Abstract
Background
In contrast to the general population, higher body mass index (BMI) is associated with greater survival in patients receiving hemodialysis (HD; “obesity paradox”). We hypothesized that this paradoxical association between BMI and death may be modified by age and dialysis vintage.
Study Design
Retrospective observational study using a large HD patient cohort.
Setting & Participants
123,383 maintenance HD patients treated in DaVita dialysis clinics between July 1, 2001, and June 30, 2006, with follow-up through September 30, 2009.
Predictors
Age, dialysis vintage, and time-averaged BMI. Time-averaged BMI was divided into 6 subgroups; <18.5, 18.5-<23.0, 23.0-<25.0, 25.0-<30.0, 30.0-<35.0, and ≥35.0 kg/m2. BMI category of 23-<25 kg/m2 was used as the reference category.
Outcomes
All-cause, cardiovascular, and infection-related mortality.
Results
Mean BMI of study participants was 27 ± 7 kg/m2. Time-averaged BMI was <18.5 and ≥35 kg/m2 in 5% and 11% of patients, respectively. With progressively higher time-averaged BMI, there was progressively lower all-cause, cardiovascular, and infection-related mortality in patients younger than 65 years. In those 65 years or older, even though overweight/obese patients had lower mortality compared with underweight/normal-weight patients, sequential increases in time-averaged BMI > 25 kg/m2 added no additional benefit. Based on dialysis vintage, incident HD patients had greater all-cause and cardiovascular survival benefit with a higher time-averaged BMI compared with the longer term HD patients.
Limitations
Causality cannot be determined, and residual confounding cannot be excluded given the observational study design.
Conclusions
Higher BMI is associated with lower death risk across all age and dialysis vintage groups. This benefit is more pronounced in incident HD patients and those younger than 65 years. Given the robustness of the survival advantage of higher BMI, examining interventions to maintain or even increase dry weight in HD patients irrespective of age and vintage are warranted.
Keywords: Obesity-mortality paradox, hemodialysis, BMI, mortality risk, ESRD
A higher body mass index (BMI) is associated with higher all-cause and cardiovascular mortality in the general population. In contrast, several epidemiologic studies in dialysis patients have demonstrated a paradoxically inverse association between obesity and mortality.1-9 A similar reverse epidemiology of obesity in other populations with chronic disease states, including the geriatric population10-12 and patients with heart failure, has been described.13,14 However, differences in mortality between different age groups of dialysis patients based on BMI have not been well studied. Previous studies that have evaluated this question were limited by their relatively smaller sample size and have yielded mixed results.15-17 A more recent study that exclusively investigated this question concluded that younger patients have a U-shaped association between mortality and BMI, whereas there was no demonstrable association between body size and mortality in older patients (aged ≥ 65 years).18
Similarly, the effect of dialysis vintage on the association between BMI and mortality is unknown. Because one of the explanations for the obesity paradox in dialysis patients is the short-term survival benefit conferred by obesity, it would be expected that in patients with a longer dialysis vintage, the lower death risk with larger body size will be attenuated, if not completely reversed. The aforementioned questions have important clinical implications. The NKF-KDOQI (National Kidney Foundation–Kidney Disease Outcomes Quality Initiative) guidelines recommend BMI to be maintained above the 50th percentile for dialysis patients (BMI > 23.6 and 24.0 kg/m2 for men and women, respectively).19 However, it currently is unclear whether the ideal BMI should vary by a patient’s age and dialysis vintage. Therefore, we undertook this study to test the hypothesis that the obesity paradox exists across all age groups, but only in patients with shorter dialysis vintages.
METHODS
Data Source
This observational cohort study uses data from maintenance hemodialysis (HD) patients treated in DaVita facilities between July 1, 2001, and June 30, 2006, linked to that from the USRDS (US Renal Data System) with follow up through September 2009. Data from DaVita were used to determine patient’s age, sex, diabetes, body weight, height, dialysis modality, and dialysis dose. The MEDEVID file from the USRDS contains data from CMS (Centers for Medicare & Medicaid Services) Medical Evidence Form 2728, which is completed for all new patients with end-stage renal disease in the United States and was used to determine the day of dialysis therapy initiation, race/ethnicity, marital status, primary insurance, and comorbid conditions (atherosclerotic heart disease, including ischemic heart disease, myocardial infarction, and cardiac arrest; other cardiac diseases, including pericarditis and cardiac arrhythmia; congestive heart failure; hypertension; cerebrovascular disease; peripheral vascular disease; chronic obstructive pulmonary disease; tobacco smoking; cancer; and HIV [human immunodeficiency virus]). The date and cause of death were obtained from the USRDS.
The initial study cohort consisted of 164,789 patients. Patients were assigned to the dialysis modality they were being treated with at the time of entry into the cohort. The following patients were excluded: patients treated with peritoneal dialysis (PD); those with missing data for dialysis modality; and those who died, underwent kidney transplantation, or were not followed up or recovered their kidney function by day 90 from dialysis therapy initiation (n = 26,433). From the entire HD cohort, patients younger than 18 years also were excluded (n = 286). Furthermore, patients with missing data for BMI (n = 9,040), age (n = 9), and dialysis vintage (n = 5,638) were excluded. Thus, the final cohort consisted of 123,383 HD patients. Table S1 (provided as online supplementary material) summarizes the differences in characteristics of HD patients older than 18 years who were included and excluded (those missing data for BMI, age, and dialysis vintage).
Post-HD dry weight for each patient during each calendar quarter was the average of up to 39 measured weight values at the end of each thrice-weekly HD treatment, measured in dialysis facilities using a standardized digital scale (Seca Digital Scale; Seca North America). These data were used to calculate the average BMI (in kg/m2) in each calendar quarter. Time-averaged BMI for each individual patient was defined as the average BMI obtained from up to 33 calendar quarters. Dialysis vintage was defined as the time between the first day of dialysis treatment and the first day that the patient entered the study cohort. The first studied quarter for each patient was the first calendar quarter in which the patient’s vintage was longer than 90 days. Dialysis dose was measured by single-pool Kt/V using urea kinetic modeling equations.
All blood samples were shipped to a single central DaVita laboratory in Deland, FL. Quarterly averages were calculated for each laboratory variable using all measurements made during that 3-month period. Subjects were divided a priori into 6 categories based on time-averaged BMI (<18.5, 18.5-<23.0, 23.0-<25.0, 25.0-<30.0, 30.0-<35.0, and ≥35.0 kg/m2).
Statistical Methods
Complete data were available for sex and diabetes. Missing data were as follows: for comorbid conditions (other than HIV), 4%; HIV status, 49%; race, 1%; insurance status, 8%; marital status and parathyroid hormone level, 19% each; and serum albumin, total iron-binding capacity, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, hemoglobin, white blood cell count, lymphocyte percentage, and normalized protein catabolic rate values, 8%-11% of the cohort. Missing data were imputed using a multiple imputation method. Data for all-cause mortality were available until only 2007.
Data are summarized as mean ± standard deviation, median (interquartile range), and proportion as appropriate. Survival analyses using Cox proportional hazard regression was performed to determine the relationships between time-averaged BMI with all-cause, cardiovascular, and infection-related mortality in: (1) the entire cohort of HD patients, (2) the cohort of HD patients categorized by age (<18, 18-<45, 45-<65, 65-<70, 70-<75, and ≥75 years), and (3) the cohort of HD patients categorized by dialysis vintage (<6 months, 6 months to <2 years, 2-<5 years, and ≥5 years). Time-averaged BMI of 23-<25 kg/m2 was used as the reference category because the NKF-KDOQI guidelines recommend that BMI of maintenance dialysis patients be maintained to at least 23.6 and 24.0 kg/m2 for men and women, respectively.19 For each regression analysis, 3 levels of adjustment were examined: (1) unadjusted model that included only mortality data and time-averaged BMI; (2) case-mix–adjusted model that included variables in the unadjusted model along with sex, race/ethnicity (whites, blacks, Hispanics, and others), dialysis dose, presence of diabetes, comorbid conditions, smoking status, primary insurance status (Medicare, Medicaid, private, and others), and marital status (divorced, married, single, and other); and (3) case-mix–and laboratory data–adjusted model that included serum total iron-binding capacity, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, albumin, bicarbonate, white blood cell count, lymphocyte percentage, hemoglobin, dialysis dose, and normalized protein catabolic rate values as additional covariates. The analyses were carried out using STATA, version 11.2 (StataCorp LP). Sigma plot graphs were used as data analyses strategies to illustrate the relationship between BMI and mortality in different age and dialysis vintage groups. Another set of analyses was carried out to model the longitudinal BMI values and time to event jointly using a joint model approach. This analysis was carried out using STATA, version 12. The study was approved by the Institutional Review Board of Los Angeles Biomedical Research Institute at Harbor–University of California Los Angeles as exempt from informed consent.
RESULTS
Patient Characteristics
Baseline characteristics of the cohort, stratified by different levels of time-averaged BMI, are summarized in Table 1. Although most patients were incident HD patients (55%), there was still a large proportion of patients with dialysis vintage of more than 6 months (45%). Patients with higher BMI were more likely to be younger and have diabetes, were less likely to have undergone dialysis for more than 5 years, and had lower delivered Kt/Vurea and serum ferritin values and higher serum creatinine and parathyroid hormone levels.
Table 1.
Patient Characteristics Stratified by BMI
| Time-Averaged BMI (kg/m2) |
|||||||
|---|---|---|---|---|---|---|---|
| Total | <18.5 | 18.5-<23.0 | 23.0-<25.0 | 25.0-<30.0 | 30.0-<35.0 | ≥35.0 | |
| No. (%) of patients | 123,383 | 6,381 (5) | 32,456 (26) | 18,933 (15) | 35,209 (29) | 17,221 (14) | 13,183 (11) |
| Age (y) | 62 ± 15 | 65 ± 17 | 63 ± 17 | 63 ± 16 | 62 ± 14 | 60 ± 13 | 56 ± 13 |
| Female sex | 45 | 56 | 43 | 38 | 41 | 50 | 58 |
| Diabetes mellitus | 58 | 38 | 47 | 55 | 62 | 70 | 74 |
| Race/ethnicity | |||||||
| White | 43 | 42 | 43 | 44 | 43 | 43 | 43 |
| Black | 33 | 35 | 31 | 30 | 31 | 35 | 40 |
| Hispanic | 15 | 10 | 14 | 16 | 17 | 1 | 12 |
| Asian | 3 | 6 | 5 | 3 | 2 | 1 | <1 |
| Other | 7 | 7 | 7 | 7 | 7 | 6 | 5 |
| Dialysis vintage | |||||||
| <6 mo | 55 | 53 | 54 | 55 | 55 | 56 | 57 |
| 6-<24 mo | 18 | 17 | 17 | 18 | 19 | 19 | 19 |
| 2-<5 y | 17 | 16 | 17 | 17 | 17 | 17 | 17 |
| ≥5 y | 10 | 14 | 12 | 10 | 9 | 8 | 7 |
| Insurance | |||||||
| Medicare | 63 | 64 | 64 | 63 | 63 | 62 | 60 |
| Medicaid | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Private | 10 | 9 | 10 | 10 | 10 | 10 | 9 |
| Other | 14 | 10 | 12 | 13 | 15 | 17 | 19 |
| Marital status | |||||||
| Married | 40 | 32 | 37 | 41 | 42 | 42 | 40 |
| Divorced | 7 | 6 | 6 | 6 | 7 | 7 | 8 |
| Single | 23 | 24 | 24 | 21 | 21 | 23 | 26 |
| Widow | 13 | 20 | 15 | 13 | 12 | 12 | 9 |
| Kt/Vurea | 1.5 ± 0.4 | 1.7 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.4 ± 0.3 |
| Comorbid conditions | |||||||
| IHD | 22 | 20 | 21 | 23 | 23 | 22 | 18 |
| CHF | 28 | 26 | 26 | 27 | 28 | 30 | 32 |
| CVA | 7 | 10 | 8 | 8 | 8 | 7 | 6 |
| HTN | 80 | 75 | 77 | 79 | 80 | 82 | 83 |
| PVD | 12 | 12 | 12 | 12 | 11 | 11 | 11 |
| COPD | 6 | 9 | 6 | 5 | 5 | 5 | 7 |
| Cancer | 5 | 6 | 6 | 5 | 4 | 4 | 3 |
| Smoker | 5 | 8 | 6 | 5 | 4 | 4 | 4 |
| HIV | 2 | 3 | 2 | 2 | 1 | 1 | 1 |
| Laboratory values | |||||||
| Albumin (g/dL) | 3.7 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.4 |
| Creatinine (mg/dL) | 8.0 ± 3.3 | 6.9 ± 2.9 | 7.9 ± 3.0 | 8 ± 3.3 | 8.2 ± 3.3 | 8.3 ± 3.4 | 8.2 ± 3.4 |
| Total CO2 (mEq/L) | 22 ± 3 | 23 ± 3 | 22 ± 3 | 22 ± 3 | 22 ± 3 | 22 ± 3 | 22 ± 3 |
| Calcium (mg/dL) | 9.2 ± 0.7 | 9.0 ± 0.8 | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.2 ± 0.7 |
| Phosphorus (mg/dL) | 5.6 ± 1.5 | 5.6 ± 1.6 | 5.5 ± 1.5 | 5.5 ± 1.5 | 5.6 ± 1.5 | 5.7 ± 1.5 | 5.8 ± 1.5 |
| TIBC (mg/dL) | 209 ± 46 | 189 ± 48 | 202 ± 46 | 208 ± 45 | 213 ± 45 | 217 ± 45 | 220 ± 45 |
| Ferritin (ng/mL) | 378 (179-709) | 474 (228-856) | 416 (199-767) | 382 (181-713) | 364 (172-683) | 345 (165-655) | 325 (158-613) |
| PTH (pg/mL) | 236 (133-411) | 205 (104-384) | 219 (122-389) | 227 (127-398) | 241 (139-411) | 258 (148-445) | 271 (160-465) |
| Hb (g/dL) | 12.0 ± 1.4 | 11.9 ± 1.5 | 12.0 ± 1.4 | 12.0 ± 1.4 | 12.0 ± 1.3 | 12.0 ± 1.3 | 12.0 ± 1.3 |
| WBC (×103/μL) | 7.5 ± 2.5 | 7.6 ± 3 | 7.4 ± 2.7 | 7.3 ± 2.5 | 7.4 ± 2.4 | 7.5 ± 2.4 | 7.9 ± 2.3 |
| Lymphocytes (%) | 20.5 ± 7.9 | 19 ± 8 | 20 ± 8 | 20 ± 7.8 | 21 ± 7.8 | 21 ± 7.8 | 21 ± 7.6 |
| nPNA (g/kg/d) | 0.95 ± 0.3 | 0.90 ± 0.3 | 0.95 ± 0.3 | 0.95 ± 0.3 | 0.95 ± 0.3 | 0.95 ± 0.3 | 0.95 ± 0.3 |
Note: Values for categorical variables are given as percentages; continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; calcium in mg/dL to mmol/L, ×0.2495; phosphorus in mg/dL to mmol/L, ×0.3229.
Abbreviations: BMI, body mass index; CHF, congestive heart failure; CO2, carbon dioxide; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; Hb, hemoglobin; HIV, human immunodeficiency virus; HTN, hypertension; IHD, ischemic heart disease; nPNA, normalized protein nitrogen appearance; PTH, parathyroid hormone; PVD, peripheral vascular disease; TIBC, total iron-binding capacity; WBC, white blood cells.
Association of BMI With Mortality
Figure 1 depicts the distribution of BMI in the study population. With progressive higher time-averaged BMI, there was progressively lower all-cause, cardiovascular, and infection-related mortality (Table 2). Hence, although there was 46%, 47%, and 48% higher risk for all-cause, cardiovascular, and infection-related mortality, respectively, in patients with time-averaged BMI < 18.5 kg/m2, the cause-specific death risk was 38%, 40%, and 27% lower in patients with time-averaged BMI > 35.0 kg/m2 (reference group, BMI of 23.0-<25.0 kg/m2).
Figure 1.
Distribution of time-averaged body mass index in patients treated with hemodialysis (n = 123,383).
Table 2.
Associations of Time-Averaged BMI With Mortality in HD Patients
| BMI Category | All-Cause | Cardiovascular | Infection-Related |
|---|---|---|---|
| <18.5 kg/m2 | 1.46 (1.41-1.51) | 1.47 (1.39-1.56) | 1.48 (1.36-1.61) |
| 18.5-<23.0 kg/m2 | 1.13 (1.10-1.16) | 1.14 (1.10-1.18) | 1.13 (1.06-1.20) |
| 23.0-<25.0 kg/m2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25.0-<30.0 kg/m2 | 0.86 (0.84-0.88) | 0.85 (0.82-0.86) | 0.90 (0.85-0.96) |
| 30.0-<35.0 kg/m2 | 0.73 (0.72-0.76) | 0.74 (0.70-0.77) | 0.83 (0.77-0.89) |
| ≥35.0 kg/m2 | 0.62 (0.60-0.64) | 0.60 (0.60-0.63) | 0.73 (0.67-0.79) |
Note: Values are given as adjusted hazard ratio (95% confidence interval). N = 123,383.
Abbreviation: BMI, body mass index.
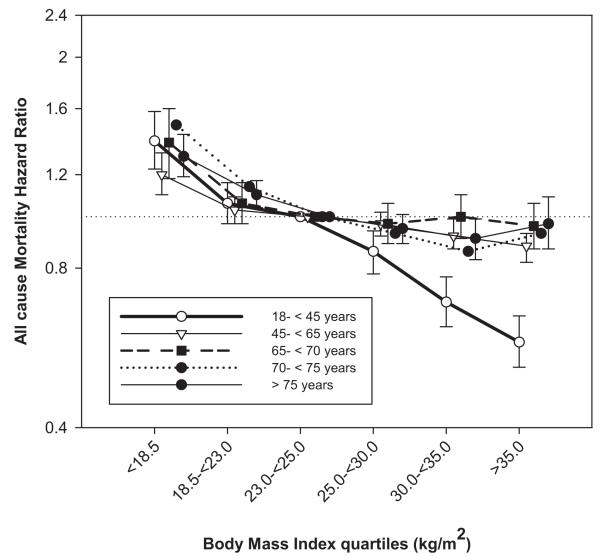
Association in Different Age Groups
With progressively higher time-averaged BMI, there was progressively lower all-cause, cardiovascular, and infection-related mortality in patients younger than 65 years. In those 65 years or older, although overweight/obese patients had lower mortality compared with underweight/normal-weight patients, sequential increases in time-averaged BMI > 25 kg/m2 added no additional benefit. In addition, in patients older than 75 years, infection-related mortality in overweight/obese patients was slightly higher compared to the reference category (Table 3; Figs 2 and 3).
Table 3.
Associations of Time-Averaged BMI With All-Cause, Cardiovascular, and Infection-Related Mortality in Patients Across Age Groups
| All-Cause Mortality |
Cardiovascular Mortality |
Infection-Related Mortality |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18-<45 y (n = 18,512) |
45-<65 y (n = 48,309) |
65-<70 y (n = 14,787) |
70-<75 y (n = 14,636) |
≥75 y (n = 27,139) |
18-<45 y (n = 18,512) |
45-<65 y (n = 48,309) |
65-<70 y (n = 14,787) |
70-<75 y (n = 14,636) |
≥75 y (n = 27,139) |
18-<45 y (n = 18,512) |
45-<65 y (n = 48,309) |
65-<70 y (n = 14,787) |
70-<75 y (n = 14,636) |
≥75 y (n = 27,139) |
|
| P for trend | 0.1 | <0.001 | 0.2 | 0.7 | <0.001 | 0.2 | 0.02 | 0.01 | 0.2 | <0.001 | 0.8 | 0.09 | 0.6 | 0.3 | 0.01 |
| BMI < 18.5 kg/m2 |
1.39 (1.23-1.58) P < 0.001 |
1.20 (1.10-1.32) P < 0.001 |
1.38 (1.18-1.60) P < 0.001 |
1.49 (1.29-1.72) P < 0.001 |
1.30 (1.19-1.43) P = 0.05 |
1.17 (0.94-1.45) P = 0.5 |
1.18 (1.01-1.37) P < 0.001 |
1.51 (1.19-1.91) P < 0.001 |
1.21 (0.95-1.55) P = 0.01 |
1.32 (1.14-1.53) P = 0.03 |
1.69 (1.25-2.28) P < 0.001 |
1.20 (0.96-1.49) P < 0.001 |
1.63 (1.15-2.31) P < 0.001 |
1.66 (1.17-2.37) P < 0.001 |
1.20 (0.94-1.52) P = 0.6 |
| BMI = 18.5- <23.0 kg/m2 |
1.06 (0.97-1.16) P = 0.4 |
1.03 (0.97-1.09) P = 0.01 |
1.06 (0.97-1.16) P < 0.001 |
1.14 (1.04-1.25) P < 0.001 |
1.10 (1.04-1.17) P = 0.5 |
0.99 (0.86-1.14) P = 0.6 |
1.01 (0.92-1.10) P = 0.02 |
1.04 (0.90-1.21) P = 0.02 |
1.04 (0.89-1.21) P = 0.6 |
1.12 (1.01-1.24) P = 0.1 |
1.12 (0.89-1.41) P = 0.4 |
0.93 (0.81-1.08) P = 0.2 |
1.03 (0.81-1.29) P = 0.4 |
1.11 (0.87-1.41) P = 0.01 |
1.13 (0.96-1.33) P = 0.8 |
| BMI = 25.0- <30.0 kg/m2 |
0.86 (0.78-0.94) P = 0.01 |
0.97 (0.92-1.02) P = 0.01 |
0.97 (0.89-1.06) P = 0.1 |
0.93 (0.85-1.02) P < 0.001 |
0.95 (0.89-1.01) P = 0.6 |
0.82 (0.71-0.97) P = 0.07 |
0.93 (0.85-1.02) P = 0.01 |
0.98 (0.85-1.12) P = 0.9 |
0.90 (0.78-1.04) P = 0.05 |
0.90 (0.81-0.99) P = 0.1 |
0.93 (0.73-1.19) P = 0.6 |
0.91 (0.80-1.04) P = 0.1 |
1.01 (0.82-1.25) P = 0.9 |
0.90 (0.71-1.13) P = 0.3 |
1.03 (0.88-1.22) P = 0.7 |
| BMI = 30.0- <35.0 kg/m2 |
0.69 (0.62-0.77) P < 0.001 |
0.92 (0.87-0.99) P < 0.001 |
1.00 (0.91-1.10) P = 0.01 |
0.86 (0.77-0.95) P < 0.001 |
0.91 (0.83-0.99) P = 0.02 |
0.65 (0.54-0.77) P < 0.001 |
0.88 (0.79-097) P = 0.01 |
1.06 (0.91-1.24) P = 0.2 |
0.77 (0.64-0.92) P = 0.03 |
0.99 (0.86-1.13) P = 0.2 |
0.78 (0.59-1.05) P = 0.2 |
0.94 (0.82-1.10) P = 0.3 |
0.85 (0.66-1.09) P = 0.9 |
0.94 (0.72-1.22) P = 0.3 |
1.05 (0.85-1.30) P = 0.2 |
| BMI≥ 35.0 kg/m2 |
0.58 (0.52-0.65) P < 0.001 |
0.88 (0.82-0.93) P = 0.01 |
0.96 (0.87-1.06) P = 0.04 |
0.93 (0.82-1.05) P = 0.06 |
0.97 (0.87-1.09) P < 0.001 |
0.50 (0.42-0.61) P = 0.01 |
0.82 (0.74-0.91) P = 0.01 |
0.98 (0.84-1.15) P = 0.5 |
0.99 (0.82-1.20) P = 0.5 |
0.89 (0.73-1.07) P = 0.1 |
0.91 (0.69-1.20) P = 0.9 |
0.88 (0.76-1.03) P = 0.5 |
0.94 (0.73-1.20) P = 0.5 |
1.01 (0.75-1.37) P = 0.2 |
1.06 (0.80-1.41) P = 0.02 |
Note: Associations given as adjusted hazard ratio (95% confidence interval); reference group is BMI of 23.0-<25.0 kg/m2. Hazard ratios were adjusted for age, sex, race and/or ethnicity, diabetes, dialysis vintage, primary insurance, dialysis dose, marital status, ischemic heart disease, congestive heart failure, other cardiac diseases, hypertension, cerebrovascular disease, peripheral vascular disease, chronic obstructive pul monary disease, cancer, current smoking, human immunodeficiency virus, and serum albumin, total iron-binding capacity, ferritin, creatinine, calcium, phosphorus, parathyroid hormone, hemoglobin, white blood cell count, lymphocyte percentage, bicarbonate, and normalized protein nitrogen appearance values.
Abbreviation: BMI, body mass index.
Figure 2.
Association of time-averaged body mass index (BMI) with mortality in hemodialysis patients across age groups (n = 123,383). Time-averaged BMI of 23.0-<25.0 kg/m2 was used as reference.
Figure 3.
Association of time-averaged body mass index (BMI) with (left) cardiovascular and (right) infection-related mortality in hemodialysis patients across age groups (n = 123,383). Time-averaged BMI of 23.0-<25.0 kg/m2 was used as reference.
Association in Different Vintage Groups
In all groups based on dialysis vintage, higher time-averaged BMI was associated with progressively lower all-cause and cardiovascular mortality. This was more pronounced for patients with dialysis vintage of less than 6 months compared with those with vintage of more than 6 months. However, for infection-related mortality, although there was a progressive decrease in mortality in patients with dialysis vintage of less than 6 months, this association became U shaped with progressive increase in time-averaged BMI (Table 4; Figs 4 and 5).
Table 4.
Associations of Time-Averaged BMI With All-Cause, Cardiovascular, and Infection-Related Mortality in Patients Across Dialysis Vintage Groups
| All-Cause Mortality |
Cardiovascular Mortality |
Infection-Related Mortality |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <6 mo (n = 68,432) |
6 mo-<2 y (n = 22,319) |
2-<5 y (n = 20,908) |
≥5 y (n = 11,724) |
<6 mo (n = 68,432) |
6 mo-<2 y (n = 22,319) |
2-5 y (n = 20,908) |
>5y (n = 11,724) |
<6 mo (n = 68,432) |
6 mo-<2 y (n = 22,319) |
2-5 y (n = 20,908) |
>5 y (n = 11,724) |
|
| P for trend | 0.01 | 0.4 | 0.8 | 0.2 | 0.6 | 0.4 | 0.4 | 0.8 | 0.01 | 0.2 | 0.7 | 0.4 |
| BMI < 18.5 kg/m2 |
1.44 (1.34-1.54) P < 0.001 |
1.10 (0.99-1.21) P < 0.001 |
1.26 (1.12-1.43) P < 0.001 |
1.22 (1.00-1.48) P < 0.001 |
1.39 (1.23-1.57) P < 0.001 |
1.01 (0.86-1.18) P < 0.001 |
1.22 (1.01-1.49) P < 0.001 |
1.16 (0.85-1.57) P < 0.001 |
1.52 (1.39-1.66) P < 0.001 |
1.10 (0.85-1.43) P < 0.001 |
1.38 (1.01-1.87) P < 0.001 |
1.68 (1.66-1.76) P = 0.08 |
| BMI = 18.5- <23.0 kg/m2 |
1.13 (1.08-1.18) P < 0.001 |
1.01 (0.94-1.07) P < 0.001 |
1.03 (0.95-1.11) P < 0.001 |
1.10 (0.96-1.26) P = 0.06 |
1.09 (1.01-1.18) P < 0.001 |
0.96 (0.87-1.07) P < 0.001 |
1.08 (0.95-1.22) P = 0.06 |
1.12 (0.91-1.38) P = 0.6 |
1.18 (1.11-1.26) P < 0.001 |
1.19 (0.92-1.29) P = 0.1 |
1.11 (0.90-1.36) P = 0.1 |
1.22 (0.88-1.70) P = 0.2 |
| BMI = 25.0- <30.0 kg/m2 |
0.92 (0.88-0.96) P < 0.001 |
0.93 (0.87-0.99) P < 0.001 |
0.92 (0.86-1.00) P < 0.001 |
0.96 (0.84-1.10) P = 0.2 |
0.86 (0.80-0.94) P < 0.001 |
0.85 (0.77-0.94) P = 0.07 |
0.93 (0.83-1.06) P < 0.001 |
0.97 (0.79-1.20) P = 0.2 |
0.84 (0.78-0.90) P = 0.05 |
1.05 (0.89-1.24) P = 0.07 |
1.11 (0.91-1.36) P = 0.4 |
1.02 (0.73-1.43) P = 0.8 |
| BMI = 30.0- <35.0 kg/m2 |
0.82 (0.78-0.87) P < 0.001 |
0.99 (0.92-1.07) P < 0.001 |
0.85 (0.78-0.93) P < 0.001 |
1.06 (0.90-1.24) P = 0.7 |
0.79 (0.72-0.87) P < 0.001 |
0.91 (0.80-1.02) P < 0.001 |
0.92 (0.79-1.06) P < 0.001 |
1.05 (0.82-1.35) P = 0.5 |
0.85 (0.77-0.93) P < 0.001 |
1.14 (0.94-1.38) P = 0.03 |
0.89 (0.70-1.14) P = 0.5 |
1.17 (0.79-1.74) P = 0.1 |
| BMI ≥ 35.0 kg/m2 |
0.70 (0.65-0.74) P < 0.001 |
0.94 (0.86-1.02) P < 0.001 |
0.89 (0.81-0.99) P < 0.001 |
0.99 (0.83-1.17) P < 0.001 |
0.65 (0.58-0.73) P < 0.001 |
0.87 (0.76-1.00) P < 0.001 |
0.93 (0.80-1.09) P < 0.001 |
0.82 (0.63-1.08) P < 0.001 |
0.76 (0.66-0.88) P < 0.001 |
0.93 (0.74-1.17) P = 0.2 |
1.07 (0.84-1.37) P = 0.2 |
1.32 (0.89-1.95) P = 0.8 |
Note: Associations given as adjusted hazard ratio (95% confidence interval); reference group is BMI of 23.0-<25.0 kg/m2. Hazard ratios were adjusted for age, sex, race and/or ethnicity, diabetes, dialysis vintage, primary insurance, dialysis dose, marital status, ischemic heart disease, congestive heart failure, other cardiac diseases, hypertension, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer, current smoking, human immunodeficiency virus, and serum albumin, total iron-binding capacity, ferritin, creatinine, calcium, phosphorus, parathyroid hormone, hemoglobin, white blood cell count, lymphocyte percentage, bicarbonate, and normalized protein nitrogen appearance values.
Abbreviation: BMI, body mass index.
Figure 4.
Association of time-averaged body mass index (BMI) with mortality in hemodialysis patients across dialysis vintage groups (n = 123,383). Time-averaged BMI of 23.0-<25.0 kg/m2 was used as reference.
Figure 5.
Association of time-averaged body mass index (BMI) with (left) cardiovascular and (right) infection-related mortality in hemodialysis patients across dialysis vintage groups (n = 123,383). Time-averaged BMI of 23.0-<25.0 kg/m2 was used as reference.
Sensitivity Analyses
Three sets of sensitivity analyses were performed to analyze the strength of the results. The first set of sensitivity analyses included only individuals with complete data for all variables (n = 32,222). We found that essentially the same qualitative trends were observed (tables a and b of Item S1). The second set of analyses looked at the relationship between baseline BMI and mortality and included only patients for whom baseline BMI data were available (n = 120,150). Again, higher BMI was associated with improved all-cause and cardiovascular mortality in all age and dialysis vintage subgroups. For infection-related mortality, a U-shaped association was seen for the age group of 18-<45 years and those with dialysis vintage of more than 5 years (Tables a and b of Item S2). In the third set of sensitivity analyses using joint modeling, it was observed that for each unit increment in BMI, mortality risk decreased by approximately 27%-29% for age groups 1 and 2 and approximately 4%-5% for age groups 3-5. Similarly, for each vintage group, a 1-unit increment in BMI was associated with a reduction in mortality risk of approximately 2%-4%. Hence, the stronger association of higher BMI with greater survival was seen in individuals younger than 65 years, which essentially was the same result observed with the conventional Cox model. However, the shortest dialysis vintage (<6 months), which showed the strongest BMI-survival among other vintage categories, did not show this contrast in the joint modeling (Table S2).
DISCUSSION
This study, based on a large contemporary cohort from the HD population in the United States, indicates that patients with higher BMI have better survival irrespective of the patient’s age and dialysis vintage. However, this benefit is more pronounced in incident dialysis patients and those younger than 65 years.
The findings in our study of the association of higher BMI with lower death risk in HD patients builds on the results of previous studies of such an association in this patient population.1-6,15,20-22 The landmark Diaphane collaborative study group was one of the first to demonstrate that low (<20 kg/m2), and not high, BMI was associated with higher overall and cardiovascular mortality in 1,453 French HD patients.23 This finding was confirmed further in 3,607 HD patients from the USRDS.24 It also has been shown that for each unit higher BMI, the relative risk of mortality is lower by 10%.1 In a few studies, a survival advantage has not been shown with higher BMI in HD patients. In a study of 116 Japanese HD patients, those with BMI < 16.9 and >23.0 kg/m2 had lower survival compared with those with BMI of 17.0-18.9 kg/m2.25 Similarly, in a much larger study in Asian Americans using the USRDS database, higher mortality risk was seen in individuals with BMI > 25 kg/m2. However, mortality risk for the whites included in the study decreased with an increase in BMI.26 Another large study (n = 109,605) that evaluated the effect of racial differences in the association of BMI and mortality concluded that the protective effect of higher BMI is strongest in blacks and Hispanics compared with whites.2 Hence, race-ethnicity may modify the effect of obesity on survival in dialysis patients.
Although there are abundant data evaluating mortality risk and BMI in HD patients, the data for effect modification by age and/or dialysis vintage are scant. Some previous studies that have evaluated this question were limited by their relatively smaller sample sizes. Our findings are consistent with those showing a similar association between body size and death risk irrespective of patient’s age in an analysis of data of 9,714 HD patients from DOPPS (Dialysis Outcomes and Practice Patterns Study).15 Kutner and Zhang16 also investigated the association of BMI and mortality in 316 HD and PD patients 60 years or older during a follow-up of 11 years. Black women, black men, and white men with higher BMI had reduced mortality risk, whereas white women had an increased risk of death.16
At least 2 previous studies have reported results contrary to our findings. The first study included 722 HD patients aged 50-75 years from NECOSAD-2 (Netherlands Cooperative Study on the Adequacy of Dialysis-2) who were followed up for 2 and 7 years. This study reported that compared with patients with normal BMI (22-25 kg/m2), those with BMI < 18.5 kg/m2 or ≥30 kg/m2 had increased mortality risk, although the risk was much higher for those with BMI < 18.5 kg/m2.17 A more recent study of 1,749 patients from the same group (with a follow-up of 7 years) concluded that in patients younger than 65 years, there was a U-shaped association between BMI and mortality: BMI < 18.5 or ≥30 kg/m2 was associated with 2.0-fold and 1.57-fold higher risks of all-cause mortality, respectively. However, there was no association between body size and death risk in patients 65 years or older.18 Potential reasons that can explain the discrepancy between the findings of the aforementioned study and our results are as follows.
In contrast to our study, the NECOSAD-2 cohort included a significant number of patients undergoing PD (50% and 22% in the age groups <65 and ≥65 years, respectively). The data for the association of body size with death risk are substantially less consistent in PD patients than those seen in HD populations.27-30
There was longer follow-up in the aforementioned study compared to this present study (7 vs 3 years). In dialysis patients with a high short-term mortality rate, obesity may be associated with better survival by virtue of better nutrition or some other mechanism,31 whereas over longer follow-up, it may exert deleterious effects.32 Results presented here demonstrate that the protective effect of higher time-averaged BMI seemed to attenuate in patients with the largest body size (BMI > 40 kg/m2) with longer dialysis vintage (>2 years).
The NECOSAD-2 cohort included incident dialysis patients only, whereas the present study included both incident and maintenance dialysis patients.
This study is the first comprehensive evaluation of the association between time-averaged BMI with mortality across different subgroups of dialysis vintage. We found that all-cause and cardiovascular mortality are related inversely to body size irrespective of the patient’s dialysis vintage. This protective effect of BMI on mortality seemed to be more marked for patients with dialysis vintage of less than 6 months. Several studies have shown that dialysis vintage co-relates negatively with the nutritional status of dialysis patients.16,25,33-35 It also has been shown that muscle mass co-relates negatively with number of years receiving dialysis therapy. Furthermore, a study done exclusively in incident HD patients has concluded that the protective effect of high BMI is seen only in patients with normal/high muscle mass and not those with high body fat, which probably can explain why the protective effect of BMI on mortality is more marked in patients with dialysis vintage of less than 6 months.36
There are several hypotheses that can explain the lower death risk with larger body size of dialysis patients. First, obese dialysis patients have more stable hemodynamic status, which allows the use of afterload-reducing agents in the setting of heart failure commonly observed in this population.37 Second, the high level of tumor necrosis factor α receptors in adipose tissue38 helps neutralize the adverse biological effects of high tumor necrosis factor α levels found in dialysis patients39 and thereby protects against cardiac injury. Third, obesity is associated with an altered stress response of the sympathetic and renin-angiotensin systems.40 Heightened sympathetic and renin-angiotensin activities are associated with a poor prognosis in patients with heart failure and those with fluid-overload states (eg, patients receiving dialysis)41; thus, decreased stress responses of these neurohormonal systems might have a favorable prognosis in obese dialysis patients. Fourth, obesity is associated with higher lipid and lipoprotein concentrations, which can actively bind and remove circulating endotoxins.42 Finally, protein-energy malnutrition and inflammation usually are common and concurrent with each other in patients receiving dialysis. This malnutrition-inflammation complex syndrome is associated with increased morbidity and cardiovascular mortality in dialysis patients. Hence, because dialysis patients have a poor survival rate, obesity is associated with better short-term survival in these patients, possibly by the aforementioned mechanisms, which transcends the long-term risks associated with obesity in the general population.
The results of our study should be interpreted in light of its limitations. First, the observational nature of this study allows us to demonstrate associations, but no inferences can be made about causality. Second, we used BMI as a surrogate for fat mass. However, although BMI does not measure body fat directly, in epidemiologic studies, it can be used as a reasonable and practical indicator of it.43 Third, information about some potential confounders, such as serum C-reactive protein level or residual kidney function, was missing. By adjusting for surrogates for inflammation and dialysis vintage, respectively, we tried to overcome this. Fourth, information was not entirely available for some variables. We performed sensitivity analyses to assess the effect of this limitation, which produced similar findings. Fifth, an important limitation of our study was that a significant proportion of the patients included (45%) were maintenance dialysis patients, which could introduce survivor bias. Finally, data for comorbid conditions were obtained from the time of dialysis therapy initiation, which may have led us to underestimate the prevalence of different comorbid conditions.
In conclusion, our study demonstrates that higher BMI is associated with lower death risk irrespective of patient’s age and dialysis vintage. This benefit is more pronounced in incident dialysis patients and those younger than 65 years. Given the consistent association between low BMI and mortality, all attempts to improve patient’s nutritional status should be made. Randomized controlled clinical trials to evaluate the effect of weight management strategies on outcomes in the dialysis population are needed.
Supplementary Material
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research (DCR) for providing the clinical data, analysis, and review for this research project.
Support: The study was supported by Dr Kalantar-Zadeh’s research grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK078106, K24 DK091419), a philanthropic grant from Mr Harold Simmons, and a research grant from DCR.
Financial Disclosure: Dr Mehrotra has received grant support and/or honoraria from Baxter Healthcare and DaVita. Dr Nissenson is an employee of DaVita Inc. Dr Kalantar-Zadeh was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA, from 2007 to 2012.
Footnotes
The other authors declare that they have no other relevant financial interests.
SUPPLEMENTARY MATERIAL
Table S1: Characteristics of included and excluded HD patients aged >18 years.
Table S2: Association of BMI with all-cause mortality across age and vintage groups using joint modeling analyses.
Item S1: Associations of time-averaged BMI with all-cause mortality across age and vintage groups.
Item S2: Associations of baseline BMI with all-cause, cardiovascular, and infection-related mortality across age and vintage groups.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2013.07.021) is available at www.ajkd.org
REFERENCES
- 1.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55(4):1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 2.Ricks J, Molnar MZ, Kovesdy CP, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58(4):574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46(3):489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83(2):202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 5.Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol. 2003;13(2):136–143. doi: 10.1016/s1047-2797(02)00251-x. [DOI] [PubMed] [Google Scholar]
- 6.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80(2):324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 7.Park J, Jin DC, Molnar MZ, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88(5):479–486. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lievense H, Kalantar-Zadeh K, Lukowsky LR, et al. Relationship of body size and initial dialysis modality on subsequent transplantation, mortality and weight gain of ESRD patients. Nephrol Dial Transplant. 2012;27(9):3631–3638. doi: 10.1093/ndt/gfs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Streja E, Molnar MZ, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175(8):793–803. doi: 10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 11.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49(7):968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 12.Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25(4):643–659. doi: 10.1016/j.cger.2009.07.005. viii. [DOI] [PubMed] [Google Scholar]
- 13.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85(7):609–617. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16(12):2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 16.Kutner NG, Zhang R. Body mass index as a predictor of continued survival in older chronic dialysis patients. Int Urol Nephrol. 2001;32(3):441–448. doi: 10.1023/a:1017581726362. [DOI] [PubMed] [Google Scholar]
- 17.de Mutsert R, Snijder MB, van der Sman-de Beer F, et al. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol. 2007;18(3):967–974. doi: 10.1681/ASN.2006091050. [DOI] [PubMed] [Google Scholar]
- 18.Hoogeveen EK, Halbesma N, Rothman KJ, et al. Obesity and mortality risk among younger dialysis patients. Clin J Am Soc Nephrol. 2012;7(2):280–288. doi: 10.2215/CJN.05700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am J Kidney Dis. 2000;35(6)(suppl 2):S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 20.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56(3):1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35(1):80–88. doi: 10.1016/S0272-6386(00)70305-2. [DOI] [PubMed] [Google Scholar]
- 22.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13(4):1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 23.Degoulet P, Legrain M, Reach I, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31(2):103–110. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 24.Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31(6):997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 25.Kaizu Y, Tsunega Y, Yoneyama T, et al. Overweight as another nutritional risk factor for the long-term survival of non-diabetic hemodialysis patients. Clin Nephrol. 1998;50(1):44–50. [PubMed] [Google Scholar]
- 26.Wong JS, Port FK, Hulbert-Shearon TE, et al. Survival advantage in Asian American end-stage renal disease patients. Kidney Int. 1999;55(6):2515–2523. doi: 10.1046/j.1523-1755.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 27.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65(2):597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 28.Aslam N, Bernardini J, Fried L, Piraino B. Large body mass index does not predict short-term survival in peritoneal dialysis patients. Perit Dial Int. 2002;22(2):191–196. [PubMed] [Google Scholar]
- 29.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14(11):2894–2901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 30.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int. 2004;65(6):2398–2408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 32.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64(5):1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann P, Smolle KH, Horina JH, Zach R, Krejs GJ. Impact of long-term hemodialysis on nutritional status in patients with end-stage renal failure. Clin Investig. 1994;72(10):754–761. doi: 10.1007/BF00180542. [DOI] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, Dunne E, Nixon K, et al. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant. 1999;14(1):169–175. doi: 10.1093/ndt/14.1.169. [DOI] [PubMed] [Google Scholar]
- 35.Pollock CA, Ibels LS, Allen BJ, et al. Total body nitrogen as a prognostic marker in maintenance dialysis. J Am Soc Nephrol. 1995;6(1):82–88. doi: 10.1681/ASN.V6182. [DOI] [PubMed] [Google Scholar]
- 36.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14(9):2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 37.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277(6, pt 1):E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Mehrotra R, Fouque D, Kopple JD. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17(6):455–465. doi: 10.1111/j.0894-0959.2004.17606.x. [DOI] [PubMed] [Google Scholar]
- 40.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37(1):169–174. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 41.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 42.Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167):1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 43.Leinig C, Pecoits-Filho R, Nascimento MM, Goncalves S, Riella MC, Martins C. Association between body mass index and body fat in chronic kidney disease stages 3 to 5, hemodialysis, and peritoneal dialysis patients. J Ren Nutr. 2008;18(5):424–429. doi: 10.1053/j.jrn.2008.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.