Abstract
Rationale
Adolescents are often described as “lacking brakes” resulting in an increase in several behaviors associated with risk for addiction. Prefrontal cortex dopamine and cortico-limbic interaction play an important role in addiction, and we have previously shown that the dopamine D1 receptor is elevated on prelimbic prefrontal output neurons in adolescent rats. We hypothesized that a constellation of risk-related behaviors are mediated by prefrontal output neuron expression of D1.
Objectives
We aimed to determine the role of the dopamine D1 receptor in behavioral and neural correlates of risk for addiction that are often observed in adolescents. Therefore high-risk behaviors as well as subcortical D2 receptor expression were investigated in adult animals with experimentally elevated D1 on prefrontal glutamatergic neurons.
Methods
A lentiviral vector that selectively expressed the D1 receptor within glutamate neurons was injected in the prelimbic prefrontal cortex of adult male rats. Place conditioning to cocaine, alcohol, and nicotine, as well as delay discounting, novelty preferences, anxiety, cocaine self-administration, and sucrose preferences were assessed.
Results
Virally-mediated D1 overexpression in adults leads to stronger drug-cue associations, greater consumption of sweet solutions, elevates bias towards immediate satisfaction rather than delaying gratification, decreases anxiety and causes rats to work harder for and take more cocaine. Furthermore elevated cortical D1 reduces D2 receptors in the accumbens (a putative risk marker).
Conclusions
Together, these data suggest a common mechanism for increased motivational drive to seek and consume substances with hedonic value, consistent with adolescent addictive processes.
Keywords: Adolescence, cocaine, D1, dopamine, impulsivity, prelimbic prefrontal cortex
Introduction
High-risk behaviors during adolescence facilitate individuation from parents during the transition to adulthood. Unfortunately, excess of these behaviors, including impulsivity, drug experimentation, and novelty-seeking, are associated with elevated addiction risk and other conditions associated with hedonically based outcomes (Chambers et al. 2003; Volkow and Wise 2005; Smith and Robbins 2013). Evidence suggests that changes within the dopamine system are likely to play a significant role in the elevated vulnerability of adolescents (Wong et al. 2013).
Adolescent rats are characteristically impulsive (Adriani et al. 1998), have high drug-taking in some (Schramm-Sapyta et al. 2011; Wong et al. 2013), but not all studies (Kantak et al. 2007; Frantz et al. 2007), show elevated novelty-seeking, and show elevated sensitivity to cocaine-associated environments (Brenhouse et al. 2008) relative to older and younger rats. These behaviors are often correlated with each other in animals (Belin et al. 2008; Molander et al. 2011) and humans (Moeller et al. 2001), and are all mediated by the prefrontal cortex (PFC) and its communication with downstream limbic structures (Jupp and Dalley 2013). Imaging studies in humans demonstrate greater reactivity within the dopamine-rich, reward-associated area of the nucleus accumbens (NAc) following reward receipt in adolescents relative to children and adults (Galvan et al. 2006). Indeed, low D2 receptors in the NAc have been associated with high impulsivity in adult animals (Dalley et al. 2007) and humans (Volkow et al. 1993), increased salience of food cues (Flagel et al. 2011), and are evident in obese individuals (Wang et al. 2004). While low D2 receptors may seem a clear choice to mediate risk during adolescence, their density does not change appreciably during this stage (Andersen et al. 2000) suggesting another mechanism may mediate these effects. D1 receptors in the prelimbic prefrontal cortex (plPFC) modulate responsiveness to drug cues (Ciccocioppo et al. 2001; McFarland et al. 2003; Brenhouse et al. 2008), and our earlier work has shown that the D1 population that is found on projections to the NAc are elevated normally during adolescence (Brenhouse et al. 2008). With no change in D1 receptor density on GABAergic neurons during adolescence (Brenhouse et al. 2008), these data suggest greater D1-mediated output – rather than less inhibition – of the plPFC during adolescence. Heightened D1 expression in the plPFC yields a ‘hypofrontal state’ (Seamans and Yang 2004) in which only potent cues, such as drugs of abuse or novelty, drive cortical excitation of the NAc. Top-down control from PFC to the NAc has been shown to affect NAc D2 activity (Scornaiencki et al. 2009). Therefore, heightened D1 expression on projection neurons in the plPFC may confer risk for drug addiction and other high-risk behaviors, including a decrease in NAc D2.
Drug self-administration is related to dopaminergic activity in the ventral tegmental area (Wong et al. 2013), and we have shown that preferences for contexts associated with cocaine can be blocked with microinjections of a D1 antagonist into the plPFC. Given that increased motivational drive is mediated by the mesocortical dopamine system (Berridge 2007) and plays a role in a number of the aforementioned behaviors, we investigated whether D1 changes could account for a constellation of high-risk behaviors that include drug and food seeking, taking, and impulsivity. While increasing evidence suggests that dopamine and plPFC excitability are involved in drug-seeking and impulsivity (Kalivas et al. 2005; Bari and Robbins 2013; Simon et al. 2013), knowledge is lacking about the causality between elevated D1 in the PFC and the correlated high-risk behaviors often seen in adolescence. For example, while trait impulsivity has been related to the maintenance and escalation of self-administration of drugs of abuse and their reinstatement (Perry et al. 2005; Dalley et al. 2007; Diergaarde et al. 2008), no study has shown a shared mechanism of action. To show causality between elevated D1 in the plPFC and behaviors such as drug-seeking, conditioned place preference, and novelty-seeking, we developed a lentiviral based gene-engineering approach for use in adult animals to minimize the influence of other developmental variables that are rapidly changing during adolescent period (Brenhouse and Andersen 2011). We report here that some high-risk behaviors that are typically seen in adolescents may be recapitulated in adults via elevated D1 receptors on glutamate neurons in the plPFC with downstream reductions in D2 receptors in the NAc.
Methods and Materials
Subjects
Sprague Dawley rats were obtained from Charles River Laboratories (Boston, MA). Unless otherwise noted male adult rats, weighing 350–375g and 90–100 days old, were used. Rats were pair-housed (2 animals per cage) in a standard cage (26 × 48 × 20 cm) with food and water available ad libitum. The animal room was kept at a temperature of 22 ± 2°C, humidity of 55 ± 25% on a 12-hr light/dark cycle (light period 0700-1900). The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH), and were approved by the Institutional Animal Care and Use Committee at McLean Hospital.
Virus production
All cloning experiments were based on standard molecular biological techniques. Virus production, concentration by ultracentrifugation, and qRT-PCR-based titering were performed according to published protocols (Sastry et al. 2002; Seo et al. 2004). Average virus titers were 106 – 107 transducing units per µl.
Surgery for lentiviral injections
Rats were anesthetized with a ketamine/xylazine mixture (80/12 mg/kg, respectively) and received 0.6 µl of virus (106 – 107 transducing units per µl) bilaterally into the plPFC at stereotaxic coordinates (AP +2.8, ML: 0.4; DV: −2.7). Assessments began between 5–10 days after surgery to allow for viral expression. Expression was stable throughout all experiments and placement within the plPFC was confirmed by histology (Figure 1). Subjects were virus was detected within the infralimbic PFC were excluded from the analyses.
Figure 1.
Cell-specific over-expression of control GFP or D1 in plPFC and effects on D2 in NAc. (a) Lentiviruses are regionally injected into the (b) plPFC driving GFP or D1 expression from the Synapsin (left) or the CamKII (right) promotor. (c) The number of D1-immunoreactive cells that are co-localized to either GABAergic (GAD67) or glutamatergic (CamKII) neurons are represented in black, with the remaining D1-IR cell counts for each label shown in white. (d) The CamKII.D1 virus activates the immediate early gene c-fos more than in CamKII.GFP controls following amphetamine. * P<0.05.
Experiment 1: Determination of lentiviral specificity and downstream effects in NAc
Subjects
A total of n=20 subjects were used in Experiment 1. With exception of control subjects, all subjects who received lentiviral injections were ultimately perfused and processed for immunohistochemistry to verify virus placement within the plPFC. For the cell counting experiments, an n= 5/group subjects were used for the Syn.GFP, Syn.D1, CamKII.GFP, and CamKII.D1 virus. For c-fos immunohistochemistry, an n=5 subjects/group was used.
Surgery for lentiviral injections
Rats were anesthetized with a ketamine/xylazine mixture (80/12 mg/kg, respectively) and received 0.6 µl of virus (106 – 107 transducing units per µl) bilaterally into the plPFC at stereotaxic coordinates (AP +2.8, ML: 0.4; DV: −2.7). Assessments began between 5–10 days after surgery to allow for viral expression. Expression was stable throughout all experiments and placement confirmed by histology as described below (Figure 1a+b).
Immunohistochemistry (IHC) and cell counting
For IHC rats were deeply anesthetized with pentobarbital, intracardially perfused with 4% formalin, and the tissue processed with standard methods. Briefly, sections were incubated overnight in either mouse CamKII IgG (1:250; Chemicon) or mouse anti-GAD67 IgG (1:2000; Chemicon) and double-labeled with rat anti-D1 DAR IgG (1:250; Sigma), washed, and incubated for 60 min with anti-mouse TRITC coupled IgG (1:200; Molecular Probes) and anti-rat Alexa 488-coupled IgG (1:200; Molecular Probes). Sections were washed, mounted on slides, and imaged with confocal microscopy. Three regions of interest were drawn within the injection bolus (visualized with FITC filter). Within each region of interest (ROI), z-series stacks were generated in the FITC (D1 immunoreactivity [IR]) and TRITC (GAD67- or CamKII-IR) channels. Within each ROI the numbers of D1-labelled cells that were co-localized with GAD67, CamKII, or “other” (D1-immunoreacive) were recorded. Three ROIs were generated per section, with four serial sections analyzed (section thickness=40µm; section interval=400µm; total cells counted~360/animal). Absolute cell numbers across all three ROIs were reported.
c-fos immunoreactivity
We used the immediate early gene c-fos as a marker of neuronal activity. CamKII.GFP and D1 subjects were injected with 1 mg/kg amphetamine, perfused 90 min later, and the tissue processed for IHC. Briefly, c-Fos (1:5000; SantaCruz) was incubated overnight, amplified with secondary (1:500; goat anti-rabbit), and visualized with DAB. Three sections per subject were counted by outlining the plPFC and counting all c-Fos-IR neurons within this area. Approximately, 200 cells/subject were counted. An unpaired t-test was used to analyze the results (P<0.05).
Experiment 2: NAc D2 dopamine receptors and Western immunoblotting
Subjects
Sprague-Dawley rats were assigned to one of the four conditions, with n=4/group: untransduced control subjects, or transduced with CamKII.GFP, CamKII.D1, or Syn.D1. Subjects received the viral vectors 8 days prior to sacrifice.
Western immunoblotting
was performed to investigate whether viral-mediated changes in the plPFC produced downstream effects in 0.98 micropunches from the NA D2 receptors. Tissue was sonicated in 1% SDS solution and protein content determined by a Bradford assay (Biorad, Hercules, CA). Fifteen micrograms of protein were loaded into a 15% Tris-HCl polyacrylamide gel and subjected to SDS-PAGE. After protein separation, the samples were transferred to a nitrocellulose membrane and probed for D2 protein using rabbit polyclonal anti-D2 IgG (1:200; Chemicon) and anti-actin (1:10,000; MP Pharmaceuticals) as a loading control. Membranes were then incubated with anti-rabbit and anti-mouse secondary antibodies conjugated with horseradish peroxidase and visualized by enhanced chemiluminescent detection (West Pico Kit; Pierce, Rockford, IL). Immunoblots were replicated in independent samples and corrected for actin values.
Experiment 2: Determination of motivational salience with place conditioning
Subjects
To first demonstrate the importance of specific D1 receptor expression on glutamate neurons, place preferences to cocaine (10 mg/kg probe dose) were assessed in Syn.D1/GFP (Figure 2b), CamKII.GFP, and CamKII.D1 transduced animals. A large number of subjects were used for the full dose-response analyses, with the final numbers excluding missed placement of the virus. The number of subjects for cocaine, ethanol, and nicotine were CamKII.GFP: 18, 19, and 16 and CamKII.D1 = 19, 16, and 17, respectively.
Figure 2.
Cell-specific over-expression of D1 in plPFC affects D2 receptor expression in NAc and place preference for cocaine. (a) Levels of actin-corrected D2 proteins from subjects that are untransduced controls, transduced with CamKII.GFP, CamKII.D1, or Syn.D1, were measured in the NAc and expressed as percent of control values. Means ± s.e.m. presented. * P<0.05. (b) Animals transduced with Syn.GFP or Syn.D1 fail to show a preference for either a 10 mg/kg cocaine or saline-associated side.
Place conditioning
An unbiased place conditioning protocol was used for cocaine and alcohol, as previously published (Cabib et al. 1996; Andersen et al. 2002; Flagel et al. 2009). Unbiased place conditioning chambers consisted of two large (24 × 18 × 33) side compartments separated by a small (12 × 18 × 33 cm) middle compartment. Screening was conducted for 30 min on day 1. Rats were placed in the middle compartment and allowed to freely explore the apparatus. Rats that demonstrated a clear preference for either side (>18 of 30 min) were eliminated from further testing. Subjects were conditioned for two days, with two 60 min sessions per day during which rats were injected with saline (1ml/kg, i.p.) in the morning and placed in one side, and 4 hrs later injected with drug (cocaine: 5, 10, and 20 mg/kg; ethanol: 0.2, 0.5, and 1.0 g/kg) and placed into the opposite side of the apparatus. On day four, rats freely explored the entire apparatus for 30 min in a drug-free state. Biased place conditioning was used for nicotine, where nicotine-paired environments were the less-preferred side during baseline screening in a two-sided chamber (Med Associates Inc, St Albans, VT). The nicotine doses were 0.04, 0.6, and 1.4 mg/kg, s.c.
To compare place conditioning of the adult virus-transduced animals with adolescent animals, we conducted place conditioning with naive adolescent (P34) male rats with a single dose (10 mg/kg cocaine; 0.6 mg/kg ethanol; and 0.6 mg/kg nicotine). Full dose-response analyses have been published by our group and others (Vastola et al. 2002; Philpot et al. 2003; Brenhouse et al. 2008).
Experiment 3: Delayed discounting as a measure of impulsivity
Subjects
Four different groups of rats were trained in a delayed discounting task: CamKII.D1, CamKII.GFP, naive control adults and naive adolescents (P34). Within those four groups three different groups of animals were tested for different delays and therefore final numbers of animals in each group were 6–7. Starting 3–5 prior to the experiment subjects were food-restricted to 85% of their free-feeding weight.
Delayed discounting
Impulsive choice was investigated with a delayed discounting paradigm according to the methods of Olmstead et al. (2006). Training and testing were conducted in a T-maze consisting of three rectangular arms (75 × 24 × 10 cm) and a clear Plexiglass guillotine door at the start box. Rats were trained to choose between one arm that has a either a small or large reward (one or four pieces of Reece’s Pieces, respectively), with the position of the large reward counterbalanced across rats. Subjects were trained for six trials per day, with 1 min between trials, until subjects reached criterion of choosing a large reward in five of six trials for two consecutive days. In other words, rats learned reward magnitude association based on arm location (e.g., left = large).
During delayed discounting testing, subjects were allowed to choose either the large or small reward arm. Subjects choosing the large reward arm were detained in the arm for 5, 10, or 15 s before gaining access to the reward. Rats choosing the small reward arm were given immediate access to the reward. Impulsivity score was calculated as the number of choices for the small reward (12 sessions in total).
Experiment 4: Locomotor activity, novelty preference, and elevated plus maze
Subjects
A total of n=24 Sprague-Dawley rats were used, representing untransduced control adult males and adolescent males (P34), CamKII.GFP, and CamKII.D1 conditions. Animals were tested in the elevated plus maze first and then tested for locomotor activity and novelty preference afterwards.
Elevated plus maze
The maze consisted of two open and two closed arms (each 10 × 50 cm) attached to a central platform, and raised 50 cm. Time in the open arms and total entries into the open and closed arms were recorded for 5 min.
Locomotor activity and novelty preference testing
The assessment for novelty preferences was conducted in a two compartment box (Med Associates Inc., St. Albans, VT) based on Marci et al. (2010). Animals were habituated for 20 min in one side of the testing apparatus (counterbalanced) each across three days, one day in the home cage, and testing on Day 5. Animals were allowed to freely explore the whole apparatus (both the familiar and the novel sides) for 20 min. Locomotor activity in the form of beam breaks was recorded for all days, and time spent in the novel chamber was recorded for the test day.
Experiment 5: Self-administration of cocaine to determine sensitivity and motivation
Subjects
Subjects underwent lentiviral surgeries first, followed by cannula placement and self-administration. After cannula placement animals had to singly-housed. The subject total was CamKII.GFP = 9 and CamKII.D1 = 15; as not all subjects went on to PR and cannula blockage resulted in subject attrition in the loss of n=4 subjects, our final numbers were CamKII.GFP = 8 and CamKII.D1 = 12.
Cannula implantation
Rats were anesthetized with a ketamine/xylazine mixture (100/10 mg/kg, i.p), and were implanted with a chronic, indwelling silastic catheter in the left jugular vein (Puhl et al. 2011). Self-administration sessions were conducted between 07:00 and 17:00, with individual sessions occurring at the same time each day. Operant chambers (Lafayette Instruments, Lafayette, IN) with a touchscreen for responding displayed two different stimuli. Responses to an active stimulus produced an intravenous (i.v.) cocaine injection of 0.2 ml delivered over 3.5 s accompanied by a tone and light cue. Cocaine infusions were followed by a 7 s time-out period, signaled by the house light turning off and the removal of the stimuli from the screen. The entire setup was controlled by MatLab (Mathworks; Natick, MA) using custom programs and the Biopsychology Toolbox (Rose et al. 2008).
Self-administration and schedules of reinforcement
Self-administration was trained with a fixed ratio (FR) schedule of reinforcement of 1 (FR1) with a 0.5 mg/kg, i.v. cocaine during 4 h sessions until intake was stable (<15% variability in intake over 3 consecutive sessions), following the methods of Larson et al. (2011). The FR requirement was increased from FR1 to FR3, re-stabilized and then increased to FR5 and stabilized to ±10% across three days of responding. A within-session dose-response assessment was conducted in five continuous, 60 min blocks of time, during which subjects could self-administer cocaine at doses of 1.0, 0.3, 0.1, 0.03, and 0 mg/kg, i.v. in descending dose-order on an FR5 schedule. A 0.2 mg/kg/injection solution was used for these studies, with adjustments made to delivery volume and time as needed. Data from sessions four and five were then averaged for data analysis.
Progressive ratio schedule of reinforcement
Responding on the FR5 schedule to 0.5 mg/kg/injection was re-stabilized to 10% across three days in daily four h sessions. Once stable, a progressive ratio (PR) schedule was implemented where each successive cocaine injection required increased responses according to the following progression (1, 2, 4, 6, 9, 12, 15, 20, etc.) as previously described (Richardson and Roberts 1996). The breakpoint was determined as the final ratio of responses/injection before a 1 h period when no further injections were earned. Consistent with Larson et al. (2011), breakpoints were determined for two consecutive days at two doses (0.25 and 0.75 mg/kg/injection) presented in counterbalanced order.
Experiment 6: Sucrose and sacharin preference test
Subjects
The first group of subjects were untransduced controls (n=6), CamKII.GFP = 7 and CamKII.D1 = 8 for the sucrose preference test. An n=8 for each group was used for the saccharin preference test.
Two bottle drinking preference test
Individually housed subjects were given a two-bottle choice between water and water at baseline, followed by water and sucrose in increasing concentrations, according to Bolaños et al. (2003). Following a four-day habituation period to two bottles, the contents of one bottle were replaced with ascending concentrations of sucrose (0, 0.25, 0.5 and 1%). Subjects were exposed to each sucrose concentration for two days, and bottles were counterbalanced to avoid side preferences. Individual bottles were weighed daily at 10 a.m. to monitor the amount of liquid consumed. A two bottle test to a 0.1% saccharin solution and water was used to determine preferences for sweet without calories at 24 and 48 h.
Statistical Analyses
All data were analyzed using the statistical software program SPSS v. 11.0. For the place conditioning analyses, a 3-way mixed analysis of variance (ANOVA: dose [3 levels] virus [2: D1/GFP] × [conditioning (pre vs. post)]) was performed, with conditioning as a repeated measure. Where significant interactions were found, Bonferonni post-hoc comparisons determined whether virus influenced the post-conditioning effects. ANOVAs were used to analyze the other behavioral measures. Comparisons between adolescent and adult control subjects were analyzed with an ANOVA.
Results
Experiment 1: Determination of lentiviral specificity and downstream effects in NAc
The CamKIID1 virus produces preferential D1 expression in glutamatergic, but not GABAergic (GAD labeled), cell types (Figure 1a+c). Transduction with the CamKII.D1 virus enhanced cfos reactivity in response to amphetamine relative to CamKII.GFP (Figure 1d) (independent t-test: t8= 2.48, P<0.05), demonstrating functional receptor expression. Importantly, D2 protein expression levels in NAC were influenced by virus expression in the plPFC (Figure 2a) (main effect of virus: F3,8=4.41, P=0.01). CamKII.D1 reduced D2 protein expression in the NAc compared to CamKII.GFP (P=0.036), suggesting a downstream effect of plPFC activity. To show functional activity, the CamKII.D1 virus activates the immediate early gene c-fos 42.5 ± 14.3% more than in CamKII.GFP controls following a 1 mg/kg injection of amphetamine.
Experiment 2: Determination of motivational salience with place conditioning
To first demonstrate the importance of specific D1 receptor expression on glutamate neurons, place preferences to a dose of cocaine we have not observed to produce place preference in adults (10 mg/kg) (Brenhouse et al. 2008) were assessed in Syn.D1/GFP (Figure 2b). As no difference was observed in place conditioning between Syn.D1 and Syn.GFP subjects, the remaining studies were conducted with the CamKII.D1 and CamKII.GFP viruses only. CamKII.D1 subjects demonstrated a significant place preference to cocaine, especially at 10 mg/kg, but reduced preferences at the higher dose of 20 mg/kg relative to CamKII.GFP subjects (dose × virus×conditioning interaction: F2,34= 5.32, P=0.01; Figure 3a). The place conditioning dose-response for nicotine also increased place preferences at the lower doses in CamKII.D1 subjects compared to controls (dose × virus: F2,29=4.68, P=0.018; conditioning was a main effect: F1,27=27.4, P=0.0001), despite the different mechanisms of action between cocaine and nicotine (Figure 3a and c). For comparison to adolescents, we illustrate that these preferences were also evident in adolescents at doses that did not produce a preference in adult CamKII.GFP subjects (Figure 3a–c). CamKII.D1 subjects tested for place conditioning to EtOH displayed a lack of aversion at a dose of EtOH that produced a place aversion in controls, however the higher dose generated a significantly stronger aversion in CamKII.D1 subjects (dose × virus × conditioning interaction: F2,29=8.05, P=0.002). Both CamKII.D1 and CamKII.GFP subjects demonstrated comparable conditioned place aversions to 1.6 and 3 mEq, 76 and 125 mg/kg lithium (conditioning main effect: F1, 12=17.4, P=0.001) as shown in Figure 3d, suggesting that CamKII.D1 does not alter fundamental drug-cue associability.
Figure 3.
Elevated D1 in cortical glutamatergic neurons increases sensitivity to cues associated with different drugs of abuse. CamKII.D1 subjects demonstrated changes in place preferences compared to CamKII.GFP subjects to (a) cocaine, (b) alcohol and (c) nicotine in a dose-dependent manner. (d) Lithium chloride produced a similar post-conditioning aversion in both CamKII.GFP and CamKII.D1 subjects relative to pre-conditioning values. Means ± s.e.m. * P<0.05 or better and # P<0.05 for adolescent versus adult control comparisons.
Experiment 3: Delayed discounting as a measure of impulsivity
No significant differences were observed between groups for the number of days required to reach criterion. The decisions for the smaller, immediate reward were influenced by delay and virus condition (delay × virus interaction: F6, 69 = 2.3, P=0.046). CamKII.D1 animals made more impulsive choices at the 5 s delay compared to control and CamKII.GFP subjects (P=0.048, P=0.02, respectively, Bonferroni corrected). In separate comparisons between adolescent and adult control, adolescents made more impulsive choices (age × delay interaction, F2,29 = 3.58, P=0.043). Post-hoc comparisons show that these differences occurred at the 10 s delay compared with all other delays (P <0.005, Bonferroni corrected) and at the 15 s delay (P=0.025, Bonferroni corrected).
Experiment 4: Anxiety, locomotor activity and novelty preferences
No significant differences in locomotor activity were observed among treatment groups in adulthood (P=0.13), whereas adolescent rats show greater activity under these circumstances compared with control adults (P=0.048, Bonferroni corrected; Figure 4b). Separation of general activity from novelty preference (Molander et al. 2011) shows a significant condition main effect (F2,19=7.32, *P=0.004; Figure 4c). CamKII.D1 subjects demonstrated greater novelty-seeking by spending more time in the novel area (P=0.004) relative to GFP controls (P=0.007; Bonferroni correction) but not adolescents (P=0.07). Thus, D1 receptors underlie novelty preferences, but not activity. The preference for novel areas is further reinforced by our observation that CamKII.D1 subjects were significantly less anxious and spent more time in the open arms of the elevated plus maze than controls (F2, 26=5.89, P=0.009) (Figure 4d).
Figure 4.
D1 over-expression in cortical glutamatergic neurons increases impulsive choice and novelty-seeking without altering locomotor activity. (a) Impulsive choice was determined in a delayed discounting task. (b) Locomotor activity across 20 min (in 5 min intervals) was not affected by D1 receptor over-expression in adults, although control adults differ from adolescents. (c) CamKII.D1 subjects spend more time in the novel area relative to controls; adolescents showed greater novelty-seeking than control adults. (d) More time in the open arm of the elevated plus maze was observed in CamKII.D1 subjects relative to controls. Means ± s.e.m. presented. * P<0.05 or better and # P<0.05 for adolescent versus adult control comparisons.
Experiment 5: Self-administration of cocaine to determine sensitivity and motivation
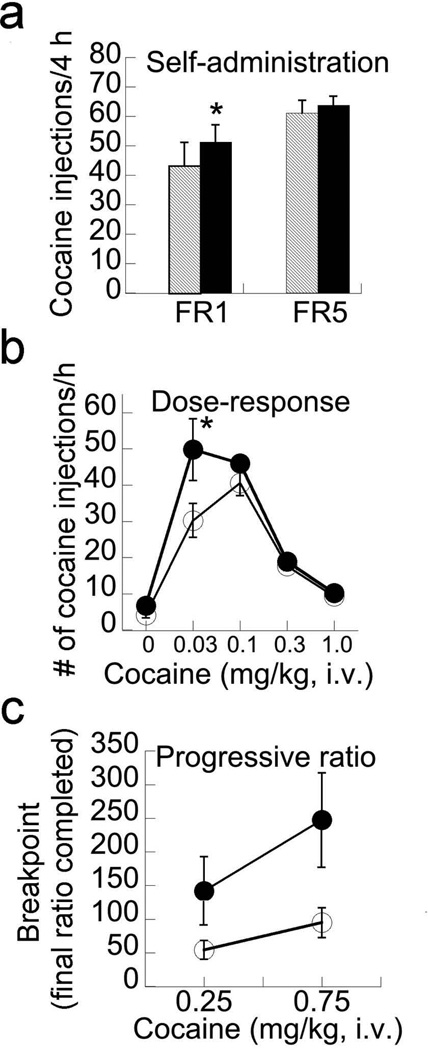
No differences in the time needed to achieve stability of responding were observed between groups (P>0.2). CamKII.D1 subjects made more responses under the FR1, but not FR5, schedule than CamKII.GFP controls (schedule × virus interaction: F1,22=5.22, P=0.03; Figure 5a). When probed for sensitivity shifts in responding where rats had to respond on an FR5 schedule for cocaine (0, 0.03, 0.1, 0.3, and 1 mg/kg per injection doses in descending order, with 60 min/dose), CamKII.D1 subjects made significantly more responses at the lower doses than CamKII.GFP subjects (dose × virus interaction: F1,22=4.43, P=0.04; Figure 5b). CamKII.D1 enhances motivational salience, as the breakpoint of PR was significantly elevated in CamKII.D1 compared to CamKII.GFP controls (schedule × virus interaction: F1,16=4.62, P=0.047); the effect was driven by the higher (0.75 mg/kg injection of cocaine) rather than the lower dose (0.25 mg/kg; Figure 5c).
Figure 5.
D1 over-expression in cortical glutamatergic neurons increases the sensitivity and motivated action to self-administer cocaine. (a) CamKII.D1 subjects took more cocaine in 4 hours on an FR1, but not an FR5, schedule relative to CamKII.GFP subjects; (b) D1 over-expressing rats were more sensitive to lower doses of cocaine; and (c) nose poked more for cocaine on a progressive ratio schedule. Means ± s.e.m.presented* P< 0.05
Experiment 6: Sucrose and saccharin preference test
CamKII.D1 subjects consumed more sucrose at lower concentrations (0.25, 0.5% versus water) relative to CamKII.GFP subjects in a two-bottle preference test, suggestive of a heightened sensitivity (virus main effect: F1,7=15.06, P=0.006; Figure 6a). Consumption of saccharin was also elevated in CamKII.D1 subjects, which may indicate that the subjects are responding to the “sweet” aspects rather than the caloric aspects of the solution (F1,12=4.84, P =0.048; Figure 6a). This “sweet” preference leads to an increased weight gain when the solution is caloric (i.e., sucrose; Figure 6b). Consistent with our hypothesis that D1 biases sensitivity to appetitive substances to influence behavior, CamKII.D1 subjects demonstrated a steeper slope of weight gain (r=5.31) compared with CamKII.GFP (r=2.85) and control subjects (r=2.39) (virus × day interaction: F2,16=7.41, P=0.005).
Figure 6.
Cortical D1 overexpression increases sensitivity to sucrose. (a) CamKII.D1 rats demonstrated an overall increase in sucrose consumption relative to CamKII.GFP. CamKII.D1 rats further preferred a 0.1% saccharin solution to water. (b) Weight gain was substantially higher across days of testing in CamKII.D1 animals versus CamKII.GFP or controls. The fit of the line (r) is presented for each group; Means ± s.e.m. * P <0.05.
Discussion
Our results demonstrate that elevated D1 dopamine receptors on glutamatergic neurons in the adult plPFC increase several behaviors associated with risk for addiction that are typically seen in adolescents. The data presented here conclusively show that overexpression of D1 in the plPFC via CamKII.D1 produces a constellation of risk-related behaviors while simultaneously reducing NAc D2 receptors, a putative risk factor for behaviors associated with addiction (Volkow et al. 1993). These data also suggest that elevated D1 receptors on glutamate plPFC neurons direct behavior towards salient environmental cues, and not a change in associative learning. If D1R over-expression facilitates associative learning in general, place conditioning to all drugs (i.e. cocaine, ethanol, nicotine and lithium) would increase. However, D1R over-expression did not produce a stronger aversion to the lithium-associated environment. Consistently, CamKII.D1 adults demonstrated shifts in sensitivity to the rewarding aspects of cocaine and nicotine at lower doses that failed to produce a preference in CamKII.GFP rats. Adolescents purportedly demonstrate similarly increased sensitivity to conditioned associations for drugs of abuse compared to typical adults (Vastola et al. 2002; Philpot et al. 2003; Brenhouse et al. 2008) that are partially explained by heightened PFC D1 (Brenhouse et al. 2008). Effects on EtOH conditioning are less clear, however it appears that higher doses of EtOH are required for CamKII.D1 rats to display conditioned aversion, compared to controls. Earlier studies in normal adult rats found place aversions at higher doses for all of the drugs tested than those used in the current study (Le Foll and Goldberg 2005; Brenhouse et al. 2008; Pelloux et al. 2009). Together, increased place preferences at low doses and place aversion at the highest dose supports the assertion that D1 overexpression produces a change in sensitivity to rewarding drug-associated cues. Increased sensitivity to drug cues is integral to greater reinstatement of cocaine self-administration associated with relapse (Kalivas et al. 2005) and has been associated with glutamatergic projections from the PFC to the NAc (McFarland et al. 2003).
While elevated D1 in adolescents may bias their sensitivity to the rewarding properties of drugs of abuse, impulsivity, and novelty-seeking, a second mechanism is probably responsible for reduced lithium aversion observed at this age (Schramm-Sapyta et al. 2006) and increased activity levels in response to a novel environment. Elevated activity has been associated with accelerated rates of self-administration (Piazza et al. 1989; Mitchell et al. 2005). A recent study has demonstrated that novelty preference is separable from the novelty-induced locomotor activity observations (Molander et al. 2011). Our study suggests that D1 is the likely mechanism associated with the former, but not the latter.
The present data suggests that elevated D1 activity on plPFC projections focuses activity on salient drug-related cues that results in prepotent responding. D1 overexpression further facilitates impulsive choice, where immediate choices were selected over delayed gratification in the current study. In addition to this disinhibited-like responding, elevated novelty preferences and decreased anxiety in high D1 expressing subjects were observed in CamKII.D1 subjects compared with controls. Notably, adolescents did not display decreased anxiety-like behavior in the elevated plus maze task compared to adults, which is consistent with previous reports on adolescent performance in the elevated plus maze (Arrant et al. 2013). Similar to virally-overexpressing adults, adolescents did display increased novelty-induced locomotion and increased impulsivity. Interestingly, adolescent impulsivity overshadowed CamKII.D1 adults at higher delays in the delayed discounting task, suggesting that plPFC D1 is but one mechanism controlling this adolescent behavior. Adults are likely to have greater regulatory control over their behavior compared with the adolescents. Overall, locomotion was also higher in adolescents compared to all adults, illustrating the limitations of D1 effects. Taken together, several related high-risk behaviors that are displayed by adolescents—but not all—can be produced by overexpression of D1 on plPFC projection neurons.
CamKII.D1 animals self-administered more cocaine at lower doses and were more motivated to do so, similar to what has been demonstrated in adolescent rats on measures such as self-administration acquisition and responses as a function of price (Wong et al. 2013; Holtz and Carroll 2013). We note, however, that the question of whether adolescent rats do self-administer cocaine more than adults is debated in the literature (Frantz et al. 2007; Schramm-Sapyta et al. 2011). We show here that adults with increased D1 expression display drug-seeking behaviors that are consistent with risk for drug addiction (Belin et al. 2008), which supports the hypothesis that motivation for drug-taking is mediated by plPFC D1. These observations further extended to sweet solutions, suggesting that D1 further encodes not only drug-cue association and novelty, but also the hedonic properties of the stimulus. Furthermore, drug-taking has been related to increased novelty-seeking and impulsivity on a delayed discounting task (Anker et al. 2009). The current data shows that these effects are mediated by D1 expression on glutamatergic outputs in the plPFC.
Our data further contribute to evidence that both drug and food share similar reward processing by the dopamine system (Volkow 2005) and highlight the potential role of “top-down” cortical control over NAc-based reward-driven behavior (Del Arco and Mora 2009; Dalley et al. 2011). The association of increased D1 receptor expression in the plPFC with a reduction of D2 density in the NAc in our study is consistent with decreased D2/D3 dopamine receptor availability in the NAc of impulsive humans or animals (Volkow et al. 1993; Dalley et al. 2007) or of obese humans with a high body mass index (Wang et al. 2004). Thus, an up-regulation of glutamatergic D1 receptors may render individuals selectively vulnerable to initiate and continue to use drugs of abuse and food compared with a normal population. In this sense, the viral-mediated approach shows that D1 receptors may be a cause – rather than a consequence – of addictive behavior (Kalivas et al. 2005). Future studies should aim at determining the downstream effects of plPFC D1 receptors on other subcortical regions and whether plPFC D1 can potentially serve as a biomarker for risk for psychopathology or provide an avenue for the development of novel treatments for substance abuse and food addiction.
Acknowledgements
The authors acknowledge the support of DA-10543 and DA-026485 (to SLA), the Leopoldina Fellowship Program LPDS 2009-40 (to NF), and NS067335 (to KCS). We also thank Dr. Joris van Arensbergen, Yenare Lee, and Noah McKenna for technical help, Dr. David Sibley (NIH) for providing the pSFD1 receptor plasmid, Dr. Karl Deisseroth (Stanford University) for the CamKII promotor, Dr. Atsushi Miyanohara (UCSD) for the Synapsin promoter, Dr. Didier Trono (EPFL, Lausanne, CH) for providing the original lentivirus vector system.
Abbreviations
- NAc
Nucleus Accumbens
- plPFC
prelimbic PFC
- CamKII
Calmodulin Kinase II
- GFP
Green Fluorescent Protein
- D1
Dopamine Receptor Type I
- D2
Dopamine Receptor Type II
- FR
Fixed Ratio
- PR
Progressive Ratio
Footnotes
Financial disclosure:
The authors declare that they have no competing financial interests.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, et al. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, et al. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm. 2009;116:941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- Arrant AE, Jemal H, Kuhn CM. Adolescent male rats are less sensitive than adults to the anxiogenic and serotonin-releasing effects of fenfluramine. Neuropharmacology. 2013;65:213–222. doi: 10.1016/j.neuropharm.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task : Possible relevance to ADHD. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, et al. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Barrot M, Berton O, et al. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S, Genua C, et al. Dose-dependent aversive and rewarding effects of amphetamine as revealed by a new place conditioning apparatus. Psychopharmacology (Berl) 1996;125:92–96. doi: 10.1007/BF02247398. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Escalation of i.v. cocaine intake in peri-adolescent vs. adult rats selectively bred for high (HiS) vs. low (LoS) saccharin intake. Psychopharmacology (Berl) 2013;227:243–250. doi: 10.1007/s00213-012-2958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Dalley JW. Behavioral endophenotypes of drug addiction: Etiological insights from neuroimaging studies. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, et al. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrì S, Laviola G, Leussis MP, Andersen SL. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology. 2010;35:392–402. doi: 10.1016/j.psyneuen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, et al. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, et al. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl) 2011;215:721–731. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Hellemans KGC, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 2006;184:221–228. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res. 2009;197:311–316. doi: 10.1016/j.bbr.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, et al. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FHE, et al. A history of bingeing on fat enhances cocaine seeking and taking. Behav Neurosci. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rose J, Otto T, Dittrich L. The Biopsychology-Toolbox: a free, open-source Matlab-toolbox for the control of behavioral experiments. J Neurosci Methods. 2008;175:104–107. doi: 10.1016/j.jneumeth.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Sastry L, Johnson T, Hobson MJ, et al. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155–1162. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cauley MC, Stangl DK, et al. Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology (Berl) 2011;215:493–504. doi: 10.1007/s00213-011-2216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornaiencki R, Cantrup R, Rushlow WJ, Rajakumar N. Prefrontal cortical D1 dopamine receptors modulate subcortical D2 dopamine receptor-mediated stress responsiveness. Int J Neuropsychopharmacol. 2009;12:1195–1208. doi: 10.1017/S1461145709000121. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seo H, Sonntag K-C, Isacson O. Generalized brain and skin proteasome inhibition in Huntington’s disease. Ann Neurol. 2004;56:319–328. doi: 10.1002/ana.20207. [DOI] [PubMed] [Google Scholar]
- Simon NW, Beas BS, Montgomery KS, et al. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37:1779–1788. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND. What do we know about drug addiction? Am J Psychiatry. 2005;162:1401–1402. doi: 10.1176/appi.ajp.162.8.1401. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, et al. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]