Abstract
The cytokines IL-4, IL-13 and TSLP, play a key role in allergic disease by virtue of their ability to initiate, maintain, and augment Th2 responses. These molecules mediate their effects through type 1 cytokine receptors which bind cytokines with a characteristic structure. Receptors are expressed on a broad array of immune cell types, and are integral to complex cytokine networks operating in health and disease. Th2-promoting cytokines bind different configurations of receptors. Receptor subunits may exist in surface bound or soluble form, as well as in isolation or in partnership with other subunits. Sharing of receptor subunits among different cytokine receptor complexes adds to the intricate landscape. This article describes the characteristics of receptors for IL-4, IL-13 and TSLP, and their respective ligands, from a structure-function perspective. We detail the mechanisms of receptor complex assembly, the interrelated nature of these receptors, and impact on allergic inflammation. The ability for novel and atypical types of receptors to modulate inflammatory processes is also discussed. We highlight current and emerging treatments that target Th2-promoting receptor complexes. Understanding molecular features of these receptors provides insight into different disease phenotypes and the variable clinical outcomes arising from targeted therapies. These considerations can be used to inform future directions for research and creative strategies for treating individual patients.
Keywords: TSLP, IL-4, IL-13, receptors, cytokines, Th2, allergy, treatment
Introduction
Cytokines are small, secreted proteins that act in an autocrine or paracrine fashion. Binding of cytokines to surface receptors allows cells to respond to external cues that are vital to immune cell function. These processes manifest as cell survival, growth, and differentiation, all of which under normal circumstances are precisely regulated biological events. The Th2-promoting cytokines, IL-4, IL-13 and TSLP are overexpressed at sites of allergic inflammation including the skin, respiratory tract, and gut. Interleukins 4 and 13 mediate a broad array of functions including IgE antibody class switching by B cells and bronchial hyperresponsiveness. In addition, they target a range of effector cell types including mast cells and basophils, which themselves release IL-4 and IL-13 upon activation. Recently, the epithelial-derived cytokine, TSLP, has been spotlighted for its ability to initiate Th2 differentiation through priming of dendritic cells.1, 2 Th2-promoting cytokines are pleiotropic and have overlapping effects. These properties arises from their ability to bind to different receptors, and by sharing of receptor subunits among different receptor complexes (Figure 1). Here we explore these molecular relationships and their influence on mechanisms of cytokine action, disease phenotype and response to treatment.
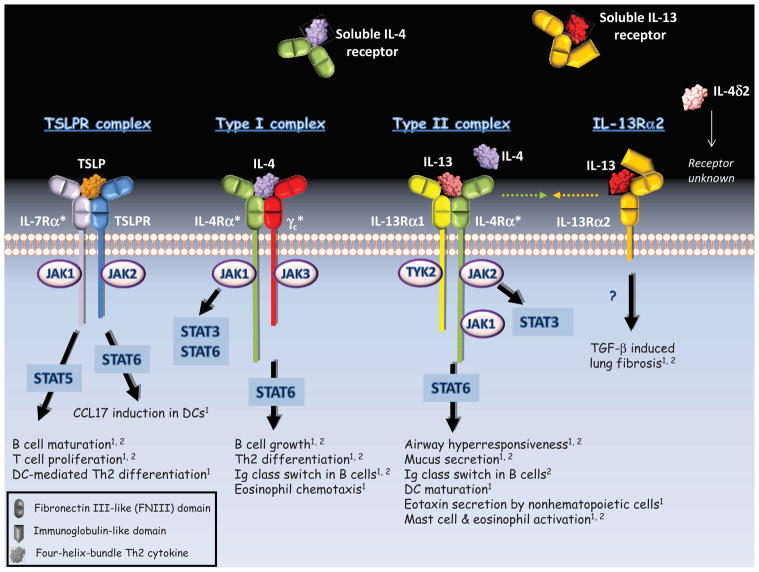
Figure 1. Receptor Configurations for TSLP, IL-4 and IL-13.
Asterisks indicate subunits shared among different receptor complexes. Green and gold arrows denote putative interactions between IL-4Rα and IL-13Rα2 to form a signaling-inert complex. Functional outcomes are listed for humans1 and mouse2.
Molecular Features of Th2-Promoting Cytokines
TSLP, IL-4 and IL-13 each belong to the IL-2 subfamily of the “four-helix-bundle” cytokine family, which also includes IL-2, IL-7, IL-9, IL-15 and IL-21. These cytokines bind their receptors through polar or charged amino acids on their surface, resulting in specific high affinity interactions and formation of stable signaling complexes.3–5 Th2-promoting cytokines exhibit limited amino acid sequence similarity (10–13% identity); however, their structures are highly conserved, comprising four short amphipathic alpha helices that organize to form a hydrophobic core (Figures 2A & E1).6 Three helices (designated A, C and D) are involved in receptor binding, and regions of high sequence similarity map to these regions (Figure E1). These conserved structural elements allow different cytokines to interact with the same receptor subunit.5
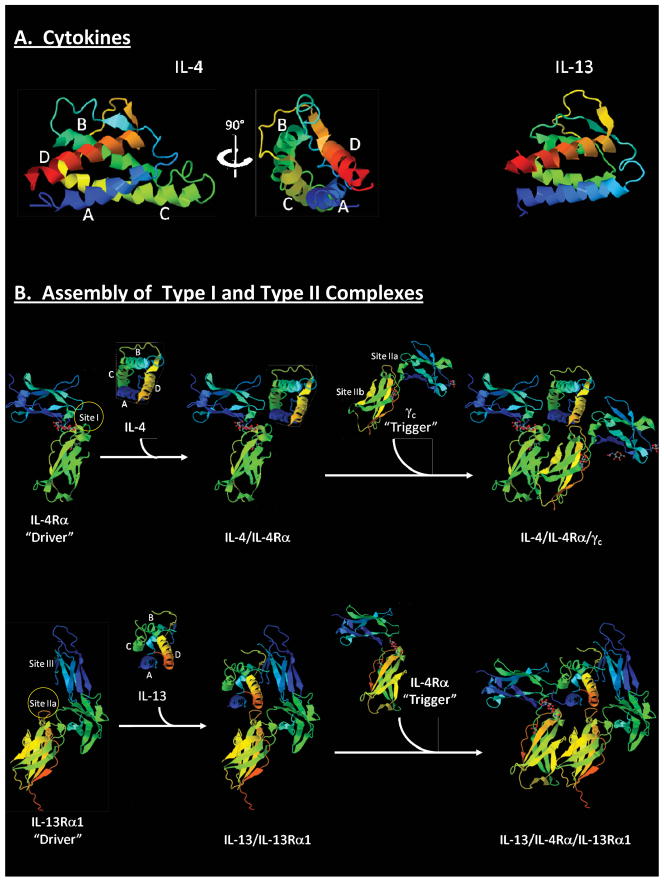
Figure 2. Assembly of Type I and Type II Receptor Complexes.
(A) Ribbon diagrams for IL-4 (PDB: 1ITM) and IL-13 (PDB: 1IJZ). (B) Assembly of type I IL-4 receptor and type II IL-13 receptor (upper and lower panels respectively). Upper panel: blue and green regions denote membrane distal and proximal FNIII domains respectively of IL-4Rα. Circle denotes Site I. Lower panel: Ig-like domain (blue), and membrane distal and proximal FNIII domains (green and mixed respectively) are shown for IL-13Rα1. Circle denotes Site IIa. Models were constructed using X-ray crystal structures of extracellular domains (IL-4/IL-4Rα/γc: PDB: 3BPL; and type II receptor complex: PDB: 3BPO).
Receptors
Extracellular Domain
Receptors for the Th2-promoting cytokines belong to the type 1 cytokine receptor family. These molecules share similarities in amino acid sequence (~20% identity) and structure. Conventional receptors for IL-4, IL-13 and TSLP exist as heterodimers which consist of an extracellular cytokine-binding domain, a transmembrane portion, and an intracellular signaling platform (Figure 1). The extracellular domain of each monomer contains tandem fibronectin III-like (FNIII) domains each comprising 7 beta strands.7 The junction of these domains forms an “elbow” configuration that is involved in cytokine binding (Figure 2B).
As is typical of type 1 cytokine receptors, each receptor subunit contains a modified WSXWS motif in the membrane-proximal FNIII domain, and four conserved cysteine residues in the membrane-distal FNIII domain. The WSXWS motif was recently reported to act as a molecular “switch” to drive dimerization subsequent to ligand binding.8 On the other hand, cysteine residues fulfill a structural role by forming disulfide bonds between different beta strands.9
Signaling Machinery
In general, Th2-promoting cytokine receptors transmit signals via pathways involving janus kinase (JAK) proteins and signal transducer and activator of transcription (STAT) proteins (Figure 1). The cytoplasmic tail of each receptor subunit contains conserved binding motifs for JAK proteins (designated Box 1 and Box 2), and tyrosine residues which are critical to signaling.10 Phosphorylation of tyrosine residues recruits STAT proteins, which in turn are phosphorylated by JAK proteins (Figure 1). This results in STAT dimerization, translocation to the nucleus, and binding to DNA elements that regulate gene transcription. A few basic principles are worth noting in relation to Th2-promoting pathways. First, the same cytokine can activate different JAK/STAT pathways resulting in different outcomes within the same cell (Figure 1)11; and second, the same cytokine may activate a different repertoire of JAK/STAT molecules depending on the cell type.2 In relation to these aspects, partnering of the same subunit with different subunits that each bind their own array of signaling molecules, allows differential signaling by receptor complexes containing shared subunits.12 Moreover, the same subunit within different receptor complexes can itself trigger discrete JAK/STAT pathways within the same cell.13 Thus, each Th2-promoting cytokine typically triggers multiple intracellular pathways that depend on the nature of the receptor complex and cell type.
Receptor Assembly
Understanding how cytokine receptor complexes form, is fundamental to the design of therapeutics for blocking Th2-promoting pathways in allergic disease (Figure 3). Receptor complexes assemble in a stepwise fashion. The conventional view is that cytokine first binds the subunit for which it has high affinity, and additional subunits are then recruited to form the functional ternary complex. This has been termed the “driver-trigger” concept. In this model, cytokine engages the first chain of the receptor complex, termed the “driver” (usually the α subunit), while interaction with the second chain provides the “trigger”.4 The driver imparts ligand specificity and initiates formation of the complex, while recruitment of the trigger into the complex establishes signaling potential. The type I receptor complex for IL-4 provides an example of this process. This receptor is a heterodimer of IL-4Rα and γc chain (Figure 1). Recent work suggests that IL-4 initially binds IL-4Rα (the “driver”), and γc chain (the “trigger”) is then recruited for ternary complex signaling (Figure 2B, upper panel).4 In this complex, IL-4Rα binds the surface of IL-4 formed by the A and C alpha helices through a region of the receptor termed “Site I”. The γc chain engages surfaces formed by the A and D alpha helices of IL-4 via “Site IIa” of the receptor, and the membrane-proximal FNIII domain of IL-4Rα (“Site IIb”).
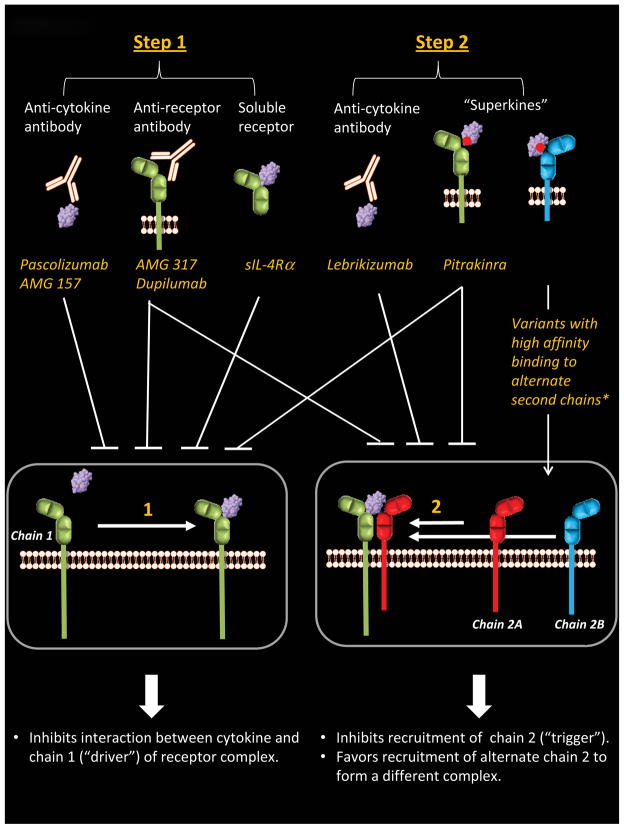
Figure 3. Treatments Targeting Th2-Promoting Pathways.
Drugs are categorized according to how they act in steps of the receptor assembly process. *Molecules in research phase.
In some cases, receptor assembly may involve recruitment of receptor chains from other types of complexes that can form in the absence of ligand. IL-4Rα, IL-7Rα, IL-2Rβ, IL-9Rα and γc, can each form homodimers independent of ligand.14–17 Furthermore, non-signaling heterodimers containing γc have been identified.17 Such “ligand-free” dimers might position receptor components in microdomains of the cell membrane for efficient recruitment to the receptor complex. Alternatively, these complexes might stabilize receptor subunits that are inactive.14, 18
Shared Receptors
Common γ Chain (γc) is Shared by Multiple Receptors
Receptor complexes for IL-4, IL-13 and TSLP each contain shared subunits. The most notable example is γc, which not only participates in IL-4 receptor, but also binds IL-2, IL-7, IL-9, IL-15 and IL-21. The overarching role of γc in the immune system is reflected in X-linked severe combined immune deficiency (X-SCID) which arises from a lack of T cells and NK cells owing to a loss of function in γc. The general view is that γc provides the “trigger” to stabilize different receptor complexes. In the receptor assembly process, cytokines that are already complexed with their high-affinity driver subunit engage with γc via a depression formed by the highly conserved D alpha helix in a configuration that has been likened to a “knob in a hole”.4, 7
Sharing of IL-4Rα Chain by Receptors that Bind IL-4 and IL-13
The IL-4Rα chain participates in two distinct receptor complexes that mediate the effects of IL-4 and IL-13, and thus are fundamental to allergic inflammatory processes. IL-4Rα partners with γc to form the type I receptor, and also couples with IL-13Rα1 to form the type II receptor (Figure 1).19 Whereas both receptors bind IL-4, the type II receptor also binds IL-13. Within the type II complex, both IL-4Rα and IL-13Rα1 can each act as both driver and trigger depending on which cytokine is bound. Thus, whereas IL-4Rα is the driver of the complex when IL-4 is bound, it acts as the trigger when IL-13 is part of the complex. Conversely, upon binding of IL-13, IL-13Rα1 acts as the driver of the type II complex but provides the trigger when IL-4 is bound. Notably, each receptor chain always binds cytokine in the same orientation. That is, IL-4Rα binds IL-4 via a “Site I” interaction and IL-13Rα1 binds IL-13 via “Site IIa” irrespective of function as driver or trigger.4
Assembly of the type II receptor complex containing IL-13 appears to be favored over IL-4. This has been attributed to the presence of an additional N-terminal Ig-like domain (Site III) within IL-13Rα1 (Figure 2B, lower panel). Specifically, this region enhances the efficiency of binding of IL-13/IL-13Rα1 (“driver”) to IL-4Rα (“trigger”) as compared to binding of IL-4/IL-4Rα (“driver”) to IL-13Rα1 (“trigger”).4, 20 These structural aspects, along with the concentration of available ligand and the relative abundance of IL-4Rα and IL-13Rα1 within the cell membrane, are key determinants in the type of receptor assembled, and thus, provide a mechanism for shaping the response to IL-4 and IL-13 in vivo.4, 21
Expression of IL-4Rα and IL-13Rα1 differs among different cell types. Only the type I receptor is expressed on hematopoietic cells such as T and B cells; however, both receptors are found on myeloid cells, including macrophages, monocytes and fibroblasts. By contrast, nonhematopoietic cells including epithelial cells and smooth muscle cells, predominantly express the type II receptor. Such tissue specificity explains, at least in part, the discrete functions of IL-4 and IL-13. In animal models, IL-4 signaling through the type I complex drives Th2 differentiation of CD4+ T cells22, IgE class switching in B cells23, and eosinophil chemotaxis.24 By contrast, IL-13 binding to the type II complex mediates the development of airway hyperresponsiveness, and mucus overproduction by epithelial cells (Figure 1).25, 26 However, the situation may be more complex in humans. Recent work using a variant of IL-4 that is engineered to bind IL-13Rα1 with high affinity revealed a role for IL-4 signaling through the type II complex in the differentiation of human monocytes into dendritic cells.27 Such observations suggest that the biologic effects of IL-4 acting through the type II complex may be underappreciated in allergic disease.
IL-7Rα Chain is Integral to TSLP Receptor Complex
IL-7Rα is a component of both IL-7 receptor (IL-7Rα/γc) and TSLP receptor complex (TSLPR/IL-7Rα).28, 29 The receptor for IL-7 is expressed on immature B cells and on T cells of various developmental stages.30, 31 By contrast, TSLP receptor complex is expressed on human dendritic cells and eosinophils, and is upregulated on activated T cells, including Th2 cells (Figure 1).1, 2, 32–34 TSLPR chain is the high-affinity ligand binding subunit of the TSLP receptor complex.35 Thus, whereas IL-7Rα is the driver of the IL-7 receptor complex, it acts as the trigger in the TSLP receptor complex. Functional overlap of IL-7 and TSLP in B cell differentiation and proliferation has been attributed to participation of IL-7Rα in receptors for each of these cytokines.36, 37
Though the structure of TSLP receptor complex remains unsolved, several observations support a model similar to the type II receptor bound to IL-13. Based on the premise that receptor chains always bind cytokines in the same orientation, IL-7Rα is predicted to engage TSLP via a Site I interaction in the TSLP receptor complex (“trigger”). By extension, TSLPR chain might be expected to bind TSLP via Site IIa (“driver”), similar to the IL-13/IL-13Rα1 interaction. Interestingly, though TSLPR chain is a homolog of γc, it also exhibits similarity with IL-13Rα1.38 Sequence similarities between IL-13 and TSLP lend credence to the proposed model of TSLP receptor complex formation. Specifically, IL-13 and TSLP each contain basic amino acids in a “hot spot” for receptor contact located within helix D (Figure E1).39, 40 Together, these aspects suggest that receptor complexes containing TSLP and IL-13 may bear resemblance.
“Alternative” Receptors and Cytokines
Soluble Receptors
Soluble cytokine receptors have the potential to modulate Th2 pathways and thus, provide targets for treatment of allergic disease. These receptors arise either from alternative splicing of pre-mRNA encoding the cytokine receptor, or from proteolytic cleavage of membrane-bound receptor extracellular domains in a process known as “shedding”. Modulation by alternative splicing is poorly defined, though recent observations indicate involvement of the RNA cleavage and polyadenylation factor CPSF-1 in humans.41 Generation of soluble receptors by shedding is regulated by matrix metalloproteinases (MMPs). Interestingly, members of this protein family have also been implicated in the pathogenesis of asthma and atopic dermatitis.42, 43
In humans, alternative splicing of IL-4Rα pre-mRNA generates a truncated soluble, secreted protein product that lacks the transmembrane and cytoplasmic tail, but retains the ability to bind IL-4 (Figure 1).44 Soluble IL-4Rα is also produced through MMP-dependent release of the IL-4Rα extracellular domain from the surface of activated human T cells.45 Studies of a recombinant soluble human IL-4Rα receptor showed its ability to enhance, or else neutralize, IL-4 activity in vitro depending on its concentration.46 However, actions of this receptor in vivo remain enigmatic. Recent work on novel soluble IL-4 receptor variants in zebrafish might shed new light on soluble forms of IL-4Rα. Soluble variants displayed tissue-specific expression profiles. Furthermore, administration of soluble IL-4Rα blocked IL-4-induced B cell proliferation and antibody production in vivo. This work implied an effect of soluble receptor on the CD154-CD40 costimulatory axis, which promotes Th2 induction by B cells.47
IL-13Rα2, which has been proposed to act as a “cytokine trap” for IL-13 (ie. a decoy receptor), can exist in membrane and soluble forms (Figure 1).48 Whereas in mice, distinct forms of this receptor are generated by alternative splicing, in humans the soluble form arises from cleavage of membrane receptor by MMP-8.49, 50 Protease allergens from dust mite can solubilize IL-13Rα2, and soluble receptor has been detected in bronchoalveolar lavage fluid of asthmatics, albeit at very low levels.51 In contrast to mice, soluble IL-13Rα2 appears to be absent in human blood.52
The affinity of IL-13Rα2 for IL-13 is several orders of magnitude higher than that of IL-13Rα1 owing to more extensive contacts with the cytokine.40 This feature, coupled with the capacity for IL-13Rα2 to efficiently internalize IL-13 without inducing signaling events53, points to an ability for IL-13Rα2 to sequester IL-13 from its signaling-competent type II receptor. Consistent with this view, an IL-13 variant (Arg110Gln) linked to bronchial asthma that binds IL-13Rα2 with reduced affinity appears to be cleared less efficiently by IL-13Rα2 in vitro.54 However, evidence that IL-13Rα2 exerts a modulatory effect in allergic disease in humans remains scant.
Adding to the complex landscape, recent evidence in mice suggests that membrane IL-13Rα2 may actually contribute to allergic inflammation.55 Thus, IL-13Rα2 may play key roles beyond its decoy activity. IL-13Rα2 can be induced on fibroblasts, smooth muscle cells, and keratinocytes.56–59 Its expression is highly regulated by IL-4 and IL-13, as well as by the type II interferon, IFN-β. Notably, induction of IL-13Rα2 in human bronchial fibroblasts by double stranded RNA through a mechanism involving IFN-β implicates this receptor in modulating responses to respiratory viruses.59 There is also evidence that binding of IL-13 and signaling through IL-13Rα2 contributes to TGF-β-induced lung fibrosis.60 Other work suggests that IL-13Rα2 can partner with IL-4Rα to form a signaling-inert complex capable of regulating the response to both IL-4 and IL-13.61 Thus, the functions of membrane-bound IL-13Rα2 may extend well beyond its ability to provide a “sink” for IL-13 in the lungs.
Cytokine Variants
Splice variants of both TSLP and IL-4 have been identified. Long and short transcripts encoding the same form of TSLP protein are expressed in human bronchial epithelial cells.62 The long transcript is preferentially upregulated in human keratinocytes by toll-like receptor ligands, and by Th2-associated cytokines.63 There is also evidence that TSLP can be modified at the post-translational level. Proteases expressed in nasal polyps generate a form of TSLP that enhances IL-5 production by mast cells, supporting a role for this molecule in the pathogenesis of chronic rhinosinusitis.64
Alternative splicing of IL-4-encoding pre-mRNA yields at least two transcripts which give rise to full-length IL-4, and a truncated form (IL-4δ2).65 The kinetics of expression of these variants differs in activated T cells from asthmatics, with IL-4 secretion peaking at 24 hours and IL-4δ2 secretion peaking later. There is evidence that these variants fulfill different functional roles and that IL-4δ2 may engage a range of receptors; however, further work is necessary to elucidate how these variants may act in allergic disease.66
Avenues for Receptor Discovery
The role of alternate forms of cytokines and receptors, as well as their participatory complexes, has largely been overlooked in allergic disease. This is striking, given that these molecules may provide valuable therapeutic targets based on their potential to counterbalance Th2-driven inflammation. IL-4δ2 provides an important example in this regard given its ability to inhibit both IL-4-induced production of polarized Th2 cells and IgE synthesis.66, 67 The availability of bioinformatics tools, including genome browsers and molecular modeling algorithms, makes it feasible to mine existing data for putative variants.68 However, distinguishing among protein variants expressed in experimental systems using available monoclonal antibodies may be problematic owing to shared epitopes. Furthermore, it is important to consider whether novel receptor configurations, including homodimers, exist, that can form functional complexes.69, 70
Establishing the relevance of “alternate” forms of Th2-promoting receptors and cytokines to the pathogenesis of allergic disease remains a significant challenge. Testing for expression of mRNA corresponding to molecular variants at inflamed sites is straightforward; however, detecting protein is problematic, especially for secreted molecules which may be rapidly degraded or sequestered at inflamed sites. In addition, certain features of cytokine receptor expression may be unique to specific cell types at sites of allergic inflammation. Single-cell transcriptomics, which involves analysis of the complete set of mRNA transcripts within a given cell, provides a powerful tool for tissue-specific receptor discovery.71 Since only a single type of cell is analyzed, this allows a determination of which gene products may partner with each other to form complexes. This is important since the tissue specificity of variants for some Th2-promoting cytokine receptor subunits may be underappreciated. Nonetheless, expression of mRNA components within the same cell does not guarantee formation of a functional receptor. Thus, receptor expression must be confirmed at the protein level, in order to establish relevance to disease.
Cytokine Networks and Allergic Disease
The Th2-promoting cytokines likely evolved to serve protective roles in the immune system. For example, IL-4 provides the principal signal for IgE class switching necessary for anti-helminthic responses. On the other hand, TSLP, which is secreted by epithelial cells in response to bacteria and viruses, is pivotal to the innate response. TSLP can regulate helminth infection and colitis through a variety of mechanisms which involve constraint of damaging effector T cells, and enhancement of regulatory T cells and inhibitory factors.72–74
The overlapping functions and multiple actions of Th2-promoting cytokines result in complex cytokine networks. A variety of regulatory mechanisms operate within these networks to control pathogenic processes. Differential expression of functional receptors on specific cell types provides one such mechanism. The integration of shared subunits into different receptor configurations capable of binding different cytokines with discrete affinities provides an additional level of molecular dexterity and control that is quite remarkable. Generation of receptor variants, including soluble forms, provides further regulation by modulating ligand engagement, or else receptor triggering. The abundance of receptor chains on the cell surface, coupled with the concentration of ligand in the milieu, are also key determinants of the type of receptor assembled, and hence, the type of signal generated.12, 21 Perturbation of these myriad processes results in exaggerated Th2-driven responses and allergic inflammation.
Dysregulation of Th2 pathways in allergic disease may be initiated at the epithelial barrier. This is supported by the propensity for bronchial epithelial cells from atopic asthmatics to express higher levels of TSLP and its receptor in response to virus, as compared with cells from non-atopic donors.96, 97 Following Th2 initiation, this process is perpetuated through upregulation of cytokine receptors on effector cells including mast cells, basophils, dendritic cells, and T cells, in response to a variety of stimuli, including allergen.77, 78 Enhancement of Th2 cytokine pathways, in turn, has the capacity to augment allergen-triggered events through upregulation of IgE receptor, thereby creating a vicious cycle of inflammation.79 While it is easy to envisage how disruption of regulatory networks may occur, their complexity presents challenges for future research and treatment strategies.
Treatments Targeting Th2-Promoting Pathways
The clinical benefits of targeting receptors for IL-4, IL-13 and TSLP are already being realized. This is accomplished using molecules that intervene in the receptor assembly process to inhibit cytokine binding, or else block the interaction of receptor subunits (Figure 3). However, not all approaches have been successful. Treatment modalities that inhibit IL-4 itself have been disappointing.80, 81 By contrast, IL-13 antagonists have proven beneficial in patients with asthma. Interestingly, the IL-13-specific monoclonal antibody, lebrikizumab, is more effective for treatment of asthma associated with high levels of periostin82, a protein implicated in asthmatic airway eosinophilia.83 Recent molecular observations indicate that the high-affinity interaction between lebrikizumab and IL-13 sterically hinders IL-13 from binding IL-4Rα in the type II complex, thereby precluding receptor assembly and signaling.84
It has been proposed that the lack of clinical benefit observed by targeting IL-4 relates, at least in part, to the redundancy between IL-4 and IL-13. Thus, drugs which inhibit both IL-4 and IL-13 pathways simultaneously (so-called dual antagonists) may be advantageous. The IL-4 variant, pitrakinra, is one such drug. Pitrakinra is engineered to contain two single point mutations which allow it to competitively bind to IL-4Rα, thereby preventing formation of both type I and type II complexes (Figure 3).85–87 Inhalation of this molecule was shown to diminish allergen-induced late phase asthmatic responses in patients with atopic asthma.88 In subsequent studies, pitrakinra inhibited asthma exacerbations in patients with moderate-to-severe asthma.89, 90 Interestingly, this latter effect was restricted to those patients who had specific single nucleotide polymorphisms in the 3 prime untranslated region of the gene encoding IL-4Rα. Thus, genetic polymorphisms, which are common in loci encoding Th2 cytokine receptors, may have predictive value for the efficacy of therapeutics which target these receptors.91 Such considerations have to be weighed when evaluating outcomes for monoclonal antibodies that target IL-4/IL-13 pathways through IL-4Rα.
Two antibodies have been developed that bind with high affinity to IL-4Rα (known as AMG 31792, 93 and dupilumab (REGN668)).94 Thus, similar to pitrakinra, these antibodies block the biologic activities of both IL-4 and IL-13 through effects on type I and type II receptor complexes (Figure 3). While AMG 317 was shown to be safe and well tolerated among patients with moderate to severe atopic asthma, no significant effect was observed in symptom score in treated patients.93 However, results in the same study showed a decrease in exacerbations in a group of patients receiving a higher dose of AMG 317, and increased response to treatment in patients with more severe asthma. More recently, dupilumab was reported to decrease asthma exacerbations among patients with persistent asthma associated with eosinophilia following withdrawal of inhaled glucocorticoids and long-acting beta agonist therapy.94 In that study, lung function was improved and markers of Th2-mediated inflammation in the blood (TARC, eotaxin-3, IgE) were significantly reduced.
As already mentioned, not all attempts to inhibit Th2-promoting pathways have proven fruitful. The use of soluble receptors is a notable example in this regard. In early studies, recombinant soluble IL-4Rα gave promising results95, 96; however, subsequent work failed to confirm beneficial effects. This might be explained by the fact that soluble IL-4Rα does not work effectively as a “cytokine trap”.97 In order for a soluble receptor to optimally block cytokine binding, it should bind cytokine with higher affinity than its membrane-bound counterpart. These molecular considerations, coupled with the overlapping effects of IL-4 and IL-13, likely conspire to undermine the efficacy of soluble IL-4 receptor as a treament modality.
Newer therapies are on the horizon that might be exploited to modify Th2-promoting pathways. Engineered cytokines that bind with high affinity to specific receptor chains are currently under development that might redirect receptor signaling by preferentially favoring the assembly of specific receptor complexes. Recent work has shown that an IL-4 variant with high affinity for IL-13Rα1 favors activation through the type II receptor (IL-4Rα/IL-13Rα1), as opposed to through the type I receptor complex (IL-4Rα/γc).27 Thus, using these so-called “superkines” to exploit the variability in numbers of second chains (ie. IL-13Rα1 and γc) in certain cell types could provide a modality to re-direct signaling both within, and among specific cell types (Figure 3).
Based on the complexity of molecules engaged in cytokine networks, it is perhaps surprising that any clinical benefit is attainable by inhibiting a single molecule. With this in mind, blocking cytokines such as TSLP which operate upstream in the allergic inflammatory cascade, might be predicted to optimize clinical outcomes. Moreover, in contrast to IL-4 and IL- 13, which appear to exert differential roles in certain asthma phenotypes, TSLP may be involved in multiple asthma phenotypes through its critical role in the innate phase of allergic responses. An anti-TSLP monoclonal antibody that blocks the interaction of TSLP with TSLP receptor (AMG 157) is currently being tested as a treatment for asthma in Phase 1b trials (Figure 3).
Conclusions and Future Directions
We have highlighted the shared and discrepant molecular features of Th2-promoting cytokines and their receptors with a view to understanding how these aspects inform their function in allergic disease, and the response to treatment. Moving forward, it will be important to understand the molecular basis of different asthma phenotypes in order to interpret variability in the response to new treatments which target Th2-promoting cytokine pathways. Genetic polymorphisms have the ability to alter the interactions between receptors and their ligands, and to modify downstream signaling events, through effects on protein structure. Alternatively, more subtle effects may occur at the post-transcriptional level which could impact the generation of splice variants, disease phenotype and therapeutic outcomes.
Environmental triggers, including allergens, feed into Th2-promoting cytokine networks by modulating receptor expression. Thus, combined therapies that mitigate the adverse effects of allergen exposure (eg. specific immunotherapy) and dampen Th2-promoting pathways (eg. receptor antagonists) may optimize clinical benefit. This dual approach could be tailored according to the individual patient’s sensitivity to allergens, and disease phenotype.
Finally, it should be noted that the Th2-promoting cytokines that contribute to the pathogenesis of allergic disease, also fulfill critical immune functions related to B cell growth and proliferation, as well as induction of regulatory T cells. Thus, long-term blockade of these cytokines may not be without risk.
In summary, the relationships among Th2-promoting cytokines and their receptors are complex. The molecular underpinnings that dictate receptor configurations, their relative abundance, and the resulting immune outcomes are shaped by genetics of the individual and environmental exposures. Studies which continue to query the nature of cytokine-receptor interactions in humans will not only serve to advance our knowledge of these structures, but also hasten the development of new treatments that provide maximal clinical benefit to the individual patient.
Supplementary Material
Key Messages.
The molecular features of Th2 cytokines and their receptors are fundamental to their biologic function, and the development of allergic inflammation and disease.
The similarities and differences among these molecules influence the response to treatment, and are important factors to consider when designing therapies to inhibit Th2 pathways.
Acknowledgments
Funding Sources: This work was supported by grants from NIH/NIAID (R01 AI-052196) and NIH/NIAMS (R01 AR-059058).
Abbreviations
- FNIII
Fibronectin III
- JAK
janus kinase
- MMP
matrix metalloproteinase
- STAT
signal transducer and activator of transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845–52. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–63. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 4.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh ST. Structural insights into the common gamma-chain family of cytokines and receptors from the interleukin-7 pathway. Immunol Rev. 2012;250:303–16. doi: 10.1111/j.1600-065X.2012.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rickert M, Wang X, Boulanger MJ, Goriatcheva N, Garcia KC. The structure of interleukin-2 complexed with its alpha receptor. Science. 2005;308:1477–80. doi: 10.1126/science.1109745. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Lupardus P, Laporte SL, Garcia KC. Structural biology of shared cytokine receptors. Annu Rev Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagil R, Knudsen MJ, Olsen JG, O’Shea C, Franzmann M, Goffin V, et al. The WSXWS motif in cytokine receptors is a molecular switch involved in receptor activation: insight from structures of the prolactin receptor. Structure. 2012;20:270–82. doi: 10.1016/j.str.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Cole AR, Hall NE, Treutlein HR, Eddes JS, Reid GE, Moritz RL, et al. Disulfide bond structure and N-glycosylation sites of the extracellular domain of the human interleukin-6 receptor. J Biol Chem. 1999;274:7207–15. doi: 10.1074/jbc.274.11.7207. [DOI] [PubMed] [Google Scholar]
- 10.Zhong J, Pandey A. Site-directed mutagenesis reveals a unique requirement for tyrosine residues in IL-7Ralpha and TSLPR cytoplasmic domains in TSLP-dependent cell proliferation. BMC Immunol. 2010;11:5. doi: 10.1186/1471-2172-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElroy CA, Holland PJ, Zhao P, Lim JM, Wells L, Eisenstein E, et al. Structural reorganization of the interleukin-7 signaling complex. Proc Natl Acad Sci U S A. 2012;109:2503–8. doi: 10.1073/pnas.1116582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kammer W, Lischke A, Moriggl R, Groner B, Ziemiecki A, Gurniak CB, et al. Homodimerization of interleukin-4 receptor alpha chain can induce intracellular signaling. J Biol Chem. 1996;271:23634–7. doi: 10.1074/jbc.271.39.23634. [DOI] [PubMed] [Google Scholar]
- 16.Pillet AH, Juffroy O, Mazard-Pasquier V, Moreau JL, Gesbert F, Chastagner P, et al. Human IL-Rbeta chains form IL-2 binding homodimers. Eur Cytokine Netw. 2008;19:49–59. doi: 10.1684/ecn.2008.0120. [DOI] [PubMed] [Google Scholar]
- 17.Malka Y, Hornakova T, Royer Y, Knoops L, Renauld JC, Constantinescu SN, et al. Ligand-independent homomeric and heteromeric complexes between interleukin-2 or -9 receptor subunits and the gamma chain. J Biol Chem. 2008;283:33569–77. doi: 10.1074/jbc.M803125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose T, Pillet AH, Lavergne V, Tamarit B, Lenormand P, Rousselle JC, et al. Interleukin-7 compartmentalizes its receptor signaling complex to initiate CD4 T lymphocyte response. J Biol Chem. 2010;285:14898–908. doi: 10.1074/jbc.M110.104232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci U S A. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Suzuki S, Kanaji S, Shiraishi H, Ohta S, Arima K, et al. Distinct structural requirements for interleukin-4 (IL-4) and IL-13 binding to the shared IL-13 receptor facilitate cellular tuning of cytokine responsiveness. J Biol Chem. 2009;284:24289–96. doi: 10.1074/jbc.M109.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, et al. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168:853–62. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller NM, Gwinn WM, Donnelly RP, Constant SL, Keegan AD. IL-4 engagement of the type I IL-4 receptor complex enhances mouse eosinophil migration to eotaxin-1 in vitro. PLoS One. 2012;7:e39673. doi: 10.1371/journal.pone.0039673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 26.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junttila IS, Creusot RJ, Moraga I, Bates DL, Wong MT, Alonso MN, et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat Chem Biol. 2012;8:990–8. doi: 10.1038/nchembio.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–80. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 29.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 30.Armitage RJ, Ziegler SF, Beckmann MP, Idzerda RL, Park LS, Fanslow WC. Expression of receptors for interleukin 4 and interleukin 7 on human T cells. Adv Exp Med Biol. 1991;292:121–30. doi: 10.1007/978-1-4684-5943-2_14. [DOI] [PubMed] [Google Scholar]
- 31.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993;90:9125–9. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–15. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 33.Reefer AJ, Hulse KE, Lannigan JA, Solga MD, Wright PW, Kelly LA, et al. Flow cytometry imaging identifies rare T(H)2 cells expressing thymic stromal lymphopoietin receptor in a “proallergic” milieu. J Allergy Clin Immunol. 2010;126:1049–58. 58 e1–10. doi: 10.1016/j.jaci.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–4. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 35.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–70. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–6. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 37.Scheeren FA, van Lent AU, Nagasawa M, Weijer K, Spits H, Legrand N, et al. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur J Immunol. 2010;40:955–65. doi: 10.1002/eji.200939419. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Wang J, Wang Q, Chen G, Zhang J, Chen T, et al. Identification of a novel type I cytokine receptor CRL2 preferentially expressed by human dendritic cells and activated monocytes. Biochem Biophys Res Commun. 2001;281:878–83. doi: 10.1006/bbrc.2001.4432. [DOI] [PubMed] [Google Scholar]
- 39.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6:15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupardus PJ, Birnbaum ME, Garcia KC. Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Structure. 2010;18:332–42. doi: 10.1016/j.str.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evsyukova I, Bradrick SS, Gregory SG, Garcia-Blanco MA. Cleavage and polyadenylation specificity factor 1 (CPSF1) regulates alternative splicing of interleukin 7 receptor (IL7R) exon 6. RNA. 2013;19:103–15. doi: 10.1261/rna.035410.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prikk K, Maisi P, Pirila E, Reintam MA, Salo T, Sorsa T, et al. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab Invest. 2002;82:1535–45. doi: 10.1097/01.lab.0000035023.53893.b6. [DOI] [PubMed] [Google Scholar]
- 43.Harper JI, Godwin H, Green A, Wilkes LE, Holden NJ, Moffatt M, et al. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br J Dermatol. 2010;162:397–403. doi: 10.1111/j.1365-2133.2009.09467.x. [DOI] [PubMed] [Google Scholar]
- 44.Kruse S, Forster J, Kuehr J, Deichmann KA. Characterization of the membrane-bound and a soluble form of human IL-4 receptor alpha produced by alternative splicing. Int Immunol. 1999;11:1965–70. doi: 10.1093/intimm/11.12.1965. [DOI] [PubMed] [Google Scholar]
- 45.Jung T, Schrader N, Hellwig M, Enssle KH, Neumann C. Soluble human interleukin-4 receptor is produced by activated T cells under the control of metalloproteinases. Int Arch Allergy Immunol. 1999;119:23–30. doi: 10.1159/000024171. [DOI] [PubMed] [Google Scholar]
- 46.Jung T, Wagner K, Neumann C, Heusser CH. Enhancement of human IL-4 activity by soluble IL-4 receptors in vitro. Eur J Immunol. 1999;29:864–71. doi: 10.1002/(SICI)1521-4141(199903)29:03<864::AID-IMMU864>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 47.Zhu LY, Pan PP, Fang W, Shao JZ, Xiang LX. Essential role of IL-4 and IL-4Ralpha interaction in adaptive immunity of zebrafish: insight into the origin of Th2-like regulatory mechanism in ancient vertebrates. J Immunol. 2012;188:5571–84. doi: 10.4049/jimmunol.1102259. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Sivaprasad U, Tabata Y, Gibson AM, Stier MT, Finkelman FD, et al. IL-13R alpha 2 membrane and soluble isoforms differ in humans and mice. J Immunol. 2009;183:7870–6. doi: 10.4049/jimmunol.0901028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO, Hershey GK. Allergy-driven alternative splicing of IL-13 receptor alpha2 yields distinct membrane and soluble forms. J Immunol. 2006;177:7905–12. doi: 10.4049/jimmunol.177.11.7905. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Tabata Y, Gibson AM, Daines MO, Warrier MR, Wills-Karp M, et al. Matrix metalloproteinase 8 contributes to solubilization of IL-13 receptor alpha2 in vivo. J Allergy Clin Immunol. 2008;122:625–32. doi: 10.1016/j.jaci.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daines MO, Chen W, Tabata Y, Walker BA, Gibson AM, Masino JA, et al. Allergen-dependent solubilization of IL-13 receptor alpha2 reveals a novel mechanism to regulate allergy. J Allergy Clin Immunol. 2007;119:375–83. doi: 10.1016/j.jaci.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Toole M, Legault H, Ramsey R, Wynn TA, Kasaian MT. A novel and sensitive ELISA reveals that the soluble form of IL-13R-alpha2 is not expressed in plasma of healthy or asthmatic subjects. Clin Exp Allergy. 2008;38:594–601. doi: 10.1111/j.1365-2222.2007.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–9. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 54.Arima K, Umeshita-Suyama R, Sakata Y, Akaiwa M, Mao XQ, Enomoto T, et al. Upregulation of IL-13 concentration in vivo by the IL13 variant associated with bronchial asthma. J Allergy Clin Immunol. 2002;109:980–7. doi: 10.1067/mai.2002.124656. [DOI] [PubMed] [Google Scholar]
- 55.Chen W, Sivaprasad U, Gibson AM, Ericksen MB, Cunningham CM, Bass SA, et al. IL-13 receptor α2 contributes to development of experimental allergic asthma. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.04.016. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshikawa M, Nakajima T, Tsukidate T, Matsumoto K, Iida M, Otori N, et al. TNF-alpha and IL-4 regulate expression of IL-13 receptor alpha2 on human fibroblasts. Biochem Biophys Res Commun. 2003;312:1248–55. doi: 10.1016/j.bbrc.2003.11.077. [DOI] [PubMed] [Google Scholar]
- 57.Moynihan BJ, Tolloczko B, El Bassam S, Ferraro P, Michoud MC, Martin JG, et al. IFN-gamma, IL-4 and IL-13 modulate responsiveness of human airway smooth muscle cells to IL-13. Respir Res. 2008;9:84. doi: 10.1186/1465-9921-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.David M, Ford D, Bertoglio J, Maizel AL, Pierre J. Induction of the IL-13 receptor alpha2-chain by IL-4 and IL-13 in human keratinocytes: involvement of STAT6, ERK and p38 MAPK pathways. Oncogene. 2001;20:6660–8. doi: 10.1038/sj.onc.1204629. [DOI] [PubMed] [Google Scholar]
- 59.Campbell-Harding G, Sawkins H, Bedke N, Holgate ST, Davies DE, Andrews AL. The innate antiviral response upregulates IL-13 receptor alpha2 in bronchial fibroblasts. J Allergy Clin Immunol. 2013;131:849–55. doi: 10.1016/j.jaci.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 60.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 61.Andrews AL, Nasir T, Bucchieri F, Holloway JW, Holgate ST, Davies DE. IL-13 receptor alpha 2: a regulator of IL-13 and IL-4 signal transduction in primary human fibroblasts. J Allergy Clin Immunol. 2006;118:858–65. doi: 10.1016/j.jaci.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 62.Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–74. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 63.Xie Y, Takai T, Chen X, Okumura K, Ogawa H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci. 2012;66:233–7. doi: 10.1016/j.jdermsci.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013 May 17; doi: 10.1016/j.jaci.2013.04.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alms WJ, Atamas SP, Yurovsky VV, White B. Generation of a variant of human interleukin-4 by alternative splicing. Mol Immunol. 1996;33:361–70. doi: 10.1016/0161-5890(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 66.Luzina IG, Lockatell V, Lavania S, Pickering EM, Kang PH, Bashkatova YN, et al. Natural production and functional effects of alternatively spliced interleukin-4 protein in asthma. Cytokine. 2012;58:20–6. doi: 10.1016/j.cyto.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arinobu Y, Atamas SP, Otsuka T, Niiro H, Yamaoka K, Mitsuyasu H, et al. Antagonistic effects of an alternative splice variant of human IL-4, IL-4delta2, on IL-4 activities in human monocytes and B cells. Cellular Immunology. 1999;191:161–7. doi: 10.1006/cimm.1998.1431. [DOI] [PubMed] [Google Scholar]
- 68.Romeo MJ, Agrawal R, Pomés A, Woodfolk JA. Human basophils express novel TSLPR variants including a putative secreted form. J Allergy Clin Immunol. 2013;131(Supp):AB 102. [Google Scholar]
- 69.Zhong J, Liu X, Pandey A. Effects of transmembrane and juxtamembrane domains on proliferative ability of TSLP receptor. Mol Immunol. 2010;47:1207–15. doi: 10.1016/j.molimm.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–92. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartfai T, Buckley PT, Eberwine J. Drug targets: single-cell transcriptomics hastens unbiased discovery. Trends in Pharmacological Sciences. 2012;33:9–16. doi: 10.1016/j.tips.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reardon C, Lechmann M, Brustle A, Gareau MG, Shuman N, Philpott D, et al. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35:223–35. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–93. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 74.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miazgowicz MM, Elliott MS, Debley JS, Ziegler SF. Respiratory syncytial virus induces functional thymic stromal lymphopoietin receptor in airway epithelial cells. J Inflamm Res. 2013;6:53–61. doi: 10.2147/JIR.S42381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee HC, Headley MB, Loo YM, Berlin A, Gale M, Jr, Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130:1187–96. e5. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hulse KE, Reefer AJ, Engelhard VH, Satinover SM, Patrie JT, Chapman MD, et al. Targeting Fel d 1 to FcgammaRI induces a novel variation of the T(H)2 response in subjects with cat allergy. J Allergy Clin Immunol. 2008;121:756–62. e4. doi: 10.1016/j.jaci.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, et al. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol. 2010;125:247–56. e1–8. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaur D, Hollins F, Woodman L, Yang W, Monk P, May R, et al. Mast cells express IL-13R alpha 1: IL-13 promotes human lung mast cell proliferation and Fc epsilon RI expression. Allergy. 2006;61:1047–53. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- 80.Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of pascolizumab (9SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol. 2002;130:93–100. doi: 10.1046/j.1365-2249.2002.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nature Reviews Drug Discovery. 2012;11:958–72. doi: 10.1038/nrd3792. [DOI] [PubMed] [Google Scholar]
- 82.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 83.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54. e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ultsch M, Bevers J, Nakamura G, Vandlen R, Kelley RF, Wu LC, et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol. 2013;425:1330–9. doi: 10.1016/j.jmb.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 85.Tomkinson A, Tepper J, Morton M, Bowden A, Stevens L, Harris P, et al. Inhaled vs subcutaneous effects of a dual IL-4/IL-13 antagonist in a monkey model of asthma. Allergy. 2010;65:69–77. doi: 10.1111/j.1398-9995.2009.02156.x. [DOI] [PubMed] [Google Scholar]
- 86.Tomkinson A, Duez C, Cieslewicz G, Pratt JC, Joetham A, Shanafelt MC, et al. A murine IL-4 receptor antagonist that inhibits IL-4- and IL-13-induced responses prevents antigen-induced airway eosinophilia and airway hyperresponsiveness. J Immunol. 2001;166:5792–800. doi: 10.4049/jimmunol.166.9.5792. [DOI] [PubMed] [Google Scholar]
- 87.Burmeister Getz E, Fisher DM, Fuller R. Human pharmacokinetics/pharmacodynamics of an interleukin-4 and interleukin-13 dual antagonist in asthma. J Clin Pharmacol. 2009;49:1025–36. doi: 10.1177/0091270009341183. [DOI] [PubMed] [Google Scholar]
- 88.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–31. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 89.Slager RE, Hawkins GA, Ampleford EJ, Bowden A, Stevens LE, Morton MT, et al. IL-4 receptor alpha polymorphisms are predictors of a pharmacogenetic response to a novel IL-4/IL-13 antagonist. J Allergy Clin Immunol. 2010;126:875–8. doi: 10.1016/j.jaci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slager RE, Otulana BA, Hawkins GA, Yen YP, Peters SP, Wenzel SE, et al. IL-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor alpha antagonist. J Allergy Clin Immunol. 2012;130:516–22. e4. doi: 10.1016/j.jaci.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maes T, Joos GF, Brusselle GG. Targeting interleukin-4 in asthma: lost in translation? Am J Respir Cell Mol Biol. 2012;47:261–70. doi: 10.1165/rcmb.2012-0080TR. [DOI] [PubMed] [Google Scholar]
- 92.Kakkar T, Sung C, Gibiansky L, Vu T, Narayanan A, Lin SL, et al. Population PK and IgE pharmacodynamic analysis of a fully human monoclonal antibody against IL4 receptor. Pharm Res. 2011;28:2530–42. doi: 10.1007/s11095-011-0481-y. [DOI] [PubMed] [Google Scholar]
- 93.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–96. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 94.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013 May 21; doi: 10.1056/NEJMoa1304048. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Borish LC, Nelson HS, Lanz MJ, Clauseen L, Whitmore JB, Agosti JM, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J respir Crit Care Med. 1999;160:1816–23. doi: 10.1164/ajrccm.160.6.9808146. [DOI] [PubMed] [Google Scholar]
- 96.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107:963–70. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 97.Mosley B, Beckmann MP, March CJ, Idzerda RL, Gimpel SD, VandenBos T, et al. The murine interleukin-4 receptor: Molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–48. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.