Abstract
Rationale
Most substance use is initiated during adolescence when substantial development of relevant brain circuitry is still rapidly maturing. Developmental differences in reward processing, behavioral flexibility, and self-regulation lead to changes in resilience or vulnerability to drugs of abuse depending on exposure to risk factors. Intervention and prevention approaches to reducing addiction in teens may be able to capitalize on malleable brain systems in a predictable manner.
Objective
To review what is known about how factors that increase vulnerability to addiction, including developmental stage, exposure to early life adversity (ranging from abuse, neglect, and bullying), drug exposure, and genetic predisposition, impact the development of relevant systems.
Results and Conclusions
Appropriate, early intervention may restore the normal course of an abnormal trajectory and reduce the likelihood of developing a substance use disorder (SUD) later in life. A considerable amount is known about the functional neuroanatomy and/or pharmacology of risky behaviors based on clinical and preclinical studies, but relatively little has been directly translated to reduce their impact on addiction in high-risk children or teenagers. An opportunity exists to effectively intervene before adolescence when substance use is likely to emerge.
Keywords: Development, substance use disorder, adolescence, delay-discounting, risk, prevention, self-regulation, behavioral flexibility, prefrontal cortex
Introduction
Adolescence is a period of life when drug use, whether for therapeutic or recreational purposes, is initiated. This period of significant structural and functional development is also when individuals are most likely to develop a substance use disorder (SUD; Wagner and Anthony 2002). The Substance Abuse and Mental Health Data Archive national sample is based on data collected from all 50 states plus the District of Columbia and includes n=70,109 respondents between five age ranges, including 12 to 17 year-olds. Based on the 2011 survey, reported alcohol use within the last 30 days rises from 1.4% of 12 year-olds, to 7.5% by 14 years, to 29.2% by 17 years of age. Marijuana use shows a similarly striking trend for use within the last 30 days: use was reported by 2.2% of teens 13 years of age and rises to 17.2% by 17 year of age. With new legislation that legalizes marijuana use for defined populations, the ensuing increased access will certainly lead to a rise in these trends.
The seriousness of teen SUD is best exemplified, however, by the fact that 64% of adult patients entering treatment for addiction started abusing drugs at age 20 or younger (Substance Abuse and Mental Health Services Administration, SAMHSA 2002). Moreover, the initiation of any drug use before age 14 leads to an approximately four-fold greater likelihood to abuse or become dependent later in life (SAMHSA 2006). Population statistics further show that specific drug class influences when teens begin to use: 50% of users started using inhalants at the age of 15 years, hallucinogens and stimulants at 17 years, and opiates at 18 years (Andersen and Teicher 2009). Consideration of the underlying mechanism of action of drug classes and their pre-pubertal development provides a window of opportunity to intervene by identifying sensitive periods of relative invulnerability. Sensitive periods are maturational stages when drugs have yet to attain any addictive effects – often during childhood and before adolescence (Andersen 2003). Refinement of optimal windows for intervention and identification of novel targets could be based on transient expression of neurotransmitters that occurs between early development and adolescence (Brenhouse and Andersen 2011a). The timing of this window of opportunity interacts with puberty (Waylen and Wolke 2004; Sinclair et al. this issue; Schramm-Sapyta et al. this issue). An earlier than usual age of puberty onset can influence the onset of SUD to an earlier age than typical (Westling et al. 2008). Strengthening the self-regulatory and reward systems during this window may be the optimal time before drugs gain their addictive potential (Figure 1). Knowledge about the neurobiology of predisposing risk factors and sensitive periods could be used to identify those vulnerable to develop an SUD and intervene early to prevent addiction.
Fig. 1. Adolescent substance abuse and its prevention through the course of developmental risk.
Normal developmental trajectory (solid line) for risk of cocaine dependence is depicted based on the work of O’Brien and Anthony (2005). Risk for developing a lifelong-dependence declines with age. A child's immature reward system prevents positive drug experiences, resulting in window of SUD prevention. By managing risk factors, facilitating better judgment in teens, and recognizing co-occurring disorders and mental health issues, a preventative trajectory may be achievable (dotted line).
To this end, a number of approaches are used to prevent and intervene in teen SUD (Figure 2). This review will briefly discuss clinical approaches and then bridge the gap between discrepancies about knowledge of addiction, its risk factors, and how preclinical and clinical/human studies in immature systems may be used to better inform prevention models. A major emphasis will be placed on the neurobiological underpinnings that lead to adolescent SUD when the transition between low-to-high risk occurs.
Fig. 2. Balancing risk with protective factors.
A number of social, behavioral, and environmental factors can cause the balance to tip to a teetering point towards either adolescent SUD or its prevention. Protective factors can outweigh risks, but the timing of the intervention needs to be developmentally appropriate.
Frameworks for prevention: universal, indicated, and selective
The initiation of substance abuse could be reduced for 1.5 million youth by simply implementing effective prevention programs (Miller and Hendrie 2008). Generally, the goal of these programs is to deter use or delay the age of initiation. Developmentally based prevention programs target specific audiences at various levels: the entire population (universal), those at moderate risk for substance use (selective), and individuals at high risk with identified vulnerabilities or currently use substances (indicated). Providing services to these three groups is advocated to prevent behavioral problems and substance use (Dishion et al. 2002; s et al. 2007). However, the enactment of these strategies comes with a cost of time, money, and valuable resources. According to SAMHSA (2008), selective and indicated programs are twice as expensive to implement versus a universal approach. The targeted effort in selective and indicated strategy leads to greater success and ultimately more cost-effective outcomes than the universal strategy. In contrast, universal approaches cast a wide net to reduce drug use in the whole population and estimates of the effectiveness of a school-based program show an $18 return for every $1 invested due to reduced SUD treatment and improved quality of life (Miller and Hendrie 2008).
Universal prevention approach
Universal prevention programs are principally carried out in school systems. These programs teach children social and emotional skills to reduce peer pressure and avoid the use of alcohol, tobacco, and marijuana. For instance, the “Just say no” initiative is an example of a universal program. More effective and validated programs that are used include the Communities Mobilizing for Change on Alcohol, the Botvin Life Skills Training Program, and the Guiding Good Choices program. The Communities Moblilizing for Change on Alcohol program provides materials for effective community-mobilizing practices that are aimed at changing policies and practices of major community institutions to reduce drug access by teenagers. The Life Skills Training Program promotes behaviors that lead to resistance of SUD: increased self-esteem, reduced anxiety, and greater resistance to peer pressure (Botvin and Kantor 2000). This commonly used training program has a cost-benefit ratio of 21:1, meaning for every dollar invested, $21 are returned in benefits to quality of life and medical costs. The third evidence-based approach is Guiding Good Choices, a multimedia substance abuse prevention program that gives parents of children ages 9–14 the knowledge and skills needed to guide their children through early adolescence. These youth development programs build positive competence and self-esteem across social, emotional, and cognitive domains (Seidman and Pedersen 2003). Such competence is linked to reductions in drug use, anxiety, and behavioral issues often found in conduct disorder (Catalano et al. 2002; Masten et al. 2005; Masten and Obradovic 2006). While various resources are necessary from early childhood throughout the teen years, parental involvement and mentor support are critical for effective intervention (discussed below). If universal programs were implemented, it is estimated that 8–12% fewer youth would engage in drinking, using marijuana, or smoking regularly (Miller and Hendrie 2008).
Selective/Indicated approaches
Selective and indicated approaches are more targeted to at-risk individuals due to a variety of circumstances. In selective prevention, vulnerable groups are identified based on factors associated with SUD. Risk factors include: drug exposure (prenatal, postnatal, or social modeling of drug use), stressful or negative life events (early life adversity that includes, but not limited to childhood maltreatment, abuse, and neglect, loss of a caregiver, exposure to a natural disaster), negative influence of peers (social defeat, bullying), and behavioral disorders (such as ADHD). An indicated prevention strategy focuses on children who already show symptoms of behavioral dysfunction and/or are more vulnerable to SUD due to potential genetic vulnerability. Training of local youth-serving professionals and volunteers to identify drug abuse in youth with the Screening, Brief Intervention, Referral and Treatment (SBIRT) model is recommended by our Department of Public Health Bureau of Substance Abuse Services in Massachusetts. Other screening approaches include the Substance Abuse Subtle Screening Inventory (SASSI) and the CRAFFT questionnaire, an acronym derived from six different questions assessing drug or alcohol use. Finally, law enforcement professionals should be trained to identify at-risk youth and to refer to appropriate social services in a preemptive, non-punitive manner.
On a neurobiological level, teens may also be well served by genetic screening for “risk” genes. The identification of genes related to drug metabolism, receptor expression, and reward-related behaviors may assist in determining vulnerability risk (e.g., CYP26 for nicotine metabolism; see Ciccioppo this issue) or treatment responsiveness (e.g., OPRM1 for naltrexone; Ducci and Goldman 2012; reviewed by Hall et al. 2013). However, whether treatment in the absence of illness (i.e., SUD typically does not appear until adolescence) is ethically reasonable or just is open for debate. On one hand, selected/indicated interventions that target high-risk youth would be the most cost-effective and most successful at reducing SUD. Alternatively, the initial up-front cost of genetic screening to identify those who would benefit most is expensive and opens Pandora’s box about ethical issues of genetic screening.
Based on human twin studies, the addictive potential of drugs correlates with heritability. The more addictive drugs are the most heritable: cocaine’s heritability index is 0.72, whereas hallucinogens have an index of 0.39 (Goldman et al. 2005; Ducci and Goldman 2012). Genotyping could be a valuable predictor to guide prevention efforts, although the field is young. Translational research can significantly impact teen SUD by modeling negative events in animals or using genetic profiling to unveil the neural mechanisms behind the etiology of SUD. As cause can often inform cure, we will briefly discuss the prevention/intervention paradigms that are effective, followed by a discussion of appropriate animal models in which to test therapeutic approaches.
Guidelines for prevention strategies
Four key guidelines are suggested when devising prevention strategies: 1) age-related patterns of competence and disorder; 2) developmental-appropriate tasks; 3) multiple contexts to increase generalization; and 4) interactions among biological, psychological, and social factors. The following sections will integrate the three types of prevention approaches within the above-mentioned guidelines. These approaches will be further informed by investigations on the developmental readiness of the underlying neurobiology in both humans and animals with a general schematic of the most relevant systems shown in Figure 3. Unique to human prevention efforts that will not be covered in this review is the need to be culturally sensitive in the approach (Resnicow et al. 2000; Hecht et al. 2003).
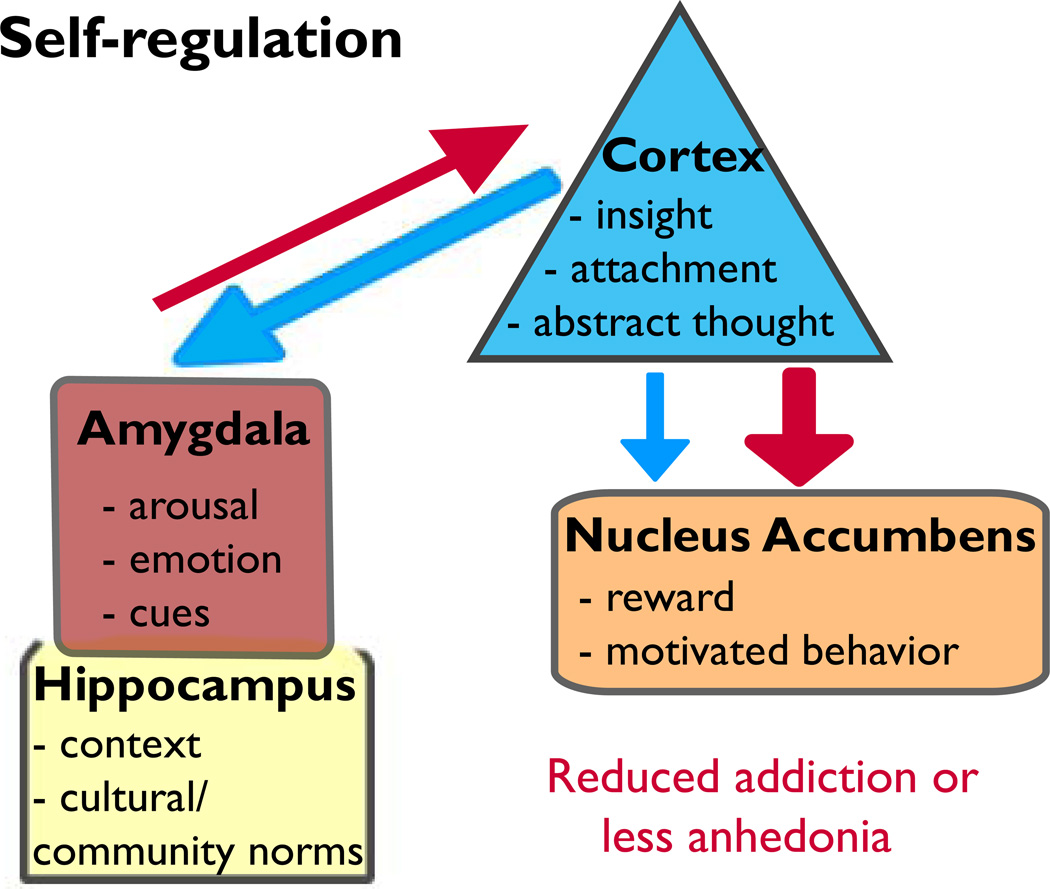
Fig. 3. Self-regulation: a brain-based hypothesis that encompasses multiple parts.
In this model, the hippocampal-amygdala-PFC pathway is susceptible in self-regulation and emotional control. Projections from the amygdala→PFC emerge prior to PFC→amygdala projections (Bouwmeester et al. 2002a,b), which are pruned in adulthood (Cressman et al. 2010). Thus, a window exists for strengthening the connections in this glutamatergic-driven system leading to increased self-regulation (i.e., better decision-making and greater cognitive flexibility). The PFC→NAc system is also maturing until adulthood (Brenhouse et al. 2008). During this developmental time, increased cortical drive leads to SUD, whereas reduced excitatory output to the NAc can reduce SUD but may produce anhedonia.
1 and 2) Age-related patterns of competence and disorder and developmental appropriateness
The first and second tenets of age-related patterns of competence suggest that programs cannot be uniform across age and need to be developmentally appropriate. All universal prevention programs emphasize the importance of self-regulation as one of the most important behaviors that can prevent substance use. While broad in concept, self-regulation includes attentional control, motivation, behavioral flexibility and inhibition, and sound decision-making and executive function (Baumeister et al. 2006).
Humans
Behavioral and magnetic resonance imaging (MRI) studies show that the underlying neural substrates of self-regulatory processes emerge across the teen years and continue to mature throughout the second decade of life (reviewed by Paus 2005). Sound decision-making and impulse control improve throughout the teen years, but their immaturity constitutes risk for SUD. Similarly, cognitive processes mature in a linear manner that gradually improves good choices, while reward-related processes show the classic inverted U shape that typifies the peak of high-risk behavior during adolescence (Casey and Jones 2010). Thus, any prevention program that facilitates the maturation of skills that strengthen risk-modulating behaviors (self-regulation and cognition) during adolescence or reduces impulsivity, sensitivity to social influences, and/or reward is likely to be effective.
Age-dependent changes in self-regulation and cognition are inversely related to impulsive behavior. Impulsive behavior is greatest in younger children and gradually decreases in a mostly linear manner into adulthood (Luna et al. 2004). Using an anti-saccade task of response inhibition, the ability of children to effectively inhibit their inclination to look at the non-target reaches adult-like levels at 14 years of age. Similar findings of linear development have been shown with a go/no-go task (Somerville et al. 2011). Part of this improved regulation serves to dampen impulsive behavior as children mature.
Other forms of impulsivity, such as delayed discounting and impulse control, are predictive of initiating substance use and developing addiction (Stanger et al. 2013a). In a delayed discounting task, a smaller reward is selected sooner more often than a larger reward is selected later. Meta analysis shows that delayed discounting is evident in individuals with clinical-level addiction (a strong effect size: Cohen’s d=0.61); the relationship is slightly weaker in non-clinical studies (d=0.45; MacKillop et al. 2011). Delayed discounting is elevated during childhood and declines markedly with age beginning at ~14 years of age (Miller et al. 1978; Scheres et al. 2006). The relevance of delayed discounting to SUD at a neurobiological level is complex and is covered more in-depth by Stanger and colleagues (2013b). Briefly, patterns of brain activation in adolescent substance abusers show reduced activity in executive structures (e.g., dorsolateral prefrontal cortex, dorsomedial PFC, inferior parietal cortex, cingulate cortex, and precuneus) on a delayed discounting task, while greater activity is observed in the amygdala, hippocampus, insula, and ventromedial prefrontal cortex as devaluation increased (Stanger et al. 2013b). The lesser inhibitory/executive control in the presence of strong reward associations and their memories may drive greater discounting in teen users more than any other age. Consistent with these teen patterns of brain activity, continuous transcranial magnetic stimulation of the dorsolateral PFC impairs delay discounting in adults (Cho et al. 2010).
For the impulse control task, participants are required to stop an already initiated motor response for a successful inhibition response (referred to as stop signal reaction time). In the largest single imaging study to date, Whelan and colleagues (2012) identified distinct brain networks activated during successful and failed attempts of impulse control in early adolescence (~14 years of age). During failed stop attempts, hypoactivity of the orbital frontal cortex was associated with substance use, while hypoactivity in the anterior cingulate region and basal ganglia network was associated with ADHD symptoms. Interestingly, genetic variation in the norepinephrine transporter gene was related to increased activity in the right inferior frontal gyrus and anterior cingulate region during stop successes. These findings implicate disparate pathways for the similar behavioral outcome of impulsivity that is found in several disorders. Thus, prevention measures should focus on gaining overall cognitive control, independent of specific disorders like SUD and ADHD that emerge during adolescence.
Of equal importance to drug use prevention is anticipating the consequences of one’s actions and one’s mistakes. Adaptive and flexible behavioral control after rewarding or punishing feedback that may be a component of insight learning (O’Doherty et al. 2003), is needed to re-evaluate the cost of a rewarding stimulus like drugs that leads to their valuation. Such learning occurs across brain networks, although the orbital frontal cortex partly determines changes in reward valence, contingency, and behavioral outcome. This region is one of the last regions in the brain to mature structurally (Gogtay et al. 2004). This protracted development, relative to the inverted-U shape of elevated reward system activity during adolescence, may drive immediacy in responding based on reward receipt rather than the final outcome (Galvan et al. 2006). The linear decline in orbital frontal cortex activation between childhood to adulthood (Galvan et al. 2006) may allow some experiential learning. However, teens have a propensity to be “sure” of their decisions no matter how incorrect they truly are (Bjork et al. 2007). Specifically, Bjork et al. found reduced activation of the posterior medial PFC in adolescents under conditions of uncertainty relative to adults, but no age difference when the outcome was predictable. How “sure” teens are of their decisions can also be targeted with an intervention by potentially focusing on future consequences and whether it will threaten their sense of self. The Above the Influence campaign takes this approach in its anti-drug advertisements and has been effective in reducing teen drug use (Slater et al. 2011).
Age-specific differences in brain responses and the effects of drugs can guide or fine-tune interventions (Geier et al. 2010). These age differences are apparent either in processing speed or the amount of brain activity required for responding. For example, activation patterns within the superior precentral sulcus show that teens require increased preparation time to respond to a stimulus (e.g., an anti-saccade task) compared to adults (Curtis and Connolly 2008). The response latency is greater in teens with SUD (Chung et al. 2011). These data suggest that relative to adults, typical teens require more – and substance-using teens require even more – time to recognize the right answer and learn from it before reducing impulsive mistakes in the future. Repeated marijuana use in adolescents activates more brain regions to process information than controls, reducing the ability to multi-task (Chung et al. 2011; Gruber et al. 2012). Another example is the finding that greater “losses,” rather than small losses, lead to adult-like responses in adolescents; in other words, varying incentive-type or magnitude may improve the shaping of appropriate responding in teens (Geier et al. 2010).
Access to peers is one of the most significant risk factors for SUD and other high-risk behaviors that is also the most readily addressable. The presence of peers in a social situation further increases risky behaviors, such as driving (Chein et al. 2011) and drug use (van Ryzin et al. 2012). Curiously, peers have less of an influence on drug use during high school age itself, but have significant influence prior to and after high school ages for alcohol, tobacco, and marijuana (van Ryzin et al. 2012). Peers increase fMRI activity in the adolescent ventral striatum during a risky decision task compared with their responses when alone (Chein et al. 2011). Ventral striatum activity in response to a stoplight task (Chein et al. 2011) or an affective facial display task inversely correlates with peer influences (Pfeifer et al. 2011). Changes in ventral striatal activity may signal the emergence of greater emotional regulation and positive affect that continues to change into adulthood (Forbes et al. 2009). In addition, ventral striatal activity is negatively coupled with amygdala activity (Pfeifer et al. 2011). Peers become less influential as the task itself is rewarding. However, as amygdala activity is elevated (or ventral striatal activity is reduced), peers may have a significant impact on vulnerability to drug use. Indeed, amygdala activity is important for drug cue processing (Wilson et al. 2004). The influence of the amygdala/ventral striatum is age-dependent, and Hare et al. (2008) demonstrated peak amygdala responsiveness to affective stimuli during mid-adolescence; the peer studies were conducted in early adolescence and adulthood. Whether the amygdala is relatively more responsive to peer influences during specific stages of adolescence is not known. Amygdala responsiveness to emotional cues is already higher in teens compared with children and adults (Guyer et al. 2008; Hare et al. 2008; reviewed by Blakemore and Robbins 2012), which could significantly affect vulnerability to SUD. Risk for poor outcomes could be reduced if adolescents were provided with an opportunity to learn social control. The Life Skills Training program provides adolescents with knowledge and skills needed to resist social influences to use cigarettes, alcohol, and other drugs by increasing general personal and social competence (Luna-Adame et al. 2013).
From a prevention viewpoint, improved self-regulation can reduce the impact of risk behaviors (e.g., impulsivity and aggression) that are associated with SUDs well before children reach adolescence and are likely to start using drugs (Thompson et al. 2013). The Good Behavior Game (Barrish et al. 1969), for example, uses a classroom-based intervention approach to teach young students to interact as a member of the classroom community. Through positive reinforcement learning, these high-risk children show reduced rates of drug-and alcohol-use that may be partially mediated by internally guided rewards that have been taught. In other words, this behavioral approach offers an SUD “vaccine” through improved self-regulation and reduced impulsivity and aggression (Embry 2002).
While self-regulatory processes can be strengthened during adolescence to reduce drug use, modulation of the highly active motivational reward systems (Casey et al. 2008; Steinberg et al. 2008) that are highly relevant for SUDs is also needed during adolescence. Functional MRI studies show greater blood flow in the nucleus accumbens (NAc) in response to a received reward in teens relative to younger and older subjects (Galvan et al. 2006; Geier et al. 2010; van Leijenhorst et al. 2010). A number of laboratory studies indicate that teens gamble with risky decisions, with the greatest proportion of risky choices made at or around age 14 (Burnett et al. 2010). The elevated activity in the NAc during adolescence positively reinforces actions relevant to SUD. Dampening the reward system, however, will have its own adverse consequences, anhedonia notwithstanding (Nestler and Carlezon 2006). Rather, prevention efforts targeting reward should include substitution-type approaches.
The reward system can be positively activated through endurance sports like running or brief aerobic exercise. Participation in school sports is associated with reduced 30-day use in tobacco, marijuana, and other illicit drugs, but not alcohol (Terry-McElrath et al. 2011). The results show that exercise delays initiation of use, rather than producing a highly significant reduction in use. As a delay in initiation is associated with reduced lifetime prevalence of addiction (SAMHSA 2006), these results are noteworthy. Exercise can also reduce craving, one of the most potent factors in relapse (Kalivas et al. 1998). In a study in adult smokers, fMRI activity in the NAc in response to smoking cues was reduced in abstinence-induced craving who were allowed 10 minutes of exercise prior to imaging (van Rensburg et al. 2012).
Animals
Substance abuse research has extensively targeted understanding reward circuitry and modifications in motivational processes (Chambers et al. 2003). The underlying neuroadaptations that occur within reward circuits during adolescent drug use are intriguing because the brain is already in a state of developmental flux (see Brenhouse and Andersen 2011a, for review). While human studies highlight brain regions involved in SUD during adolescence because most utilize functional magnetic resonance imaging (fMRI), which is an indirect measure of blood flow, they are limited in what they can tell us about the underlying neurochemistry that could facilitate pharmacological intervention. Animal studies fill this gap, although the time is approaching where practitioners might use this information to find ways to reduce SUD rather than describing its nature and risk factors.
In general, the biggest influence on teen SUD may be the inverted U peak of the reward system that occurs during adolescence. The NAc, ventral tegmental area (VTA), and PFC are principal regions of the brain reward circuitry, and the reinforcing effects of most drugs of abuse are, directly or indirectly, due to the modulatory effects of dopamine neurotransmission (Kalivas and Volkow 2005). The glutamatergic pyramidal cells in the ventral mPFC (prelimbic and infralimbic regions) predominately receive dopaminergic efferents from the VTA (Lindvall et al. 1978).
The developmental increase in dopaminergic (and serotonergic) inputs to the PFC (Kalsbeek et al. 1988; Leslie et al. 1991; Rosenberg and Lewis 1994) plays a prominent role in regulating motivational systems that are relevant to SUD. More specifically, D1 dopamine receptors on glutamate neurons influence sensitivity to drug cues, their extinction, and their reinstatement – all factors that influence drug-seeking and relapse by elevating motivational salience (Kalivas 2005; Rebec and Sun 2005; Badanich et al. 2006; Ventura et al. 2007; Andersen et al. 2008; Brenhouse et al. 2008). Dopamine receptors are overproduced in the medial PFC during adolescence (Andersen et al. 2000), with D1 receptor increases localized to glutamatergic projection neurons – including those projecting to the NAc (Brenhouse et al. 2008). The peak expression of this specific receptor population during this stage is likely to motivate adolescents to seek hedonic-related substances more than any other age group and also plays a role in novelty preferences and impulsive choice (Sonntag et al., this issue). These preclinical data suggest that altering motivational salience in a vulnerable population with a highly targeted intervention may be a crucial step in being able to prevent SUD.
Impulsivity is another important risk factor that contributes to elevated risk for SUD. Impulsivity itself may be sub-divided into three main areas (Dalley et al. 2008; Fineberg et al. 2010): deficiencies in delaying rewards, response inhibition (i.e., stop signal reaction test), and decision-making (i.e., 5-choice serial reaction time test; Besson et al. 2010). Glutamatergic input from the PFC into the NAc is a point of convergence for impulsivity (Winstanley et al. 2004a; Fineberg et al. 2010), although each form of impulsivity seems to have one principal receptor or neurotransmitter associated with the behavior. What little is known about mechanistic changes underlying impulsivity specifically during adolescence will be presented, although a paucity of data exists due to the long training periods needed in tasks assessing operant behavior. Thus, most of what is presented is based on studies in adult animals.
Behavioral evidence in immature animals shows that delayed discounting is high during adolescence (Laviola et al. 2003). Delayed discounting is modulated by both dopamine and serotonin (5-HT). Elevated activity at the D1 receptor in the plPFC increases discounting in adult rats (reviewed by Dalley et al. 2008); D1 receptors are elevated in the plPFC during adolescence (Brenhouse et al. 2008). Excitotoxic lesions of the basolateral amygdala (BLA) increase discounting, while lesions of the OFC have the opposite effect (Winstanley et al. 2004b). If BLA activity is reduced with a GABA agonist (e.g., baclofen or muscimol) infusion, risk aversion increases while preferences for large rewards with high cost increase (Ghods-Sharif et al. 2009). The BLA encodes and maintains the representation of the reward. Developmentally, high levels of discounting in juveniles are likely to be more attributable to reduced executive control. In rats, projections from the BLA to the PFC (BLA→PFC) emerge at an earlier time point than top-down regulation brought by PFC→BLA projections, which emerge a week later (Bouwmeester et al. 2002a,b). The BLA→PFC continues to mature through adolescence (Cunningham et al. 2002) limiting the capacity of the juvenile to effectively recall a large reward with a delay. However, the PFC→BLA system prunes after adolescence (Cressman et al. 2010), as does the number of cells in both regions (Markham et al. 2007; Rubinow et al. 2009, respectively). The PFC→NAc system is also maturing until adulthood; however, sufficiently salient rewards can activate this pathway (Brenhouse et al. 2008). The emotionally activating aspects of peers elevate the rewarding nature of cocaine and nicotine in adolescent rats (Thiel et al. 2008; 2009) perhaps by this network. Responses to peers and other social stimuli are also likely to be modified by the hippocampus and its interactions with the amygdala, PFC, and the NAc (O’Donnell and Grace 1995). Both animal and human imaging studies show the involvement of the hippocampus in stress, emotional regulation, and contextual processing (O’Mara et al. 2009; Fanselow and Dong 2010). Together, modulating discounting behavior in juveniles or adolescents should capitalize on increasing the size of reward to reduce impulsivity. Pharmacological interventions that may achieve this result are discussed below under #4.
In addition to DA, serotonin receptors (5-HTRs) are also involved in impulsivity. 5-HTRs have both inhibitory and excitatory influences and play a role in controlling impulsive choice (Zhang 2003; Beique et al. 2004; Kirby et al. 2011). Both the 5-HT1A and the 5-HT2C receptors are implicated in choice behavior, but with opposing actions (reviewed more completely by Cunningham and Anastasio 2013). For example, the 5-HT1A agonist 8-hydroxy-2-dipropylaminotetralin increases delay discounting (Winstanley et al. 2005; Stanis et al. 2008), whereas antagonism of the 5-HT2C is needed to increase impulsivity (Winstanley et al. 2004c). 5-HTRs mature earlier than DA receptors (Lambe et al. 2000) and have distinct subtypes, localization (pyramidal neurons versus interneurons), and differ in expression levels across development (reviewed by Andersen and Navalta 2011). Studies on changes in adolescent rodent 5-HTRs have yet to be conducted; however, a recent study by Lambe and colleagues (2011) examined mRNA expression of 5-HTRs in human postmortem brains from infancy to adulthood. Within the PFC, 5-HT2A shows an inverted U pattern of expression that peaks during childhood and adolescence; 5-HT2C demonstrates a more linear course than 5-HT2A, with a slight down-regulation from adolescence to adulthood. 5-HT1A shows linear development; however, pre- and post-synaptic distribution of this receptor subtype may change differentially across age. Because impulsive choice is directly related to 5-HT2A manipulations (Winstanley et al. 2004c), this receptor may provide a unique target for adolescent treatment.
Impulsive action, or impairment in the ability to inhibit a pre-potent motor response, is measured by the stop signal reaction time test (Robinson et al. 2008; Eagle et al. 2008; Fineberg et al. 2010). No developmental data are available on this task in animal models due to the extensive training; however, human data show increased speeding in responses during childhood, followed by a gradual slowing into adulthood (Bedard et al. 2002). This task is mediated by norepinephrine (α2A receptor) within the OFC through projections to the NAc (Chamberlain et al. 2006; Chamberlain et al. 2007a,b; Bari et al. 2009). Lesions of the orbital frontal cortex slow stop signal reaction times (Eagle et al. 2008). Work by Arnsten (Arnsten, 2009; Arnsten and Pliszka 2011) suggests that α2A receptors are co-localized on the dendrites with D1 receptors. Neurons that respond to similar stimuli can be further activated by α2A receptor agonism, aiding in attentional control and focus; neuronal firing to dissimilar features is modulated by D1 receptor agonism and increases distractibility and impulsivity. Given that D1 receptors peak during adolescence and α2A receptors are still maturing through adulthood (Happe et al. 2004), performance is likely to be biased towards distractibility. Together, the development of dopamine, serotonin, and norepinephrine systems during adolescence contributes to elevated impulsivity that increases risk for SUD.
Norepinephrine dysfunction is also directly linked to SUD (Weinshenker and Schroeder 2007). Specifically, noradrenergic receptors play a role in drug reward and reinstatement of drug-seeking behavior (Drouin et al. 2002; Ventura et al. 2003; Lee et al. 2004; Shepard et al. 2004; Zhang and Kosten 2005) and impulsivity (reviewed by Chamberlain and Robbins 2013; Bari and Robbins 2013). In adult animals, reinstatement can be blocked by antagonizing α1 with prazosin (Zhang and Kosten 2005) or induced by antagonizing 2 receptors with yohimbine (Lee et al. 2004; Shepard et al. 2004). Given that SUD risk changes in an age-dependent manner, we can speculate on the importance of α1 and α2a receptors driving actions during adolescence. The PFC continues to receive noradrenergic input through late adolescence (measured by noradrenergic transporter binding; Moll et al. 2000; Sanders et al. 2005) and indirectly affects dopamine transmission in the NAc. Nontraditional interactions also exist between norepinephrine and dopamine, as noradrenergic fibers via the noradrenergic transporter can take up, package, and release both dopamine and norepinephrine in the PFC (Morrison et al. 1981; DeVoto et al. 2001). This is presumably one mechanism of action of atomoxetine, a drug used to treat ADHD (Bymaster et al. 2002). In stark contrast to dopamine and 5-HT receptors, the developmental expression of noradrenergic receptors has not been fully examined in rats. A linear increase occurs in α2a receptors across development in both the PFC and amygdala, although adolescence was not assessed (Happe et al. 2004; also see review by Murrin et al. 2007). In the non-human primate, α2a receptors increase monotonically in the cortex with age-related changes in laminar distribution (Lidow and Rakic 1992). The α2A receptor is the prevailing noradrenergic mechanism in impulsivity and cognitive flexibility (Lapiz and Morilak 2006; Caetano et al. 2012; Kim et al. 2012; Chamberlain and Robbins 2013).
Importantly, multiple aspects of self-regulation are linked to these aforementioned underlying neurobiological processes. Self-regulation can be behaviorally characterized in animals, although more studies have focused in adults rather than in adolescents due to the timing and complexity of operant training necessary to measure behavior (e.g., Chudasama and Muir 1997; Birrell and Brown 2000; Dalley et al. 2004; Floresco 2013). For the sake of this review, we will focus on several aspects of self-regulation that have been studied in adolescent animals. First, behavioral flexibility, defined as the ability to change behavior in response to different environmental task conditions, gradually improves during the transition to adulthood (Weed et al. 2008). Second, self-regulatory skills can also be measured with decision-making tasks.
Behavioral flexibility tasks in rats have focused primarily on attentional set-shifting (i.e., Wisconsin Card Sorting Task) and reversal learning. Adolescent rats show a lack of attentional and cognitive flexibility. Rats show impairment when they have to attend to a once irrelevant dimension for a predicted reward (Newman and McGaughy 2011) and respond after inhibiting a previous response (Simon et al. 2013). However, the positive cognitive consequences of a juvenile developmental state suggest that juveniles might be suited to changing environments (i.e., reversal learning; Johnson and Wilbrecht 2011). While adolescent rats eventually learn, previous findings suggest that the amount of distractibility should be monitored and minimized, and the pressure of multi-tasking should be reduced. Promisingly, deficits could be remediated with the administration of the noradrenergic reuptake inhibitor, atomoxetine (Cain et al. 2011), suggesting a critical role for noradrenergic, or as discussed above, dopaminergic, function.
Likewise, decision-making, including working memory and impulsive choice, improves during the transition from adolescence to adulthood (Koss et al. 2011). Adolescent rats generally commit more errors and are less accurate than adults on delay-dependent tasks that are highly sensitive to changes in PFC function (such as delayed alternation or matching to sample; Koss et al. 2011). Work in adult animals has focused on glutamatergic (Wang et al. 2013) and dopaminergic function (Vijayraghavan et al. 2007; reviewed in depth by Arnsten and Pliska 2011; Floresco 2013).
Similar to exercise in humans, animal studies (e.g., rats) have shown that exercise reduces drug-reinforced behaviors in adolescents compared to adults. Chronic exercise (treadmill running for six weeks, up to one hour/day) reduces the rewarding properties of cocaine compared to sedentary rats (Thanos et al. 2010). Wheel running also reduces drug-taking behaviors in rats that were concurrently trained to self-administer cocaine during long access sessions (Zlebnik et al. 2012). Some of these exercise effects in adolescent rats may modulate the potency of cues. Adolescent rats that self-administered nicotine showed a reduction in drug-seeking during abstinence if they had the opportunity to exercise, although exercise did not have a clear effect on reinstatement (Sanchez et al. 2013).
3) Multiple contexts to increase generalization of intervention
Exposure to drug-associated cues elicits strong drug-seeking behavior even after a long period of abstinence (Shaham and Stewart 1995; Kalivas and McFarland 2003; Taylor et al. 2009). For this reason, interventions need to be long lasting and flexible. One goal of prevention research is to reduce drug-seeking behavior by strengthening extinction learning (i.e., when the drug-paired stimuli no longer result in the delivery of reward). Extinction can influence reinstatement or relapse (Kelamangalath et al. 2007).
Humans
Multiple contexts of learning prevention are more effective in reducing drug use versus merely trying to avoid drug-related cues altogether. Cue exposure therapy is one such clinical method, although the effects of this therapy as a stand-alone treatment strategy have been relatively weak (d=0.08; Conklin and Tiffany 2002). Combining cue exposure therapy with cognitive-enhancing drugs holds promise for adolescents, and a number of agents are available (Sofuoglu et al. 2013). The partial NMDA agonist d-cycloserine enhances extinction and reduces reinstatement of drug conditioning and taking (reviewed by Myers and Carlezon 2012). Rather than targeting extinction processes, a recent study in both humans and rats demonstrated that altering the reconsolidation process may be an effective strategy (Xue et al. 2012). In this study, exposure to drug cues (a “reactivation”) followed by a second exposure to drug cues in the absence of drugs 10 min later, but not 6 hours later, produced enduring decreases in craving. A third methodology aimed at reducing the potency of drug-associated cues is to pre-expose the individual to reward-based cues that s/he may encounter during the course of the day (Peters and Buchel 2010). Finally, devaluing future rewards as “nothing now, but something later” also reduces impulsive choice with the potential to reduce relapse (Magen et al. 2008).
Animals
Adolescent rats are up to 75% more resistant to extinction of cocaineconditioned associations than adults in a place conditioning procedure (Brenhouse and Andersen 2008). Although once the drug cues were extinguished, adult rats showed greater cue-based reinstatement than adolescents, while adolescents are more sensitive to stress and drug primes (Li and Frantz 2009; Anker and Carroll 2010). Orexin A antagonists block stress- and cue-induced, but not drug-primed, reinstatement of both cocaine and alcohol self-administration in adult rats (Boultrel et al. 2013; Mahler et al. 2013). Their effectiveness in addictive processes in developing animals has yet to be determined.
Distinct behavioral and pharmacological strategies in the adolescent population have been proven effective, suggesting that the competition between strong memories of potent rewards and new memories can be overcome (Brenhouse et al. 2010). Extinction can be greatly facilitated in adolescent rats by explicitly pairing the drug-associated cues with the absence of the reinforcer (Brenhouse et al. 2010). If the context is more vague (e.g., unexplicit pairing of no drug and cues), adolescent rats require significantly more time than adults to extinguish (Brenhouse and Andersen 2008). The non-explicit contextual pairing might be more “real-world” where treatment needs to generalize to multiple contexts to be effective. Pharmacological manipulations with a D1 agonist SKF38393 or a noradrenergic reuptake inhibitor atomoxetine facilitate extinction in the unpaired condition. Moreover, these treatments blocked drug-primed reinstatement of cocaine conditioning.
4) Interactions among biological, psychological, and social factors
Humans
Prevention/intervention programs have to address the special needs of children and teens that are biologically vulnerable to develop an SUD. This group includes those who are exposed to adversity early in life where SUD is reported in up to 26% (Molnar et al. 2001); drug-exposed (which will not be reviewed here; but see the following papers in this issue: Leslie et al.; Izenwasser et al.; Ciccocioppo); or children with a disorder, such as ADHD or conduct disorder/oppositional defiant disorder where SUD occurs in nearly 10% of these populations (Polanczyk et al. 2007). A number of clinical studies recognize neurobiological differences between teens that initiate drug use earlier than their non-using peers (Stanger et al. 2013a), suggesting that a biological predisposition exists.
a) Exposure to early adversity
For the majority of these at-risk children, the importance of greater structure and positive stimulation as an intervention cannot be underestimated. Because environments can widely differ between children or teens in the same peer groups, selective or indicated approaches may be more appropriate and effective to address specific environmental deficits or liabilities. One of the most relevant outcomes of early life stress relevant to SUD is the recent observation of reduced and slower incentive processing in exposed teens compared with typical controls (Mueller et al. 2012). Home visits to more-at-risk, low-income families not only prevent the likelihood of child abuse, but visitation that continues into the teen years also reduces SUD (Karoly et al. 1998). Similarly, more structured programs, such as the Strengthening Families Program, work towards increasing family support, parental monitoring, and stable friends and also mitigate effects on teen substance use. High parental monitoring reduces use by nearly 4-fold in 14–16 year-olds relative to low parental monitoring (Fallu et al. 2010). Based on Sher’s model (Sher 1991), greater parental monitoring can further guide friend choice from affiliation with more deviant peers to more conventional peers with a more positive influence.
Animal
Less is known about the effects of prevention/intervention in a number of high-risk animal models during development (Andersen and Navalta 2011). One important exception is the role of early environmental enrichment in animal models of early adversity. While exposure to stress can occur at any age, exposure during development has unique and enduring effects on neurobiology depending on the age of exposure (Andersen and Teicher 2008; Teicher et al. 2006; Leussis et al. 2008; Novick et al. 2011; Kolb et al. 2012; Muhammad et al. 2012). The effects of exposure to post-weaning (e.g., Gipson et al. 2011) and adolescent stress are reviewed by Burke and Miczek (this issue) and will not be discussed here. Animal models and the human condition show that both the risk and protective factors are familial for younger children, whereas teens are more vulnerable or supportive by their peers.
One approach to model early life adversity in rats is to separate neonatal pups from their mothers before weaning (Smotherman et al. 1977; Lehmann and Feldon 2000). Early maternal separation stress (MS) has been studied with a variety of paradigms, ranging from MS between postnatal day (P) 2–9, P2–20, P9–16 and for time periods ranging from short (5–15 min; Stanton and Levine 1985) to long (180–360 min; Andersen and Teicher 2004; Plotsky et al 2005). While differences do exist in their enduring effects, the increased drug use that has been observed may be more predictable – and preventable – based on the underlying neurobiological effects. For example, the long MS rats preferred and consumed more alcohol in adulthood than the normally reared or short MS subjects (Huot et al. 2001; Ploj et al. 2003).
Alterations in dopamine and serotonin systems are widely implicated in the neurobiological consequences of MS (see Miczek et al. 2008). For example, studies show down-regulation of striatal DAT (Meaney et al. 2002), decreased D3 binding in the striatum (Brake et al. 2004), and decreased levels of serotonin in the amygdala in both rats (Vicentic et al. 2006; Oreland et al. 2009) and non-human primates (Ichise et al. 2006). These data suggest that the striatal dopamine system is over-active, although the behavioral data show that MS blunts reward processing of stimulants and sucrose in adulthood producing anhedonia (Matthews and Robbins 2003; Stoker et al. 2012). Within this framework, the MS paradigm where pups are individually isolated (i.e., removed from the dam and littermates) increases cocaine self-administration in adulthood (Kosten et al. 2000, 2004; Moffett et al. 2006; Vazquez et al. 2006).
In the case of MS, we have recently shown a selective decline in DA D1 receptors on the plPFC → NAc (core, specifically; Brenhouse et al. 2013). Given the predominant role of this receptor in motivated behavior, its loss may partially play a role in the reduced motivation for rewarding substances. One possibility for an intervention approach is to increase motivation by increasing plPFC activity by social stimulation in animals with a deprivation history. Consistent with MS attenuation in the plPFC→NAc pathway, reduced plPFC activity by pharmacological inactivation decreased the duration of social play in adolescent rats (Van Kerkhof et al. 2013). Similar to the effectiveness of home visits to reduce SUD in the clinical setting discussed above (e.g., exposure to early adversity), developing rats also respond positively to improved living conditions and show less drug use. Environmental enrichment beginning post-weaning in rats reduced intake of cocaine in a low unit dose of amphetamine (1 hour/day escalation procedure) in adulthood that may be more attributable to the presence of social cohorts than novel objects (Gipson et al. 2011). While these studies were conducted in normal animals, a social intervention is likely to restore some of the motivational deficits in animals with a deprivation history.
While improved living conditions for children exposed to early adversity would be ideal, pharmacological approaches may be a more realistic intervention. MS rats show more depressive-like effects that are associated with GABA cell loss in the adolescent, but not the juvenile, PFC (Leussis et al. 2012). As alcohol acts as positive allosteric modulator, MS subjects may be more likely to consume alcohol to compensate. Moreover, MS reduces social behaviors (reviewed by Bardo et al. 2013), which maybe the result of elevated N-methyl-D-aspartate receptor (NMDA) 2A (NR2A) receptors in MS rats (Wieck et al. 2013). Pharmacological intervention with a NR2A antagonist, such as PEAQX, increases social interactions (Morales et al. 2013; Morales et al. this issue). Therefore, NR2A antagonism may serve as a potential intervention to reduce the likelihood of alcohol consumption in this population.
A third novel intervention approach to reducing SUD in this population capitalizes on the link between neuroinflammatory processes, aberrant glutamatergic drive, and MS (Brenhouse and Andersen 2011b; Wieck et al. 2013). The proactive administration of an anti-inflammatory cytokine (IL-10) during the juvenile period prevents the loss of parvalbumin-positive GABAergic interneurons in adolescents that were exposed to MS. This same treatment also modulates MS-induced upregulation of NMDA receptors. Pascual et al. (2007) showed that the non-steroidal anti-inflammatory compound, indomethacin (an inhibitor of cyclooxygenase-2 activity; Shibata et al. 2003), reduced ethanol-associated effects on behavior. As MS adolescents have elevated cyclooxygenase-2 level in the plPFC (Brenhouse and Andersen 2011b), these data suggest a potential intervention to reduce SUD. While human studies are limited, evidence that relates neuroinflammatory processes to childhood adversity and psychiatric disorders is emerging (Dennison et al. 2012).
b) Defined clinical populations that are at-risk for SUD
Human
Medication plays an important role in prevention paradigm for children that have been clearly identified as high-risk. For example, children with ADHD, especially with comorbid Conduct Disorder, were almost 3 times more likely to develop a SUD compared with the typical adolescent (Wilens et al. 2011). Onset of SUD is also earlier in these teens, which is associated with an exacerbation of severity and duration of ADHD (Wilens and Biederman 2006). Medication may significantly reduce SUD in a subset of high-risk children when given before puberty (Wilens et al. 2008; Mannuzza et al. 2008).
Animal
Early pharmacological intervention for children that have clearly identifiable clinical disorders should target relevant neurobiological risk mechanisms during a sensitive period of invulnerability (Andersen and Navalta 2011). Clinical observations of reduced drug use have been recapitulated in animal models (Andersen et al. 2002; Bolanos et al. 2003). The importance of these animal studies lies in their ability to dissociate psychosocial factors in the clinical domain (e.g., concerned parents or treatment) from the underlying neurobiology that can be manipulated as to reduce SUD risk. As children transition to adulthood, the use and abuse of different substances rises (Andersen and Teicher 2009). As many of the papers in this Special Edition show (Bolanos et al.; Sinclair et al.), the progressive development of neurotransmitter systems implies that they are still programmable (Andersen 2003). Intervention during this stage may prevent problems later in life by selectively locking-in this invulnerable state.
Sensitive periods of invulnerability to SUD can be manipulated to protect at-risk children from developing an addiction (Andersen 2005). However, such an approach requires certainty in diagnosis before treatment with the sole purpose of protection (versus other, more immediate reduction in symptoms) is initiated. Pre-pubertal treatment with methylphenidate, cocaine, or amphetamines reduces preferences for cocaine-associated cues in late adolescence (Andersen et al. 2002; Mague et al. 2003). While such an intervention seems paradoxical (e.g., to administer an addictive substance to reduce addiction), the brain is still being programmed to respond to its environment. If a drug is given for a period of time and then withdrawn, a deficit state is produced that requires more drug to achieve a normal state. In normal animals, this deficit state appears as anhedonia (Bolanos et al. 2003), but in children with ADHD this could normalize function (Andersen 2005).
Drugs such as atomoxetine and guanfacine are widely prescribed in children for the treatment of ADHD disorders (Biederman et al. 2008; Sallee et al. 2009). Unfortunately, very little is known about their effects in animals. Atomoxetine (Straterra) blocks the norepinephrine transporter and reduces impulsive choice, but not impulsive action, in rats treated during adolescence and tested in adulthood (Sun et al. 2012). Atomoxetine also reduces set-shifting, but not initial discrimination learning, in adolescent normal rats (Cain et al. 2011) and Spontaneously Hypertensive rats (SHR; a model for ADHD; Harvey et al. 2013). For atomoxetine, the single juvenile study suggests 1 mg/kg reduces hyperactivity, with similar effects in non-impulsive adults (Robinson et al. 2008). Like atomoxetine, a paucity of preclinical information exists about the effectiveness of guanfacine, an α2 agonist, to reduce impulsivity. To our knowledge, no studies exist in developing animals. Doses of 0.1–0.7 mg/kg that improved cognition in normal mice (Franowicz et al. 2002; Bari et al. 2009) found 0.3 mg/kg effective in the stop signal reaction time task. A reduction in impulsivity is similar to the effects of guanfacine in the treatment of children and adolescents with ADHD (Garnock-Jones and Keating 2009). Unlike methylphenidate exposure (e.g., Andersen et al. 2002), juvenile exposure to guanfacine does not have sustained effects on reducing SUD (e.g., preferences for cocaine-associated cues) in adulthood (Lukkes et al. 2013).
c) General prevention approaches
More promising pharmacotherapy on prevention involves vaccination against stimulant, nicotine, and opiate use (Orson et al. 2008; Kosten and Domingo 2013). Vaccines stimulate the production of antibodies that bind to the drug, preventing it from entering into the brain. While vaccination would be convenient to administer during the window of sensitivity, research is in the early stages. A number of critical questions exist: 1) are the amount of circulating antibodies sufficient for blocking substance use later in life; 2) will vaccines be more effective after initial drug use rather than prophylactically; and 3) how many or what kinds of substances should be targeted? The use of vaccines warrants concern in regard to the potential “slippery slope” of escalation intake, and for the most part, does not differ from a number of medications that are readily available to directly stop substance abuse in adults. Most of the current medications are aimed at treating withdrawal, cravings, and blocking metabolism: naltrexone, acamprosate, and disulfiram (for alcohol), replacement (for nicotine), and methadone and buprenorphine (for opiates).
Conclusion
Prevention of the initiation of substance use is ultimately the best way to reduce addiction. Efforts to reduce risk need to be multifaceted and occur across the course of development. Neuroscience research can play a key role in informing other fields involved in addiction prevention and treatment (e.g., clinicians, social workers, preventionists, education specialists, and parents) through education about optimal windows to intervene and which neural systems to target at a given stage in development. Similarly, neuroscientists can benefit from learning more about what works in the real world and find ways for improvement. In this review, we discussed what is known clinically and preclinically about windows of invulnerability and windows of vulnerability when the developmental risk factors for addiction, including impulsivity, poor self-regulation and decision-making, peak during adolescence.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Napierata L, Brenhouse HC, Sonntag KC. Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur J Neurosci. 2008;27:2962–2972. doi: 10.1111/j.1460-9568.2008.06254.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Annual Research Review: New frontiers in developmental neuropharmacology: can long-term therapeutic effects of drugs be optimized through carefully timed early intervention? J Child Psychol Psychiatry. 2011;52:476–503. doi: 10.1111/j.1469-7610.2011.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacol. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse (NY) 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. J Pediatr. 2009;154:I-S43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology. 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobio. 2013 doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Barrish HH, Saunders M, Wolf MM. Good behavior game: effects of individual contingencies for group consequences on disruptive behavior in a classroom. J Appl Behav Anal. 1969;2:119–124. doi: 10.1901/jaba.1969.2-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Gailliot M, DeWall CN, Oaten M. Self-regulation and personality: how interventions increase regulatory success, and how depletion moderates the effects of traits on behavior. J Pers. 2006;74:1773–1801. doi: 10.1111/j.1467-6494.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Tannock R. Methylphenidate improves Stroop naming speed, but not response interference, in children with attention deficit hyperactivity disorder. J Child Adol Psychop. 2002;12:301–309. doi: 10.1089/104454602762599844. [DOI] [PubMed] [Google Scholar]
- Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacol. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spect. 2008;13:1047–1055. doi: 10.1017/s1092852900017107. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nat Neurosci. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiat. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Botvin GJ, Kantor LW. Preventing alcohol and tobacco use through life skills training. Alcohol Res Health. 2000;24:250–257. [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Steiner N, Halfon O. The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci. 2013;7:59. doi: 10.3389/fnbeh.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J Comp Neurol. 2002a;450:241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol. 2002b;442:239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011a;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiat. 2011b;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Dumais K, Andersen SL. Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience. 2010;169:628–636. doi: 10.1016/j.neuroscience.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Lukkes JL, Andersen SL. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3:143–158. doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Bault N, Coricelli G, Blakemore SJ. Adolescents' heightened risk-seeking in a probabilistic gambling task. Cognitive Dev. 2010;25:183–196. doi: 10.1016/j.cogdev.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacol. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Caetano MS, Jin LE, Harenberg L, Stachenfeld KL, Arnsten AF, Laubach M. Noradrenergic control of error perseveration in medial prefrontal cortex. Front Integ Neurosci. 2012;6:125. doi: 10.3389/fnint.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA. Atomoxetine facilitates attentional set shifting in adolescent rats. Dev Cogn Neurosci. 2011;1:552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann NY Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano RF, Hawkins JD, Berglund ML, Pollard JA, Arthur MW. Prevention science and positive youth development: competitive or cooperative frameworks? J Adolescent Health. 2002;31:230–239. doi: 10.1016/s1054-139x(02)00496-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiat. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Robbins TW. Noradrenergic modulation of cognition: Therapeutic implications. J Psychopharmacol (Oxford, England) 2013;27:694–718. doi: 10.1177/0269881113480988. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Robbins TW, Sahakian BJ. The neurobiology of attention-deficit/hyperactivity disorder. Biol Psychiat. 2007;61:1317–1319. doi: 10.1016/j.biopsych.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiat. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Ko JH, Pellecchia G, Van Eimeren T, Cilia R, Strafella AP. Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stim. 2010;3:170–176. doi: 10.1016/j.brs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, Thatcher D, Clark DB. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug Alcohol Depen. 2011;115:43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Beh. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dennison U, McKernan D, Cryan J, Dinan T. Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychol Med. 2012;42:1865–1871. doi: 10.1017/S0033291712000074. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatr. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Kavanagh K, Schneiger A, Nelson S, Kaufman NK. Preventing early adolescent substance use: a family-centered strategy for the public middle school. Prev Sci. 2002;3:191–201. doi: 10.1023/a:1019994500301. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiat Clin N Am. 2012;35:495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Embry DD. The Good Behavior Game: a best practice candidate as a universal behavioral vaccine. Clin Child Fam Psychol Rev. 2002;5:273–297. doi: 10.1023/a:1020977107086. [DOI] [PubMed] [Google Scholar]
- Fallu JS, Janosz M, Briere FN, Descheneaux A, Vitaro F, Tremblay RE. Preventing disruptive boys from becoming heavy substance users during adolescence: a longitudinal study of familial and peer-related protective factors. Addict Behav. 2010;35:1074–1082. doi: 10.1016/j.addbeh.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix ED, Furlong MJ, Sharkey JD, Osher D. Implication for evaluating multi-component complex prevention initiatives: Taking guidance from the safe school/health students initiative. J School Viol. 2007;6:3–22. [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacol. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an "inverted-U" toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Pediatr Drugs. 2009;11:203–226. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214(2):557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. P Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. 2012;511:89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiat. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Jain S, Uhl GR. Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong? Pharmacol Ther. 2013;140(3):267–279. doi: 10.1016/j.pharmthera.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe HK, Coulter CL, Gerety ME, Sanders JD, O'Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiat. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology. 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht ML, Marsiglia FF, Elek E, Wagstaff DA, Kulis S, Dustman P, Miller-Day M. Culturally grounded substance use prevention: an evaluation of the keepin' it R.E.A.L. curriculum. Prev Sci. 2003;4:233–248. doi: 10.1023/a:1026016131401. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev Cogn Neurosci. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiat. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Karoly LA, Greenwood PW, Everingham SS, Hoube J, Kilburn MR, Rydell CP, Sanders M, Chiesa J. Santa Monica, Calif.: RAND Corportation, MR-898-TCWF; 1998. Investing in our children: what we know and don’t know about the costs and benefits of early childhood interventions. [Google Scholar]
- Kelamangalath L, Swant J, Stramiello M, Wagner JJ. The effects of extinction training in reducing the reinstatement of drug-seeking behavior: involvement of NMDA receptors. Behav Brain Res. 2007;185:119–128. doi: 10.1016/j.bbr.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bobeica I, Gamo NJ, Arnsten AF, Lee D. Effects of alpha-2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology. 2012;219:363–375. doi: 10.1007/s00213-011-2520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacol. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. P Natl Acad Sci USA. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Dev Psychobiol. 2011;53:724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res. 2004;151:137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB. Can you vaccinate against substance abuse? Expert Opin Biol Ther. 2013;13:1093–1097. doi: 10.1517/14712598.2013.791278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Fillman SG, Webster MJ, Shannon Weickert C. Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. Plos One. 2011;6:e22799. doi: 10.1371/journal.pone.0022799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacol. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Brain Res Dev Brain Res. 1991;62:109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev Neurosci. 2012;34:210–217. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Lawson K, Stone K, Andersen SL. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse (NY) 2008;62:185–192. doi: 10.1002/syn.20483. [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology. 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cereb Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Moore RY, Stenevi U. Mesencephalic dopamine neurons projecting to neocortex. Brain Res. 1974;81:325–331. doi: 10.1016/0006-8993(74)90947-0. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Sonntag KC, Andersen SL. Enduring effects of pharmacotherapies on female behavior and catecholamine receptors in an animal model of ADHD. SFN Abstracts. 2013;142.12 [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna-Adame M, Carrasco-Gimenez TJ, Rueda-Garcia Mdel M. Evaluation of the effectiveness of a smoking prevention program based on the 'Life Skills Training' approach. Health Educ Res. 2013;28:673–682. doi: 10.1093/her/cyt061. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen E, Dweck CS, Gross JJ. The hidden-zero effect: representing a single choice as an extended sequence reduces impulsive choice. Psychol Sci. 2008;19:648–649. doi: 10.1111/j.1467-9280.2008.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Andersen SL, Carlezon WA., Jr Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiat. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiat. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Masten AS, Obradovic J. Competence and resilience in development. Ann NY Acad Sci. 2006;1094:13–27. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- Masten AS, Roisman GI, Long JD, Burt KB, Obradovic J, Riley JR, Boelcke-Stennes K, Tellegen A. Developmental cascades: linking academic achievement and externalizing and internalizing symptoms over 20 years. Dev Psychol. 2005;41:733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Jones JL, Takahashi YK, Schoenbaum G. Learning theory: A driving force in understanding orbitofrontal function. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrino. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Therapeut. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T, Henrie D. Substance abuse prevention analysis. 2008 DHHS Pub. No. SMA 07-4298. [Google Scholar]
- Miller DT, Weinstein SM, Karniol R. Effects of age and self-verbalization on children’s ability to delay discounting. Dev Psychol. 1978;14:569–570. [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317:1210–1218. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91:753–760. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behav Brain Res. 2013;250:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Molliver ME, Grzanna R, Coyle JT. The intra-cortical trajectory of the coeruleo-cortical projection in the rat: a tangentially organized cortical afferent. Neuroscience. 1981;6:139–158. doi: 10.1016/0306-4522(81)90051-8. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, Peloso E, Maheu FS, Pine DS, Ernst M. Incentive effect on inhibitory control in adolescents with early-life stress: an antisaccade study. Child Abuse Neglect. 2012;36:217–225. doi: 10.1016/j.chiabu.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-cycloserine effects on extinction of conditioned responses to drug-related cues. Biol Psychiat. 2012;71:947–955. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Pychiat. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick AM, Forster GL, Tejani-Butt SM, Watt MJ. Adolescent social defeat alters markers of adult dopaminergic function. Brain Res Bull. 2011;86:123–128. doi: 10.1016/j.brainresbull.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacol. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mara SM, Sanchez-Vives MV, Brotons-Mas JR, O'Hare E. Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Prog Neuro-Psychoph. 2009;33:782–790. doi: 10.1016/j.pnpbp.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Oreland S, Pickering C, Gokturk C, Oreland L, Arborelius L, Nylander I. Two repeated maternal separation procedures differentially affect brain 5-hydroxytryptamine transporter and receptors in young and adult male and female rats. Brain Res. 2009;1305(Suppl):S37–S49. doi: 10.1016/j.brainres.2009.08.069. [DOI] [PubMed] [Google Scholar]
- Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. Substance abuse vaccines. Ann NY Acad Sci. 2008;1141:257–269. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacol. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiat. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicow K, Soler R, Braithwaite RL, Ahluwalia JS, Butler J. Cultural sensitivity in substance use prevention. J Community Psychol. 2000;28(3):271–290. [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacol. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiat. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology. 2013;227:403–411. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Seidman E, Pedersen S. Holistic contextual perspectives on risk, protection, and competency among urban adolescents. In: Luthar SS, editor. Resilience and vulnerability: Adaptation in the context of childhood adversities. Cambridge, England: Cambridge University Press; 2003. pp. 318–342. [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiat. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Overview of research methods and findings. Recent Dev Alcohol. 1991;9:301–326. [PubMed] [Google Scholar]
- Shibata H, Katsuki H, Nishiwaki M, Kume T, Kaneko S, Akaike A. Lipopolysaccharide-induced dopaminergic cell death in rat midbrain slice cultures: role of inducible nitric oxide synthase and protection by indomethacin. J Neurochem. 2003;86:1201–1212. doi: 10.1046/j.1471-4159.2003.01929.x. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater MD, Kelly KJ, Lawrence FR, Stanley LR, Comello ML. Assessing media campaigns linking marijuana non-use with autonomy and aspirations: "Be Under Your Own Influence" and ONDCP's "Above the Influence". Prev Sci. 2011;12(1):12–22. doi: 10.1007/s11121-010-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Brown CP, Levine S. Maternal responsiveness following differential pup treatment and mother-pup interactions. Horm Behav. 1977;8:242–253. doi: 10.1016/0018-506x(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cognitive Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Bickel WK. A developmental perspective on neuroeconomic mechanisms of contingency management. Psychol Addict Behav. 2013a;27:403–415. doi: 10.1037/a0028748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Elton A, Ryan SR, James GA, Budney AJ, Kilts CD. Neuroeconomics and adolescent substance abuse: individual differences in neural networks and delay discounting. J Am Acad Child Adolesc Psychiatry. 2013b;52:747–755. e6. doi: 10.1016/j.jaac.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiol Behav. 1985;34(6):1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology. 2012;221:317–327. doi: 10.1007/s00213-011-2578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Applied Studies; 2002. National and state estimates of the drug abuse treatment gap: 2000 national household survey on drug abuse. NSDUH Series H-14, DHHS Publication No. SMA 02-3640. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of applied studies; 2006. Results from the 2005 National Survey on Drug Use and Health: national findings. NSDUH Series H-30, DHHS Publication No. SMA 06-4194. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of applied studies; 2008. Results from the 2007 National Survey on Drug Use and Health: national findings. NSDUH Series H-34, DHHS Publication No. SMA 08-4343. [Google Scholar]
- Substance Abuse and Mental Health Services Administration; National Survey on Drug Use and Health. Ann Arbor, MI: Inter-university consortium for political and social research; 2011. ICPSR34481-v2. [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann NY Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM, Johnston LD. Exercise and substance use among American youth, 1991–2009. Am J Prev Med. 2011;40:530–540. doi: 10.1016/j.amepre.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, Volkow ND. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010;215:77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EA, Connelly CD, Thomas-Jones D, Eggert LL. School difficulties and co-occurring health risk factors: substance use, aggression, depression, and suicidal behaviors. J Child Adolesc Psychiatr Nurs. 2013;26:74–84. doi: 10.1111/jcap.12026. [DOI] [PubMed] [Google Scholar]
- van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJ. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacol. 2013;38(10):1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- van Rensburg K, Taylor A, Benattayallah A, Hodgson T. The effects of exercise on cigarette cravings and brain activation in response to smoking-related images. Psychopharmacology. 2012;221:659–666. doi: 10.1007/s00213-011-2610-z. [DOI] [PubMed] [Google Scholar]
- van Ryzin MJ, Fosco GM, Dishion TJ. Family and peer predictors of substance use from early adolescence to early adulthood: an 11-year prospective analysis. Addict Behav. 2012;37:1314–1324. doi: 10.1016/j.addbeh.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Giros B, Dauge V. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behav Pharmacol. 2006;17:715–724. doi: 10.1097/FBP.0b013e3280116e6f. [DOI] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23:1879–1885. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. P Natl Acad Sci USA. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacol. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waylen A, Wolke D. Sex 'n' drugs 'n' rock 'n' roll: the meaning and social consequences of pubertal timing. Eur J Endocrinol. 2004;151(Suppl 3):U151–U159. doi: 10.1530/eje.0.151u151. [DOI] [PubMed] [Google Scholar]
- Weed MR, Bryant R, Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157:22–28. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacol. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Westling E, Andrews JA, Hampson SE, Peterson M. Pubertal timing and substance use: the effects of gender, parental monitoring and deviant peers. J Adolesc Health. 2008;42:555–563. doi: 10.1016/j.jadohealth.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Buchel C, Byrne M, Cummins TD, Fauth-Buhler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, Stephens DN, Struve M, Thyreau B, Vollstaedt-Klein S, Robbins TW, Schumann G, Garavan H. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav Immun. 2013;28:218–226. doi: 10.1016/j.bbi.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adamson J, Monuteaux MC, Faraone SV, Schillinger M, Westerberg D, Biederman J. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediat Adol Med. 2008;162:916–921. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J. Alcohol, drugs, and attention-deficit/ hyperactivity disorder: a model for the study of addictions in youth. J Psychopharmacol. 2006;20:580–588. doi: 10.1177/0269881105058776. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, Biederman J. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. 2011;50:543–553. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacol. 2004a;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004b;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004c;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacol. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiat. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang ZW. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci. 2003;23:3373–3384. doi: 10.1523/JNEUROSCI.23-08-03373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology. 2012;224:387–400. doi: 10.1007/s00213-012-2760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]