Abstract
Purpose
To analyze the cleavage patterns in dipronuclear (2PN) and tripronuclear (3PN) embryos in relation to fertilization method.
Method
Time-lapse analysis.
Results
Compared to 2PN, more 3PN IVF embryos displayed early cleavage into 3 cells (p < 0.001), displayed longer duration of the 3-cell stage (p < 0.001), and arrested development from the compaction stage and onwards (p < 0.001). For the IVF embryos, the 2nd and 3rd cleavage cycles were completed within the expected time frame. However, timing of the cell divisions within the cleavage cycles differed between the two groups. In contrast, the completion of the 1st, 2nd, and 3rd cleavage cycle was delayed, but with a similar division pattern for 3PN ICSI compared with the 2PN ICSI embryos. 3PN, more often than 2PN ICSI embryos, displayed early cleavage into 3 cells (p = 0.03) and arrested development from the compaction stage and onwards (p = 0.001). More 3PN IVF than ICSI embryos displayed early cleavage into 3 cells (p < 0.001).
Conclusions
This study reports differences in cleavage patterns between 2PN and 3PN embryos and for the first time demonstrates differences in the cleavage pattern between 3PN IVF and ICSI embryos.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-014-0178-3) contains supplementary material, which is available to authorized users.
Keywords: Cell division, Embryo, Humans, Triploidy, Time-lapse
Introduction
Triploidy may originate from either digynic or diandric fertilizations [1–3]. Triploidy is estimated to be present in 1–2 % of pregnancies, which makes it one of the most common chromosome abnormalities in human gestation. Triploid pregnancies nearly always result in early spontaneous abortion and account for 10 % of these [4]. Occasionally, the triploid pregnancy however persists with very diverse but always pathological phenotypes in the form of hydatidiform mole, abnormal fetus that die in utero, or, rarely, a live born infant surviving only shortly [5].
Triploidy is also found in vitro during the course of fertility treatment and occurs in 4–7 % of IVF inseminated embryos [2,6,7] and in 6 % of ICSI embryos [2]. The vast majority of triploid embryos can be recognized at the zygotic stage by the appearance of 3 pronuclei (PN) instead of the normal 2. Otherwise, 3PN and 2PN embryos have the same morphological appearance [8]. Diandric triploids are estimated to account for approximately 80 % of 3PN IVF inseminated embryos [9] and over 80 % of these are due to dispermy [3]. The vast majority of 3PN embryos from the ICSI treatment are estimated to be digynic due to failure in the second meiotic division and non-extrusion of the second polar body [1,2].
Previous analyses of the cleavage patterns in 3PN IVF and ICSI embryos have focused on the chromosomal contents rather than the dynamics and timing of various cell cycle events [2,8,10–14]. An abnormal 1st cleavage in 3PN embryos has been described by observing 10 cases [15]. In 3PN IVF embryos, 2 pairs of centrioles are present in over 80 % of the cases compared with 3PN ICSI embryos where only one pair of centrioles is found [16,17]. As the first mitotic division is controlled by the paternally inherited centriole [16,17], we hypothesize that there are differences in the cleavage pattern of 3PN IVF and 3PN ICSI embryos.
The aim of the present study was therefore to compare the timing of cell-cycle completions and cleavage patterns of 3PN IVF, 3PN ICSI, 2PN IVF, and 2PN ICSI embryos using time-lapse monitoring.
Material and methods
Participants
Between February 2010 and September 2012, all patients undergoing treatment at the Fertility Clinic, Aarhus University Hospital were asked for permission to include their 3PN embryos in the study. All patients consented to time-lapse monitoring and culturing of the embryos in the EmbryoScope®. The 2PN group consisted of 2PN embryos from women, where no 3PN embryos were observed in the treatment cycle in which they were recruited. The 3PN group consisted of 3PN embryos from women where one or more 3PN embryos were observed in the treatment cycle in which they were recruited. In the 2PN group, all 2PN embryos completing the 1st cleavage were evaluated. In the 3PN group, only the 3PN embryos were annotated and evaluated.
Ethical approval
Written informed consent was obtained from all participants before inclusion. The Regional Committees on Biomedical Research Ethics in Southern Denmark, the Central Region of Denmark, and the Danish Data Protection Agency approved the study.
In vitro fertilization and embryo culture
Ovarian stimulation and oocyte retrieval were performed according to standard procedures as previously described [18]. Following retrieval, oocytes were fertilized using conventional IVF or ICSI procedures according to treatment indications. Immediately after injection, ICSI fertilized embryos were placed in individual wells (EmbryoSlide®, Unisense FertiliTech, Aarhus, Denmark) in a tri-gas time-lapse incubator (EmbryoScope®, Unisense FertiliTech, Aarhus, Denmark) [18]. IVF fertilized embryos were cultured for 18 h in a conventional incubator under oil at 37 °C, 20 % O2, and 6 % CO2, followed by transfer to EmbryoSlides™ for further culturing in the time-lapse incubator. In the time-lapse incubator, IVF and ICSI embryos were cultured under oil at 37 °C, 5 % O2 and 6 % CO2, except for 21 3PN IVF embryos that were cultured at 37 °C, 20 % O2 and 6 % CO2 exclusively. All embryos were cultured in a sequential culture medium (Sydney IVF Fertilization/Cleavage/Blastocyst Medium, COOK®, Sydney, Australia). Embryos were cultured until day 5 or 6. The media were changed in the morning of day 3 and 5. Twenty-nine 3PN embryos were excluded from the analysis of developmental arrest since they were removed from the incubator at random time-points before 115 h of culture.
Time-lapse monitoring and annotation
Images were automatically recorded every 20 min. in seven planes (15 μm intervals, 1280 × 1024 pixels, 3 pixels per μm, monochrome, 8-bit < 0.5 s per image, using single 1 W red LED). For ICSI embryos, the time of fertilization (T0) was defined as the time of injecting the sperm into the oocyte. For IVF embryos, T0 was defined as the time of adding the sperm to the dish. Time-points (hours after fertilization) were recorded for key events such as cellular divisions and blastocyst kinetics. Embryos were all annotated manually in the time-lapse system by the same observer according to definitions previously described [19]. Intra- and inter-observer variations were evaluated in a separate study [20].
The time-point of cellular division was defined as the first time-point when two daughter cells were completely separated by a cytoplasmic membrane, i.e. time of 1st division was annotated as the time of the first image where two separate cells were observed, time of 2nd division as the time of the first image where three separate cells were observed, and so forth. If more than one division occurred in the interval between image recordings, those particular divisions were annotated with the same time-point. The time-point of compaction was recorded as the time of the first image where blurred boundaries of at least two cells were observed. The time-point of the early blastocyst was recorded as the time of the first image where a blastocoel was visible and the time-point of full blastocyst as the time of the first image where the blastocoel filled out the embryo, just prior to expansion.
The 1st cytokinesis was categorized as aberrant if one or more of the following features were observed: A unipolar cleavage furrow, membrane ruffling, and/or multiple cleavage furrows [15]. The 1st cytokinesis was categorized as prolonged if the duration was over half an hour. Duration (hours) of events such as the 1st cytokinesis, the cellular stages, and the cleavage cycles were calculated.
Cleavage from 1 to 3 cells and from 3 to 5 cells was defined by duration of the 2-cell stage and 4-cell stage, respectively. Early cleavage was defined arbitrary as a duration of 2-cell stage or 4-cell stage <1 h. A cleavage cycle refers to the cluster of developmentally consistent mitotic divisions, where the cell number is doubled [15,21,22]. The first three cleavage cycles yield a 2-cell embryo (1st cleavage cycle), a 4-cell embryo (2nd cleavage cycle) and an 8-cell embryo (3rd cleavage cycle), respectively. Embryos in which one or more of the blastomeres showed no further cleavage were categorized according to the number of cell cycles observed in the healthy blastomeres (i.e., an embryo with only 6 blastomeres, where 2 blastomeres had arrested development, would be defined as having completed the third cleavage cycle). Time-points refer to the time where an image was recorded and are reported as hours after fertilization (T0).
Statistical Analyses
Wilcoxon rank-sum test was used to test the hypothesis of no difference between timing of events in the 3PN embryos and the control embryos. The estimates are reported as medians with interquartile ranges. For categorical data, Fisher’s exact test was used to test the hypothesis of no difference between the two groups. All analyses were performed with STATA for Mac, version 11.0 (StataCorp, USA). Two-sided p-values less than 0.05 were considered significant. Figures were created using Graph Pad version 5.01 (graphpad.com).
Results
Fifty-five (n = 55) patients contributed with 78 (57 IVF and 21 ICSI) 3PN embryos to the 3PN group. The control group consisted of 764 (224 IVF and 540 ICSI) 2PN embryos from 121 patients. There was no significant difference between the age distributions in the two groups.
The division pattern was altered for both IVF and ICSI 3PN embryos compared with control embryos. Furthermore, the alterations in timing of development differed between 3PN IVF and ICSI embryos, which will therefore be described separately.
IVF fertilized embryos
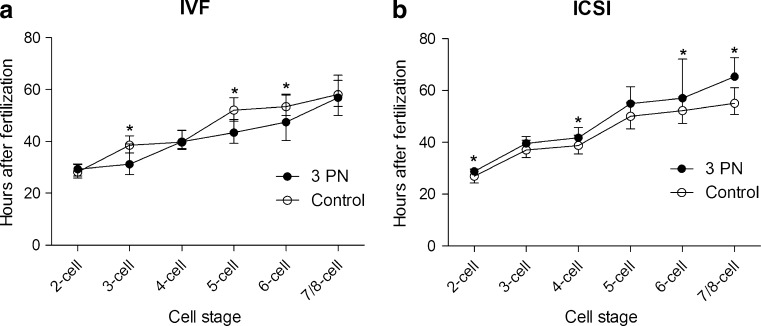
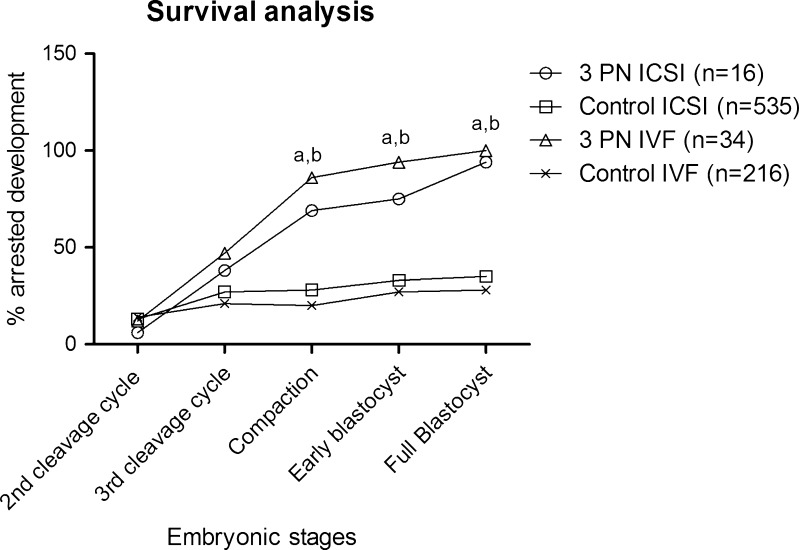
A significantly higher proportion of the 3PN IVF embryos displayed a prolonged and aberrant 1st cytokinesis compared with 2PN IVF embryos (p < 0.001 and p = 0.02) (Table 1). More 3PN IVF than 2PN IVF embryos displayed early cleavage to the 3-cell stage (p < 0.001) and had a longer duration of the 3-cell stage (3PN: 11.3 (0.6;12.6) hours and 2PN 0.7 (0.3;1.7) hours, median (interquartile range)) p < 0.001). The 2nd and 3rd cleavage cycles (4-cell stage and 7/8-cell stage, respectively; Table 1) were completed within the expected time frame. However, timing of the cell divisions within the cleavage cycles differed between the two groups as 3PN embryos completed the 3-, 5, and 6-cell stage at significantly earlier time-points than 2PN embryos (Table 1). The differences between timing and duration of the cell stages between the two groups are illustrated in Fig. 1. There was a higher but non-significant difference in the proportion of 3PN IVF embryos showing multinucleation at the 2-cell stage compared with 2PN IVF embryos (p = 0.06) (Table 1). The proportion of embryos that arrested development at the compaction stage or later were significantly higher in the 3PN IVF group compared with the 2PN IVF group. During the 1st cleavage cycle, however, no significant differences in the proportions of embryos with arrested development were seen between the two groups (Fig. 2).
Table 1.
Cleavage characteristics (A) and time-points for cleavage events (B) in 3PN and 2PN embryos
| IVF | ICSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A* | 3PN | 2PN | p-value | 3PN | 2PN | p-value | ||||
| Ncases/Nanalyzed (%) | Ncases/Nanalyzed (%) | Ncases/Nanalyzed (%) | Ncases/Nanalyzed (%) | |||||||
| Aberrant 1st cytokinesis | 34/55 (62) | 28/184 (15) | <0.001 | 7/20 (35) | 100/515 (19) | 0.09 | ||||
| 1st cytokinesis, >30 min (Prolonged 1st cytokinesis) | 20/55 (36) | 29/186 (16) | 0.002 | 3/21 (14) | 80/477 (17) | 1.0 | ||||
| Cleavage 1 to 3 cells <1 h (Early cleavage to 3 cells) | 40/54 (74) | 16/183 (9) | <0.001 | 5/20 (25) | 41/464 (9) | 0.03 | ||||
| Cleavage 3 to 5 cells <1 h (Early cleavage to 5 cells) | 28/45 (62) | 18/181 (10) | <0.001 | 2/18 (11) | 49/430 (11) | 1.0 | ||||
| Multi-nucleation at the 2-cell stagea. | 4/17 (24) | 17/208 (8) | 0.06 | 5/16 (31) | 59/499 (12) | 0.04 | ||||
| B** | 3PN | 2PN | p-value | 3PN | 2PN | p-valuec | ||||
| N | Time-point/hoursb | N | Time-point/hoursb | N | Time-point/hoursb | N | Time-point/hoursb | |||
| 2-cell stage (1st division/1st cleavage cycle) | 55 | 29.3 (26.7;31.8) | 189 | 28.2 (25.9;31.3) | 0.05 | 21 | 28.8 (26.8;30.2) | 479 | 26.9 (24.3;29.7) | 0.02 |
| 3-cell stage (2nd division) | 55 | 31.2 (27.2;39.0) | 199 | 38.6 (35.6;42.2) | <0.001 | 20 | 39.6 (35.9;42.3) | 476 | 37.0 (34.1;40.8) | 0.13 |
| 4-cell stage (3rd division/2nd cleavage cycle)/hours. | 51 | 39.9 (37.2;44.2) | 192 | 39.8 (36.9;44.3) | 0.87 | 20 | 41.8 (39.2;45.8) | 468 | 38.8 (35.5;42.8) | 0.01 |
| 5-cell stage (4th division) | 45 | 43.4 (39.3;48.4) | 192 | 52.1 (47.8;56.9) | <0.001 | 18 | 54.9 (47.7;61.5) | 431 | 50.0 (45.2;55.5) | 0.06 |
| 6-cell stage (5th division) | 43 | 47.5 (40.4;57.9) | 181 | 53.4 (50.0;58.3) | <0.001 | 16 | 57.0 (51.1;72.1) | 408 | 52.2 (47.2;57.5) | 0.01 |
| 7/8-cell stage (6th/7th division/3rd cleavage cycle) | 29 | 56.9 (50.0;63.5) | 165 | 58.1 (53.5;65.6) | 0.14 | 13 | 65.3 (60.6;72.7) | 352 | 55.0 (50.7;61.0) | 0.003 |
T0 was defined as time of adding sperm to the dish for IVF embryos and time of injection for ICSI embryos. IVF and ICSI time-points are therefore not entirely comparable. Ncases refers to number of embryos where the event was observed. Nanalyzed refers to the number of embryos that was evaluated. Nanalyzed became smaller with time as some embryos arrested development in almost each cell cycle
A*. The hypotheses of no difference between the fractions for 3 PN and 2 PN embryos were tested with Fishers exact test. a: Embryos with early cleavage from 1 to 3 cells (nIVF = 56 an nICSI = 46) were not included in this evaluation
B**. b: Time-points are reported as medians with quartiles. c: The hypotheses of no difference between the median time-points for 3PN and 2 PN embryos were tested with Wilcoxon rank sum test. The time points for completion of 6th and 7th divisions were grouped together, as these divisions were often difficult to distinguish
Fig. 1.
The differences between timing and duration of the cell stages for 3PN IVF (a) and 3PN ICSI (b) compared to their respective 2PN controls. Time-points are reported as medians marked with interquartile range. Wilcoxon rank-sum test was used to test the hypothesis of no difference between timing of events in the 3PN embryos and the control embryos. Significant differences (p < 0.05) between 3 PN embryos and the 2 PN embryos are marked with an asterisk
Fig. 2.
Survival analysis of 3PN IVF or ICSI compared to their respective 2PN controls. Fisher’s exact test was used to test the hypothesis of no difference between the 3 PN and 2 PN groups. Significant differences (p < 0.05) between 3 PN embryos and the 2 PN embryos are marked with a for the IVF embryos and b for the ICSI embryos, respectively
See Online Resource 1 for an example of time-lapse imagining of 1st division with early cleavage to 3-cells.
ICSI fertilized embryos
In contrast to the 3PN IVF embryos, the majority of 3PN ICSI embryos displayed a normal 1st cytokinesis. No difference was observed between the proportions 2PN and 3PN ICSI embryos where the 1st cytokinesis was aberrant or prolonged. 3PN ICSI embryos were more often than the 2PN ICSI control embryos observed to cleave early from 1 to 3 cells (p = 0.03) (Table 1); whereas, in contrast to the 3PN IVF embryos, there was no significant difference in duration of the 3-cell stage between the 3PN and 2PN ICSI embryos ((3PN: 0.6 (0.0;2.6) hours and 2PN 0.7 (0.3;2.0) hours, median (interquartile range)) p = 0.98). The completions of the 1st, 2nd, and 3rd cleavage cycles were delayed compared with control 2PN embryos (Fig. 1 and Table 1), but 3PN ICSI had a division pattern similar to 2PN ICSI, as all the cell divisions within the cleavage cycle were delayed (Fig. 1). Multi-nucleation at the 2-cell stage was observed significantly more often in 3PN ICSI embryos compared with 2PN ISCI embryos (Table 1). Similarly to the 3PN IVF embryos, a significantly higher proportion of 3PN ICSI embryos arrested development before the blastocyst stage compared to control 2PN ICSI embryos (p = 0.001) (Fig. 2).
Comparing the proportion of early cleavage to 3 cells between IVF and ICSI embryos directly, significantly more 3PN IVF embryos cleaved early to 3 cells compared to 3PN ICSI embryos (40/54 vs. 5/20, p < 0.001).
Discussion
Embryo development has traditionally been evaluated at discrete time-points with long intervals between the observations, which limits the information obtained. The principle of time-lapse is that images are recorded automatically with a fixed time-interval between the image acquisitions. Therefore, using time-lapse instruments, the monitoring is not entirely continuous, as continuous light exposure is harmful to the embryos [23]. Hence, when studying development in human embryos, the only option of uninterrupted semi-continuous monitoring is time-lapse imagining.
In the present study, we studied embryos with early cleavage from 1 to 3 cells, arbitrarily defined as when the duration of a possible 2 cell-stage was less than 1 h. It can be argued that this assumption is reasonable, since it is unlikely that any DNA and centriole replication [24] could take place within this hour. Furthermore the duration of the first cytokinesis in 2PN embryos is only 14.3 + 6.0 min, and the interval between the end of the first mitosis and the initiation of the second (hence the timeframe where DNA and centriole replication occur) is 11.1 + 2.2 h[15].
Using the surrogate measurement of time-points and our definition of early cleavage to 3, we have shown that – in contrast to control 2PN IVF and 3PN ICSI embryos − 3PN IVF embryos more often display early cleavage into 3 cells. However, we have also shown that a fraction of 3PN ICSI, 2PN ICSI, and 2PN IVF embryos display early cleavage into 3 cells even though they (theoretically) only have a single centriole pair.
The first mitotic division of 3PN IVF embryos has been observed and analyzed by Kola et al. [11], who reported that out of 29 3PN IVF zygotes, 18 (62 %) cleaved directly to 3 cells. Divergent results have been reported from other studies where the cleavage pattern of 3PN IVF embryos was evaluated by reporting the number of cells on day 2; Balakier et al. reported that out of 32, 3PN IVF embryos 6 (19 %) was at the 3-cell stage [25] and in a study by Pang et al., it was found that 7 out of 32 (22 %) 3PN IVF embryos were at the 3-cell stage [14]. Staessen et al. reported on 323 3PN embryos and found significantly more 3-cell embryos on day 2 from 3PN IVF embryos compared with 2PN IVF embryos [2] and suggested that this indicated direct cleavage from 1 to 3 cell embryos. However, the embryos were not observed continuously. In agreement with the observations by Kola et al. [11], we found early cleavage to 3 cells in 76 % of the 3PN IVF embryos. Comparing our observations in 3PN IVF embryos with the 2PN IVF control embryos, we found that significantly more 3PN IVF embryos cleaved early to 3 cells. This is also in agreement with the day 2 observations by Staessen et al. [2].
Only Staessen et al. [2] has investigated the early cleavage pattern of 3PN ICSI embryos, reporting that the majority of 3PN ICSI embryos were at the 2-cell or 4-cell stage on day 2. However, a larger proportion of 3PN ICSI embryos were at the 3-cell stage compared to 2PN ICSI embryos. This rather unexpected finding was hypothesized to be caused by either an asynchronous cleavage from 2-cell to 4-cell stage resulting in a relatively long duration of the 3-cell stage or direct cleavage into 3-cells. In agreement, we found that more 3PN ICSI embryos cleave early into 3 cells when compared to 2PN ICSI embryos.
Staessen et al. [2] compared the number of cells of 3PN IVF and ICSI embryos on day 2, but found no significant difference in the percentages of 3 cell stage embryos from IVF and ICSI. Staessen et al. argued that their single observation on day 2 could not identify asynchronous cleavage from 2 to 4 cells. In the present study, we observed that significantly more 3PN IVF embryos display early cleavage into 3 cells compared with 3PN ICSI embryos. We also observed that, compared to their respective controls, 3PN IVF displayed a different division pattern whereas 3PN ICSI embryos displayed a delayed, but similar division pattern compared to the control embryos (Fig. 1). The delay observed for 3PN ICSI embryos might explain the differences between the results of the present study and the study by Staessen et al. since the solitary day 2 observations did not allow for a detailed analysis of the division pattern. The detailed analysis performed in our study underlines the strength of time-lapse monitoring when analyzing development.
Centrioles, together with the pericentriolar matrix, organize the mitotic spindle. The enucleation experiments by Palermo et al. (1994) provided evidence that the sperm centrioles control the first mitotic division [16]. Excess centrioles in the zygote have been shown to facilitate abnormal cleavage directly into 3 cells if the excess centriole does not remain dormant [3,7,16,17]. Santhananthan et al. (1991) demonstrated that the centrioles are paternally inherited from the midpiece of the spermatozoa [17]. Thus, dispermy introduces 2 pairs of centrioles into the oocyte. The significantly higher frequency of early cleavage into 3 cells and the higher frequency of asynchronous cleavages observed in the 3PN IVF embryos compared to both the 2PN IVF embryos and the 3PN ICSI embryos, might be explained by a higher frequency of two pairs of centrioles in IVF embryos, caused by dispermic fertilization. Our study does not allow for any final conclusions on this part as the settings did not allow for direct visualization of centrioles and pericentriolar matrix during the time lapse monitoring.
The hypothesis that an extra paternal pair of centrioles affects cleavage does not, however, explain why some 3PN ICSI and some normal 2PN embryos display early cleavage into 3 cells. As argued above, it may be assumed that no DNA and centriole replication occurred in the limited time between annotations and therefore at least two different scenarios may be hypothesized. If the oocyte forms a spindle pole without a centriole, as seen in parthenogenetic activated human oocytes (reviewed in [26]), a third spindle pole could be formed in zygotes with a normal centriole content. Alternatively, an extra centriole might organize a third spindle pole. An extra centriole may come in place if the normal degradation of the maternal centriole is lacking, or a double duplication of the paternal centriole occurs during the S-phase in the first cell cycle, or errors in the male gametogenesis results in a sperm cell with two pairs of centrioles. The present study does not allow for discrimination between these possibilities. Since 2PN embryos that cleave directly into 3 cells have a significantly lower implantation rate compared to embryos with a normal cleavage pattern [27], the question of how and why there is a formation of a third spindle pole remains a subject of key interest.
To the best of our knowledge, the duration of key cellular events in 3PN embryos has not been studied by time-lapse imaging before.
We found that the 3PN IVF embryos had a prolonged 1st cytokinesis compared to 2PN IVF embryos. We hypothesize that the delay is due to the complicated construction of the mitotic spindle in the 3PN embryo. As a consequence of the tripolarity, mitotic microtubules bind to the centromeric plate from 3 points resulting in an abnormal Y-shaped metaphase plate [7,28]. It is easily imagined that this complicated division pattern takes longer time to establish compared with normally fertilized embryos.
Even though 3PN ICSI embryos also displayed early cleavage into 3 cells more often than 2PN ICSI embryos the 1st first cytokinesis was not prolonged compared to 2PN ICSI embryos. Although the numbers are small, one could hypothesize that the early cleavage into 3 cells has different etiology in 3PN IVF and 3PN ICSI embryos. One could also speculate that the 3PN ICSI embryos cleave to 2 cells and then immediately, without DNA and centriole replication, subsequently cleave into 3 cells avoiding the possible time-consuming formation of a Y-shaped metaphase Plate.A prolonged 3-cell stage was observed for 3PN IVF compared to 2PN IVF embryos. Intuitively, this is not surprising as the interphase is longer than the mitosis, and the 3 cell stage could, in the case of 3PN IVF, be viewed as the first complete cell cycle. This hypothesis is also supported by the different timing and pattern of the following cell divisions between 3PN IVF and 2PN IVF embryos, where 3PN IVF reaches the 6 cell stage before 2PN IVF (Fig. 1).
3PN ICSI embryos displayed, apart from the initial delay and early cleavage to 3, timing and division patterns similar to 2PN ICSI embryos (Fig. 1). As the initial early cleavage into 3 does not seem to affect the developmental pattern compared to 2PN ICSI, it supports the hypothesis that the initial early cleavage into 3 cells has different etiology than 3PN IVF.
Regardless of the fertilization method, a high frequency of developmental arrest was observed at the compaction stage when compared to normal 2PN embryos. As the arrest occurs approximately at the time of activation of the embryonic genome [29], we hypothesize that the arrest is either due to the imbalance between maternal and paternal genomes or activation of genes controlling cell cycle checkpoints/apoptosis.
The aim of the present study was to compare the timing of cell-cycle completions and cleavage patterns of 3PN and 2PN embryos. As pathogenesis behind the formation of 3PN embryos is poorly understood, we could not eliminate the possibility that 3PN embryos included in this study were the result of genetic errors, for example dispermy as a result of a defect in the cortical reaction [30,31], or digyny as a result of maternal meiotic errors [1,2]. We therefore decided to use 2PN embryos from couples without 3PN embryos as controls instead of the 2PN embryos from the couples with 3PN embryos.
Due to laboratory procedures, embryos from IVF patients were cultured for ~18 h under 20 % O2 in a conventional incubator followed by transfer to a time-lapse incubator for culture under 5 % O2. The ~18-h delay in transfer to the time-lapse incubator was introduced to allow for fertilization at normal conditions (intact cumulus) before the necessary denudation of IVF embryos performed to achieve optimal image acquisition. Emerging evidence suggests that high oxygen influences the timing of development, in particular by delaying the pre-compaction stage [32,33]. Furthermore, direct comparison of timing between IVF and ICSI embryos are complicated by the difference in T0. In the majority of analyses, we therefore do not compare IVF and ICSI directly, but compare 3 PN with 2 PN within the IVF and ICSI groups only. Based on the observation, that differences in the division pattern for the 3 PN embryos compared with the 2 PN embryos are fundamentally different between IVF and ICSI embryos (as discussed above) we conclude that the division pattern is different between 3 PN IVF and ICSI embryos. This is supported by the only analysis where IVF and ICSI embryos were compared directly, and where the proportion of early cleavage to 3 cells was found to be larger for 3 PN IVF than 3 PN ICSI embryos. As the impact of different oxygen conditions has been related to the third cleavage cycle only [32,33], there is no evidence that a temporary exposure to high oxygen would result in an altered pattern in terms of early cleavage to three cells, we therefore do not find it plausible that the clearly divergent pattern observed in the 3PN embryos would be explained by differences in oxygen concentration. Twenty-one (n = 21) 3PN IVF embryos were cultured exclusively under high oxygen. We evaluated any possible effect of differences in oxygen concentration in a sub-analysis, where the 21 3PN IVF embryos cultured exclusively under high oxygen were excluded. Although the number of embryos analyzed in this sub-analysis was smaller, the results support our interpretation that the observed differences in cleavage pattern were not the result of the difference in oxygen concentration as the overall results were unchanged (see Online Resource 2). Therefore, we concluded that the 21 (n = 21) 3PN IVF embryos that were cultured exclusively under high oxygen could be included in the 3PN IVF group.
In conclusion, this observational study demonstrates for the first time significant differences in the cleavage pattern between 3PN IVF and 3PN ICSI embryos compared to their respective controls. We speculate that an extra centriole increases the risk of early cleavage into 3 cells and might also explain the different division pattern as observed in the 3PN IVF embryos when compared to both 2PN IVF and 3PN ICSI.
Electronic supplementary material
(MPEG 13782 kb)
(PDF 31 kb)
Acknowledgments
The authors wish to thank the clinical, paramedical, and laboratory team of the Fertility Clinic, Aarhus University Hospital. The study was supported by Aarhus University, University of Southern Denmark, Aase and Einar Danielsen Foundation, Lipperts Foundation, the Augustinus Foundation and the Toyota Foundation. Research at the Fertility Clinic is funded by an unrestricted grant from Ferring and MSD.
Conflict of interest
M.W.J., J.H., L.B., L.S., H.J.I, and K.K, declare no conflicts of interest. I.A. works part-time as a scientific consultant for Unisense FertiliTech and holds stocks in the company.
Footnotes
Capsule Tripronuclear embryos display early cleavage into three cells more often than dipronuclear embryos, and tripronuclear IVF embryos display early cleavage into three cells more often than tripronuclear ICSI embryos.
References
- 1.Grossmann M, Calafell JM, Brandy N, Vanrell JA, Rubio C, Pellicer A, et al. Origin of tripronucleate zygotes after intracytoplasmic sperm injection. Hum Reprod. 1997;12:2762–2765. doi: 10.1093/humrep/12.12.2762. [DOI] [PubMed] [Google Scholar]
- 2.Staessen C, Van Steirteghem AC. The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1997;12:321–327. doi: 10.1093/humrep/12.2.321. [DOI] [PubMed] [Google Scholar]
- 3.Yu SL, Lee RK, Su JT, Chih YF, Tsai YC, Lin MH, et al. Distinction between paternal and maternal contributions to the tripronucleus in human zygotes obtained after in vitro fertilization. Taiwan J Obstet Gynecol. 2006;45:313–316. doi: 10.1016/S1028-4559(09)60249-7. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs PA, Szulman AE, Funkhouser J, Matsuura JS, Wilson CC. Human triploidy: relationship between parental origin of the additional haploid complement and development of partial hydatidiform mole. Ann Hum Genet. 1982;46:223–231. doi: 10.1111/j.1469-1809.1982.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 5.Jauniaux E, Brown R, Rodeck C, Nicolaides KH. Prenatal diagnosis of triploidy during the second trimester of pregnancy. Obstet Gynecol. 1996;88:983–989. doi: 10.1016/S0029-7844(96)00330-4. [DOI] [PubMed] [Google Scholar]
- 6.Macas E, Imthurn B, Rosselli M, Keller PJ. The chromosomal complements of multipronuclear human zygotes resulting from intracytoplasmic sperm injection. Hum Reprod. 1996;11:2496–2501. doi: 10.1093/oxfordjournals.humrep.a019147. [DOI] [PubMed] [Google Scholar]
- 7.Plachot M, Crozet N. Fertilization abnormalities in human in-vitro fertilization. Hum Reprod. 1992;7(Suppl 1):89–94. doi: 10.1093/humrep/7.suppl_1.89. [DOI] [PubMed] [Google Scholar]
- 8.Angell RR, Templeton AA, Messinis IE. Consequences of polyspermy in man. Cytogenet Cell Genet. 1986;42:1–7. doi: 10.1159/000132242. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbusch B, Schneider M, Sterzik K. The chromosomal constitution of multipronuclear zygotes resulting from in-vitro fertilization. Hum Reprod. 1997;12:2257–2262. doi: 10.1093/humrep/12.10.2257. [DOI] [PubMed] [Google Scholar]
- 10.Coonen E, Harper JC, Ramaekers FC, Delhanty JD, Hopman AH, Geraedts JP, et al. Presence of chromosomal mosaicism in abnormal preimplantation embryos detected by fluorescence in situ hybridisation. Hum Genet. 1994;94:609–615. doi: 10.1007/BF00206952. [DOI] [PubMed] [Google Scholar]
- 11.Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37:395–401. doi: 10.1095/biolreprod37.2.395. [DOI] [PubMed] [Google Scholar]
- 12.Lim AS, Goh VH, Su CL, Yu SL. Microscopic assessment of pronuclear embryos is not definitive. Hum Genet. 2000;107:62–68. doi: 10.1007/s004390000335. [DOI] [PubMed] [Google Scholar]
- 13.Pieters MH, Dumoulin JC, Ignoul-Vanvuchelen RC, Bras M, Evers JL, Geraedts JP. Triploidy after in vitro fertilization: cytogenetic analysis of human zygotes and embryos. J Assist Reprod Genet. 1992;9:68–76. doi: 10.1007/BF01204118. [DOI] [PubMed] [Google Scholar]
- 14.Pang MG, Jee BC, Kim SH, Ryu BY, Oh SK, Suh CS, et al. Chromosomal constitution of embryos derived from tripronuclear zygotes studied by fluorescence in situ hybridization using probes for chromosomes 4, 13, 18, 21, X, and Y. Gynecol Obstet Invest. 2005;59:14–18. doi: 10.1159/000080522. [DOI] [PubMed] [Google Scholar]
- 15.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 16.Palermo G, Munne S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–1225. doi: 10.1093/oxfordjournals.humrep.a138682. [DOI] [PubMed] [Google Scholar]
- 17.Sathananthan AH, Kola I, Osborne J, Trounson A, Ng SC, Bongso A, et al. Centrioles in the beginning of human development. Proc Natl Acad Sci U S A. 1991;88:4806–4810. doi: 10.1073/pnas.88.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkegaard K, Hindkjaer JJ, Grondahl ML, Kesmodel US, Ingerslev HJ. A randomized clinical trial comparing embryo culture in a conventional incubator with a time-lapse incubator. J Assist Reprod Genet. 2012;29:565–572. doi: 10.1007/s10815-012-9750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–1285. doi: 10.1093/humrep/des079. [DOI] [PubMed] [Google Scholar]
- 20.Sundvall L, Ingerslev HJ, Breth Knudsen U. Kirkegaard K. Hum Reprod: Inter- and intra-observer variability of time-lapse annotations; 2013. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales DS, Pinheiro JC, Bavister BD. Prediction of the developmental potential of hamster embryos in vitro by precise timing of the third cell cycle. J Reprod Fertil. 1995;105:1–8. doi: 10.1530/jrf.0.1050001. [DOI] [PubMed] [Google Scholar]
- 22.Grisart B, Massip A, Dessy F. Cinematographic analysis of bovine embryo development in serum-free oviduct-conditioned medium. J Reprod Fertil. 1994;101:257–264. doi: 10.1530/jrf.0.1010257. [DOI] [PubMed] [Google Scholar]
- 23.Oh SJ, Gong SP, Lee ST, Lee EJ, Lim JM. Light intensity and wavelength during embryo manipulation are important factors for maintaining viability of preimplantation embryos in vitro. Fertil Steril. 2007;88:1150–1157. doi: 10.1016/j.fertnstert.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Azimzadeh J, Bornens M. Structure and duplication of the centrosome. J Cell Sci. 2007;120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- 25.Balakier H. Tripronuclear human zygotes: the first cell cycle and subsequent development. Hum Reprod. 1993;8:1892–1897. doi: 10.1093/oxfordjournals.humrep.a137955. [DOI] [PubMed] [Google Scholar]
- 26.Schatten H, Sun QY. The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol Hum Reprod. 2009;15:531–538. doi: 10.1093/molehr/gap049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–1463. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 28.Gu YF, Lin G, Lu CF, Lu GX. Analysis of the first mitotic spindles in human in vitro fertilized tripronuclear zygotes after pronuclear removal. Reprod Biomed Online. 2009;19:745–754. doi: 10.1016/j.rbmo.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 30.McFadden DE, Jiang R, Langlois S, Robinson WP. Dispermy–origin of diandric triploidy: brief communication. Hum Reprod. 2002;17:3037–3038. doi: 10.1093/humrep/17.12.3037. [DOI] [PubMed] [Google Scholar]
- 31.Zaragoza MV, Surti U, Redline RW, Millie E, Chakravarti A, Hassold TJ. Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am J Hum Genet. 2000;66:1807–1820. doi: 10.1086/302951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril 2013; 99:738–744 e734. [DOI] [PubMed]
- 33.Wale PL, Gardner DK. Time-lapse analysis of mouse embryo development in oxygen gradients. Reprod Biomed Online. 2010;21:402–410. doi: 10.1016/j.rbmo.2010.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MPEG 13782 kb)
(PDF 31 kb)