Abstract
Purpose
Antioxidant and anti-apoptotic effects of melatonin on development of in vitro fertilization (IVF)/vitrified two-cell mouse embryos were evaluated in this study.
Methods
The IVF two-cell embryos were vitrified by cryotop, and were cultured in KSOM medium in different concentrations of melatonin (10−6, 10−9, 10−12 M) and without melatonin. The blastocyst cell number, apoptotic cells and glutathione (GSH) level were evaluated by differential, TUNEL and cell tracker blue staining, respectively. The expression of Bax and Bcl-xl genes was evaluated by qPCR. The expression of melatonin receptors (Mtnr1a and Mtnr1b) in mouse 2-cell embryos and blastocysts was evaluated by RT-PCR.
Results
Melatonin increased the rate of cleavage and blastulation at 10−12 M concentration (p < 0.05). The number of trophectoderm and inner cell mass showed a significant increase (p < 0.05) in 10−9 M melatonin. The 10−9 M and 10−12 M melatonin treatments significantly reduced (p < 0.05) the apoptotic index. The significant increase in the expression of Bcl-xl observed at 10−9 M concentration however, reduced expression of Bax was not statistically significant. The levels of GSH in 10−9 and 10−12 M groups were significantly improved relative to the control group (p < 0.05). The Mtnr1a was expressed in 2-cell embryos and blastocysts in all groups, but the expression of Mntr1b was not detected.
Conclusion
Melatonin may have a special role against oxidative stress in protection of IVF/vitrified embryos.
Keywords: Melatonin, Vitrification, IVF, Reactive oxygen species, Apoptosis
Introduction
Frozen embryos may be one solution to overcome many of the problems associated with in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). The problems that occur include ovarian hyperstimulation syndrome, increased risk of breast and uterine cancers, and elevated rate of multiple births [1, 2].
Vitrification is the best and most rapid method for cryopreservation of embryos. The high success rate of vitrification minimizes the period of embryo exposure to cryoprotectants such as ethylene glycol (EG) and dimethyl sulfoxide (DMSO); these reduce osmotic stress and prevent formation of ice crystals [3]. However, vitrification also has harmful effects on survival and development of embryos [4, 5]. Thus, low rate of preimplantation embryo development is the main problem due to creation of reactive oxygen species (ROS) during the process [6]. ROS causes damage to the embryo including membrane lipid peroxidation, amino acid and nucleic acid oxidation, adenosine triphosphate (ATP) depletion, and mitochondrial dysfunction [7]. In addition, ROS induces the mitochondria-dependent apoptotic response [8]. In the response to oxidative stress, the functional balance of Pro-apoptotic (Bax, Bak, Bad) and anti-apoptotic (Bcl-2, Bcl-w, Bcl-xl) mitochondrial proteins are altered which eventually lead to apoptosis [9].
Harmful effects of ROS can be controlled or inhibited by intracellular antioxidant systems such as glutathione (GSH), ascorbic acid, and enzymes such as superoxide dismutase and catalase [10]. GSH is a major intracellular free thiol group that protects cells from toxicants and ROS [11, 12]. GSH is required for embryo development until blastocyst stage after IVF [12]. It appears that in vitro culture of mammalian embryos, especially after vitrification, produces more free radicals that overwhelm the antioxidant capacity of the embryos. Under normal conditions, embryos have a defensive capacity against ROS but this support may be reduced following vitrification of embryos [13]. Attention has been focused on addition of antioxidants to the culture medium of preimplantation embryos as a means of protecting ROS [2]. Various systems of free radical scavengers have been studied for in vitro culture of mammalian embryos, of which melatonin is one of the most important scavengers [14, 15].
Melatonin (N-acetyl-5-methoxytryptamine) is the most important multi-functional indole which is produced by the pineal gland and other organs [16, 17]. Melatonin and its metabolites are powerful free radical scavengers with antioxidants with anti-apoptosis properties [18, 19]. In comparison to other known scavengers, due to its lipophilic properties, melatonin is considered as a hydrophobic antioxidant. This property enables it to easily pass morphophysiological barriers without receptors or a specific location [20]. In particular, melatonin plays a protective role in mitochondrial function and homeostasis, reducing and preventing mitochondrial oxidative stress, which leads to a reduction in apoptosis [15, 21, 22].
Although melatonin protects different cells by powerful scavenger actions, melatonin membrane receptors [MT1 (Mtnr1a), MT2 (Mtnr1b) and MT3] play an important role in cell protection [23]. Melatonin MT1 receptors have been localized in various tissues but melatonin MT2 receptors are mainly found in the brain, although their presence has also been detected in other tissues [24, 25].
Several studies have investigated the effects of melatonin on reproductive efficacy. In the porcine oocyte culture, melatonin at physiological concentrations (10−9 M), significantly increased the rate of oocyte maturation and blastulation [21, 22]. Beneficial effects of melatonin on the development of in vivo two-cell mouse embryos indicated an elevated development rate [26]. The results of Abecia and co-workers [27] in which sheep blastocysts were vitrified and thawed showed that the hatching rate of blastocysts was increased while the rate of embryo decline was decreased.
To our knowledge, there have been no reports on the effect of melatonin in the culture medium of IVF/vitrified 2-cell mouse embryo during in vitro preimplantation development. Therefore, the aim of the present study was to investigate the effect of melatonin on the development of IVF/vitrified 2-cell mouse embryos in vitro.
Materials and methods
All chemicals were purchased from Sigma Chemical Corporation (St. Louis, MO) except otherwise specified.
Oocyte and sperm collection and in vitro fertilization
Six to eight week-old NMRI female mice (Pasteur Institute, Tehran, Iran) were superovulated with 10 IU of pregnant mare serum gonadotropin (PMSG), followed by 10 IU of human chorionic gonadotropin (HCG) 48 h later. Metaphase II (MII) oocytes were collected from the oviductal ampullae 13 or 14 h after injection of HCG. The oocytes cumulus complexes were released into FHM medium. Subsequently, they were placed in 50 μl droplets of KSOM fertilization medium containing 15 mg/ml bovine serum albumin (BSA), and were covered with mineral oil.
Epididymal sperm from 10-week-old NMRI mice (Pasteur Institute, Tehran, Iran) was used for IVF of oocytes of the same strain. Cauda epididymides and vas deferens were excised using a pair of small scissors. Blood and adipose tissue were removed, and then a dense mass of sperm was squeezed out by using sharply pointed forceps. This sperm mass was placed in 0.5 ml Ham’s F10 medium supplemented with 4 mg/ml BSA in a petri dish. The sperm suspension was left at 37 °C for 10 min to allow the spermatozoa to disperse into the medium. After incubation for 10 min, the sperm suspension (free of excess tissue and aggregates) was centrifuged at 3,000 rpm for 3 min. The supernatant was decanted, and 0.5 ml of warm Ham’s F10 medium was added to pellet and placed at 37 °C, 5 % CO2 for 45 min. After swim-up period, 5 × 106 sperms were added to 50 μl of fertilization media droplets containing 10 oocytes and incubated at 37 °C under 5 % CO2 in humidified air for 6 h.
Presumptive zygotes were washed 3 times in FHM medium and then transferred into fresh KSOM and cultured for 24 h at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air.
Vitrification of mouse embryos by cryotop
Mouse embryos in 2-cell stage were vitrified by a two-step procedure with the KITAZATO Vitrification KIT (Kitazato Biopharmaceuticals, Shizuoka, Japan) using the cryotop (Kitazato) as carrier. The test was performed based on the procedure described by Kuwayama et al. [28].
Embryos were initially equilibrated in equilibration solution (ES) consisting of 7.5 % (v/v) EG and 7.5 % (v/v) DMSO at room temperature for 2 min, and were placed in vitrification solution (VS) consisting of 15 % (v/v) EG, 15 % (v/v) DMSO and 0.5 mol/l sucrose and then washed 3 times. Within less than 60s, three to five embryos in minimal VS (<1 μl) were placed onto the inner surface of the cryotop carrier. The cryotop was plunged vertically into liquid nitrogen, and inserted into a protective straw-cap prior to cryo-storage within liquid nitrogen.
Warming
Vitrified embryos were kept in liquid nitrogen for 10–14 days. The embryos were warmed using a four-step dilution procedure (Kitazato Biopharmaceuticals, Shizuoka, Japan). Briefly, the cryotop containing the embryos was removed from the protective straw-cap and dipped into warming solution containing 0.5 mol/L sucrose at 37 °C. After 1 min equilibration, the embryos were transferred into diluent solutions containing 0.3 M and 0.1 M sucrose respectively for 3 min. Subsequently, the embryos were transferred to 0 M sucrose washing solution 1 for 3 min. The survival rate of embryos was assessed by observing the intactness of blastomeres and zona pellucida.
Melatonin treatment of embryos
To assess the effect of melatonin on embryo development, the recovered embryos were cultured to the blastocyst stage (96 h) in KSOM, KSOM containing DMSO and KSOM containing three different concentrations of melatonin (KSOM containing 10−6, 10−9 and 10−12 M melatonin). The concentration of DMSO is equal to 10−6 M in KSOM medium (the same amount as the embryos receiving melatonin treatment in 10−6 M group). The number of two-cell, four-cell, morula embryos and blastocysts were recorded 1, 2, 3 and 4 days later. Blastocyst rate, blastocyst cell number, trophectoderm (TE), inner cell mass (ICM), apoptotic cells and gene expression were evaluated 96 h after culture [29].
Differential staining
The number of TE and ICM were evaluated by differential staining using Kaidi protocol [30]. Briefly, IVF/vitrified 2-cell embryos reaching the blastocyst stage were freed of zona pellucida by using acid Tyrode’s solution and were incubated in rabbit anti-mouse serum in FHM (1:2) solution for 30 min at 37 °C. Afterwards, they were briefly washed in FHM and incubated in 30 % guinea pig complement in FHM supplemented with 10 μg/ml propidium iodide (PI) and 10 μg/ml Hoechst H33342 for 30 min. After incubation in complement, blastocysts were fixed in ethanol and transferred to glycerol on glass slides, and were flattened with coverslips. They were immediately observed and photographed to count the nuclei under a fluorescent microscope.
TUNEL assay
TUNEL staining was done by in situ cell death detection kit TMR red (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Embryos were transferred to permeabilisation solution (0.1 % triton-x100 +0.1 % sodium citrate) for 2 min. Fixed embryos were incubated in TUNEL reaction medium for 1 h at 37 °C in the dark. After the reaction was stopped, the embryos were washed and transferred to DAPI (10 μg/ml) for 30 s at room temperature in the dark . The embryos were washed three times and mounted on slides. The number of apoptotic nuclei and total number of nuclei were determined from optical image of whole-mount embryos under a fluorescence microscope [31].
Measurement of intracellular glutathione (GSH) level
To assess the effect of melatonin on GSH level, the recovered 2-cell embryos were cultured up to 1 h in KSOM, KSOM containing DMSO and KSOM containing three different concentrations of melatonin (KSOM containing 10−6, 10−9 and 10−12 M melatonin concentrations). Intracellular GSH levels were determined by previously reported methods [14, 32]. Briefly, cell tracker blue CMF2HC (4-chloromethyl-6, 8-difluoro-7-hydroxycoumarin; Molecular probe, Life technology) was used to detect intracellular GSH level by blue fluorescence. Ten embryos from each treatment group were washed with FHM containing 0.1 % PVA, and were incubated for 30 min (in the dark) in KSOM supplemented with 10 μM cell tracker blue. After incubation, the embryos were washed using FHM containing 0.1 % PVA, and the fluorescence was observed by epifluorescence microscope (Nikon, Japan) with UV filters (371 nm). Fluorescent images were saved, and the fluorescence intensities of embryos were analyzed using Image J software (Version 1.45 s; National Institutes of Health, USA) as mean values of fluorescence.
Simultaneous RNA extraction and cDNA synthesis
Preimplantation embryos at blastocyst stages of development were transferred to Eppendorf tube containing 1.5 μl lysis buffer [33]. 2 μl random hexamer and 5 μl water was added to each 2 μl embryo sample. The samples were placed in a thermocycler for 5 min at 75 °C for the reaction to take place. After this time, the tubes were placed on ice, and 5× RT Buffer, 200 u RT Enzyme, 10 mM dNTP, 10 u RNase inhibitor was added to the reaction. Both RT and PCR reactions were performed on an applied Bio Rad thermocycler. The amplification program for the reverse transcription step was as follows: 25 °C for 10 min, 37 °C for 15 min, 42 °C for 45 min and 72 °C for 10 min. After the reverse transcriptase reaction, the samples were kept at 4 °C overnight; then, PCR mixture was added to each sample: 1.25 μl Taq Polymerase, 20.75 μl Master Mix 2 μl cDNA and 2 μl specific primer (Table 1). The endogenous control (Hprt1) and the two investigated genes were amplified with the following PCR cycle program: 94 °C for 3 min (denaturation), 94 °C for 30 s (denaturation), 60 °C for 45 s (annealing), and 72 ° C for 45 s (extension) followed by 40 cycles. Final elongation step was performed at 72 °C for 7 min. Ten microliters of PCR product was mixed with 1 ml loading buffer and electrophoresed on a 2 % agarose gel in TAE for 25 min. The products were visualized under short wave UV. Mouse diencephalon was used as a positive control for melatonin receptors. Total RNA from diencephalon was extracted by using Qiazol reagent (Qiagen). Complementary DNA synthesized using first strand cDNA synthesis kit (Fermentas) according to the manufacture’s instruction.
Table 1.
Primers used in this study in RT-PCR and real time quantitative PCR
| Genes | Primer sequence | Accession no |
|---|---|---|
| Bax | Forward: CAG CGG CAG TGA TGG AC | NM_007527.3 |
| Reverse: TCC TGG ATG AAA CCC TGT AG | ||
| Bcl-xl | Forward: CAG TCA GCC AGA ACC TTA TC | NM_009743.4 |
| Reverse: AAC ACC TGC TCA CTT ACT GG | ||
| Hprt1 | Forward: TCC CAG CGT CGT GAT TAG | NM_013556.2 |
| Reverse: CGA GCA AGT CTT TCA GTC C | ||
| Mtnr1a | Forward: GAC ATT CTG GGC AAC CTG | NM_008639.2 |
| Reverse: GTC AGC ACC AAG GGA TAA | ||
| Mtnr1b | Forward: TAC AGT AGT CGT CGT CAC CAC | NM_145712.2 |
| Reverse: CAA GGC TAT CAC CAA GTC AG |
Quantitative PCR analysis
Real-time quantitative PCR was performed to assess the expression of Bax and Bcl-xl genes using Rotor Gene Q instrument (QIAGEN). Real time PCR reactions were carried out in a total volume of 13 μl according to the manuals for DNA Master SYBR Green I mix (Roche Applied Sciences). The primer concentrations were adjusted to 1 μM for each gene. The cycling parameters were 5 s at 95 °C, 3 min at 95 °C for denaturation, 15 s at 60 °C, 10 s at 72 °C for amplification and 40 cycles of extension. The specificity of all individual amplification reactions was confirmed by melting curve analysis. The assays used Hprt1 as the endogenous internal house-keeping gene. Three replications were performed and the mRNA level of each sample was normalized to that of Hprt1 mRNA level.
Statistical analysis
All statistical analyses were performed using SPSS ver. 16 software (SPSS, Chicago, IL, USA). The means of cleavage and developmental ratio to the blastocyst stage were compared by non-parametric analysis test (Kruskal-Wallis). Glutathione levels, average number of cells in blastocysts and TUNEL positive cells in blastocyst obtained under each culture conditions were compared by ANOVA (Turkey’s HSD post-hoc test). The relative levels of mRNA were analyzed by REST 2009 Software (Qiagen). Data were expressed as means ± SD. A statistically significant difference was accepted at P < 0.05.
Results
A total of 570 oocytes were used for IVF; 451 oocytes were fertilized. Thus, the fertilization rate was 79 %. After IVF, 2- cell embryos were vitrified; this was associated with survival rate of 95 %. The effect of melatonin supplementation on cleavage rates and blastocyst formation of IVF/Vitrified 2- cell mouse embryo was evaluated; these data are shown in Table 2. The cleavage and blastocyst rates in 10−12 M melatonin and development to blastocyst stage in 10−9 M melatonin were significant improved relative to the control group (p < 0.05).
Table 2.
Development of IVF/vitrified 2-cell mouse embryo, cultured in the medium supplemented with different concentrations of melatonin.
| Group | Two-cell stage | Four-cell stage | Eight-cell stage | Morula | Blastocysts |
|---|---|---|---|---|---|
| Control | 77 | 67 (88.84 %) | 61 (80.96 %) | 58 (75.96 %) | 51 (65.92 %) |
| DMSO | 82 | 69(84.85 %) | 64 (75.36 %) | 58 (69.36 %) | 55 (66.21 %) |
| Melatonin 10−6 M | 78 | 64(80 %) | 63 (78.8 %) | 59(74.46 %) | 54 (68.44 %) |
| Melatonin 10−9 M | 77 | 66 (86.24 %) | 64 (83.33 %) | 61(79.70 %) | 61(79.70 %)* |
| Melatonin 10−12 M | 81 | 76 (95.26 %) | 72 (90.98 %) | 71(89.98 %)* | 71(89.98 %)* |
*P < 0.05 versus the control group
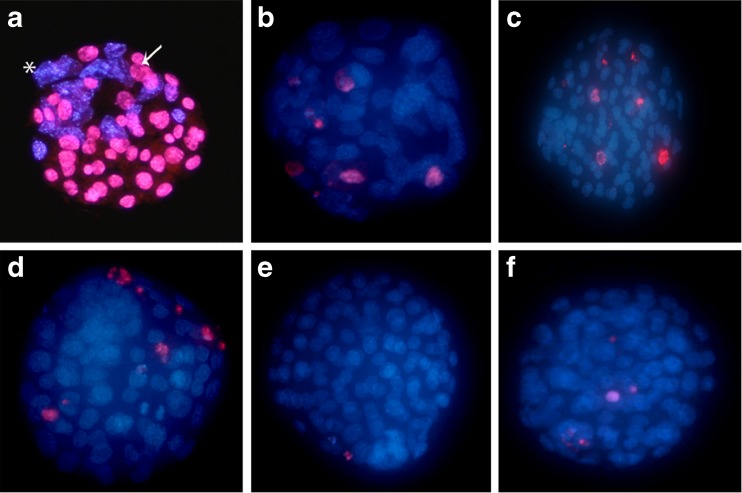
Blastocysts from each group were double stained to determine the cell number of ICM and TE (Fig. 1a). Table 3 summarizes the results of cell number in each group. The group supplemented with 10−9 M melatonin showed a significantly higher (p < 0.05) cell number in ICM, TE and total number of cells in comparison to control group. The average percent of ICM cells was 14.60 ± 3.13 and 22.70 ± 5.88 in control and melatonin 10−9 M groups, respectively. The average percent of TE cells was 38.60 ± 16.35 and 59.60 ± 6.31 in control and melatonin 10−9 M groups, respectively.
Fig. 1.
a Fluorescence micrograph of a differentially labeled mouse blastocyst showing trophectoderm nuclei (arrow) and inner cell mass nuclei (star). b–f Apoptosis in IVF/vitrified mouse blastocysts. Nuclei are stained with DAPI (blue) and the apoptotic nuclei stained with TUNEL (red). b Control, c DMSO, d 10-6 M melatonin, e 10−9 M melatonin and f 10−12 M melatonin groups
Table 3.
The average number of total, ICM and TE cells and percentage of apoptotic cell in blastocysts of each group
| Group | No. of ICM | No. of TE | No. of Total cell | Apoptotic cell rate (% ± S.D) |
|---|---|---|---|---|
| (mean ± S.D) | (mean ± S.D) | (mean ± S.D) | ||
| Control | 14.60 ± 3.13 a | 38.60 ± 16.35 a | 53.20 ± 17.86 a | 10.25 ± 6.01 a,c |
| DMSO | 19.33 ± 4.38 a,b | 49.55 ± 11.42 a,b | 68.66 ± 11.32 a,b | 11.07 ± 5.94 a |
| Melatonin 10−6 M | 12.88 ± 5.92 a | 48.33 ± 14.29 a,b | 61.22 ± 14.72 a | 7.55 ± 3.24 a,b |
| Melatonin 10−9 M | 22.70 ± 5.88 b | 59.60 ± 6.31 b | 82.30 ± 11.45 b | 4.49 ± 1.81 b |
| Melatonin 10−12 M | 15.60 ± 4.94 a | 39.10 ± 12.53 a | 54.30 ± 15.62 a | 5.86 ± 4.58 a,b |
Within the same column, values with different letters were significantly different (P < 0.05)
To determine the frequency of apoptosis, the TUNEL assay was carried out (Fig. 1b-f). The 10−9 M and 10−12 M melatonin treatments significantly reduced (p < 0.05) the apoptotic index compared to those cultured in the absence of melatonin (Table 3).
The relative transcript abundance of the anti-apoptotic gene Bcl-xl and the proapoptotic gene Bax in blastocysts of IVF/vitrified mouse 2-cell cultured in the presence or absence of melatonin was measured by qPCR (Fig. 2). Expression of Bcl-xl mRNA was significantly (p < 0.05) enhanced in the blastocysts with 10−9 and 10−12 M melatonin treatments compared to the controls. The expression of Bax transcripts in blastocysts cultured with 10−9 and 10−12 M melatonin did not differ from control blastocysts. The Bcl-xl expression in 10−9 M melatonin group was significantly higher than other groups except 10−12 M melatonin treatment. Expression levels of both Bax and Bcl-xl genes in 10−6 M melatonin treatment were significantly reduced compared to control (Fig. 2). Bcl-xl expression in 10−12 M melatonin was significantly higher than 10−6 M melatonin treatment.
Fig. 2.
Relative expression levels of mouse Bcl-xl and Bax genes in the IVF/Vitrified cultured with or without melatonin. The mRNA levels of the genes were analyzed with qPCR. The mRNA level of each sample was normalized to that of Hprt1 mRNA level. ** Significant down regulation and * significant up regulation compared to the control
The effect of melatonin supplementation on GSH levels of IVF/Vitrified 2- cell mouse embryos was evaluated (Fig. 3). The intracellular levels of GSH in 10−9 (235.80 ± 4.87) and 10−12 M (233.20 ± 4.42) groups were significantly improved relative to the control group (219.09 ± 8.84) (p < 0.05).
Fig. 3.
Glutathione level of IVF/Vitrified 2-cells mouse embryos cultured in different concentrations of melatonin. Melatonin in 10−9 M and 10−12 M concentrations had significantly higher GSH levels compared with control group (*p < 0.05)
The expression of melatonin receptors (Mtnr1a and Mtnr1b) in mouse 2-cell embryos and blastocysts was evaluated by RT-PCR (Figs. 4 and 5). The Mtnr1a was expressed in mouse 2-cell embryos and blastocysts in all groups, but the expression of Mntr1b was not detected (Fig. 5).
Fig. 4.
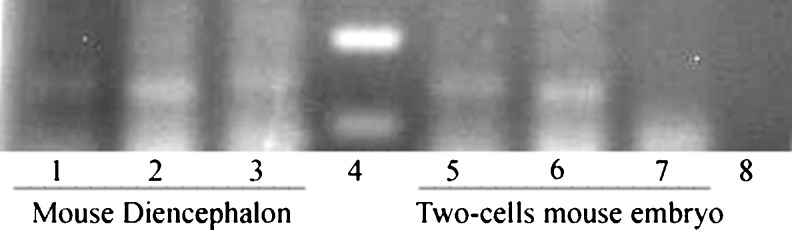
The expression of melatonin receptors (Mtnr1a, Mtnr1b) and the housekeeping gene (Hprt1) in mouse diencephalon (lanes 1, 2 and 3) and IVF/Vitrified 2-cell mouse embryos (lanes 5, 6 and 7). Lane 4 shows DNA molecular weight marker (100 bp ladder), and the lanes 1 and 5 indicate Hprt1. Using Mtnr1a and Mtnr1b primers, 137 bp and 143 bp bands corresponding to the expected size for the amplified products of Mtnr1a were detected in mouse diencephalon and IVF/Vitrified 2-cell mouse embryos (lanes 2 and 6). Mtnr1b band was observed in diencephalon (lane 3), but this band was undetectable in 2 cell embryos (lane 7). Lane 8 shows the PCR reaction without cDNA substrate as negative control
Fig. 5.
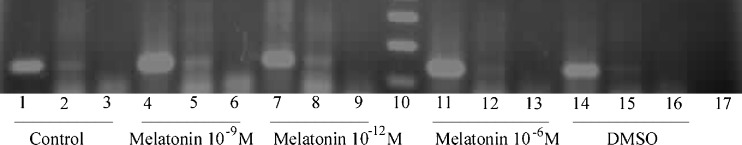
RT-PCR analysis of mRNA expression of Mtnr1a and Mtnr1b melatonin receptors in the IVF/Vitrified mouse blastocysts cultured in different concentrations of melatonin. Lane 10 shows DNA molecular weight marker (100 bp ladder), and the housekeeping gene Hprt1 was used as an internal control (lanes 1, 4, 7, 11 and 14). Lanes 2, 5, 8, 12 and 15 show the expression of Mtnr1a in all groups; however, melatonin receptor Mtnr1b was not expressed in embryo samples (lanes 3, 6, 9, 13 and 16). Lane 17 is negative control
Discussion
This study shows that vitrification of two-cell mouse embryos derived from IVF using cryotop achieved a 95 % rate of survival. Several studies on mammalian embryos have proven that the best tool for vitrification is cryotop [28, 34–36]. In the Zhang study [36], the vitrified mouse embryos derived in vivo with cryotop showed survival rates of 96 % for two-cell vitrified embryos. Yan and coworkers [37] vitrified two-cell mouse embryos derived from IVF with OPS, and reported the survival rate of 51 %. Manipulation of gametes and embryos during IVF and vitrification induces high levels of ROS [38]. It has been shown that intracellular GSH protects embryo from ROS [12]. Previous study has shown that the level of GSH decreased after vitrification and addition of melatonin increases the GSH levels [14]. The results of present study showed that the intracellular levels of GSH in 10−9 and 10−12 M groups were significantly improved relative to the control group. It has been shown that increase in GSH levels improved development of preimplantation embryo [11]. Furthermore, exposure of preimplantation embryos to GSH oxidizing agent decreases intracellular GSH levels and development of embryo to blastocyst stage [12]. Thus, melatonin increases the GSH level and improved development of 2-cell IVF/vitrified mouse embryos to blastocyst stage.
Previous studies have shown the beneficial effects of melatonin on mammalian embryo development [21, 22, 26, 27, 39, 40]. However, there is still the question of whether melatonin improves development of mouse two-cell embryos derived from IVF/vitrification from two-cell to blastocyst stages. Our results showed that 10−9 M and 10−12 M concentrations of melatonin had the best impact on development of 2-cell IVF/vitrified mouse embryos to blastocyst stage while the concentration of 10−6 M had no effect on embryo development compared to control group. The results of this study supports previous findings on the development of embryos cultured with melatonin.
Although the number of embryos developed to the blastocyst stage is a good criterion for determining embryo development, use of other methods to determine the quality of the embryos cultured after thawing such as counting ICM and TE cells, total number of blastocyst cells, the rate of apoptosis and expression of apoptosis genes were also examined. The results of double staining suggested that melatonin at a concentration of 10−9 M led to a rise in the total, ICM and TE cells of blastocysts. Thus, melatonin, with antioxidant and anti-apoptotic properties, prevented cell death [39]. Tian and colleaguse [26] demonstrated that melatonin at 10−9 M concentrations leads to an increase in porcine blastocysts blastomeres, which is related to the antioxidant and anti-apoptotic properties of this indole.
Antioxidant and anti-apoptotic activity of melatonin and the supportive role of this molecule were studied in preimplantation embryo culture by Reiter et al. [41] and Gao et al. [14]. Mitochondrial DNA is a major target of free radicals. Mitochondrial DNA is among the known targets of melatonin in prevention of damage. Melatonin is involved in regulating the expression of antioxidant genes, and causes an increase of SOD expression [42]. In addition, metabolites of melatonin are also free radical scavengers [43]. Rodriguez-Osorio et al. [15] showed that developmental rate of IVM/IVF porcine embryos cultured with a concentration of 10−9 M melatonin did not significantly increase but blastocyst cell number was elevated significantly, while melatonin at 10−3 M concentration was lethal to embryos. Ishizuka et al. [21] suggested that the concentration of melatonin in mouse embryo development has a positive effect on 10−6 M while Rodriguez-Osorio et al. [15] claimed no significant difference in the cleavage stage of porcine embryo or blastocyst development in 10−6 M concentration. Gao et al. [14] vitrified two-cell mouse embryos obtained in vivo via OPS method, and found that melatonin at concentrations of 10−5 to 10−11 shows significant differences in the development of two-cell vitrified embryos to the blastocyst and increases the number of cells.
Survival of frozen embryos is usually determined by growth in medium after warming. However, the potential viability of the cell surface is not just determined by morphology. A reliable method for evaluation of cell survival is determination of the apoptosis rate doing by the TUNEL method. Our results indicate that melatonin concentrations of 10−9 and 10−12 M significantly reduced the number of apoptotic cells and improved the blastocysts quality.
In the present study, the expression of Bax did not change while expression of Bcl-xl increased significantly 4.99-fold at 10−9 M melatonin respect to control group. Increased expression (1.3 fold) of Bax at 10−12 M melatonin was not significant respect to control group; however Bcl-xl showed significant expression (3.17 fold) respect to control group. The expression of Bax and Bcl-xl are regulated by melatonin, although many studies have documented the effects of melatonin on downregulation of Bax and upregulation of Bcl-xl [31, 44].
Bcl-2 and other anti-apoptotic members (Bcl-2, Bcl-w, Bcl-xl) prevent apoptosis through functionally inhibiting pro-apoptotic proteins Bax and Bak. Death signals lead to changes in mitochondrial membrane permeability by releasing of cytochrome c, formation of apoptosome complex, pro- caspase activation and apoptosis cascade [31, 45].
Jang et al. in 2005 showed that melatonin can reduce the expression levels of caspase-3 and Bax while increasing the expression levels of Bcl-2 [45]. The inhibitory effect of melatonin has several mechanisms. Wang and his colleagues [46] demonstrated that melatonin, as a powerful antioxidant, removes toxic hydroxyl radicals and plays an important role in the survival of neurons. Transcription signals promoted via melatonin receptors stimulate anti-apoptotic effects of this indole [47]. This activates stat3 and leads to Bcl-2 and Bcl-xl expression, thus inhibiting the mitochondrial-dependent apoptotic pathway [48].
Melatonin also increases the expression of Bcl-2, while decreasing intracellular glutamate and increasing levels of gamma-amino butyric acid [49].
Previous studies have shown the expression of melatonin MT1 receptors in oocytes, cumulus cells and bovine blastocysts [50, 51]. Studies on other species have shown the presence of MT1 receptor transcripts in porcine cumulus cells and granulosa cells but not in oocytes [22]. Studying Kunming mice strain has not shown the transcript of MT1 and MT2 receptors in blastocysts [14]. The results of present study for the first time showed the expression of mRNA of MT1 receptors in 2-cell embryos and blastocysts of NMRI mice. However, RT-PCR analysis failed to detect the expression of MT2 receptors in 2-cell embryos and blastocysts of NMRI mice. Jin et al. showed that MT1 receptor mediates the majority of melatonin response at low concentrations of this hormone, but MT2 mediated response occurs at higher concentrations [52]. Failure to detect the MT2 receptors might be due to the melatonin concentration in our study or absence of these receptors in embryo. Gao and colleagues have reported that the beneficial effects of melatonin on vitrified mouse 2-cell embryos are not mediated by melatonin membrane receptors, and suggested that the potent free radical scavenging and antioxidant capacity of melatonin accounts for its protective effects on the development of vitrified 2-cell embryos [14]. However, expression of MT1 receptors in 2-cell embryos and blastocysts in the present study suggested that melatonin acts through its receptors and antioxidant capacity. The signal transduction pathways for melatonin receptors are varied among cell types [24, 25]. The MT1 receptors are coupled to different G proteins that mediate adenylyl cyclase (cAMP) inhibition and phospholipase C beta activation, leading to activation of a large variety of G proteins [24, 25]. However, in some cells, melatonin causes stimulation of cAMP, and this stimulatory effect is independent of G proteins [53]. Activation of MT1 receptor stimulates phosphoinositide turnover and inhibits the guanylate cyclase enzyme and can induce PKC activation by Ca2+ waves, in parallel with the inhibition of the cAMP pathways [22, 50, 51]. Cellular signaling of melatonin receptors needs further investigations to understand the melatonin role in preimplantation development.
In conclusion, low concentrations of melatonin improved the development and quality of embryos after vitrification. Anti-apoptotic activity and reduced expression of apoptosis genes may lead to increased survival and better quality of blastocyst embryos derived from two -cell IVF/vitrified embryos. Melatonin treatment may have a beneficial effect on embryo development after vitrification and could be a therapy for improving vitrified embryos in infertile patients. The usefulness of melatonin administration for improving the development of human vitrified embryos for clinical outcome of IVF and ICSI needs to be evaluated.
Acknowledgments
This paper was taken from the master’s thesis research project by Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical sciences.
Footnotes
Capsule Exogenous melatonin has been found to affect BCL-xl expression and enhance in vitro developmental potenial of mice embryos after vitrification.
References
- 1.Englert Y, Moens E, Vannin AS, Liesnard C, Emiliani S, Delbaere A, et al. Impaired ovarian stimulation during in vitro fertilization in women who are seropositive for hepatitis C virus and seronegative for human immunodeficiency virus. Fertil Steril. 2007;88(3):607–11. doi: 10.1016/j.fertnstert.2006.11.177. [DOI] [PubMed] [Google Scholar]
- 2.Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002;17(10):2686–93. doi: 10.1093/humrep/17.10.2686. [DOI] [PubMed] [Google Scholar]
- 3.Tsang WH, Chow KL. Mouse embryo cryopreservation utilizing a novel high-capacity vitrification spatula. Biotechniques. 2009;46(7):550–2. doi: 10.2144/000113125. [DOI] [PubMed] [Google Scholar]
- 4.Azadbakht M, Valojerdi MR. Development of vitrified-warmed mouse embryos co-cultured with polarized or non-polarized uterine epithelial cells using sequential culture media. J Assist Reprod Genet. 2008;25(6):251–61. doi: 10.1007/s10815-008-9231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuleshova LL, Lopata A. Vitrification can be more favorable than slow cooling. Fertil Steril. 2002;78(3):449–54. doi: 10.1016/s0015-0282(02)03305-8. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzuka T, Sakamoto N, Ozawa M, Ushitani A, Hirabayashi M, Kanai Y. Alleviation of maternal hyperthermia-induced early embryonic death by administration of melatonin to mice. J Pineal Res. 2005;39(3):217–23. doi: 10.1111/j.1600-079X.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Liu D, Wang J, Liu S, Gao M, Ling EA, et al. Cytoprotective effects of melatonin on astroglial cells subjected to palmitic acid treatment in vitro. J Pineal Res. 2012;52(2):253–64. doi: 10.1111/j.1600-079X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 8.Juknat AA, Mendez Mdel V, Quaglino A, Fameli CI, Mena M, Kotler ML. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res. 2005;38(2):84–92. doi: 10.1111/j.1600-079X.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 9.Maity P, Bindu S, Dey S, Goyal M, Alam A, Pal C, et al. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J Pineal Res. 2009;46(3):314–23. doi: 10.1111/j.1600-079X.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Falcone T, Attaran M, Goldberg JM, Agarwal A, Sharma RK. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78(6):1272–7. doi: 10.1016/s0015-0282(02)04236-x. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa M, Nagai T, Fahrudin M, Karja NW, Kaneko H, Noguchi J, et al. Addition of glutathione or thioredoxin to culture medium reduces intracellular redox status of porcine IVM/IVF embryos, resulting in improved development to the blastocyst stage. Mol Reprod Dev. 2006;73(8):998–1007. doi: 10.1002/mrd.20533. [DOI] [PubMed] [Google Scholar]
- 12.Salmen JJ, Skufca F, Matt A, Gushansky G, Mason A, Gardiner CS. Role of glutathione in reproductive tract secretions on mouse preimplantation embryo development. Biol Reprod. 2005;73(2):308–14. doi: 10.1095/biolreprod.104.038307. [DOI] [PubMed] [Google Scholar]
- 13.Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59(3–4):939–49. doi: 10.1016/s0093-691x(02)01125-1. [DOI] [PubMed] [Google Scholar]
- 14.Gao C, Han HB, Tian XZ, Tan DX, Wang L, Zhou GB, et al. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res. 2012;52(3):305–11. doi: 10.1111/j.1600-079X.2011.00944.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Osorio N, Kim IJ, Wang H, Kaya A, Memili E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J Pineal Res. 2007;43(3):283–8. doi: 10.1111/j.1600-079X.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 16.Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebesteny T, et al. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res. 2011;51(1):17–43. doi: 10.1111/j.1600-079X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 17.Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52(2):217–27. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 18.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoo YM, Jung EM, Choi KC, Jeung EB. Effect of melatonin on mRNA expressions of transcription factors in murine embryonic stem cells. Brain Res. 2011;1385:1–7. doi: 10.1016/j.brainres.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Kucukakin B, Gogenur I, Reiter RJ, Rosenberg J. Oxidative stress in relation to surgery: is there a role for the antioxidant melatonin? J Surg Res. 2009;152(2):338–47. doi: 10.1016/j.jss.2007.12.753. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res. 2000;28(1):48–51. doi: 10.1034/j.1600-079x.2000.280107.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang JT, Koo OJ, Kwon DK, Park HJ, Jang G, Kang SK, et al. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res. 2009;46(1):22–8. doi: 10.1111/j.1600-079X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 23.Espino J, Ortiz A, Bejarano I, Lozano GM, Monllor F, Garcia JF, et al. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil Steril. 2011;95(7):2290–6. doi: 10.1016/j.fertnstert.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 24.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–80. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–53. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Tian XZ, Wen Q, Shi JM, Liang W, Zeng SM, Tian JH, et al. Effects of melatonin on in vitro development of mouse two-cell embryos cultured in HTF medium. Endocr Res. 2010;35(1):17–23. doi: 10.3109/07435800903539607. [DOI] [PubMed] [Google Scholar]
- 27.Abecia JA, Forcada F, Zuniga O. The effect of melatonin on the secretion of progesterone in sheep and on the development of ovine embryos in vitro. Vet Res Commun. 2002;26(2):151–8. doi: 10.1023/a:1014099719034. [DOI] [PubMed] [Google Scholar]
- 28.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Salehi M, Kato Y, Tsunoda Y. Effect of melatonin treatment on developmental potential of somatic cell nuclear-transferred mouse oocytes in vitro. Zygote. 2013:1–5. doi:10.1017/S0967199413000336. [DOI] [PubMed]
- 30.Kaidi S, Bernard S, Lambert P, Massip A, Dessy F, Donnay I. Effect of conventional controlled-rate freezing and vitrification on morphology and metabolism of bovine blastocysts produced in vitro. Biol Reprod. 2001;65(4):1127–34. doi: 10.1095/biolreprod65.4.1127. [DOI] [PubMed] [Google Scholar]
- 31.Choi J, Park SM, Lee E, Kim JH, Jeong YI, Lee JY, et al. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol Reprod Dev. 2008;75(7):1127–35. doi: 10.1002/mrd.20861. [DOI] [PubMed] [Google Scholar]
- 32.Kere M, Siriboon C, Lo NW, Nguyen NT, Ju JC. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J Reprod Dev. 2012;59(1):78–84. doi: 10.1262/jrd.2012-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuccotti M, Boiani M, Ponce R, Guizzardi S, Scandroglio R, Garagna S, et al. Mouse Xist expression begins at zygotic genome activation and is timed by a zygotic clock. Mol Reprod Dev. 2002;61(1):14–20. doi: 10.1002/mrd.1126. [DOI] [PubMed] [Google Scholar]
- 34.Graves-Herring JE, Boone WR. Blastocyst rate and live births from vitrification and slow-cooled two-cell mouse embryos. Fertil Steril. 2009;91(3):920–4. doi: 10.1016/j.fertnstert.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 35.Hiraoka K, Kinutani M, Kinutani K. Case report: successful pregnancy after vitrification of a human blastocyst that had completely escaped from the zona pellucida on day 6. Hum Reprod. 2004;19(4):988–90. doi: 10.1093/humrep/deh177. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Cui J, Ling X, Li X, Peng Y, Guo X, et al. Vitrification of mouse embryos at 2-cell, 4-cell and 8-cell stages by cryotop method. J Assist Reprod Genet. 2009;26(11–12):621–8. doi: 10.1007/s10815-009-9370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan CL, Yang QE, Zhou GB, Hou YP, Zhao XM, Fan ZQ, et al. Open-pulled straw (OPS) vitrification of in vitro fertilised mouse embryos at various stages. Acta Vet Hung. 2008;56(2):245–53. doi: 10.1556/AVet.56.2008.2.12. [DOI] [PubMed] [Google Scholar]
- 38.Leon J, Acuna-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38(1):1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Zhang L, Zhang H, Liu B, Wu Z, Zhao W, et al. Exogenous melatonin modulates apoptosis in the mouse brain induced by high-LET carbon ion irradiation. J Pineal Res. 2012;52(1):47–56. doi: 10.1111/j.1600-079X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 40.Papis K, Poleszczuk O, Wenta-Muchalska E, Modlinski JA. Melatonin effect on bovine embryo development in vitro in relation to oxygen concentration. J Pineal Res. 2007;43(4):321–6. doi: 10.1111/j.1600-079X.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- 41.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50(4):1129–46. [PubMed] [Google Scholar]
- 42.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 43.Tan DX, Poeggeler B, Reiter RJ, Chen LD, Chen S, Manchester LC, et al. The pineal hormone melatonin inhibits DNA-adduct formation induced by the chemical carcinogen safrole in vivo. Cancer Lett. 1993;70(1–2):65–71. doi: 10.1016/0304-3835(93)90076-l. [DOI] [PubMed] [Google Scholar]
- 44.Jang HY, Ji SJ, Kim YH, Lee HY, Shin JS, Cheong HT, et al. Antioxidative effects of astaxanthin against nitric oxide-induced oxidative stress on cell viability and gene expression in bovine oviduct epithelial cell and the developmental competence of bovine IVM/IVF embryos. Reprod Domest Anim. 2010;45(6):967–74. doi: 10.1111/j.1439-0531.2009.01469.x. [DOI] [PubMed] [Google Scholar]
- 45.Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, et al. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci Lett. 2005;380(1–2):26–31. doi: 10.1016/j.neulet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Sauer MV. In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag. 2006;2(4):355–64. doi: 10.2147/tcrm.2006.2.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S. Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J Pineal Res. 2011;50(4):374–80. doi: 10.1111/j.1600-079X.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 48.Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S, Sugimachi M, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78(3):326–30. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, Shi Z, Yuan F, Li G, Sun Y, Zhang Y, et al. Melatonin modulates the effects of diethylstilbestrol (DES) on the anterior pituitary of the female Wistar rat. Folia Histochem Cytobiol. 2010;48(2):278–83. doi: 10.2478/v10042-010-0023-1. [DOI] [PubMed] [Google Scholar]
- 50.El-Raey M, Geshi M, Somfai T, Kaneda M, Hirako M, Abdel-Ghaffar AE, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev. 2011;78(4):250–62. doi: 10.1002/mrd.21295. [DOI] [PubMed] [Google Scholar]
- 51.Sampaio RV, Conceicao S, Miranda MS, Sampaio Lde F, Ohashi OM. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod Biol Endocrinol. 2012;10:103. doi: 10.1186/1477-7827-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23(3):1054–60. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuster C, Williams LM, Morris A, Morgan PJ, Barrett P. The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction pathway. J Neuroendocrinol. 2005;17(3):170–8. doi: 10.1111/j.1365-2826.2005.01288.x. [DOI] [PubMed] [Google Scholar]