Abstract
Arteriovenous malformations occur when abnormalities of vascular patterning result in the flow of blood from arteries to veins without an intervening capillary bed. Recent work has revealed the importance of the Notch and TGF-β signaling pathways in vascular patterning. Specifically, Notch signaling has an increasingly apparent role in arterial specification and suppression of branching, whereas TGF-β is implicated in vascular smooth muscle development and remodeling under angiogenic stimuli. These physiologic roles, consequently, have implicated both pathways in the pathogenesis of arteriovenous malformation. In this review, we summarize the studies of endothelial signaling that contribute to arteriovenous malformation and the roles of genes implicated in their pathogenesis. We further discuss how endothelial signaling may contribute to vascular smooth muscle development and how knowledge of signaling pathways may provide us targets for medical therapy in these vascular lesions.
Keywords: Arteriovenous malformation, Notch, TGF-β, DLL4, ALK1, ENG, RASA1, Endo–MT
Introduction
Arteriovenous malformations (AVMs) are vascular developmental anomalies wherein the blood flow through an artery or arteriole is directed into a vein or venule without an intervening capillary bed. The existence of a shunt from the high-pressure arterial system into the normally low-pressure venous system exposes these vascular lesions to the risk of hemorrhagic rupture. AVMs can be found anywhere in the body, including the brain and lungs, where a spontaneous bleed can result in grave consequences.
Patients who present with AVMs most commonly do so between 30 and 40 years of age [1–4]. While genetically normal individuals may present with this pathology, AVMs are particularly common in patients with the disease hereditary hemorrhagic telangiectasia (HHT). Up to 35 % of individuals with HHT harbor at least one AVM, although multiple AVMs occur in these patients [5–7]. The sporadic and hereditary nature of AVM suggests that both inherited signaling abnormalities and environmental exposures are involved in their pathogenesis.
Arteriovenous malformations can spontaneously disappear or recur after treatment with surgery, radiosurgery or endovascular therapy. This plastic nature indicates that such lesions are actively remodeling [1]. Therefore, they must be dependent on continuous abnormal cell signaling for their maintenance and elaboration. In particular, the signaling defects of AVMs likely culminate in failures of appropriate arterial and venous fate specification as well as microvascular branching and remodeling.
In this review, we outline the abnormalities in endothelial cell (EC) signaling associated with AVM that have been used to create animal models or observed in primary human samples, with a focus on the Notch and TGF-β pathways.
Notch signaling
Notch signaling is an evolutionarily conserved pathway that has been implicated in cell fate specification in many tissues, wherein signals are transmitted by direct cell-to-cell interaction. Notch family cell-surface receptors bind their cell-surface ligands—including Delta-Like Ligands (DLL) and JAGGED (JAG)—on neighboring cells. Binding induces conformational change in NOTCH receptors. This leads to the cleavage of the Notch intracellular domain (NICD) from the full-length receptor. NICD is then translocated to the nucleus, where it binds RBP-J (also known as CSL, CBF1 and Suppressor of hairless (SUH)) and potentiates transcription of effector molecules, including HES and HEY transcription factors [8–10]. Notch pathway alterations have an established role in the human diseases CADASIL (mutant NOTCH3) and Alagille syndrome (mutant JAGGED1) in which vascular smooth muscle and biliary epithelial cell development, among other processes, are compromised [11, 12]. In the endothelium, Notch signaling has a particularly important role in the control of arterial fate specification and vascular patterning [10]. Both of these functions are involved in the pathogenesis of AVMs.
Interestingly, models that induce both constitutive activity of Notch signaling as well as complete or partial interruption of the pathway have proven sufficient to induce AVMs in animal models. The former result in AVMs with increased arterial diameter, whereas the latter produce AVMs with decreased growth. Both types illustrate functions of Notch in the vasculature, but models with constitutively active Notch signaling more closely mimic AVMs in humans [13–18]. We will discuss NOTCH1 and NOTCH4 receptors and the ligand DLL4 in the context of AVM (Table 1).
Table 1.
Notch pathway mutants that exhibit AVMs
| References | Gene | Mod. | Sp. | Description (time of embryonic lethality, if applicable) |
|---|---|---|---|---|
| Krebs et al. [13] | Notch1 | KO | Mm | (E9.5) Narrow, collapsed vessels with poor remodeling of primitive vasculature |
| Notch4 | KO | Mm | Moderate changes suggestive of a functional overlap with Notch1 | |
| Notch1 and Notch4 | KO | Mm | (E9.5) Changes similar to, but more pronounced than Notch1 KO | |
| Lawson et al. [20] | SuH, mib | DN | Dr | Abnormal arterial markers, ectopic venous markers, and AV shunts |
| Krebs et al. [16] | Notch1 | CA | Mm | Defective remodeling, fusion of dorsal aorta and common cardinal vein, similar to RBPJ-KO and ephrinB2/EphB4-KO mice |
| Uyttendaele et al. [22] | Notch4 | EC–CA | Mm | (E10) Diminished branching, particularly in the brain, with associated tissue necrosis, dilated vasculature without appropriate microvasculature |
| Carlson et al. [17] | Notch1 and Notch4 | EC–CA | Mm | Adult activation induced lethal AVMs that can be suppressed with target suppression |
| Murphy et al. [23] | Notch4 | CA | Mm | Activation at birth induced AVMs, decreased capillary branching, and hemorrhage |
| Murphy et al. [18] | Notch4 | CA | Mm | Intravital repression of active Notch4 caused AVM regression, return of capillary flow and the re-establishment of appropriate vessel identity |
| Gale et al. [26] | Dll4 | Het | Mm | (E9.5) Fusion of the arterial and venous systems decreased body size, diffuse tissue necrosis |
| Duarte et al. [25] | Dll4 | KO | Mm | (E9.5) Poor arteriogenesis, great vessel atresia, absence of arterial markers, ubiquitous venous markers |
| Krebs et al. [27] | Dll4 | KO | Mm | (E9.5) Fistulization between dorsal aortae and posterior cardinal veins |
| Benedito et al. [31] | Dll4 | KO | Mm | Diffusely reduced arterial caliber. Abnormal EC morphology with dysfunctional basement membrane. Increased EC migration and proliferation contributing to AVM formation |
| Trindade et al. [32] | Dll4 | OE | Mm | (E9.0) Excessive arterialization with VSMC and basal laminae, ectopic arterial markers |
SuH Suppressor of Hairless, mib mindbomb, EC endothelial cell, KO knockout, DN dominant negative, CA constitutive activity, Het heterozygous, OE overexpressed, Mm Mus musculus, Dr Danio rerio
Notch1 and Notch4 receptors
Notch1 homozygous deletion leads to embryonic lethality in mice at embryonic day 9.5 (E9.5) with defects in vascular patterning that include diffuse vascular underdevelopment, hypersprouting, and poor remodeling of the primitive vascular plexus [19]. These mice exhibit direct anastomoses between arterial and venous circulations within the embryo, a feature that is characteristic of AVM [13, 16]. Mice with Notch4 homozygous deletion, however, develop without abnormality, survive through adulthood and are fertile. Notch1/Notch4 double-null mutants also exhibit lethality at E9.5, but display a more severe phenotype than mice with only Notch1 gene deletion. The amplification of defects in combined-null mutants suggests overlap in the developmental responsibilities of Notch1 and Notch4 [13] (Table 2).
Table 2.
TGF-β pathway mutants that exhibit AVMs
| References | Gene | Mod. | Sp. | Description (time of embryonic lethality, if applicable) |
|---|---|---|---|---|
| Johnson et al. [60] | ACVRL1 | N/A | Hs | Responsibility of ACVRL1 in HHT2 |
| Oh et al. [54] | acvrl1 | KO | Mm | (Mid-gestation) fusion of capillary plexi, vascular dilation |
| Urness et al. [74] | Acvrl1 | KO | Mm | (E9.5–11.5) shunting from aortic arches to cardinal veins, poor VSMC recruitment, reduced arterial markers, hemogenic venous endothelium |
| Srinivasan et al. [65] | Acvrl1 | Het | Mm | Survive to adulthood, age-dependent incidence of AVMs, unpredictable organ involvement |
| Seki et al. [67] | Acvrl1 | Het + LacZ | Mm | Demonstrates arterial specificity of ALK1, notably in developing/remodeling vessels |
| Park et al. [36] | Acvrl1 | EC–KO | Mm | (E18.5) diminished arterial wall development, irregular VSMC, shunts |
| Hao et al. [78] | Acvrl1 + Eng | Het + VEGF | Mm | Stimulation with VEGF and vasodilator produces neurovascular dysplasia, EC proliferation, greater sensitivity of Endoglin mutants |
| Park et al. [75] | Acvrl1 | KO | Mm | Adult deactivation sufficient for organ AVMs. Skin lesions required injury also |
| Walker et al. [112] | Acvrl1 | KO + VEGF | Mm | Local deactivation of ALK1 and VEGF stimulation produces AVMs and abnormal arterial and venous markers |
| Corti et al. [68] | acvrl1 | KO | Dr | AVMs secondary to stabilization of normally transient shunts, enlarged arterial caliber |
| Milton et al. [76] | Acvrl1 | SM–KO | Mm | Age-dependent brain and spinal cord AVMs leading to paralysis in 10–15 weeks of life |
| Larrivée et al. [83] | Acvrl1 | Fc-ALK1 | Mm | Soluble ALK1 receptor, blockade of BMP9, dense dilated plexi with poor remodeling |
| McAllister et al. [59] | ENG | N/A | Hs | Responsibility of ENG in human HHT1 |
| Li et al. [91] | Eng | KO | Mm | (E11.5) poor VSMC development, atretic major vessels, poor remodeling |
| Bourdeau et al. [64] | Eng | Het | Mm | Develop clinical HHT over months; telangiectases, hemorrhage, and poor weight gain |
| KO | Mm | (E10) diffuse hemorrhage, poor remodeling, vessel rupture | ||
| Jonker and Arthur [90] | Eng | LacZ | Mm | Expression begins E6.5, widespread in endothelium |
| Satomi et al. [94] | Eng | Het | Mm | Cerebral AVMs develop in endoglin heterozygotes, not homozygous-wild type |
| Torsney et al. [95] | Eng | Het | Mm | 6 % of animals with large AVM, 25 % lethal, 44 % with hemorrhage, infertility, symptomatic at 36 weeks, many uterine AVMs |
| Sorensen et al. [107] | Eng | KO | Mm | Hemogenic venous endothelium, endoglin null does not change in ephrinB2 |
| Mahmoud et al. [92] | Eng | KO | Mm | Poor remodeling of retinal plexi, endothelial proliferation, decreased capillary investment of gel matrix implant |
| Choi et al. [97] | Eng + Acvrl1 | KO | Mm | Local knockout of endoglin with VEGF is more dysplastic than the same for ALK1 |
| Cole et al. [61] | HHT3 | N/A | Hs | Identification of locus mutated in HHT3 |
| Bayrak-Toydemir et al. [58] | HHT4 | N/A | Hs | Identification of locus mutated in HHT4 |
| Gallione et al. [62, 63] | SMAD4 | N/A | Hs | Identification of SMAD4 as gene responsible for JP–HHT |
EC endothelial cell, KO knockout, LoF loss of function, CA constitutive activity, Het heterozygous, OE overexpressed, Hs Homo sapiens, Mm Mus musculus, Dr Danio rerio
Complete interruption of Notch signaling in zebrafish with dominant negative Notch downstream mediators induced the downregulation of arterial markers and upregulation of venous markers, as well as shunting of blood from the arterial to venous system [20]. Interestingly, the alteration of EC identity was observed prior to the onset of blood flow, which suggested that EC fate specification is a primary process mediated by Notch signaling [20].
The discovery that Notch determines EC fate led to further study with the aid of the constitutively active Notch4-based oncogene int3 [21]. In light of the fact that Notch1 and Notch4 have overlapping effects in ECs, int3 has been employed to assay the effects of activated Notch signaling. Mice that expressed int3 in the endothelium to stimulate Notch signaling exhibited embryonic lethality at E10 with diffuse AVMs [22]. Early postnatal [23] and adult expression [17] of int3 induced AVMs, followed by lethality, within weeks. As opposed to Notch knockout models, wherein venous markers were induced in natively arterial cells, constitutive Notch activation produced vascular enlargement, increased expression of arterial marker EPHRINB2 in natively venous endothelial cells and increased recruitment of vascular smooth muscle cells (VSMCs). This was associated with increased endogenous Notch activity (i.e., Notch activity via int3-independent signaling) through Hey1, endogenous Notch4 and Dll4 [17].
The neurovasculature in this animal model was particularly notable. The presence of constitutively active Notch4 signaling induced AVMs in mouse brains accompanied by neurologic deficits and hemorrhage. Brains were specifically noted to harbor dilated vessels with poor tissue capillary infiltration and associated tissue necrosis, reminiscent of human cerebral AVM [4, 22–24]. In one study, hemorrhage was observed in all transgenic mice and was always secondary to the presence of an AVM [23].
Arteriovenous malformations have also been observed secondary to the overexpression of Notch1-ICD. These AVMs demonstrated dilatation unlike hypoplastic AVMs in Notch1 knockout mice. Systemic AVMs were observed in these animals as early as E9.5 and closely mirrored AVMs observed in EphrinB2 and EphB4 knockout mice [16].
Postdevelopmental alterations of Notch signaling cause remodeling of the vasculature and may also result in AVMs. Int3 activation in the neonatal and adult mouse induced AVMs with subsequent hemorrhage, neurologic sequelae, and ultimately death. Furthermore, regression of AVMs was achieved upon the suppression of int3- [17, 23]. AVM regression was observed with in vivo microscopy that revealed the restoration of capillaries, the regression of shunt enlargement and re-establishment of arterial and venous endothelial markers. Interestingly, marker restoration and shunt regression occurred without a change in endothelial cell number. The observed change in the arterial/venous marker expression, without a change in their number, suggests that Notch signaling is sufficient for cell fate specification [18].
Decreased branching, consistent with well-characterized anti-angiogenic functions of Notch signaling, is also observed in animals with endothelial expression of int3 [22]. This may contribute to AVM development due to reduced size and growth of the capillary bed, along with vascular enlargement, which potentiate direct communication between arteries and veins.
While it is true that both Notch pathway inhibition and activation can induce vascular shunting, AVMs from patients typically demonstrate NOTCH1 activation as suggested by histological features and greater expression of effector molecule HES1 compared to non-diseased vessels. This latter finding was mirrored by int3-driven animal models of Notch activation, which suggests similarity in the underlying signaling [14]. Another series of human primary samples demonstrated NOTCH1 activation in ECs as well as VSMCs with elevated levels of JAG1, DLL4, and HES1 [15]. While findings in diseased tissues do not demonstrate the causality of Notch activity, their histological and molecular similarity to mouse models of Notch activity is convincing enough to suggest a parallel mechanism of AVM.
Delta-like ligand 4 (DLL4)
Delta-like ligand 4 is a ligand for both NOTCH1 and NOTCH4 [13, 22]. It is involved in arterial and venous specification during development in addition to its established role in tip cell formation in the remodeling vasculature [25–27]. VEGF signaling induces DLL4 expression, which is used by tip cells to suppress tip fate of neighboring ECs in order to prevent excessive vessel branching [28–30]. Dll4 is expressed in arterial endothelia during development, but only in remodeling small arteries and microvessels in the adult mice, where it has a “salt and pepper” expression pattern. These patterns are suggestive of its role in both primary vascular morphogenesis and signal-driven remodeling [29].
Heterozygous or homozygous-null Dll4 mutant mice exhibit a phenotype similar to, but more extensive than, Notch1-null mice. Mice that are homozygous-null or heterozygous for Dll4 die at approximately E9.5 (depending on genetic background). At this time point, mutants exhibit a decrease in somite number, atretic vasculature and tissue necrosis [26]. Shunting in AVMs was specifically identified in only one of three studies that initially described Dll4 haploinsufficiency [27], but findings in these three studies were similar in many other respects [25, 26].
Apart from the aforementioned abnormalities, EC identity is also compromised in Dll4 mutants. Arterial markers such as EPHRINB2 and CONNEXIN40 are not detectable in arterial EC, while the venous marker EPHB4 is expressed in endothelial cells of arteries and veins despite normal expression of lineage-independent EC markers. These changes were observed prior to the onset of blood flow [25–27].
Flow-dependent structural abnormalities have also been noted. Impaired branching and remodeling of the vessels were accompanied by diminished arterial caliber, and atresia or fusion of the aorta and other large vessels [25–27]. On the cellular level, Dll4 mutants also displayed underdeveloped basement membranes and EC adhesion [31]. In each of these studies, it appeared that none of these defects were attributable to abnormal vasculogenesis.
As one might expect, mice in which Dll4 was overexpressed in the endothelium exhibited excessive arterialization [32]. EPHRINB2 and CONNEXIN37 were strongly expressed in both arterial and venous endothelia while EPHB4 expression was diminished in veins. These mice also displayed enlarged vessels, shunting through AVMs, and died by E9. Increased extracellular matrix deposition was also observed with increased ECM protein synthesis and decreased matrix metalloprotease production [32].
In summary, developmental effects of the ligand DLL4 are broad and reciprocate the effects of its receptors NOTCH1 and NOTCH4. These encompass vascular patterning, endothelial cell identity and function. Such findings imbue the reviewed studies with internal consistency. The endothelial roles of Dll4 further display acute sensitivity to gene dosing, which suggests the importance of its time- and site-specific regulation in the developing vasculature.
Insights from notch findings
What do the phenotypes of Notch1/4 and Dll4 mutants teach us about Notch signaling in the vasculature and the development of AVMs? One set of findings concerns EC identity and the achievement of arterial and venous specification. Notably, the expression of arterial markers correlates with the degree of Notch activity: upregulation induces arterial markers in natively venous endothelia, whereas downregulation suppresses their expression in natively arterial endothelia. Several groups noted that altered expressions of arterial and venous endothelial markers were observed before the onset of blood flow. The timing of these aberrations suggests that vascular identity is intrinsically determined, at least in part, by Notch signaling [25, 31, 32]. EPHRINB2 and EPHB4 are expressed in arterial and venous endothelia, respectively, as a consequence of Notch activity. They are markers, rather than determinants, of cellular identity. Expression of EPHRINB2, for instance, is also controlled by Notch signaling in cardiac development [20, 33]. Blood flow, which has been shown to influence intracellular signaling and secondary vascular specification [34], may also be involved in vascular remodeling, but seems uninvolved in the initial determination of vascular cell fate.
The timing of embryonic lethality in Notch mutants is likely attributable to its role in vascular remodeling. Until around E8.5, Notch pathway mutants are histologically indistinguishable from wild-type mice, but all prove lethal around E10. By this point vasculogenesis, or the de novo formation of the vasculature, is complete. Arteriogenesis, the process of remodeling, branching and elaboration of these vessels, is initiated at this time and is critical to sustain perfusion of the growing fetus [35]. Notch pathway mutants, with either gain- or loss-of-function, share this time point of embryonic lethality because of defective arteriogenesis.
Notch activation requires an appropriate middle ground with respect to its effects on vascular branching and remodeling. Tissues need appropriate investment with vasculature for sustenance. Excessive Notch activation limits capillary sprouting, thereby limiting adequate blood vessel network formation. This results in tissue necrosis in the area surrounding an AVM. Conversely, Notch hypoactivity produces vessels that are too atretic to provide vascular supply. Interestingly, the degree of endothelial Notch activity correlates with arterial caliber [33]. Notch gain-of-function mutants, either via constitutive receptor activity [17] or DLL4 overexpression [36] demonstrate increased arterial lumen diameter which may predispose to rupture.
While the exact mechanism of Notch-induced lumen enlargement remains unclear, several mechanisms can be considered. Diminished endothelial cell migration may leave parent vessels with greater numbers of endothelial cells, thereby producing enlargement [36]. Alternatively, alterations in the balance of the endothelial cell population between arterial and venous fate may lead to direct arteriovenous connection with subsequent lumen enlargement [37]. In a mouse model, gain-of-function Notch mutations as well as loss of EphrinB2 or EphB4 suggested that reciprocal changes in size of arteries and veins occur secondary to distribution shifts in ECs, without a change in population size [37]. Similar changes were seen in a mouse model of constitutive RAF1/ERK activation that leads, among other things, to increased DLL4 expression. In the mutants, a shift in arterial and venous identities resulted in formation of enlarged arteries and diminished veins [38].
Notch may also contribute to the determination of individual cell size. Intravital regression of AVMs observed after interruption of constitutively active Notch signaling is not associated with a change in cell number [18]. In animals with DLL4 overexpression, as in animals with constitutive Notch activity, arteries are found to be enlarged [32]. When hyperactivation of Notch signaling is reversed, however, enlarged arteries show regression to normal size [18]. Thus, cellular density and cell size must also be determined, in part, by Notch activity.
Insufficient Notch activation, conversely, results in atretic vasculature with poor remodeling from primitive plexi. This leads to formation of AVMs via the persistence of multiple small-vessel anastomoses between arteries and veins [16] as well as increased venous markers at the expense of arterial markers [20, 25–27, 37]. While there may appear to be an excess of vascular quantity in such tissues, such vessels fail to adequately perfuse tissues to support their growth and survival [39, 40].
Taken together, these results speak to pleiotropic functions of the Notch pathway in vascular development: the establishment of EC identity, vascular branching and microvascular investment, EC migration and population determination. So how do Notch abnormalities produce AVMs? AVMs may occur secondary to the elimination of the capillary bed normally interposed between the arterial and venous circulations as a consequence of Notch’s potential for microvascular suppression. They may occur due to arterial enlargement and the subsequent need for vascular outflow, given Notch’s effects on arterial growth and cell migration. Lastly, AVMs may occur due to rerouting of blood flow due to abnormalities in cell identity precipitated by Notch and EphrinB2/EphB4 signaling. To assign AVM to any of these specific malfunctions is not possible at the present. Further work is needed to determine whether these are distinct functions and, if so, to identify the mechanisms by which each function is influenced by Notch.
TGF-β signaling and Hereditary Hemorrhagic Telangiectasia
Transforming growth factor-β (TGF-β) signaling is critical to numerous aspects of development and includes a large family of molecules. In mammals, ligands of this family consist of closely related proteins designated TGF-β1-3, as well as a number of related proteins that include Bone Morphogenetic proteins (BMPs). TGF-β and BMP family members elicit their effects by activating serine/threonine kinase receptor heterotetrameric complexes composed of two type I and two type II receptors. There are seven type I receptors, named activin receptor-like kinase (genes Acvrl1 to Acvrl7, which encode the receptors ALK1–7) and five type II receptors (ACTRIIA, ACTRIIB, BMPRII, TGF-βRII, and AMHRII) [41]. In addition there are a number of accessory receptors including, ENDOGLIN (ENG; CD105) and TGFBR3, which do not transmit signals directly, but influence TGF-β cascade activity by their interaction with TGF-β receptors. Type I and type II receptors display similar structural properties, comprised of a relatively short extracellular domain, a single membrane-spanning domain and an intracellular domain containing a serine-threonine kinase domain. Upon ligand binding, the type II receptors phosphorylate and activate the type I receptors [42].
Classically, TGF-β signaling involves phosphorylation and nuclear translocation of SMAD transcription factors, but there are also SMAD-independent pathways. Activated type I receptors recruit and phosphorylate receptor-regulated SMADs (R-SMADs); SMADs 2 and 3 are specifically activated by TGF-β type I receptors (ALK4, ALK5, ALK7), whereas SMADs 1, 5, and 8 are activated by BMP type I receptors (ALK1, ALK2, ALK3, ALK6) [43–47]. R-SMADs then form complexes with SMAD4 (Co-SMAD) and translocate to the nucleus, where they regulate the transcription of target genes [48]. SMAD6 and SMAD7, the inhibitory SMADs (I-SMADs) inhibit signaling downstream of TGF-β type I receptors, thereby acting as negative regulators of signaling mediated by TGF-β [49, 50]. SMAD6 is known to be more specific for BMP type I receptor-mediated signaling, while SMAD7 is able to block signaling mediated by multiple TGF-β type I receptors, including type I receptors for BMP and TGF-β/ACTIVIN [51]. In addition, I-SMADs can compete with SMAD4 for association with R-SMADs, resulting in an inactive complex [52].
Transforming growth factor-β family members can also elicit SMAD-independent signaling that leads to activation of MAP kinases (ERK, P38, JNK), PI3K/AKT, TAK1 and RHO GTPases [52, 53]. The effects of these pathways in the vasculature are broad and unclear, complicated in no small part by the numerous molecules that participate in signal transduction with pleiotropic and antagonistic effects [54].
Inadequate TGF-β signaling, and BMP signaling via its known dependence on TGF-β-pathway molecules, has been associated with vascular pathologies that can lead to the formation of AVMs. Hereditary hemorrhagic telangiectasia (HHT, also known as Osler-Weber-Rendu disease) is an autosomal-dominant disorder characterized by the age-dependent development of focal AVMs, capillary telangiectasia and vascular dysplasia [7, 55, 56]. HHT affects one in five thousand people [56] and is diagnosed by the presence of three out of four possible traits: an affected kindred, recurrent nosebleeds, multiple mucocutaneous telangiectasias, or AVMs on major organs [57]. At least four forms of HHT have been described [55, 56, 58]. HHT1 and HHT2 develop in individuals who have defects in genes encoding components of the TGF-β signaling pathway: ENG [59], and ACVRL1, respectively [60]. The loci of genes abnormal in HHT3 [61] and HHT4 have been identified, but remain uncharacterized [58]. An additional disease, Juvenile Polyposis with HHT (JP–HHT) has been characterized as a result of mutation in SMAD4 [62, 63].
Animal models of Acvrl1 and Eng mutations have shown that abnormal TGF-β signaling is sufficient to induce certain features of HHT, namely vascular fragility, hemorrhage and the production of age-dependent AVMs [64, 65]. Endothelial cell-specific deletion of Smad4 has not been found to induce AVM, but it does produce blood vessel fragility, increased endothelial cell proliferation and defective pericyte recruitment [66].
Activin-like receptor kinase 1 (Acvrl1; ALK1)
Expression of ALK1 in mice is limited to the arterial endothelium in the settings of vascular development and remodeling but is induced during angiogenesis, following inflammation or wound healing [67–69]. ALK1 signaling is initiated by TGF-β [70] and more potently by BMP9 and BMP10 [71, 72]. With the latter two, it acts to inhibit VEGF expression and its angiogenic sequelae [71–73]. Genetic mouse models have shown that Acvrl1 deficiency results in early embryonic lethality at E11.5 as a result of defects in the remodeling of the primary capillary plexus into a functional network of arteries, capillaries, and veins [54, 74]. Prominent shunts exist between the dorsal aorta and posterior cardinal vein. Further vascular defects include poor VSMC recruitment, diminished arterial EPHRINB2 expression and the appearance of hemogenic venous endothelium [74]. Interestingly, AVMs following loss of Acvrl1 more closely mirror those found in Notch gain-of-function than Notch loss-of-function [17].
When Acvrl1 deletion is limited to the endothelium in an Acvrl1-Cre, Acvrl1 fl/fl mouse lethality was delayed to E18.5 [36]. This is likely due, at least in part, to the delayed complete endothelial expression of this Cre as well as the absence of its endocardial expression. Notwithstanding, these animals still display AVM characteristics with poorly developed and disordered vascular smooth muscle within a grossly tortuous arterial system [36]. Endothelial-specific deletions of Acvrl1 in postnatal and adult mice prove lethal at 5 and 9–21 days, respectively, secondary to diffuse AVMs and hemorrhage [75].
Acvrl1 heterozygous mice survive into adulthood, but greater than 40 % of these animals developed AVMs within 1 year of age. AVMs and shunt-associated tissue necrosis in these animals can result in high-output cardiac failure [65]. Arguably, this genotype best mirrors the natural history of HHT2 by demonstrating multi-organ AVMs with an age-dependent incidence in the manner of other autosomal dominant diseases.
Interestingly, Sm22α-directed knockout of Acvrl1, which preferentially deleted Acvrl1 in VSMCs, did not produce embryonic lethality. It did, however, induce AVMs in the central nervous system that were lethal in a fraction of each litter, between 2 and 15 weeks of age, due to inconsistent Cre activation [76]. This model suggests that VSMCs may also be implicated in AVM pathogenesis, but further work is required to better evaluate the contribution of VSMCs to AVM.
When defects in ALK1 signaling are combined with stress, such as VEGF stimulation or wound healing, vasodilation or injury and inflammation, blood vessels take on dysplastic traits that can result in AVMs [75]. Remarkably, the use of an anti-VEGF agent was shown to reduce vascular dysplasia in an adult Acvrl1 knockout model [77]. It appears that dysplastic traits brought on by vascular remodeling stimuli in Acvrl1 heterozygotes represent a precursor lesion to the development of large AVMs that are observed in the setting of disordered ALK1 signaling, such as in HHT2 [75, 78, 79].
Recent studies have attempted to elucidate the downstream cellular effects mediated by ALK1 signaling, but show conflicting results. One model states that ALK1 and ALK5 may balance the effects of TGF-β family ligands in response to different ligand concentrations, with ALK1 promoting the initiation of angiogenesis, and ALK5 promoting its resolution [80, 81]. However, it has also been reported that ALK1 and ALK5 have mutually exclusive expression patterns in blood vessels, which suggests that each type I receptor has a physically isolated function during vascular development [67]. Importantly, endothelial Tgfbr1 (encoding ALK5) and Tgfbr2 deletions in mice and zebrafish ECs do not phenocopy Acvrl1 deletions [36]. Because ALK5 is reportedly required for ALK1 signaling via TGF-β, there is limited support for a balance model in which the ALK5 kinase activity is required for appropriate ALK1 activation as it pertains to AVM [36].
Other studies show that ALK1 signaling can promote endothelial quiescence in response to BMP9 stimulation. Reports indicate that BMP9 and BMP10 prevent EC migration in an ALK1-dependent manner [71]. Furthermore, zebrafish studies in which acvrl1 has been mutated show that loss of receptor function leads to increased numbers of ECs in cranial vessels, suggesting that acvrl1 may normally act to prevent EC proliferation [69]. Studies in mice, using LacZ reporter genes, also show that ALK5 is more restricted to the vessel media, particularly VSMCs, while ALK1 is expressed in the endothelium [36]. Therefore, it appears that ALK1 may act as a suppressor of endothelial cell migration and possibly proliferation, maintaining a quiescent endothelial state. Further recent in vitro studies have supported this interpretation [72, 82, 83]. ALK1 may also regulate the behavior of ECs and participate in the prevention of AVM through its regulation of gene expression. For example, in cultured ECs and zebrafish, BMP9/ALK1 reportedly upregulates expression of the potent vasoconstrictor Endothelin-1 [68, 84] and downregulates expression of Cxcr4 and Apelin [83, 85], which are involved in migration and proliferation of ECs. These may contribute to the phenotypic changes, such as vessel enlargement, observed in conditions of reduced ALK1 signaling. Therefore, it is possible that gene dysregulation, due to loss of ALK1 signaling in HHT2 patients, is also involved in AVM pathogenesis.
Additionally, AVMs associated with ALK1 signaling deficiency may also arise as a consequence of blood vessel enlargement. For example, zebrafish with acvrl1 deficiency have increased cranial blood vessel diameter, which may allow the persistence of normally transient arteriovenous connections, resulting in AVMs [69]. It has been suggested that alterations of blood flow in the setting of such persistent vasculature dilation might contribute to AVM [68], but given the strength of blood flow in animals at this stage, as well as the frequency of low-shear stress pulmonary AVMs in HHT patients, it is unclear if AVM might be assigned to abnormal flow conditions.
ALK1 may also exert its effects on angiogenesis in cooperation with Notch signaling [83]. Inhibition of ALK1 and Notch signaling in retinas resulted in increased endothelial tip cell proliferation and excessive angiogenesis; a finding consistent with the previously-noted importance of BMP9 and BMP10 signaling in retinal development [86]. Combined Notch and ALK1 inhibition produced more profound vascular abnormality than blockade of these pathways individually. These defects were rescued by BMP9 in a SMAD-dependent manner. Furthermore, ALK1 signaling resulted in upregulation of several factors involved in Notch signaling, such as Hey1, Hey2, and Jag1. These findings indicate that Notch activity in the setting of mutant Acvrl1, such as in HHT2, may contribute to AVM directly through pathway crosstalk [83].
Endoglin
Endoglin (ENG) is the gene that encodes the transmembrane glycoprotein and accessory receptor for TGF-β ligands which is mutated in HHT1 [59]. While HHT1 is similar to HHT2, there is a particular enrichment of cerebral and pulmonary AVMs in HHT1 patients [7, 87]. Developmentally, ENG is expressed throughout the endothelium whereas ALK1 is limited to the arterial circulation [67]. It appears sequentially in the vasculature, beginning with major vessels and progressing into distal vessels. Adult expression of ENG is mostly restricted to the quiescent arterial endothelium, but can be re-expressed under conditions of endothelial stress [88, 89]. Notably, ENG has a relatively higher expression in the primitive neurovasculature [90].
Homozygous global deletion of Eng results in embryonic lethality at E10.5 [64, 91]. Animals appear normal until E9.5, but by E10.5 there is a drastic reduction in body size relative to wild-type mice. There are no defects in vascular differentiation, but arterial and venous endothelia display abnormalities in their respective cell identities before the onset of blood flow. These mice also demonstrate defective vascular remodeling, wherein blood vessels fail to remodel from a primitive plexus to a segmental and branched vasculature. Additionally, there is visible hemorrhage within the embryo. As in Acvrl1 mutants, Eng mutants displayed smooth muscle cells were differentiated, but they were underdeveloped and disorganized [64, 91].
Neonatal Eng knockout also results in AVM formation, increased endothelial cell proliferation, decreased migration and poor vascular remodeling [92]. AVMs in these animals express venous markers EPHB4 and Apelin receptor (APLNR), but not arterial markers JAG1 or EPHRINB2. Interestingly, these changes occurred without alteration in levels of activated SMAD2 or SMAD1/5/8 [92]. This indicates that SMAD-independent pathways downstream of ENG may also play a role in the defects associated with ENG dysfunction, and HHT1 pathogenesis.
Eng heterozygous mice developed normally, but older animals displayed clinical signs of HHT, such as nosebleeds and telangiectasia [64, 93]. These animals develop AVMs of the brain as early as 25 weeks of age [94, 95]. Such vascular morphology is correlated with a poorly organized and underdeveloped smooth muscle layer, a 70 % reduction in smooth muscle coverage and an increase in mast cell-driven inflammation [95].
Like ALK1, ENG is upregulated in the setting of vascular injury or repair [89]. Expression of ENG is also upregulated in states of hypoxia in mouse brain endothelium where its expression is driven by ERK, P38 and JNK signaling [96]. In endothelial cells that are not remodeling, however, ENG is weakly expressed or altogether undetectable [88]. VEGF is capable of inducing capillary dysplasia in Eng heterozygotes in a similar way, but more intensely, than in Acvrl1 heterozygotes [79]. Dysplasia was increased by 33 % in Eng heterozygotes [79], but when normalized to efficacy of transduction, a later study reported an 800 % increase in dysplasia [97]. This greater sensitivity may explain the increase in frequency of manifestations of HHT1 over HHT2, but its biological origin remains unclear [7, 79].
In sporadic (HHT-unrelated) AVMs, ENG protein is observed at levels comparable to those in non-diseased endothelium [98]. ENG levels in AVMs of HHT1 patients, given the heterozygosity of these individuals at the ENG locus, and decreased by half from levels observed in tissues of non-diseased patients. [99–101]. Importantly, however, these levels are consistent throughout the endothelium, which suggests that there is not a complete absence of ENG within AVMs of HHT1 [101], but there may exist foci of “second hits” in genes with parallel function that potentiate AVM formation at an individual site [98].
Other TGF-β family members
Smad 4
SMAD4 mutation leads to the syndrome of juvenile polyposis: a portion of patients with this syndrome also present with the signs of HHT, producing a compound disease state (JP–HHT) [62, 63]. The full extent of clinical variability and frequency of features is not well understood, as clinical information on SMAD4 mutation carriers is relatively limited (SMAD4 mutations represent 2–3 % of cases of HHT). Although it had been suspected that variability in the presentation of JP–HHT correlated with mutation in exons 8, 9 or 11 of SMAD4, it now appears that causative mutations may occur in any exon of the gene [102]. Published studies show that a significant proportion of JP–HHT patients develop AVMs of the lung and liver, capillary telangiectases of the brain, and oral telangiectases [62].
The pathogenesis of JP–HHT might be due to different effects of lowered levels of SMAD4 within endothelial cells, leading to decreased TGF-β/BMP signaling, which may produce vascular dysplasia. Deletion of Smad4 in mouse cerebrovascular ECs demonstrated impaired interaction with pericytes and increased EC proliferation. Interestingly, Smad4 appears to stabilize cerebrovascular EC-pericyte interactions by regulating the transcription of N-cadherin through its association with Notch transcriptional machinery at the N-cadherin promoter [66]. Despite its modeling of a human mutation and evidence for cooperation between TGF-β and Notch signaling, this animal notably lacked AVM and resemblance Alk1/Eng mutants.
Matrix GLA protein
The knockout of the BMP antagonist Matrix Gla protein (Mgp) has also been shown to induce AVMs in lungs and kidneys. MGP is a small, gamma-carboxylated protein that binds and inhibits BMP2 and BMP4. Mgp knockout produced elevated BMP activity, elevated Alk1/2/5 expression, SMAD1/5/8 activation in lung epithelial cells and stimulated their VEGF production, with similar effects in the renal mesangium [103]. In endothelial cells, MGP natively increases VEGF expression through TGF-β, associates with ALK1 and provides context-dependent stimulation and inhibition of ALK1 expression and signaling [104–106].
At this time, the mechanism by which Mgp mutations induce AVM remains unclear. Simply put, MGP appears to have context-dependent effects on TGF-β signaling. These context-dependent effects directly alter native ALK1/5 signaling, which appear to serve as an entry point for AVM pathogenesis. Further investigation into the function of MGP in AVM and its potential to modulate TGF-β signaling will be important and useful, as MGP represents another link in a pathogenic signaling pathway that already includes receptors (i.e., ALK1, ENG) and a transcription factor (SMAD4).
Insights from TGF-β-driven models
These models leave us with several questions about the vascular functions of ALK1, ENG and TGF-β signaling in addition to some insight. Although it appears that ALK1 and ENG share some transduction pathways, the observed differences between mutants for these receptors are not clearly understood, both in animal models and in patients with HHT. In humans, heterozygosity for ENG results in more pulmonary and cerebral AVMs, while heterozygosity for ACVRL1 results in more hepatic AVMs [7, 94]. Furthermore, mouse models demonstrate that Eng mutants do not display the degree of dilatation observed in Acvrl1 mutants, show vascular shunts later than Acvrl1 mutants, and do not display the downregulation of arterial EPHRINB2 [107]. It is unclear whether these manifestations carry across species or if there is an organ-specific variation in the expression of these proteins that manifests in differential AVM appearance. There is also little insight into how these differences are contingent on signaling, and this area requires further investigation. To some extent, differences in signaling may be attributed to the known interaction of ALK1 signaling with the Notch pathway [83, 86], that is apparently absent for ENG signaling, given that Notch is known to have effects on dilatation and the expression of EphrinB2 (discussed above).
It is important to note that Notch gain-of-function, rather than loss-of-function studies, more closely mirror TGF-β loss-of-function mutants [17]. This may indicate that AVMs formed following disruption of ALK1/ENG and Notch signaling occur through different mechanisms. Several studies have described interplay between the Notch and SMAD-dependent cascades, but many questions remain as to the specific roles of such crosstalk in AVM. For instance, Notch intracellular domain has been shown to interact with the SMAD transcriptional complex [108]. Studies by different groups have also shown reduced expression of Notch downstream targets (Hey1, Hey2, Hes1) following inhibition of SMAD signaling [83, 109, 110]. Furthermore, Notch has been shown to partially compensate for loss of ALK1 signaling in restoring endothelial gene expression of arterial markers such as EPHRINB2, and in preventing excessive sprouting [83, 109]. Lastly, a mouse model demonstrated reduced Notch signaling in Acvrl1 knockout embryos [111]. Further studies will be needed to understand the consequences of disrupted ALK1 signaling on Notch activation, the effects of inadequate Notch signaling on ALK1 signaling, and the counterpart alterations with changes in ENG signaling.
One may also speculate that the differences in ALK1- and ENG-driven AVMs can be accounted for by differences in sensitivity to VEGF as a dysplasia-inducing agent. The features of vascular dysplasia—increased cellularity, dilatation and tortuosity—appear to be a precursor lesion of AVM, as mutants manifest these features secondary to vascular insult or comparable stimuli. VEGF signaling has many downstream effectors, some of which are implicated in TGF-β-induced SMAD-independent signaling. Perhaps there exists crosstalk between these VEGF-dependent pathways and the SMAD-independent downstream signals of ALK1 and ENG. This is likely given that abnormalities in neonatal Eng knockout mice appeared to have no change in SMAD activation patterns [92]. More work is required in this area to clarify the contextually-relevant downstream effectors of these receptors, as well as to identify the physiologic ligand(s) whose signaling is disturbed in TGF-β pathway-driven AVMs.
The finding that TGF-β receptor mutants are susceptible to dysplasia with VEGF stimulation is notable as these experiments likely represent an expedited recapitulation of the mouse models of heterozygosity for Acvrl1 and Eng and, perhaps, HHT patients. Dysplasia seems to serve as an early manifestation of the full AVM disease state. On a background of defective TGF-β signaling (namely receptor heterozygosity), endothelial stress that stimulates VEGF signaling produces vascular dysplasia [112]. Homozygous mutations of Acvrl1 and Eng result in AVMs in the setting of endothelial stimulation: such stimulation may take the form of VEGF or comparable signaling, as occurs during development, injury or inflammation. If both ALK1 and ENG are physiologically upregulated in times of such stress, and the aforementioned dysplasia occurs because of stress in individuals carrying mutations for these genes, then we can infer that the role of TGF-β signaling, in the simplest terms, is to stabilize vessels as they endure angiogenic remodeling.
These findings can be extrapolated to speculate on the origin of AVMs in HHT patients. The fact that AVMs are rare in infants has caused some to contend that they cannot be developmental anomalies [112]. Data from these models would argue against this notion. One may consider the growth of the body as a robust angiogenic stimulus. Such a stimulus, in the setting of TGF-β pathway mutations, may be sufficient to produce an AVM. While such an AVM might develop postnatally, it would still have formed secondary to vascular development. This explanation for AVM reconciles the notions that AVMs form either as consequences of development [23] or as a response-to-injury [113]; the latter being a common explanation for the age-dependent incidence of AVM.
Given these facts, what is particular about HHT biology that results in frequent AVMs? This propensity may be explained by the accumulation of a “second hit” due to an environmental insult [75]; an idea which may be extended to the development of a genetic mutation at the locus of the HHT mutation or partner signaling molecule. Notably, animals heterozygous for Acvrl1 or Eng only demonstrated stress-induced vascular dysplasia or age-dependent incidence of AVMs. Homozygous-null mutants, however, displayed AVMs soon after endothelial stress. If this is true, then it may also be the case that humans genotypically normal for ALK1 and ENG accumulate “first hits” at these loci, but these hits are silent. Only when two hits are accumulated at a particular locus, or loci with parallel function, might an AVM manifest. Examples of such parallel loci are numerous, and might include Mgp, Smad4, Smad1/5/8 or any of the TGF-β type II receptors. For example, mutations in the human BMP9 gene have been found to produce a disorder in the spectrum of HHT [114].
Soluble factors apart from VEGF may also contribute to vascular dysplasia. The soluble ENG receptor (sENG), a protein observed at high levels in women with preeclampsia [115], was also observed at high levels in human-derived AVMs [116]. When expressed in mouse brain vasculature, sENG also produced vascular dysplasia with VEGF coadministration [116]. Similar results have been achieved with the synthetic protein soluble ALK1 (sALK1) [83]. Interruption of signaling upstream of membrane-bound receptors suggests that the phenotypes observed are consequences of ligand-binding of ENG and ALK1, rather than an effect at the cell surface such as co-receptor interaction. Additionally, TNF-α has been shown to cause downregulation of ENG levels, reducing surface expression by 50 % [117]. This functional ENG knockdown may directly lead to vascular dysplasia in the absence of this protein’s native function [92], but also in the presence of the associated inflammatory milieu. Alternatively, local induction of VEGF signaling [118–120] in the setting of injury may produce AVMs after TNF-α-associated decrease of endoglin.
RASA1
Capillary malformation-arteriovenous malformation (CM–AVM) is a newly-recognized familial disease wherein patients have cutaneous capillary malformations and large organ AVMs [121]. AVMs in CM–AVM can reside in any tissue and produce similar end-organ effects, such as epilepsy, vein of Galen malformation, various tumors and high-output heart failure [121–123].
This phenotype is defined by heterozygous loss-of-function mutations in the RASA1 gene, which encodes p120-RasGTPase activating protein (RasGAP) [121]. CM–AVM is caused by polyallelic mutations in RASA1, with greater than forty recognized mutations. One-third of individuals with RASA1 mutations display AVMs [123]. The native function of this protein is to negatively regulate cellular growth and proliferation downstream of receptor tyrosine kinases by accelerating hydrolysis of guanosine triphosphate associated with RAS [124, 125]. Deactivating mutations of GAPs can augment signaling of the Ras oncogene, which is evidenced by mutations of such proteins in basal cell carcinoma and neurofibromatosis [121]. RASA1 mutations also link the pathogenesis of AVMs to ERK signaling, as RAS is upstream of ERK and diminished RAS deactivation engenders increased ERK activity.
Mice mosaic for RASA1 deletion show abnormal angiogenesis in conjunction with limited ability to remodel their primitive vasculature [126]. Alterations observed in these vessels could be due to interactions of p120-RasGAP with p190-RhoGAP, which has effects on cytoskeletal remodeling and cell motility [127].
Inflammatory markers
Inflammatory signaling is developing importance in the AVM literature. Animal models and study of primary human samples reveal involvement of inflammatory cells and cytokines, especially TNF-α and IL-1β [76, 95, 128–130]. This correlates with increased tissue markers of inflammation, such as matrix metalloproteases and reactive oxygen species [116, 131]. Inflammation and manipulation of AVMs by treatment also contribute to proliferation, abnormal cellular morphology and alterations in cell adhesion molecules [118–120, 132–136].
Defects in endothelial signaling and smooth muscle development
The manner by which defective endothelial TGF-β signaling leads to disordered development of smooth muscle cells, in spite of their adequate differentiation, remains to be established. It has been previously determined that endothelial cells signal to smooth muscle cells via paracrine signaling in a SMAD2-dependent pathway, but it has not been explained how ALK1 and ENG are involved in the establishment of these smooth muscle architecture [137].
One possible explanation for the smooth muscle effects of Acvrl1 and Eng knockout in endothelium is that these knockouts disrupt endothelial-mesenchymal transition (Endo–MT); a process that has been implicated in both cardiovascular development and disease [138]. In Endo–MT endothelial cells are converted to mesenchymal cells including vascular smooth muscle cells and fibroblasts. The trigger seems to be sustained activation of endothelial TGF-β signaling, which is natively suppressed by fibroblast growth factor (FGF) signals. In the setting of inflammatory cascades such as those involved in TNF-α, IL-1β, and IFNγ, FGF signaling is suppressed and TGF-β is allowed to induce Endo–MT [139]. Notch has also been shown to independently induce Endo–MT [140]. Furthermore, crosstalk between Notch and TGF-β has been implicated in Endo–MT and the development of the vascular smooth muscle cell phenotype [141–144].
A recent model developed for another form of cerebrovascular disease, cerebral cavernous malformation (CCM), demonstrates the importance of Endo–MT in its pathogenesis as well as the reliance of this process on TGF-β/BMP and Notch signaling. A knockout of proteins known to cause CCM induced dysplastic changes to vascular structure, expression of mesenchymal markers in the endothelium, increased sensitivity to BMP6 and suppression of Notch signaling [145]. Significant alterations in the implicated pathways suggest that there might be parallels in the pathogeneses of CCM and AVM. Certain features of AVM, such as increased neointima and perivascular fibrosis may well be direct consequences of Endo–MT [139]. Endo–MT is an evolving subject of investigation. Studies to determine the extent to which this process contributes to AVM would certainly be of interest.
Intravital signaling as a therapeutic target
As the adult knockout models described above suggest, there appears to be a sustained requirement for appropriate Notch and TGF-β signaling in the vasculature. This implies that perturbations in these signals potentiate the formation of AVMs. In animal models, such perturbations are achieved by genetic manipulation. In humans, they occur by mutation or dysregulation of cell signals. Because the vasculature remodels throughout life, it is placed at risk for such mutations in adulthood and not only during development. Remodeling of the adult vasculature, for instance, takes part in the formation of AVMs or AV fistulae at surgical sites and within large wounds.
Promising observations pertaining to treatment of disordered signaling have been made in both Notch and TGF-β animal models. Interruption of hyperactive Notch signaling in AVMs, by targeting either the Notch receptors or ligands, might be effective to restore vascular identity, improve tissue blood supply, and possibly induce lesion regression [18]. Also, anti-VEGF agents cause regression in AVMs of disordered TGF-β signaling both in animal models and [77, 112], at least initially, in studies of patients with HHT [146]. If intravital regression is possible by disrupting pathologic cell signals, then targeted medical therapy might prove sufficient to treat AVMs. Such therapy may one day complement, or replace, current open surgical, endovascular and radiation therapies.
Summary
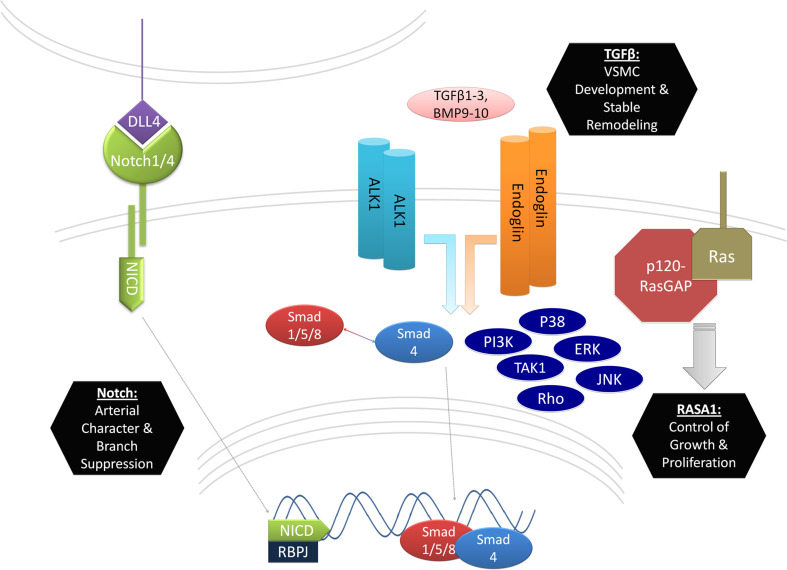
Abnormalities in endothelial signaling contribute to the development of arteriovenous malformations. The Notch and TGF-β pathways appear to be particularly central to the development of these lesions. Animal models have enlightened our understandings of the physiological roles of these pathways and how they might contribute to AVM. Abnormal activation or inactivation of receptors NOTCH1 and NOTCH4, as well as their ligand DLL4, implicate these molecules in the regulation of endothelial cell identity and branching morphogenesis (Fig. 1, left). TGF-β pathway elements are similarly implicated, but by a different mechanism. These genes appear to be critical to the regulation of vascular smooth muscle development and remodeling, especially in response to angiogenic stressors (Fig. 1, center). New data is also emerging on endothelial functions of other signaling cascades such as those involving p120RasGAP (Fig. 1, right) and inflammatory cytokines. It is possible that AVM represents abnormal signaling in these pathways separately, but also a disruption in the interdependence of these pathways. Considerable work remains to characterize endothelial functions of these pathways and to perhaps realize this investigation in the form of medical therapy.
Fig. 1.
Summary of major endothelial signaling pathways implicated in AVM. AVM signaling in the endothelium is characterized by abnormalities in Notch and TGF-β signaling with growing contributions from other findings that include abnormalities in Ras signaling. Notch signaling defects in AVMs include those from Notch ligand DLL4 and its receptors NOTCH1 and NOTCH4. Notch receptor intracellular domains (NICD) are cleaved upon ligand binding and translocated to the nucleus where they become transcriptionally active with binding partner RBP-J. Functions of this receptor-ligand combination that are pertinent to AVM formation include the determination of arterial character and the suppression of small vessel branching. TGF-β mutants for ALK1, ENG, and SMAD4 correlate with the incidence in AVMs in humans. Mutants for the ALK1 and ENG also produce AVMs in animal models. These TGF-β receptors produce transcriptional activity of SMAD1/5/8 in association with SMAD4 and the activation of SMAD-independent pathways (PI3K, TAK1, RHO, P38, ERK and JNK). Mutants for these genes have abnormal development of VSMCs and marked abnormalities in vascular remodeling. Mutations in p120RasGAP have also been shown to induce AVMs, presumably by failing to limit endothelial cell growth and proliferation
Acknowledgments
We would like to thank Yong Deng (Yale University) for discussion. This review was supported in part by NIH Grants 1RO1HL111504-01 (A.E.), HL084619 (M.S.), the Edward N. and Della L Thome Memorial Foundation (A.E.), INSERM (B.L., A.E.), the Howard Hughes Medical Institute Medical Research Fellowship (D.A.) and the Leducq Foundation Transatlantic Network Grant (B.L., A.E., M.S.).
References
- 1.Laakso A, Hernesniemi J. Arteriovenous malformations: epidemiology and clinical presentation. Neurosurg Clin N Am. 2012;23(1):1–6. doi: 10.1016/j.nec.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, Ko NU, Achrol AS, Lawton MT, Higashida RT, Young WL. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38(9):2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr, Kondziolka D, Rosenwasser R, Young WL, Hademenos G. AHA scientific statement: recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32(6):1458–1471. doi: 10.1161/01.str.32.6.1458. [DOI] [PubMed] [Google Scholar]
- 4.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359(9309):863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 5.Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, Faughnan ME. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. Am J Neuroradiol. 2000;21(6):1016–1020. [PMC free article] [PubMed] [Google Scholar]
- 6.Willemse RB, Mager JJ, Westermann CJ, Overtoom TT, Mauser H, Wolbers JG. Bleeding risk of cerebrovascular malformations in hereditary hemorrhagic telangiectasia. J Neurosurg. 2000;92(5):779–784. doi: 10.3171/jns.2000.92.5.0779. [DOI] [PubMed] [Google Scholar]
- 7.Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, Westermann CJ. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43(4):371–377. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18(8):901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokubo H, Miyagawa-Tomita S, Johnson RL. Hesr, a mediator of the Notch signaling, functions in heart and vessel development. Trends Cardiovasc Med. 2005;15(5):190–194. doi: 10.1016/j.tcm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 12.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16(3):235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 13.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14(11):1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Investig. 2009;89(9):971–982. doi: 10.1038/labinvest.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, Chen G, Chen Y, Lawton MT, Young WL, Greenberg DA, Jin K. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132(Pt 12):3231–3241. doi: 10.1093/brain/awp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs LT, Starling C, Chervonsky AV, Gridley T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis. 2010;48(3):146–150. doi: 10.1002/dvg.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, Wang R. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci USA. 2005;102(28):9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy PA, Kim TN, Lu G, Bollen AW, Schaffer CB, Wang RA (2012) Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci Transl Med 4(117):117ra118. doi:10.1126/scitranslmed.3002670 [DOI] [PMC free article] [PubMed]
- 19.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8(6):707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 20.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128(19):3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 21.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122(7):2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- 22.Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA. 2001;98(10):5643–5648. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, Carlson TR, Wang RA. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci USA. 2008;105(31):10901–10906. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704–2712. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 25.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18(20):2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101(45):15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18(20):2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 29.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104(9):3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedito R, Trindade A, Hirashima M, Henrique D, da Costa LL, Rossant J, Gill PS, Duarte A. Loss of Notch signalling induced by Dll4 causes arterial calibre reduction by increasing endothelial cell response to angiogenic stimuli. BMC Dev Biol. 2008;8:117. doi: 10.1186/1471-213X-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trindade A, Kumar SR, Scehnet JS, Lopes-da-Costa L, Becker J, Jiang W, Liu R, Gill PS, Duarte A. Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood. 2008;112(5):1720–1729. doi: 10.1182/blood-2007-09-112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16(2):222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128(17):3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- 35.Carlson TR, Hu H, Braren R, Kim YH, Wang RA. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development. 2008;135(12):2193–2202. doi: 10.1242/dev.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111(2):633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development. 2008;135(22):3755–3764. doi: 10.1242/dev.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y, Larrivee B, Zhuang ZW, Atri D, Moraes F, Prahst C, Eichmann A, Simons M. Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood. 2013;121(19):3988–3996. doi: 10.1182/blood-2012-12-474601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirziu D, Jaba IM, Yu P, Larrivee B, Coon BG, Cristofaro B, Zhuang ZW, Lanahan AA, Schwartz MA, Eichmann A, Simons M. Endothelial nuclear factor-kappaB-dependent regulation of arteriogenesis and branching. Circulation. 2012;126(22):2589–2600. doi: 10.1161/CIRCULATIONAHA.112.119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cristofaro B, Shi Y, Faria M, Suchting S, Leroyer AS, Trindade A, Duarte A, Zovein AC, Iruela-Arispe ML, Nih LR, Kubis N, Henrion D, Loufrani L, Todiras M, Schleifenbaum J, Gollasch M, Zhuang ZW, Simons M, Eichmann A, le Noble F. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development. 2013;140(8):1720–1729. doi: 10.1242/dev.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20(3):203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75(4):671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 43.Kretzschmar M, Liu F, Hata A, Doody J, Massague J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11(8):984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 44.Nakao A, Roijer E, Imamura T, Souchelnytskyi S, Stenman G, Heldin CH, ten Dijke P. Identification of Smad2, a human Mad-related protein in the transforming growth factor beta signaling pathway. J Biol Chem. 1997;272(5):2896–2900. doi: 10.1074/jbc.272.5.2896. [DOI] [PubMed] [Google Scholar]
- 45.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393(6687):786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238(2):574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- 47.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118(Pt 16):3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 48.Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383(6603):832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 49.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389(6651):622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 50.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389(6651):631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 51.Yan X, Liu Z, Chen Y. Regulation of TGF-beta signaling by Smad7. Acta Biochim Biophys Sin (Shanghai) 2009;41(4):263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20(9):556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19(1):71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97(6):2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006;43(2):97–110. doi: 10.1136/jmg.2005.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 2010;24(6):203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000;91(1):66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 58.Bayrak-Toydemir P, McDonald J, Akarsu N, Toydemir RM, Calderon F, Tuncali T, Tang W, Miller F, Mao R. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am J Med Genet Part A. 2006;140(20):2155–2162. doi: 10.1002/ajmg.a.31450. [DOI] [PubMed] [Google Scholar]
- 59.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8(4):345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 60.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13(2):189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 61.Cole SG, Begbie ME, Wallace GM, Shovlin CL. A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J Med Genet. 2005;42(7):577–582. doi: 10.1136/jmg.2004.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4) Lancet. 2004;363(9412):852–859. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- 63.Gallione CJ, Richards JA, Letteboer TG, Rushlow D, Prigoda NL, Leedom TP, Ganguly A, Castells A, Ploos van Amstel JK, Westermann CJ, Pyeritz RE, Marchuk DA. SMAD4 mutations found in unselected HHT patients. J Med Genet. 2006;43(10):793–797. doi: 10.1136/jmg.2006.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Investig. 1999;104(10):1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, Marchuk DA. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12(5):473–482. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 66.Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev cell. 2011;20(3):291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93(7):682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 68.Corti P, Young S, Chen CY, Patrick MJ, Rochon ER, Pekkan K, Roman BL. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development. 2011;138(8):1573–1582. doi: 10.1242/dev.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, Garrity DM, Moon RT, Fishman MC, Lechleider RJ, Weinstein BM. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129(12):3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- 70.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.David L, Mallet C, Vailhe B, Lamouille S, Feige JJ, Bailly S. Activin receptor-like kinase 1 inhibits human microvascular endothelial cell migration: potential roles for JNK and ERK. J Cell Physiol. 2007;213(2):484–489. doi: 10.1002/jcp.21126. [DOI] [PubMed] [Google Scholar]
- 72.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 73.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114(10):2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 75.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, Oh SH, Walter G, Raizada MK, Sorg BS, Oh SP. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Investig. 2009;119(11):3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milton I, Ouyang D, Allen CJ, Yanasak NE, Gossage JR, Alleyne CH, Jr, Seki T. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke. 2012;43(5):1432–1435. doi: 10.1161/STROKEAHA.111.647024. [DOI] [PubMed] [Google Scholar]
- 77.Walker EJ, Su H, Shen F, Degos V, Jun K, Young WL. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43(7):1925–1930. doi: 10.1161/STROKEAHA.111.647982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, Young WL, Yang GY. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295(6):H2250–H2256. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, Young WL. VEGF induces more severe cerebrovascular Dysplasia in Endoglin than in Alk1 mice. Trans Stroke Res. 2010;1(3):197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12(4):817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 81.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13(7):301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 82.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev cell. 2012;22(3):489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park JE, Shao D, Upton PD, Desouza P, Adcock IM, Davies RJ, Morrell NW, Griffiths MJ, Wort SJ. BMP-9 induced endothelial cell tubule formation and inhibition of migration involves Smad1 driven endothelin-1 production. PLoS ONE. 2012;7(1):e30075. doi: 10.1371/journal.pone.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young K, Conley B, Romero D, Tweedie E, O’Neill C, Pinz I, Brogan L, Lindner V, Liaw L, Vary CP. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120(20):4263–4273. doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119(25):6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berg JN, Guttmacher AE, Marchuk DA, Porteous ME. Clinical heterogeneity in hereditary haemorrhagic telangiectasia: are pulmonary arteriovenous malformations more common in families linked to endoglin? J Med Genet. 1996;33(3):256–257. doi: 10.1136/jmg.33.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11(1):79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 89.Ma X, Labinaz M, Goldstein J, Miller H, Keon WJ, Letarte M, O’Brien E. Endoglin is overexpressed after arterial injury and is required for transforming growth factor-beta-induced inhibition of smooth muscle cell migration. Arter Thromb Vasc Biol. 2000;20(12):2546–2552. doi: 10.1161/01.atv.20.12.2546. [DOI] [PubMed] [Google Scholar]
- 90.Jonker L, Arthur HM. Endoglin expression in early development is associated with vasculogenesis and angiogenesis. Mech Dev. 2002;110(1–2):193–196. doi: 10.1016/s0925-4773(01)00562-7. [DOI] [PubMed] [Google Scholar]
- 91.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 92.Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106(8):1425–1433. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- 93.Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, Breant C, Mathivet T, Larrivee B, Thomas JL, Arthur HM, Westermann CJ, Disch F, Mager JJ, Snijder RJ, Eichmann A, Mummery CL. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16(4):420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 94.Satomi J, Mount RJ, Toporsian M, Paterson AD, Wallace MC, Harrison RV, Letarte M. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34(3):783–789. doi: 10.1161/01.STR.0000056170.47815.37. [DOI] [PubMed] [Google Scholar]
- 95.Torsney E, Charlton R, Diamond AG, Burn J, Soames JV, Arthur HM. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation. 2003;107(12):1653–1657. doi: 10.1161/01.CIR.0000058170.92267.00. [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y, Sun Y, Xie L, Jin K, Sheibani N, Greenberg DA. Hypoxic induction of endoglin via mitogen-activated protein kinases in mouse brain microvascular endothelial cells. Stroke. 2003;34(10):2483–2488. doi: 10.1161/01.STR.0000088644.60368.ED. [DOI] [PubMed] [Google Scholar]
- 97.Choi EJ, Walker EJ, Shen F, Oh SP, Arthur HM, Young WL, Su H. Minimal homozygous endothelial deletion of eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33(6):540–547. doi: 10.1159/000337762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsubara S, Bourdeau A, terBrugge KG, Wallace C, Letarte M. Analysis of endoglin expression in normal brain tissue and in cerebral arteriovenous malformations. Stroke. 2000;31(11):2653–2660. doi: 10.1161/01.str.31.11.2653. [DOI] [PubMed] [Google Scholar]
- 99.Cymerman U, Vera S, Pece-Barbara N, Bourdeau A, White RI, Jr, Dunn J, Letarte M. Identification of hereditary hemorrhagic telangiectasia type 1 in newborns by protein expression and mutation analysis of endoglin. Pediatr Res. 2000;47(1):24–35. doi: 10.1203/00006450-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Pece N, Vera S, Cymerman U, White RI, Jr, Wrana JL, Letarte M. Mutant endoglin in hereditary hemorrhagic telangiectasia type 1 is transiently expressed intracellularly and is not a dominant negative. J Clin Investig. 1997;100(10):2568–2579. doi: 10.1172/JCI119800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, Becker L, Letarte M. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156(3):911–923. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gallione C, Aylsworth AS, Beis J, Berk T, Bernhardt B, Clark RD, Clericuzio C, Danesino C, Drautz J, Fahl J, Fan Z, Faughnan ME, Ganguly A, Garvie J, Henderson K, Kini U, Leedom T, Ludman M, Lux A, Maisenbacher M, Mazzucco S, Olivieri C, Ploos van Amstel JK, Prigoda-Lee N, Pyeritz RE, Reardon W, Vandezande K, Waldman JD, White RI, Jr, Williams CA, Marchuk DA. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am J Med Genet Part A. 2010;152A(2):333–339. doi: 10.1002/ajmg.a.33206. [DOI] [PubMed] [Google Scholar]
- 103.Yao Y, Jumabay M, Wang A, Bostrom KI. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Investig. 2011;121(8):2993–3004. doi: 10.1172/JCI57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bostrom K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem. 2004;279(51):52904–52913. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- 105.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281(45):33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 106.Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276(17):14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 107.Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261(1):235–250. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 108.Itoh F, Itoh S, Goumans MJ, Valdimarsdottir G, Iso T, Dotto GP, Hamamori Y, Kedes L, Kato M, ten Dijke Pt P. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J. 2004;23(3):541–551. doi: 10.1038/sj.emboj.7600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim JH, Peacock MR, George SC, Hughes CC. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis. 2012;15(3):497–509. doi: 10.1007/s10456-012-9277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell. 2012;22(3):501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Somekawa S, Imagawa K, Hayashi H, Sakabe M, Ioka T, Sato GE, Inada K, Iwamoto T, Mori T, Uemura S, Nakagawa O, Saito Y. Tmem100, an ALK1 receptor signaling-dependent gene essential for arterial endothelium differentiation and vascular morphogenesis. Proc Natl Acad Sci USA. 2012;109(30):12064–12069. doi: 10.1073/pnas.1207210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69(6):954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92. doi: 10.1007/978-3-7091-0693-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wooderchak-Donahue WL, McDonald J, O’Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fulop GT, Langa C, Morrell NW, Botella LM, Bernabeu C, Stevenson DA, Runo JR, Bayrak-Toydemir P. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013 doi: 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 116.Chen Y, Hao Q, Kim H, Su H, Letarte M, Karumanchi SA, Lawton MT, Barbaro NM, Yang GY, Young WL. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66(1):19–27. doi: 10.1002/ana.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li C, Guo B, Ding S, Rius C, Langa C, Kumar P, Bernabeu C, Kumar S. TNF alpha down-regulates CD105 expression in vascular endothelial cells: a comparative study with TGF beta 1. Anticancer Res. 2003;23(2B):1189–1196. [PubMed] [Google Scholar]
- 118.Koizumi T, Shiraishi T, Hagihara N, Tabuchi K, Hayashi T, Kawano T. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery. 2002;50(1):117–124. doi: 10.1097/00006123-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 119.Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD, Bien S, Bertalanffy H. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg. 2001;94(6):972–977. doi: 10.3171/jns.2001.94.6.0972. [DOI] [PubMed] [Google Scholar]
- 120.Jabbour MN, Elder JB, Samuelson CG, Khashabi S, Hofman FM, Giannotta SL, Liu CY. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. Neurosurgery. 2009;64(1):139–146. doi: 10.1227/01.NEU.0000334417.56742.24. [DOI] [PubMed] [Google Scholar]
- 121.Eerola I, Boon LM, Mulliken JB, Burrows PE, Dompmartin A, Watanabe S, Vanwijck R, Vikkula M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73(6):1240–1249. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boon LM, Mulliken JB, Vikkula M. RASA1: variable phenotype with capillary and arteriovenous malformations. Curr Opin Genet Dev. 2005;15(3):265–269. doi: 10.1016/j.gde.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 123.Revencu N, Boon LM, Mulliken JB, Enjolras O, Cordisco MR, Burrows PE, Clapuyt P, Hammer F, Dubois J, Baselga E, Brancati F, Carder R, Quintal JM, Dallapiccola B, Fischer G, Frieden IJ, Garzon M, Harper J, Johnson-Patel J, Labreze C, Martorell L, Paltiel HJ, Pohl A, Prendiville J, Quere I, Siegel DH, Valente EM, Van Hagen A, Van Hest L, Vaux KK, Vicente A, Weibel L, Chitayat D, Vikkula M. Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008;29(7):959–965. doi: 10.1002/humu.20746. [DOI] [PubMed] [Google Scholar]
- 124.Gibbs JB, Marshall MS, Scolnick EM, Dixon RA, Vogel US. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP) J Biol Chem. 1990;265(33):20437–20442. [PubMed] [Google Scholar]
- 125.Clark GJ, Quilliam LA, Hisaka MM, Der CJ. Differential antagonism of Ras biological activity by catalytic and Src homology domains of Ras GTPase activation protein. Proc Natl Acad Sci USA. 1993;90(11):4887–4891. doi: 10.1073/pnas.90.11.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Henkemeyer M, Rossi DJ, Holmyard DP, Puri MC, Mbamalu G, Harpal K, Shih TS, Jacks T, Pawson T. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377(6551):695–701. doi: 10.1038/377695a0. [DOI] [PubMed] [Google Scholar]
- 127.Kulkarni SV, Gish G, van der Geer P, Henkemeyer M, Pawson T. Role of p120 Ras-GAP in directed cell movement. J Cell Biol. 2000;149(2):457–470. doi: 10.1083/jcb.149.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59(1):72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 129.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang GY, Young WL. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62(6):1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Storer KP, Tu J, Karunanayaka A, Morgan MK, Stoodley MA. Inflammatory molecule expression in cerebral arteriovenous malformations. J Clin Neurosci. 2008;15(2):179–184. doi: 10.1016/j.jocn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 131.Toporsian M, Jerkic M, Zhou YQ, Kabir MG, Yu LX, McIntyre BA, Davis A, Wang YJ, Stewart DJ, Belik J, Husain M, Henkelman M, Letarte M. Spontaneous adult-onset pulmonary arterial hypertension attributable to increased endothelial oxidative stress in a murine model of hereditary hemorrhagic telangiectasia. Arter Thromb Vasc Biol. 2010;30(3):509–517. doi: 10.1161/ATVBAHA.109.200121. [DOI] [PubMed] [Google Scholar]
- 132.Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Investig Dermatol. 1990;95(4):422–427. doi: 10.1111/1523-1747.ep12555569. [DOI] [PubMed] [Google Scholar]
- 133.Sammons V, Davidson A, Tu J, Stoodley MA. Endothelial cells in the context of brain arteriovenous malformations. J Clin Neurosci. 2011;18(2):165–170. doi: 10.1016/j.jocn.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 134.Sato S, Kodama N, Sasaki T, Matsumoto M, Ishikawa T. Perinidal dilated capillary networks in cerebral arteriovenous malformations. Neurosurgery. 2004;54(1):163–168. doi: 10.1227/01.neu.0000097518.57741.be. [DOI] [PubMed] [Google Scholar]
- 135.Sure U, Butz N, Siegel AM, Mennel HD, Bien S, Bertalanffy H. Treatment-induced neoangiogenesis in cerebral arteriovenous malformations. Clin Neurol Neurosurg. 2001;103(1):29–32. doi: 10.1016/s0303-8467(01)00112-3. [DOI] [PubMed] [Google Scholar]
- 136.Tu J, Stoodley MA, Morgan MK, Storer KP. Ultrastructure of perinidal capillaries in cerebral arteriovenous malformations. Neurosurgery. 2006;58(5):961–970. doi: 10.1227/01.NEU.0000210248.39504.B5. [DOI] [PubMed] [Google Scholar]
- 137.Carvalho RL, Jonker L, Goumans MJ, Larsson J, Bouwman P, Karlsson S, Dijke PT, Arthur HM, Mummery CL. Defective paracrine signalling by TGFbeta in yolk sac vasculature of endoglin mutant mice: a paradigm for hereditary haemorrhagic telangiectasia. Development. 2004;131(24):6237–6247. doi: 10.1242/dev.01529. [DOI] [PubMed] [Google Scholar]
- 138.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125(14):1795–1808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2(6):1684–1696. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, Setiadi A, Smrz J, Kyle A, Minchinton A, Marra M, Hoodless PA, Karsan A. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell. 2011;21(2):288–300. doi: 10.1016/j.devcel.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 141.Tang Y, Urs S, Boucher J, Bernaiche T, Venkatesh D, Spicer DB, Vary CP, Liaw L. Notch and transforming growth factor-beta (TGFbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J Biol Chem. 2010;285(23):17556–17563. doi: 10.1074/jbc.M109.076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol. 2008;182(2):315–325. doi: 10.1083/jcb.200710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23(5):1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fu Y, Chang A, Chang L, Niessen K, Eapen S, Setiadi A, Karsan A. Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J Biol Chem. 2009;284(29):19452–19462. doi: 10.1074/jbc.M109.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498(7455):492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 146.Kanellopoulou T, Alexopoulou A (2013) Bevacizumab in the treatment of hereditary hemorrhagic telangiectasia. Expert Opin Biol Ther. doi:10.1517/14712598.2013.813478 [DOI] [PubMed]