Abstract
Short 50-kilohertz (kHz) range frequency-modulated ultrasonic vocalizations (USVs) produced by rats and mice are unconditionally elicited by drugs of abuse or electrical stimulation that increase dopamine activity in the nucleus accumbens, and it has been suggested that they reflect “positive affect” or incentive motivational states associated with appetitive behavior. The repeated administration of amphetamine is known to not only produce “psychomotor” sensitization, but also to facilitate a number of appetitive behaviors, including conditioned drug pursuit behavior. We were interested, therefore, in whether amphetamine-induced 50-kHz USVs would also increase with repeated drug exposure. USV recordings were made during 5-min sessions immediately after a saline infusion, and again 4-5 hours later, after 1.0 mg/kg intravenous amphetamine exposure. These sessions took place every other day over a 5-day period. A challenge dose of 1.0 mg/kg amphetamine was administered 2 weeks later to determine whether sensitization would persist. The initial amphetamine infusion increased 50-kHz USVs relative to the saline infusion. This effect was enhanced over trials and during the amphetamine challenge two weeks later. Classification of 50-kHz range call types revealed that complex frequency-modulated trill calls were sensitized by amphetamine, but not flat 50-kHz calls. It is possible that 50-kHz USV recordings could provide a potentially valuable behavioral measure of sensitization linked to enhanced incentive salience and increased tendency to self-administer drugs of abuse.
1. Introduction
Repeated intermittent administration of amphetamine or cocaine produces enduring neuroadaptations that render the brain hypersensitive to subsequent drug exposure at the original dosage. Stimulant drug-induced sensitization is often assessed as a progressive increase in psychomotor behavior that can be influenced by drug-associated context [2, 26, 27, 30, 36]. Sensitization is accompanied by chronic structural, neurochemical and other brain events including increases in dendritic arborization and spine density in the nucleus accumbens and frontal cortex [31] and enhanced reactivity in dopamine neurons that project from the ventral tegmental area to the nucleus accumbens and prefrontal cortex [18, 25, 38-40]. However, in addition to “psychomotor sensitization” repeated drug treatment also enhances the rewarding and/or incentive motivational effects of drugs and other rewards, perhaps through the process of “incentive-sensitization” [27-29]. Once produced, both psychomotor and incentive-sensitization, persist long after drug treatment is discontinued, and may contribute to the propensity to relapse. Given that repeated treatment with drugs like amphetamine alter the operation of brain systems that mediate reward and incentive motivation, other indices indicative of activation of such brain systems may also undergo sensitization-like changes as a consequence of repeated exposure to such drugs.
It has been suggested that short frequency-modulated 50-kHz range ultrasonic vocalizations (USVs) produced by adult rats reflect a state of positive affect associated with appetitive behavior (in contrast to long simple 22-kHz calls that have been associated with negative affect) [6, 8, 21, 32] and 50-kHz USVs are modulated by midbrain dopamine activity [7, 9-11, 13, 14]. Rats produce 50-kHz USVs in response to unconditioned natural rewards such as food or sexual encounters, to acute administration of drugs of abuse, as well as to conditioned cues that predict reward [5, 8, 9, 11, 19, 20]. Acute administration of amphetamine, either systemically or directly into the nucleus accumbens elicits dose-dependent increases in 50-kHz USVs that are blocked by dopamine antagonists [9, 37, 41]. Amphetamine microinjection into the shell of the nucleus accumbens elicts a greater increase in 50-kHz USVs than injection into the core [37], which is consistent with the role of the shell region as an important site for drug self-administration [15, 16]. Moreover, the number of 50-kHz USVs produced in response to an unconditional reward predict the subsequent development of pursuit behavior. For example, the number of USVs elicited by acute electrical brain stimulation applied to the ventral tegmental area or lateral hypothalamus predicts the later acquisition of self-stimulation behavior, and USVs elicited by an initial injection of the μ-opioid agonist DAMGO into the ventral tegmental area predict the development of conditioned place preference for DAMGO [11]. Conversely, dopamine depletion by neurotoxin delivery into the ventral tegmental area or medial forebrain bundle reduces the number of USVs elicited by a social reward situation [11], or degrades the acoustic quality of the calls, even when the depletion is unilateral [13, 14].
Given that 50-kHz USVs are related to the activation of brain systems known to be “sensitized” by repeated amphetamine treatment, and that they are thought to reflect the activation of positive appetitive states, also known to sensitize, we hypothesized that repeated exposure to amphetamine may increase (sensitize) the ability of amphetamine to elicit 50-kHz USVs. Three intravenous (i.v.) infusions of 1.0 mg/kg amphetamine were administered every other day for 5 days, and USVs were recorded for five minutes following infusions. An i.v. route of administration was chosen based on evidence that a single, rapidly infused i.v. dose of cocaine produces psychomotor sensitization and activation of mesocorticolimbic structures [33, 34]. To determine whether drug-induced changes in USVs persist long-term, a challenge dose of 1.0 mg/kg i.v. amphetamine was given two weeks after the last infusion.
2. Method
2.1. Animals
Nine male Long-Evans rats weighing 380-540 grams (Charles River) were used in both saline and amphetamine trials. Rats were singly housed in standard polycarbonate cages with a reversed 12:12 hr light:dark cycle, and all testing took place during the dark phase of the cycle. Food and water were available ad libitum, and rats were handled daily for 5 days prior to surgery as well as throughout the experiment. All experiments were approved by the University of Texas Animal Care and Use Committee.
2.2. Catheter Surgery
To enable intravenous delivery of amphetamine, jugular catheterization was performed. Rats underwent the surgical procedure while anesthetized by a mixture of oxygen (0.8 L/min, Airgas Southwest, Corpus Christi, TX) and isoflurane (2.5-4%; AErrane, Baxter Healthcare, Deerfield, IL) delivered through a gas delivery system (VetEquip Inc., Pleasanton, CA). Rats received the anti-inflammatory agent, Rimadyl (5 mg/kg, s.c.), post-surgically to alleviate pain. Catheters were constructed from 8.5 mm silastic tubing (0.64 mm o.d.) with one end connected to a cannula endpiece (Plastics One, Roanoke, VA). The catheter was inserted into the right jugular vein and the cannula endpiece was passed subcutaneously to an incision on the head. The catheter was anchored to the top of the head with four stainless steel screws and dental acrylic cement. For the first week after surgery, catheters were flushed daily with 0.1 m of 0.9% saline that contained 1 U/ml heparin and 67.0 mg/ml Timentin. Animals continued receiving the same solution daily without the Timentin component through the duration of the experiment to maintain catheter patency.
2.3. USV recordings and drug infusions
Rats were given three intermittent i.v. infusions of amphetamine during the pre-exposure phase, followed by a challenge dose of amphetamine two weeks later to test for sensitization. To establish baseline rates of vocalization and to test for the expression of conditioned vocalization, saline recordings were obtained prior to amphetamine recordings. Ultrasonic vocalizations were recorded during the first 5 minutes after each i.v. saline (0.1 ml heparinized saline) or i.v. amphetamine infusion (1.0 mg/kg in 1.0 mg/ml solution, weight of d-amphetamine sulfate salt; Sigma). All rats received both saline and amphetamine on trial days, with saline infusions occurring in the mornings, and amphetamine infusions occurring 4-5 hrs later in the afternoon. Amphetamine and saline trials took place every other day over a 5 day period, with Trials 1, 2, and 3 occurring on Days 1, 3, and 5 respectively, and the 2 week Challenge occurring on day 19. On days 2, 4, and 6-18 rats were left undisturbed except for the daily flushing of catheters with heparinized saline.
During saline and amphetamine trials, rats remained in their home cages and were transferred from the colony room to a recording environment in a separate room. Cage tops containing food and water were replaced with a modified cage top holding the microphone panel. Discrete social olfactory cues were provided to help animals distinguish between saline and amphetamine conditions, as cues associated with a drug are known to play an important role in the induction and expression of sensitization [1-3, 30]. Odor cues consisted of 1-inch-round wooden beads (CraftWorks, Seattle, WA) that were impregnated with different social odors [for details see 35]. Odor cues that were distinct for saline vs. amphetamine conditions were presented 1 minute prior to infusion and remained in the cage throughout the trial, except for their brief removal and replacement at the start of the saline or drug delivery and every 5 minutes to prevent sensory adaptation. Infusions were administered by the experimenter and the rate of infusion was recorded, with infusion durations for amphetamine ranging from 10-15 seconds.
A model CM16 ultrasonic microphone (Avisoft, Germany) mounted in a Plexiglas panel was placed over the cage as previously described [13], and USVs were recorded for 5 minutes after infusions. Animals remained in the testing room for 15 min after recording procedures in an attempt to ensure that environmental cues were associated with the peak effects of amphetamine. Two weeks after the third trial, rats with intact catheters (n = 5) were again given 0.1 ml heparinized saline and then 1.0 mg/kg amphetamine following the same infusion and recording procedures.
2.4. Data analysis and statistics
Analog USV recordings were digitized with an A/D card (National Instruments, USA) at a 200-kHz sampling rate with 16-bit resolution. Sonograms were generated and analyzed using Saslab Pro (Avisoft, Germany), with a 512 FFT-length and 75% overlap frame setup. A trained observer, blind to treatment conditions, counted the number of USVs produced in 5 minutes following saline and amphetamine, and categorized calls as flat, freqency-modulated, or harmonic, based on the presence or absence of rapid fluctuations in frequency (“trill” components) or harmonic components (see Fig. 1) [11, 13, 14]. All USVs recorded were found to be in the 50-kHz range, with no 22-kHz calls detected. Since few harmonic calls (< 2% of total calls) were observed in both saline and amphetamine conditions, this call type is not discussed further.
Fig. 1.
Example sonograms of a) frequency-modulated “trill” 50-kHz call, and b) flat 50-kHz call. Colors indicate relative level of signal intensity (dB). Audio samples of 50 kHz calls (slowed down 10 ×) can be heard at the lab website: www.schallertlab.org
To examine the effects of repeated amphetamine, each rat’s amphetamine-elicited calls were compared to its saline-elicited calls recorded earlier in the day. A 2 × 3 (Drug × Trial) repeated measures ANOVA followed by within-subjects contrasts was used to examine amphetamine vs. saline USVs on Trial 2 compared to Trial 1, and on Trial 3 compared to Trial 1. Recordings from the Challenge day were compared to Trial 1 using a 2 × 2 (Drug × Trial) repeated measures ANOVA.
3. Results
3.1. Amphetamine effects on 50-kHz frequency modulated and flat calls combined
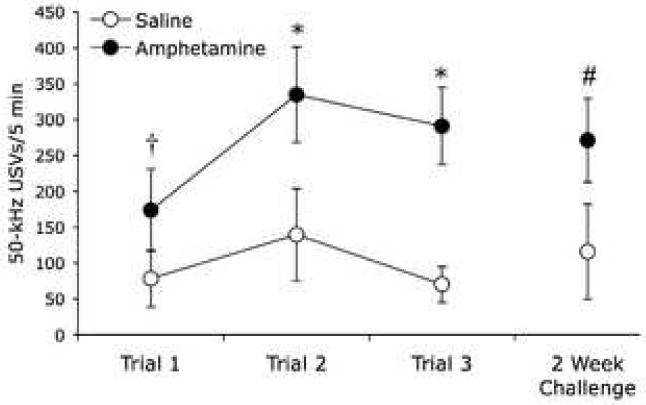
The initial exposure to amphetamine elicited significantly more 50-kHz USVs than saline in 8 of the 9 animals (Fig. 2). One rat did not produce USVs in response to saline or amphetamine, and was consequently excluded from the study, as the goal of the experiment was to examine changes in unconditioned USV responses to amphetamine across multiple exposures. However, inclusion of this rat’s data did not change the statistical significance of the outcome. In the remaining rats, repeated amphetamine elicited a significantly greater increase in 50-kHz USVs than the initial exposure to amphetamine, and this elevated response was present 2 weeks after the cessation of drug treatment (Fig. 2). For the number of 50-kHz USVs (all types) produced in 5 min post-infusion, a repeated measures ANOVA revealed a significant main effect of Trial (F(2,14) = 9.34, p = .003), a significant main effect of Drug (F(1,7) = 40.53, p < .001), and a significant Trial × Drug interaction (F(2,14) = 5.19, p = .021). Within subjects contrasts revealed that the amphetamine-induced increase in 50-kHz USVs was significantly greater on Trials 2 and 3 compared to Trial 1 (F(1,7) = 11.39, p = .012; F(1,7) = 11.02, p = .013 respectively). This effect was observed 2 weeks later, as the challenge dose of amphetamine elicited significantly more 50-kHz USVs than the initial exposure (Trial, F(1,4) = 213.89, p < .001; Drug, F(1,4) = 14.55, p = .019; Trial × Drug, F(1,4) = 12.87, p = .023).
Fig. 2.
Repeated amphetamine increases the production of 50-kHz USVs. Results are mean ± SEM of number of 50-kHz USVs recorded in 5 minutes after i.v. infusions of saline and amphetamine. In Trial 1, acute amphetamine elicited more 50-kHz USVs than the preceding saline infusion († paired samples t-test, t = −3.18; p = .015, two-tailed). In Trial 2 and Trial 3 amphetamine elicited a greater increase in 50-kHz USVs than on Trial 1 (* p < .05; N = 8). Two weeks after Trial 3 (day 19), a challenge dose of amphetamine elicited a greater increase in calls than on Trial 1 (# p < .05; n = 5).
3.2. Amphetamine sensitization of frequency modulated but not flat 50-kHz calls
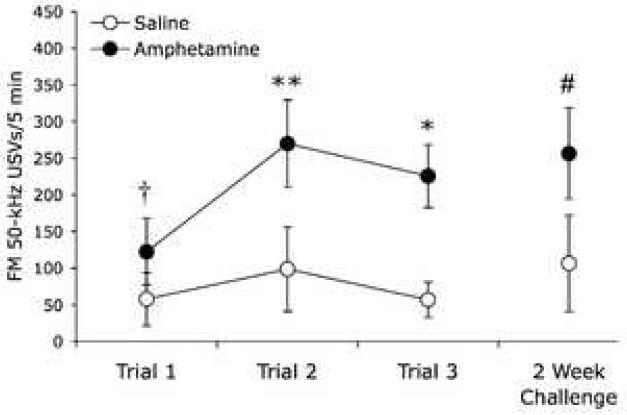
The additional 50-kHz USVs elicited by amphetamine were predominantly of the frequency-modulated (trill) type, whereas the number of flat 50-kHz USVs was not detectably changed by amphetamine (Figs. 3 and 4). Repeated amphetamine significantly increased the production of frequency-modulated 50-kHz USVs, with a significant main effect of Trial (F(2,14) = 9.28, p = .003), a significant main effect of Drug (F(1,7) = 49.72, p < .001), and a significant Trial × Drug interaction (F(2,14) = 5.76, p = .015). The increase in amphetamine-induced frequency-modulated 50-kHz USVs was significantly greater on Trials 2 and 3 compared to Trial 1 (F(1,7) = 16.15, p = .005; F(1,7) = 11.86, p = .011 respectively), and this effect persisted with the amphetamine challenge (Trial, F(1,4) =147.69, p < .001; Drug, F(1,4) =13.44, p = .021; Trial × Drug, F(1,4) = 14.37, p = .019).
Fig 3.
Frequency-modulated 50-kHz USVs are increased by repeated amphetamine. Results are mean ± SEM of the number of 50-kHz USVs categorized as frequency-modulated (trill type calls). In Trial 1, amphetamine elicited more frequency-modulated calls than saline († paired samples t-test, t = −3.28; p = .014, two-tailed). Re-exposure to amphetamine in Trials 2 and 3 elicited a greater increase in frequency-modulated 50-kHz USVs than Trial 1 (* p < .05, ** p < .01; N = 8). Two weeks after Trial 3, a challenge dose of amphetamine elicited a greater increase in frequency-modulated calls than the initial exposure (# p < .05; n = 5).
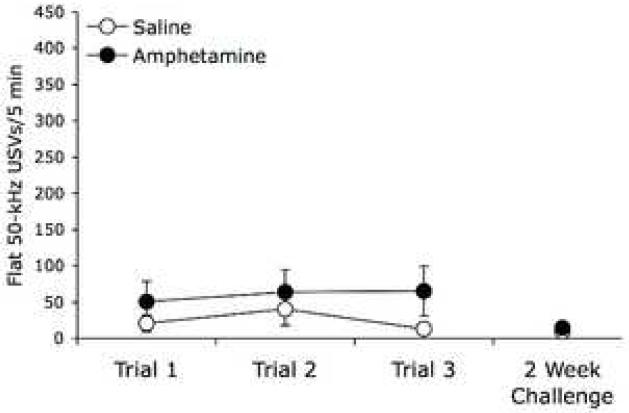
Fig 4.
Flat 50-kHz USVs were not significantly increased by the initial exposure to amphetamine or by multiple exposures to amphetamine. Results are mean ± SEM of the number of 50-kHz USVs categorized as flat.
Conversely, the number of flat calls remained unchanged on all test days, including the 2 week challenge (Fig. 4). No significant effects of repeated amphetamine were observed in the first 3 trial days (Trial, F(2,14) = 1.68, p = .222; Drug, F(1,7) = 3.76, p = .094; Trial × Drug, F(2,14) = 1.58, p = .241), nor following the amphetamine challenge (Trial, F(1,4) = 2.9, p = .164; Drug, F(1,4) = .64, p = .468; Trial × Drug, F(1,4) = .06, p = .811). As expected, 22-kHz long flat calls linked to aversive states were never observed.
3.3. Amphetamine sensitization of locomotor activity
Repeated exposure to amphetamine also increased locomotor activity, measured as the percentage of time spent rearing (Fig. 5). A 2 × 2 (Trial × Drug) repeated measures ANOVA revealed that the amphetamine-induced increase in rearing was significantly greater in pre-exposure Trial 3 compared to Trial 1 (Trial, F(1,7) = 47.19, p < .001; Drug, F(1,7) = 9.45, p = .018; Trial × Drug, F(1,7) = 6.87, p = .034). Behavior was not videotaped on the 2 week challenge day.
Fig 5.
Mean (± SEM) of percentage of time spent rearing in 5 min post-infusion. More rearing behavior was elicited by amphetamine in Trial 3 compared to Trial 1 (* p < .05, N = 8).
4. Discussion
The results of this study indicate that (1) i.v. amphetamine elicits 50-kHz USVs, (2) repeated intermittent treatment with amphetamine facilitates (“sensitizes”) its ability to elicit 50-kHz USVs, and (3) this effect persists for at least 2 weeks. To our knowledge, this is the first report both of USVs elicited by intravenous amphetamine, as well as changes in USV responses across repeated drug treatments [although see 12]. The development of amphetamine sensitization is supported by ratings of locomotor activity. Re-exposure to amphetamine increased the amount of time rats spent engaged in rearing and upward exploratory sniffing, behaviors that have been reported to sensitize to psychostimulants [23]. Although there are no rearing data from the amphetamine challenge, previous studies have found that locomotor sensitization persists for weeks or months after the discontinuation of drug treatment [23, 24].
That repeated treatment with amphetamine increased the ability of amphetamine to elicit 50-kHz USVs is consistent with the modulatory role of dopamine on the rate and quality of 50-kHz USV production [9, 11, 37, 41]. Frequency-modulated call types may be particularly sensitive to dopamine activity and appetitive states. For example, an elevated ratio of trill calls to flat calls has been observed in situations that elicit approach behavior, and lesions of midbrain dopamine neurons or pre-treatment with dopamine antagonists selectively block the frequency-modulated calls evoked by a reinforcing stimulus, without affecting flat 50-kHz calls or aversive 22-kHz calls [10, 11]. Furthermore, situations that elicit 50-kHz USVs but are not considered “reward-related” (such as exposure to a novel environment or brief separation from a cage mate) elicit primarily flat 50-kHz calls [42].
The finding that 50-kHz USVs are sensitized by repeated amphetamine suggests that these calls may possibly provide a valuable behavioral measure of key processes underlying addiction. In particular, USVs may provide insight into the development of incentive-sensitization, as they are suggested to reflect affective states associated with appetitive behavior, and are influenced by prior experiences and associative learning. For example, both male and female rats emit large numbers of 50-kHz USVs during sexual encounters [4, 13, 14, 22]. Male rats also vocalize in response to the odors of estrous females, and greater numbers of odor-elicited calls are associated with previous sexual experience and increased sexual motivation [5, 13]. Juvenile rats emit 50-kHz calls during social interactions and play with conspecifics, as well as in response to cues that predict access to conspecifics [19], and rats previously exposed to amphetamine or morphine emit 50-kHz calls when placed in the drug-associated context [20]. The appetitive states produced by drugs of abuse have been suggested to activate dopamine mediated incentive-salience processes, and strengthen the associations between drug-related cues and motivation to obtain the drug [27, 28, 29]. Thus, the relationship between 50-kHz USVs and emotional/motivational states may provide unique insights into changes in incentive-motivation that occur with chronic drug exposure.
Acknowledgements
This work was supported by the Davis Phinney Foundation and grants NS 19608, HD 02023, and NS042345. The authors would like to thank Ame Wongsa for technical assistance with i.v. catheter surgery, Michelle Ciucci for helpful comments on an earlier version of this manuscript, and Michael Spinetta and Larry Cormack for their advice and expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- [2].Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine induced psychomotor sensitization. Neuropsychopharmacol. 2002;26(6):703–15. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- [3].Badiani A, Camp DM, Robinson TE. Enduring enhancement of amphetamine sensitization by drug-associated environmental stimuli. J Pharmacol Exp Ther. 1997;282:787–94. [PubMed] [Google Scholar]
- [4].Barfield RJ, Auerbach P, Geyer LA, McIntosh TK. Ultrasonic vocalizations in rat sexual behavior. Am Zool. 1979;19:469–80. [Google Scholar]
- [5].Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–90. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- [6].Brudzynski SM. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev. 2001;25:611–17. doi: 10.1016/s0149-7634(01)00058-6. [DOI] [PubMed] [Google Scholar]
- [7].Brudzynski SM. Principles of rat communication: Quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35(1):85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- [8].Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–7. [PubMed] [Google Scholar]
- [9].Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50 kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–4. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- [10].Burgdorf J, Pankepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [11].Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–83. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- [12].Chehayeb D, Sorge RE, Franklin KB, Clarke PBS. Ultrasonic vocalizations and psychomotor stimulant reward in adult rats. Society for Neuroscience abstract. 2007 [Google Scholar]
- [13].Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: A preliminary study. Behav Brain Res. 2007;82(2):284–9. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson’s disease: Deficit-targeted training. Parkinson’s Dis Related Disord. 2008 doi: 10.1016/j.parkreldis.2008.04.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- [16].Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacol. 2004;47:227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- [17].Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Bio. 2005;3(12):e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- [19].Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- [20].Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalization index conditioned pharmacological reward. Physiol Behav. 1999;66:639–43. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- [21].Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective state in rats. Psychol Bul. 2002;128:961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- [22].McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol Behav. 2003;80:81–8. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- [23].Nordquist RE, Vanderschuren LJMJ, Jonker AJ, Bergsma M, de Vries TJ, Pennartz CMA, Voorn P. Expression of amphetamine sensitization is associated with the recruitment of a reactive neuronal population in the nucleus accumbens core. Psychopharmacology. 2008;198:113–26. doi: 10.1007/s00213-008-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–92. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- [26].Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- [27].Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- [28].Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- [29].Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Phil Trans R Soc B. 2008 doi: 10.1098/rstb.2008.0093. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Robinson TE, Brownman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–54. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- [31].Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- [32].Sadananda M, Wöhr M, Schwarting RKW. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett. 2008;435:17–23. doi: 10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [33].Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioural plasticity: implications for addiction. J Neurosci. 2004;24:6362–70. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26(2):82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- [35].Spinetta MJ, Woodlee MJ, Cormack LK, Schallert K, Stroud C, Schallert T. Ethanol-induced retrograde memory impairment in rats: Prevention by caffeine. Psychopharmacol. 2008 doi: 10.1007/s00213-008-1294-5. in press. [DOI] [PubMed] [Google Scholar]
- [36].Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- [37].Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: A quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [38].Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacol. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- [39].Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [40].White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–53. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- [41].Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70:317–23. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- [42].Wöhr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93(4-5):766–76. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]