Abstract
Rationale
Research on adolescence and drug abuse increased substantially in the past decade. However, drug-addiction related behaviors following stressful experiences during adolescence are less studied. We focus on rodent models of adolescent stress cross-sensitization to drugs of abuse.
Objectives
Review the ontogeny of behavior, dopamine, corticotropin-releasing factor (CRF), and the hypothalamic pituitary adrenal (HPA) axis in adolescent rodents. We evaluate evidence that stressful experiences during adolescence engender hypersensitivity to drugs of abuse and offer potential neural mechanisms.
Results and Conclusions
Much evidence suggests that final maturation of behavior, dopamine systems, and HPA axis occurs during adolescence. Stress during adolescence increases amphetamine- and ethanol-stimulated locomotion, preference, and self-administration under many conditions. The influence of adolescent stress on subsequent cocaine- and nicotine-stimulated locomotion and preference is less clear. The type of adolescent stress, temporal interval between stress and testing, species, sex, and the drug tested are key methodological determinants for successful cross-sensitization procedures. The sensitization of the mesolimbic dopamine system is proposed to underlie stress cross-sensitization to drugs of abuse in both adolescents and adults through modulation by CRF. Reduced levels of mesocortical dopamine appear to be a unique consequence of social stress during adolescence. Adolescent stress may reduce the final maturation of cortical dopamine through D2 dopamine receptor regulation of dopamine synthesis or glucocorticoid-facilitated pruning of cortical dopamine fibers. Certain rodent models of adolescent adversity are useful for determining neural mechanisms underlying the cross-sensitization to drugs of abuse.
Keywords: Adolescence, Stress, Drug Abuse, Addiction, Amphetamine, Cocaine, Nicotine, Ethanol, Dopamine, Hypothalamic Pituitary Axis
Preamble
Adverse experiences during adolescence predict increased use of illicit substances (DeWit et al. 1999; Dube et al. 2006; Nelson et al. 1995; Somaini et al. 2011; Sullivan et al. 2006; Tharp-Taylor et al. 2009; Topper et al. 2011) and addiction (Dube et al. 2002; Dube et al. 2003; Hoffmann et al. 2000; Kaltiala-Heino et al. 2000). Substance use during adolescence is associated with addiction, depression, conduct disorders, and cognitive impairments (Clark et al. 1998; Viveros et al. 2012). Furthermore, beginning substance use during adolescence shortens the transition from the first drug exposure to addiction (Clark et al. 1998). Basic research models are urgently needed to investigate the neural mechanisms responsible for the connection between adolescent stress and the development of substance use disorder (Marco et al. 2011; Sullivan et al. 2006).
While research on drugs of abuse and adolescence in animal models has increased in the past decade (Figure 1 white bars), experiments investigating drug abuse, adolescence, and stress have received less attention (Figure 1 black bars). This review focuses on rodent models of adolescent stress and subsequent behavioral and neural cross-sensitization to drugs of abuse. Application of stressful procedures to the adolescent rodent can increase drug-stimulated locomotion, drug preference, and voluntary drug intake. However, the type of stress, interval between stress and testing, species, sex, and the drug tested are critical factors to consider. We hypothesize dopamine (DA) D2 receptors, extrahypothalamic corticotropin-releasing factor (CRF), and glucocorticoids as potential neural mechanism underlying sensitized mesolimbic DA and reduced cortical DA.
Figure 1.
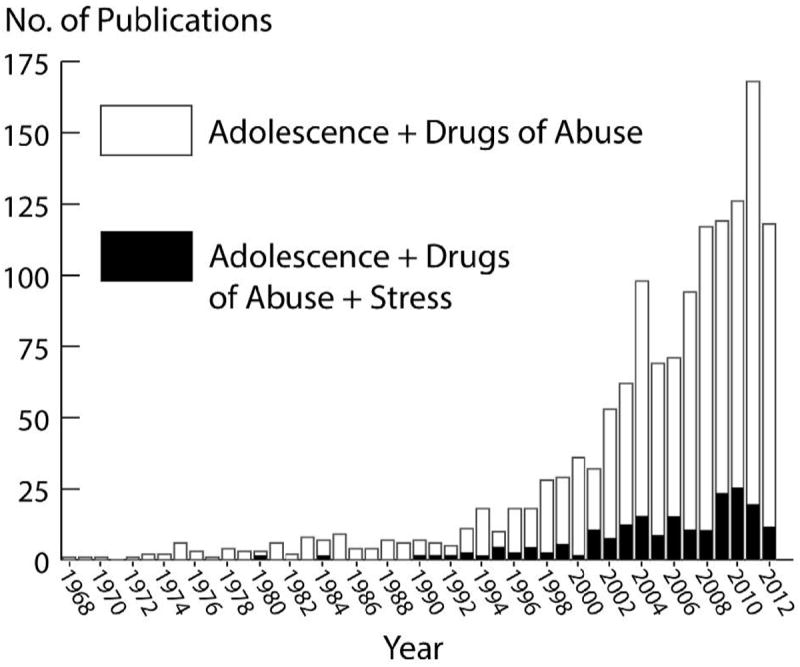
Number of publications since 1968 on the topic “adolescence and drugs of abuse” (white bars) and “adolescence and drugs of abuse and stress” (black bars). Scopus.com search1 suggest an 8-fold increase in articles published from 1997 to 2011 on adolescence and the most abused drugs. What has received relatively less attention is the effect of stress on adolescent drug abuse. In fact, adding “stress” to these search criteria reduced the search results by 7-fold (black bars).
Footnote: 1Searches conducted at www.scopus.com (Elsevier B.V.) and data were downloaded on June 3rd, 2013. Search terms entered into the field for “Adolescent + Drugs of Abuse” were, “TITLE-ABS-KEY(((rat OR mouse OR monkey OR hamster OR animal) AND (juvenile OR adolescence) AND (cocaine OR heroin OR morphine OR amphetamine OR methamphetamine OR marijuana OR thc OR alcohol OR mdma OR nicotine)))”. Search terms for “Adolescence + Drugs of Abuse + Stress” were, “TITLE-ABS-KEY(((rat OR mouse OR monkey OR hamster OR animal) AND (juvenile OR adolescence) AND (cocaine OR heroin OR morphine OR amphetamine OR methamphetamine OR marijuana OR thc OR alcohol OR mdma OR nicotine) AND stress))”.
1. Adult stress cross-sensitization to drugs of abuse
Reports from emergency rooms, the criminal justice system, and epidemiological data all link drug abuse with violent crimes, stress, and post-traumatic stress disorder (Substance Abuse and Mental Health Services Administration 2010; Sinha 2009; Sinha et al. 2006). Basic research seeks to model the link between stress and drug abuse. A “stressor” must disrupt homeostasis, initiate anticipatory behavioral and physiological responses after repeated exposure and exceed the adaptive capacity and regulatory range of the individual allostasis (reviewed in Koolhaas et al. 2011; Miczek et al. 2008; Ulrich-Lai and Herman 2009). When prior stress increases subsequent behavioral or neural responsiveness to a drug, drug preference, or drug self-administration it is termed “cross-sensitization”. Many stress models have been shown to cross-sensitize to drugs of abuse. For instance, tail pressure (Antelman et al. 1980), brief pulses of foot-shock (Herman et al. 1984; Robinson et al. 1985), particularly when uncontrolled (MacLennan and Maier 1983), and context specific immobilization stress (Robinson et al. 1985) can increase drug-induced locomotion and stereotypy. Stressful procedures increase many drug abuse-related behaviors such as behavioral sensitization (e.g., Covington and Miczek 2001; Kalivas and Duffy 1989; Sorg and Kalivas 1991), conditioned place preference (CPP) (e.g., McLaughlin et al. 2006; Rozeske et al. 2009), intravenous self-administration (e.g., Beck and O’Brien 1980; Goeders and Guerin 1996; Haney et al. 1995; Tidey and Miczek 1997), and reinstatement of drug seeking (Erb et al. 1996; Shaham and Stewart 1996). It is quite clear that many rodent models of adverse adult experiences can cross-sensitize with the behavioral effects of abused drugs, particularly psychostimulants.
There is a wide range of intensity between types of stressful procedures applied to rodents, implying that not all stressors are equal. Animals habituate to some stressors, such as restraint (Kirschbaum et al. 1995), but not to others such as social stress (Covington and Miczek 2005). Controllability and predictability are key factors to consider when developing models of stress in animals (Koolhaas et al. 2011). These factors also apply stress cross-sensitization to abused drugs in animal models. For instance, chronic unpredictable stress increases cocaine-induced locomotion and CPP, while chronic predictable stress does not (Haile et al. 2001). Social defeat, like unpredictable stress, cross-sensitized to drugs of abuse (Miczek et al. 2008). Repeated social defeat of adult rats shortens the latency to acquire (Haney et al. 1995), increases the rate of self-administration under limited and extended access conditions (Covington et al. 2008), and abolishes the circadian pattern of cocaine self-administration (Miczek et al. 2004). Threat of social defeat also increases the reinstatement of drug seeking behavior (Funk et al. 2005; Ribeiro Do Couto et al. 2006). Thus, social defeat appears to be a stressor of significance for adult stress cross-sensitization to cocaine.
Sensitization of mesocorticolimbic DA system has been implicated in stress cross-sensitization to drugs for more than three decades (Sorg and Kalivas 1991) and is still a major focus (e.g., Garcia-Keller et al. 2013). Both restraint and threat of social defeat are known to increase DA release (Imperato et al. 1989; Tidey and Miczek 1996). These stressors also increase subsequent amphetamine (Amph) or cocaine-stimulated extracellular DA in the nucleus accumbens (NAc) (Miczek et al. 2011; Pacchioni et al. 2007; Tidey and Miczek 1996). While greater NAc DA sensitivity after stress is important, other neural mechanisms such as brain-derived neurotropic factor (BDNF), corticotropin-releasing factor type 1 receptor (CRF-R1) (Boyson et al. 2011; Miczek et al. 2011), and NAc actin cytoskeleton protein (Esparza et al. 2012) are also implicated in this temporal connection between stress and abused drugs. Overall, the neural system common to adult stress-induced sensitization to drugs of abuse is the mesocorticolimbic DA system. This system develops during adolescence and exposure to stress during adolescence may alter the final maturation of brain DA (Adriani and Laviola 2004; Andersen 2003; Andersen and Teicher 2009; Crews et al. 2007; Hall 1998; O’Donnell 2010; Rodrigues et al. 2011; Spear 2000).
2. Adolescence and relevant maturation
2.1. Adolescence and puberty
Most (~93%) neurodevelopmental preclinical studies use rat and mouse models (Clancy et al. 2007). Adolescent rodents exhibit some similar behavioral characteristics to human adolescents (Adriani and Laviola 2004; Andersen 2003; Laviola et al. 2003; Lukkes et al. 2009c; Spear 2000; Spear and Brake 1983). The specific postnatal days (P) when a rodent is considered an adolescent are quite variable between studies (e.g. Yetnikoff et al. 2013). The period 7-10 days prior to puberty and a few days after puberty are referred to as periadolescence (Adriani and Laviola 2000; Spear and Brake 1983). The term juvenile is used to refer to rats between P21 and P28 (Brenhouse et al. 2008). In the broadest sense, P21 through P59 are considered “adolescence” in rodents (Laviola et al. 2003; Tirelli et al. 2003), while some suggest immaturity persists even longer (Adams and Boice 1983; Brenhouse and Andersen 2011). Here, rodent adolescence is separated into the following stages as shown in Figure 2: early adolescence (P21-34), mid-adolescence (P34-46), and late adolescence (P46-59). These stages are in line with other reports (Laviola et al. 2003; Lukkes et al. 2009c; Tirelli et al. 2003) to correspond to early (10-14 years), middle (15-17 years), and late (18-21 years) human adolescence (e.g. Braet et al. 2013; Wills et al. 1996). Even though these particular ages are approximations and individuals mature at different rates, the precise age of a rodent is most often the basis for experimental design and methodology in rodents.
Figure 2.
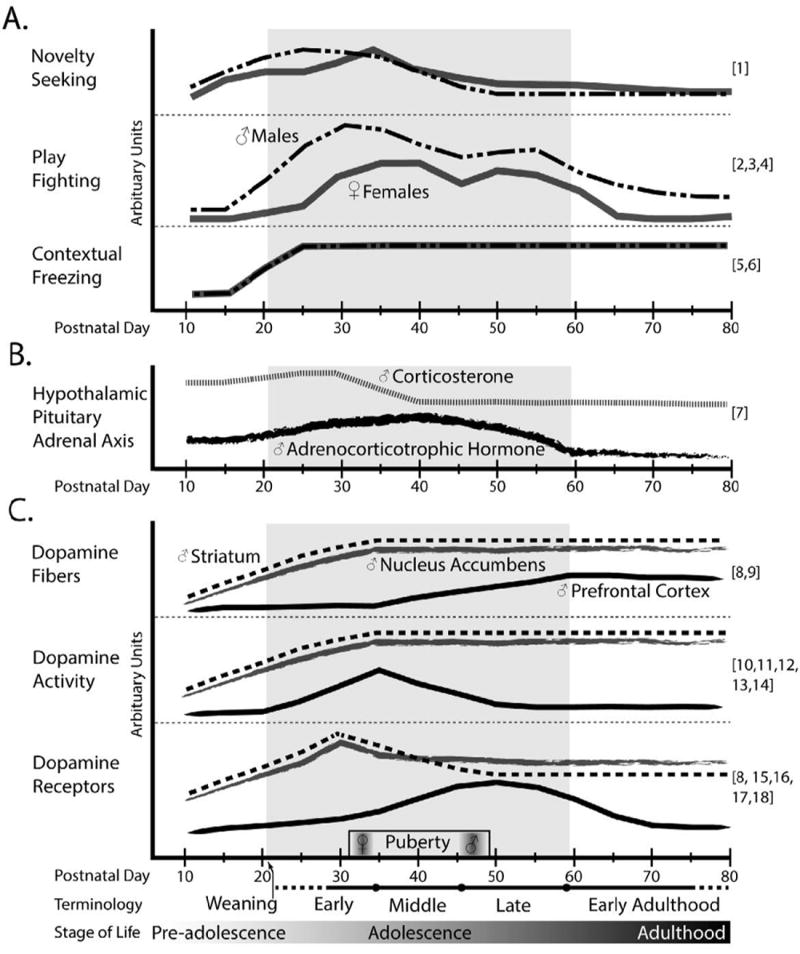
Schematic of adolescence and adolescent maturation of behavior (A), hypothalamic pituitary axis (B), and mesocorticoaccumbal dopamine system (C). The lines estimate the relative changes during adolescent development based on the references cited. To improve the accuracy of the ontogenic estimates, only studies that analyzed the dependent variable at more than two time points during adolescence were included in the creation of the lines (see text for details and further citations). (A) Estimated adolescent ontogeny of novelty seeking, play fighting, and contextual freezing behaviors for males (intermittent dashed black line) and females (solid gray line). (B) Estimated ontogeny of the hormones corticosterone (dashed gray line) and adrenocorticotrophic hormone (rough black line) in response to restraint stress during adolescence. (C) Estimated ontogeny of dopamine fibers, dopamine activity, and dopamine receptors for the striatum (dashed black line), nucleus accumbens (rough gray line) and prefrontal cortex (solid black line). References: [1] Vetter-O’Hagen and Spear 2012b; [2] Bolles and Woods 1964; [3] Meaney and Stewart 1981; [4] Pellis and Pellis 1990; [5] Schiffino et al. 2011; [6] Akers et al. 2012; [7] Foilb et al. 2011; [8] Naniex et al. 2012; [9] Tarazi et al. 1998a; [10] Andersen et al. 1997a; [11] Teicher et al. 1993; [12] Andersen and Gazzara 1993; [13] Leslie et al. 1991; [14] Badanich et al. 2006; [15] Andersen et al. 2000; [16] Tarazi and Baldessarini 2000; [17] Tarazi et al. 1998b; [18] Tarazi et al. 1999.
While there is no physical marker for the temporal boundaries of adolescence, the onset of puberty can be measured. Puberty is triggered by the hypothalamic gonadal axis during adolescence and influences physiological, behavioral, and central nervous system maturation (Sisk and Foster 2004). Puberty in mice and hamsters appears at an age similar to rats (Safranski et al. 1993; Vomachka and Greenwald 1979), which is often considered adolescence (e.g., Lopez et al. 2011; Wommack et al. 2004). Measurement of preputial separation and vaginal opening in 678 Sprague Dawley male and female rats, respectively, suggests that males reach puberty between P45-48 and female puberty is around P32-34 (Lewis et al. 2002). However, the range of pubertal onset among individuals varies widely, occurring from P40-76 in males and P28-41 in females (Lewis et al. 2002). In one report, male rat body weight at the typical onset of puberty (P45-48) ranged from 154 to 242 grams, while rats weighing between 250-274 grams ranged in age from P49 to P70 (McCutcheon and Marinelli 2009). This suggests that weight is a poor indicator of age. Furthermore, housing males in close proximity to females can accelerate the onset of female puberty (Khan et al. 2008; Vandenbergh 1967; Vandenbergh et al. 1976). Overall, there are many social and environmental determinants of the highly variable onset of puberty. Although sexual maturation and reproductive ability rapidly, much brain and behavioral maturation encompasses a more extensive developmental trajectory triggered by separate neural mechanisms (Sisk and Foster 2004).
2.2. Behavior
The adolescent rodent exhibits peaks in behaviors reminiscent of human adolescents (Adriani et al. 2004; Brenhouse and Andersen 2011; Brenhouse et al. 2008; Laviola et al. 2003; Marco et al. 2011; Spear 2000; Spear and Brake 1983). Novelty directed behavior is greater in adolescence compared to adults (Adriani et al. 1998; Douglas et al. 2003; Laviola et al. 2003). The peak in novelty seeking around P32-36 (Vetter-O’Hagen and Spear 2012b) is greater in males than in females (Figure 2A top). Interestingly, Vetter-O’Hagen and Spear (2012a; b) find that gonadal hormones are not involved in the adolescent peak in novelty-directed behavior. Goal-directed behavior matures during adolescence (Naneix et al. 2012; Naneix et al. 2013) and adolescents exhibit greater preference for social stimuli than adults (Douglas et al. 2004; Yates et al. 2013). Play fighting begins to rise as early as P18 (Bolles and Woods 1964) and peaks between P30 and P40 (Meaney and Stewart 1981; Panksepp 1981; Panksepp et al. 1984; Pellis and Pellis 1990) (Figure 2A middle). Play fighting within the same sex is greater in males whether housed in groups or individually (Meaney and Stewart 1981; Pellis and Pellis 1990) (Figure 2A middle). Adolescents exhibit greater novelty exploration (Stansfield and Kirstein 2006) and time spent on the unprotected arms of the elevated plus maze (Macrı et al. 2002), which are interpreted as risk-taking behaviors (McCourt et al. 1993). This cannot be explained by underdeveloped fear memory since freezing in response to a context previously paired with foot-shock appears as early as P14 in mice (Akers et al. 2012), and around P18-23 in rats (Brasser and Spear 2004; Raineki et al. 2010; Rudy and Morledge 1994; Schiffino et al. 2011) (Figure 2A lower). Increased adolescent behaviors (e.g., novelty seeking, play fighting, and social preference) are thought to be evolutionarily adaptive by encouraging explorative behaviors, reducing the possibility of inbreeding, discovering new potentially essential resources, and developing social skills pertinent to being functional in adulthood (Casey et al. 2008; Feixa 2011; Spear 2000).
2.3. Hypothalamic Pituitary Adrenal Axis
The hypothalamic-pituitary-adrenal (HPA) axis is an important component of the stress response and is implicated in stress-induced escalations of drug seeking behaviors (Goeders 2002a; b; Steiner and Wotjak 2008). The paraventricular nucleus of the hypothalamus (PVN) releases CRF into the anterior pituitary portal system, which in turn causes the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary (Antoni 1986; Rivier et al. 1982). This hormone stimulates the production of glucocorticoids from the adrenal cortex (Antoni 1986; Herman et al. 2003), which regulates the release of further ACTH and CRF through a negative feedback mechanism (Keller-Wood and Dallman 1984). Early adolescent rats exhibit prolonged and elevated levels of the glucocorticoid, corticosterone, in response to restraint compared to adults (Gomez et al. 2002). This exaggerated HPA response peaks in early adolescence and restraint-induced corticosterone is the same as adult levels by P40, while stimulated adrenocortocotropic hormone levels mature by P60 (Foilb et al. 2011) (Figure 2B). Early adolescents exposed to repeated restraint secrete more corticosterone than adults exposed to the same procedure (Romeo et al. 2006). This adolescent maturation of the HPA axis is not regulated by pubertal increases testosterone in adolescent male rats (Romeo et al. 2004). Adolescent females exhibit prolonged elevation similar to males in response to restraint stress, but release more corticosterone than males at the apex of the response (Romeo 2010). Adolescent males have greater CRF mRNA expression in the PVN, while the opposite was reported in adolescent females (Romeo et al. 2007; Viau et al. 2005). These studies are in agreement with other reports that the HPA axis is hyper-responsive during adolescence (reviewed in Klein and Romeo 2013; McCormick 2010). This effect is attributed to underdeveloped negative feedback (Sapolsky et al. 1985; Vazquez and Akil 1993), which is a characteristic that may increase vulnerability to stress cross-sensitization to drugs of abuse (Deroche-Gamonet et al. 2004).
2.4. Brain Dopamine and CRF
During postnatal development, components of neurocircuitry are overproduced in early life followed by substantial elimination, or pruning, of this circuitry during adolescence. White matter in the human brain increases linearly throughout development into adulthood (Gogtay et al. 2004). The overall change in gray matter during adolescence follows an inverted U-shaped function, rising during adolescence and declining to adult levels at the end of adolescence (Giedd 2004; Giedd et al. 1999), which is supported by synaptic density measurements in humans (Glantz et al. 2007) and rats (Andersen and Teicher 2004). The peak of neural activity in subcortical regions during adolescence may underlie the increased risk taking and novelty seeking behaviors, which are normally inhibited by cortical control regions that undergo a much slower adolescent maturation than subcortical regions (Casey et al. 2008). Although many rodent neural systems mature during adolescence (Brenhouse and Andersen 2011; McCutcheon and Marinelli 2009; Sisk and Foster 2004; Spear 2000), the maturation of DA neurons in the VTA that project to the medial prefrontal cortex (mPFC) and NAc is perhaps the most critical neural system relevant to processing salient events including responses to psychostimulants (reviewed in Everitt and Wolf 2002; Schultz 2002; Wise 1996). Thus, the adolescent reorganization of the DA system is very instructive (Laviola et al. 2003; Spear 2000).
2.4.1. Dopamine Fibers
Much evidence from rodent studies demonstrates the adolescent maturation of the mesocorticolimbic DA system. Measuring DA transporters as a marker for innervation density (Ciliax et al. 1995) suggests that these molecules increase rapidly until P21 then gradually increase until P35 in the striatum and NAc (Tarazi et al. 1998a), while tyrosine hydroxylase labeled fibers reach a stable level between P25 and P70 (Naneix et al. 2012). In contrast, dopaminergic innervation of the mPFC continues to increase progressively throughout adolescence until about P60 (Benes et al. 2000; Kalsbeek et al. 1988; Naneix et al. 2012). The evidence suggests that DA fibers in the striatum and NAc mature early while DA projections to mPFC finish final maturation in late adolescence (Figure 2C top).
2.4.2. Dopamine Activity
There is increased mPFC DA synthesis and turnover in mid-adolescent rats (P30) compared to adults (Andersen et al. 1997a; Teicher et al. 1993), suggesting a peak in early adolescence. In the NAc and striatum, DA levels appear to increase to adult levels between P28 and P42 (Andersen and Gazzara 1993; Leslie et al. 1991), possibly between P35 and P45 (Badanich et al. 2006). The rate-limiting enzyme in DA synthesis, tyrosine hydroxylase, is higher in adulthood than in adolescence in the NAc core and cingulate cortex, but not the striatum (Mathews et al. 2009). However, others find that basal rates of DA transients in the NAc core are the same during early adolescence and adulthood (Robinson et al. 2011). Generally, DA activity rises during early to mid-adolescence with a clear peak in the mPFC that declines by adulthood (Figure 2C middle).
2.4.3. Dopamine Receptors
Both D1-like and D2-like dopamine (D1; D2) receptors are coupled to Gs and Gi proteins, respectively, and found in the mPFC, NAc, and striatum (Centonze et al. 2002; Fishburn et al. 1995). In the rat mPFC, levels of D1 and D2 DA receptors peak around P40 to P60 (Andersen et al. 2000; Naneix et al. 2012; Tarazi and Baldessarini 2000) and then decline as the rat ages (Andersen et al. 2000; Naneix et al. 2012). In the NAc and striatum, the density of D1 and D2 receptors peak at P28 and then decline in adulthood (Tarazi et al. 1998b; 1999), with an adolescent peak of DA receptor mRNA in the striatum (Naneix et al. 2012). The mid-adolescent peak in D1 receptors occurs in mPFC neurons innervating the NAc (Brenhouse et al. 2008). The ability of D2 autoreceptors to modulate DA synthesis declines with maturation, and this effect is most pronounced in the mPFC where D2 receptor regulation of DA synthesis is almost completely lost by adulthood (Andersen et al. 1997a). In sum, these results indicate an early adolescent peak in striatum and NAc DA receptors, with a late adolescent peak in DA receptors in the mPFC (Figure 2C bottom). The overproduction of corticolimbic DA receptors during adolescence continues unhindered by gonadectomy (Andersen et al. 2002). The peak in DA receptors is much less dramatic in females than males (Andersen et al. 1997b). Typically, only males are investigated when characterizing the ontogeny of the adolescent DA system. This could be due to the complexities in assessing the hormonal state of females.
Further evidence implicates stress in early NAc DA maturation and delayed mPFC DA maturation. Lyss et al. (1999) found that neuronal activation occurred mostly in the NAc when stress was applied in early adolescence, but in the mPFC when stress was applied in late adolescence. Pruning and fine-tuning of DA systems in late adolescent is thought essential for proper development into adulthood (Andersen 2003; Spear 2000; Weinberger 1987), since alterations to the pruning of DA systems may underlie the appearance of psychiatric disorders in adulthood (Lipska and Weinberger 2002; Thompson et al. 2004) and even increase susceptibility to drug addiction (Wise 1996).
2.4.4. Corticotropin-releasing factor
In addition to activating CRF in the PVN, stress also increases CRF in the VTA, which stimulates VTA DA neurons and local DA release under certain conditions (Wanat et al. 2008; Wang et al. 2005). CRF is a neuropeptide that signals through the CRF-R1 and the type 2 receptor (CRF-R2) in the VTA, NAc and mPFC, among other regions (Lemos et al. 2012; Rodaros et al. 2007; Swanson et al. 1983; Ungless et al. 2003; Van Pett et al. 2000). Genetic deletions implicate CRF-R1 signaling in anxiety-like behavior and CRF-R2 signaling as anxiolytic (Bale et al. 2002). In much of the brain, CRF receptor levels peak prior to adolescence (Avishai-Eliner et al. 1996; Insel et al. 1988). However, levels of CRF mRNA in the central nucleus of the amygdala are greater in adulthood than early-adolescence (Viau et al. 2005). Binding to CRF-R2 in the amygdala at P30 is lower than in P98 male rats (Weathington and Cooke 2012). In contrast, CRF-R2 mRNA levels are greater in adolescence compared to adulthood in the basolateral amygdala (BLA) (Eghbal-Ahmadi et al. 1998). Thus, some evidence supports adolescent maturation of the CRF system in the amygdala.
3. Models of adolescent stress and subsequent exposure to drugs of abuse
Many of the same procedures applied to adult rodents to model stress are also used for studying the outcomes of adolescent stress (i.e. electric shock, restraint, social defeat, chronic variable stress). Some stressors have unique consequences when applied during adolescence (i.e. individual housing). The type of test used to assess behavioral sensitivity to drugs of abuse is critical and the direction of the effect does not always agree between methods. For instance, high levels of cocaine-stimulated locomotion do not always predict high levels of cocaine self-administration (Thomsen and Caine 2011). The following studies are organized by the method used to assess behavioral sensitivity to abused drugs after some form of adolescent stress.
3.1. Acute drug-stimulated locomotion
Repeated episodes of restraint stress in adulthood can increase motor activity in response to an acute drug challenge (e.g., Cruz et al. 2012; Doremus-Fitzwater et al. 2010), an effect attributed to activity of NAc DA (Johnson et al. 1996; Pijnenburg and van Rossum 1973). Less research has investigated adolescent stress cross-sensitization to acute drug-stimulated locomotion.
However, this is the method most heavily studied following adolescence stress.
3.1.1. Amphetamine and Cocaine
During adolescence, repeated restraint increases the acute motor response to psychostimulants in male and female rats when tested approximately two days after stress termination (Cruz et al. 2012; Doremus-Fitzwater et al. 2010; Lepsch et al. 2005) (Table 1). Some stressful procedures, however, fail to cross sensitize to acute drug challenge. For example, adult and mid-adolescent “social instability” fails to alter acute Amph-stimulated locomotion when data are collapsed across sexes (Mathews et al. 2008) (Table 1). Female rats exhibit greater locomotion in response to higher, but not lower doses, of acute Amph after early adolescent restraint stress (Doremus-Fitzwater et al. 2010) (Table 1). This could be explained by a graded effect of stress duration, whereby 4 days of 1 hour restraint was not sufficient to cause increased acute Amph-heightened locomotion at the low dose in female rats. In addition, there is no cross-sensitization when the acute response to Amph was tested more than two days after social instability or restraint (Doremus-Fitzwater et al. 2010; Mathews et al. 2008). Thus, restraint stress, but not social instability, during early adolescence or adulthood increases acute Amph-stimulated locomotion when tested soon after stress termination.
Table 1.
Abused drug-stimulated locomotion following stress during adolescence
| Species Strain | Sex | Age and Stress | Age and Drug Challenge | Outcome | Reference |
|---|---|---|---|---|---|
|
Amphetamine
| |||||
| Rats S.D. | M | P28-56 variable social or other environmental stress for 2 h 1x/d for 28 d | P76 Amph (1 mg/kg, ip) every 3 d for 12 d with Amph (0.5 mg/kg, ip) challenge 7 d later | ↓ locomotion at challenge dose after social stress only | Kabbaj et al. 2002 |
| Rats L.E. | M F | P35-45 social isolation 1 hr 1x/d & rehoused with new cagemate | P47 or P70 acute Amph (1.0 mg/kg, ip) and challenge 9 days later P60 or P83 | ø in acute Amph, ↑ locomotion in males and females as 1 group in response to challenge at P63 and P83 | Mathews et al. 2008 |
| Rats S.D. | M | P35-39 social defeat, 1x/d for 5 d | P58 acute Amph (2.5 mg/kg, ip) | ø acute Amph-stimulated locomotion, ø in stereotypy | Burke et al. 2010 |
| Rats S.D. | F | P23-26 or P65-68 restraint in plastic tube for 1 h/d for 4 d | P28 and P49 or P71 and P92 acute Amph (1.5 & 3.0 mg/kg) | ↑ acute Amph (3.0)-stimulated locomotion 2 d after adolescent or adult restraint only; ø in Amph-induced stereotypy | Doremus-Fitzwater et al. 2010 |
| Mice C57BL/6 | M | P25-29 chronic variable stress, 1 stress/d for 5 d | P45-54 or P87-138 acute Amph (2.5 mg/kg) | ↑ acute Amph-stimulated locomotion when measured in adulthood, but not in adolescence | Peleg-Raibstein & Feldon 2011 |
| Rats Wistar | M | P28-34 or P60-66 restraint 2 h/d for 7 d | P37 or P69 acute Amph (1 mg/kg ip.) | ↑ acute Amph-stimulated locomotion in adolescents and adults | Cruz et al. 2012 |
| Rats S.D. | M | P35-39 social defeat, 1x/d for 5 d | P56 acute or P62 repeated daily for 6 d Amph (1 mg/kg, ip) | ↑ acute Amph-stimulated locomotion, ø in Amph-stimulated locomotion after repeated Amph | Burke et al. 2013 |
|
Cocaine
| |||||
| Hamster Syrian | M | Between P32 and P37 single episode of social defeat | ≈P58 cocaine (20 mg/kg, ip) for 7 d and challenge 12 days later (5 mg/kg, ip) | ↓ acute cocaine-stimulated locomotion after social defeat, no effect of stress on expression | Trzcinska et al. 2002 |
| Rats Wistar | M | P25-35 Restraint 2 hr 2x/d or chronic variable stress 2x/d, both for 10 d | P37 cocaine (10 mg/kg, ip) | ↑ acute cocaine-stimulated locomotion after both restraint and variable stress | Lepsch et al. 2005 |
|
Nicotine
| |||||
| Rats L.E. | M F | P33-48 social isolation 1 hr 1x/d & rehoused with new cagemate | P80 nicotine (0.5 mg/kg, sc) 1x/d for 5 days plus challenge 3 d later | ↑ acute nicotine-stimulated locomotion and ↑ nicotine-stimulated locomotion on 5th & 6th injection in females only | McCormick et al. 2004 |
| Rats L.E. | M F | P33-48 or P65-80 social isolation 1 hr 1x/d & rehoused with new cagemate | P69 or P101 nicotine (0.25 mg/kg, sc) 1x/d for 5 days plus Amph (0.5 mg/kg, sc) challenge 1 d later | ø nicotine locomotion ↑Amph-stimulated locomotion upon challenge in females following adolescent stress only | McCormick et al. 2005 |
| Rats L.E. | M F | P30-45 Social isolation 1 hr 1x/d & rehoused with new cagemate or the original cagemate | P46-58 Nicotine (0.5 mg/kg, sc) 1x every 2 d for 14 d | ↓ nicotine-stimulated locomotion in females after isolation & new cagemate | McCormick & Ibrahim, 2007 |
| Rats Wistar | M | P28-34 or P60-66 restraint 2 h/d for 7 d | P37 or P69 Acute nicotine (0.4 mg/kg sc)- | ø acute nicotine locomotion after adolescent stress or adult stress | Cruz et al. 2008 |
| Rats Wistar | M | P28-34 or P60-66 saline or nicotine (0.4 mg/kg, sc) injection then restraint for 2 h/d for 7 d | P37 or P69 nicotine-(0.4 mg/kg, sc) | ø acute nicotine locomotion after adolescent stress or adult stress, ↑ nicotine sensitization after nicotine then stress in adolescents only | Zago et al. 2012 |
|
Ethanol
| |||||
| Rats S.D. | M | P28 or P70 restraint 90 mins 1x. Socially isolated in standard cage for 90 mins or 180 mins | P28 or P70 Ethanol (3.5 g/kg ig) | ↑ ethanol-stimulated locomotion in adults only and only immediately after social isolation | Acevedo et al. 2013 |
Abbreviations: increased (↑), decreased (↓), no change in (ø), postnatal day (P), amphetamine (Amph), occurrence (x), hour (h), day (d), intragastric (ig), intraperitoneal (ip), subcutaneous (sc), Sprague-Dawley (S.D.), Long Evans (L.E.)
Social defeat is a stressor that elicits a sensitizing and non-habituating corticosterone response in adolescent (Watt et al. 2009) and adult rats (Covington and Miczek 2005), respectively. Rats defeated in adolescence exhibit increased locomotion in response to Amph (1.0 mg/kg, ip.) in adulthood (Burke et al. 2013) (Table 1). However, at higher doses of psychostimulants adolescent social defeat decreases drug-stimulated locomotion compared to controls (Burke et al. 2010; Trzcinska et al. 2002) (Table 1). The increase in Amph-heightened locomotion after adolescent social defeat (Burke et al. 2013) (Table 1) may only be apparent at the low dose of psychostimulants because DA release and reuptake have not yet exceeded an upper limit (Cain et al. 2005; Cain et al. 2004; Klebaur and Bardo 1999; Klebaur et al. 2001). High doses of Amph are hypothesized to cause a maximal behavioral response by activating all mesocorticolimbic DA regions and abolishing individual differences (Beckmann et al. 2011; Freed and Yamamoto 1985; Sharp et al. 1987). Given the regional differences in adolescent development of DA systems, adolescent stress may have an impact on region-specific sensitivity to psychostimulants. Future studies should investigate multiple doses of psychomotor stimulants to help differentiate these possible mechanisms.
Chronic variable stress (CVS) or chronic mild stress (Willner 1997) is a procedure that presents multiple stressors in an irregular order to avoid habituation to repeated stress. Lepsch et al. (2005) (Table 1) compared the effects of CVS and repeated restraint administration for ten days during adolescence. They found that while both stressors increase acute cocaine-stimulated locomotion when tested later in adolescence, the CVS groups exhibit a greater degree of cross-sensitization to cocaine compared to those exposed to restraint. Peleg-Raibstein and Feldon (2011) (Table 1) exposed C57BL/6J mice to a different stressor each day for five days during adolescence. When tested in adulthood, but not adolescence, the acute behavioral response to 2.5 mg/kg Amph increases. The lack of cross-sensitization when tested in adolescence may contrast with Lepsch et al (2005) because the CVS lasted longer and persisted overnight for seven out of ten days, whereas Peleg-Raibstein and Feldon (2011) administered a brief stressor once per day for five days. The ability of adolescent stress to elicit an effect at this dose of Amph might contrast with studies in rats (Burke et al. 2010) because C57BL/6J mice show greater psychostimulant-heightened motor activation than rats (Thomsen and Caine 2011). Overall, cross-sensitization due to adolescent stress persists into adulthood after social defeat or CVS (Burke et al. 2013; Peleg-Raibstein and Feldon 2011), but not after restraint (Doremus-Fitzwater et al. 2010). These data suggest that non-habituating stressors cause long lasting crosssensitization to the motor-stimulating effects of psychostimulants in adolescence, whereas stressors characterized by habituation do not.
3.1.2. Nicotine
Fewer studies have tested the effects of adolescent stress on the response to acute nicotine challenge. Restraint stress in male rats does not alter acute nicotine-stimulated locomotion tested three days later regardless of whether the stress occurred in adolescence or adulthood (Cruz et al. 2008; Zago et al. 2012) (Table 1). In contrast, female rats exposed to social instability from P33-48 exhibit increased acute nicotine-stimulated locomotion compared to males and non-stressed controls when measured at P80 (McCormick et al. 2004) (Table 1). So far, the limited data suggest that adolescent stress increases the acute motor response to nicotine in adult females, but not males.
3.1.3. Ethanol
The motor response to acute ethanol was examined in adolescent and adult Sprague Dawley rats immediately after a single exposure to restraint or single housing (Acevedo et al. 2013) (Table 1). Adult single housing, but not restraint, increases the motor response to intragastric ethanol, but if this type of social stress occurred in adolescence there is no change in ethanol-stimulated motor activity. In adult rats, behavioral and neural sensitization are found after 21 drug free days, but not after three or seven drug free days (Paulson and Robinson 1995). A stress-free time segment may be required for adolescent stress to permanently alter the brain in a manner that cross-sensitizes to acute drug-stimulated locomotion.
3.2. Drug-stimulated locomotion after repeated exposure
Behavioral sensitization typically refers to an augmented motor response after repeated exposures to the drug (Robinson and Becker 1986; Segal 1975). Stressful experiences in adulthood often increase behavioral sensitization to drugs of abuse (e.g., Covington and Miczek 2001; Haile et al. 2001; Kalivas and Duffy 1989; Sorg and Kalivas 1991). Less is known about how adolescent stress affects behavioral sensitization.
3.2.1. Amphetamine
In an initial study, Kabbaj et al. (2002) dissociated the effects of social (isolation, defeat, rehousing, crowding, novelty, litter shifting) and other environmental stress (cold, forced swim, loud noise, ether, restraint) throughout adolescence on behavioral sensitization to Amph (Table 1). Handled controls and non-socially stressed rats exhibit a sensitized locomotor response to Amph, whereas the socially stressed rats did not (Kabbaj et al. 2002). Similarly, mid-adolescent social defeat does not increase psychostimulant-heightened locomotion after repeated dosing whether tested immediately or after a drug free period (Burke et al. 2013; Trzcinska et al. 2002) (Table 1). In contrast, social instability in adolescence increases Amph-stimulated motor activity relative to the acute Amph response, whereas non-stressed controls exhibit no such behavioral sensitization (Mathews et al. 2008) (Table 1). Mathews et al (2008) also report that regardless of whether social instability occurs during adolescence or adulthood, it enhances behavioral sensitization to Amph. Therefore, a few studies suggest that adolescent social stress does not increase behavioral sensitization to Amph or cocaine (Burke et al. 2013; Kabbaj et al. 2002; Trzcinska et al. 2002), while others suggest an increase comparable to that seen after adult social stress (Mathews et al. 2008).
3.2.2. Nicotine
Social instability stress applied to mid-adolescent females increased the motor response to nicotine in adulthood on the fifth day of repeated injections and after three drug free days compared to males and non-stressed controls (McCormick et al. 2004) (Table 1). This was not observed at a lower dose of nicotine on P69 (McCormick et al. 2005) (Table 1). Following repeated nicotine injections in adulthood, females stress during adolescence were injected with Amph and exhibited increased motor activity (McCormick et al. 2005). These results agree with the effects of social instability on adult acute Amph locomotion (Mathews et al. 2008), but are at variance with other effects of social stress on adult behavioral sensitization (Burke et al. 2013; Kabbaj et al. 2002; Trzcinska et al. 2002). Female rats concomitantly injected with nicotine and exposed to social instability in adolescence causes decreased motor response to repeated nicotine when tested in adolescence (McCormick and Ibrahim 2007) (Table 1). Overall, adolescent social instability appears not to cross-sensitize to repeated nicotine when tested in adolescence. However, when the psychostimulants are administered in adulthood, adolescent social instability appears to cross-sensitize to nicotine only at higher doses and only in females.
3.3. Drug conditioned place preference
The CPP test measures preference for drug-paired cues/context to assess motivation to prefer a context paired with a drug experience (Bardo and Bevins 2000; Tzschentke 1998). While adult stress increases the preference for drug-paired cues (e.g., McLaughlin et al. 2006; Rozeske et al. 2009), drug CPP after adolescent stress is much less studied.
3.3.1. Preference for amphetamine and cocaine
Adult male rats exposed to social defeat in adolescence exhibit increased Amph CPP, but the experience of electric foot-shock at matched times fails to increase adult Amph CPP (Burke et al. 2011) (Table 2). This suggests differential effects of social versus non-social stress on preference for drug-paired cues, consistent with other data (Kabbaj et al. 2002). One two-hour restraint session, an arguably less intense stressor than repeated social defeat actually reduces CPP for Amph in adulthood (Richtand et al. 2012) (Table 2). Similarly, Thanos et al. (2010) (Table 2) find that forced wheel running also reduces cocaine CPP when tested one day later. Overall, these studies demonstrate that some stress (i.e. social defeat) sensitizes, while others can protect (i.e. single restraint) against psychostimulant CPP, even when the stressor is very brief. The persistence of these effects later in life is unclear based on these limited studies.
Table 2.
Preference for drugs of abuse following stress during adolescence
| Species Strain | Sex | Age and Stress | Age and Conditioning | Outcome | Reference |
|---|---|---|---|---|---|
|
Amphetamine
| |||||
| Rats L.E. | M F | P35-45 social isolation 1 h 1x/d & rehoused with new cagemate | P52 or P76 test, conditioned with Amph (0.5 or 1.0 mg/kg, ip) | ↑ trend (P=0.05) Amph CPP in P52 females at 1.0 mg/kg only, not males and not at P76 | Mathews et al. 2008 |
| Rats S.D. | M | Social defeat, 1x/d for 5 d 10 0.5 mA foot-shock 1x/d for 5 d | P70 test, conditioned with Amph (1.0 mg/kg, ip) | ↑ preference for Amph-paired cues after defeat, not foot-shock | Burke et al. 2011 |
| Rats S.D. | M | P35 one episode of restraint 1x 2 h | P98 test, conditioned with Amph (0.5 or 2.0 mg/kg, sc) | ↓ preference for 0.5 mg/kg Amph after stress, ø 2.0 mg/kg preference after stress | Richtand et al. 2012 |
|
Cocaine
| |||||
| Mice OF1 | M F | P21-35 or P60-73 crowded housing condition (8 mice per cage) for 15 days prior to and during cocaine training (social isolation - additional variable) | P36 or P74 test, conditioned with cocaine (50, 12.5, 3.125 mg/kg ip) | ø cocaine (25 mg/kg, ip) primed reinstatement after 50 mg/kg training after adolescent stress, but in controls and stressed adults cocaine (25, 12.5, and 6.25 mg/kg ip) primed reinstatement was found | Ribiero Do Couto et al. 2009 |
| Rats Lewis | M F | P42-93 forced wheel running, 60 mins/d 5 d/week | P93 test, conditioned with cocaine (25 mg/kg, ip) | ↓ preference for cocaine after forced exercise in M & F | Thanos et al. 2010 |
|
Nicotine
| |||||
| Rats S.D. | M | P28 intermittent 10 0.8 mA foot-shocks in one episode | P31 test, conditioned with nicotine (0.2, 0.4, 0.6 mg/kg, sc) | ↑ preference for 0.2 and 0.4 mg/kg nicotine after stress | Brielmaier et al. 2012 |
|
Ethanol
| |||||
| Mice Kunming | M | P28 or P28-34 versus P56 or P56-62 20 0.6 mA foot-shock for 1 (acute) or 7 (chronic) d | P29 or P35 versus P57 or P63 test, conditioned with ethanol (1.0 or 2.0 g/kg, ip) | ↑ preference for 2.0 g/kg ethanol after chronic, not acute, adolescent stress, ø ethanol CPP in stressed adults. | Song et al. 2007 |
| Rats S.D. | M | P29-33 restraint for 90 mins 1x/d for 5 d | P35 or P75 ethanol (1.0 mg/kg, ip) | ø to ethanol-elicited taste aversion due to stress | Anderson et al. 2012 |
| Rats S.D. | M | P30-34 or P70-74 restraint 90 mins 1x/d for 5 d Chronic variable stress 1-2 stressors/d for 5 d | P36 or P76 ethanol (0.25, 0.50, 0.75 g/kg, ip) | ↑ ethanol-induced social preference in restraint stressed adults only | Wiley & Spear 2013 |
Abbreviations: increased (↑), decreased (↓), no change in (ø), postnatal day (P), amphetamine (Amph), occurrence (x), hour (h), day (d), intraperitoneal (ip), subcutaneous (sc), Sprague-Dawley (S.D.), Long Evans (L.E.)
Only exhibit Amph CPP when tested in adolescence following social instability, but this stress-sensitizing effect disappears by early adulthood (Mathews et al. 2008) (Table 2). These results suggest that females are more sensitive to social instability in adolescence than males (McCormick and Ibrahim 2007; McCormick et al. 2004; McCormick et al. 2005), and this effect is short lasting. It appears that several determinants for increased CPP after stress in adolescence are important: the interval between stress and CPP, the sex, and ethanol versus Amph CPP.
3.3.2. Preference for nicotine and ethanol
In the earliest investigation of the effects of adolescent stress on drug CPP, Song et al. (2007) report that electric foot-shock applied repeatedly to adolescent mice promotes ethanol CPP at 2 g/kg when measured 24 hours after stress (Table 2). No ethanol preference is observed in non-shocked and acute electric shocked mice. Meanwhile, in adult mice neither acute nor chronic foot-shock elicits an ethanol CPP to 1 g/kg ethanol (Song et al. 2007). Likewise, foot-shock in adolescence just prior to nicotine conditioning caused CPP at lower doses, while non-stressed rats do not exhibit CPP for lower doses of nicotine (Brielmaier et al. 2012). Thus, foot-shock in adolescence can reduce the dose at which a preference to nicotine or ethanol is established, but only within a couple days of stress termination.
3.4. Drug Self-administration
Self-administration is the most useful technique for measuring voluntary intake of drugs (O’Brien et al. 1998; Piazza and Deroche-Gamonet 2013). Adult stress has been found to increase oral self-administration and intravenous self-administration of drugs of abuse (Funk et al. 2005; Goeders and Guerin 1996; Tidey and Miczek 1997). The effects of episodic adolescent stress on subsequent intravenous self-administration have not been investigated to date, with limited studies measuring oral intake.
3.4.1 Ethanol self-administration
A few studies have investigated ethanol and cocaine voluntary consumption and preference using the two bottle choice task (Table 3). Chester et al (2008) (Table 3) reports that adolescent foot-shock stress increases ethanol preference in both male and female adult High Alcohol Preferring (HAP1) mice. This preference for ethanol was not observed when the same foot-shock procedure was administered to adult mice. This suggests that adolescence is a particularly vulnerable period for foot-shock to have negative effects on ethanol preference in adulthood whether intake is voluntary (Chester et al. 2008) or forced (Song et al. 2007). Regardless of whether alcohol preference or total grams of alcohol per body weight ingested were analyzed, Chester et al. (2008) found that foot-shock in adolescence increases ethanol preference and intake in adulthood. In contrast, Brunell and Spear (2005) (Table 3) found that the heightened ethanol intake in non-stressed adolescents compared to adults was not reproduced when investigating preference for ethanol over water. The authors attribute this to the greater fluid consumption in adolescent rats relative to adults.
Table 3.
Self-administration of drugs of abuse following stress during adolescence
| Species Strain | Sex | Age and Stress | Age and Drug | Outcome | Reference |
|---|---|---|---|---|---|
|
Cocaine
| |||||
| Rats Wistar | M | P30-40 bright light, noise, and cold stress 10 mins 1x/d for 10 d | P90 cocaine (0.1 mg/ml) | ø cocaine intake and preference in two bottle choice task and absence of progressive increase in preference over time | Marquardt et al. 2004 |
|
Ethanol
| |||||
| Rats S.D. | M | P25-34 or P71-80 chronic (1x/d for 8 d) or acute (1x/d for 1 d) 60 1 s. 1.0 mA foot-shocks VI 30 schedule | P25-34 or P71-80 sweetened ethanol (10%; 0.1% saccharin) | ↓ ethanol intake during chronic adolescent stress as measured by two bottle choice task, ø ethanol intake after acute adolescent stress or adult stress | Brunell & Spear 2005 |
| Mice HAP1 | M F | P36-47 or P72-77 15 0.2 mA foot-shock 1x/d for 10 d | ≈P69-70 or ≈P106-111 ethanol (10% solution) | ↑ ethanol intake and preference in both sexes after adolescent foot-shock, but not adult foot-shock in two bottle choice task | Chester et al. 2008 |
| Mice C57BL/6 | M F | P35-50 chronic variable stress 2x/d for 14 d (also isolation rearing) | P63 ethanol (15% solution) | ↑ ethanol consumption in both sexes after chronic variable stress in two bottle choice task | Lopez et al. 2011 |
Abbreviations: increased (↑), decreased (↓), no change in (ø), postnatal day (P), occurrence (x), day (d), Sprague-Dawley (S.D.)
Brunell and Spear (2005) were the first to investigate alcohol intake during stress in adolescent pair-housed rats that were fitted with a transmitter to determine the contact with the bottle by each individual. Ethanol intake during adolescence is not exacerbated by concurrent foot-shock during 16 days of access to a sweetened ethanol solution (Brunell and Spear 2005). Under these conditions, foot-shock for eight days reduces simultaneous and post-stress ethanol intake (Brunell and Spear 2005). This reduction appears to be a specific avoidance of ethanol because stress did not decrease saccharin intake (Brunell and Spear 2005). This conclusion is further supported by Anderson et al (2012) (Table 2), who reported that early adolescent restraint stress does not affect ethanol-elicited taste aversion. It is possible that ethanol-increased social contact influenced sweetened ethanol consumption. Willey and Spear (2013) tested the effects of restraint stress or variable stress during early adolescence on ethanol-heightened social contact in adolescent and adult pair-housed rats (Table 2). Adolescents exposed to early adolescent stress do not spend more time investigating a novel conspecific under the influence of ethanol. However, adults with a history of restraint stress exhibit increased social contact under the influence of alcohol. A fruitful new avenue of investigation would be to test the effect of other adolescent stressors (i.e. social defeat) on ethanol intake and preference. Overall, it is unclear why adolescent stress reduces concurrent ethanol intake in rodent models. This finding contradicts what is observed in human studies where stress increases ethanol consumption (e.g., Dube et al. 2002), reducing the validity of current procedures for linking adolescent stress-induced sensitization to ethanol.
Lopez et al. (2011) found that CVS during mid-late adolescence (Table 3) increases ethanol drinking in a two bottle choice task in group and individually housed adolescent mice. This finding agrees with Chester et al. (2008), but is at odds with Brunell and Spear (2005). The combination of individual housing conditions does not exacerbate the CVS-induced increase in ethanol drinking (Lopez et al. 2011). However, individual housing alone increases ethanol intake, but only if mice were housed individually during adolescence. This further highlights adolescence as a sensitive period of development.!!
4. Isolation rearing and subsequent exposure to drugs of abuse
The most commonly used model of adversity in adolescent rodents is isolation rearing (Fone and Porkess 2008; Hall 1998; Lukkes et al. 2009c). Isolation rearing is conducted by housing rats individually immediately after weaning. If this procedure begins in adulthood, it is usually referred to as isolation housing (Hall 1998; Lu et al. 2003). Individual housing alters anxiety and drug-elicited behavior only after the individual housing is initiated at weaning, referred to as isolation rearing (Einon and Morgan 1977; Lopez et al. 2011; Ribeiro Do Couto et al. 2009; Robbins et al. 1996; Schenk et al. 1985; Schenk et al. 1990). These effects are attributed to the deprivation of social contact and play fighting during adolescence (Einon et al. 1978), which normally peak in adolescence (Section 2.2.). This type of stressor differs from stressful events, such as electric foot-shock pulses or even 1 hr of isolation, because withholding social contact for the entire adolescent period causes neither repeated episodes of physiological arousal, sudden surges of stress hormones, nor mesocorticolimbic DA release that are all thought to be critical for cross-sensitization (Sorg and Kalivas 1991).
The effects of individual housing on responses to drugs of abuse have been extensively reviewed (Bardo et al. 2013; Fone and Porkess 2008; Hall 1998; Lu et al. 2003; Neisewander et al. 2012; Robbins et al. 1996). Initially, higher doses of Amph and morphine were required for producing the same effects in isolates (Chance 1946; Katz and Steinberg 1970). More recently, isolation rearing is reported to cross-sensitize to the motor activating effects of psychostimulants (Ahmed et al. 1995; Bardo et al. 1995; Robbins et al. 1996; Sahakian et al. 1975; but see Weiss et al. 2001). With regard to psychostimulant self-administration and CPP, there have been several reports that isolation rearing increases (Howes et al. 2000; Schenk et al. 1987), decreases (e.g., Schenk et al. 1986) and does not change (e.g., Bowling and Bardo 1994) preference or operant responding reinforced by psychostimulants. Hall and colleagues suggested that isolation rearing increases drug self-administration during acquisition, but the effect disappears after repeated drug taking episodes (Lu et al. 2003). In contrast, most studies report a reduced preference for opiates after isolation rearing (e.g., Schenk et al. 1985; Wongwitdecha and Marsden 1996; but see Raz and Berger 2010), but greater opiate CPP was found when measured during adolescence (Kennedy et al. 2012). Meanwhile, most studies find that isolation rearing increases ethanol intake (e.g., Lopez et al. 2011; Wolffgramm 1990; but see Pisu et al. 2011). Overall, the effects of isolation rearing on subsequent responses to drugs of abuse are inconsistent (Fone and Porkess 2008), perhaps due to variations in the duration of individual housing (i.e. a range from about 21 days to 140 days), amount of experimenter handling, drug investigated, and the method used to assess drug preference, reinforcement, or behavioral activation (Hall 1998).
One of the reasons for such inconsistency could be explained by whether the animal is tested in a socially deprived state or after a period of rehousing (Lukkes et al. 2009c). Rats isolated from P21 to P42 and resocialized P42 into adulthood exhibit social anxiety-like behavior (Lukkes et al. 2009a; Lukkes et al. 2009b) and greater impulsivity (Baarendse et al. 2013), suggesting that isolation during early- and mid-adolescence followed by group housing for the rest of adolescence is sufficient to cause negative outcomes. Whitaker, et al. (2013) recently reported that isolation rearing from P21-42, but not P21-28 or P42-63, increases Amph and ethanol CPP. They discovered that this effect is mediated by glutamate receptor-dependent longterm potentiation in the VTA, which is exacerbated by resocialization from P42-56 (Whitaker et al. 2013). It should be noted that resocialization abolishes the isolation rearing-induced conditioned place aversion to morphine (Coudereau et al. 1997). There continue to be exceptions to the generalizations about whether individual housing changes responses to abused drugs. The most consistent finding is that isolation housing and isolation rearing cause hyperactivity to novelty, a behavioral trait long associated with greater drug responsiveness (Hooks et al. 1991; Orsini et al. 2004; Piazza et al. 1989).
5. Possible neural mechanisms: focus on dopamine
5.1. Sensitization of the mesolimbic dopamine system
Adult Amph injection elicits a larger increase in NAc core DA tissue content after mid-adolescent social defeat stress, while there is no change in serotonin (5HT), norepinephrine, or metabolites in the dorsal striatum, hippocampus, NAc shell, or medial prefrontal cortex (Burke et al. 2010) (Table 4). Similarly, early adolescent stress, but not adult stress, increases DA content in the NAc of Amph injected mid-adolescent rats (Cruz et al. 2012) (Table 4). These results suggest that stress during early to mid-adolescence sensitizes the NAc DA neurons immediately (Cruz et al. 2012), and this effect persists into early adulthood (Burke et al. 2010). These results suggest a common neural substrate for adult and adolescent stress cross-sensitization.
Table 4.
Dopaminergic response to drugs of abuse following stress during adolescence
| Species Strain | Sex | Age and Stress | Age and Drug | Outcome | Reference |
|---|---|---|---|---|---|
|
Amphetamine
| |||||
| Rats S.D. | M | P35-39 social defeat, 1x/d for 5 d | P58 second Amph (2.5 mg/kg, ip) experience with sampling 30 mins later | ↑ dopamine tissue content % change from saline injected group in NAc core, ↓ dopamine tissue content % change from saline injected group in mPFC | Burke et al. 2010 |
| Rats Wistar | M | P28-34 or P60-66 restraint 2 h/d for 7 d | P37 or P69 acute Amph (1 mg/kg ip.) with sampling 60 mins later | ↑ dopamine tissue content in NAc of adolescents, but not adults exposed to stress, ↑ dopamine turnover in VTA of adolescents, but not adults exposed to stress | Cruz et al. 2012 |
| Rats S.D. | M | Social defeat, 1x/d for 5 d 10 0.5 mA foot-shock 1x/d for 5 d | P58 Amph (1.0 mg/kg, ip) every other d for 14 d with sampling on d 16 | ↓ D2 dopamine receptor protein in NAc core after foot-shock and control treatment, but ø socially defeated NAc core samples, ø in mPFC | Burke et al. 2011 |
| Rats S.D. | M | P35-39 social defeat, 1x/d for 5 d | P56 “acute” or P62 repeated Amph (1 mg/kg, ip) daily for 6 d with continuous sampling for 200 mins after injection | ↓ extracellular dopamine levels in mPFC in response to acute Amph of adults exposed to adolescent stress, ø in NAc core | Burke et al. 2013 |
Abbreviations: increased (↑), decreased (↓), no change in (ø), nucleus accumbens (NAc), medial prefrontal cortex (mPFC), ventral tegmental area (VTA) postnatal day (P), occurrence (x), day (d), hour (h), Sprague-Dawley (S.D.)
Rats defeated in mid-adolescence that exhibit a sensitized motor response to Amph fail to also show sensitized extracellular DA levels in the NAc core (Burke et al. 2010) (Table 4). Perhaps, NAc shell DA release would be sensitized after adolescent defeat, as observed following adult defeat (Miczek et al. 2011). The VTA is also a primary target for adult social defeat-induced cross-sensitization to cocaine (Boyson et al. 2011; Miczek et al. 2011).
Dopamine turnover in the VTA when measured in adolescence is greater following restraint stress in early-adolescence (Cruz et al. 2012). Further studies are needed to determine whether VTA DA cells are similarly hypersensitive to drugs of abuse after adolescent stress as observed following adult stress, and whether this effect is long lasting.
Some evidence suggests that 20 brief foot-shocks per day for one-week results in ethanol CPP in adolescents, but not adults (Song et al. 2007). Since experimenter administered ethanol causes a greater DA release in the NAc shell in adolescents compared to adults (Pascual et al. 2009), this is an attractive neural mechanism underlying the idea that adolescents are more sensitive to stress cross-sensitization than adults (Andersen and Teicher 2008). However, stressinduced increases in ethanol-induced motor activity are observed in adults, but not adolescents (Acevedo et al. 2013). This could be explained by the finding that adolescent mice exhibit reduced corticosterone in response to mild stressors compared to adults (Adriani and Laviola 2000), supporting the idea of adolescent resilience to stress (Buwalda et al. 2011). Neural mechanisms that underlie the apparent increase in voluntary ethanol intake after adolescent stress (Chester et al. 2008; Lopez et al. 2011) need further investigation.
Sensitization of the mesocorticolimbic DA system may also explain the aforementioned sex differences (Sections 3.1.1., 3.1.2, 3.2.2. and 3.3.1). Namely, cross-sensitization to psychostimulants after adolescent social instability occurs in females more than males and this effect was transient (Mathews et al. 2008; McCormick et al. 2004; McCormick et al. 2005). Adolescent females exhibit less severe decreases in NAc DA during nicotine withdrawal (Natividad et al. 2010) and are hypothesized to exhibit greater DA release in response to nicotine compared to adult females and males of any age (O’Dell and Torres 2013). Females may also exhibit greater DA in response to adolescent stress, which could be a key component of increased female vulnerability to social stress-heightened nicotine-stimulated locomotion compared to males stressed in adolescence.
5.1.1. CRF and VTA
The CRF system in the VTA has been implicated in adult stress cross-sensitization to drugs of abuse. Rats that receive a systemic or intra-VTA CRF-R1 antagonist, CP-154,526, prior to adult social defeat stress, are protected against social stress-stimulated sensitization and escalated cocaine self-administration (Boyson et al. 2011). This effect is most likely mediated by suppression of stress-induced CRF release that stimulates VTA DA neurons (Wanat et al. 2008; Wang et al. 2005). In addition, the social defeat stress-sensitized DA release in the NAc shell in response to cocaine is blocked by CP-154,526 infusion into the VTA prior to each defeat episode (Boyson et al. unpublished). This suggests that CRF-R1 is critical for adult stress to increase cocaine-stimulated DA release when tested ten days later in adulthood. Concerning adolescent stress, systemic CP-154,526 administration prior to early-adolescent stress also blocks cross-sensitization to nicotine CPP (Brielmaier et al. 2012). Other evidence suggests that the shift from an enriched environment during adolescence to standard housing is stressful and cross-sensitizes to cocaine CPP (Nader et al. 2012). Chronic administration of a CRF-R1 antagonist (antalarmin) blocks the environmental stress-heightened cocaine CPP (Nader et al. 2012). These data suggest that stress-stimulated VTA CRF signaling through CRF-R1 is involved in stress cross-sensitization to psychostimulants in both adolescents and adults. If CRF-R1 agonist infusion into the VTA also causes behavioral and neural hypersensitivity to drugs of abuse, then the role of CRF-R1 would be confirmed.
5.1.2. Amygdala CRF
Amygdala CRF is implicated in the adult stress response and is hypothesized to underlie age differences in stress cross-sensitization to drugs of abuse. Uncontrollable adult stress increases CRF mRNA in the BLA and medial amygdala (Fekete et al. 2009; Fernandez Macedo et al. 2013) and urocortin-stimulated BLA CRF receptors increases anxiety-like behavior (Rainnie et al. 2004). How adolescent stress affects the still developing CRF system in the amygdala (Section 2.3.4.) remains to be determined. CRF in the BLA is implicated in enhancing memory consolidation of drug-paired cues that initiate reinstatement of drug seeking behavior (Carelli et al. 2003; Roozendaal et al. 2008). Stress may differentially affect the CRF-R2 in the BLA in adolescents versus adults because binding to CRF-R2 is less in adolescents than adults (Weathington and Cooke 2012). Conceivably, disrupted adolescent maturation of CRF-R2 by stress could explain the absence of cocaine cue-induced reinstatement after adolescent stress as opposed to adult stressed groups (Table 2) (Ribeiro Do Couto et al. 2009). Furthermore, adolescent stress fails to cross-sensitize to a drug challenge after withdrawal from repeated injections of psychostimulants (Table 1) (Burke et al. 2013; Kabbaj et al. 2002; Trzcinska et al. 2002), which disagrees with studies conducted in adults. Amygdala CRF increases during withdrawal from ethanol or cocaine (Zorrilla et al. 2001) and CRF-R2 is implicated in the motor response to methamphetamine after withdrawal from repeated injections (Giardino et al. 2011). It is possible that the immature amygdala CRF system during adolescent stress could explain the lack of cross-sensitization after adolescent stress. Determining how adolescent stress affects the CRF cells in the central nucleus of the amygdala and bed nucleus of the stria terminalis would give insight into the differential effects of adolescent versus adult stress cross-sensitization to drugs of abuse.
5.2. Reduced cortical dopamine
Adolescent social defeat stress reduces basal (Watt et al. 2009) and acute Amph-stimulated DA tissue content and extracellular levels in the mPFC (Burke et al. 2013; Burke et al. 2010) (Table 4). Mid- to late-adolescent isolation housing also reduces “frontal cortex” DA tissue content and basal extracellular levels in mice that were at a genetic risk (Dominant-negative disrupted in schizophrenia 1) (Niwa et al. 2013). In addition, isolation rearing (P21-42) followed by resocialization abolishes the sensitivity of mPFC pyramidal neurons to DA (Baarendse et al. 2013). These results provide evidence that social stress during adolescence can disrupt the maturation of mPFC DA. This effect is probably specific to adolescence since adult social defeat does not alter mPFC basal DA levels (Tidey and Miczek 1996). Thus, reduced mPFC DA may be a unique consequence of adolescent social stress, but further research on social stress is warranted that directly compares adolescents to adults.
Dopamine in the mPFC can modulate drug-stimulated motor activity through cortical regulation of the limbic system. In fact, Amph-heightened locomotion negatively correlates with extracellular DA levels in the mPFC, whereby reduced mPFC DA predicts greater amphetamine-induced locomotion (Burke et al. 2013). The mPFC sends glutamatergic projections to the NAc and striatum (McGeorge and Faull 1989; Spence et al. 1985). Dopamine release in the mPFC inhibits glutamate neurons in the mPFC (Thierry et al. 1986) and glutamate antagonists injected in the NAc reduce amphetamine-elicited increases in locomotion (Pulvirenti et al. 1989). Thus, the reduction of mPFC DA activity by adolescent social stress may allow for enhanced mPFC glutamatergic input to the NAc to increase NAc DA in response to appetitive stimuli (Mitchell and Gratton 1992) and underlie drug-stimulated behavior (Freed and Yamamoto 1985; Meredith et al. 2008). As no relationship between DA release in the NAc core and locomotion in response to Amph was observed (Burke et al. 2013), it will be of interest to investigate other motor control regions that receive mPFC glutamatergic projections such as the NAc shell, striatum, or subthalamic nuclei (Humphries and Prescott 2010; Ikemoto 2007). Glutamate release in the NAc could also mediate heightened drug-stimulated motor activity after adolescent social stress (Table 1).
5.2.1. D2 dopamine receptors
Adult threats of social defeat and witnessing an emotional response to stress both increase mPFC DA (Kaneyuki et al. 1991; Tidey and Miczek 1996). A potential mechanism underlying the adolescent stress-induced reduction in mPFC DA is D2 DA autoreceptor activation because mPFC DA peaks during adolescence (Figure 2C) and social stress may cause excess mPFC DA release during episodes of adolescent stress. Presumably, stress in adolescence also increases mPFC DA release and these repeated phasic increases in DA may also underlie the reduction in mPFC DA content and release following adolescent social defeat (Burke et al. 2013). While the adult mPFC does not contain synthesis-regulating autoreceptors and relies on release modulating autoreceptors to dampen DA release (Galloway et al. 1986; Wolf and Roth 1990), the adolescent mPFC contains synthesis-regulating autoreceptors (Andersen et al. 1997a). Activation of presynaptic Gi-coupled D2 receptors inhibits tyrosine hydroxylase, the rate-limiting enzyme for DA synthesis (Lindgren et al. 2003; Picetti et al. 1997). Thus, the repeated activation of D2 autoreceptors during adolescence by excess stress-induced DA release may decrease DA synthesis. Furthermore, decreased DA may be permanent because the mPFC DA system is still undergoing development and pruning of unused neurons. Systemic adolescent activation of D2 autoreceptors via quinpirole (D2 agonist) reduces adult tyrosine hydroxylase fibers (Naneix et al. 2013). This same treatment also reduces D1 and D2 DA receptors restricted to the mPFC (Naneix et al. 2013). This adolescent treatment also reduces DA content in the mPFC, NAc, and striatum. When quinpirole is infused directly into the mPFC during mid-adolescence, it also reduces mPFC DA (Watt et al. 2012). Furthermore, the adolescent defeat-induced reduction in cortical DA is blocked by the intra-mPFC D2 antagonist amisulpride (Watt et al. 2012). However, mPFC D2 receptors are not changed by adolescent social defeat (Burke et al. 2011) (Table 4). Future studies should demonstrate that adolescent defeat induced cross-sensitization to psychomotor stimulants is abolished by blocking D2 DA autoreceptors.
5.2.2. Glucocorticoids
Glucocorticoid receptors that bind corticosterone are expressed in the mPFC (Diorio et al. 1993) and on VTA DA neurons that project to the mPFC (Harfstrand et al. 1986). The adolescent mPFC undergoes late adolescent maturation and the HPA axis is hyper-responsive to stress possibly due to underdeveloped negative feedback (Section 2.3. & 2.4.). In addition, social defeat increases corticosterone within the mPFC (Croft et al. 2008). Glucocorticoid administration increases DA activity in the NAc and mPFC (Imperato et al. 1989; Rothschild et al. 1985), increases amphetamine self-administration (Piazza et al. 1991), and mice with a genetic deletion of glucocorticoid receptors do not exhibit defeat-induced NAc DA release (Barik et al. 2013). Glucocorticoid agonists and corticosterone reduce glutamatergic pyramidal neuron apical dendrite size in the mPFC (Cerqueira et al. 2007), suggesting stress-increased corticosterone could reduce synaptic contacts between mesocortical DA projections and mPFC pyramidal cells. Assuming the adolescent mPFC functions similarly, cortical DA terminals that do not make synaptic connections with mPFC pyramidal cells (or GABA interneurons) would likely be pruned or undergo axonal regression during final adolescent mPFC development (Low and Cheng 2006; Luo and O’Leary 2005). In support of this hypothesis, early to mid-adolescent rats exposed to chronic restraint stress have decreased dendritic complexity in the mPFC (Eiland and Romeo 2012). The excess corticosterone response to social stress in adolescence may underlie the reduced mPFC DA. Blocking the corticosterone response to adolescent defeat or administering corticosterone during adolescence may block or mimic, respectively, the effects of adolescent social stress on mPFC DA.
6. Future directions
It is imperative to compare, within the same study, the effects of adolescent versus adult stress (Buwalda et al. 2011) on the response to drugs of abuse (e.g. Laviola et al. 2002). Several studies discussed above directly compared adolescents to adults (Chester et al. 2008; Cruz et al. 2012; Doremus-Fitzwater et al. 2010; McCormick et al. 2005; Ribeiro Do Couto et al. 2009; Song et al. 2007; Willey and Spear 2013; Zago et al. 2012) to determine if adolescents are vulnerable or resilient to stress cross-sensitization to abused drugs compared to adults. While there is compelling evidence that adolescents are resilient to the negative effects of social stress compared to adults (Buwalda et al. 2013; Jankord et al. 2011; Willey and Spear 2013), four studies found increased vulnerability to stress in adolescence versus adulthood on cross-sensitization to drugs of abuse (Chester et al. 2008; McCormick et al. 2005; Song et al. 2007; Zago et al. 2012). However, a few studies find that age has no effect on whether the stress cross-sensitizes to drugs of abuse (Cruz et al. 2008; Cruz et al. 2012; Doremus-Fitzwater et al. 2010).
Very few studies investigate stress-induced changes in brain and behavior restricted to the temporally distinct stages of adolescence as Whitaker et al. (2013) did. It would be instructive to compare the detrimental effects of early adolescent verses mid-adolescent stress, for instance, on subsequent behavioral responses to drugs of abuse within the same study. This type of experimental design is often used to elegantly demonstrate the peak in adolescent behaviors, activity of HPA axis, DA systems, and cocaine CPP (Brenhouse et al. 2008; Foilb et al. 2011; Naneix et al. 2012; Vetter-O’Hagen and Spear 2012b). The persistence of the negative effects of adolescent stress is also important. For example, adolescent social stress increases the locomotor response to nicotine in adulthood (McCormick and Ibrahim 2007), but not when measured during adolescence, and adult social defeat cross-sensitization to cocaine is still present 70 days after the first defeat (Covington et al. 2005). Studies that find a clear difference in brain and behavior as a result of adolescent stress should investigate whether these effects are short lasting, long-lasting, or perhaps not apparent until adulthood, after adolescent maturation is complete.
In adult rats, brief exposure to a stressor has a very different outcome than continuous exposure to adversity. While brief intermittent stress augments the motor stimulating effects of psychostimulants (Section 1.), prolonged uncontrollable stress can cause reward dysfunction (Shimamoto et al. 2011; Willner 1997). Brief episodes of social defeat cause a sensitized NAc DA system and increased cocaine taking, while after continuous subordination stress the opposite occurs (i.e., less NAc DA and cocaine taking) (Miczek et al. 2011). Thus, an apparent paradox exists where longer duration of stress actually elicits less sensitization to drugs. For adolescents rodents, CVS that persists for a longer duration than the stress-free period causes greater cross-sensitization to drugs of abuse (Lepsch et al. 2005; Lopez et al. 2011), while brief variable stressors increase (Peleg-Raibstein and Feldon 2011) or block (Kabbaj et al. 2002) cross-sensitization to psychostimulants. The reward impairing effects of continuous stress on ensuing drug abuse-related measurements might be age-dependent.
There are sex differences in the effects of early life stress on subsequent response to drugs of abuse during adolescence (Viveros et al. 2011). However, very few studies have investigated sex differences in adolescent stress cross-sensitization to drugs of abuse. Only eight out of the 26 studies cited in Tables 1-3 investigate females. Very few studies have characterized the basal changes in the DA system and HPA axis during female adolescence (Andersen et al. 1997b; Romeo et al. 2004). Figure 2 B and C summarize the suspected changes in the dopaminergic system throughout male adolescence because data for females are very limited. Future studies should investigate the effects of adolescent stress on drugs of abuse in females and males.
Intravenous operant self-administration is the most informative preclinical method to assess salient aspects of drug abuse, such as the transition from impulsive to compulsive intake, motivation to work for a drug, and controllability of intake (O’Brien et al. 1998; Piazza and Deroche-Gamonet 2013). As adult social defeat and other stressors can escalate subsequent cocaine self-administration behaviors (Section 1.), future experiments should test voluntary i.v. self-administration following social defeat in adolescence, as currently investigated (Burke and Miczek, unpublished). Since reduced acute Amph-induced mPFC DA content and increased Amph-stimulated locomotion following adolescent social defeat is observed after acute Amph, but not repeated Amph injections, adolescent defeat may exert its greatest impact on Amph responses during the acquisition phase (Burke et al. 2013). Likewise, adolescent defeat may elicit greater preference for drug-paired cues after a single pairing with Amph.
So far, most efforts to study the effects of social defeat in adulthood on alcohol and heroin consumption are unsuccessful in demonstrating cross-sensitization to these substances (van Erp and Miczek, 2001; Quadros and Miczek, 2009). These data are at odds with results of other drugs (Section 1.). Adolescent social stress in humans is associated most often with increased nicotine, alcohol, marijuana, and inhalant use (Sullivan et al. 2006; Tharp-Taylor et al. 2009; Topper et al. 2011). However, no preclinical studies to date have investigated adolescent stress cross-sensitization to marijuana or inhalants, for instance. Demonstrations of adolescent stress cross-sensitization to other drugs of abuse would support the applicability of this basic research to humans.
7. Conclusions
The recent rise in research on adolescence and drug use often overlooks the impact of stress during adolescence. Rodent models are useful for determining the neural mechanisms that underlie the connection between adolescent adversity and illicit drug use. The final maturation of many behaviors, DA, CRF, and HPA axis occurs during adolescence. The acute motor response to psychostimulants is increased after adolescent restraint stress, but this effect does not persist into adulthood unless a non-habituating stressor is applied. In contrast, stress during adolescence does not increase drug-stimulated motor behavior after repeated psychostimulant injections. Only females exhibit greater motor responses to nicotine after adolescent stress. Adolescent stress most often increases later ethanol and Amph CPP. Few studies investigate voluntary drug self-administration after adolescent stress, but ethanol consumption was greater in adulthood after adolescent stress in two extensive studies. Isolation rearing is fundamentally different from episodic stress, but often causes cross-sensitization to drugs of abuse and disrupts brain maturation. Some evidence suggests sensitization of the mesolimbic DA system after adolescent stress similar to that observed after stress in adulthood. The CRF system in the VTA, especially CRF-R1s, has a role to sensitize VTA DA cells to drugs of abuse regardless of age. Given the late maturation of mPFC, stress during adolescence may uniquely reduce mPFC DA levels to underlie cross-sensitization to drugs of abuse. Dopamine D2 receptors and glucocorticoids are potential neural mechanism underlying stress-induced reductions in cortical DA. Further research is warranted to determine the precise role of DA, CRF, and HPA axis in adolescent stress cross-sensitization to abused drugs.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants F32DA032226 to A.R.B. and R01DA031734 and R01AA013983 to K.A.M.
Footnotes
The authors declare no conflicts of interest.
References
- Acevedo MB, Pautassi RM, Spear NE, Spear LP. Age-dependent effects of stress on ethanol-induced motor activity in rats. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams N, Boice R. A longitudinal study of dominance in an outdoor colony of domestic rats. Journal of Comparitive Psychology. 1983;97:24–33. [Google Scholar]
- Administration SAaMHS. Results from the 2009 National Survey on Drug Use and Health: Summary of national findings. I. US Department of Health and Human Services; Rockville, MD: 2010. [Google Scholar]
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29:869–78. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–46. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Stinus L, Le Moal M, Cador M. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1995;117:116–24. doi: 10.1007/BF02245106. [DOI] [PubMed] [Google Scholar]
- Akers KG, Arruda-Carvalho M, Josselyn SA, Frankland PW. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn Mem. 2012;19:598–604. doi: 10.1101/lm.027581.112. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/-)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol. 1997a;356:173–81. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Gazzara RA. The ontogeny of apomorphine-induced alterations of neostriatal dopamine release: effects on spontaneous release. J Neurochem. 1993;61:2247–55. doi: 10.1111/j.1471-4159.1993.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997b;8:1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–24. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–91. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–9. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience. 2012;249:214–22. doi: 10.1016/j.neuroscience.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–31. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986;7:351–78. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Brain Res Dev Brain Res. 1996;91:159–63. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJ, Counotte DS, O’Donnell P, Vanderschuren LJ. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38:1485–94. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–9. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–5. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Beck SG, O’Brien JH. Lethal self-administration of morphine by rats. Physiol Behav. 1980;25:559–64. doi: 10.1016/0031-9384(80)90122-5. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–65. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Anim Behav. 1964;12:427–441. [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–64. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–69. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet C, Vlierberghe LV, Vandevivere E, Theuwis L, Bosmans G. Depression in early, middle and late adolescence: differential evidence for the cognitive diathesis-stress model. Clin Psychol Psychother. 2013;20:369–83. doi: 10.1002/cpp.1789. [DOI] [PubMed] [Google Scholar]
- Brasser S, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiol Learn Mem. 2004;81:46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, McDonald CG, Smith RF. Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: a role for corticotropin-releasing factor 1 receptors. Psychopharmacology (Berl) 2012;219:73–82. doi: 10.1007/s00213-011-2378-1. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of Stress on the Voluntary Intake of a Sweetened Ethanol Solution in Pair-Housed Adolescent and Adult Rats. Alcoholism: Clinical and Experimental Research. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Burke AR, Forster GL, Novick AM, Roberts CL, Watt MJ. Effects of adolescent social defeat on adult amphetamine-induced locomotion and corticoaccumbal dopamine release in male rats. Neuropharmacology. 2013;67:359–69. doi: 10.1016/j.neuropharm.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Renner KJ, Forster GL, Watt MJ. Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res. 2010;1352:147–56. doi: 10.1016/j.brainres.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL. Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience. 2011;197:269–79. doi: 10.1016/j.neuroscience.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Geerdink M, Vidal J, Koolhaas JM. Social behavior and social stress in adolescence: a focus on animal models. Neurosci Biobehav Rev. 2011;35:1713–21. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: A study on the consequences of social stress in rats. Neuroscience. 2013;35:1713–21. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–75. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology (Berl) 2004;176:129–38. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci. 2003;23:8204–11. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Usiello A, Gubellini P, Pisani A, Borrelli E, Bernardi G, Calabresi P. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology. 2002;27:723–6. doi: 10.1016/S0893-133X(02)00367-6. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Chance MR. Aggregation as a factor influencing the toxicity of sympathomimetic amines in mice. J Pharmacol Exp Ther. 1946;87:214–129. [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–94. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–23. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–21. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Coudereau JP, Debray M, Monier C, Bourre JM, Frances H. Isolation impairs place preference conditioning to morphine but not aversive learning in mice. Psychopharmacology (Berl) 1997;130:117–23. doi: 10.1007/s002130050218. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–21. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–98. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–40. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–16. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, O’Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res. 2008;1238:12–22. doi: 10.1016/j.brainres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addict Biol. 2008;13:63–9. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Marin MT, Leao RM, Planeta CS. Stress-induced cross-sensitization to amphetamine is related to changes in the dopaminergic system. J Neural Transm. 2012;119:415–24. doi: 10.1007/s00702-011-0720-8. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, MacDonald K, Offord DR. Childhood stress and symptoms of drug dependence in adolescence and early adulthood: social phobia as a mediator. Am J Orthopsychiatry. 1999;69:61–72. doi: 10.1037/h0080382. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Edwards VJ, Croft JB. Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav. 2002;27:713–725. doi: 10.1016/s0306-4603(01)00204-0. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood Abuse, Neglect, and Household Dysfunction and the Risk of Illicit Drug Use: The Adverse Childhood Experiences Study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF. Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. J Adolesc Health. 2006;38:444 e1–10. doi: 10.1016/j.jadohealth.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner S, Chalmers DT, Baram TZ. The developmental profile of the corticotropin releasing factor receptor (CRF2) in rat brain predicts distinct age-specific functions. Brain Res Dev Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–32. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ, Kibbler CC. Brief periods of socialization and later behavior in the rat. Dev Psychobiol. 1978;11:213–25. doi: 10.1002/dev.420110305. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–12. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Esparza MA, Bollati F, Garcia-Keller C, Virgolini MB, Lopez LM, Brusco A, Shen HW, Kalivas PW, Cancela LM. Stress-induced sensitization to cocaine: actin cytoskeleton remodeling within mesocorticolimbic nuclei. Eur J Neurosci. 2012;36:3103–17. doi: 10.1111/j.1460-9568.2012.08239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feixa C. Past and present of adolescence in society: the ‘teen brain’ debate in perspective. Neurosci Biobehav Rev. 2011;35:1634–43. doi: 10.1016/j.neubiorev.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Macedo GV, Cladouchos ML, Sifonios L, Cassanelli PM, Wikinski S. Effects of fluoxetine on CRF and CRF1 expression in rats exposed to the learned helplessness paradigm. Psychopharmacology (Berl) 2013;225:647–59. doi: 10.1007/s00213-012-2859-x. [DOI] [PubMed] [Google Scholar]
- Fishburn CS, Elazar Z, Fuchs S. Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J Biol Chem. 1995;270:29819–24. doi: 10.1074/jbc.270.50.29819. [DOI] [PubMed] [Google Scholar]
- Foilb AR, Lui P, Romeo RD. The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol. 2011;210:391–8. doi: 10.1530/JOE-11-0206. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Freed CR, Yamamoto BK. Regional brain dopamine metabolism: a marker for the speed, direction, and posture of moving animals. Science. 1985;229:62–5. doi: 10.1126/science.4012312. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–9. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Galloway MP, Wolf ME, Roth RH. Regulation of dopamine synthesis in the medial prefrontal cortex is mediated by release modulating autoreceptors: studies in vivo. J Pharmacol Exp Ther. 1986;236:689–98. [PubMed] [Google Scholar]
- Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, Cancela LM. Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. Eur J Neurosci. 2013;37:982–95. doi: 10.1111/ejn.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Pastor R, Anacker AM, Spangler E, Cote DM, Li J, Stenzel-Poore MP, Phillips TJ, Ryabinin AE. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes Brain Behav. 2011;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–91. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002a;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002b;301:785–9. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–48. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143:2852–62. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl) 2001;154:213–20. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–62. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986;83:9779–83. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Stinus L, Le Moal M. Repeated stress increases locomotor response to amphetamine. Psychopharmacology (Berl) 1984;84:431–5. doi: 10.1007/BF00555227. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP, Cerbone FG, Su SS. A growth curve analysis of stress and adolescent drug use. Subst Use Misuse. 2000;35:687–716. doi: 10.3109/10826080009148417. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–70. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol. 1989;165:337–8. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–8. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–38. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Churchill L, Klitenick MA, Hooks MS, Kalivas PW. Involvement of the ventral tegmental area in locomotion elicited from the nucleus accumbens or ventral pallidum. J Pharmacol Exp Ther. 1996;277:1122–31. [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25:913–28. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Rimpela M, Rantanen P, Rimpela A. Bullying at school--an indicator of adolescents at risk for mental disorders. J Adolesc. 2000;23:661–74. doi: 10.1006/jado.2000.0351. [DOI] [PubMed] [Google Scholar]
- Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M, Tanaka M. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain Res. 1991;557:154–61. doi: 10.1016/0006-8993(91)90129-j. [DOI] [PubMed] [Google Scholar]
- Katz DM, Steinberg H. Long-term isolation in rats reduces morphine response. Nature. 1970;228:469–71. doi: 10.1038/228469a0. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Runckel PA, Lahvis GP. Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology (Berl) 2012;219:923–32. doi: 10.1007/s00213-011-2421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Berger RG, DeCatanzaro D. The onset of puberty in female mice as reflected in urinary steroids and uterine/ovarian mass: interactions of exposure to males, phyto-oestrogen content of diet, and ano-genital distance. Reproduction. 2008;135:99–106. doi: 10.1530/REP-07-0314. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–74. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63:131–6. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–75. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Klein ZA, Romeo RD. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Horm Behav. 2013;64:357–63. doi: 10.1016/j.yhbeh.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002;130:117–25. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–6. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepsch LB, Gonzalo LA, Magro FJ, Delucia R, Scavone C, Planeta CS. Exposure to chronic stress increases the locomotor response to cocaine and the basal levels of corticosterone in adolescent rats. Addict Biol. 2005;10:251–6. doi: 10.1080/13556210500269366. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Brain Res Dev Brain Res. 1991;62:109–14. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Lewis EM, Barnett JF, Jr, Freshwater L, Hoberman AM, Christian MS. Sexual maturation data for Crl Sprague-Dawley rats: criteria and confounding factors. Drug Chem Toxicol. 2002;25:437–58. doi: 10.1081/dct-120014794. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci U S A. 2003;100:4305–9. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. A neurodevelopmental model of schizophrenia: neonatal disconnection of the hippocampus. Neurotox Res. 2002;4:469–475. doi: 10.1080/1029842021000022089. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–64. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Cheng HJ. Axon pruning: an essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc Lond B Biol Sci. 2006;361:1531–44. doi: 10.1098/rstb.2006.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–91. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009a;55:248–56. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009b;158:845–55. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009c;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–56. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Lyss PJ, Andersen SL, LeBlanc CJ, Teicher MH. Degree of neuronal activation following FG-7142 changes across regions during development. Brain Res Dev Brain Res. 1999;116:201–3. doi: 10.1016/s0165-3806(99)00069-3. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Maier SF. Coping and the stress-induced potentiation of stimulant stereotypy in the rat. Science. 1983;219:1091–3. doi: 10.1126/science.6681679. [DOI] [PubMed] [Google Scholar]
- Macr S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim Behav. 2002;64:541–546. [Google Scholar]
- Marco EM, Adriani W, Ruocco LA, Canese R, Sadile AG, Laviola G. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci Biobehav Rev. 2011;35:1722–39. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Marquardt AR, Ortiz-Lemos L, Lucion AB, Barros HM. Influence of handling or aversive stimulation during rats’ neonatal or adolescence periods on oral cocaine self-administration and cocaine withdrawal. Behav Pharmacol. 2004;15:403–12. doi: 10.1097/00008877-200409000-00015. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Mills RG, McCormick CM. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol. 2008;50:451–9. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Waters P, McCormick CM. Changes in hyporesponsiveness to acute amphetamine and age differences in tyrosine hydroxylase immunoreactivity in the brain over adolescence in male and female rats. Dev Psychobiol. 2009;51:417–28. doi: 10.1002/dev.20381. [DOI] [PubMed] [Google Scholar]
- McCormick CM. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiol Behav. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Ibrahim FN. Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacol Biochem Behav. 2007;86:92–102. doi: 10.1016/j.pbb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm Behav. 2004;46:458–66. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McCourt WF, Gurrera RJ, Cutter HS. Sensation seeking and novelty seeking. Are they the same? J Nerv Ment Dis. 1993;181:309–12. doi: 10.1097/00005053-199305000-00006. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–37. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. A descriptive study of social development in the rat (rattus norvegicus) Anim Behav. 1981;29:34–45. [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or Suppressed Cocaine Reward, Tegmental BDNF, and Accumbal Dopamine Caused by Episodic versus Continuous Social Stress in Rats. J Neurosci. 2011;31:9848–57. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–28. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A. Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci. 1992;12:3609–18. doi: 10.1523/JNEUROSCI.12-09-03609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Chauvet C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology. 2012;37:1579–87. doi: 10.1038/npp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 2012;32:16223–32. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Pichon A, Pape JR, Coutureau E. Adolescent Stimulation of D2 Receptors Alters the Maturation of Dopamine-dependent Goal-Directed Behavior. Neuropsychopharmacology. 2013;38:1566–74. doi: 10.1038/npp.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Tejeda HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–45. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Higginson GK, Grant-Worley JA. Physical abuse among high school students. Prevalence and correlation with other health behaviors. Arch Pediatr Adolesc Med. 1995;149:1254–8. doi: 10.1001/archpedi.1995.02170240072011. [DOI] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S, Ozaki N, Nabeshima T, Sawa A. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–9. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.055. http://dx.doi.org/10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed]
- O’Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–12. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172:264–70. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–92. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Differential effects of post-weaning juvenile stress on the behaviour of C57BL/6 mice in adolescence and adulthood. Psychopharmacology (Berl) 2011;214:339–51. doi: 10.1007/s00213-010-1991-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–31. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A multi-step general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Saiardi A, Abdel Samad T, Bozzi Y, Baik JH, Borrelli E. Dopamine D2 receptors in signal transduction and behavior. Crit Rev Neurobiol. 1997;11:121–42. doi: 10.1615/critrevneurobiol.v11.i2-3.20. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, van Rossum JM. Letter: Stimulation of locomotor activity following injection of dopamine into the nucleus accumbens. J Pharm Pharmacol. 1973;25:1003–5. doi: 10.1111/j.2042-7158.1973.tb09995.x. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Mostallino MC, Dore R, Maciocco E, Secci PP, Serra M. Effects of voluntary ethanol consumption on emotional state and stress responsiveness in socially isolated rats. Eur Neuropsychopharmacol. 2011;21:414–25. doi: 10.1016/j.euroneuro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti L, Swerdlow NR, Koob GF. Microinjection of a glutamate antagonist into the nucleus accumbens reduces psychostimulant locomotion in rats. Neurosci Lett. 1989;103:213–8. doi: 10.1016/0304-3940(89)90578-8. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 2009;206:109–20. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20:1037–46. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz S, Berger BD. Effects of fluoxetine and PCPA on isolation-induced morphine self-administration and startle reactivity. Pharmacol Biochem Behav. 2010;96:59–66. doi: 10.1016/j.pbb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Lluch J, Rodriguez-Arias M, Minarro J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology (Berl) 2009;207:485–98. doi: 10.1007/s00213-009-1678-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 2006;185:459–70. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn PS, Chambers B, Davis J, Benoit S. Effects of prenatal immune activation and peri-adolescent stress on amphetamine-induced conditioned place preference in the rat. Psychopharmacology (Berl) 2012;222:313–24. doi: 10.1007/s00213-012-2646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Brownstein M, Spiess J, Rivier J, Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982;110:272–8. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Jones GH, Wilkinson LS. Behavioural and neurochemical effects of early social deprivation in the rat. J Psychopharmacol. 1996;10:39–47. doi: 10.1177/026988119601000107. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Smith KJ, Spear LP. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Angus AL, Becker JB. Sensitization to stress: the enduring effects of prior stress on amphetamine-induced rotational behavior. Life Sci. 1985;37:1039–42. doi: 10.1016/0024-3205(85)90594-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rodrigues AJ, Leao P, Carvalho M, Almeida OF, Sousa N. Potential programming of dopaminergic circuits by early life stress. Psychopharmacology (Berl) 2011;214:107–20. doi: 10.1007/s00213-010-2085-3. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31:232–40. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2007;85:199–206. doi: 10.1159/000102950. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–51. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild AJ, Langlais PJ, Schatzberg AF, Miller MM, Saloman MS, Lerbinger JE, Cole JO, Bird ED. The effects of a single acute dose of dexamethasone on monoamine and metabolite levels in rat brain. Life Sci. 1985;36:2491–501. doi: 10.1016/0024-3205(85)90145-6. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology. 2009;34:834–43. doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–34. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Safranski TJ, Lamberson WR, Keisler DH. Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol Reprod. 1993;48:669–73. doi: 10.1095/biolreprod48.3.669. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain Res. 1975;84:195–205. doi: 10.1016/0006-8993(75)90975-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–73. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- Schenk S, Ellison F, Hunt T, Amit Z. An examination of heroin conditioning in preferred and nonpreferred environments and in differentially housed mature and immature rats. Pharmacol Biochem Behav. 1985;22:215–20. doi: 10.1016/0091-3057(85)90380-6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–6. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav. 1986;24:1793–6. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–31. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95:190–8. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Segal DS. Behavioral characterization of d- and l-amphetamine: neurochemical implications. Science. 1975;190:475–7. doi: 10.1126/science.1166317. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996;125:385–91. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–30. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl) 2011;218:271–9. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol Psychiatry. 2009;66:100–1. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Somaini L, Donnini C, Manfredini M, Raggi MA, Saracino MA, Gerra ML, Amore M, Leonardi C, Serpelloni G, Gerra G. Adverse childhood experiences (ACEs), genetic polymorphisms and neurochemical correlates in experimentation with psychotropic drugs among adolescents. Neurosci Biobehav Rev. 2011;35:1771–8. doi: 10.1016/j.neubiorev.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res. 2007;31:2001–5. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spence SJ, Silverman JA, Corbett D. Cortical and ventral tegmental systems exert opposing influences on self-stimulation from the prefrontal cortex. Behav Brain Res. 1985;17:117–24. doi: 10.1016/0166-4328(85)90024-5. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2006;48:10–5. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Sullivan TN, Farrell AD, Kliewer W. Peer victimization in early adolescence: association between physical and relational victimization and drug use, aggression, and delinquent behaviors among urban middle school students. Dev Psychopathol. 2006;18:119–37. doi: 10.1017/S095457940606007X. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett. 1998a;254:21–4. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res. 1998b;110:227–33. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–9. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Barber NI, Gelbard HA, Gallitano AL, Campbell A, Marsh E, Baldessarini RJ. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology. 1993;9:147–56. doi: 10.1038/npp.1993.53. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, Volkow ND. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010;215:77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34:561–7. doi: 10.1016/j.addbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Le Douarin C, Penit J, Ferron A, Glowinski J. Variation in the ability of neuroleptics to block the inhibitory influence of dopaminergic neurons on the activity of cells in the rat prefrontal cortex. Brain Res Bull. 1986;16:155–60. doi: 10.1016/0361-9230(86)90027-4. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011;19:321–41. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–9. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Topper LR, Castellanos-Ryan N, Mackie C, Conrod PJ. Adolescent bullying victimisation and alcohol-related problem behaviour mediated by coping drinking motives over a 12 month period. Addict Behav. 2011;36:6–13. doi: 10.1016/j.addbeh.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Trzcinska M, Bergh J, DeLeon K, Stellar JR, Melloni RH., Jr Social stress does not alter the expression of sensitization to cocaine. Physiol Behav. 2002;76:457–63. doi: 10.1016/s0031-9384(02)00727-8. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-Releasing Factor Requires CRF Binding Protein to Potentiate NMDA Receptors via CRF Receptor 2 in Dopamine Neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–11. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Effect of the presence of a male on the sexual maturation of female mice. Endocrinology. 1967;81:345–9. doi: 10.1210/endo-81-2-345. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG, Finlayson JS, Dobrogosz WJ, Dills SS, Kost TA. Chromatographic separation of puberty accelerating pheromone from male mouse urine. Biol Reprod. 1976;15:260–5. doi: 10.1095/biolreprod15.2.260. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res. 1993;34:646–53. doi: 10.1203/00006450-199311000-00017. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012a;227:224–32. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012b;54:523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–46. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, de Fonseca FR. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol. 2012;26:164–76. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, Lopez-Gallardo M, Garcia-Segura LM, Wagner EJ. Framework for sex differences in adolescent neurobiology: a focus on cannabinoids. Neurosci Biobehav Rev. 2011;35:1740–51. doi: 10.1016/j.neubiorev.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomachka AJ, Greenwald GS. The development of gonadotropin and steroid hormone patterns in male and female hamsters from birth to puberty. Endocrinology. 1979;105:960–6. doi: 10.1210/endo-105-4-960. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–70. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Roberts CL, Scholl JL, Meyer DL, Miiller LC, Barr JL, Novick AM, Renner KJ, Forster GL. Decreased prefrontal cortex dopamine activity following adolescent social defeat in male rats: role of dopamine D receptors. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3353-9. doi:10.1007/s00213-013-3353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Cooke BM. Corticotropin-releasing factor receptor binding in the amygdala changes across puberty in a sex-specific manner. Endocrinology. 2012;153:5701–5. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Domeney AM, Moreau JL, Russig H, Feldon J. Dissociation between the effects of pre-weaning and/or post-weaning social isolation on prepulse inhibition and latent inhibition in adult Sprague--Dawley rats. Behav Brain Res. 2001;121:207–18. doi: 10.1016/s0166-4328(01)00166-8. [DOI] [PubMed] [Google Scholar]
- Whitaker LR, Degoulet M, Morikawa H. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron. 2013;77:335–45. doi: 10.1016/j.neuron.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Spear LP. Effects of acute ethanol administration and chronic stress exposure on social investigation and 50kHz ultrasonic vocalizations in adolescent and adult male Sprague-Dawley rats. Pharmacol Biochem Behav. 2013;105:17–25. doi: 10.1016/j.pbb.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G, Hirky AE. Escalated substance use: a longitudinal grouping analysis from early to middle adolescence. J Abnorm Psychol. 1996;105:166–80. doi: 10.1037//0021-843x.105.2.166. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–51. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–43. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–9. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Salinas A, Melloni RH, Jr, Delville Y. Behavioural and neuroendocrine adaptations to repeated stress during puberty in male golden hamsters. J Neuroendocrinol. 2004;16:767–75. doi: 10.1111/j.1365-2826.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Effect of social isolation on the reinforcing properties of morphine in the conditioned place preference test. Pharmacol Biochem Behav. 1996;53:531–4. doi: 10.1016/0091-3057(95)02046-2. [DOI] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alcohol Depend. 2013;129:240–6. doi: 10.1016/j.drugalcdep.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Reichard RA, Schwartz ZM, Parsely KP, Zahm DS. Protracted maturation of forebrain afferent connections of the ventral tegmental area in the rat. J Comp Neurol. 2013 doi: 10.1002/cne.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago A, Leão RM, Carneiro-de-Oliveira PE, Marin MT, Cruz FC, Planeta CS. Effects of simultaneous exposure to stress and nicotine on nicotine-induced locomotor activation in adolescent and adult rats. Braz J Med Biol Res. 2012;45:33–37. doi: 10.1590/S0100-879X2011007500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like- immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]


