Abstract
Background
While stressful life events can enhance the risk of mental disorders, positive social interactions can propagate good mental health and normal behavioral routines. Still, the neural systems that promote these benefits are undetermined. Oxytocin is a hormone involved in social behavior and stress; thus, we focus on the impact that social buffering has on the stress response and the governing effects of oxytocin.
Methods
Female prairie voles (Microtus ochrogaster) were exposed to 1 hr immobilization stress then recovered alone or with their male partner to characterize the effect of social contact on the behavioral, physiological, and neuroendocrine stress response. In addition, we treated immobilized females recovering alone with oxytocin, or vehicle, and females recovering with their male partner with a selective oxytocin receptor antagonist, or vehicle. Group sizes varied from 6 to 8 voles (n = 98 total).
Results
We found that 1 hr immobilization increased anxiety-like behaviors and circulating levels of corticosterone, a stress hormone, in females recovering alone, but not the females recovering with their male partner. This social buffering by the male partner on biobehavioral responses to stress was accompanied by increased oxytocin release in the paraventricular nucleus (PVN) of the hypothalamus. Intra-PVN oxytocin injections reduced behavioral and corticosterone responses to immobilization whereas injections of an oxytocin receptor antagonist blocked the effects of the social buffering.
Conclusions
Together, our data demonstrate that PVN oxytocin mediates the social buffering effects on the stress response, and thus may be a target for treatment of stress-related disorders.
Keywords: HPA axis, Corticosterone, Immobilization stress, Elevated plus maze, Pair-bond, Social buffering
INTRODUCTION
Stressful life events (e.g., divorce or death of a spouse) are deleterious to adult mental health in humans (1, 2), and the social environment can either propagate or attenuate these effects. For example, depression is concomitant with the lack of social support following a stressful life event (2). In contrast, close relationships ameliorate stress-induced biobehavioral responses and reduce the risk of psychological disorders in humans (3, 4). For instance, the natural occurrence of physical touch with an infant during pre-feeding behaviors and nursing is sufficient to reduce the anxiety experienced by mothers during physical and psychological stress (5). This social buffering effect has also been observed through contact with a committed partner, reducing the negative impact of stress (e.g., suffering from a panic disorder or psychological distress) (3, 4). Still, the neuroendocrine mechanisms underlying social buffering via committed partnerships are not well understood. Further, there is limited modeling of this phenomenon in animal research as less than 3% of mammalian species display social bonding between partners.
The prairie vole (Microtus ochrogaster) is a socially monogamous rodent that forms long-term pair bonds between partners (6). These bonds may protect against the aversive effects of stress by attenuating the action of the hypothalamic-pituitary-adrenal (HPA) axis (7). For example, while prolonged social separation elevates basal levels of corticosterone and increases a depression-like response to acute psychological stressors (8), social pairing can reduce basal corticosterone levels (9, 10). Further, reunion with a social partner can attenuate the HPA axis response associated with separation (11). Several neuroendocrine systems including oxytocin (OT), vasopressin (AVP), and corticotrophin-releasing hormone (CRH) are involved in prairie vole pair bonds (4, 6). Interestingly, these systems are vital to the regulation of the stress-induced HPA axis activation. In response to stress, the paraventricular nucleus (PVN) of the hypothalamus releases CRH and AVP to promote a signaling cascade, leading to an increase in circulating corticosterone and an induction in psychological and behavioral pathologies (4). In contrast, OT released from the PVN in rats acts as an anxiolytic—suppressing HPA axis function (12). Therefore, the neuroendocrine systems that are impetuses to adult social bonds may also regulate the social buffering of the stress response.
We investigated the biobehavioral effects and underlying neuroendocrine mechanisms of social buffering in female prairie voles. We established a behavioral paradigm demonstrating recovery with a male partner following immobilization stress decreases the biobehavioral stress response while promoting activity of the OT system in the PVN. We then focused on the functional role of the OT system in social buffering and demonstrated that social recovery following stress facilitates OT release in the PVN to promote social buffering.
MATERIALS AND METHODS
Detailed methods and experimental design are provided in Supplement 1.
RESULTS
Social support attenuated behavioral and hormonal responses to immobilization stress
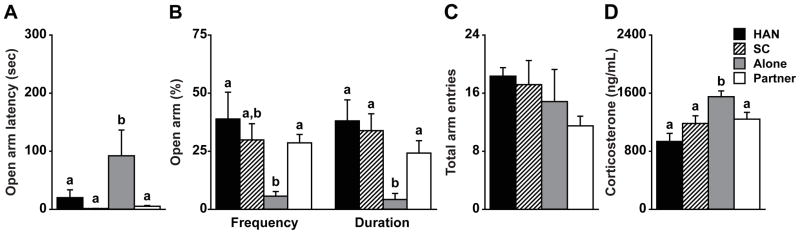
We characterized the impact of recovering with a male partner following immobilization on the behavioral and hormonal response in female prairie voles. Pair-bonded females received 1 hr immobilization, recovered either alone or with their male partner for 30 min, and were examined for their anxiety-like behaviors in an elevated plus maze (EPM) test and circulating levels of corticosterone. Immobilized females recovering alone displayed a substantial increase in EPM anxiety-like behaviors, including delayed open arm latency (F (3, 17) = 4.93, p < 0.05), fewer open arms entries (F (3, 22) = 3.86, p < 0.05), and reduced open arm duration (F (3, 22) = 5.14, p < 0.01), compared to the handled controls (Figure 1A,B), similar to our previous reports (13). The former also had a rise in circulating corticosterone compared to the latter (F (3, 21) = 6.32, p < 0.01; Figure 1D). By contrast, recovering with a male partner following immobilization attenuated anxiety-like behaviors and blunted the rise in circulating corticosterone, mirroring responses in the handled controls. This effect was behavior-specific as locomotor behavior was similar for all groups (Figure 1C). Furthermore, as prairie voles are sensitive to social separation, which may affect HPA axis function and EPM performance (8, 14, 15), we included a cohort of non-immobilized females that were removed from their male partner during the 30 min recovery period (i.e., social control). These females did not differ from the handled controls in anxiety-like behaviors or circulating levels of corticosterone (Figure 1). Together, these data demonstrate that the immobilization-induced stress response can be buffered by a bonded partner.
Figure 1.
Social support attenuated the behavioral and hormonal stress response 30 min post-immobilization. (A,B) Immobilized females recovering alone (Alone) displayed a substantial increase in EPM anxiety-like behaviors, including delayed open arm latency, fewer open arms entries, and reduced open arm duration. By contrast, females recovering with their social partner (Partner) displayed low anxiety-like behavior similar to the handle controls (HAN). (C) These effects seemed to be behavior-specific as total arm entries, a locomotor measure, did not vary between groups. (D) In addition to elevated EPM anxiety-like behavior, immobilized females recovering alone displayed a rise in circulating corticosterone concentrations, but females recovering with their male social partner had corticosterone levels similar to HAN controls. Social controls (SC) are non-immobilized females removed from their social partner for 30 min prior to the EPM test. Bars labeled with different letters differ significantly by post hoc SNK test in which a significant main effect was detected in the ANOVA (p < 0.05). Data are expressed as mean ± SEM.
Social support suppressed female stress-related behaviors and promoted dyadic interaction
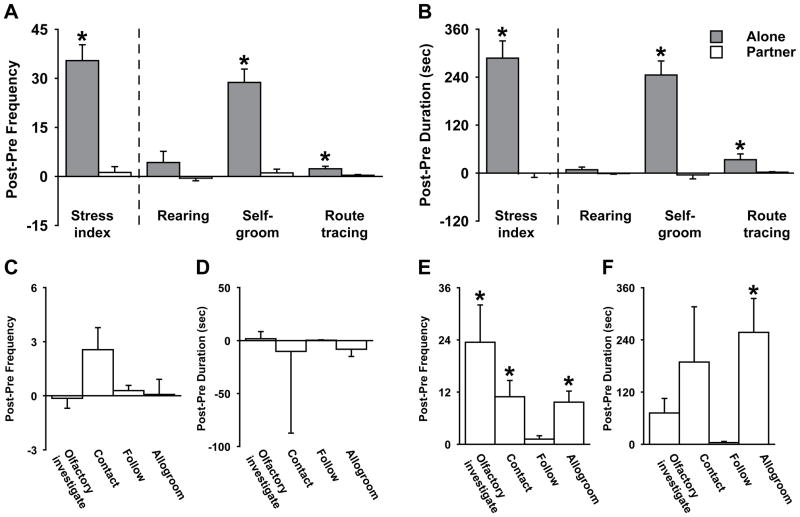
We further evaluated the behavioral stress response by comparing the occurrence of stress-related behaviors (i.e., rearing, repetitive autogrooming, and route tracing) for 1 hr while females remained undisturbed in their home enclosures with their male partner and again for 1 hr post-immobilization while they recovered alone or with their male partner. We observed immobilization-induced changes in stress-related behaviors in the recovery chamber compared to baseline conditions that were dependent on the social environment (stress index frequency, t (11) = 4.00, p < 0.005; stress index duration, t (11) = 3.83, p < 0.005; Figure 2A,B). Specifically, females that recovered alone displayed a significant increase in these stress-related behaviors, namely route tracing (frequency, t (5) = 3.07, p < 0.05; duration, t (5) = 2.70, p < 0.05) and repetitive autogrooming (frequency, t (5) = 4.37, p < 0.01; duration, t (5) = 4.82, p < 0.005). These effects were noted during the first 30 min following immobilization, returning to baseline levels 30 min later (Table S1 in Supplement 1). We observed no changes in stress-related behaviors initially among immobilized females recovering with their male partner (Figure 2A,B). After interacting with their male partner for 30 min, autogrooming behavior was suppressed (frequency, t(6) = −2.73, p < 0.05; duration, t (6) = −3.06, p < 0.05; Table S1 in Supplement 1). Route tracing behavior was not different at this latter period compared to baseline (Table S1 in Supplement 1). However, this null effect may be due to the fact that seven of the eight females recovering with their male partner never displayed this behavior during the baseline condition (inducing a flooring effect). These data suggest that the occurrence of stress-related behaviors was augmented by immobilization and suppressed via social recovery.
Figure 2.
During the initial 30 min of recovery after immobilization, social support reduced female stress-related behavior, but only males increased social behavior following immobilization. (A,B) Immobilized females recovering alone displayed significant increases in stress-related behavior, including rearing, self-grooming, and route tracing as well as a composite score that accounts for all stress-related behaviors (i.e., stress index), values represent a raw change score in female stress-related behaviors (post-stress minus pre-stress values). (C,D) Females did not change their social behavior after being immobilized, values represent a raw change score in female social behaviors. (E,F) Males increased their display of social behaviors when their female partners returned after experiencing immobilization, values represent a raw change score in male social behaviors. Bars labeled with asterisks indicate a significant change between pre- and post-stress values as determined by a one-sample or paired t-test (p < 0.05). Data are expressed as mean ± SEM.
In humans and other gregarious mammals, stress can stimulate social seeking behaviors (16). Further, group members can reduce anxiety by increased prosocial behaviors—behavior that is performed for the benefit of others— directed toward distressed individuals (4). In the current study, we did not observe a substantive change in female social behaviors after immobilization (Figure 2C,D and Table S1 in Supplement). However, males augmented their social behaviors during the first 30 min when their female partner returned from immobilization—approaching (frequency, t (6) = 2.91, p < 0.05), sniffing (frequency, t (6) = 2.74, p < 0.05), and grooming (frequency, t (6) = 3.80, p < 0.01; duration, t (6) = 3.31, p < 0.05) the female more often (Figure 2E,F). Such effects were not observed in the subsequent 30 min (Table S1 in Supplement 1). Only one of the eight pairs was observed mating during the pre- and post-stress periods. Therefore, as females recover from immobilization with their male partner, they experience an enhanced social display from their male partner and concomitantly display less stress-related behaviors.
Immobilization affected neuropeptide receptor content in a brain region-specific manner
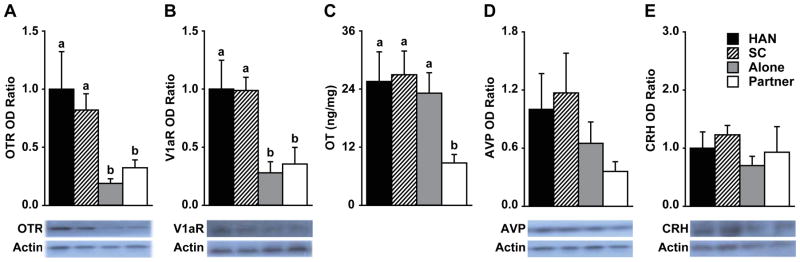
Western blotting revealed the 1 hr immobilization reduced the content of OT receptors (OTR; F (3, 19) = 5.37, p < 0.01; Fig. 3a) and AVP V1a receptors (V1aR; F (3, 9) = 5.88, p < 0.05; Figure 3B) in the PVN, regardless of whether the female voles recovered alone or with a male partner. Such effects on OTR and V1aR were not found in other brain areas, including the nucleus accumbens (NAcc) and the medial (MeA) and central (CeA) nuclei of the amygdala, indicating that the effect was brain region-specific (Table S2 in Supplement 1). Data from other rodent species have shown that stress can increase OT and AVP release in the PVN (17), and persistent OT and AVP release can lead to receptor desensitization and internalization within 30 min (18, 19). Subsequently, internalized OTRs undergo endocytosis and some V1aRs are not recycled back to the cell surface (20). Therefore, decreased OTR and V1aR expression in the PVN may indicate immobilization-induced activation of both systems.
Figure 3.
Neurochemical changes occurred as a result of immobilization and social support in the PVN. (A) Immobilized females had significantly lower optical density (OD) of oxytocin receptor (OTR) in the PVN. (B) V1aR expression was also lower in the PVN following immobilization. Social controls (SC) are non-immobilized females removed from their social partner for 30 min prior to sacrifice and tissue collection. (C) Recovering from immobilization with a social partner induced a reduction in the oxytocin (OT) content in the PVN. (D,E) No changes were observed in vasopressin (AVP) or CRH content. Bars labeled with different letters differ significantly by post hoc SNK test in which a significant main effect was detected in the ANOVA (p < 0.05). Data are expressed as mean ± SEM.
OT was released in the PVN during immobilization and social buffering
We measured the total content of OT via enzyme-immunoassay (ELISA) as well as AVP and CRH via Western blotting in the PVN (Figure 3C–E), NAcc, MeA, and CeA (Table S2 in Supplement 1). Immobilized females that recovered with a male partner had a significantly lower level of OT in the PVN compared to other groups (F (3, 22) = 4.47, p < 0.05; Figure 3C). As OT content was not detectable in the NAcc or amygdalar nuclei, we also measured OT in the supraoptic nucleus (SON) of the hypothalamus (Table S2 in Supplement 1), for a control region. No group differences were found in the OT, AVP, or CRH content in any of the other brain regions measured (Figure 3C–E, Table S2 in Supplement 1). Therefore, the social buffering-induced decrease in the PVN OT was brain region- and peptide-specific.
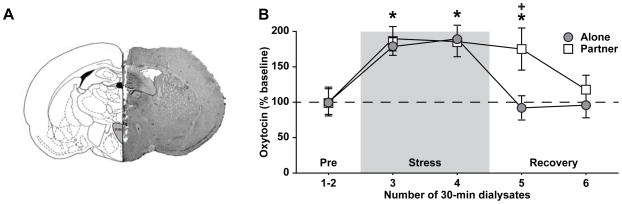
A decrease in the PVN OT content may be due to changes in OT synthesis, release, or both. In female rats, OT mRNA expression in the PVN is significantly increased 30 min following immobilization (21), and social contact facilitates OT activity (22). Therefore, a decrease in PVN OT content following immobilization and subsequent social buffering may be more likely due to a change in OT release. We used brain microdialysis with ELISA to measure extracellular OT concentrations in the PVN in immobilized female voles that recovered alone or with their male partner (Figure 4A). As compared to baseline sample concentrations, immobilization significantly increased OT concentrations in the PVN in all females (F (4, 44) = 24.76, p < 0.0001; Figure 4B), similar to reports in rats (23, 24). When immobilized females recovered alone, PVN OT concentrations returned to baseline levels, demonstrating no lasting release associated with the stress. Intriguingly, when females recovered with their male partner, PVN OT concentrations were substantially increased above baseline levels as well as concentrations in females recovering alone at the same time period (F (4, 44) = 4.48, p < 0.005; Figure 4B). Therefore, recovering with a male partner following immobilization reduced OT content by promoting its release in the PVN.
Figure 4.
Social support promotes oxytocin release in the PVN. (A) Schematic drawing (left, adapted from (50)) and representative photomicrograph of vole brain section (right) illustrates location of microdialysis probe placement in the PVN. (B) Microdialysis was used to measure extracellular oxytocin concentrations when immobilized females recovered alone or with their social partner. Immobilization significantly increased oxytocin outflow in the PVN in all females as compared to baseline. However, during the recovery period, extracellular oxytocin concentrations only increased above baseline levels when immobilized females recovered with their social partner. Asterisks indicate differences from baseline while plus signs indicate group differences at a specific time point, determined by post hoc SNK test in which a significant main effect or interaction was detected in the ANOVA (p < 0.05). Data are expressed as mean ± SEM.
PVN OT mediated the social buffering effect
Social buffering is an effective anxiolytic in humans before a stressor (25) or afterward (16). In rodents, intra-PVN microinjections of OT can reduce stress-induced rises of circulating corticosterone and anxiety-like behavior to acute psychological stressors (4). As our data demonstrate that social recovery with a male partner alleviates the behavioral and hormonal stress response and promotes the local release of OT in the PVN, we hypothesized that PVN OT mediates the social buffering effect on the stress response in female prairie voles.
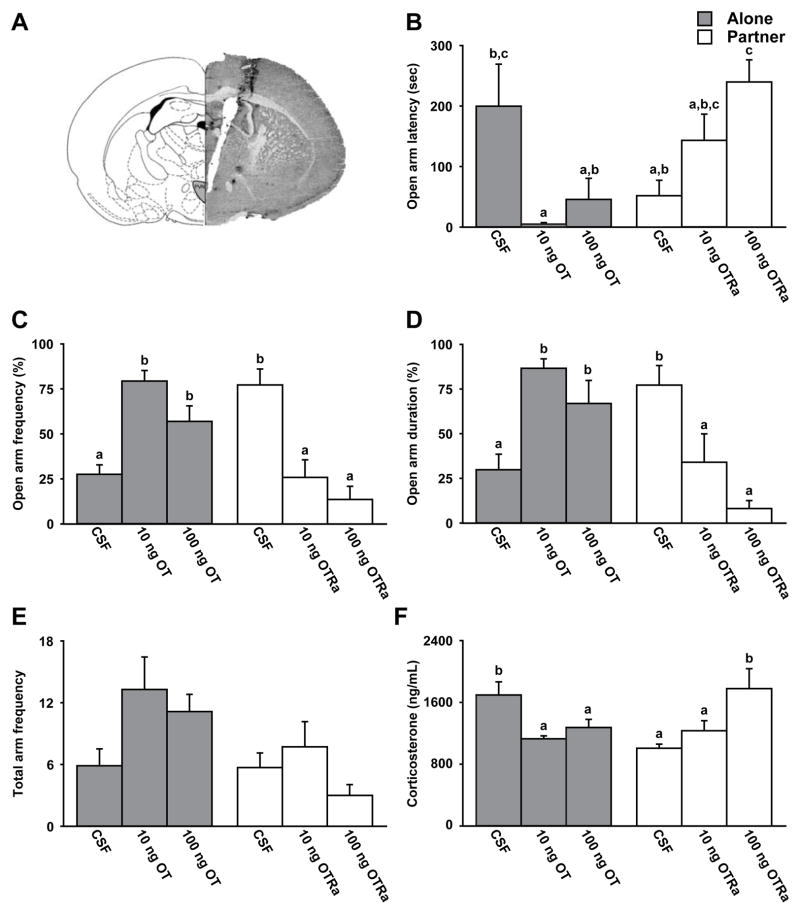
Female voles received bilateral stereotaxic implantations of cannulae aimed to the PVN (Figure 5A). After 1 hr immobilization, they were randomly assigned into one of two groups that recovered alone or with their male partner. Immediately prior to the recovery period, females recovering alone received bilateral intra-PVN microinjections of a vehicle (artificial cerebrospinal fluid or aCSF; 200nl/side) or aCSF containing a low (10ng/200nl/side) or a high (100ng/200nl/side) dose of OT. Females recovering with their male partner received intra-PVN microinjections of aCSF or aCSF containing a low (10ng/200nl/side) or a high (100ng/200nl/side) dose of a selective OTR antagonist.
Figure 5.
Oxytocin is sufficient to reduce the stress response and necessary for the social buffering effect. (A) Schematic illustrations (left, adapted from (50)) and representative photomicrograph of vole brain section (right) demonstrating location of micro-injections in the PVN. All females received bilateral implantation of guide cannulae in the PVN. Females were then immobilized, injected with a vehicle (artificial cerebrospinal fluid; CSF), oxytocin (OT; 10 ng or 100 ng/200nl/side), or a selective oxytocin receptor antagonist (OTRa; 10 ng or 100 ng/200nl/side), and recovered alone or with their male partner. (B–D) Females recovering alone displayed elevated EPM anxiety-like behavior unless they received an intra-PVN oxytocin injection. By contrast, females recovering with their partner displayed reduced EPM anxiety-like behavior compared to females recovering alone. When females recovering with their partner received an intra-PVN injection of a selective oxytocin receptor antagonist, no social buffering effects were observed on behavior. (E) No group differences were observed in total arm entries. (F) Social support and oxytocin injections reduced circulating corticosterone concentrations as compared to females recovering alone. However, when females recovering with their social partner receiving an intra-PVN selective oxytocin receptor antagonist injection, corticosterone levels were elevated, similar to females recovering alone. Bars labeled with different letters differ significantly by post hoc SNK test in which a significant main effect or interaction was detected in the ANOVA (p < 0.05). Data are expressed as mean ± SEM.
Similar to the data reported in Figure 1, immobilized voles recovering with a male partner displayed significantly lower levels of anxiety-like behaviors, including delayed open arm latency (F (5, 40) = 5.12, p < 0.001), fewer open arms entries (F (5, 40) = 12.78, p < 0.0001) and reduced open arm duration (F (5, 40) = 8.32, p < 0.0001), as well as a reduced level of circulating corticosterone (F (5, 40) = 5.27, p < 0.001), compared to the voles recovering alone (Figure 5B–F). Microinjections of OT in the PVN decreased anxiety-like behaviors and reduced circulating levels of corticosterone in the female voles recovering alone, indicating that OT is anxiolytic. In contrast, intra-PVN injections of an OTR antagonist increased anxiety-like behaviors and elevated circulating levels of corticosterone in the female voles recovering with a male partner. Together, these data suggest that PVN OT is both necessary and sufficient for mediating social buffering effects on biobehavioral responses to stress in female prairie voles.
DISCUSSION
Previous research has demonstrated the beneficial impact of social ties in adulthood on the physical, psychological, emotional, and behavioral responses toward a stressful event (3, 4). The current study provides evidence for a neural mechanism underlying the social buffering effect from a partner in pair-bonded female prairie voles. Female voles displayed buffered anxiety-like behavior and levels of corticosterone when recovering from psychological stress with their male partner. Furthermore, OT was released in the PVN when immobilized females recovered with their male partner, and released OT mediated the social buffering effects on biobehavioral responses to stress. Together, these data demonstrate that PVN OT is one mechanism through which social buffering occurs in female prairie voles.
Social buffering: An impetus for social living
The driving forces that facilitate social attachments, such as pair bonding, include reinforcing social behaviors through activating the brain reward centers; the consequences of disruption or separation of close social bonds on an individual’s well-being and emotional state; and the beneficial effects of close social contact to improve mental and physical health (26). Previous research has documented that pair bonding in prairie voles is facilitated by the brain reward circuitry reinforcing bond-related behaviors and the consequences associated with bond absence or loss. For example, dopamine transmission within the NAcc promotes pair bonds in prairie voles (6). In addition, the absence of social contact in prairie voles can promote a disruption to normal HPA axis activity and behavioral routines that mimic symptomatology of depression and anxiety disorders in humans (14, 15). Moreover, prolonged social separation from a bonded partner can elevate basal levels of corticosterone in circulation, CRH mRNA expression in the brain, and depression-like behavioral response to acute psychological stressors in prairie voles (8). The current study focuses on social buffering and demonstrates that social support attenuates the stress response as the immobilization-induced increase in stress-related behaviors and circulating corticosterone levels were eliminated when females recovered with their male partner. This extends results from previous research showing that social housing with a male, but not a female, can reduce the baseline HPA axis activity in female prairie voles (9). Therefore, interactions with a male partner seem to alleviate stress and benefit the emotional state and well-being of female prairie voles that live in a fundamentally social environment.
Researchers have found that experience of a stressful event can attract group members and social partners together (16), and this can lead to a reduction in their stress levels (4). For example, rats and humans are more likely to affiliate when under duress or following a stressful event. Thus, one potent stress buffering strategy is to seek or utilize support provided by a social partner or social network. While we did not observe a substantive change in social seeking behavior in female prairie voles after experiencing psychological stress, their male partners augmented their social behavior. Particularly, after olfactory investigation, males established contact with the female and engaged in social grooming. From rats to humans, individuals will act to attenuate distress in group members and significant others. For example, rats will free cagemates from restraints, acting deliberately to extinguish distress in another (27). Consoling distressed individuals following social conflicts seems to be a critical behavior for stress coping in greater apes (28). Furthermore, women who receive more hugs and other forms of “warm” physical contact from a committed partner (e.g., cohabiting partner or husband) can experience a suppression in the stress response (29). Thus, like humans and other highly social mammals, female prairie voles may experience social buffering effects through received or enacted support from a primary group member, in this case their pair-bond partner.
Efficacy of OT to promote the social buffering effect and potential therapeutic benefits
While a number of biological pathways undoubtedly contribute to the social buffering of the stress response, the convergence of evidence denotes a role for OT in social bond-promoting behaviors and their buffering effects on the stress response. The beneficial effects of social interaction on stress reduction seem to be associated with a release of OT, and disruption of OT action can inhibit the social buffering effect. For example, positive communication and “warm” physical contact between married couples are associated with higher plasma OT levels (30), which, in turn, are correlated with decreased levels of blood pressure and cardiovascular activity (29). In male rats, mating releases OT in the PVN and facilitates a reduction in anxiety-like behavior during psychological stress (31). Our data depict a similar story as social recovery promotes the release of OT in the PVN while reducing the stress response in female prairie voles. However, unlike male rats (31), the OT release in the PVN in female prairie voles seems to be independent of sexual interaction as such OT release was significantly elevated in all eight females recovered with the male partner although only one dyad engaged in mating behavior. Sexual encounters have been demonstrated to evoke OT release in female prairie voles (e.g., NAcc (32)); however, the lack of mating in the current study suggests that another aspect of the social interaction with their male partner evoked the OT release in the PVN. The natural occurrence of skin-to-skin contact, a source of cutaneous warmth and tactile stimulation, that occurs between the mother and infant during infant pre-feeding behaviors and nursing is sufficient to evoke OT release in newborn rat pups (33) and postpartum female rats (34), which is thought to originate from centrally projecting PVN neurons (35). In our study, male partners established side-by-side contact with and groomed the female voles more often after the female returned from the immobilization. This increased social contact may have provided the chemosensory or somatosensory cues necessary to evoke an increased release of OT in the PVN in female prairie voles.
In addition, the social buffering effect seems to rely on OT action as pharmacological blockade of OTR in the brain, via intracerebroventricular injections of an OTR antagonist, can disrupt alleviation of depression-like and anxiety-like behaviors induced by social housing in mice and mating in rats (31, 36). Our data demonstrate that despite recovering with a social partner after psychological stress, female prairie voles do not display stress relief if concomitantly receiving an injection of a selective OTR antagonist. This is the first evidence to demonstrate that the social buffering effect by a committed social partner requires the activation of OTR in the PVN, providing site-specificity to the model. These data coincide with other reports that inhibition of OT action in brain regions that release OT during psychological stress (e.g., PVN (37) and CeA (38)) can attenuate the stress-induced biobehavioral response (38). Thus, our findings support the notion that OT is one mechanism that propagates social buffering.
No drugs currently approved for psychiatric use directly target or address the social symptoms or components that accompany many stress-related psychiatric conditions. Given the prosocial effects of OT, this neurohypophysial hormone has become a target for therapeutic treatment of a wide range of psychiatric disorders, with dozens of clinical trials under way (39). OT gives impetus to social and sexual behavior (40), and it seems that through this action, OT can inhibit stress-induced activity in the HPA axis. In humans, social interactions can activate OT action. Specifically, OT levels are elevated when individuals engage in positive social communication with their spouse (41, 42). Reciprocally, intranasal OT administration reduces the conflict-promoted cortisol response and increases positive social communication (43). Further, social support can reduce stress-induced increases in cortisol levels and subjective response (e.g., calmness and anxiety), and intranasal OT administrations potentiate these effects (25, 44). Still, before OT can be normally prescribed as a therapeutic agent, more knowledge is necessary regarding the effects of OT in individuals in different psychological states. Several recent studies using rodent models have provided some evidence that OT may be a valuable target when treating stress-related disorders that manifest within a social context. Analogous to social support, OT appears to function as an anxiolytic agent. Social isolation activates the HPA axis and promotes depression- and anxiety-like behaviors in socially monogamous (e.g., hamsters and prairie voles) and gregarious (e.g., rats and mice) rodents (14, 15, 31, 36, 45). By contrast, OT administered during periods of social isolation can inhibit many of these negative effects (14, 15, 36, 45). For example, female prairie voles display more depression-like behavior in response to psychological stress and symptoms of anhedonia (e.g., a diminished sucrose preference) when living in social isolation; yet, subcutaneous administration of OT during social isolation can eliminate these depression-like symptoms (14). Our data demonstrate in the absence of a social partner, the female prairie vole stress response can be alleviated via an intra-PVN OT injection. Therefore, the negative impact of stress can be attenuated by OT treatments, even without social contact.
Another major concern for clinical use of OT is the lack of mechanistic details. There is a variety of anatomical and functional evidence to suggest that the effects of social buffering, as mediated by OT, may be localized within the PVN. First, there are data demonstrating a functional anatomical connection in the PVN between OT-expressing neurons and CRH-expressing neurons—which regulate HPA axis activity. There are synaptic connections between OT- and CRH-expressing neurons, and OT from the PVN can have paracrine effects locally (46), causing alterations in neuronal firing rates following OT administration. The production and release of OT originates primarily from the PVN, and OTR is colocalized on CRH-expressing neurons in the PVN (47). Second, from our data and others, psychosocial stress and social interactions can promote PVN OT release (17, 37), which includes dendritic release (46). Third, like social housing, local OT injections in the PVN can reduce stress-induced activation of CRH-expressing neurons and intracellular signaling in rats (48, 49). Furthermore, in the absence of social contact, the behavioral and hormonal response to psychological stress can be attenuated by local injections of OT in the PVN (48, 49), indicating that the suppressive effects of OT on the HPA axis seem to be mediated within the PVN itself. For the first time, our data demonstrates that even in the presence of a social partner, social buffering does not occur if OT action in the PVN is inhibited by an intra-PVN injection of a selective OTR antagonist. Taken together, these data strongly support the notion that social buffering can reverse the aversive effects of psychological stress on behavior, physiology, and neurochemistry through local activation of the OT system in the PVN.
Conclusion
The socially monogamous prairie vole has emerged as a unique model system to study the neurobiological mechanisms regulating the dynamic relationship between the social environment and stress. The absence of social contact can disturb normal HPA axis activity and increase stress-related behaviors in prairie voles (14, 15). The current study demonstrates that the enduring pair bond in female-male pairs provides a source of social buffering, which may be promoted through augmented post-stress social contact similar to effects observed in humans and other gregarious species (28, 29). Furthermore, we provide direct evidence that social support recruits the OT system and that site-specific OT action in the PVN is required to promote socially-mediated stress relief in female prairie voles. Together, our data expand upon previous reports in humans that note stress reduction through comfort from a committed partner is associated with peripheral OT release (30), providing site-specificity and functionality to this model. Therefore, OT may serve as a common regulatory element of the social environment and the stress response, and may be a targeted agent when studying the etiology, treatment, and prevention of stress-related disorders via social buffering.
Supplementary Material
Acknowledgments
Special thanks to R. Altshuler, L. Linton, M. Butler, C. Smith, and L. Rouser for assistants in data collection and behavioral analyses. We also thank Dr. Y. Liu, Dr. H. Wang, C. Lieberwirth, K. Lei, S. Huth, and A. Colombo for their critical reading of an early version of this manuscript. This work was supported by the National Science Foundation Graduate Research Fellowship and National Institutes of Health grant NIMHF31-095464 to AS and the NIH grant NIMHR01-058616 to ZW.
Footnotes
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest. The authors alone are responsible for the content and writing of the paper.
AUTHOR CONTRIBUTIONS STATEMENT
AS collected and analyzed the data, wrote the main manuscript text, and prepared all figures and tables. AS and ZW designed the experiments, reviewed and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown GW. Life events and illness. London: Unwin Hyman; 1989. [Google Scholar]
- 2.Dalgard OS, Dowrick C, Lehtinen V, Vazquez-Barquero JL, Casey P, Wilkinson G, et al. Negative life events, social support and gender difference in depression: A multinational community survey with data from the ODIN study. Soc Psych Psych Epid. 2006;41:444–451. doi: 10.1007/s00127-006-0051-5. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 4.Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Annals of the New York Academy of Sciences: ACTH and Related Peptides: Structure, Regulation, and Action. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- 6.Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): An animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- 7.DeVries AC, Craft TKS, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuroendocrinology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVries AC, DeVries MB, Taymans SE, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci USA. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVries AC, Taymans SE, Carter CS. Social modulation of corticosteroid responses in male prairie voles. Annals of the New York Academy of Sciences: ACTH and Related Peptides: Structure, Regulation, and Action. 1997;807:494–497. doi: 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- 11.Carter CS, DeVries AC, Taymans SE, Roberts RL, Williams JR, Chrousos GP. Adrenocorticoid hormones and the development and expression of mammalian monogamy. Annals of the New York Academy of Sciences: ACTH and Related Peptides: Structure, Regulation, and Action. 1995;771:82–91. doi: 10.1111/j.1749-6632.1995.tb44672.x. [DOI] [PubMed] [Google Scholar]
- 12.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith AS, Lieberwirth C, Wang Z. Behavioral and physiological responses of female prairie voles to various stressful conditions. Stress: The International Journal on the Biology of Stress. doi: 10.3109/10253890.2013.794449. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simsek Y, Celik O, Karaer A, Yilmaz E, Gul M, Ozerol E, et al. Elevated cardiac oxidative stress in newborn rats from mothers treated with atosiban. Arch Gynecol Obstet. 2012;285:655–661. doi: 10.1007/s00404-011-2069-5. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Frontiers in Neuroendocrinology. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Evans JJ, Forrest-Owen W, McArdle CA. Oxytocin receptor-mediated activation of phosphoinositidase C and elevation of cytosolic calcium in the gonadotrope-derived 3T3–1 cell line. Endocrinology. 1997;138:2049–2055. doi: 10.1210/endo.138.5.5138. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga Si, Setoguchi S, Hirasawa A, Tsujimoto G. Monitoring ligand-mediated internalization of G protein-coupled receptor as a novel pharmacological approach. Life Sci. 2006;80:17–23. doi: 10.1016/j.lfs.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:630–668. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 21.Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Annals of the New York Academy of Sciences: ACTH and Related Peptides: Structure, Regulation, and Action. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- 22.Neumann ID. Brain oxytocin mediates beneficial consequences of close social interactions: From maternal love and sex. In: Pfaff D, Kordon C, Chanson P, Christen Y, editors. Hormones and social behaviour. Heidelberg: Springer-Verlag Berlin; 2008. pp. 81–101. [Google Scholar]
- 23.Jezová D, Michajlovskij N, Kvetnanský R, Makara GB. Paraventricular and supraoptic nuclei of the hypothalamus are not equally important for oxytocin release during stress. Neuroendocrinology. 1993;57:776–781. doi: 10.1159/000126436. [DOI] [PubMed] [Google Scholar]
- 24.Babygirija R, Bulbul M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci. 2012;167:56–60. doi: 10.1016/j.autneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 26.Neumann ID. The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Frontiers in Neuroendocrinology. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Bartal IB-A, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser ON, Stahl D, Aureli F. Stress reduction through consolation in chimpanzees. Proc Natl Acad Sci USA. 2008;105:8557–8562. doi: 10.1073/pnas.0804141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunbar RIM. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci Biobehav R. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Developmental cognitive neuroscience. 2011;1:471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J Neuroendocrinol. 2012;24:831–840. doi: 10.1111/j.1365-2826.2012.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress: The International Journal on the Biology of Stress. 2002;5:259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- 36.Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, DeVries AC. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: A potential role for oxytocin. Psychosom Med. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 37.Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behaviour and the release of excitatory amino acids. Neuropsychopharmacology. 2004;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- 39.Miller G. Neuroscience. The promise and perils of oxytocin. Science. 2013;339:267–269. doi: 10.1126/science.339.6117.267. [DOI] [PubMed] [Google Scholar]
- 40.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. In: Neumann ID, Landgraf R, editors. Advances in Vasopressin and Oxytocin: From Genes to Behaviour to Disease. New York: Elsevier; 2008. pp. 331–336. [Google Scholar]
- 41.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- 42.Gouin J-P, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 47.Dabrowska J, Hazra R, Ahern TH, Guo J-D, McDonald AJ, Mascagni F, et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blume A, Bosch OJ, Miklos S, Luz T, Wales L, Waldherr M, et al. Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic; 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.