Natl Acad Sci U S A. 1994;91:331–334. doi: 10.1073/pnas.91.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macqueen AJ, Roeder GS. Fpr3 and Zip3 ensure that initiation of meiotic recombination precedes chromosome synapsis in budding yeast. Current biology : CB. 2009;19:1519–1526. doi: 10.1016/j.cub.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C, Davis RW. Meiotic disjunction of circular minichromosomes in yeast does not require DNA homology. Proc Natl Acad Sci U S A. 1986;83:6017–6019. doi: 10.1073/pnas.83.16.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Perez E, Shaw P, Moore G. The Ph1 locus is needed to ensure specific somatic and meiotic centromere association. Nature. 2001;411:204–207. doi: 10.1038/35075597. [DOI] [PubMed] [Google Scholar]
- Martinez-Perez E, Shaw P, Reader S, Aragon-Alcaide L, Miller T, Moore G. Homologous chromosome pairing in wheat. J Cell Sci. 1999;112(Pt 11):1761–1769. doi: 10.1242/jcs.112.11.1761. [DOI] [PubMed] [Google Scholar]
- Naranjo T, Corredor E. Nuclear architecture and chromosome dynamics in the search of the pairing partner in meiosis in plants. Cytogenetic and genome research. 2008;120:320–330. doi: 10.1159/000121081. [DOI] [PubMed] [Google Scholar]
- Newnham L, Jordan P, Rockmill B, Roeder GS, Hoffmann E. The synaptonemal complex protein, Zip1, promotes the segregation of nonexchange chromosomes at meiosis I. Proc Natl Acad Sci U S A. 2010;107:781–785. doi: 10.1073/pnas.0913435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB, Koch CA. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J Cell Biol. 1969;43:40–50. doi: 10.1083/jcb.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso D, Dawson DS. Temporal characterization of homology-independent centromere coupling in meiotic prophase. PloS one. 2010;5:e10336. doi: 10.1371/journal.pone.0010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TR, Feingold E, Yu K, Cheung V, Tinker S, Yadav-Shah M, Masse N, Sherman SL. New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet. 2008;4:e1000033. doi: 10.1371/journal.pgen.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östergren G. The mechanism of co-orientation in bivalents and multivalents. Hereditas. 1951;37:85–156. [Google Scholar]
- Page J, Viera A, Parra MT, de la Fuente R, Suja JA, Prieto I, Barbero JL, Rufas JS, Berrios S, Fernandez-Donoso R. Involvement of synaptonemal complex proteins in sex chromosome segregation during marsupial male meiosis. PLoS Genet. 2006;2:e136. doi: 10.1371/journal.pgen.0020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Nibau C, Wnetrzak J, Jenkins G. High resolution analysis of meiotic chromosome structure and behaviour in barley (Hordeum vulgare L.) PLoS One. 2012;7:e39539. doi: 10.1371/journal.pone.0039539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P, Santos AP, Moore G, Shaw P. Chromosomes associate premeiotically and in xylem vessel cells via their telomeres and centromeres in diploid rice (Oryza sativa) Chromosoma. 2004;112:300–307. doi: 10.1007/s00412-004-0274-8. [DOI] [PubMed] [Google Scholar]
- Qiao H, Chen JK, Reynolds A, Hoog C, Paddy M, Hunter N. Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis. PLoS genetics. 2012;8:e1002790. doi: 10.1371/journal.pgen.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SW. The transformation of the Synaptonemal Complex into the 'elimination chromatin' in Bombyx mori oocytes. Chromosoma. 1977;60:205–221. doi: 10.1007/BF00329771. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Qiao H, Yang Y, Chen JK, Jackson N, Biswas K, Holloway JK, Baudat F, de Massy B, Wang J, et al. RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nature Genetics. 2013;45:269–278. doi: 10.1038/ng.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronceret A, Doutriaux MP, Golubovskaya IN, Pawlowski WP. PHS1 regulates meiotic recombination and homologous chromosome pairing by controlling the transport of RAD50 to the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20121–20126. doi: 10.1073/pnas.0906273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LO, Rankin S, Shuster MF, Dawson DS. Effects of homology, size and exchange of the meiotic segregation of model chromosomes in Saccharomyces cerevisiae. Genetics. 1996;142:79–89. doi: 10.1093/genetics/142.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuno T, Tanaka K, Hauf S, Watanabe Y. Repositioning of aurora B promoted by chiasmata ensures sister chromatid mono-orientation in meiosis I. Developmental cell. 2011;21:534–545. doi: 10.1016/j.devcel.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Scherthan H, Weich S, Schwegler H, Heyting C, Harle M, Cremer T. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol. 1996;134:1109–1125. doi: 10.1083/jcb.134.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonn MA, McCarroll R, Murray AW. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science. 2000;289:300–303. doi: 10.1126/science.289.5477.300. [DOI] [PubMed] [Google Scholar]
- Sourirajan A, Lichten M. Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes & Development. 2008;22:2627–2632. doi: 10.1101/gad.1711408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed RM. The effects of ageing on the meiotic chromosomes of male and female mice. Chromosoma. 1977;64:241–254. doi: 10.1007/BF00328080. [DOI] [PubMed] [Google Scholar]
- Stewart MN, Dawson DS. Potential roles for centromere pairing in meiotic chromosome segregation. Cell Cycle. 2004;3:1232–1234. doi: 10.4161/cc.3.10.1193. [DOI] [PubMed] [Google Scholar]
- Storlazzi A, Xu L, Schwacha A, Kleckner N. Synaptonemal complex (SC) component Zip1 plays a role in meiotic recombination independent of SC polymerization along the chromosomes. Proc Natl Acad Sci U S A. 1996;93:9043–9048. doi: 10.1073/pnas.93.17.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant AH, Beadle GW. The Relations of Inversions in the X Chromosome of Drosophila Melanogaster to Crossing over and Disjunction. Genetics. 1936;21:554–604. doi: 10.1093/genetics/21.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Handel MA. Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes. Chromosoma. 2008;117:471–485. doi: 10.1007/s00412-008-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ide N, Tanaka I. Immunocytochemical visualization of the centromeres during male and female meiosis in Lilium longiflorum. Chromosoma. 1997;106:435–445. doi: 10.1007/s004120050265. [DOI] [PubMed] [Google Scholar]
- Sym M, Engebrecht JA, Roeder GS. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993;72:365–378. doi: 10.1016/0092-8674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- Sym M, Roeder GS. Zip1-induced changes in synaptonemal complex structure and polycomplex assembly. J Cell Biol. 1995;128:455–466. doi: 10.1083/jcb.128.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S, Lake CM, Morais-de-Sa E, Sunkel CE, Hawley RS. Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in Drosophila oocytes. Current biology : CB. 2011;21:1845–1851. doi: 10.1016/j.cub.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Tanneti NS, Landy K, Joyce EF, McKim KS. A pathway for synapsis initiation during zygotene in Drosophila oocytes. Current Biology: CB. 2011;21:1852–1857. doi: 10.1016/j.cub.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Tarsounas M, Pearlman RE, Moens PB. Meiotic activation of rat pachytene spermatocytes with okadaic acid: the behaviour of synaptonemal complex components SYN1/SCP1 and COR1/SCP3. J Cell Sci. 1999;112(Pt 4):423–434. doi: 10.1242/jcs.112.4.423. [DOI] [PubMed] [Google Scholar]
- Tease C, Hartshorne GM, Hulten MA. Patterns of meiotic recombination in human fetal oocytes. Am J Hum Genet. 2002;70:1469–1479. doi: 10.1086/340734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi T, Macqueen AJ, Roeder GS. Initiation of meiotic chromosome synapsis at centromeres in budding yeast. Genes Dev. 2008;22:3217–3226. doi: 10.1101/gad.1709408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi T, Roeder GS. A synaptonemal complex protein promotes homology-independent centromere coupling. Science. 2005;308:870–873. doi: 10.1126/science.1108283. [DOI] [PubMed] [Google Scholar]
- Tung KS, Roeder GS. Meiotic chromosome morphology and behavior in zip1 mutants of Saccharomyces cerevisiae. Genetics. 1998;149:817–832. doi: 10.1093/genetics/149.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez J, Belmont AS, Sedat JW. The dynamics of homologous chromosome pairing during male Drosophila meiosis. Current Biology: CB. 2002;12:1473–1483. doi: 10.1016/s0960-9822(02)01090-4. [DOI] [PubMed] [Google Scholar]
- Wang K, Wang M, Tang D, Shen Y, Miao C, Hu Q, Lu T, Cheng Z. The role of rice HEI10 in the formation of meiotic crossovers. PLoS Genet. 2012;8:e1002809. doi: 10.1371/journal.pgen.1002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y. Geometry and force behind kinetochore orientation: lessons from meiosis. Nature reviews Molecular cell biology. 2012;13:370–382. doi: 10.1038/nrm3349. [DOI] [PubMed] [Google Scholar]
- Watts FZ, Hoffmann E. SUMO meets meiosis: an encounter at the synaptonemal complex: SUMO chains and sumoylated proteins suggest that heterogeneous and complex interactions lie at the centre of the synaptonemal complex. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:529–537. doi: 10.1002/bies.201100002. [DOI] [PubMed] [Google Scholar]
- Westermann S, Drubin DG, Barnes G. Structures and functions of yeast kinetochore complexes. Annu Rev Biochem. 2007;76:563–591. doi: 10.1146/annurev.biochem.76.052705.160607. [DOI] [PubMed] [Google Scholar]
- Wolf KW. How meiotic cells deal with non-exchange chromosomes. BioEssays : news and reviews in molecular, cellular and developmental biology. 1994;16:107–114. doi: 10.1002/bies.950160207. [DOI] [PubMed] [Google Scholar]
- Yu HG, Koshland D. Chromosome morphogenesis: condensin-dependent cohesin removal during meiosis. Cell. 2005;123:397–407. doi: 10.1016/j.cell.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. The leptotene-zygotene transition of meiosis. Annual Review Of Genetics. 1998;32:619–697. doi: 10.1146/annurev.genet.32.1.619. [DOI] [PubMed] [Google Scholar]