Abstract
Endometrial cancer, the most common gynecologic malignancy, is a hormonally-regulated tumor. Response to progestin-based therapy correlates positively with progesterone receptor (PR) expression. However, many endometrial tumors have low levels or loss of PR, limiting the clinical application of progestin. We evaluated the ability of epigenetic modulators to restore functional PR expression in Type I endometrial cancer cells with low basal PR. Treatment with the histone deacetylase inhibitor (HDACi) LBH589 induced a profound upregulation of PR mRNA. LBH589 restored PR protein expression at 24 hours and sustained expression for 72 hours, even in the presence of progesterone. LBH589 promoted a dose-dependent increase in PR protein levels, with an obvious increase with 10 nM LBH589. To investigate if the restored PR is functional as a transcription factor, we examined PR nuclear localization and expression of PRE- or Sp1-containing target genes. After treatment with LBH589 in the absence or presence of progesterone, PR nuclear expression was increased as demonstrated by Western blotting of nuclear fractions and immunostaining. Next, restored PR upregulated FoxO1, p21, and p27 and downregulated cyclin D1 in a ligand-dependent manner. Finally, LBH589 treatment induced cell cycle arrest in G1 that was further augmented by progesterone. Regulation of PR target genes was also achieved with other HDAC inhibitors, indicating that agents in this class work similarly with respect to PR. Our findings reveal that epigenetic modulators can restore endogenous functional PR expression in endometrial cancer cells and suggest that strategies to re-establish PR expression will resensitize endometrial tumors to progestin therapy.
Keywords: Progesterone, progesterone receptor, endometrial cancer, epigenetic modulation, HDAC inhibitor, PRE-containing genes, cell cycle arrest
Introduction
Endometrial cancer is the most common female reproductive cancer and the fourth most common cancer among women, with a predicted 47,130 new cases and 8,010 deaths in 2012 (1). In the normal cycling endometrium, estrogen promotes proliferation of endometrial epithelial cells, while progesterone counteracts effects of estrogen by inducing differentiation. Progesterone functions as a tumor suppressor in the endometrium based on its role in differentiation, cell cycle arrest, and apoptosis, which lead to inhibition of invasion and inflammation (2). A majority of endometrial tumors arise in the setting of excess estrogen unopposed by progesterone, signifying the exquisite sensitivity of the endometrium to hormonal regulation (2). Not surprisingly, expression of the progesterone receptor (PR) is gradually lost with progression of endometrial cancer (3), thus abrogating the tumor suppressive properties of progesterone.
A majority of endometrial tumors are classified as Type I endometrial cancer. These are generally well-differentiated and expresses estrogen receptor (ER) and PR (2). By contrast, Type II tumors are poorly differentiated and most often devoid of steroid hormone receptors. Given the differentiating ability of progesterone, progestin-based therapy has long been studied as a treatment modality for endometrial cancer (4). Patient response to progestin therapy strongly correlates with expression of PR. For example, in the Gynecologic Oncology Group (GOG) study #81, the response rate to progestin therapy was 37% in patients with PR-positive tumors as compared to only 8% in patients with tumors lacking detectable PR expression (5). Results from this study therefore demonstrate that the success of progestin therapy necessitates sustained expression of PR. Thus, new strategies to promote and maintain PR expression in endometrial tumors are urgently needed.
Several mechanisms have been proposed to explain silencing of PR in endometrial cancers, including epigenetic modifications that repress the transcription of PR (2). Towards restoring PR expression as a therapeutic strategy, three studies in endometrial cancer cell lines have reported that increased PR mRNA and protein expression can be achieved via epigenetic modulation, either with treatment with a DNA methyl transferase inhibitor (DNMTi) (6) or the combination of a DNMTi with a histone deacetylase inhibitor (HDACi) (7, 8). While this combination strategy has been shown to induce apoptosis of endometrial cancer cells upon addition of progesterone (8), a limitation of these studies is the absence of molecular analyses that validate transcriptional activity of the restored PR.
Using endometrial cancer cell lines with low basal PR expression as a model for Type I tumors, our objective in this study was to determine whether epigenetic modification restores functional PR expression. Our data reveal that treatment with an HDACi alone results in re-expression of PR at the mRNA and protein levels, nuclear localization of this restored PR, and transcriptional regulation of PR target genes, culminating in cell cycle arrest upon addition of progesterone. These data have important implications in designing future clinical trials of hormone therapy for endometrial cancers with modest baseline PR expression.
Materials and Methods
Reagents
5-aza-2′-deoxycytidine (5-aza-dC), progesterone, and the β-actin antibody (#A1978) were obtained from Sigma Aldrich. LBH589, SAHA, MS275 and PXD101 were purchased from Selleck Chemicals and resuspended in DMSO. Antibodies against PRA/B (#3153), PRB (#3157), p27 (#3686), FoxO1 (#2880), cyclin D1 (#2926), histone H3 acetylated at lysine 9 (H3K9Ace, #9671), H3K18Ace (#9675), and p21 (#2947) were from Cell Signaling. The antibody against Hsp90 (ADI-SPA-835) was from Enzo. PR antibody (sc-539) was from Santa Cruz Biotechnology.
Cell lines
ECC-1 endometrial cancer cell line was purchased from ATCC and grown according to the recommended guidelines. Ishikawa H endometrial cancer cell line (gifts from Dr. Erlio Gurpide, New York University) was grown in DMEM media supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Gibco).
Real-time PCR
Total RNA was extracted from cultured cells using the miRvana miRNA Isolation kit (Ambion, Life Technologies). RNA yield and purity was assessed using a NanoDrop Model 1000 spectrophotometer (Thermo Scientific). Total RNA (500 ng) was oligo-dT reverse transcribed with SuperScript III (Invitrogen, Life Technologies). Real-time PCR was performed in triplicate on an Applied Biosystems Model 7900 Genetic Analyzer under standard conditions using the following primer sequences: progesterone receptor (PR) PRFOR: 5′ATCCTACAAACACGTCAGTGGGCA-3′ (NM_000926, pos.3041-3064) and PRREV: 5′-ACTGGGTTTGACTTCGTAGCCCTT-3′ (NM_000926, pos.3264-3241). Results were quantitated using the comparative cycle threshold (ΔΔCt) method (9). 18S rRNA was measured in the same samples (18SFOR: 5′-AACTTTCGATGGTAGTCGCCG-3′ (NR_003286, pos.363-383) AND 18SREV: 5′-CCTTGGATGTGGTAGCCGTTT-3′ (NR_003286, pos.467-447) and used to correct for variations in RNA content among samples.
Western blotting
Following treatment, cells were solubilized in cold NP-40 cell lysis buffer (150 mM NaCl, 50 mM Tris/HCl, pH 7.4, 1% NP-40 with a protease and phosphatase inhibitor cocktail from Pierce) and then sonicated to release nuclear proteins. In some experiments, cytoplasmic and nuclear fractions were extracted using a nuclear extraction kit (Cat # 40010, Active Motif). Lysates were analyzed by Western blotting with specific primary and HRP-conjugated secondary antibodies. For detection of PR protein, a combination of the PRA/B (#3153, Cell Signaling) and PRB (#3157, Cell Signaling) antibodies at a 1:1000 dilution each was used for Western blotting.
Immunostaining
Cells were grown on glass coverslips and treated with indicated reagents. After fixation with 2% paraformaldehyde and permeabilization with 1% Triton X-100, cells were labeled with a PR antibody (sc-539, Santa Cruz) followed by Alexa Fluor-488 (ECC1 cells) or Alexa Fluor-555 (Ishikawa H cells) conjugated secondary antibody. Images were visualized by fluorescence microscopy and acquired with an Olympus BX51 camera.
Cell cycle analysis
Cells were grown for 24 h in normal growth media followed by treatment for an additional 72 h. Floating cells were collected with the media, and attached cells were collected by trypsinization. After centrifugation, the cell pellets were fixed in 70% cold ethanol, treated with RNAase A, stained with propidium iodide, and prepared for flow cytometry. FACScan (BD Bioscience) was used to detect the samples and ModFit was used to analyze the cell cycle alterations.
Results
PR gene expression is restored by epigenetic modulation in endometrial cancer cell lines
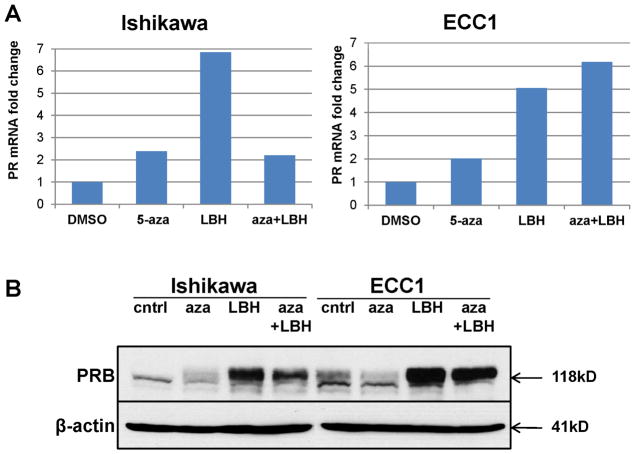
Our objective was to understand the impact of epigenetic modulation in Type I endometrial cancer with modest baseline PR expression as modeled by Ishikawa H and ECC1 cells. Based on two previous studies that examined how epigenetic regulation alters PR expression in endometrial cancer cells (7, 8), we first tested the ability of a DNMTi, 5-aza-2′deoxycytidine (5-aza-dC), either alone or in combination with a potent HDACi, LBH589, to restore PR mRNA levels by quantitative RT-PCR (qRT-PCR). Whereas treatment with 1 μM 5-aza-dC promoted a modest 2-fold upregulation of PR mRNA levels in both Ishikawa H and ECC1 cells, a substantial 5–7-fold increase in PR levels were observed in response to 10 nM LBH589 (Fig. 1A). The combination of 5-aza-dC and LBH589 did not synergistically increase PR mRNA levels (Fig. 1A). Next, we confirmed that epigenetic modification restored PR protein expression by Western blotting in both cell lines (Fig. 1B). Consistent with effect of LBH589 on mRNA levels, we also detected higher PR expression in cells treated with 10 nM LBH589 as compared to 5-aza-dC alone or in combination with LBH589 (Fig. 1B). Of note, the PR isoform that was predominantly restored was PRB. Based on these data, subsequent experiments focused on the role of the HDACi LBH589 in restoring PR expression.
Figure 1. HDAC inhibitor LBH589 but not DNMT inhibitor 5-aza-dC restores PR mRNA and protein expression in Type I endometrial cancer cells with modest basal PR expression.
(A) Ishikawa H or ECC1 cells were treated with DMSO control, 1 μM 5-aza-dC (5-aza) for 72 h, 10 nM LBH589 (LBH) for 24 h, or 1 μM 5-aza-dC for 48 h then 10 nM LBH589 for an additional 24h (aza+LBH). PR mRNA expression was normalized to 18S, and data are displayed as fold-change relative to the DMSO control. (B) Ishikawa H or ECC1 cells were treated as in (A) and PRB protein expression (118 kDa) was determined by Western blotting. β-actin, loading control.
Re-expression of PR is sustained at low doses of LBH589
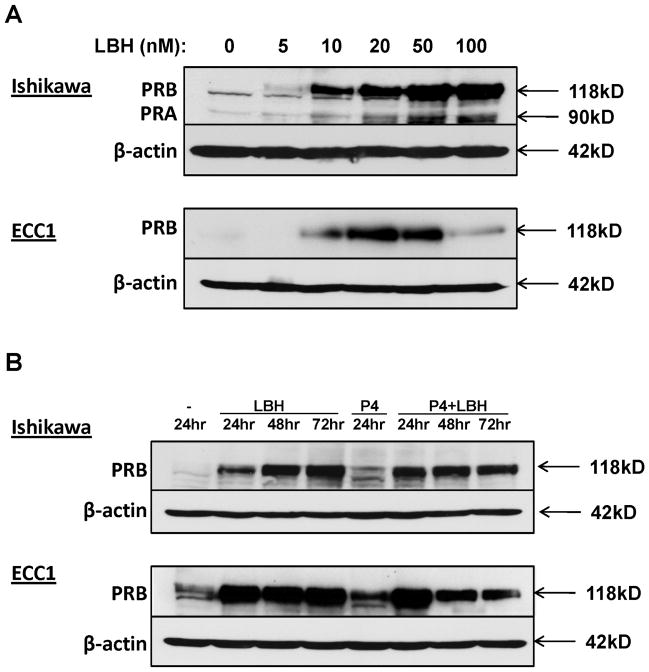
To determine the minimal LBH589 dose required to induce PR protein expression, we conducted a dose response experiment in Ishikawa H and ECC1 cells. PRB expression was apparent at doses as low as 10 nM LBH589, with expression peaking at 50 nM–100 nM for Ishikawa H cells and 20 nM for ECC1 cells (Fig. 2A). The decrease in PR expression at high doses in ECC1 cells may be due to toxicity at doses greater than 20 nM LBH589 (data not shown). Next, we investigated the stability of re-expressed PR by performing a time course experiment with LBH589. Cells were treated with 10 nM LBH589 once and harvested every day for three days. Strikingly, PRB expression was sustained at time points as late as 72 hours in both cell lines (Fig. 2B). Given that PR undergoes ligand-dependent proteosomal downregulation (10), we also tested if the LBH589-induced PR expression can be maintained in the presence of progesterone. As compared to treatment with LBH589 alone, the combination of progesterone with LBH589 for 72 h resulted in only a slight decrease in PR levels in Ishikawa H and ECC1 cells (Fig. 2B). This result suggests that re-expression of PR by treatment with LBH589 can be sustained for up to three days in the presence of ligand.
Figure 2. LBH589-mediated upregulation of PR is dose-dependent and sustained at 72h in the presence of progesterone.
(A) Cells were treated with the indicated concentrations of LBH589 for 24 h. Expression of PRB (118 kDa) and PRA (90 kDa) was assessed by Western blotting. (B) Ishikawa H or ECC1 cells were treated with 10 nM LBH589 in the absence or presence of 100 nM progesterone (P4) for the indicated times. β-actin, loading control.
Restored PR localizes to the nucleus and is functional
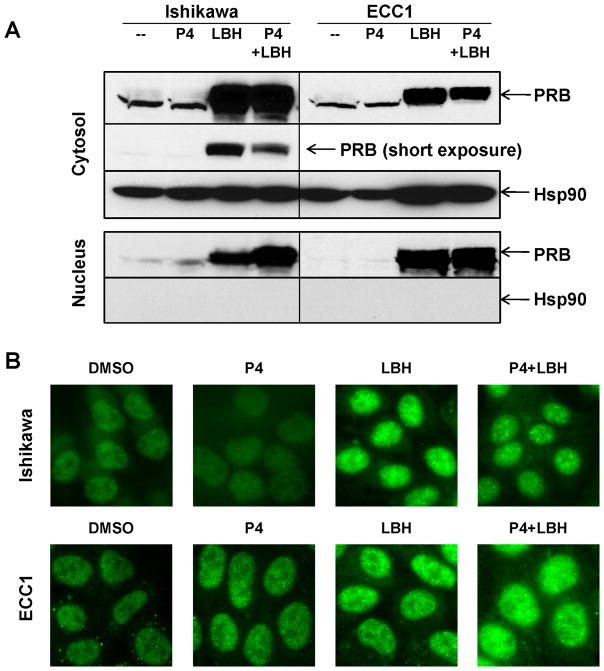
As a transcription factor, PR translocates to the nucleus, where it controls downstream gene transcription. PRA is predominantly nuclear, whereas PRB is often cytosolic but translocates to the nucleus in response to ligand binding (11). Since we detected selective expression of the PRB isoform in response to LBH589 treatment, we next examined the subcellular distribution of restored PR in Ishikawa H and ECC1 cells. First, we conducted a cell fractionation assay and found that treatment with LBH589 alone for 24 hours resulted in a significant increase in the amount of PR in both the nucleus and the cytosol, with a predominant cytosolic localization (Fig. 3A). Combination of progesterone with LBH589 promoted a further increase in the proportion of nuclear PR in Ishikawa H cells. This effect was less evident in ECC1 cells, though we did observe a decrease in the cytosolic PR in both cell lines (see short exposure of Ishikawa H cells, cytosolic fraction). To validate these observations, we assessed PR distribution by immunostaining. In both cell types, PR staining was stronger in the nucleus of cells treated with LBH589, though cytosolic staining was also apparent (Fig. 3B). Treatment with progesterone in combination with LBH589 did not significantly alter the amount of nuclear PR. Taken together, these data indicate that the restored PR localizes to the nucleus.
Figure 3. Treatment with HDACi LBH589 promotes nuclear PR expression.
(A) Ishikawa H or ECC1 cells were treated with 100 nM P4, 10 nM LBH589, or the combination for 24 h, and then expression of PR was evaluated in the cytosolic or nuclear fractions by Western blotting. Hsp90 serves as a control for purity of the cytosolic fraction. (B) Immunostaining of PR in Ishikawa H (upper panels) or ECC1 (lower panels) cells after treatment as in (A).
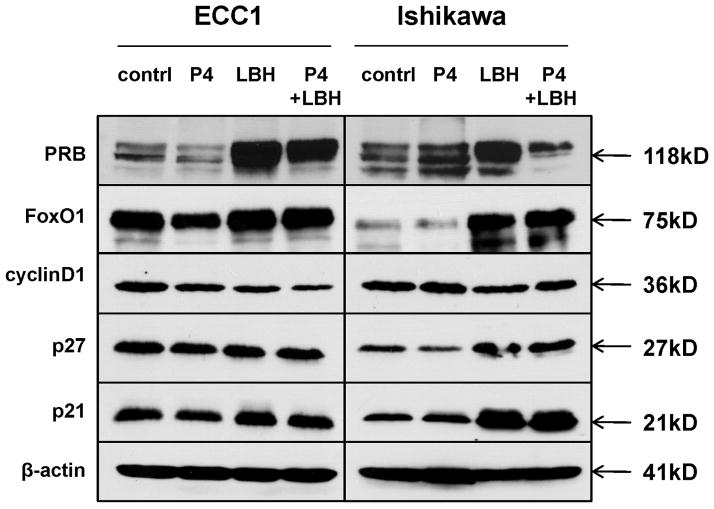
Next, to determine whether the restored nuclear PR is functional, we examined protein expression of several PR target genes, including FoxO1 and cyclin D1, which contain progesterone-response elements (PREs), and p27 and p21, which contain SP1 sites that are also controlled by progesterone (2). Treatment with LBH589 alone resulted in an increase in FoxO1, p21, and p27 and a decrease in cyclin D1 (Fig. 4), consistent with established patterns of PR transcriptional regulation (2). Addition of progesterone did not diminish these effects on PR transcriptional activity (Fig. 4). Together with the nuclear localization, these data provide strong evidence that the restored PR is functional as a transcription factor.
Figure 4. Restored PR is functional as a transcription factor.
Protein levels of PR downstream target genes FoxO1, cyclin D1, p27, and p21 were evaluated by Western blotting after treatment with 100 nM P4, 10 nM LBH589, or the combination for 24 h.
Effects of a panel of HDACi on PR expression and function
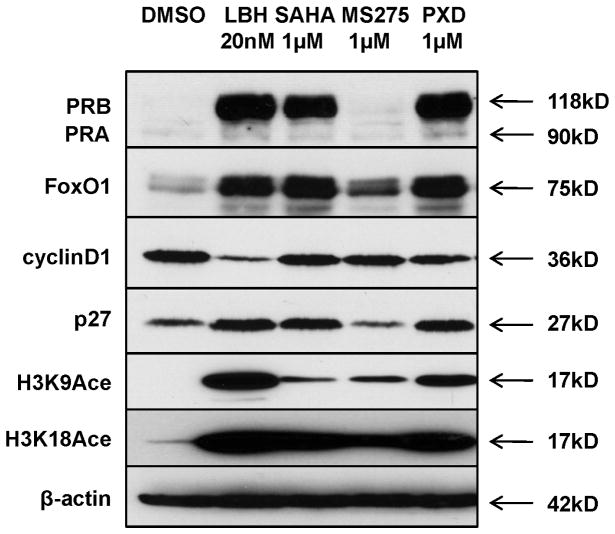
We expanded our study to other HDAC inhibitors to determine the generality of our data. LBH589, SAHA, and PXD101 are pan-HDAC inhibitors, whereas MS-275 selectively inhibits a subset of class I HDACs (12). Using Ishikawa H cells, we first compared the effect of each of these HDAC inhibitors on PR expression and found that PXD101 restores PR levels to those observed with LBH589 (Fig. 5). SAHA also induced PR expression, but at lower levels than LBH589, and MS-275 had no effect on PR expression. It is important to note that, while PXD101 and SAHA both have the ability to restore PR, much higher doses were necessary to achieve PR expression as compared to LBH589. We also examined expression of PR transcriptional target genes and found an association between the extent to which PR was restored with each HDACi and regulation of PR target gene expression (Fig. 5). Specifically, PXD101 promoted increased expression of FoxO1 and p27 to a greater degree than SAHA, and MS-275 had little-to-no effect on the PR target genes. As a marker for drug efficacy, we also assessed histone H3 acetylation on lysine 9 (K9) and lysine 18 (K18). Consistent with the effects on PR expression, histone acetylation was greatest in cells treated with LBH589 and PXD101 (Fig. 5). These data identify LBH589, PXD101, and potentially SAHA as potent inducers of functional PR expression in Type I endometrial cancer cells.
Figure 5. Multiple HDAC inhibitors have the capacity to induce functional PR expression.
Functional expression of PR was examined in Ishikawa H cells in response to treatment with different HDAC inhibitors LBH589, SAHA (vorinostat), MS-275 (MS, entinostat), or PXD101 (PXD, belinostat) for 24 h at the indicated concentrations. H3K9Ace: histone H3 Lys9 acetylation; H3K18Ace: histone H3 Lys9 acetylation.
Progesterone augments G1 cell cycle arrest induced by LBH589
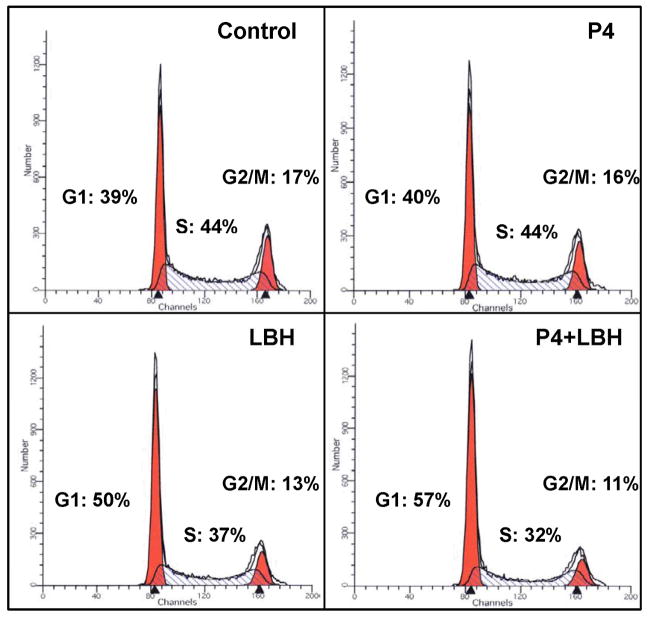
We next sought to understand whether the functional PR expression in response to HDACi treatment resulted in phenotypic cellular changes. It is well-established that treatment of cells expressing PR with progesterone results in arrest in G1, which contributes to the differentiating effects of progesterone on the normal endometrium. We therefore examined the effect of LBH589 in the absence or presence of progesterone on cell cycle progression. Treatment of Ishikawa H cells with progesterone alone had no impact on cell cycle (Fig. 6), consistent with the notion that PR must be expressed to achieve an effect with ligand. Next, in response to LBH589 as a single agent, we observed a significant 10% increase in arrest in G1, consistent with our previous findings (13). Combining LBH589 with progesterone further increased the percentage of cells in G1 to 57% with a concomitant reduction in the percentage of cells in other phases of the cell cycle (5% decrease in S phase and 2% decrease in G2/M vs. LBH589 alone). The additional benefit achieved by progesterone and LBH589 combination treatment suggests that cell cycle arrest is mediated by the restored PR.
Figure 6. Activation of re-expressed PR with progesterone augments LBH589-induced G1 cell cycle arrest.
Ishikawa H cells were treated with 100 nM P4, 10 nM LBH589, or 100 nM P4 and 10 nM LBH589 for 72 h, and DNA content was analyzed by flow cytometry.
Discussion
In comparison to chemotherapy, hormone therapy represents a safe treatment strategy for cancers that are hormonally dependent. However, with progression of endometrial cancer, PR expression is gradually lost, thus limiting the application of progestin therapy in this disease. In this study, we identify histone deacetylase inhibition as one approach to restore expression and functionality of PR in Type I endometrial cancer cell lines with a low baseline level of PR. Specifically, treatment with LBH589 induced robust expression of PR at both the mRNA and protein levels, with appreciable PR levels detected at doses as low as 10 nM. Expression of PR in response to HDAC inhibition was sustained at 72 hours in both the presence and absence of ligand, suggesting minimal ligand-mediated receptor downregulation. Importantly, nuclear localization and gene expression studies revealed that the restored PR is functional as a transcription factor, with expected changes in canonical PR target genes cyclin D1, p21, p27, and FoxO1. Similar results were observed with a panel of HDAC inhibitors, though certain agents demonstrated greater efficacy than others in restoring functional PR expression. In physiologic studies, combination treatment of LBH589 and progesterone induced cell cycle arrest in G1 to a greater extent that either treatment alone, providing further evidence for ligand-mediated PR effects. Taken together, these data represent the first report to demonstrate that treatment with an epigenetic modulator results in re-expression of a transcriptionally active PR.
The most important finding in this study is that the restored PR localizes to the nucleus and is functional as demonstrated by regulation of PR target genes. It has previously been reported that epigenetic modulation results in re-expression of PR in endometrial cancer cells (6–8). However, it remained unclear whether the effects in these studies were dependent on PR re-expression or simply an effect of the drugs independent of PR. We attempted to answer this question by examining the functional effects of PR at the molecular level in endometrial cancer cells. As a nuclear transcription factor, PR belongs to the steroid nuclear receptor family and controls many downstream signaling pathways through its transcriptional activity (2). We choose four representative downstream signaling molecules to validate that the restored PR is an active transcription factor: FoxO1 and cyclin D1, which contain PRE within their promoter region, and p21 and p27, which contain SP1 elements in their promoters. While it is possible that the cellular responses we observed could be attributed to PR-independent effects, combining LBH589 with progesterone resulted in a further increase in cell cycle arrest as compared to LBH589 alone. In addition, all experiments were conducted in serum-containing media with a predicted progesterone concentration of 0.9 nM, which may contribute to PR activity in response to LBH589 in the absence of exogenous progesterone. We conclude that epigenetic modulation induces cellular events through restored functional PR expression.
In previously reported studies in endometrial cancer cell lines, combination of an HDACi with a DNMTi was necessary to achieve substantial PR re-expression (7, 8). However, we did not observe an additive or synergistic effect on PR mRNA or protein levels in the cell lines used in our study. One potential reason for this lack of synergistic effect of LBH589 with 5-aza- deoxycytidine may be due to the apparent absence of methylation on the promoter of either Ishikawa H or ECC1 cells (data not shown). This result is in contrast to the previous studies that detected methylation of the PR promoter in models of both Type I and Type II endometrial cancer cell lines (6–8). The differential methylation patterns may be attributed to differences in culture conditions or approaches to measure promoter methylation.
Our data indicate that the HDAC inhibitors have varying efficacies in restoring PR expression and transcriptional activity. One potential explanation for this result is that the HDAC inhibitors used in our study have different reported affinities for HDACs. For example, the Kd values of LBH589, PXD101, and SAHA for Class I HDACs (HDACs 1, 2, and 3) are in the low nanomolar range (100–350 nM), whereas the Kd of MS-275 for these HDACs is in the low micromolar range (3–8 μM) (12). In addition, this panel of tested HDACi displays preferential affinity for particular Class II HDACs. LBH589 has a greater affinity for HDAC10 than PXD101, and the converse is true for HDAC6. MS-275 has no apparent affinity for either HDAC6 or 10. Thus, the failure of MS-275 to restore PR suggests that HDACs which are not targeted by this agent but are by LBH589, PXD101, or SAHA may be responsible for the histone deacetylation driving PR suppression in endometrial cancer. Furthermore, the differences in expression of PR target genes in response to LBH589, PXD101, or SAHA may also shed light on which HDACs are responsible for silencing PR expression. Future studies are warranted to determine whether expression of particular HDACs may serve as biomarkers for response to HDACi and progesterone combination therapy.
Conclusion
In summary, our data demonstrate that combining a histone deacetylase inhibitor with progesterone may represent a new treatment option to restore functional PR expression in Type I endometrial cancer cells. These findings serve as a platform for designing future clinical trials to examine whether treatment with an HDACi resensitizes endometrial tumors to progestin.
Acknowledgments
This work was supported by NIH R01CA99908 (KKL) and the Department of Obstetrics and Gynecology Research Development Fund (KKL).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Cancer Facts and Figures. American Cancer Society; 2011. [November 21, 2011]; Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf. [Google Scholar]
- 2.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145–52. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortel R, Zaino R, Satyaswaroop PG. Heterogeneity and progesterone-receptor distribution in endometrial adenocarcinoma. Cancer. 1984;53(1):113–6. doi: 10.1002/1097-0142(19840101)53:1<113::aid-cncr2820530120>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Thiel KW, De Geest K, Leslie KK. Endometrial cancer: reviving progesterone therapy in the molecular age. Discov Med. 2011;12(64):205–12. [PubMed] [Google Scholar]
- 5.Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17(6):1736–44. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki M, Dharia A, Oh BR, Tanaka Y, Fujimoto S, Dahiya R. Progesterone receptor B gene inactivation and CpG hypermethylation in human uterine endometrial cancer. Cancer Res. 2001;61(1):97–102. [PubMed] [Google Scholar]
- 7.Xiong Y, Dowdy SC, Gonzalez Bosquet J, Zhao Y, Eberhardt NL, Podratz KC, et al. Epigenetic-mediated upregulation of progesterone receptor B gene in endometrial cancer cell lines. Gynecol Oncol. 2005;99(1):135–41. doi: 10.1016/j.ygyno.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Liu X, Ma D, Feng Y, Zhong N. Down-regulation of the progesterone receptor by the methylation of progesterone receptor gene in endometrial cancer cells. Cancer Genet Cytogenet. 2007;175(2):107–16. doi: 10.1016/j.cancergencyto.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97(3):1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie KK, Stein MP, Kumar NS, Dai D, Stephens J, Wandinger-Ness A, et al. Progesterone receptor isoform identification and subcellular localization in endometrial cancer. Gynecol Oncol. 2005;96(1):32–41. doi: 10.1016/j.ygyno.2004.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nature Biotechnol. 2011;29(3):255–65. doi: 10.1038/nbt.1759. [DOI] [PubMed] [Google Scholar]
- 13.Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, et al. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6(6):e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]