Abstract
Background
Major depressive disorder (MDD) affects more than 15% of the population across their lifespan. In this study, we used the well-characterized unpredictable chronic mild stress (CMS) model of depression to examine this condition.
Methods
Sprague-Dawley rats were presented randomly with mild stressors for four weeks, with body weight and sucrose intake monitored weekly. Locomotor activity and elevated plus maze test/forced swim test were conducted on week 5; ventral tegmental area (VTA) dopamine (DA) neuron activity was assessed within a week after the behavioral test using three indices: DA neuron population activity (defined as the number of spontaneously firing DA neurons), mean firing rate, and percent burst firing (i.e., the proportion of action potentials occurring in bursts).
Results
Consistent with previous studies, we found that, compared to controls, rats that underwent the CMS procedure were slower in gaining body weight, and developed anxiety- and despair-like behavior. We now report a significant decrease in DA neuron population activity of CMS rats, and this decrease is restored by pharmacologically attenuating the activity of either the basolateral nucleus of the amygdala (BLA), or the ventral pallidum (VP). Moreover, pharmacological activation of the amygdala in non-stressed rats decreases DA neuron population activity similar to that with CMS, which is reversed by blocking the BLA-VP pathway.
Conclusions
The CMS rat depression model is associated with a BLA-VP-VTA inhibition of DA neuron activity. This information can provide insight into the circuitry underlying MDD and serve as a template for refining therapeutic approaches to this disorder.
Keywords: unpredictable chronic mild stress, rat, ventral tegmental area, amygdala, ventral pallidum, dopamine
Introduction
Major depressive disorder (MDD) is a complex disorder involving anhedonia, anxiety, and behavioral despair, each of which is associated with alterations in the dopamine (DA) system (1-7). Nonetheless, evidence implicating the DA system in MDD is only recently emerging (8-10). In addition, the basolateral nucleus of the amygdala (BLA) shows increased volume (11-13) and hyper-responsiveness to stress and aversive emotional stimuli (14, 15) in MDD. Previously, we demonstrated that acute restraint stress induced a delayed decrease in DA system responsivity that is reversed by pharmacologically attenuating BLA activity (16). Together, these data suggest that the BLA may be involved in a diminished VTA DA neuron response in MDD.
The unpredictable chronic mild stress (CMS) procedure is a validated animal model of human depression (17). Physical and psychological mild stressors in this procedure induce anxiety-, despair-, and anhedonia-like behaviors in rodents. We used the CMS procedure to study the changes in VTA DA responsivity under depression. We propose that the CMS-induced decrease in VTA DA activity is mediated via the BLA-ventral pallidum (VP)-VTA pathway, given that the BLA projects to the VP (18) and the VP potently inhibits VTA DA neuron population activity (19).
Methods and materials
Subjects and materials
Male Sprague-Dawley rats (300-400g; Harlan Laboratories) were housed for at least five days in pairs in a temperature (22°C)- and humidity (47%)-controlled facility upon arrival on a 12 h light/dark cycle (lights on at 7:00 a.m.) with food and water available ad libitum. Animals were handled in accordance with the guidelines outlined in the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Unpredictable chronic mild stress (CMS) procedure
All rats that underwent the CMS procedure were single housed. Stressors were randomly presented each week (3-4 per week depending on the week) over four weeks (20, 21) (Figure 1). The stressors included food deprivation, water deprivation followed by 1-hr of empty bottle presentation, overnight illumination, homecage tilting, damp bedding, foreign intruder, strobe light illumination, and presentation of predator odor. Age and body weight matched control rats were housed in pairs over the equivalent period of time, except a subgroup that were single housed in order to sample individual intake of sucrose solution.
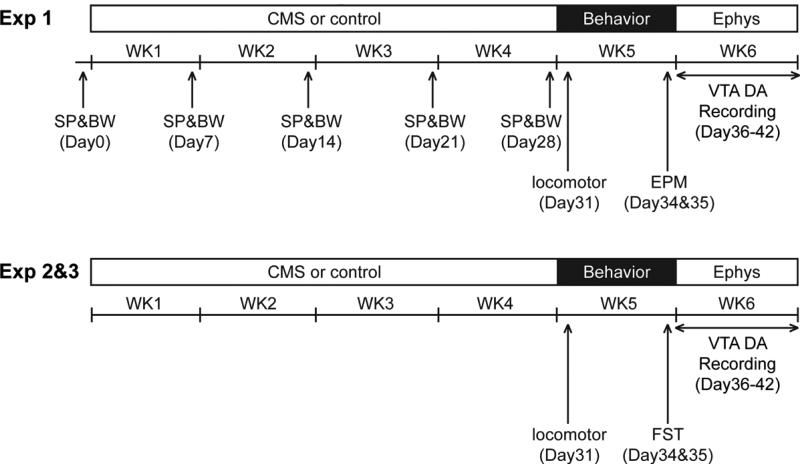
Figure 1.
Experimental timeline for rats that underwent the CMS procedure. SP, sucrose preference test; BW, body weight; EPM, elevated plus maze test; FST, forced swim test; Ephys, electrophysiology.
Behavioral assays
Body weight (BW) and sucrose preference test (SP)
Rats acclimated for five days were presented with two drinking bottles, one with 1% sucrose solution and one with water (22). Body weight and percentage of 1% sucrose solution intake over 24 hrs (9 a.m. to 9 a.m. the next day) were set as baseline (BL; Day 0), and rats were divided into two matched groups: One subjected to CMS procedure (CMS), and one control (CON). Two drinking bottles were present at all times with the position alternated daily. At the end of each week (Days 7, 14, 21, 28), the percentage of 1% sucrose solution intake over 24 hrs was sampled, and body weight was measured.
Locomotor activity
General locomotor activity indexed as the total distance traveled (cm) was measured (Day 31; 9 a.m. to 12 p.m.) by placing the rat into an open-field arena (Coulbourn Instruments). Spontaneous activity was monitored for 10 min by beam breaks using TruScan software (Coulbourn Instruments).
Elevated plus maze (EPM)
One day before the test (Day 34), all rats were handled and acclimated for two hours (12 p.m. to 2 p.m.) to the elevated plus maze (Med Associates Inc.) room. On the test day (Day 35; 9 a.m. to 12 p.m.), each rat was placed onto the maze facing one of the closed arms. Over five minutes, the total time spent in the open arms as the percentage of total time spent in the open plus closed arms, and the open arm entries as the percentage of total entries in open plus closed arms, were measured (23).
Forced swim test (FST)
The forced swim test took place in the cylinder (50 cm in height × 20 cm in diameter) filled with water (23-25 °C) to 30 cm. One day before the test (Day 34; 9 a.m. to 5 p.m.), a pre-exposure of 15 min swimming was given to ensure that the rats quickly adopt an immobile posture on the test day. On the test day (Day 35; 9 a.m. to 5 p.m.), each rat was placed in the cylinder for 5 min, and the total time spent immobile was measured (24).
Electrophysiological recordings
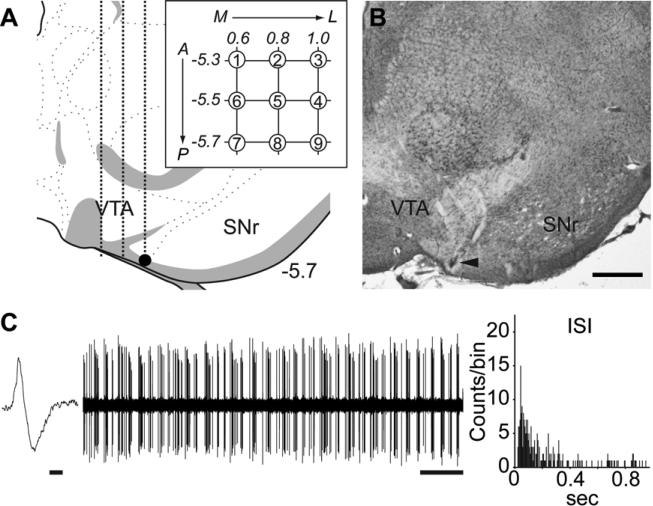
Single-unit extracellular recordings were performed on rats (9 a.m. to 6 p.m.) anesthetized with 8% chloral hydrate (400 mg/kg, i.p.) and VTA recordings [from bregma: AP −5.3 to −5.7 mm; ML −0.6 to −1.0 mm; ventral from brain surface: −6.5 to −9.0 mm] were made as previously described (16). DA neurons were identified with open filter setting (50-16k Hz bandpass) and distinguished by their unique long-duration waveform (> 2.5 ms), slow irregular firing rate, and other well-established criteria (25, 26), which enabled accurate identification of the large majority of DA neurons recorded (27). Three parameters of VTA DA neuron activity were calculated. (1) Population activity, or the relative number of spontaneously firing DA neurons, was assessed by passing the electrode through the VTA in a predetermined grid pattern of 9 tracks separated by 200 μm; all spontaneously active DA neurons encountered per electrode track were counted (Figure 2A and 2B). For each DA neuron, 3 min of activity was recorded to determine the (2) firing rate and (3) percentage burst firing (Figure 2C).
Figure 2.
Evaluation of VTA DA neuron activity states. (A) Glass electrodes filled with 2% Pontamine sky blue in 2 M NaCl with in situ impedance of 4–10 MΩ measured at 1kHz, were lowered into the VTA [from bregma: AP −5.3 to −5.7 mm; ML −0.6 to −1.0 mm; from brain surface: DV −6.5 to −9.0 mm]. Population activity was assessed by passing the electrode through the VTA in a predetermined grid pattern (3 × 3) of nine tracks separated by 0.2 mm (inset). All spontaneously active DA neurons encountered per electrode track were counted. The final location of the last track was marked by electrophoretic ejection of dye (filled dot) for histological verification. (B) A representative Pontamine sky blue deposit. Arrowhead indicates the final location of the electrode. Scale bar: 500 μm. (C) 3 min of spontaneous activity was recorded for every DA neurons isolated to determine firing rate and percentage burst firing. Figures show a representative waveform of DA neuron (Scale bar: 1 ms) and 1 min segment of spontaneous activity (Scale bar: 5 s). Percentage burst firing was quantified by examining the proportion of action potentials occurring in bursts, with bursts defined as the occurrence of two spikes with an interspike interval (ISI) of <80 ms indicating the initiation of a burst, and two spikes with an ISI >160 ms signaling burst termination. Burst firing was robust for the DA neuron. Coronal brain section image in (A) adapted from Swanson (52).
For Exp 1-3 (CMS), the recordings were done on Days 36-42, with the recording order counterbalanced among groups. For Exp 4 and 5, recordings were conducted on non-stressed, naïve rats.
Drug administration
For local drug administration, a 28 gauge stainless-steel cannula (Plastic One) was lowered into the BLA [relative to bregma: AP −2.9 mm; ML +5.0 mm; DV −8.6 mm] or the VP [relative to bregma: AP −0.3 mm; ML +2.0 mm; DV −7.8 mm]. All drugs were freshly mixed in Dulbecco's PBS buffer (VEH) (Sigma), with the doses chosen based on previously published reports. Depending on the design of each experiment, 0.2 μl of tetrodotoxin (TTX; 1 μM) (Sigma) (28), N-Methyl-D-aspartic acid (NMDA; 0.75 μg) (Sigma) (28), or VEH, was infused into the BLA, while 0.5 μl of kynuneric acid (KYN; 5 μg) (Sigma) (28) or VEH was infused into the VP. Drugs were delivered at 0.2 μl/min, and another one minute was allowed for diffusion. The cannula was then removed, immediately followed by single unit recordings of VTA DA neurons.
Data analysis and statistics
Single-unit neuron activity was analyzed using Powerlab (AD Instruments) and Nex (NEX Technologies) software. All data are represented as the mean ± SEM and were submitted to analysis of variance (ANOVA) or t-test. Post hoc comparisons using Fisher's LSD test was performed for ANOVAs that achieved a significance of p < 0.05. All statistics were calculated using SPSS (IBM) or SigmaStat (Systat Software Inc.).
Results
Exp 1: CMS-treated rats exhibited slower weight gain, anxiety-like behavior, and an attenuation of VTA DA neuron population activity
Rats were exposed to the CMS procedure (n=14) or controls (n=14), with sucrose preference measured in a subgroup of rats (n = 8; CON and CMS, respectively) (Figure 1).
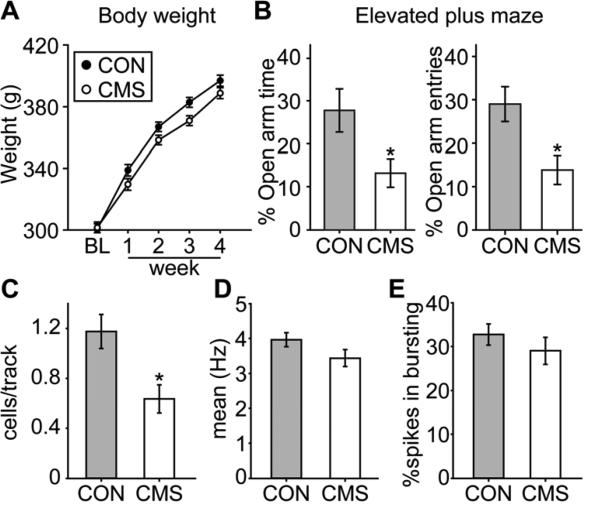
CMS rats were slower in gaining body weight over the four weeks of the CMS procedure (Figure 3A; ANOVA significant main effect of “week” [F(4,104) = 790.86, p < 0.001] and a significant interaction between “group” and “week” [F(4, 104) = 3.63, p = 0.008]). We did not find an alteration in sucrose preference (index of anhedonia) in that the percentage of 1% sucrose solution intake was always above 90% for both groups (no difference between the main effect of “group” [F(1,14) = 1.12, p = 0.307], “week” [F(4, 56) = 1.26, p = 0.297], or the interaction between “group” and “week” [F(4,56) < 1]). CMS rats did not differ from controls in their locomotor activity measured by the distance travelled in 10 min [CON = 2409.9 ± 73.2, CMS = 2341.2 ± 84.4; t(26) = 0.62, p = 0.544]. By the end of the procedure, CMS rats developed anxiety-like behavior as indexed by the elevated plus maze test (Figure 3B). CMS rats spent less time in the open arms over 5 min [Figure 3B left; t(26) = 2.43, p = 0.022], and had fewer entries into the open arms [Figure 3B right; t(26) = 2.91, p = 0.007]. The total visits of different arms were equivalent between groups [CON = 14.3 ± 0.7, CMS = 14.5 ± 0.7; t(26) = 0.07, p = 0.943], suggesting that the lower percentage of open arm entries was not due to decreased movement.
Figure 3.
Rats that underwent the CMS procedure were slower in gaining body weight and developed anxiety-like behavior, and there was a significant decrease in VTA DA neuron population activity. (A) CMS rats (n = 14) were significantly slower in gaining body weight compared to controls (CON; n = 14) (p < 0.05), and (B) developed anxiety-like behavior with less total time spent in the open arms (left) and fewer entries into the open arms (right) on the elevated plus maze (*, p < 0.05). (C) DA neuron population activity in CMS rats (n = 14, 80 neurons) was significantly lower than CON rats (n = 13, 134 neurons) (*, p < 0.05), with no significant differences in (D) mean firing rates or (E) bursting activity.
One rat was excluded from data analysis due to electrode misplacement, yielding the final group sizes: CON (n = 13; 134 neurons) and CMS (n = 14; 80 neurons). CMS-exposed rats had 50% fewer DA neuron population activity [Figure 3C; t(25) = 3.06, p = 0.005]. No differences in average firing rate [Figure 3D; t(212) = 1.64, p = 0.102] or percent of spikes in bursts [Figure 3E; t(212) = 0.94, p = 0.35] were observed.
Exp 2: CMS-exposed rats showed deficits in forced swim and decreased VTA activity; attenuation of BLA restored DA neuron activity
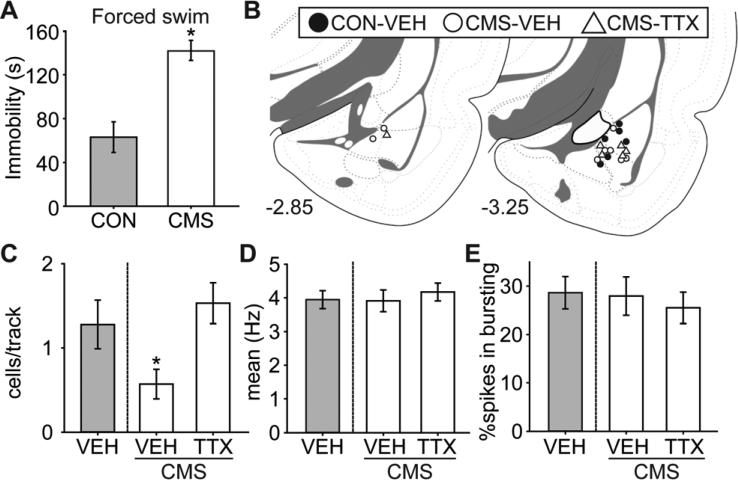
CMS-exposed (n=16) or control (n=8) rats were first tested in the forced swim test (Figure 1). We then tested whether the CMS-induced decrease in DA neuron population activity was restored by attenuating BLA activity with local infusion of the sodium channel blocker, tetrodotoxin (TTX).
Compared to controls, CMS rats did not show fatigue; indeed, the rats actually showed higher levels of locomotor activity. CMS rats travelled a longer distance in 10 min compared to controls [CON = 2162.9 ± 89.5, CMS = 2448.2 ± 66.4; t(22) = 2.52, p = 0.02]. By the end of the CMS exposure, rats developed despair-like behavior as assessed by the forced swim test (Figure 4A), in that they spent more time in immobility over the 5 min test period [t(22) = 4.90, p < 0.001].
Figure 4.
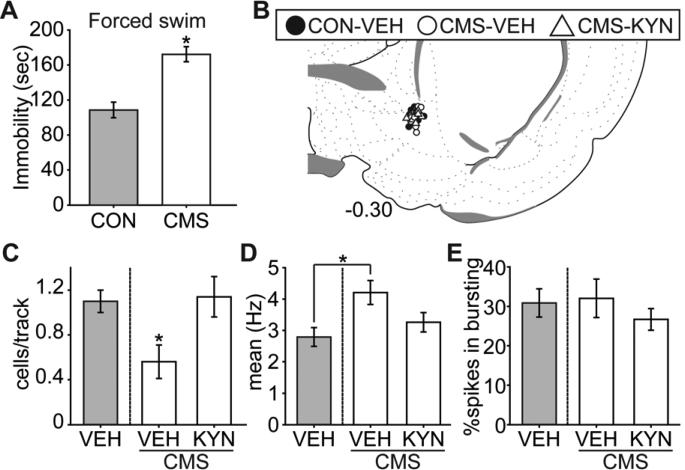
Rats that underwent the CMS procedure developed deficits in the forced swim test, which has been associated with despair-like behavior, and there was a significant decrease in VTA DA neuron population activity that was restored by attenuating BLA activity. (A) CMS rats (n = 16) exhibited more time spent in immobility compared to controls (CON; n = 8) in the forced swim test (*, p < 0.05). CMS rats were then divided into two groups matched for forced swim behavior (n = 8 each), and received local BLA infusion of tetrodotoxin (TTX) or vehicle (VEH; CON and CMS) immediately before DA recordings. (B) Cannulae placements for all rats included in this study. (C) DA neuron population activity in CMS rats (VEH, white bar; n = 8, 41 neurons) was significantly lower than CON rats (VEH, gray bar; n = 6, 69 neurons) (*, p < 0.05), which was restored to control level by attenuating BLA activity with TTX (n = 6, 81 neurons) infusion. There was no significant difference among groups in (D) mean firing rates or (E) bursting activity. Coronal brain section images in (B) adapted from Swanson (52).
CMS rats were divided into two groups (n = 8 each) matched for time spent in immobility in the FST, and assigned to local BLA infusion (Figure 4B) of vehicle or tetrodotoxin immediately before recording VTA DA neuron activity. Given the small injection volume compared to other relevant studies (29, 30), the spread of the infused drug is estimated to remain within the BLA. Rats with cannulae and/or electrode misplacement were excluded from data analysis, yielding the final group sizes: CON-VEH (n = 6; 69 neurons), CMS-VEH (n = 8; 41 neurons), and CMS-TTX (n = 6; 81 neurons). CMS-exposed rats had 50% fewer DA neuron population activity, which was restored to control levels following attenuation of BLA activity with tetrodotoxin (Figure 4C; ANOVA significant main effect of “group” [F(2,19) = 5.00, p = 0.02]). Post hoc analysis revealed that the DA neuron population activity was significantly lower in the CMS-VEH group compared to others (both ps < 0.05), with no significant difference between CON-VEH and CMS-TTX. No differences in mean firing rate [Figure 4D; F(2,190) < 1] or percent of spikes fired in bursts [Figure 4E; F(2, 190) < 1] were seen .
Exp 3: CMS-exposed rats showed deficits in forced swim and decreased VTA activity; blockade of VP glutamatergic inputs restored DA neuron activity
CMS-exposed (n=16) or control (n=8) rats were first tested in the FST (Figure 1). We then tested whether the CMS-induced decrease in DA neuron population activity was restored by blocking glutamatergic inputs onto VP, a brain region that receives glutamatergic BLA afferents (18) and that attenuates VTA DA neuron population activity, using the broad-spectrum glutamate receptor antagonist, kynurenic acid (KYN) (28, 31).
Data of one rat was lost due to computer error, leading to the final group sizes: CON (n = 8) and CMS (n = 15). CMS rats did not differ from controls in their locomotor activity measured by the distance travelled in 10 min [CON = 2272.18 ± 190.99, CMS = 2275.93 ± 66.19; t(21) = 0.02, p = 0.982]. By the end of the CMS exposure, CMS rats spent more time in immobility over the 5 min test period in FST [Figure 5A; t(22) = 4.61, p < 0.001].
Figure 5.
The decrease in VTA DA neuron population activity observed in CMS rats was restored by blockade of VP glutamatergic inputs. (A) As in Figure 4, CMS rats (n = 16) exhibited more time spent in immobility compared to controls (CON; n = 8) in the forced swim test (*, p < 0.05). CMS rats were then divided into two groups matched for forced swim behavior (n = 8 each), and received local VP infusion of kynurenic acid (KYN) or vehicle (VEH; CON and CMS) immediately before DA recordings. (B) Cannulae placements for all rats included in this study. (C) DA neuron population activity in CMS rats (VEH, white bar; n = 7, 35 neurons) was significantly lower than CON rats (VEH, gray bar; n = 7, 69 neurons) (*, p < 0.05), and was restored to control level by blockade of VP glutamate inputs with KYN (n = 8, 82 neurons) infusion. (D) The mean firing rate of CMS-VEH was significantly higher than CON-VEH, but there was no significant difference in (E) bursting activity. Coronal brain section image in (B) adapted from Swanson (52).
CMS rats were divided into two groups (n = 8 each) matched for time spent in immobility, and assigned to local VP infusion (Figure 5B) of vehicle or kynurenic acid immediately before VTA DA neuron recording. The small volume of VP injection is comparable to other relevant studies (32), and the spread of the infused drug is estimated to remain within the confines of the VP. Rats with misplaced cannulae/electrodes were excluded, yielding the final group sizes: CON-VEH (n = 7; 69 neurons), CMS-VEH (n = 7; 35 neurons), and CMS-KYN (n = 8; 82 neurons). CMS-exposed rats had 50% fewer DA neuron population activity that was restored to control levels following kynurenic acid infusion into VP (Figure 5C; ANOVA significant main effect of “group” [F(2,19) = 4.67, p = 0.022]). Post hoc analysis revealed that the DA neuron population activity was significantly lower in the CMS-VEH group compared to others (both ps < 0.05), with no significant difference between CON-VEH and CMS-KYN. We observed a significant difference in mean firing rate among groups [Figure 5D; F(2, 183) = 3.43, p = 0.035], with the firing rate of CMS-VEH significantly higher than CON-VEH. No difference in percent of spikes fired in bursts was seen [Figure 5E; F(2,183) < 1].
Exp 4 and 5: The BLA-VP pathway mediates the BLA-driven decrease in VTA DA neuron population activity
The CMS-induced decrease in VTA DA neuron population activity appears to be mediated by the BLA, a region known to be activated by stress (33), which has direct projections onto the VP (18), a brain region that then has inhibitory regulation on the VTA DA neuron population activity (19). To further delineate the pathway, we used pharmacological manipulation in naïve, non-stressed rats, to test: 1) how direct augmentation or attenuation of BLA activity using N-methyl-D-aspartic acid (NMDA) or TTX infusion, respectively, alters VTA DA neuron activity (Exp 4), and 2) whether the BLA-VP pathway is critical in modulating BLA-driven alterations in DA neuron population activity (Exp 5).
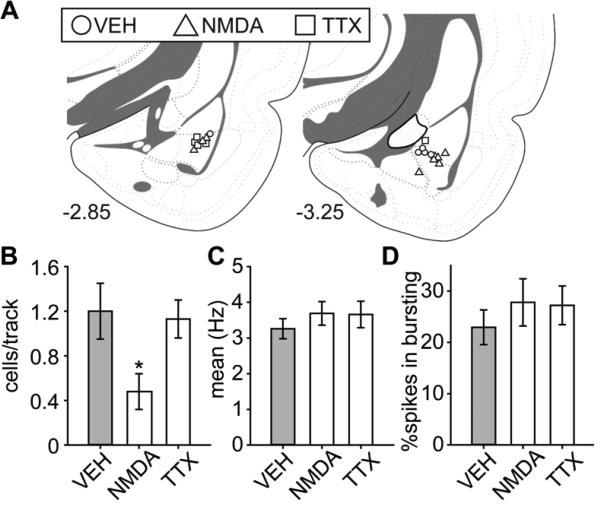
Figure 6A summarized the infusion site of BLA; excluding rats with cannulae/electrode misplacements yielded final group sizes: VEH (n = 5; 54 neurons), NMDA (n = 9; 39 neurons), and TTX (n = 5; 49 neurons). NMDA-induced activation of the BLA decreased VTA DA neurons firing spontaneously by 60%, which is consistent with that observed following CMS; BLA inactivation with TTX had no effect (Figure 6B; ANOVA significant main effect of “group” [F(2,16) = 5.04, p = 0.02]). Post hoc tests revealed that DA neuron population activity was significantly lower in the NMDA group compared to VEH and TTX (both ps < 0.05), with no significant difference between VEH and TTX. No differences in the average firing rate [Figure 6C; F(2,139) < 1] or percent of spikes in bursts [Figure 6D; F(2,139) < 1] were observed.
Figure 6.
Activation of the BLA decreased VTA DA neuron population activity. (A) Cannulae placements for all rats included in this study. (B) Activation of the BLA with NMDA (n = 9, 39 neurons) significantly decreased VTA DA neuron population activity compared to controls (VEH; n = 5, 54 neurons) (*, p < 0.05), with no significant difference between (C) mean firing rates or (D) bursting activity. Blocking BLA activity with TTX (n = 5, 49 neurons) had no effect on DA neuron activity. Coronal brain section images in (A) adapted from Swanson (52).
We next explored whether the BLA-VP pathway is critical in modulating BLA-driven decreases in DA neuron population activity. The BLA was activated with NMDA as above and the broad-spectrum glutamate antagonist kynurenic acid (KYN) was locally infused into the VP.
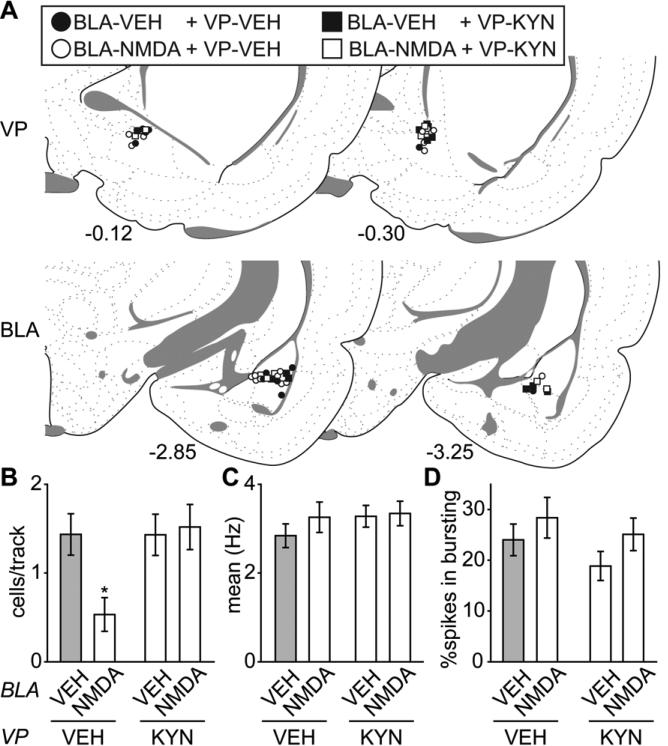
Excluding rats with cannulae/electrode misplacements yielded final group sizes: “BLA-VEH + VP-VEH” (n = 6; 71 neurons), “BLA-NMDA + VP-VEH” (n = 9; 43 neurons), “BLA-VEH + VP-KYN” (n = 6; 85 neurons), and “BLA-NMDA + VP-KYN” (n = 5; 67 neurons) (cannula placements in Figure 7A). Compared to controls (“BLAVEH + VP-VEH”), activation of the BLA with NMDA (“BLA-NMDA + VP-VEH”) produced a 65% decrease in VTA DA neuron population activity. However, blocking the glutamate inputs in the VP with KYN (“BLA-NMDA + VP-KYN”) completely prevented the BLA-mediated decrease in population activity (Figure 7B; ANOVA significant main effect of “VP (KYN/VEH)” [F(1,22) = 4.59, p = 0.044] and a significant interaction between “BLA (NMDA/VEH)” and “VP (KYN/VEH)” [F(1,22) = 4.68, p = 0.042]). Post hoc tests revealed that the DA neuron population activity was significantly lower in the “BLA-NMDA + VP-VEH” group compared to all other groups (all ps < 0.05). Local infusion of KYN itself had no effects on VTA DA neuron population activity. There was no difference between group of “BLA-VEH + VP-KYN” and control group of “BLA-VEH + VP-VEH”. Moreover, no differences in the average firing rate [Figure 7C; all Fs (1,262) < 1] or percent of spikes in bursts [Figure 7D; “BLA (NMDA/VEH)” F(1,262) = 2.53, p = 0.11; “VP (KYN/VEH)” F(1,262) = 1.60, p = 0.21; “interaction” F(1,262) < 1] were observed.
Figure 7.
The BLA-VP pathway mediates the BLA activation-induced decrease in VTA DA neuron population activity. (A) Cannulae placements for all rats included in this study. (B) Activation of the BLA with NMDA (BLA-NMDA + VP-VEH; n = 9, 43 neurons) significantly decreased VTA DA neuron population activity compared to controls (BLA-VEH + VP-VEH; n = 6, 71 neurons) (*, p < 0.05), which was restored to baseline by blocking VP glutamatergic drive with kynurenic acid infusion (KYN) (BLANMDA + VP-KYN; n = 5, 67 neurons). Local infusion of KYN itself (BLA-VEH + VPKYN; n = 6, 85 neurons) had no effect on VTA DA neuron population activity. There was no significant difference among groups for (C) mean firing rates or (D) bursting activity. Coronal brain section images in (A) adapted from Swanson (52).
Discussion
In this study, we found that the CMS model of depression resulted in a slower body weight gain and development of anxiety- and despair-like behaviors. There was a significant decrease in VTA DA neuron population activity, which was mediated through the BLA-VP pathway. Such decreases in the number of spontaneously firing DA neurons would be expected to greatly lessen the amplitude of the DA response to stimuli (34), which is proposed to decrease the rewarding value of external stimuli.
CMS procedure is proposed to mimic socio-environmental stressors in inducing symptoms that share some characteristics of human depression, such as increased fearful/anxiety-like behavior (20, 35), decreased consumption of palatable food and physiological changes (36), and loss of motivation (8, 36). Importantly, the deteriorated performance of the tasks due to CMS procedure improves with chronic anti-depressant medication treatment, supporting its validity as an animal model (37, 38). Emotionality in rodents is best characterized by the use of complementary behavioral assays (20). In this study, we assessed multiple dimensions of depressive behavioral phenotypes using tasks including long-term monitoring of the body weight (physiological changes), sucrose preference test (anhedonia), locomotor activity, elevated plus maze test (anxiety-like behavior), and forced swim test (motivation/despair-like behavior). We found that rats chronically exposed to these stressful conditions were slower in gaining body weight. They were more anxious (20), in that they were less willing to explore or stay in the open arms on the elevated plus maze. They also showed a sign of behavioral despair (24), in that they spent more time in immobility in the forced swim test. These behavioral deteriorations were not due to differences in motor ability. Compared to controls, the CMS rats either did not show differences (Exp 1 and 3), or were even more agitated (Exp 2), in locomotor activity. Moreover, both control and CMS procedure groups showed equivalent total number of entries into different arms on the elevated plus maze, showing that motor impairment was not a factor. Although no deficit in sucrose preference was observed, the robustness of the sucrose preference test or its validity in tests of depression has been a subject of controversy (39, 40); furthermore, the less intense CMS procedure used here compared to those that presented different stressors twice daily for up to 8-12 weeks (8) may be a factor. Given these results, it is unclear whether a more severe CMS procedure (8) would have had a more significant impact on sucrose preference as compared to the standard procedure used here (21, 41), or whether anhedonia was indeed induced but not detected because the sucrose preference test itself was not sufficiently sensitive. It is worth noting that convergent, rather than consistent, symptoms are the core of human depression (20, 42), and in several instances the measures are opposite in nature (e.g., decreased food intake/increased food intake) (43). Nonetheless, we demonstrated that a set of behavioral assays covering different dimensions of depressive behavioral phenotypes are the consequence of CMS exposure in our experiments.
There are two major activity states of DA neurons relevant to their function. DA neurons exhibit burst firing when the behaving animals encounter a salient stimulus, such as one predicting reward (44). However, in order to exhibit burst discharge, the DA neuron must be firing spontaneously (19, 32). Thus, the proportion of DA neurons firing spontaneously, the population activity, determines the amplitude of the DA phasic burst response, serving as an amplification factor of the salience signal (34). We found that for CMS rats, there was a significant decrease in VTA DA neuron population activity. This would attenuate the amplitude of the phasic burst firing response (19, 34) when the behaving animals encounter a salient stimulus (44), making these individuals less capable of attending to a positive affective response to external, normally salient or rewarding events. This is consistent with what has been reported in depression (45-47). In Exp 3 (Figure 5), rats did show higher firing rates in CMS-VEH vs CON-VEH, which was not present in other experiments. While the origin of this discrepancy is unclear, it is interesting to note that KYN VP-infusion did restore firing rate to baseline.
One region likely to be involved in CMS-induced changes is the BLA because of its well-known involvement in aversive (stressful) learning (48, 49). Indeed, the BLA exhibits hyper-responsiveness to stress and aversive emotional stimuli (14, 15) in MDD. Our data suggested that attenuating BLA activity reversed the CMS-induced decrease in DA neuron population activity. Moreover, activation of the BLA in non-stressed, naïve rats with NMDA local infusion significantly decreased VTA DA neuron population activity without affecting average firing rate or firing pattern, which mimicked that observed in the CMS rats. There are several pathways through which the BLA can indirectly exert potent actions on VTA DA neurons. We specifically explored the BLA-VP-VTA pathway, given that the BLA has direct excitatory projections to the VP (18), and previous studies showed that the VP exerts specific effects on VTA DA neuron population activity without substantially affecting average firing rate or burst firing (34). We showed that the BLA activation-induced decrease in DA neuron population activity was prevented by blocking glutamate inputs into the VP. Although we cannot rule out the possibility that glutamatergic afferents from other regions activated by the amygdala, such as the infralimbic cortex (50), also drive the VP, our data confirm that the VP is at least one critical component involved in BLA modulation of the VTA. The VP is thus placed in a strategic position in that its inhibition (via the ventral subiculum-nucleus accumbens pathway) will increase VTA DA neuron population activity (19), whereas its activation (via the BLA and/or other excitatory inputs) will attenuate VTA DA neuron population activity.
These data extend previous findings of stress, DA and depression. We have shown that acute stressors activate the DA system (51), which is followed 24 hours later by potently diminished DA neuron activity and attenuated response to amphetamine (16). The initial increase in DA activity is also consistent with data showing DA activation facilitates induction of depressed behavior (9), and that activation of the VTA reverses behavioral depression (8). Therefore, the DA system appears to play a role both in the induction and expression of some depressive behaviors. These results provide valuable information regarding the neural circuitry underlying MDD, which is critical in the development of potential new and more effective pharmacotherapeutic approaches that selectively target these key regions in MDD.
Acknowledgements
This work was supported by USPHS MH57440 (A. A. G.). We thank Nicole MacMurdo for her assistance in histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
C.-h. C. and A. A. G. designed the research; C.-h. C. performed the research; C.-h. C. analyzed data; C.-h. C. and A. A. G. wrote the paper.
Financial disclosures
Dr. Chang reports no biomedical financial interests or potential conflicts of interest. Dr. Grace received funds from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, and Asubio.
References
- 1.Huang AC, Hsiao S. Haloperidol attenuates rewarding and aversively conditioned suppression of saccharin solution intake: reevaluation of the anhedonia hypothesis of dopamine blocking. Behav Neurosci. 2002;116:646–650. doi: 10.1037//0735-7044.116.4.646. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli S, Drago A, Fabbri C, Serretti A. Mechanisms of antidepressant action: an integrated dopaminergic perspective. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1532–1543. doi: 10.1016/j.pnpbp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem Soc Trans. 2009;37:313–317. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- 4.Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- 5.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci. 2012;35:1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savitz J, Hodgkinson CA, Martin-Soelch C, Shen PH, Szczepanik J, Nugent A, et al. The Functional DRD3 Ser9Gly Polymorphism (rs6280) Is Pleiotropic, Affecting Reward as Well as Movement. PLoS One. 2013;8:e54108. doi: 10.1371/journal.pone.0054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A, et al. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165:872–880. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eijndhoven P, van Wingen G, van Oijen K, Rijpkema M, Goraj B, Jan Verkes R, et al. Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry. 2009;65:812–818. doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 15.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 16.Chang CH, Grace AA. Amygdala beta-Noradrenergic Receptors Modulate Delayed Downregulation of Dopamine Activity following Restraint. J Neurosci. 2013;33:1441–1450. doi: 10.1523/JNEUROSCI.2420-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maslowski-Cobuzzi RJ, Napier TC. Activation of dopaminergic neurons modulates ventral pallidal responses evoked by amygdala stimulation. Neuroscience. 1994;62:1103–1119. doi: 10.1016/0306-4522(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 19.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31. doi: 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung M, Lu L, Hughes AM, Treit D, Dickson CT. FG7142, yohimbine, and betaCCE produce anxiogenic-like effects in the elevated plus-maze but do not affect brainstem activated hippocampal theta. Neuropharmacology. 2013;75C:47–52. doi: 10.1016/j.neuropharm.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 25.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 26.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Ungless MA, Grace AA. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;27:27. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmslie KS, Yoshikami D. Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord. Brain Res. 1985;330:265–272. doi: 10.1016/0006-8993(85)90685-7. [DOI] [PubMed] [Google Scholar]
- 32.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 33.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 34.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 35.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 36.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 37.Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid Receptor and FKBP5 Expression Is Altered Following Exposure to Chronic Stress: Modulation by Antidepressant Treatment. Neuropsychopharmacology. 2012;21:225. doi: 10.1038/npp.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss JM. Does decreased sucrose intake indicate loss of preference in CMS model? Psychopharmacology (Berl) 1997;134:368–370. doi: 10.1007/s002130050472. discussion 371-367. [DOI] [PubMed] [Google Scholar]
- 40.Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- 41.Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 43.First MB, Frances A, Pincus HA. DSM-IV-TR Guidebook. American Psychiatric Publishing; 2004. [Google Scholar]
- 44.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Wang CE, Brennen T, Holte A. Decreased approach motivation in depression. Scand J Psychol. 2006;47:505–511. doi: 10.1111/j.1467-9450.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 46.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Percept Mot Skills. 2001;92:857–868. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]
- 48.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 49.Johnson LR, Hou M, Prager EM, Ledoux JE. Regulation of the Fear Network by Mediators of Stress: Norepinephrine Alters the Balance between Cortical and Subcortical Afferent Excitation of the Lateral Amygdala. Front Behav Neurosci. 2011;5:23. doi: 10.3389/fnbeh.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 51.Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanson LW. Brain maps: structure of the rat brain. 1 ed. Elsevier Inc; New York: 1992. [Google Scholar]