Abstract
Purpose
Polycystic ovary syndrome (PCOS) is associated with oxidative stress (OS) and serum superoxide dismutase (SOD) activity has been reported with mixed results. The objective of this study was to examine the activity of SOD both in the serum and FF from women with PCOS undergoing ICSI, as well as the expression of Cu/Zn-SOD mRNA in the cells recovered from the FF.
Methods
Forty women undergoing an ICSI trial were divided into: group I, included 20 PCOS cases, group II included 20 age-matched controls with tubal factor infertility. Both groups were similarly stimulated. A total of 204 metaphase II (MII) oocytes were aspirated; (108) from PCOS, and (96) from the control group. SOD activities in the serum and FF, as well as Cu/Zn-SOD (SOD1) mRNAs in follicular fluid (FF) cells were analyzed.
Results
There was a statistically highly significant decrease (p < 0.001) both in the mean serum SOD (45.56 ± 18.06) and FF SOD activity (42.49 ± 11.46) in PCOS than the control group (77.38 ± 7.82), (74.37 ± 6.15) respectively. The mean relative levels of Cu, Zn SOD mRNAs was significantly lower (p < 0.001) in cells isolated from the FF in PCOS (0.36 ± 0.14) than the control group (0.81 ± 0.15). SOD activity in FF had no effects on fertilization rate (p > 0.05), or embryo quality after intracytoplasmic sperm injection (ICSI).
Conclusion
Although decreased SOD activity in FF has no effect on fertilization rate and/or embryo quality, serum SOD activity could be a clinical parameter for determining systemic oxidative stress in PCOS.
Keywords: Follicular fluid, PCOS, Superoxide dismutase, Gene expression
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine abnormality in reproductive-age women, its etiology remains unclear, there is an increasing evidence for an oxidative stress (OS) in PCOS that induce genomic and mitochondrial DNA damage that leads directly to reduced fertility [1, 2] (Fig. 1).
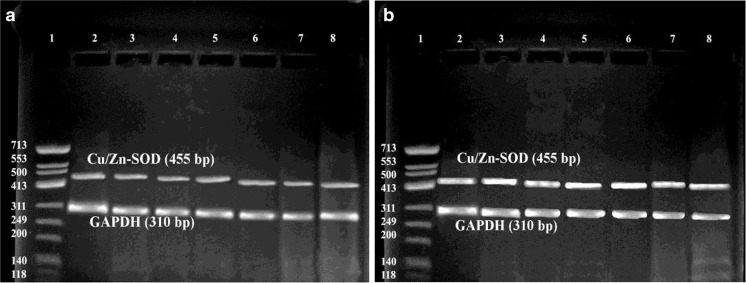
Fig. 1.
Cu/Zn-SOD gene expression in follicular fluid cellular pellets versus internal control (GAPDH); a PCOS, b Control samples. Lane 1: DNA marker. Lanes 2–8: Cu/Zn-SOD and GAPDH genes expression in follicular fluid cellular pellets
SOD is responsible for catalyzing the conversion of superoxide to elemental oxygen and hydrogen peroxide. This transformation is called dismutation, hence the enzyme’s name. Dismutation is a reaction between two identical molecules in which one is reduced and the other oxidized, here 2 molecules of superoxide anions were converted into hydrogen peroxide and molecular oxygen. Three forms of superoxide dismutase are present in humans. SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular. The first is a dimer (consists of two units), whereas the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc, whereas SOD2, the mitochondrial enzyme, has manganese in its reactive center [3]. Keeping SODs at reduced concentrations within the follicular fluid milieu may provide the appropriate balance of superoxide anion and hydrogen peroxide for normal cell function. Superoxide dismutase (SOD) dismutates the superoxide anion to hydrogen peroxide and is the first line of defence in antioxidant reactions against reactive oxygen species (ROS).
In PCOS patients, serum SOD activity has been reported with mixed results [4, 5]. Follicular fluid (FF) is easily available during oocyte pick-up and provides a very important microenvironment for the development of oocytes. A threshold level of ROS in the FF may be correlated with fertilization, embryo quality, pregnancy rate and outcome [6]. The SOD activity is attributed to the extracellular secreted isoform of SOD, the cytosolic copper/zinc SOD (Cu/Zn-SOD) are located within both granulosa and theca cells [7, 8]. Genetic expression of Cu/Zn-SOD in cells recovered from FF in PCOS has not been addressed before. It is unknown whether SOD activity affects fertilization and/or pregnancy rates in PCOS undergoing intracytoplasmic sperm injection (ICSI). In the present study, we examined the activity of SOD both in the serum and FF from women with PCOS undergoing ICSI, as well as the expression of Cu/Zn-SOD mRNA in the cells recovered from the FF. The research question was “Does follicular fluid SOD activity correlate with oocyte quality and subsequently the embryo quality in PCOS? We were looking for a biochemical markers in addition to the morphological assessment of oocyte in PCOS patients.
Because morphological assessment of the oocyte bears no relation to the quality of oocytes. At the same time, it is well known that PCOS produces a large number of oocytes during ovarian stimulation for ICSI, and fining a biochemical assessment of the enclosed microfllicular environment may allow better selection of good quality oocytes and hence possible good quality embryos.
Materials and methods
Patient selection
This study was carried out as a case control study; patients were enrolled from the Fertility care unit, Mansoura University Hospital, Mansoura, Egypt, during the period from July 2011 to May 2013. Forty women undergoing ICSI were divided into: group I, included 20 cases of PCOS, group II included 20 age-matched control group diagnosed with tubal factor. All subjects had provided written informed consent according to the guidelines of the research ethical committee at Mansoura University. PCOS patients, included in the study, were diagnosed according to the revised 2003 consensus on diagnostic criteria and long-term health risks related to PCOS. Patients with hyperprolactinemia, hypothyroidism, Cushing disease, androgen-secreting neoplasms, associated endometriosis, current cigarette smokers, diabetes mellitus, or cardiovascular diseases as hypertension were excluded. Also, couples with male factors were excluded.
Ovarian stimulation
In both groups, ovarian stimulation started by down-regulation in the luteal phase with a gonadotrophin-releasing hormone agonist triptorelin (0.1 mg/day; Decapeptyl®, Ferring, Kiel, Germany), followed by administration of recombinant human FSH (Gonal-f®, Serono, Switzerland) with doses ranging from 75 to 225 IU/day. Follicular growth was monitored by serum E2 levels and transvaginal sonography. When at least 3 follicles ≥16 mm in diameter and the leading follicle reached a mean diameter of 18 mm, human chorionic gonadotrophin (hCG; Choriomon®, IBSA, Switzerlan) was administered.
Samples collection
Blood samples for estimation of SOD activity were obtained from all included subjects on the day of oocyte retrival that was performed 34–36 h after hCG injection using transvaginal ultrasound. Follicles size was determined immediately before retrival, only follicles ≥18–22 mm in diameter were included, each follicle was aspirated individually and FF were collected in a separate tube. Flushing of the aspiration catheter was made with standard volumes of buffered solution in order to obtain a known dilution of FF substances. The cumulus-oocyte complexes were removed, oocytes were numbered in the same order as for FF, only metaphase II (M II) oocytes were injected, metaphase I oocytes (M I) were injected if they changed to M II within 6 h of collection. Other types of oocytes were excluded. A total of 204 metaphase II (MII) oocytes were injected (108 from PCOS, and 96 from the control group), 1–3 embryos were transferred for each case, other remaining embryos were cryothawed.
Estimation of SOD activity
Blood samples were centrifuged at 1000 g for 10 min, clear serum were frozen and stored at −80 °C until analysis. FF samples were centrifuged at 5000 g at 4 °C for 10 min and clear supernatants as well as the cellular pellets were frozen and stored at −80 °C until analysis. SOD activity was assayed in both serum and FF supernatants. It relies on the ability of the enzyme to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye [9]. Purified SOD inhibit the initial rate of activated phenazinemethosulphate mediated reduction of O2 to O2- which then reduces nitroblue tetrazolium. % of inhibition was calculated and compared to standard of 0.5 μg of the purified enzyme that produced 80 % inhibition (activity = 3.000 units/mg protein). The final SOD activity levels were expressed as units of enzymatic activity per mg of protein contained in the samples (U/mg protein).
Expression of Cu/Zn-SOD mRNA in FF cells
-
Extraction of RNA from the FF cellular pellets:
Total RNA extraction from cellular pellet was carried out using TriFast TM reagent (PeqLab. Biotechnologie GmbH, Carl-Thiersch St. 2B 91052 Erlongen, Germany, Cat. No.30-2010) according to the manufacturer’s instructions. The remaining DNA was removed by digestion with DNase I (Sigma). The concentration of isolated RNA was determined spectrophotometrically by measuring the optical density at 260 nm (Jenway, Genova Model, UK). 10 ul of each sample was added to 990 ul of DEPC treated water and quantified by measuring the absorbance at 260 nm as RNA yield (ug/ml) = A260 × 40 × 100 (dilution factor) [9]. The purity of RNA was determined by gel electrophoresis using formaldehyde agarose gel electrophoresis and ethidium bromide staining to show two sharp purified bands representing 28S and 18S ribosomal RNA.
-
RT-PCR for extracted RNA:
Semi quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed using the QIAGEN One-step RT-PCR kit (QIAGEN Inc. 28159 Avenue Stanford, Valencia, USA) according to the method of McPherson and Moller [10]. It utilizes omniscript and sensiscript reverse transcriptase enzymes and HotStarTaq DNA polymerase to generate a PCR product from RNA template. Each test is optimized to allow the first strand cDNA synthesis and PCR reaction to proceed sequentially as a single tube, single step reaction. A reaction mix (40 μl/reaction) was prepared as 2 μl of dNTP mix; 3 μl containing 30 pmol of PCR sense primer, 3 μl containing 30 pmol of PCR anti-sense primer, 2 μl One-step RT-PCR enzyme mix, 10 μl 5× one-step RT-PCR buffer and 20 μl of DEPC-treated water to obtain a total volume of 40 μl. The reaction mix was mixed thoroughly by pipetting few times. Then, 10 μl of each template RNA were added in each tube and mixed well. One tube was prepared as a negative control reaction to test for DNA contamination. The reactions were transferred to the thermal cycler (Techne C-312) and incubated at 50 °C for 30 min for synthesis of cDNA followed by incubation at 95 °C for 15 min to inactivate the reverse transcriptase and completely denature the template. Gene specific primers were purchased from Biolegio. BV, PO Box 91, 5600 AB Nijmegen, Netherlands. The oligonucleotide primers for Cu, Zn-SOD; sense: 5′-CGA GCA GAA GGA AAG TAA TG-3′ and antisense: 5′-TAG CAG GAT AAC AGA TGA GT-3′ were designed on the basis of the human Cu/Zn-SOD (455 bp) [11]. Two oligonucleotide primers: sense 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ and anti-sense 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′ were also used to amplify GAPDH as an internal control (310 bp). Thermal cycling reaction was performed using thermal cycler (Techne C-312) with the following program: 30 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min then final extension at 72 °C for 10 min.
-
Detection of amplified RT-PCR products:
Specific PCR products were subjected to agarose gel electrophoresis using 2 % agarose stained with ethidium bromide and visualized via light UV Transilluminator (Model TUV-20, OWI. Scientific, Inc. 800 242-5560) and photographed under fixed conditions (the distance, the light and the zoom). No products were detected when the RT-PCR step was carried out with no added RNA, indicating that all reagents were free target sequence contamination. Assessment of the amount of Cu/Zn-SOD mRNA expression was performed comparatively using GAPDH mRNA expression as a control.
The primary outcome was the embryo quality for proper selection of good oocytes for ICSI in PCOS patients.
Statistical analysis
Statistical analysis was performed using SPSS software (Inc., Chicago, IL). Sample size for 50 follicle were needed in each group so as to gain a significant difference of 22 % in fertilization rate at a significant level of 5 % and a power of 80 %. Intergroup differences were evaluated with unpaired t-test and Pearson’s correlation between different variables. Statistical significance was set at p < 0.05.
Results
In the present study, there were no statistical significant differences noted between both groups regarding the age and BMI. There was no significant difference in the duration of infertility (4.21 + 1.68 vs. 4.34 + 1.89 years). The mean levels of LH and FSH were significantly higher (p < 0.001) in women with PCOS, that was physiologically expected than the control group. The SOD activity were detected in all samples, there was a statistically significant decrease (p < 0.001) in the mean serum SOD activity (45.56 ± 18.06) in PCOS than the control group (77.38 ± 7.82). However, the mean serum SOD activity was higher in both groups than the FF SOD activity (Table 1).
Table 1.
Clinical and Laboratory criteria of the studied groups
| PCOS (N° = 20 cases) | Control (N° = 20 cases) | P value | |
|---|---|---|---|
| Age (yrs) | 31.2 ± 1.10 | 29.4 ± 2.1 | 0.35 |
| BMI (kg/m2) | 31.3 ± 3.2 | 29.2 ± 3.6 | 0.41 |
| FSH (IU/L) | 9.4 ± 1.9 | 5.2 ± 2.1 | < 0.001** |
| LH (IU/L) | 11.2 ± 2.3 | 6.1 ± 1.1 | < 0.001** |
| E2 on the day of HCG administration (pg/ml) | 2978 ± 325 | 1439 ± 437 | < 0.001** |
| Serum SOD activity (U/mg protein) | 45.56 ± 18.06 | 77.38 ± 7.82 | < 0.001** |
| N° of injected oocytes | 54 | 48 | |
| SOD activity in FF samples (U/mg protein) | 42.49 ± 11.46 | 74.37 ± 6.15 | <0.001** |
| SOD mRNA expression in FF cells | 0.36 ± 0.14 | 0.81 ± 0.15 | <0.001** |
| Fertilization Nº (%) | 90 (83.3 %) | 88 (91.6 %) | 0.26 |
| Embryo quality | |||
| A | 50 | 56 | 0.22 |
| B | 26 | 28 | |
| C | 14 | 4 | |
| N° of transferred embryo | 33 | 30 | |
| N° of pregnancies | 6 (30 %) | 8 (40 %) | 0.84 |
Results were expressed as mean ± SD, frequency. *Indicates statistical significance at P < 0.05, **indicates statistical significance at P < 0.001
In PCOS, fertilization was successful in 90 out of 108 oocytes (83.3 %), while in the control group, fertilization occurred in 88 out of 96 oocytes (91.6 %). The mean FF SOD activity were statistically lower (42.49 ± 11.46) in PCOS cases than the control group (74.37 ± 6.51); (p < 0.001). However, there was no significant difference in the mean FF SOD activity in relation to embryo quality as well as the pregnancy rates between both groups (Table 1). Moreover, there was non-significant difference in the mean FF SOD activity between fertilized and non fertilized oocytes in both groups (Table 2).
Table 2.
Comparison between FF SOD activity of fertilized & non fertilized oocytes in both groups
| Oocytes in PCOS cases (N° = 108) | Oocytes in control cases (N° = 96) | |||
|---|---|---|---|---|
| Fertilized (N° = 90) | Non fertilized (N° = 18) | Fertilized (N° = 88) | Non fertilized (N° = 8) | |
| SOD activity in FF | 45.22 ± 11.17 | 39.15 ± 13.12 | 68.03 ± 11.23 | 59.13 ± 8.01 |
| t | 1.11 | 1.54 | ||
| p | 0.25 | 0.13 | ||
Results were expressed as mean ± SD
The mean Cu/Zn-SOD mRNA expression in FF cells was statistically lower in PCOS cases than the control group (p < 0.001). Also, non-significant differences in the mean SOD mRNA expression between fertilized and non fertilized oocytes were found (Table 3). 1–3 embryos at day 3 were transferred for each case, 23 embryos were transferred in PCOS cases, and 20 embryos in the control group. There was a significant positive correlation between serum SOD activity and FF SOD activity (p = 0.012) in both groups. There was a highly significant positive correlation between FF SOD activity and Cu/Zn-SOD mRNA expression in cells isolated from the FF in both groups (Table 4).
Table 3.
Comparison between Cu/Zn-SOD mRNA expression in FF cells of fertilized & non fertilized oocytes in both groups
| Oocytes in PCOS cases (N° = 108) | Oocytes in control cases (N° = 96) | |||
|---|---|---|---|---|
| Fertilized (N° = 90) | Non fertilized (N° = 18) | Fertilized (N° = 88) | Non fertilized (N° = 8) | |
| SOD mRNA expression in FF cells | 0.37 ± 0.91 | 0.33 ± 0.12 | 0.82 ± 0.11 | 0.79 ± 0.01 |
| t | 1.31 | 1.12 | ||
| p | 0.17 | 0.35 | ||
Results were expressed as mean ± SD
Table 4.
Pearson correlation coefficient between SOD parameters in PCO patients
| Serum SOD activity | FF SOD activity | SOD mRNA expression in FF cells | ||
|---|---|---|---|---|
| Serum SOD activity | r p |
– | 0.579 0.012* |
0.152 0.547 |
| FF SOD activity | r p |
0.579 0.012* |
– | 0.690 0.002** |
| SOD mRNA expression in FF cells | r p |
0.152 0.547 |
0.690 0.002** |
– |
*Correlation is significant at the 0.05 level (2-tailed)
**indicates statistical significance at P < 0.001
Discussion
In the present study, there was a statistically highly significant decrease (p < 0.001) in the mean serum SOD activity in PCOS than the control group. These results were in agreement with Zhang et al. [12]. This systemic decrease in SOD activity may be due to utilization of SOD in response to augmented production of ROS due to both hyperglycemia and excess free fatty acids. It was reported that OS in PCOS induces a proinflammatory state that may contribute to co-morbidities as abdominal obesity, endothelial dysfunction, dyslipidemia, hyperandrogenism, elevated insulin levels, and insulin resistance [13, 14].
Follicular fluid provides a very important microenvironment for the development of oocytes, some biochemical constituents of the FF may play a critical role in determining oocyte quality and the subsequent potential to achieve fertilization and embryo development. Studies have suggested that the health and function of the granulosa cells and cumulus cells may be reflective of the health status of the enclosed oocyte [4, 6]. Although, follicular fluid SOD activity was significantly lower in PCOS cases than the control group, it has no significant effect on either fertilization rate or embryo quality. The existence of SOD in the microfollicular environment as an attempt to counteract the excessive production of ROS to protect the oocytes from ROS-dependent apoptosis [15, 16]. Also, decreased SOD activity has been shown to influence cell functions by decreasing the levels of the second messenger cGMP [17].
If H2O2 produced by SOD, is not removed immediately it will react with super oxide radicals giving rise to highly reactive hydroxyl radicals, also the accumulation of hydrogen peroxide lowers both cAMP dependent and non-cAMP dependent steroidogenesis, decreases gonadotropin action and inhibition of progesterone secretion [11]. Serum SOD activity was higher in both groups than FF SOD (Table 2), the results were in contrary to that reported by others [4].
FF is a product of both the transfer of blood plasma constituents that cross the blood follicular barrier and of the secretory activity of granulosa and theca cells. There was a significant positive correlation between serum SOD activity and FF SOD activity (p = 0.012*) in both groups. This indicates that the main source of FF SOD may be the degenerated GCs [18], while diffusion of SOD from the systemic circulation play a minor role.
Decreased FF SOD activity and SOD mRNA in granulosa cells may signal the existence of OS in the microfollicular environment, these may be derived from degenerating or dead granulosa cells, about 20 % of granulosa cells in women undergoing ovarian stimulation are apoptotic [19], these may indicate its role in the oocyte maturation process, but it has no effect on fertilization rate as well as the embryo quality. Due to our small sample size, further study with the inclusion of a larger sample of women in both groups, is needed to extend these findings.
Carbone et al. (2003), reported that follicular fluid from older women exhibited a higher level of SOD activity, and reduced levels of glutathione peroxidase, that could impair ROS scavenging efficiency in the follicular environment [20]. Variations in SOD activity emphasize the importance of oxidative stress in the oocyte maturation process, and are suggested to be a potential biomarker of ART success [21]. Up to the best of our knowledge, this is the first report on SOD mRNA in GCs in humans, other investigators measure it in bovine (Combles) with the high content in FF from small diameter follicles [22]. Keeping SODs at reduced concentrations within the follicular fluid milieu may provide the appropriate balance of superoxide anion and hydrogen peroxide for normal oocyte function.
Conclusion
Serum SOD activity could become a clinical parameter for determining systemic OS in PCOS. Although FF SOD activity and expression were lowered, they have no effect on fertilization rate and embryo quality.
Footnotes
Capsule Serum superoxide dismutase (SOD) activity could be a clinical parameter for determining systemic oxidative stress in PCOS.
References
- 1.Kuscu NK, Var A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2009;88(5):612–617. doi: 10.1080/00016340902859315. [DOI] [PubMed] [Google Scholar]
- 2.Sabuncu T, Vural H, Harma M, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34(5):407–413. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 3.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24(8):1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 4.Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril. 1999;72(6):1027–1034. doi: 10.1016/s0015-0282(99)00411-2. [DOI] [PubMed] [Google Scholar]
- 5.Verit FF, Erel O. Oxidative stress in nonobese women with polycystic ovary syndrome: correlations with endocrine and screening parameters. Gynecol Obstet Invest. 2008;65(4):233–239. doi: 10.1159/000113046. [DOI] [PubMed] [Google Scholar]
- 6.Jana SK, Babu NK, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29(4):447–451. doi: 10.1016/j.reprotox.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Chin-Kun BL, Sajal G, Nabil N, Ashok A. Role of oxidative stress in polycystic ovary syndrome. Curr Women’s Health Rev. 2010;6(2):96–107. [Google Scholar]
- 8.Nishikimi M, Roa NA, Yogi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 9.Raha S, Ling M, Merante F. Extraction of total RNA from tissues and cultured cells. In: Rapley R, Walker JM, editors. Molecular biomethods handbook. Humana Press Inc., Totowa, NJ. Ch. 1; 1998. p. 1–8.
- 10.McPherson MJ, Moller SG. Analysis of gene expression. In: McPherson MJ, Moller SG, editors. PCR. BIOS Scientific Publishers Ltd, Oxford. Ch. 8; 2000. p. 183–211.
- 11.Sogino N, Takiguchi S, Kaschida S, Karube A, Nakamura Y, Kato H. Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Mol Hum Reprod. 2000;6(1):19–25. doi: 10.1093/molehr/6.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Luo WY, Liao H, Wang CF, Sun Y. The effects of oxidative stress to PCOS. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39(3):421–423. [PubMed] [Google Scholar]
- 13.Rajendran S, Willoughby SR, Chan WP, Liberts EA, Heresztyn T, Saha M, et al. Polycystic ovary syndrome is associated with severe platelet and endothelial dysfunction in both obese and lean subjects. Atherosclerosis. 2009;204(2):509–514. doi: 10.1016/j.atherosclerosis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species induced oxidative stress in the development of insulin resistance and hyperandrogenismin polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(1):336–340. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 15.Bausenwein J, Serke H, Eberle K, Hirrlinger J, Jogschies J, Abu Hmeidan F, et al. Elevated levels of oxidized low-density lipoprotein and of catalase activity in follicular fluid of obese women. Mol Hum Reprod. 2010;16(2):117–124. doi: 10.1093/molehr/gap078. [DOI] [PubMed] [Google Scholar]
- 16.Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Zeev Reznick A, Ishai D, et al. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8(Suppl 1):S40–S42. doi: 10.1016/s1071-5576(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 18.Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9(11):639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- 19.Centurione L, Giampietro F, Sancilio S, Piccirilli M, Artese L, Tiboni GM, et al. Morphometric and ultrastructural analysis of human granulosa cells after gonadotrophin-releasing hormone agonist or antagonist. Reprod BioMed Online. 2010;20(5):625–633. doi: 10.1016/j.rbmo.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Carbone MC, Tatone M, Monache SD, et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age‐dependent changes MHR: basic science of reprod. Medicine. 2003;9(11):639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- 21.Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod. 2009;15(7):411–419. doi: 10.1093/molehr/gap034. [DOI] [PubMed] [Google Scholar]
- 22.Combelles CMH, Holick AE, Paolella JL, Walker CD, Wu Q. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction. 2010;139:871–881. doi: 10.1530/REP-09-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]