Abstract
Background
Identifying potentially modifiable risk factors is critically important for reducing the burden of chronic kidney disease. We sought to examine the association of body mass index (BMI) with kidney function decline in a cohort of young adults with preserved glomerular filtration at baseline.
Study Design
Longitudinal cohort.
Setting & Participants
2,891 black and white young adults with cystatin C-based estimated glomerular filtration rate (eGFRcys) >90 ml/min/1.73 m2 taking part in the year-10 examination (in 1995–1996) of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
Predictor
BMI, categorized as 18.5–24.9 (reference), 25.0–29.9. 30.0–39.9, and ≥40.0 kg/m2.
Outcomes
Trajectory of kidney function decline, rapid decline (>3% per year), and incident eGFRcys <60 ml/min/1.73 m2 over 10 years of follow-up.
Measurements
GFRcys estimated from the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation for calibrated cystatin C at CARDIA years 10, 15, and 20.
Results
At year 10, participants had a mean age of 35.1 years, median eGFRcys of 114 ml/min/1.73 m2, and 24.5% had BMI ≥30.0 kg/m2. After age 30 years, average eGFRcys was progressively lower with each increment of BMI after adjustment for baseline age, race, sex, hyperlipidemia, smoking status, and physical activity. Higher BMI category was associated with successively higher odds of rapid decline (for 25.0–29.9, 30.0–39.9, and ≥40.0 kg/m2, the adjusted ORs were 1.50 [95% CI, 1.21–1.87], 2.01 [95% CI, 1.57–2.87], and 2.57 [95% CI, 1.67–3.94], respectively). Eighteen participants (0.6%) had incident eGFRcys <60 ml/min/1.73 m2. In unadjusted analysis, higher BMI category was associated with incident eGFRcys <60 ml/min/1.73 m2 (for 25.0–29.9, 30.0–39.9, and ≥40.0 kg/m2, the ORs were 5.17 [95% CI, 1.10–25.38], 7.44 [95% CI, 1.54–35.95], and 5.55 [95% CI, 0.50–61.81], respectively); adjusted associations were no longer significant.
Limitations
Inability to describe kidney function before differences by BMI category were already evident. Absence of data on measured GFR or GFR estimated from serum creatinine.
Conclusions
Higher BMI categories are associated with greater declines in kidney function among a cohort of young adults with preserved GFR at baseline. Clinicians should vigilantly monitor overweight and obese patients for evidence of early kidney function decline.
Chronic kidney disease (CKD) affects an estimated 14% of U.S. adults and is associated with significant morbidity and mortality.1 Identification of potentially modifiable risk factors for CKD is essential for reducing its burden.
Although several longitudinal studies have demonstrated an association between obesity and incident CKD, these observations have been made primarily in cohorts of older individuals2–4 or among individuals with established kidney disease.2,4 Because obesity affects an estimated 17% of children and adolescents, nearly triple its prevalence in the 1980s,5,6 determining the association of obesity with declining kidney function in younger populations as well as understanding the association of obesity on the continuum of incipient kidney disease throughout the life course is critical.
Within a cohort of young adults with preserved glomerular filtration at baseline, we examined the association of body mass index (BMI) categories with kidney function decline as measured by cystatin C, an alternative biomarker used to estimate glomerular filtration rate (GFR) and thought to be particularly useful for detecting reductions in kidney function at earlier stages (estimated GFR [eGFR] >60ml/min/1.73m2).7 We hypothesized that individuals with higher BMI would have faster decline in kidney function and more progression to eGFR <60 ml/min/1.73m2 than their counterparts with normal BMI.
METHODS
Study Design and Population
We conducted a longitudinal analysis of CARDIA (Coronary Artery Risk Development in Young Adults) Study participants; CARDIA is a multicenter cohort study to evaluate the development and determinants of cardiovascular risk factors and disease in young adults. From 1985 to 1986, 5,115 asymptomatic young adults aged 18–30 years were recruited from 4 U.S. cities (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). The study design, protocol, and recruitment process have been previously described in detail.8 The institutional review boards at each site approved the examination protocol, and written informed consent was obtained at every examination. The cohort was designed for balance by race (black and white), sex, age, and education, and there were 52% black participants, 55% women, and 40% with ≤12 years of formal education. Follow-up examinations were completed at study years 2, 5, 7, 10, 15, 20, and 25. The retention rate of the surviving cohort was 79% at year 10, 74% at year 15, and 72% at year 20. All measures were obtained on all available participants at every study visit except for cystatin C, which was measured as part of an ancillary study to CARDIA on all available stored plasma samples from participants attending the year-10, year-15, and year-20 examinations.
Of the 3,944 participants who completed the year-10 study visit, we excluded 58 without a year-10 BMI measurement, 76 with BMI <18.5 kg/m2 at year 10, and 118 without a cystatin C measurement at year 10. We excluded 295 participants with eGFR estimated from cystatin C (eGFRcys) <90 ml/min/1.73m2at year 10 in order to examine the cohort with preserved kidney function at baseline (year 10). We excluded 538 additional participants with no year-15 cystatin C measure because consecutive kidney function measurements were required for our rapid eGFRcys decline and incident CKD outcomes. The participants excluded because of missing year-15 cystatin C had similar BMI subgroup prevalences compared with the 2,839 participants with available data who were included in this study (p=0.09).
Predictor
Our primary predictor was BMI (in kg/m2), which we categorized as 18.5–24.9 (reference, normal weight), 25.0–29.9 (overweight), 30.0–39.9 (obese), or ≥40.0 kg/m2 (extremely obese).9 The BMI was calculated from body weight measured with light clothing to the nearest 0.2 lb and body height without shoes to the nearest 0.5 cm.
Main Outcome
Our outcome of interest was kidney function decline, which we evaluated in three ways: (1) trajectory of eGFRcys (in ml/min/1.73 m2) using repeated eGFRcys measurements at study years 10, 15, and 20; (2) rapid eGFRcys decline (>3% per year in the intervals between years 10, 15, and 20); and (3) incident eGFRcys <60 ml/min/1.73m2 at study years 15 or 20. Our definition of rapid decline represents a magnitude of change three times the expected rate previously described in aging population studies10 and has been utilized in several studies.11–13 Serum creatinine was also measured at all study years on all available participants; however, as in prior CARDIA Study analyses, we estimated kidney function using cystatin C, as it has been shown to perform better in evaluating kidney function among persons at higher eGFR ranges7,14,15 and may detect changes in kidney function earlier than creatinine.16 Furthermore, creatinine values in the CARDIA Study have been measured using different assays over time, thus rendering examination of trajectories in kidney function uninterpretable.
Cystatin C was measured as part of an ancillary study on all stored frozen plasma from study years 10, 15, and 20 by nephelometry using the N Latex Cystatin C kit (Dade Behring), and later calibrated to most recent cystatin C standardization. Kidney function (eGFR in ml/min/1.73m2) was then estimated by the 2012 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equation, which is for use with calibrated cystatin C.17
Covariates
Age, race, sex, smoking status, and hyperlipidemia were defined using year 10 data and were measured on all available CARDIA participants. Age, race (white or black), sex, and smoking status were obtained by self-report. We defined smoking status as never, past, or current use. Hyperlipidemia was defined by a low density lipoprotein cholesterol (LDL-C; derived by the Friedewald equation)18 measurement >130mg/dL or self-reported lipid lowering medication use.
We defined diabetes by a fasting serum glucose level ≥126mg/dL or self-reported hypoglycemic medication use using data collected at year 10, 15, and 20 examinations. Serum glucose was measured using hexokinase coupled to glucose-6-phosphate dehydrogenase by Linco Research (St. Louis, Missouri). Three seated systolic and diastolic blood pressure measurements were obtained with a random-zero sphygmomanometer. We used the mean of second and third readings at year 10, 15, and 20 examinations and considered systolic and diastolic blood pressures separately as continuous variables.
Albuminuria was determined from a single, untimed (spot) urine sample collected at year 10, 15 and 20 examinations.19 Urine albumin concentrations were measured using a nephelometric procedure with a specific anti-albumin monoclonal antibody, and creatinine was assessed using the Jaffe method.19 Urine albumin-creatinine ratios were standardized to sex and race and expressed in milligrams per gram of creatinine.20 Albuminuria was defined as urine albumin-creatinine ratio > 30 mg/g.
Serum highly sensitive C-reactive protein (CRP, in mg/L) was measured at the year 7, 15, and 20 examinations with a BNII nephelometer (Dade Behring, Deerfield, IL). Since CRP was not measured at year 10, we used the year 7 data as the baseline measure. Because values were not normally distributed, we log2 transformed them to down-weight noisier values in the higher ranges.
Physical activity was defined by an interviewer-administered questionnaire at each CARDIA examination. This validated questionnaire asked about frequency of participation in 13 categories of moderate and vigorous recreational sports, exercise, leisure, and occupational activities over the previous 12 months.21 We used log-transformed physical activity scores, which were calculated in exercise units based on frequency and intensity of each activity.
Statistical Analyses
We estimated the trajectory of population mean eGFRcys across the entire age spectrum from 30 to 50 years, using linear mixed models in which age effects were captured using 5-knot restricted cubic splines, specific to each BMI group. Individual departures from the trajectory of the population mean were modeled using random intercepts and cubic spline components. We examined population mean eGFRcys graphically, by age, for each BMI group separately, which included a total of 8,146 eGFRcys measurements over 26,535 person-years. We tested for differences in the slopes across BMI categories of the eGFRcys trajectories adjusting for baseline age (to control for possible cohort effects), race, sex, smoking status, hyperlipidemia, and physical activity as potential confounders. Since physical activity was defined by repeated measures, it was considered as a time dependent covariate in all models.
We used repeated-measures logistic regression with robust standard errors to examine associations of BMI categories with rapid eGFRcys decline (>3% per year) in the intervals between years 10, 15, and 20, and a pooled logistic model to assess associations with incident eGFRcys <60 ml/min/1.73m2 at year 15 or 20, adjusting for age, race, sex, smoking status, hyperlipidemia, and physical activity (multivariate model). Since cystatin C is produced by all nucleated cells, including adipose cells, we performed a sensitivity analysis for each outcome in which we additionally adjusted multivariate models for changes in BMI over time. Because participants with no year 15 cystatin C were excluded to examine BMI association with rapid eGFR decline and incident eGFR <60 ml/min/1.73m2, we performed an additional sensitivity analysis in which we repeated the analysis for mean eGFRcys trajectory including these participants.
For all outcomes, we then performed a series of analyses on the multivariate model, sequentially adding incident diabetes, blood pressure, CRP, and albuminuria. These covariates were added individually and then all simultaneously to determine if the associations of BMI with kidney function decline were mediated by these factors. Since incident diabetes, systolic blood pressure, diastolic blood pressure, albuminuria, and CRP were defined by repeated measures, they were considered as time dependent covariates in the models. Finally, we tested the multivariate model of each outcome for interactions of BMI with race and sex. Analyses were performed using SAS 9.2 (SAS Institute Inc)and Stata 13 (StataCorp LP).
RESULTS
The mean BMI in the study sample at baseline (CARDIA year 10) was 27.2 ± 5.9 (standard deviation) kg/m2 and 33.0%, 20.5%, and 4.0% had BMIs of 25.0–29.9, 30.0–39.9, and ≥40.0kg/m2, respectively. The median eGFRcys was 114 ((interquartile range [IQR], 107–119) at baseline, 112 (IQR, 105–117) at year 15, and 106 (IQR, 96–112) ml/min/1.73m2 at year 20 among participants overall. Participant characteristics by BMI category are shown in Table 1. Mean age of participants included in the study was 35.1 years at baseline and was not different by BMI group. A BMI ≥40.0kg/m2 was present among 9.5%, 2.7%, 2.1%, and 1.3% of black women, black men, white men, and white women, respectively. Mean systolic and diastolic blood pressure and prevalence of baseline diabetes, hyperlipidemia, never smoking, and albuminuria was higher in the higher BMI categories. The median CRP level was 10 times as high in the group with BMI ≥40kg/m2 compared with BMI 18.5–24.9kg/m2.
Table 1.
Baseline participant characteristics by BMI group.
| BMI 18.5–24.9 (n=1207) | BMI 25.0–29.9 (n=936) | BMI 30.0–39.9 (n=583) | BMI ≥40 (n=113) | p-valuec | |
|---|---|---|---|---|---|
| eGFRcys (ml/min/1.73 m2) | 115 [109–120] | 114 [107–119] | 112 [105–118] | 110 [100–114] | <0.001 |
| Age (y) | 35.0 (3.6) | 35.2 (3.6) | 35.1 (3.7) | 35.02 (3.9) | 0.7 |
| Race and sex | <0.001 | ||||
| Black women | 213 (17.7) | 214 (22.9) | 249 (42.7) | 71 (62.8) | |
| Black men | 189 (15.7) | 214 (22.9) | 131 (22.5) | 15 (13.3) | |
| White women | 491 (40.7) | 184 (19.7) | 101 (17.3) | 17 (15.0) | |
| White men | 314 (26.0) | 324 (34.6) | 102 (17.5) | 10 (8.9) | |
| Smoking status | 0.03 | ||||
| never | 503 (41.7) | 416 (44.4) | 282 (48.4) | 61 (54.0) | |
| past | 424 (35.1) | 300 (32.0) | 164 (28.2) | 31 (27.4) | |
| current | 280 (23.2) | 220 (23.5) | 136 (23.4) | 21 (18.6) | |
| Hyperlipidemia* | 183 (15.2) | 261 (27.9) | 176 (30.2) | 31 (27.4) | <0.001 |
| Physical activity (exercise units) | 5.7 [5.0–6.2] | 5.7 [5.0–6.2] | 5.3 [4.5–5.9] | 5.3 [4.4–5.9] | <0.001 |
| Diabetes** | 41 (3.4) | 38 (4.1) | 69 (11.8) | 26 (23.0) | <0.001 |
| Systolic BP | 106.1 (11.2) | 110.6 (11.3) | 114.1 (12.8) | 116.4 (14.4) | <0.001 |
| Diastolic BP | 69.7 (9.0) | 72.9 (9.4) | 75.9 (10.2) | 77.2 (9.3) | <0.001 |
| C-reactive protein (mg/L) | 0.65 [0.30–1.58] | 0.96 [0.51–2.3]) | 2.60 [1.20–4.80] | 6.76 [3.97–10.20] | <0.001 |
| Albuminura >30 mg/g | 31 (2.9) | 24 (2.8) | 32 (6.1) | 10 (10.2) | <0.001 |
Note: N=2,839. Baseline indicates CARDIA year 10 (except baseline CRP, which was measured at CARDIA year 7 but not year 10). BMI expressed in kg/m2. Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range].
BMI, body mass index; BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults; eGFRcys, serum cystatin C–based estimated glomerular filtration rate;
p-value is analysis of variance (age, systolic BP, diastolic BP), Wilcoxon (eGFRcys, physical activity, C-reactive protein), or Chi-square t- test (all other) of association between BMI category and characteristic.
Defined as low-density lipoprotein cholesterol >130mg/dL or receiving lipid medication.
Defined as fasting serum glucose >126 mg/dL or receiving diabetes medication.
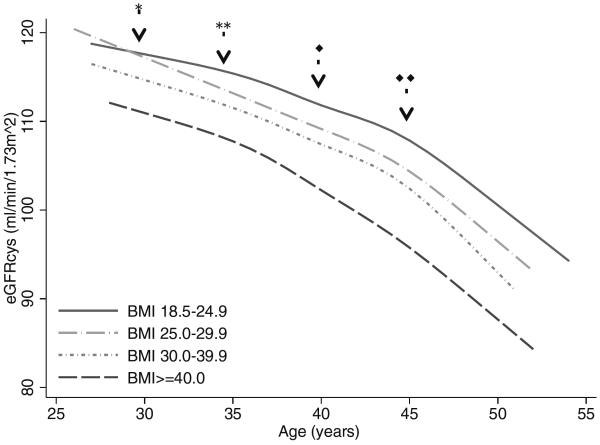
The overall association between BMI category and eGFRcys trajectory is shown in Figure 1. Detailed numeric results are shown in Table S1 (provided as online supplementary material). After age 30, average eGFRcys was progressively lower with each increment of BMI category after adjustment for confounders as well as potential mediators. Across all ages, the rate of eGFRcys decline was nominally slowest for the group with BMI 18.5–24.9kg/m2, but differences were not statistically significant for most comparisons. Adjustment for change in BMI over time modestly attenuated between-group differences in eGFRcys decline (Table S2). Results of a sensitivity analysis including participants with no year 15 cystatin C measurement (who were excluded for the rapid eGFR decline and incident eGFRcys<60ml/min/1.73m2 outcomes) were not substantively different. Although associations between BMI categories and eGFRcys trajectory varied significantly by sex (p<0.001) and race (p<0.001), BMI ≥25.0 kg/m2 was associated with faster eGFRcys decline in all four race/sex subgroups (Figure S1).
Figure 1.
Trajectory of eGFRcysa declineb by age and BMIa category
a eGFRcys=cystatin C estimated glomerular filtration rate, ml/min/1.73m2, BMI= body mass index, kg/m2
bAdjusted for baseline age, race, sex, hyperlipidemia, smoking status, and physical activity.
*Difference in eGFRcys slope BMI 25.0–29.9 vs. BMI 18.5–24.9, p=0.01
**Difference in eGFRcys slope BMI 25.0–29.9 vs. BMI 18.5–24.9, p=0.002
◆Difference in eGFRcys slope BMI ≥40 vs. BMI 18.5–24.9, p=0.02
◆◆Difference in eGFRcys slope BMI 30.0–39.9 vs. BMI 18.5–24.9, p=0.002
Overall, rapid eGFRcys decline (>3% per year) was observed in 569 (10.7%) of the 5, 307 5-year person-intervals included in the analysis. Results of the primary analyses for the rapid eGFRcys decline outcome are shown in Table 2. Relative to the BMI 18.5–24.9 kg/m2 referent group, each of the BMI categories were significantly associated with successively higher likelihood of rapid eGFRcys decline in the unadjusted and confounder adjusted models. Additional adjustments for changes in BMI over time, incident diabetes, albuminuria, and CRP did not appreciably change the association. There was evidence that the association between BMI categories and rapid eGFRcys decline was significantly stronger among women than men (p for interaction=0.005) and somewhat stronger among whites than blacks (p for interaction=0.1) (Table 3).
Table 2.
Odds of rapid eGFRcys decline by BMI category
| Model | BMI 25.0–29.9 (203/1713 [11.5%]) | BMI 30.0–39.9 (153/1078 [14.2%]) | BMI≥40.0 (34/208 [16.3%]) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Unadjusted | 1.53 (1.24–1.89) | <0.001 | 1.98 (1.57–2.49) | <0.001 | 2.36 (1.57–3.56) | <0.001 |
| Adjusted MV modelb | 1.50 (1.21–1.87) | <0.001 | 2.01 (1.57–2.87) | <0.001 | 2.57 (1.67–3.94) | <0.001 |
| MV model + BMI change | 1.49 (1.19–1.87) | <0.001 | 2.00 (1.56–2.58) | <0.001 | 2.74 (1.79–4.19) | <0.001 |
| MV model + incident diabetes | 1.51 (1.21–1.88) | <0.001 | 2.05 (1.60–2.64) | <0.001 | 2.65 (1.71–4.11) | <0.001 |
| MV model + repeated systolic and diastolic BP measurements | 1.44 (1.15–1.79) | 0.001 | 1.85 (1.44–2.37) | <0.001 | 2.33 (1.58–3.54) | <0.001 |
| MV model + repeated CRP measures | 1.48 (1.17–1.86) | 0.001 | 1.96 (1.48–2.59) | <0.001 | 2.46 (1.52–4.00) | <0.001 |
| MV model + repeated albuminuria measures | 1.56 (1.25–1.96) | <0.001 | 2.02 (1.57–2.61) | <0.001 | 2.36 (1.51–3.70) | <0.001 |
| Fully adjusted (includes confounders + all potential mediatorsc) | 1.50 (1.18–1.91) | 0.001 | 1.95 (1.45–2.61) | <0.001 | 2.27 (1.36–3.78) | 0.002 |
Note: Rapid eGFRcys decline defined as >3% decline per year. BMI expressed in kg/m2; column headings also give n/N [%], which corresponds to number of events per number of 5-year person-intervals, with the corresponding percentage. Reference group is BMI of 18.5–24.9 kg/m2 (179/2263 [7.9%]).
BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; eGFRcys, serum cystatin C–based estimated glomerular filtration rate; MV multivariate; OR odds ratio; CI. confidence interval
Adjusted for potential confounding by age, race, sex, hyperlipidemia, smoking status, and physical activity
Potential mediators include incident diabetes, interim systolic and diastolic BP measurements, repeated CRP, and repeated albuminuria measures
Table 3.
Odds of rapid eGFRcys decline by BMI category, stratified by race and sex
| Subgroup | BMI 18.5–24.9 | BMI 25.0–29.9 | BMI 30.0–39.9 | BMI≥40.0 | p-interactione | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eventsc | OR (95% CI) | eventsc | OR (95% CI) | p-valued | eventsc | OR (95% CI) | p-value | eventsc | OR (95% CI) | p-value | ||
| Men | 96/924 (10.4%) | 1.00 (reference) | 119/973 (12.2%) | 1.39 (0.92–1.63) | 0.2 | 56/410 (13.7%) | 1.45 (1.01–2.07) | 0.04 | 9/44 (20.4%) | 2.27 (1.09–4.73) | 0.03 | 0.005 |
| Women | 83/1339 (6.2%) | 1.00 (reference) | 78/740 (10.5%) | 1.88 (1.34–2.63) | <0.001 | 90/628 (14.3%) | 2.74 (1.92–3.92) | <0.001 | 22/152 (14.5%) | 3.06 (1.76–5.33) | <0.001 | |
| Blacks | 63/724 (8.7%) | 1.00 (reference) | 77/788 (9.8%) | 1.17 (0.81–1.68) | 0.4 | 84/669 (12.6%) | 1.62 (1.13–2.31) | 0.009 | 23/150 (15.3%) | 2.28 (1.33–3.89) | 0.004 | 0.1 |
| Whites | 116/1539 (7.5%) | 1.00 (reference) | 120/925 (13.0%) | 1.74 (1.31–2.31) | <0.001 | 62/369 (16.8%) | 2.52 (1.78–3.57) | <0.001 | 8/46 (17.4%) | 2.66 (1.18–5.97) | 0.02 | |
Note: Rapid eGFRcys decline defined as >3% decline per year (adjusted for age, race, sex, hyperlipidemia, smoking status, and physical activity). BMI expressed in kg/m2.
BMI, body mass index; eGFRcys, serum cystatin C–based estimated glomerular filtration rate; OR, odds ratio; CI. confidence interval
number of events per number of 5-year person-intervals, with the corresponding percentage, within subgroup
p-value for difference in rate of decline compared to reference BMI category
p for interaction for BMI category and subgroup
Only 18 (0.6%) participants developed incident eGFRcys <60ml/min/1.73m2 over the 10-year study period. Compared to the 18.5–24.9 kg/m2 reference group, in the unadjusted model higher BMI category was associated with an increased likelihood of incident eGFRcys <60ml/min/1.73m2 for the BMI 25.0–29.9 (odds ratio [OR], 5.17; 95% confidence interval [CI], 1.10–25.38; p=0.04) and 30.0–39.9kg/m2 (OR, 7.44; 95% CI, 1.54–35.95; p=0.01) groups, but did not reach statistical significance for the ≥40.0 kg/m2 group (OR, 5.55; 95% CI, 0.50–61.81; p=0.2). These findings were similar after adjusting for age, race, sex, hyperlipidemia, smoking status, and physical activity, although the association remained statistically significant only for the BMI 30.0–39.9 kg/m2 group (adjusted OR, 6.10; 95% CI, 1.08–34.41; p=0.04). The small number of outcomes prevented testing of interactions between BMI category and race and sex.
DISCUSSION
In a cohort of black and white young adults free of kidney disease at baseline, we found that increasing BMI was significantly associated with lower kidney function throughout the 10-year study period, with some evidence of faster kidney function decline throughout the age spectrum. Furthermore, we found that higher BMI categories were strongly associated with a higher likelihood of rapid kidney function decline, even after adjustment for important confounders and potential mediating factors.
Prior investigation of participants in the Framingham Offspring Study who had a mean age of 43 years and were free of kidney disease at baseline found that each standard deviation increase in BMI was associated with roughly a 20% increased likelihood of incident CKD over a mean 18.5-year follow-up.3 Our study builds upon our understanding of the potential effect of obesity over the life course of kidney function by examining younger participants and by additionally examining the trajectory of kidney function decline and rapid kidney function decline. By using cystatin C to estimate GFR we are able to describe declines in kidney function that may occur within the normal GFR range. We confirmed the importance of BMI as a risk factor for declining kidney function and determined that the association increases with successively higher BMI from 25.0 kg/m2, can be observed in early adulthood, and is related to rapid decline in eGFR within the normal GFR range. Furthermore, we have extended understanding in this area by examining a cohort comprising blacks and whites, and confirmed that the association between BMI and kidney function is observed among both men and women, and blacks and whites.
To our knowledge, only two prior studies have examined the association between obesity and kidney function in a younger cohort. Vivante et al22 found obesity was associated with a 3-fold increased risk of incident end-stage renal disease (ESRD) over 25 years in a cohort of Israeli Army volunteers who were 17 years old at baseline. In a British cohort, Silverwood et al23 found that those who became overweight as defined by BMI >25.0 at age 26 or 36 years were approximately twice as likely to have CKD (decreased eGFR and abnormal albuminuria) at age 60–64 years than those who never became overweight. However, lacking interim measures for kidney function or potential mediators, neither study can describe the trajectory of kidney function decline over time or potential pathways for obesity's effect on kidney function decline. An important strength of our study is that we were able to examine interval development of diabetes and hypertension as potential mediators of the association between BMI and kidney function decline.
We also examined albuminuria and inflammation as potential mediators of our findings, given that obesity is thought to lead to direct kidney injury through a glomerulopathy resembling focal segmental glomerulosclerosis with enlarged glomeruli24 —and because obesity is also recognized as a state of low-grade inflammation,25 with several studies26–28 implicating inflammatory markers in the progression of kidney disease in older and elderly populations. However, a significant association between BMI and kidney function remained after simultaneously accounting for all potential mediators, suggesting the presence of other mechanisms for the potential negative effect of BMI on kidney function.
We determined that the slope of decline in eGFR may differ significantly among higher BMI categories compared to normal BMI, which may have important implications for monitoring and early interventions in this high-risk population. However, our finding that obese (BMI 30–39.9 kg/m2) and extremely obese (BMI ≥40.0kg/m2) participants in their late twenties already have a lower eGFR than their normal weight (BMI 18.5–24.9kg/m2) counterparts suggests that obesity may have an important impact on kidney function at even younger ages. Further study of even younger adults and adolescents is needed to inform this question of when increased BMI may begin affecting eGFR. Only one study to date has examined the association between cystatin C and BMI among adolescents and found that cystatin C was positively correlated with BMI.29 Because BMI cannot distinguish muscle mass from fat mass, it has been questioned as a less accurate measure of adiposity, and therefore, obesity, than waist circumference.30 However, additional adjustment for waist circumference fully attenuated our findings, suggesting significant overlap between the two measures and reinforcing that both are good measures of obesity for this analysis. Further study examining waist circumference as a predictor of kidney function decline would be interesting. Regardless, the impact of obesity of kidney function early in life is of critical importance given the high prevalence of obesity in childhood.
Cystatin C has properties that make it superior to creatinine for estimating GFR because it is not affected by muscle mass, but rather is made by all nucleated cells, including adipose cells. Therefore some have questioned its accuracy among obese patients.31 However, Schuck et al32 found that obesity had no effect on the relationship between cystatin C and GFR measured by inulin clearance in adults with CKD. Regardless, these concerns are unlikely to affect the overall interpretation of our study findings as we observed trajectories in decline over time among all BMI categories. Further, adjusting for change in BMI over time resulted in an expected modest attenuation of associations, suggesting that ongoing weight gain explains a portion of the observed kidney function decline.
Although replication of our findings using creatinine-based GFR estimating equations would further strengthen confidence in our cystatin C-based findings, we were unable to do so within the CARDIA Study due to changes in creatinine measurement assays over time. Future confirmatory studies using creatinine-based equations are needed.
Progressive decrease in kidney function has a widespread effect on health, including on the functioning of the heart and brain; on hormonal balance, anemia, and bone and mineral metabolism; and on ability to resist infections.33 Our finding of independent associations between BMI and kidney function decline among a cohort of young adults with preserved glomerular filtration at study baseline underscores a need for effective obesity interventions early in life. While dramatic weight loss following bariatric surgery may improve GFR,34–36 a recent meta-analysis25 found that modest non-surgical weight loss did not improve creatinine-based estimates of GFR among those with significantly decreased kidney function, suggesting that weight loss may have been too late to improve kidney function or that a threshold effect for weight loss intervention may exist. Further study is needed to determine the effect of various degrees of weight loss on reversing or stabilizing potential obesity-related declines throughout the spectrum of kidney function. Because individual lifestyle modification is often short-lived and prone to relapse,37 evidence of early kidney function decline may help motivate sustained weight loss to prevent deleterious CKD outcomes among overweight and obese individuals.
In conclusion, higher BMI is associated with greater declines in kidney function among a cohort of young adults with preserved glomerular filtration at study baseline. Given the growing epidemic of obesity among adults and children, the long-term impact of these findings could be substantial. Clinicians should vigilantly monitor overweight and obese patients for evidence of early kidney function decline.
Supplementary Material
Acknowledgements
Support: Dr Grubbs was supported by grant 1K23DK093710-01A1 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation. Dr Bibbins-Domingo was in part supported by grant R01DK078124 from the NIDDK, grant N01HC48050 from the National Heart, Lung and Blood Institute (NHLBI), and from the Center for Health and Risk in Minority Youth and Adults (CHARM) grant 1P60MD006902 from the National Institute on Minority Health and Health Disparities, Comprehensive Centers of Excellence, and from grant P30-DK092924 from the NIDDK. This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through UCSF-CTSI grant KL2 TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The CARDIA Study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the NHLBI and the Intramural Research Program of the National Institute on Aging. The manuscript was reviewed by CARDIA for scientific content prior to submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
REFERENCES
- 1.U.S. Renal Data System . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: 2012. [Google Scholar]
- 2.de Boer IH, Katz R, Fried LF, et al. Obesity and change in estimated GFR among older adults. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Dec;54(6):1043–1051. doi: 10.1053/j.ajkd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA : the journal of the American Medical Association. 2004 Feb 18;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 4.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005 Nov;46(5):871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention [Accessed March 14, 2013];Overweight and obesity. 2012 http://www.cdc.gov/obesity/childhood/index.html.
- 6.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA : the journal of the American Medical Association. 2010 Jan 20;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Annals of internal medicine. 2006 Aug 15;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 9.National Institues of Health NH, Lung, and Blood Institute [Accessed May 6, 2013];Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks. http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/bmi_dis.htm.
- 10.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985 Apr;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 11.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008 Nov 10;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. Journal of the American Society of Nephrology : JASN. 2009 Dec;20(12):2625–2630. doi: 10.1681/ASN.2009050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C as an endogenous marker of renal function in patients with mild to moderate impairment of kidney function. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006 Jul;21(7):1855–1862. doi: 10.1093/ndt/gfl073. [DOI] [PubMed] [Google Scholar]
- 15.White C, Akbari A, Hussain N, et al. Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. Journal of the American Society of Nephrology : JASN. 2005 Dec;16(12):3763–3770. doi: 10.1681/ASN.2005050512. [DOI] [PubMed] [Google Scholar]
- 16.Herget-Rosenthal S, Pietruck F, Volbracht L, Philipp T, Kribben A. Serum cystatin C--a superior marker of rapidly reduced glomerular filtration after uninephrectomy in kidney donors compared to creatinine. Clin Nephrol. 2005 Jul;64(1):41–46. doi: 10.5414/cnp64041. [DOI] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 19.Murtaugh MA, Jacobs DR, Jr., Yu X, Gross MD, Steffes M. Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2003 Oct 1;158(7):676–686. doi: 10.1093/aje/kwg208. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs DR, Jr., Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002 Jun 15;155(12):1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 21.Sidney S, Jacobs DR, Jr., Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991 Jun 15;133(12):1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 22.Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012 Nov 26;172(21):1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverwood RJ, Pierce M, Thomas C, et al. Association between younger age when first overweight and increased risk for CKD. Journal of the American Society of Nephrology : JASN. 2013 Apr;24(5):813–821. doi: 10.1681/ASN.2012070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration The facts about weight loss products and programs. [Accessed March 14, 2013];DHHS Publication No (FDA) 92-1189. 2006 http://web.archive.org/web/20060926035920/http://www.cfsan.fda.gov/~dms/wgtloss.html.
- 25.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clinical journal of the American Society of Nephrology : CJASN. 2009 Oct;4(10):1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2009 Apr;53(4):596–605. doi: 10.1053/j.ajkd.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erlinger TP, Tarver-Carr ME, Powe NR, et al. Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am J Kidney Dis. 2003 Aug;42(2):256–263. doi: 10.1016/s0272-6386(03)00650-4. [DOI] [PubMed] [Google Scholar]
- 28.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004 Dec;15(12):3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 29.Retnakaran R, Connelly PW, Harris SB, Zinman B, Hanley AJ. Cystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youth. Pediatr Nephrol. 2007 Jul;22(7):1007–1013. doi: 10.1007/s00467-007-0471-9. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher D, Visser M, Sepúlveda D, Pierson R, Harris T, Heymsfield S. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 31.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P. Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Jun;53(6):993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuck O, Teplan V, Stollova M, Skibova J. Estimation of glomerular filtration rate in obese patients with chronic renal impairment based on serum cystatin C levels. Clin Nephrol. 2004 Aug;62(2):92–96. doi: 10.5414/cnp62092. [DOI] [PubMed] [Google Scholar]
- 33.National Kidney Foundation I [Accessed May 11, 2013];KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. 2002 http://www.kidney.org/professionals/kdoqi/guidelines_commentaries.cfm.
- 34.Hou CC, Shyu RS, Lee WJ, Ser KH, Lee YC, Chen SC. Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 2013 Mar-Apr;9(2):202–206. doi: 10.1016/j.soard.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Jose B, Ford S, Super P, Thomas GN, Dasgupta I, Taheri S. The Effect of Biliopancreatic Diversion Surgery on Renal Function-a Retrospective Study. Obes Surg. 2013 May;23(5):634–637. doi: 10.1007/s11695-012-0851-5. [DOI] [PubMed] [Google Scholar]
- 36.Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. Journal of the American Society of Nephrology : JASN. 2006 Dec;17(12 Suppl 3):S213–217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA. Low-carbohydrate diets and realities of weight loss. JAMA : the journal of the American Medical Association. 2003 Apr 9;289(14):1853–1855. doi: 10.1001/jama.289.14.1853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.