Abstract
Receiving care at multiple clinics may compromise the therapeutic patient-provider alliance and adversely affect the treatment of people living with HIV. We evaluated 12,759 HIV-infected adults in Philadelphia, PA between 2008 and 2010 to determine the effects of using multiple clinics for primary HIV care. Using generalized estimating equations with logistic regression, we examined the relationship between receiving care at multiple clinics (≥1 visit to two or more clinics during a calendar year) and two outcomes: (1) use of ART and (2) HIV viral load ≤200 copies/mL for patients on ART. Overall, 986 patients (8 %) received care at multiple clinics. The likelihood of attending multiple clinics was greater for younger patients, women, blacks, persons with public insurance, and for individuals in their first year of care. Adjusting for sociodemographic factors, patients receiving care at multiple clinics were less likely to use ART (AOR = 0.62, 95 % CI 0.55–0.71) and achieve HIV viral suppression (AOR = 0.78, 95 % CI 0.66–0.94) than individuals using one clinic. Qualitative data are needed to understand the reasons for visiting multiple clinics.
Keywords: HIV care, HIV clinics, Use of multiple clinics, HIV outcomes, Viral suppression
Introduction
Continuity of care, or developing a sustained patient-provider partnership to cooperatively manage a patient’s health, is a fundamental aspect of primary care [1–3]. Data indicate that continuity is valued by both patients and providers, and is associated with improved outcomes, decreased emergency care utilization, and higher patient satisfaction [4–7]. Individuals with chronic conditions, such as HIV infection, public insurance, and at the ends of the age spectrum appear to value provider continuity more than their counterparts [1].
For people living with HIV infection (PLWH), maintaining a continuous relationship with a provider is associated with receiving antiretroviral therapy (ART), fewer HIV-related complications, and lower risk of HIV transmission to others [8–14]. The Centers for Disease Control and Prevention (CDC) estimate that only 77 % of PLWH successfully link to care after diagnosis and less than 75 % of those remain in continuous care [15–17]. Data on how patients participate in outpatient care is needed to improve linkage rates and retention in care.
Most prior studies have compared patients consistently engaged in care to those without a regular source of care [10, 13, 15, 18, 19]. However, patients can continuously participate in care either by seeking care at a single clinic or by moving between clinics as needed or desired. Receiving care at multiple clinics may adversely affect the treatment of PLWH; increasing unnecessary testing and contributing to higher healthcare costs [5, 20, 21]. In addition, lack of coordination among providers or inconsistent advice from different sources may lead to medication errors, drug toxicity, and compromise the therapeutic patient-provider alliance [22–25]. To our knowledge, no data on patients receiving care at multiple clinics has been reported. Therefore, we aimed to determine the effect of using multiple sites of care on clinical HIV outcomes in a large urban area.
Methods
Study Design and Participants
We conducted a retrospective cohort study of HIV-infected adults in care at Ryan White Program funded clinics in Philadelphia, PA between 2008 and 2010. Twenty-six HIV clinics treat adults and receive Ryan White funding, representing approximately 71 % of all PLWH in care in Philadelphia (unpublished data, City of Philadelphia Department of Public Health). All HIV-infected adults (age ≥18 years) engaged in care, defined by having at least two primary HIV visit and one CD4 test in a calendar year, between January 1, 2008 and December 31, 2010 were eligible for inclusion.
Data Collection
Data were extracted from CAREWare, a Health Resources and Services Administration (HRSA) recommended data management system containing demographic, laboratory, pharmacy, and health service utilization information for all patients seen at Philadelphia Ryan White Program funded clinics. Clinics abstract patient-level information from medical records of all patients in care, not only those covered under the Ryan White Program. After quality control and verification, data are sent to the City of Philadelphia Department of Public Health and combined across clinics to produce a uniform database. Each patient in the database has a unique identifier, independent of personal information or site of care, to allow monitoring even when care is provided at multiple sites. Periodic chart reviews and site visits are undertaken to verify the accuracy and completeness of data abstraction and entry. The study was approved by the Institutional Review Boards of the University of Pennsylvania and the City of Philadelphia Department of Public Health.
Sociodemographic and Clinical Variables
For each year of observation, patients’ age as of January 1 was divided into four groups: 18–29, 30–39, 40–49, and over 50 years old. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other/unknown. Self-reported HIV transmission behavior was grouped into heterosexual, men who had sex with men (MSM), injection drug use (IDU), and other/unknown. Patients who had IDU in combination with another risk factor (e.g. MSM, heterosexual transmission) were classified as IDU. Insurance coverage in each year was categorized as private, Medicaid, Medicare, uninsured, or other/unknown. Patients whose care was funded by Ryan White were considered to be uninsured. Annual household income was divided into <$10,000, $10,000–$19,999, $20,000–$49,999 and ≥$50,000 according to CDC classifications [26]. Median CD4 cell count in each year was grouped as ≤350 or >350 cells/mm3 based on differential indications for starting ART [27]. Patients in their first year of care were distinguished from those already engaged in care as outcomes vary between the first and later years in care [16, 28, 29].
Primary HIV visit data, including number of completed visits and site of care, were collected on an individual basis over each calendar period. Primary HIV visits were defined according to HRSA criteria: a visit to an outpatient provider with prescribing privileges (not include nurses, pharmacists, social workers, or other support services providers) in an HIV care setting [30]. The number of visits for primary HIV care in each year was categorized as 2, 3, 4, 5, 6, 7 or ≥8.
Main Exposure Variable
Patients were classified as receiving care at multiple clinics if they completed at least one primary HIV visit to two or more clinics in a calendar year; individuals could be classified as receiving care at multiple clinics in 1 year and classified as receiving care at a single clinic in another year. Three clinic utilization patterns were determined for patients attending multiple clinics: (1) one-time visit to a second clinic; (2) transfers (changes) in care; and (3) alternating visits between multiple clinics. The principal clinic was defined as the clinic visited most frequently. For patients with an equal number of visits to multiple clinics, the principal clinic was classified as the last clinic visited in the year.
Outcome Variables
Outcomes of interest were use of ART and HIV suppression in the calendar year. Patients were considered to be on ART if they received three antiretroviral drugs (excluding ritonavir) at the last outpatient visit in each calendar year. HIV suppression for patients receiving ART was classified as last annual HIV viral load ≤200 copies/mL.
Statistical Analyses
The patient-year was the unit of analysis. Each patient could contribute one observation per calendar year. For each calendar year, data for only those patients meeting the inclusion criteria (at least two primary HIV visit and one CD4 test) were included. Thus, the number of patient-years was not constant across patients. We excluded 2,817 patient-years in which individuals had only one primary HIV visit in a calendar year, since these individuals were ineligible to experience the main exposure of visiting multiple HIV clinics in the same calendar year. In addition, we excluded an additional 305 patient-years in which individuals died, as they did not provide adequate time to measure use of ART or viral suppression. After excluding these patient-years, 14 % of the sample contributed 1 year of data; 29 % contributed 2 years; and 57 % contributed 3 years.
Statistical comparisons of demographic and clinical characteristics of the sample across calendar years were made using the χ2 test for trend. We assessed the relation between number of patients using multiple clinics and calendar year using the χ2 test of independence. The Mann–Whitney test was used to compare the number of visits to the principal clinic (ordinal variable with non-normal distribution) and number of clinics attended in a year. Multivariate logistic regression examined demographic (age, sex, race/ethnicity, HIV transmission behavior, insurance coverage, annual household income) and clinical factors (median CD4 cell count, first year in care) associated with attending more than one HIV clinic a year, adjusting for the number of primary HIV visits and calendar year. For those attending multiple clinics, we used the χ2 test of independence to identify difference in patient demographic and clinical characteristics across the three clinic utilization patterns.
Multivariate logistic regression assessed the relationship between receiving care at multiple HIV clinics and two clinical HIV outcomes: (1) use of ART and (2) HIV viral suppression for patients receiving ART. In secondary analyses, we evaluated use of ART for only patients recommended to receive ART (CD4 cell count ≤350 cells/mm3). The variables considered for both use of ART and HIV viral suppression were age, sex, race/ethnicity, HIV transmission behavior, insurance coverage, annual house-hold income, first year in care, median CD4 cell count, number of primary HIV visits, and calendar year.
Analyses were designed to ensure that the main exposure variable preceded the outcome of interest. Receipt of ART was assessed at the last outpatient visit in each calendar year. Consequently, all primary HIV visits occurred before or at the time of ART measurement. HIV viral suppression was assessed at the last annual HIV viral load test. We identified 75 patients, contributing 78 patient-years, who had a visit to a second clinic after their last HIV viral load test in the year. Since the exposure of receiving care at multiple clinics occurred after the outcome of HIV viral suppression for these patient-years, we removed them from the HIV viral suppression analysis. In addition, we excluded 338 patient-years with missing viral load data from the HIV viral suppression analysis: 318 patient-years (1.25 %) of persons attending one clinic and 20 patient-years (1.74 %) of those using multiple clinics in a year.
Because patients contributed data in multiple years, we used generalized estimating equations, clustered on patient, with exchangeable working correlation and robust standard errors to address the correlation across years for individual patients. Two-sided testing was used, with a P value of <0.05 considered significant. Analyses were conducted using STATA 12.1 (College Station, TX).
Results
Between 2008 and 2010, 12,759 HIV-infected patients were followed for a total of 26,574 patient-years (Table 1). Yearly sample size increased from 7,461 patients in 2008 to 9,960 patients in 2010. The percentage of patients who were 50 or older increased over time (from 28 to 34 %; P <0.01), as did the percentage of males (from 64 to 66 %; P <0.01). The proportion of blacks decreased but remained higher than whites and Hispanics (P <0.01). Similarly, the proportion of patients with heterosexual and IDU transmission risk decreased from 49 to 46 % (P <0.01) and 18–16 % (P <0.01), respectively. There was an increase in patients with private insurance (from 16 to 19 %; P < 0.01), with a corresponding decrease in those with Medicaid (from 67 to 64 %; P <0.01). The proportion of study participants in their first year of care decreased (from 18 to 13 %; P < 0.01). Use of ART increased (from 80 to 84 %; P < 0.01), with the percentage of patients with median CD4 count >350 cells/mm3 and those achieving HIV viral suppression increasing from 66 to 70 % (P < 0.01) and 62–75 % (P < 0.01), respectively.
Table 1.
Sample demographic and clinical characteristics by calendar year
| Characteristic | Calendar year |
P value* | ||
|---|---|---|---|---|
| 2008 N = 7,461 (%) | 2009 N = 9,153 (%) | 2010 N = 9,960 (%) | ||
| Age (years) | ||||
| 18–29 | 919 (12.32) | 1,164 (12.72) | 1,289 (12.94) | <0.01 |
| 30–39 | 1,532 (20.53) | 1,694 (18.51) | 1,721 (17.28) | |
| 40–49 | 2,900 (38.87) | 3,503 (38.27) | 3,605 (36.19) | |
| ≥50 | 2,110 (28.28) | 2,792 (30.50) | 3,345 (33.58) | |
| Sex | ||||
| Male | 4,742 (63.56) | 5,972 (65.25) | 6,590 (66.16) | <0.01 |
| Female | 2,719 (36.44) | 3,181 (34.75) | 3,370 (33.84) | |
| Race/ethnicity | ||||
| White | 1,161 (15.56) | 1,625 (17.75) | 1,887 (18.95) | <0.01 |
| Black | 5,001 (67.03) | 6,030 (65.88) | 6,347 (63.72) | |
| Hispanic | 1,033 (13.85) | 1,197 (13.08) | 1,341 (13.46) | |
| Other/unknown | 266 (3.57) | 301 (3.29) | 385 (3.87) | |
| HIV risk factor | ||||
| Heterosexual | 3,679 (49.31) | 4,312 (47.11) | 4,624 (46.43) | <0.01 |
| MSM | 2,106 (28.23) | 2,867 (31.32) | 3,238 (32.51) | |
| IDU | 1,345 (18.03) | 1,526 (16.67) | 1,605 (16.11) | |
| Other/unknown | 331 (4.44) | 448 (4.89) | 493 (4.95) | |
| Insurance | ||||
| Private | 1,112 (14.90) | 1,542 (16.85) | 1,807 (18.14) | <0.01 |
| Medicaid | 3,895 (52.20) | 4,571 (49.94) | 4,931 (49.51) | |
| Medicare | 962 (12.89) | 1,331 (14.54) | 1,539 (15.45) | |
| Ryan White/uninsured | 1,353 (18.13) | 1,593 (17.40) | 1,586 (15.92) | |
| Other/unknown | 139 (1.86) | 116 (1.27) | 97 (0.97) | |
| Income ($) | ||||
| <10,000 | 5,453 (73.09) | 5,876 (64.20) | 6,020 (60.44) | <0.01 |
| 10,000–19,999 | 1,282 (17.18) | 1,962 (21.44) | 2,332 (23.41) | |
| 20,000–49,999 | 636 (8.52) | 1,108 (12.11) | 1,298 (13.03) | |
| ≥50,000 | 90 (1.21) | 207 (2.26) | 310 (3.11) | |
| Median CD4 cell count (cell/mm3) | ||||
| ≤350 | 2,645 (35.45) | 3,086 (33.72) | 3,016 (30.28) | <0.01 |
| >350 | 4,816 (64.55) | 6,067 (66.28) | 6,944 (69.72) | |
| Use of ART | ||||
| No | 1,524 (20.43) | 1,593 (17.40) | 1,600 (16.06) | <0.01 |
| Yes | 5,937 (79.57) | 7,560 (82.60) | 8,360 (83.94) | |
| Last HIV RNA in year (copies/mL) | ||||
| ≤200 | 2,712 (36.35) | 2,756 (30.11) | 2,454 (24.64) | <0.01 |
| >200 | 4,603 (61.69) | 6,286 (68.68) | 7,425 (74.55) | |
| Missing | 146 (1.96) | 111 (1.21) | 81 (0.81) | |
| First year in care | ||||
| No | 6,135 (82.23) | 7,752 (84.69) | 8,685 (87.20) | <0.01 |
| Yes | 1,326 (17.77) | 1,401 (15.31) | 1,275 (12.80) | |
| No. of primary HIV visits | ||||
| 2 | 1,105 (14.81) | 1,359 (14.85) | 1,552 (15.58) | <0.01 |
| 3 | 1,141 (15.29) | 1,602 (17.50) | 1,766 (17.73) | |
| 4 | 1,216 (16.30) | 1,481 (16.18) | 1,635 (16.42) | |
| 5 | 925 (12.40) | 1,187 (12.97) | 1,268 (12.73) | |
| 6 | 734 (9.84) | 942 (10.29) | 969 (9.73) | |
| 7 | 591 (7.92) | 641 (7.00) | 690 (6.93) | |
| ≥8 | 1,749 (23.44) | 1,941 (21.21) | 2,080 (20.88) | |
ART antiretroviral therapy, HET heterosexual transmission, HIV human immunodeficiency virus, IDU injection drug use, MSM men who have sex with men
Statistical comparisons of demographic and clinical characteristics of the sample across calendar years were made using the χ2 test for trend
Over all 3 years, 986 patients (8 % of 12,749) received care at more than one HIV clinic. The number of patients using multiple HIV clinics in a year fluctuated over the study period, with 328 patients visiting multiple clinics in 2008, 428 in 2009, and 393 in 2010 (χ2 test of independence =6.28, P = 0.04). This resulted in a total of 1,149 patient-years with attendance at more than one HIV clinic in a year; 49 % of these patient-years were a one-time visit to a second clinic, 36 % were transfers in care, and 15 % represented a pattern of alternating visits between multiple clinics. Of the 422 patient-years where transfers in care occurred, 33 (8 %) represented a transition from a pediatric/adolescent to an adult HIV clinic. Patients with an alternating clinic utilization pattern were significantly more likely to have IDU as an HIV risk factor, had a greater number of primary HIV visits per year, and were less often new to care (i.e. in their first year of care), compared to those with other clinic utilization patterns. In addition, although they more commonly received ART, they were less likely to achieve viral suppression (Table 2).
Table 2.
Demographic and clinical characteristics for patients receiving care at multiple clinics by clinic pattern
| Characteristic | Clinic pattern |
P value* | ||
|---|---|---|---|---|
| One-time visit N = 561 PY (%) |
Alternating visits N = 171 PY (%) |
Transfers in care N = 417 PY (%) |
||
| Age (years) | ||||
| 18–29 | 118 (21.03) | 28 (16.37) | 91 (21.82) | 0.35 |
| 30–39 | 113 (20.14) | 42 (24.56) | 82 (19.66) | |
| 40–49 | 200 (35.65) | 51 (29.82) | 145 (34.77) | |
| ≥50 | 130 (23.17) | 50 (29.24) | 99 (23.74) | |
| Sex | ||||
| Male | 324 (57.75) | 95 (55.56) | 247 (59.23) | 0.71 |
| Female | 237 (42.25) | 76 (44.44) | 170 (40.77) | |
| Race/ethnicity | ||||
| White | 63 (11.23) | 23 (13.45) | 47 (11.27) | 0.34 |
| Black | 403 (71.84) | 109 (63.74) | 301 (72.18) | |
| Hispanic | 69 (12.30) | 32 (18.71) | 54 (12.95) | |
| Other/unknown | 26 (4.63) | 7 (4.09) | 15 (3.60) | |
| HIV risk factor | ||||
| Heterosexual | 303 (54.01) | 76 (44.44) | 205 (49.16) | <0.01 |
| MSM | 128 (22.82) | 39 (22.81) | 119 (28.54) | |
| IDU | 85 (15.15) | 47 (27.49) | 68 (16.31) | |
| Other/unknown | 45 (8.02) | 9 (5.26) | 25 (6.00) | |
| Insurance | ||||
| Private | 48 (8.56) | 13 (7.60) | 31 (7.43) | 0.93 |
| Medicaid | 336 (59.89) | 101 (59.06) | 260 (62.35) | |
| Medicare | 64 (11.41) | 22 (12.87) | 51 (12.23) | |
| Ryan white/uninsured | 99 (17.65) | 28 (16.37) | 63 (15.11) | |
| Other/unknown | 14 (2.50) | 7 (4.09) | 12 (2.88) | |
| Income ($) | ||||
| <10,000 | 393 (70.05) | 115 (67.25) | 296 (70.98) | 0.56 |
| 10,000–19,999 | 124 (22.10) | 39 (22.81) | 94 (22.54) | |
| 20,000–49,999 | 39 (6.95) | 17 (9.94) | 25 (6.00) | |
| ≥50,000 | 5 (0.89) | 0 (0.00) | 2 (0.48) | |
| Median CD4 cell count (cell/mm3) | ||||
| ≤350 | 233 (41.53) | 65 (38.01) | 150 (35.97) | 0.20 |
| >350 | 328 (58.47) | 106 (61.99) | 267 (64.03) | |
| Use of ART | ||||
| No | 194 (34.58) | 42 (24.56) | 125 (29.98) | 0.03 |
| Yes | 367 (65.42) | 129 (75.44) | 292 (70.02) | |
| Last HIV RNA in year (copies/mL) | ||||
| ≤200 | 258 (45.99) | 52 (30.41) | 152 (36.45) | <0.01 |
| >200 | 291 (51.87) | 118 (69.01) | 258 (61.87) | |
| Missing | 12 (2.14) | 1 (0.58) | 7 (1.68) | |
| First year in care | ||||
| No | 323 (57.58) | 112 (65.50) | 221 (53.00) | 0.02 |
| Yes | 238 (42.42) | 59 (34.50) | 196 (47.00) | |
| No. of primary HIV visits | ||||
| 2 | 66 (11.76) | 0 (0.00) | 0 (0.00) | <0.01 |
| 3 | 83 (14.80) | 0 (0.00) | 0 (0.00) | |
| 4 | 94 (16.76) | 11 (6.43) | 35 (8.39) | |
| 5 | 64 (11.41) | 6 (3.51) | 56 (13.43) | |
| 6 | 80 (14.26) | 14 (8.19) | 50 (11.99) | |
| 7 | 38 (6.77) | 23 (13.45) | 49 (11.75) | |
| ≥8 | 136 (24.24) | 117 (68.42) | 227 (54.44) | |
ART antiretroviral therapy, HET heterosexual transmission, HIV human immunodeficiency virus, IDU injection drug use, MSM men who have sex with men, PY patient-year
Statistical comparisons of demographic and clinical characteristics of those using multiple clinics by clinic pattern were made using the χ2 test of independence
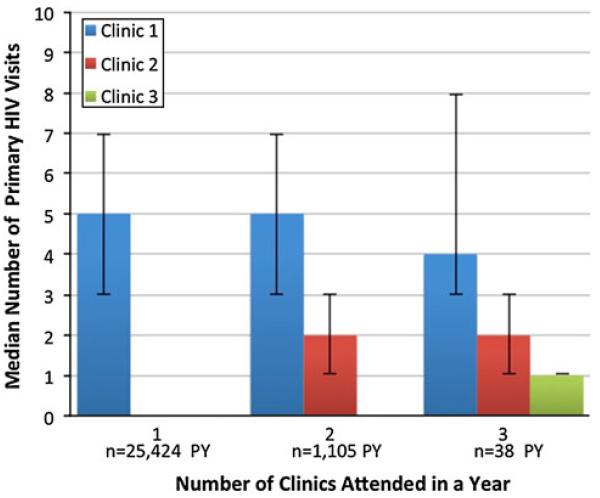
Figure 1 presents the relationship between the number of clinics attended and the number of visits made to each clinic in a year. Among PLWH attending multiple clinics, 96 % had visits to two clinics, 4 % had visits to three clinics, and less than 1 % had visits to 4–6 clinics. Patients who used two clinics in the year had a similar number of visits to their principal clinic as those who attended only one clinic in a year (median 5 vs. 5 visits, respectively, P = 0.47, Mann–Whitney test). Similarly, patients using three clinics were equally likely to visit their principal clinic as those who attended only one clinic in a year (median 4 vs. 5 visits, respectively, P = 0.52, Mann–Whitney test).
Fig. 1.
Relationship between number of primary HIV visits and number of clinics attended
We examined repeat use of multiple clinics during the study period. Of the 328 patients utilizing multiple clinics in 2008, 68 (21 %) continued to attend more than one clinic in 2009. Similarly, among the 428 patients accessing multiple sites of care in 2009, 84 (20 %) visited multiple clinics in 2010. In multivariate analysis, the likelihood of attending more than one clinic in a year was higher for women [adjusted odds ratio (AOR) =1.23, 95 % confidence interval (CI) 1.04–1.45], blacks (AOR = 1.35, 95 % CI 1.09–1.68), and those with Medicaid (AOR = 1.82, 95 % CI 1.49–2.36), Medicare (AOR = 1.62, 95 % CI 1.19–2.21), or no health insurance (AOR = 1.34, 95 % CI 1.01–1.78). Individuals in their first year of care had higher odds of using multiple clinics than those already engaged in care (AOR = 5.12, 95 % CI 4.48–5.86). Whereas, patients 40–49 years old (AOR = 0.76, 95 % CI 0.63–0.93) and those ≥50 years old (AOR = 0.66, 95 % CI 0.53-0.82) were less likely to attend multiple clinics than younger patients (Table 3).
Table 3.
Factors associated with use of multiple HIV clinics in a year
| Characteristic | No. of patients using 1 clinic N = 25,425 PY (%) |
No. of patients using ≥2 clinics N = 1,149 PY (%) |
AOR (95 % CI) |
|---|---|---|---|
| Age (years) | |||
| 18–29 | 3,135 (12.33) | 237 (20.63) | 1.00 (Ref) |
| 30–39 | 4,710 (18.53) | 237 (20.63) | 0.87 (0.70–1.08) |
| 40–49 | 9,612 (37.81) | 396 (34.46) | 0.76 (0.63–0.93) |
| ≥50 | 7,968 (31.34) | 279 (24.28) | 0.66 (0.53–0.82) |
| Sex | |||
| Male | 16,638 (65.44) | 666 (57.96) | 1.00 (Ref) |
| Female | 8,787 (34.56) | 483 (42.04) | 1.23 (1.04–1.45) |
| Race/ethnicity | |||
| White | 4,540 (17.86) | 133 (11.58) | 1.00 (Ref) |
| Black | 16,565 (65.15) | 813 (70.76) | 1.35 (1.09–1.68) |
| Hispanic | 3,416 (13.44) | 155 (13.49) | 1.21 (0.92–1.59) |
| Other/unknown | 904 (3.56) | 48 (4.18) | 1.29 (0.89–1.87) |
| HIV risk factor | |||
| Heterosexual | 12,031 (47.32) | 584 (50.83) | 1.00 (Ref) |
| MSM | 7,925 (31.17) | 286 (24.89) | 0.92 (0.75–1.13) |
| IDU | 4,276 (16.82) | 200 (17.41) | 1.13 (0.93–1.37) |
| Other/unknown | 1,193 (4.69) | 79 (6.88) | 1.20 (0.91–1.58) |
| Insurance | |||
| Private | 4,369 (17.18) | 92 (8.01) | 1.00 (Ref) |
| Medicaid | 12,700 (49.95) | 697 (60.66) | 1.82 (1.40–2.36) |
| Medicare | 3,695 (14.53) | 137 (11.92) | 1.62 (1.19–2.21) |
| Ryan white/uninsured | 4,342 (17.08) | 190 (16.54) | 1.34 (1.01–1.78) |
| Other/unknown | 319 (1.25) | 33 (2.87) | 2.80 (1.78–4.41) |
| Income ($) | |||
| <10,000 | 16,545 (65.07) | 804 (69.97) | 1.00 (Ref) |
| 10,000–19,999 | 5,319 (20.92) | 257 (22.37) | 1.08 (0.92–1.27) |
| 20,000–49,999 | 2,961 (11.65) | 81 (7.05) | 0.85 (0.65–1.12) |
| ≥50,000 | 600 (2.36) | 7 (0.61) | 0.56 (0.25–1.26) |
| Median CD4 cell count (cell/mm3) | |||
| ≤350 | 8,299 (32.64) | 448 (38.99) | 1.00 (Ref) |
| >350 | 17,126 (67.36) | 701 (61.01) | 1.01 (0.89–1.15) |
| First year in care | |||
| No | 21,916 (86.20) | 656 (57.09) | 1.00 (Ref) |
| Yes | 3,509 (13.80) | 493 (42.91) | 5.12 (4.48–5.86) |
| No. of primary HIV visits per year | |||
| 2 | 3,950 (15.54) | 66 (5.74) | 1.00 (Ref) |
| 3 | 4,426 (17.41) | 83 (7.22) | 1.30 (0.93–1.80) |
| 4 | 4,192 (16.49) | 140 (12.18) | 2.46 (1.82–3.33) |
| 5 | 3,254 (12.80) | 126 (10.97) | 2.85 (2.10–3.87) |
| 6 | 2,501 (9.84) | 144 (12.53) | 4.05 (2.99–5.49) |
| 7 | 1,812 (7.13) | 110 (9.57) | 4.08 (2.96–5.62) |
| ≥8 | 5,290 (20.81) | 480 (41.78) | 6.53 (4.98–8.58) |
| Year | |||
| 2008 | 7,133 (28.06) | 328 (28.55) | 1.00 (Ref) |
| 2009 | 8,725 (34.32) | 428 (37.25) | 1.20 (1.04–1.38) |
| 2010 | 9,567 (37.63) | 393 (34.20) | 1.11 (0.95–1.28) |
AOR adjusted odds ratio, ART antiretroviral therapy, HET heterosexual transmission, HIV human immunodeficiency virus, IDU injection drug use, MSM men who have sex with men, PY patient-year
Over the study period, 69 % of patients seeking care at multiple clinics received ART, with 68 % suppressing HIV viral load ≤200 copies/mL. Comparably, 83 % of patients in care at a single clinic were on ART, with 78 % achieving virologic suppression. Table 4 reports multivariate associations between patient factors and the two out-come measures. Adjusting for demographic and clinical factors, patients receiving care at multiple HIV clinics were less likely to use ART (AOR = 0.62, 95 % CI 0.55–0.71) and achieve HIV viral suppression (AOR = 0.78, 95 % CI 0.66–0.94) compared to individuals with only one care site. Analyses stratified by first year in care demonstrated similar results (Appendix Tables 5, 6). In a secondary analysis restricted to only PLWH with a strong indication to receive therapy (CD4 cell count ≤350 cells/mm3), the likelihood of using ART continued to be lower for patients attending multiple clinics (AOR = 0.40, 95 % CI 0.32–0.51).
Table 4.
Factors associated with use of ART and HIV virologic suppression
| Characteristic | Use of ART AOR (95 % CI) |
HIV viral suppression AOR (95 % CI) |
|---|---|---|
| No. of clinics visited per year | ||
| 1 | 1.00 (Ref) | 1.00 (Ref) |
| ≥2 | 0.62 (0.55–0.71) | 0.78 (0.66–0.94) |
| Age (years) | ||
| 18–29 | 1.00 (Ref) | 1.00 (Ref) |
| 30–39 | 1.67 (1.48–1.88) | 1.53 (1.33–1.76) |
| 40–49 | 2.44 (2.18–2.73) | 1.85 (1.63–2.11) |
| ≥50 | 2.80 (2.47–3.18) | 2.64 (2.30–3.04) |
| Sex | ||
| Male | 1.00 (Ref) | 1.00 (Ref) |
| Female | 0.86 (0.77–0.95) | 0.85 (0.77–0.94) |
| Race/ethnicity | ||
| White | 1.00 (Ref) | 1.00 (Ref) |
| Black | 0.97 (0.87–1.09) | 0.69 (0.61–0.78) |
| Hispanic | 1.07 (0.92–1.24) | 0.96 (0.82–1.12) |
| Other/unknown | 0.75 (0.62–0.91) | 0.92 (0.73–1.16) |
| HIV risk factor | ||
| Heterosexual | 1.00 (Ref) | 1.00 (Ref) |
| MSM | 0.97 (0.86–1.08) | 1.23 (1.10–1.38) |
| IDU | 0.92 (0.81–1.04) | 0.81 (0.73–0.91) |
| Other/unknown | 0.78 (0.66–0.92) | 0.97 (0.80–1.16) |
| Insurance | ||
| Private | 1.00 (Ref) | 1.00 (Ref) |
| Medicaid | 1.10 (0.98–1.23) | 0.69 (0.61–0.79) |
| Medicare | 1.27 (1.10–1.47) | 0.73 (0.63–0.85) |
| Ryan white/uninsured | 0.73 (0.65–0.83) | 0.67 (0.58–0.78) |
| Other/unknown | 0.60 (0.48–0.76) | 0.71 (0.50–1.01) |
| Income ($) | ||
| <10,000 | 1.00 (Ref) | 1.00 (Ref) |
| 10,000–19,999 | 1.06 (0.97–1.16) | 1.08 (0.98–1.18) |
| 20,000–49,999 | 0.99 (0.88–1.12) | 1.34 (1.15–1.55) |
| ≥50,000 | 1.32 (1.00–1.73) | 1.40 (1.01–1.93) |
| Median CD4 cell count (cell/mm3) | ||
| ≤350 | 1.00 (Ref) | 1.00 (Ref) |
| >350 | 0.58 (0.53–0.63) | 4.30 (3.98–4.64) |
| First year in care | ||
| No | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 0.39 (0.36–0.42) | 0.77 (0.70–0.85) |
| No. of primary HIV visits per year | ||
| 2 | 1.00 (Ref) | 1.00 (Ref) |
| 3 | 1.20 (1.10–1.32) | 1.13 (1.00–1.27) |
| 4 | 1.50 (1.36–1.65) | 1.30 (1.15–1.47) |
| 5 | 1.62 (1.46–1.80) | 1.46 (1.28–1.66) |
| 6 | 1.62 (1.45–1.82) | 1.57 (1.36–1.80) |
| 7 | 2.10 (1.83–2.40) | 1.61 (1.38–1.87) |
| ≥8 | 1.95 (1.76–2.16) | 1.51 (1.35–1.70) |
| Year | ||
| 2008 | 1.00 (Ref) | 1.00 (Ref) |
| 2009 | 1.23 (1.15–1.30) | 1.25 (1.16–1.35) |
| 2010 | 1.38 (1.29–1.47) | 1.48 (1.37–1.60) |
AOR adjusted odds ratio, ART antiretroviral therapy, HET heterosexual transmission, HIV human immunodeficiency virus, IDU injection drug use, MSM men who have sex with men
Discussion
In this study of HIV-infected adults seeking care at multiple clinics, 8 % of patients attended more than one clinic between 2008 and 2010. While this represents a minority of patients, this group is of particular interest to HIV providers and public health officials. For providers, it is critical to document care received at other locations, as this can lead to ART medication errors and unrecognized drug–drug interactions, resulting in harmful side effects and development of drug resistance [31, 32]. On the public health level, receiving care at multiple clinics can lead to duplicative and unnecessary services, resulting in higher health care costs [33–37]. In addition, attending multiple clinics impacts how public health departments track and monitor the care of PLWH. Current measures of retention in care are based solely on primary HIV visits and do not distinguish visits completed at different clinic [15, 16]. As such, a patient may have one visit to two separate clinics in a 12-month period and be considered “retained in care” by national standards. Our observation that approximately one in ten PLWH receives care at multiple clinics should serve as the basis for a large discussion on how best to assess continuity and retention in care—two related, but distinct processes.
Patients seen at multiple sites primarily made a one-time visit to a second clinic (47 %), with fewer patients changing sites of care (36 %) or alternating between multiple clinics (15 %). Yet, this pattern of multiple clinic use continued year to year for one-fifth of patients and was significantly associated with lower use of ART and lack of virologic suppression. There are several possible explanations for these findings. Comorbidity, difficulty accepting HIV diagnosis and coping with stigma, the need to access clinical and social services unavailable at their primary clinic, and searching for patient-provider concordance may be reasons for patients visiting multiple clinics, while lack of provider continuity, difficulty managing HIV and other comorbid diseases, and conspiracy beliefs regarding medical recommendations may contribute to worse outcomes.
Prior studies have described an association between comorbidity and outpatient utilization [38–41]. Among 13,806 adults with chronic conditions, patients with three or more comorbid diseases were more likely to use out patient services compared to those with a single chronic disease [38]. Data on comorbid diseases were not consistently collected from all sites in our study; as such, we were unable to investigate the impact of comorbidity on primary HIV care utilization. Future studies should evaluate how mental illness, substance abuse, and other comorbid conditions influence use of multiple clinics. Denial of HIV diagnosis, belief that one is too healthy, and inability to cope with HIV stigma and disclosure may also lead to use of multiple clinics, particularly for younger patients and those in their first year of care [42, 43].
Patients may deliberately visit multiple sites of care to access clinical and social services unavailable onsite at their primary clinic. This can include treatment for hepatitis C virus (HCV) infection and opioid dependence, as well as nutrition counseling, food services, housing and transportation assistance, and medical case management [44]. Individuals with Medicaid and Medicare, who may use certain social services more frequently than those with private insurance, were more likely to visit multiple clinics in our cohort. The patient-centered medical home model, which seeks to provide high-quality, comprehensive, patient centered care that is both accessible and coordinated, may help reduce the use of multiple sites of care [45].
In addition, searching for patient-provider concordance may lead to individuals attending multiple clinics. Patient-provider concordance, defined as shared identities (e.g. gender, social class, race, ethnicity, language, sexual orientation, beliefs about health and illness) between patients and providers, is associated with increased patient satisfaction and improved health outcomes [46–48]. Among 1,241 adults receiving HIV care from 287 providers, racial concordance between patients and providers resulted in earlier receipt of ART [48]. Similarly, high levels of patient-provider concordance in HIV treatment decision-making was associated with greater adherence to therapy, improved quality of life, and higher CD4 counts [47]. Searching for providers more like them may partly explain why blacks, women, and those in their first year of care were more likely to visit multiple clinics compared to their counterparts.
Lastly, visiting multiple clinics may be a marker of dissatisfaction with the clinic, provider and health system distrust, and doctor shopping (visiting multiple providers to obtain opioid prescriptions) [49-52]. However, most patients using multiple sites of care only made a one-time visit to a second clinic and less than 3 % of the total sample switched primary clinics. These results suggest that dissatisfaction with the clinic, provider and health system distrust, and doctor shopping were not the primary drivers of clinic utilization.
In our cohort, patients who used more than one clinic had a similar number of visits to their principal clinic as those receiving care at a single site. This observation identifies a group of patients who are high users of health care (‘super-utilizers’). Prior studies have documented that super-utilizers have less social supports, greater numbers of medical and psychological diseases, and lower self-perceived health status than regular users of care [53–56]. In addition, this group is more likely to be unemployed and/or on disability [55, 57]. Providers should be aware that regular receipt of care at one site does not preclude the use of care services at other locations, and that use of multiple care sites may be a marker of other conditions. Asking patients where else they receive their health care maybe a simple screening tool that providers can use to identify this population.
Lack of provider continuity may contribute to worse outcomes for patients using multiple HIV clinics [4–7]. In a double-blinded randomized trial evaluating the impact of outpatient provider continuity on patient satisfaction and health service utilization, patients randomized to the dis-continuity group (i.e. had follow-up visits scheduled with differing providers in the same health system) were more likely to be admitted to the hospital, had longer length of stays, and were less satisfied with their medical care [6]. Gaps in healthcare continuity may be particularly important for patients switching providers and those who alternate between multiple clinics.
Patients’ ability to self-care and manage their HIV infection and other comorbid diseases is critical for medication adherence, implementation of healthy lifestyle behaviors, and ongoing problem-solving to overcome potential barriers to care [58–61]. Variations in self-management may have contributed to differences in use of ART and virologic suppression for patients using multiple sites of care compared to those attending a single clinic. Moreover, patients attending multiple clinics may be more suspicious of the healthcare system and HIV care in general than patients with one provider. These beliefs may negatively influence their acceptance of ART and treatment adherence [62, 63].
The current analysis has several limitations. First, as a retrospective cohort study, we were unable to interview patients on the reasons for visiting multiple HIV clinics. Second, this study involved patients receiving care at urban clinics in Philadelphia, potentially limiting the generalizability of results to rural or suburban settings. Third, it is possible that patients received care at clinics outside of the Philadelphia Ryan White system. As such, the proportion of patients utilizing multiple HIV clinics may be higher than reported. Lastly, this study did not collect data on potentially relevant factors that may influence the use of multiple HIV clinics, including number of comorbid diseases, prescription of opioids, and type of clinical and social services used by patients. Future studies should investigate how these factors influence utilization of multiple HIV clinics.
This study reports outcome for patients using multiple clinics for primary HIV care. Overall, 8 % of patients used more than one HIV clinic; these individuals were significantly less likely to use ART and to achieve virologic suppression. Qualitative data are needed to better understand the reasons for visiting multiple HIV clinics. Patients attending multiple clinics were equally likely to visit their principle clinic. Providers and clinic administrators should be aware that regular use of outpatient services does not preclude utilization of other sites for care.
Acknowledgments
We are grateful to all the patients, physicians, investigators, and staff involved in the Philadelphia Ryan White System. We would like to acknowledge the staff of the Philadelphia Department of Health AIDS Activities Coordinating Office including Jane Baker, Coleman Terrell, Marlene Matosky, and Ethan Schofer. The study was supported by the Penn Center for AIDS Research (P30 AI 045008). BRY and JPM were supported by the National Institutes of Health (K23-MH097647-01A and K24-AI073957-05, respectively). SCK was supported by the Agency for Healthcare Research and Quality (400-4239-4-555854-2446-2192).
Appendix
Table 5.
Factors associated with use of ART stratified by first year in care
| Characteristic | Use of ART AOR (95 % CI) |
|
|---|---|---|
| First year in care | Not in first year | |
| No. of clinics visited per year | ||
| 1 | 1.00 (Ref) | 1.00 (Ref) |
| ≥2 | 0.64 (0.52–0.80) | 0.57 (0.48–0.68) |
| Age (years) | ||
| 18–29 | 1.00 (Ref) | 1.00 (Ref) |
| 30–39 | 1.34 (1.09–1.64) | 1.83 (1.59–2.11) |
| 40–49 | 1.91 (1.57–2.31) | 2.65 (2.32–3.03) |
| ≥50 | 1.93 (1.55–2.41) | 3.19 (2.75–3.70) |
| Sex | ||
| Male | 1.00 (Ref) | 1.00 (Ref) |
| Female | 0.92 (0.77–1.10) | 0.80 (0.71–0.90) |
| Race/ethnicity | ||
| White | 1.00 (Ref) | 1.00 (Ref) |
| Black | 0.91 (0.74–1.11) | 0.98 (0.86–1.12) |
| Hispanic | 0.96 (0.73–1.25) | 1.14 (0.96–1.37) |
| Other/unknown | 0.62 (0.44–0.89) | 0.83 (0.67–1.05) |
| HIV risk factor | ||
| Heterosexual | 1.00 (Ref) | 1.00 (Ref) |
| MSM | 0.87 (0.71–1.06) | 0.94 (0.82–1.08) |
| IDU | 0.86 (0.68–1.09) | 0.90 (0.78–1.04) |
| Other/unknown | 0.69 (0.52–0.91) | 0.83 (0.68–1.02) |
| Insurance | ||
| Private | 1.00 (Ref) | 1.00 (Ref) |
| Medicaid | 1.22 (0.97–1.54) | 1.07 (0.94–1.22) |
| Medicare | 1.67 (1.21–2.30) | 1.19 (1.01–1.40) |
| Ryan white/uninsured | 0.63 (0.50–0.80) | 0.77 (0.67–0.89) |
| Other/unknown | 0.92 (0.60–1.42) | 0.49 (0.37–0.67) |
| Income ($) | ||
| <10,000 | 1.00 (Ref) | 1.00 (Ref) |
| 10,000–19,999 | 1.01 (0.85–1.21) | 1.08 (0.97–1.20) |
| 20,000–49,999 | 0.87 (0.67–1.13) | 1.04 (0.90–1.20) |
| ≥50,000 | 1.09 (0.61–1.93) | 1.30 (0.95–1.77) |
| Median CD4 cell count (cell/mm3) | ||
| ≤350 | 1.00 (Ref) | 1.00 (Ref) |
| >350 | 0.29 (0.25–0.33) | 0.67 (0.61–0.73) |
| No. of primary HIV visits per year | ||
| 2 | 1.00 (Ref) | 1.00 (Ref) |
| 3 | 1.25 (1.00–1.55) | 1.17 (1.05–1.30) |
| 4 | 1.87 (1.48–2.36) | 1.38 (1.23–1.55) |
| 5 | 2.32 (1.80–2.99) | 1.46 (1.29–1.66) |
| 6 | 2.38 (1.82–3.12) | 1.43 (1.25–1.64) |
| 7 | 3.69 (2.67–5.10) | 1.78 (1.53–2.08) |
| ≥ 8 | 3.13 (2.48–3.95) | 1.69 (1.50–1.90) |
| Year | ||
| 2008 | 1.00 (Ref) | 1.00 (Ref) |
| 2009 | 1.24 (1.04–1.47) | 1.25 (1.16–1.34) |
| 2010 | 1.15 (0.97–1.37) | 1.47 (1.36–1.58) |
AOR adjusted odds ratio, ART antiretroviral therapy, HET heterosexual transmission, HIV human immunodeficiency virus, IDU injection drug use, MSM men who have sex with men
Table 6.
Factors associated with HIV viral suppression stratified by first year in care
| Characteristic | HIV viral suppression AOR (95 % CI) |
|
|---|---|---|
| First year in care | Not in first year | |
| No. of clinics visited per year | ||
| 1 | 1.00 (Ref) | 1.00 (Ref) |
| ≥2 | 0.68 (0.50–0.91) | 0.76 (0.61–0.96) |
| Age (years) | ||
| 18–29 | 1.00 (Ref) | 1.00 (Ref) |
| 30–39 | 1.42 (1.07–1.90) | 1.58 (1.35–1.85) |
| 40–49 | 1.61 (1.23–2.10) | 1.95 (1.68–2.25) |
| ≥50 | 2.03 (1.50–2.74) | 2.82 (2.41–3.29) |
| Sex | ||
| Male | 1.00 (Ref) | 1.00 (Ref) |
| Female | 1.12 (0.90–1.41) | 0.81 (0.73–0.90) |
| Race/ethnicity | ||
| White | 1.00 (Ref) | 1.00 (Ref) |
| Black | 0.76 (0.59–0.99) | 0.68 (0.59–0.77) |
| Hispanic | 1.19 (0.82–1.71) | 0.93 (0.78–1.10) |
| Other/unknown | 0.79 (0.48–1.31) | 0.98 (0.76–1.27) |
| HIV risk factor | ||
| Heterosexual | 1.00 (Ref) | 1.00 (Ref) |
| MSM | 1.48 (1.14–1.92) | 1.18 (1.04–1.34) |
| IDU | 1.10 (0.82–1.47) | 0.77 (0.69–0.87) |
| Other/unknown | 1.13 (0.76–1.67) | 0.96 (0.78–1.19) |
| Insurance | ||
| Private | 1.00 (Ref) | 1.00 (Ref) |
| Medicaid | 0.70 (0.50–0.96) | 0.70 (0.60–0.80) |
| Medicare | 0.64 (0.43–0.97) | 0.75 (0.63–0.88) |
| Ryan white/uninsured | 0.56 (0.40–0.79) | 0.67 (0.59–0.82) |
| Other/unknown | 0.77 (0.42–1.43) | 0.71 (0.46–1.11) |
| Income ($) | ||
| <10,000 | 1.00 (Ref) | 1.00 (Ref) |
| 10,000–19,999 | 1.07 (0.85–1.35) | 1.08 (0.97–1.20) |
| 20,000–49,999 | 1.13 (0.78–1.63) | 1.36 (1.16–1.60) |
| ≥50,000 | 1.30 (0.59–2.90) | 1.36 (0.96–1.92) |
| Median CD4 cell count (cell/mm3) | ||
| ≤350 | 1.00 (Ref) | 1.00 (Ref) |
| >350 | 3.46 (2.84–4.21) | 4.49 (4.14–4.87) |
| No. of primary HIV visits per year | ||
| 2 | 1.00 (Ref) | 1.00 (Ref) |
| 3 | 1.06 (0.77–1.46) | 1.11 (0.97–1.26) |
| 4 | 1.79 (1.29–2.49) | 1.21 (1.06–1.38) |
| 5 | 1.66 (1.19–2.32) | 1.43 (1.24–1.65) |
| 6 | 2.12 (1.48–3.02) | 1.46 (1.25–1.71) |
| 7 | 2.87 (1.94–4.24) | 1.42 (1.20–1.68) |
| ≥8 | 2.89 (2.12–3.93) | 1.34 (1.18–1.52) |
| Year | ||
| 2008 | 1.00 (Ref) | 1.00 (Ref) |
| 2009 | 1.37 (1.10–1.71) | 1.23 (1.13–1.33) |
| 2010 | 1.51 (1.20–1.89) | 1.44 (1.33–1.57) |
AOR adjusted odds ratio, ART antiretroviral therapy, HET heterosexual transmission, HIV human immunodeficiency virus, IDU injection drug use, MSM men who have sex with men
Contributor Information
Baligh R. Yehia, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 1021 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104, USA; Philadelphia Veterans Affairs Center for Health Equity Research and Promotion, Philadelphia, PA, USA
Asher J. Schranz, Department of Medicine, New York University, New York, NY, USA
Florence Momplaisir, Department of Medicine, Temple University School of, Medicine, Philadelphia, PA, USA.
Sara C. Keller, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 1021 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104, USA
Robert Gross, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 1021 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104, USA; Philadelphia Veterans Affairs Center for Health Equity Research and Promotion, Philadelphia, PA, USA; Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Ian Frank, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 1021 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104, USA.
Joshua P. Metlay, General Medicine Division, Massachusetts General Hospital, Boston, MA, USA
Kathleen A. Brady, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 1021 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104, USA
References
- 1.Nutting PA, Goodwin MA, Flocke SA, Zyzanski SJ, Stange KC. Continuity of primary care: to whom does it matter and when? Ann Fam Med. 2003;1(3):149–55. doi: 10.1370/afm.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney KG, Gray DP. Patients who do not receive continuity of care from their general practitioner-are they a vulnerable group? Br J Gen Pract. 1995;45(392):133–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Primary Care: America’s Health in a New Era. National Academy Press; Washington DC: 1996. [Google Scholar]
- 4.Fletcher RH, O’Malley MS, Fletcher SW, Earp JA, Alexander JP. Measuring the continuity and coordination of medical care in a system involving multiple providers. Med Care. 1984;22(5):403–11. doi: 10.1097/00005650-198405000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sundararajan V, Konrad TR, Garrett J, Carey T. Patterns and determinants of multiple provider use in patients with acute low back pain. J Gen Intern Med. 1998;13(8):528–33. doi: 10.1046/j.1525-1497.1998.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasson JH, Sauvigne AE, Mogielnicki RP, Frey WG, Sox CH, Gaudette C, et al. Continuity of outpatient medical care in elderly men. A randomized trial. JAMA. 1984;252(17):2413–7. [PubMed] [Google Scholar]
- 7.Parchman ML, Pugh JA, Noel PH, Larme AC. Continuity of care, self-management behaviors, and glucose control in patients with type 2 diabetes. Med Care. 2002;40(2):137–44. doi: 10.1097/00005650-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23(1):41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg MB, Safren SA, Mimiaga MJ, Grasso C, Boswell S, Mayer KH. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS Care. 2005;17(7):902–7. doi: 10.1080/09540120500101658. [DOI] [PubMed] [Google Scholar]
- 10.Giordano TP, White AC, Jr, Sajja P, Graviss EA, Arduino RC, Adu-Oppong A, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32(4):399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37(8):1112–8. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 12.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 13.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metsch LR, Pereyra M, Messinger S, Del Rio C, Strathdee SA, Anderson-Mahoney P, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis. 2008;47(4):577–84. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 15.Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, Berry SA, et al. Comparing different measures of retention in outpatient HIV care. AIDS. 2012;26(9):1131–9. doi: 10.1097/QAD.0b013e3283528afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60(3):249–59. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Signs Vital HIV Prevention Through Care and Treatment—United States. MMWR Morb Mortal Wkly Rep. 2011;2(60):1618–23. [PubMed] [Google Scholar]
- 18.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131(2):81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 19.Giordano TP, Visnegarwala F, White AC, Jr, Troisi CL, Fran-kowski RF, Hartman CM, et al. Patients referred to an urban HIV clinic frequently fail to establish care: factors predicting failure. AIDS Care. 2005;17(6):773–83. doi: 10.1080/09540120412331336652. [DOI] [PubMed] [Google Scholar]
- 20.Valenstein P, Leiken A, Lehmann C. Test-ordering by multiple physicians increases unnecessary laboratory examinations. Arch Pathol Lab Med. 1988;112(3):238–41. [PubMed] [Google Scholar]
- 21.Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother. 2007;5(1):31–9. doi: 10.1016/j.amjopharm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Elstad E, Carpenter DM, Devellis RF, Blalock SJ. Patient decision making in the face of conflicting medication information. Int J Qual Stud Health Well-being. 2012;7:1–11. doi: 10.3402/qhw.v7i0.18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter DM, DeVellis RF, Fisher EB, DeVellis BM, Hogan SL, Jordan JM. The effect of conflicting medication information and physician support on medication adherence for chronically ill patients. Patient Educ Couns. 2010;81(2):169–76. doi: 10.1016/j.pec.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21(6):661–5. doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apollo A, Golub SA, Wainberg ML, Indyk D. Patient-provider relationships, HIV, and adherence: requisites for a partnership. Soc Work Health Care. 2006;42(3-4):209–24. doi: 10.1300/J010v42n03_13. [DOI] [PubMed] [Google Scholar]
- 26.DiNenno E, Denning P. [Accessed 25 Mar 2013];Communities in crisis: is there a generalized HIV epidemic in impoverished Urban areas of the United States? 2013 http://www.cdc.gov/hiv/topics/surveillance/resources/other/poverty.htm.
- 27.Panel on Antiretroviral Guidelines for Adults and Adolescents [Accessed 25 Mar 2013];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013 http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 28.Giordano TP, White AC, Jr, Sajja P, Graviss EA, Arduino RC, Adu-Oppong A, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32(4):399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 30.Health Services and Resources Administration [Accessed 25 Mar 2013];HAB HIV core clinical performance measures for adult/adolescent clients: group 1 2008. 2013 http://hab.hrsa.gov/deliverhivaidscare/files/habgrp1pms08.pdf.
- 31.Yehia BR, Mehta JM, Ciuffetelli D, Moore RD, Pham PA, Metlay JP, et al. Antiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adults. Clin Infect Dis. 2012;55(4):593–9. doi: 10.1093/cid/cis491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLorenze GN, Follansbee SF, Nguyen DP, Klein DB, Horberg M, Quesenberry CP, Jr, et al. Medication error in the care of HIV/AIDS patients: electronic surveillance, confirmation, and adverse events. Med Care. 2005;43(9 Suppl):63–8. doi: 10.1097/01.mlr.0000175622.81335.4d. [DOI] [PubMed] [Google Scholar]
- 33.Lewis S, Foreman J. Low-cost diagnostic technologies and clinical outcomes. The impact of inappropriate utilization. International journal of technology assessment in health care. 1997;13(4):501–11. doi: 10.1017/s0266462300009971. Fall. [DOI] [PubMed] [Google Scholar]
- 34.van Walraven C, Naylor CD. Do we know what inappropriate laboratory utilization is? A systematic review of laboratory clinical audits. JAMA. 1998;280(6):550–8. doi: 10.1001/jama.280.6.550. [DOI] [PubMed] [Google Scholar]
- 35.May TA, Clancy M, Critchfield J, Ebeling F, Enriquez A, Gallagher C, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126(2):200–6. doi: 10.1309/WP59-YM73-L6CE-GX2F. [DOI] [PubMed] [Google Scholar]
- 36.Vegting IL, van Beneden M, Kramer MH, Thijs A, Kostense PJ, Nanayakkara PW. How to save costs by reducing unnecessary testing: lean thinking in clinical practice. Eur J Intern Med. 2012;23(1):70–5. doi: 10.1016/j.ejim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Kwok J, Jones B. Unnecessary repeat requesting of tests: an audit in a government hospital immunology laboratory. J Clin Pathol. 2005;58(5):457–62. doi: 10.1136/jcp.2004.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westert GP, Satariano WA, Schellevis FG, van den Bos GA. Patterns of comorbidity and the use of health services in the Dutch population. Eur J Public Health. 2001;11(4):365–72. doi: 10.1093/eurpub/11.4.365. [DOI] [PubMed] [Google Scholar]
- 39.Himelhoch S, Weller WE, Wu AW, Anderson GF, Cooper LA. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Med Care. 2004;42(6):512–21. doi: 10.1097/01.mlr.0000127998.89246.ef. [DOI] [PubMed] [Google Scholar]
- 40.Broemeling AM, Watson DE, Prebtani F. Population patterns of chronic health conditions, co-morbidity and healthcare use in Canada: implications for policy and practice. Healthc Q. 2008;11(3):70–6. doi: 10.12927/hcq.2008.19859. [DOI] [PubMed] [Google Scholar]
- 41.Struijs JN, Baan CA, Schellevis FG, Westert GP, van den Bos GA. Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv Res. 2006;6:84. doi: 10.1186/1472-6963-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajabiun S, Mallinson RK, McCoy K, Coleman S, Drainoni ML, Rebholz C, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDs. 2007;21(Suppl 1):S20–9. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 43.Tobias CR, Cunningham W, Cabral HD, Cunningham CO, Eldred L, Naar-King S, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDs. 2007;21(6):426–34. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 44.City of Philadelphia Department of Public Health AIDS Activities Coordinating Office [Accessed 20 Feb 2013];Program services Philadelphia. 2012 http://www.phila.gov/health/aaco/AACOProgramServices.html.
- 45.Berenson RA, Hammons T, Gans DN, Zuckerman S, Merrell K, Underwood WS, et al. A house is not a home: keeping patients at the center of practice redesign. Health Aff (Millwood) 2008;27(5):1219–30. doi: 10.1377/hlthaff.27.5.1219. [DOI] [PubMed] [Google Scholar]
- 46.Cooper L. Disparities in patient experiences, health care processes, and outcomes: the role of patient-provider racial, ethnic, and language concordance. The Commonwealth Fund; New York: 2004. [Google Scholar]
- 47.Clucas C, Harding R, Lampe FC, Anderson J, Date HL, Johnson M, et al. Doctor-patient concordance during HIV treatment switching decision-making. HIV Med. 2011;12(2):87–96. doi: 10.1111/j.1468-1293.2010.00851.x. [DOI] [PubMed] [Google Scholar]
- 48.King WD, Wong MD, Shapiro MF, Landon BE, Cunningham WE. Does racial concordance between HIV-positive patients and their physicians affect the time to receipt of protease inhibitors? J Gen Intern Med. 2004;19(11):1146–53. doi: 10.1111/j.1525-1497.2004.30443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman S, Boehmer U, Kanaya F, Grasso C, Tan J, Bradford J. Retention challenges for a community-based HIV primary care clinic and implications for intervention. AIDS Patient Care STDs. 2007;21(9):691–701. doi: 10.1089/apc.2006.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97(7):1283–9. doi: 10.2105/AJPH.2005.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall MA, Zheng B, Dugan E, Camacho F, Kidd KE, Mishra A, et al. Measuring patients’ trust in their primary care providers. Med Care Res Rev. 2002;59(3):293–318. doi: 10.1177/1077558702059003004. [DOI] [PubMed] [Google Scholar]
- 52.Peirce GL, Smith MJ, Abate MA, Halverson J. Doctor and pharmacy shopping for controlled substances. Med Care. 2012;50(6):494–500. doi: 10.1097/MLR.0b013e31824ebd81. [DOI] [PubMed] [Google Scholar]
- 53.Pearson SD, Katzelnick DJ, Simon GE, Manning WG, Helstad CP, Henk HJ. Depression among high utilizers of medical care. J Gen Intern Med. 1999;14(8):461–8. doi: 10.1046/j.1525-1497.1999.06278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lefevre F, Reifler D, Lee P, Sbenghe M, Nwadiaro N, Verma S, et al. Screening for undetected mental disorders in high utilizers of primary care services. J Gen Intern Med. 1999;14(7):425–31. doi: 10.1046/j.1525-1497.1999.07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savageau JA, McLoughlin M, Ursan A, Bai Y, Collins M, Cashman SB. Characteristics of frequent attenders at a community health center. J Am Board Fam Med. 2006;19(3):265–75. doi: 10.3122/jabfm.19.3.265. [DOI] [PubMed] [Google Scholar]
- 56.Neal RD, Heywood PL, Morley S. Frequent attenders’ consulting patterns with general practitioners. Br J Gen Pract. 2000;50(461):972–6. [PMC free article] [PubMed] [Google Scholar]
- 57.Scaife B, Gill P, Heywood P, Neal R. Socio-economic characteristics of adult frequent attenders in general practice: secondary analysis of data. Fam Pract. 2000;17(4):298–304. doi: 10.1093/fampra/17.4.298. [DOI] [PubMed] [Google Scholar]
- 58.Chou FY, Holzemer WL. Linking HIV/AIDS clients’ self-care with outcomes. J Assoc Nurses AIDS Care. 2004 Jul-Aug;15(4):58–67. doi: 10.1177/1055329003255592. [DOI] [PubMed] [Google Scholar]
- 59.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 60.Gifford AL, Groessl EJ. Chronic disease self-management and adherence to HIV medications. J Acquir Immune Defic Syndr. 2002;15(31 Suppl 3):S163–6. doi: 10.1097/00126334-200212153-00016. [DOI] [PubMed] [Google Scholar]
- 61.Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care. 2009;21(10):1321–34. doi: 10.1080/09540120902803158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaston GB, Alleyne-Green B. The Impact of African Americans’ Beliefs About HIV Medical Care on Treatment Adherence: a Systematic Review and Recommendations for Interventions. AIDS Behav. 2012;17(1):31–40. doi: 10.1007/s10461-012-0323-x. [DOI] [PubMed] [Google Scholar]
- 63.Bogart LM, Wagner G, Galvan FH, Banks D. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among african american men with HIV. J Acquir Immune Defic Syndr. 2010;53(5):648–55. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]