Abstract
Objective We review our institution's experience with the treatment of inverted papilloma (IP) with emphasis on the implications of surgical margins for disease control.
Design Retrospective chart review of patients with IP treated at the University of Michigan from 1996 to 2011.
Setting Tertiary care center.
Participants Patients undergoing surgical resection with curative intent for IP.
Main Outcome Measures Overall survival, disease-specific survival, and locoregional control were used as main outcome measures.
Results We studied 129 patients including 19 with carcinoma arising from IP. Disease-free rates at 2, 3, and 5 years were 79.7%, 77.9%, and 61%, respectively. Overall, 10 of 18 recurrences were detected > 2 years from follow-up, with recurrences detected up to 8 years from surgery. For benign disease, obtaining tissue margins outside of the primary specimen for margin control did not affect disease control rates.
Conclusion IP is a disease that requires significant follow-up periods beyond 2 years. For IP without carcinogenesis, acquiring margins outside of the tumor specimen did not appear to affect disease control rates in this study. No clear predictors of malignancy were seen in this study, which highlights the need for further research to predict this phenomenon.
Keywords: inverted papilloma, sinonasal tumors, paranasal sinuses, staging systems
Introduction
Inverted papilloma (IP) is a usually benign but locally aggressive tumor of the nasal cavity and paranasal sinuses, occurring most commonly in white men 40 to 70 years of age.1 2 3 4 Representing between 0.4% and 4.7% of all sinonasal neoplasms, the overall incidence of these usually unilateral tumors is between 0.74 and 1.5 cases per 100,000 persons per year.3 4 Risk factors for developing IP include outdoor and industrial occupations.5
Derived from sinonasal Schneiderian mucosa, IPs are characterized histologically by hyperplastic Schneiderian mucosa enclosed in basement membrane that grows endophytically into the underlying stroma. This neoplasm can have unlimited growth potential3 and can be difficult to resect for cure.1 4 6 7 8 Furthermore, IPs are known to degenerate into or simultaneously harbor squamous cell carcinoma in ∼ 15% of cases.6 9 Regardless of endoscopic or open transfacial approaches for resection of these tumors, the literature demonstrates an overall unsatisfactory recurrence rate ranging from 5.7% to 32%.7 8 10 11 12
Although surgeons agree that complete tumor extirpation is the treatment of choice for IP, no current established standard exists regarding resection margins and management of surrounding structures. Some authors13 14 recommend drilling of underlying bone structures at the sites of mucosal attachment, but the benefit of this additional procedure has never been proven. The development of endoscopes has improved visualization and the surgeon's ability to discern disease from normal mucosa, perhaps changing the paradigm of necessary negative mucosal margins taken from adjacent tissue required in most head and neck tumor ablations. The additional benefit of margins must thus be investigated.
We review our institution's experience with IP treatment and the relationship of surgical margins to rates of recurrence.
Methods
The University of Michigan institutional review board approved this study. Between 1996 and 2011, 147 patients were diagnosed with IP at the University of Michigan. These patients were identified from the pathology archive, using the search target “inverted papilloma” for exact matches. This study required a stringent pathology review of all patient blocks by a dedicated head and neck pathologist (JM). To ensure consistency of pathology and patient reporting, other Schneiderian papillomas such as fungiform papillomas and cylindrical/oncocytic papillomas were excluded for data analysis. We included any IP with or without dysplasia or carcinomatous degeneration.
Patient charts were reviewed for demographic data, smoking status, information pertaining to number of previous surgical procedures, follow-up duration, recurrence, and presence of malignancy. Smoking status was recorded as current smoker at diagnosis, previous smoker, and never-smoker. Only patients who underwent definitive treatment with curative intent were included. Operative details were reviewed for site of tumor origin as well as whether additional margins were taken. If additional tissue margins were taken outside of the primary specimen for margin control, patients were placed into group 1. Patients in whom no additional tissue margins were taken were placed in group 2. Surgical approaches were defined as endoscopic, open (midfacial degloving, subcranial approach, and lateral rhinotomy), or combined endoscopic and open exposure. For survival analysis, a reliable online search engine for the Social Security death index was used (http://www.genealogybank.com/gbnk/ssdi/). Censor date was set to December 31, 2011. A total of 129 patients met the criteria for data analysis.
Staging information was determined from previous documentation or after thorough perusal of operative findings and radiographic imaging. The Krouse staging system15 was used to categorize disease extent. Briefly, stage I disease is limited to the nasal cavity alone, stage II includes ethmoid and medial and the superior portion of maxillary sinus involvement, stage III indicates lateral or inferior aspect of maxillary sinus extension or into the frontal and sphenoid sinuses, and stage IV is reserved for malignancy or tumor spread outside of paranasal sinuses. Staging is assigned for each patient based on the initial operative intervention. For example, if a patient presents with a stage III tumor that recurs 12 months after resection and is restaged as stage IV due to new finding of squamous cell carcinoma, the initial stage (III) was recorded.
SPSS software v.19 was used for all statistical calculation and analysis. The product limit method of Kaplan and Meier was used to calculate rates of locoregional control, overall survival, and disease-specific survival.
Results
Patient Demographics
Of the 129 patients with IP included in this study, 101 were male and 28 were female. The average age at initial diagnosis was 51.8 years. Patient ethnicities consisted of 106 whites, 16 blacks, 1 Hispanic, and 7 Asians. The median follow-up was 51.8 months.
The mean overall survival was 182.8 months, and the mean disease-specific survival was 199.3 months. Among the 129 patients, 28 recurrences were recorded. Although most of the recurrences occurred within the first 2 years of follow-up (18 of 28 recurrences [64.3%]), recurrence was detected up to 8 years postresection. The median locoregional control rate was 95.6 months. Tumor control rates at 2, 3, and 5 years were 79.7%, 77.9%, and 61%, respectively. The median time to recurrence was 17.1 months. The impact of patient demographics (sex, age, and ethnicity) was analyzed, and no factor significantly affected tumor control or patient survival (Table 1).
Table 1. Significance of factors influencing overall survival, disease-specific survival, and locoregional control.
| OS | DSS | LRC | |
|---|---|---|---|
| Sex | NS | NS | NS |
| Race | NS | NS | NS |
| Smoking status | p = 0.013 (worse survival for smokers) | NS | NS |
| Tumor subsite | NS | NS | NS |
| Primary/Reoperation | NS | NS | NS |
| Stage | < 0.05 (worse survival for stage IV) | < 0.05 (worse survival for stage IV) | NS |
| Additional margins | NS | NS | NS |
| Presence of malignancy | p < 0.001 | p < 0.001 | NS |
Abbreviations: DSS, disease-specific survival; LRC, locoregional control; NS, not significant; OS, overall survival.
Pathology
The pathologic diagnosis was IP in 105 patients (81.4%) (Table 2). Five patients (3.9%) harbored dysplasia in the final surgical specimen, and 19 patients (14.7%) were diagnosed with malignancy arising in IP. Of the 19 cases of carcinoma, 16 were conventional keratinizing squamous cell carcinomas. One patient developed mucoepidermoid carcinoma, one patient harbored cylindrical cell (nonkeratinizing) carcinoma, and one patient's pathology showed combined spindle cell carcinoma and adenocarcinoma. Overall, 11 (57.8%) of these 19 patients presented with carcinoma at the initial consultation (synchronous tumors); 8 patients (42.2%) developed malignancy during the follow-up period (metachronous tumors). Among patients who developed malignancy on follow-up, the mean interval between initial resection and diagnosis of malignancy was 73 months. The number of recurrences or surgical resections per patient did not correlate with the chance of developing malignancy, and patient demographics also did not significantly predict future malignancy during the follow-up period.
Table 2. Pathology results for all patients in study.
| Pathology | n |
|---|---|
| IP | 105 |
| IP and dysplasia | 5 |
| IP and carcinoma | 19 |
Abbreviation: IP, inverted papilloma.
Survival analysis based on tumor pathology was performed and is shown in Fig. 1. The 5-year overall survival for IP, IP with dysplasia, and IP with carcinoma was 95%, 100%, and 62%, respectively. This finding is statistically significant for poorer survival with carcinoma (p < 0.001). The 5-year disease-specific survival for IP, IP with dysplasia, and IP with carcinoma was 98.6%, 100%, and 66%, respectively. This is also statistically significant for poorer survival for patients with carcinoma (p < 0.001). The locoregional control rates for IP, IP with dysplasia, and IP with carcinoma were 78%, 100%, and 77% at 2 years and 60%, 100%, and 65% at 5 years. There was no statistical significance in this analysis.
Fig. 1.
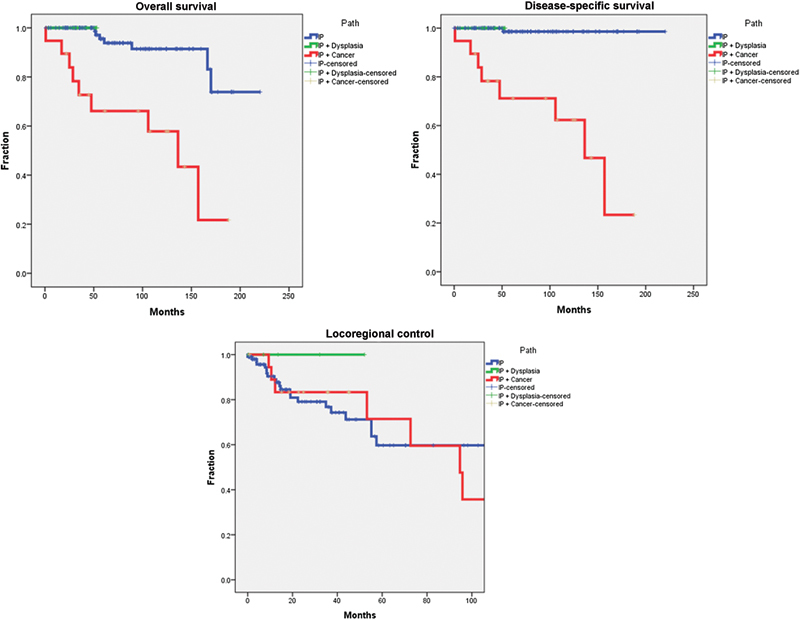
Kaplan-Meier survival curves for overall survival, disease-specific survival, and locoregional control based on the presence of dysplasia or carcinoma. IP, inverted papilloma.
Staging
Initial staging details were available for all 129 patients. The distribution of stages is shown in Table 3; survival analysis illustrated in Fig. 2. Most patients presented with stage III disease (57 of 129 [44.2%]). The 5-year overall survival and disease-specific survival for stages I to IV at presentation were 88, 88, 100, and 64.2%, respectively, for overall survival and 100, 92, 100, and 63.7%, respectively, for disease-specific survival. Stage IV significantly affected both overall survival and disease-specific survival. Staging did not significantly affect locoregional control rates in this study (Table 1).
Table 3. Staging distribution for all patients.
| Stage | n |
|---|---|
| I | 16 |
| II | 35 |
| III | 57 |
| IV | 21 |
| Total | 129 |
Fig. 2.
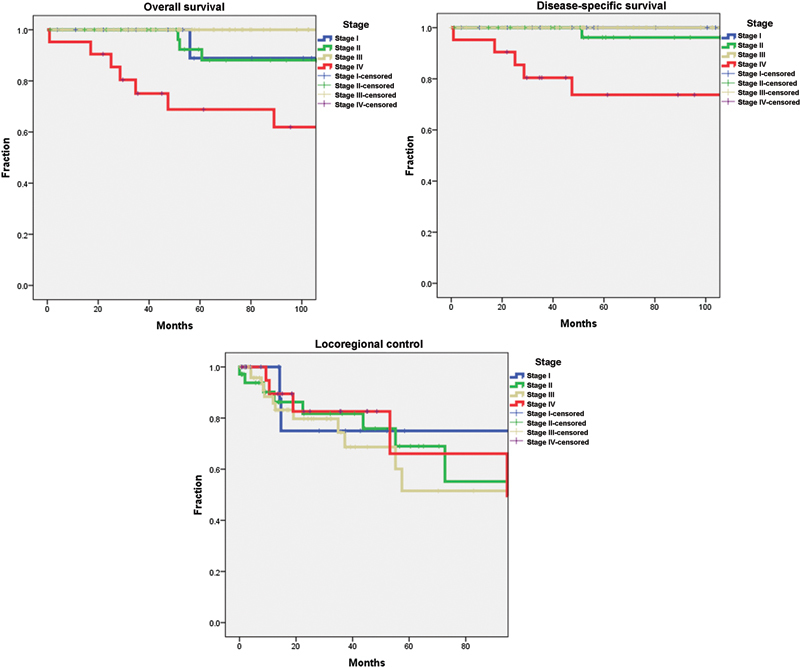
Kaplan-Meier survival curves for overall survival, disease-specific survival, and locoregional control based on stage.
Surgical Approach
Overall, 72 cases were performed with endoscopic resection, 40 cases with the open approach, and 17 cases with combined approaches. The open approach was more likely to be used in patients whose disease harbored malignancy (16 of 40 cases with malignancy versus 2 of 65 with malignancy for endoscopic and 1 of 16 for the combined approach; p = 0.05). Analyzing recurrence rates for benign disease alone, 2-year locoregional control rate was 82% for endoscopic approach, 74% for the open approach, and 67% for the combined approach (not significant).
Margins at Surgery
All tumors were resected in their entirety. Additional surgical margins were obtained for 64 of 129 patients (group 1) from surrounding tissue for confirmation of negative margins. These 64 patients included 14 cases of carcinoma. Only 5 of 64 patients whose surgery did not include additional margins (group 2) had carcinoma. This difference was statistically significant (p = 0.05). Thus for outcome analysis, we analyzed only the 110 cases of benign disease for locoregional control. The two groups were similar demographically as were the distribution of surgical approaches chosen (Table 4). Local control rate was 80% at 2 years for group 1 and 78% for group 2. The differences in locoregional control rates between groups were not significant (Fig. 3).
Table 4. Surgical approach for inverted papilloma without carcinogenesis separated by presence and absence of additional surgical margins.
| Additional margins: group 1 | No additional margins: group 2 | |
|---|---|---|
| Age, y | 47.8 | 52.8 |
| Sex | M = 40; F = 10 | M = 46; F = 14 |
| Race | 80% white | 83% white |
| Smoking | 60% never, 14% previous, 26% current | 39% never, 33% previous, 28% current |
| Approach | Endoscopic = 31; open = 15; combined = 4 | Endoscopic = 39; open = 9; combined = 12 |
| Total | 50 | 60 |
Fig. 3.
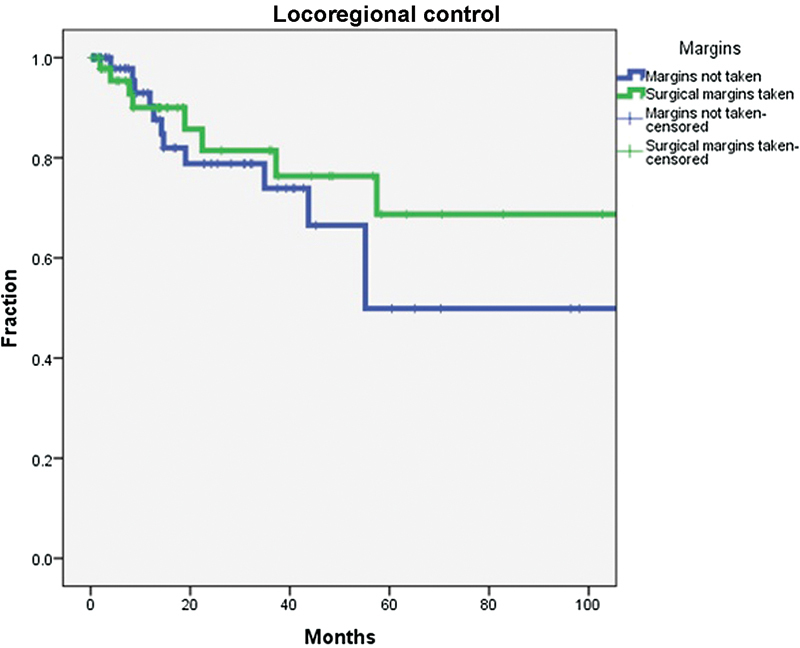
Local control rates for inverted papilloma without carcinogenesis based on the presence or absence of additional surgical margins. The difference between groups was not statistically significant.
Smoking
Medical records were definitive for patient smoking history in 128 of 129 patients. Overall, 56 were never-smokers, 34 were previous smokers, and 38 were smokers at tumor diagnosis. Smoking significantly affected overall survival (p = 0.013), with current smokers (p = 0.009) and previous smokers (p = 0.007) both performing worse than never-smokers (Table 2). However, on disease-specific survival analysis, the smoking status was not significant. Smoking also did not affect locoregional control rates. The Fisher exact test demonstrates only a trend (p = 0.1) that active smoking may predict the chance of malignant degeneration compared with previous smoking and never-smokers combined.
Tumor Location
Table 5 details the distribution of tumor sites of origin. The most common location for tumorigenesis is the lateral nasal wall (45 of 129 [35%]), followed by the maxillary (25 of 129 [19.4%]) and ethmoid sinuses (24 of 129 [18.6%]). Overall survival, disease-specific survival, and locoregional control were not statistically significant for this study, and the location of the tumor was also not predictive of malignancy at presentation or on follow-up.
Table 5. Distribution of primary tumor sites.
| Site | n |
|---|---|
| Lateral nasal wall/medial maxillary sinus | 45 |
| Nasal cavity | 15 |
| Ethmoid sinus | 24 |
| Maxillary sinus | 25 |
| Frontal sinus | 8 |
| Sphenoid sinus | 2 |
| Septum | 2 |
| Unknown | 8 |
| Total | 129 |
Primary Resection versus Secondary Resection
A total of 97 patients underwent primary resection of their tumor; 32 patients were treated after previous resection with recurrence performed outside our institution. Primary resection and secondary resection showed similar results in regard to overall survival, disease-free survival, and locoregional control. No statistical significance was detected (Table 1).
Discussion
Although otolaryngologists have treated sinonasal IP for many decades, the significant rates of recurrence continue to be well documented and present significant challenges for the head and neck or endoscopic surgeon.1 7 8 10 11 12 16 17 This clinical review highlights the importance of follow-up in these patients given the high recurrence rates of 20.3%, 22.1%, and 39% at 2, 3, and 5 years of follow-up. Similar to previous studies,3 8 18 19 20 most tumors recur during the first 2 years of follow-up (18 of 28 recurrences). However, longer follow-up periods are recommended given multiple recurrences after 2 years in this study and recurrences up to 8 years of follow-up. In the largest retrospective review of IPs to date, Kim et al21 divided patients into a group with < 3 years of follow-up and another with at least 3 years of follow-up. The authors were able to detect recurrence more often when following patients for at least 3 years (26.1% and 8.5%, respectively).
Multiple authors12 15 21 22 23 have found staging systems for IP to be helpful for reporting and predicting treatment outcome. The stages proposed by Krouse15 in 2000 continue to be the most widely used today. This system was refined by Cannady et al23 in 2007 by staging patients based on recurrence rates. By grouping Krouse stages I and II into a single category (stage A) and maintaining Krouse stage III as stage B and Krouse stage IV as stage C, the authors demonstrated a significant difference in recurrence rates between the three groups. Given that almost all stage IV patients in this study harbored carcinoma, it is not surprising that survival was significantly decreased in this study group. However, similar to the study by Kim et al,21 we did not find staging to be a significant predictor of locoregional control. The lack of statistical significance may be explained by the overall high recurrence rates for tumors of all subsites as well by the lack of power to detect small differences in this study. Similar arguments can be used to explain the lack of statistical significance for tumor origin as a predictor of overall survival, disease survival, and locoregional recurrence.
Endoscopic resection has become the procedure of choice for the treatment of the vast majority of cases of IP, with many authors8 12 21 22 24 reporting comparable control rates compared with transfacial and other open approaches. Similarly, we did not find any statistical difference in recurrence rates between endoscopic, open, and combined approaches for IP. Cases with malignancy were excluded in this particular analysis because open approaches in this study were much more likely to be used for malignant pathology. Endoscopic resection has replaced open surgery in our institution for almost all IPs except for cases with massive frontal or supraorbital extension.
Obtaining separate surgical margins outside of the resected tumor specimen did not affect recurrence rates in this study. The two study groups were evenly divided including a similar distribution of surgical approaches used for tumor extirpation. As expected, we were more likely to obtain surgical margins in those patients with malignancy. For this reason, we excluded malignant cases in this analysis to create more uniform and comparable groups. Mortuaire et al25 evaluated the quality of surgical margins in 65 patients treated with open or endoscopic approaches but did not elaborate on specific criteria for specimen quality or whether margins were taken from surgical specimen or outside the specimen after resection. They concluded that “safe” margins are ideal but did not affect the rate of local recurrence. Our study is the first to define and analyze the effect of obtaining separate surgical margins for IP. Although separate surgical margins did not affect local control in our analysis, the retrospective nature of the study makes this finding more difficult to interpret. The exact reason for or against separate surgical margins could not be defined in each individual case. The decision of whether extra tissue margin is necessary likely depends on the surgeons comfort with the resection. Our data show that if the surgeon feels confident with a particular resection, additional tissue margins are unnecessary for benign disease. As in any clinical data for rare disease, there is always a question of sample size, and a multi-institutional trial of a larger cohort of patients may further clarify the necessity of separate tissue margins. The number of malignant tumors from IP in this study was not sufficient to comment on the effect of separate surgical margins on patient outcome, but it remains our protocol to obtain margins under frozen section control for all malignant tumors.
Our analysis failed to show any difference in survival or recurrence between patients undergoing primary or secondary operation for tumor recurrence. Higher recurrence rates have been reported26 for recurrent IP compared with primary cases, with other authors8 12 27 noting its importance at least in surgical planning given the skewed anatomical landmarks, scarring resulting in difficult visualization, and reactive bone sclerosis. Lawson and Patel12 reported a higher rate of adjuvant procedures such as the Caldwell-Luc procedure and lateral rhinotomy for secondary surgery, but this association was not investigated in our report.
Aside from the high rates of recurrence, the malignant potential of IP remains a major concern. The malignancy rate in this study of 14.7% is congruent with the literature.6 9 27 Lesperance and Esclamado9 reported a mean interval of 63 months of follow-up before metachronous malignancy is detected, compared with 73 months in this study. The high rates of synchronous tumors in this analysis and in the literature likely represent the bias of studying these rare tumors in tertiary referral centers. Despite close follow-up for tumor recurrence and aggressive treatment with curative intent when malignancy is detected, the survival among patients with malignancy remains poor (66% disease-specific survival at 5 years). Virtually no patients succumbed to IP in this study if no malignancy was detected. The rate of dysplasia without malignancy was low in this cohort (5 of 129), and although no recurrence or deaths were detected among this population, no clear conclusions can be drawn for this subgroup given the small numbers.
The predictors of malignancy continue to be elusive for IP,4 18 28 and we similarly did not find additional high-risk factors for this feared sequela of the disease. Active smoking in this study demonstrated only a trend to eventual carcinomatous progression, and tobacco use did not affect disease-specific survival or locoregional control of IP. The number of recurrences and surgical resections did not correlate with eventual development of malignancy, a finding that may be surprising but is consistent with previous reports.3 28 Regardless, our preference continues to be complete definitive resection using any approach necessary for complete tumor extirpation given the possibility of malignancy, local aggressive growth, and biopsy sample error for larger tumors and tumors with radiologic aggressiveness. The likelihood of malignancy during follow-up is likely inherent in the tumor biology. Human papillomavirus16 17 29 and chromosomal aneuploidy30 have been suggested as markers, but further investigations are needed to confirm these findings.
Conclusion
IP is a locally aggressive tumor with malignant potential that requires prolonged follow-up. Because additional surgical margins taken outside of tumor specimens did not change disease recurrence in this study, we conclude that sending separate tissue margins is unnecessary for IP if the surgeon deems a resection to be adequate. Similarly, tumor site of origin and surgery for recurrence do not clearly affect outcome. Stage IV tumors are associated with worse survival, but staging criteria did not affect recurrence rates in this study. Because no clear predictors of malignancy were seen in this study, further research to predict this phenomenon is needed.
Footnotes
Disclaimer The authors have no financial interests, disclosures, or conflicts of interest regarding the content of this original manuscript.
References
- 1.Vrabec D P. The inverted Schneiderian papilloma: a 25-year study. Laryngoscope. 1994;104(5 Pt 1):582–605. doi: 10.1002/lary.5541040513. [DOI] [PubMed] [Google Scholar]
- 2.Hyams V J. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80(2):192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15(3):279–297. doi: 10.1038/modpathol.3880524. [DOI] [PubMed] [Google Scholar]
- 4.Pensak M L. New York, NY: Thieme; 2001. Controversies in Otolaryngology. [Google Scholar]
- 5.Sham C L, Lee D L, van Hasselt C A, Tong M C. A case-control study of the risk factors associated with sinonasal inverted papilloma. Am J Rhinol Allergy. 2010;24(1):e37–e40. doi: 10.2500/ajra.2010.24.3408. [DOI] [PubMed] [Google Scholar]
- 6.Myers E N, Schramm V L Jr, Barnes E L Jr. Management of inverted papilloma of the nose and paranasal sinuses. Laryngoscope. 1981;91(12):2071–2084. doi: 10.1288/00005537-198112000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Lawson W, Ho B T, Shaari C M, Biller H F. Inverted papilloma: a report of 112 cases. Laryngoscope. 1995;105(3 Pt 1):282–288. doi: 10.1288/00005537-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Lawson W, Kaufman M R, Biller H F. Treatment outcomes in the management of inverted papilloma: an analysis of 160 cases. Laryngoscope. 2003;113(9):1548–1556. doi: 10.1097/00005537-200309000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Lesperance M M, Esclamado R M. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105(2):178–183. doi: 10.1288/00005537-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi D, Tomenzoli D, Buttà L. et al. Limitations and complications of endoscopic surgery for treatment for sinonasal inverted papilloma: a reassessment after 212 cases. Head Neck. 2011;33(8):1154–1161. doi: 10.1002/hed.21589. [DOI] [PubMed] [Google Scholar]
- 11.Sham C L, Woo J K, van Hasselt C A, Tong M C. Treatment results of sinonasal inverted papilloma: an 18-year study. Am J Rhinol Allergy. 2009;23(2):203–211. doi: 10.2500/ajra.2009.23.3296. [DOI] [PubMed] [Google Scholar]
- 12.Lawson W, Patel Z M. The evolution of management for inverted papilloma: an analysis of 200 cases. Otolaryngol Head Neck Surg. 2009;140(3):330–335. doi: 10.1016/j.otohns.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe S G, Schlosser R J, Bolger W E, Lanza D C, Kennedy D W. Endoscopic and endoscope-assisted resections of inverted sinonasal papillomas. Otolaryngol Head Neck Surg. 2004;131(3):174–179. doi: 10.1016/j.otohns.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Pasquini E, Sciarretta V, Farneti G, Modugno G C, Ceroni A R. Inverted papilloma: report of 89 cases. Am J Otolaryngol. 2004;25(3):178–185. doi: 10.1016/j.amjoto.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Krouse J H. Development of a staging system for inverted papilloma. Laryngoscope. 2000;110(6):965–968. doi: 10.1097/00005537-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Beck J C, McClatchey K D, Lesperance M M, Esclamado R M, Carey T E, Bradford C R. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113(5):558–563. doi: 10.1177/019459989511300506. [DOI] [PubMed] [Google Scholar]
- 17.Beck J C, McClatchey K D, Lesperance M M, Esclamado R M, Carey T E, Bradford C R. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113(1):49–55. doi: 10.1016/s0194-5998(95)70144-3. [DOI] [PubMed] [Google Scholar]
- 18.von Buchwald C, Bradley P J. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg. 2007;15(2):95–98. doi: 10.1097/MOO.0b013e3280803d9b. [DOI] [PubMed] [Google Scholar]
- 19.Weissler M C, Montgomery W W, Turner P A, Montgomery S K, Joseph M P. Inverted papilloma. Ann Otol Rhinol Laryngol. 1986;95(3 Pt 1):215–221. doi: 10.1177/000348948609500301. [DOI] [PubMed] [Google Scholar]
- 20.Woodworth B A, Bhargave G A, Palmer J N. et al. Clinical outcomes of endoscopic and endoscopic-assisted resection of inverted papillomas: a 15-year experience. Am J Rhinol. 2007;21(5):591–600. doi: 10.2500/ajr.2007.21.3086. [DOI] [PubMed] [Google Scholar]
- 21.Kim D Y, Hong S L, Lee C H. et al. Inverted papilloma of the nasal cavity and paranasal sinuses: a Korean multicenter study. Laryngoscope. 2012;122(3):487–494. doi: 10.1002/lary.22495. [DOI] [PubMed] [Google Scholar]
- 22.Carta F, Verillaud B, Herman P. Role of endoscopic approach in the management of inverted papilloma. Curr Opin Otolaryngol Head Neck Surg. 2011;19(1):21–24. doi: 10.1097/MOO.0b013e3283425213. [DOI] [PubMed] [Google Scholar]
- 23.Cannady S B, Batra P S, Sautter N B, Roh H J, Citardi M J. New staging system for sinonasal inverted papilloma in the endoscopic era. Laryngoscope. 2007;117(7):1283–1287. doi: 10.1097/MLG.0b013e31803330f1. [DOI] [PubMed] [Google Scholar]
- 24.Sautter N B, Cannady S B, Citardi M J, Roh H J, Batra P S. Comparison of open versus endoscopic resection of inverted papilloma. Am J Rhinol. 2007;21(3):320–323. doi: 10.2500/ajr.2007.21.3020. [DOI] [PubMed] [Google Scholar]
- 25.Mortuaire G, Arzul E, Darras J A, Chevalier D. Surgical management of sinonasal inverted papillomas through endoscopic approach. Eur Arch Otorhinolaryngol. 2007;264(12):1419–1424. doi: 10.1007/s00405-007-0401-2. [DOI] [PubMed] [Google Scholar]
- 26.Tufano R P, Thaler E R, Lanza D C, Goldberg A N, Kennedy D W. Endoscopic management of sinonasal inverted papilloma. Am J Rhinol. 1999;13(6):423–426. doi: 10.2500/105065899781329665. [DOI] [PubMed] [Google Scholar]
- 27.Mirza S, Bradley P J, Acharya A, Stacey M, Jones N S. Sinonasal inverted papillomas: recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007;121(9):857–864. doi: 10.1017/S002221510700624X. [DOI] [PubMed] [Google Scholar]
- 28.Lawson W, Le Benger J, Som P, Bernard P J, Biller H F. Inverted papilloma: an analysis of 87 cases. Laryngoscope. 1989;99(11):1117–1124. doi: 10.1288/00005537-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa M, Deng Z, Maeda H. et al. Human papillomavirus load and physical status in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology. 2012;50(1):87–94. doi: 10.4193/Rhino.11.106. [DOI] [PubMed] [Google Scholar]
- 30.Klemi P J, Joensuu H, Siivonen L, Virolainen E, Syrjänen S, Syrjänen K. Association of DNA aneuploidy with human papillomavirus-induced malignant transformation of sinonasal transitional papillomas. Otolaryngol Head Neck Surg. 1989;100(6):563–567. doi: 10.1177/019459988910000607. [DOI] [PubMed] [Google Scholar]


