Abstract
Purpose
The arrangement of the blastomeres within the 4-cell stage embryo reflects the orientation of the cleavage planes during the second division. To examine their relevance, the developmental capacity and the pregnancy rate were compared between tetrahedral-shaped and non-tetrahedral-shaped 4-cell stage human embryos.
Methods
The study included 3,546 4-cell stage embryos. The arrangement of the blastomeres at the 4-cell stage was annotated as being tetrahedral or non-tetrahedral on day 2 of preimplantation development. Embryo quality was compared on day 3 and day 5. Pregnancy rates were calculated per single embryo transfer on day 3 or day 5.
Results
In total, 2,803 4-cell stage embryos (79 %) displayed a tetrahedral arrangement and 743 (21 %) displayed a non-tetrahedral arrangement. Tetrahedral-shaped embryos developed more into high-quality embryos on day 3 (p < 0.001) and day 5 (p = 0.036) and had a higher blastulation rate (p = 0.009). Though, the number of high-quality embryos selected for transfer did not differ between both groups on day 3 (p = 0.167) and day 5 (p ~ 1). Three hundred thirty single embryo transfers were analysed. No significant difference in clinical pregnancy was found between both groups after transfer on day 3 (p = 0.209) and day 5 (p = 0.653).
Conclusions
The arrangement of the blastomeres according to their previous cleavage planes was correlated to the developmental potential of the 4-cell stage embryo up to the blastocyst stage. If embryo transfers are performed on day 3 and day 5 of development using embryos of adequate quality, the blastomere arrangement at the 4-cell stage had no predictable value regarding pregnancy success.
Keywords: Embryo morphology, Blastomere arrangement, Tetrahedron, Embryo selection, Cleavage plane
Introduction
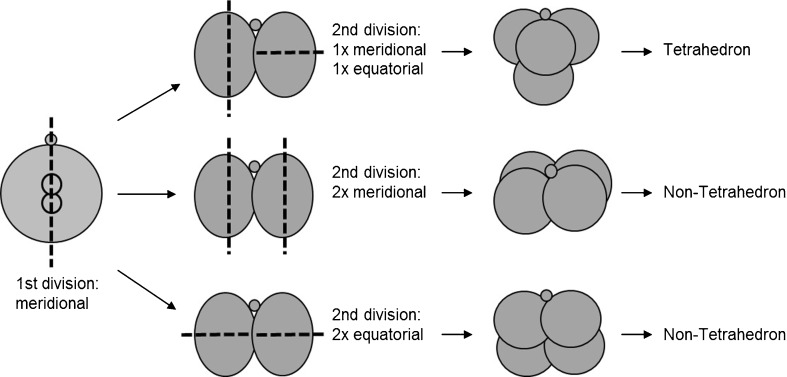
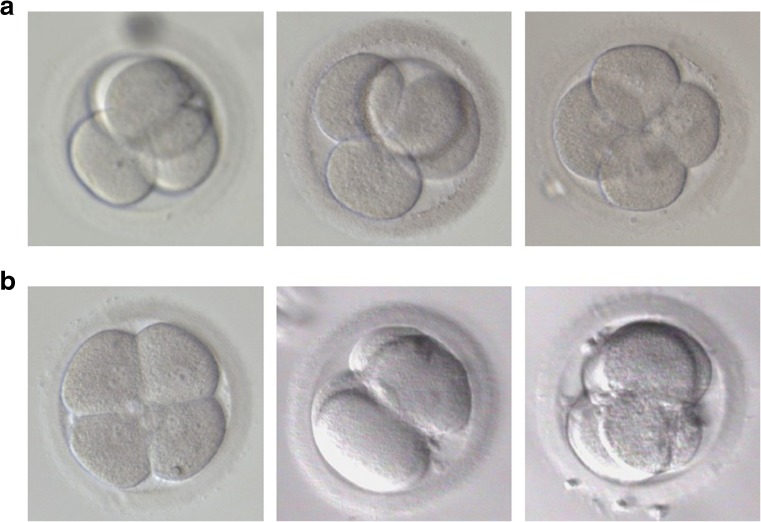
In 1975, Gulyas demonstrated that the blastomeres of 4-cell stage rabbit embryos are arranged crosswise in a tetrahedron [1]. This arrangement results of a first division along the meridional axis, followed by a second division in which one daughter cell divides meridionally and the other equatorially, perpendicular to the meridional axis (rotational cleavage) (Fig. 1) [2]. Like in other mammals, the four cells of the human embryo too are normally arranged in a tetrahedron (Fig. 2a) [3, 4]. However, in some cases, the blastomeres are organized in a non-tetrahedral way displaying a planar arrangement (Fig. 2b). This arrangement is the result of similar orientations of the cleavage planes during the second division, i.e. two consecutive meridional or equatorial divisions (Fig. 1).
Fig. 1.
Schematic presentation of the generation of tetrahedral and non-tetrahedral-shaped embryos. The second polar body is presented by the small circle. Dash line: orientation of the cleavage plane
Fig. 2.
Pictures of human 4-cell stage embryos on day 2 of preimplantation development. a. Tetrahedral-shaped embryos. b. Non-tetrahedral-shaped embryos
Mammalian oocytes consist of an animal pole, marked by the second polar body and a vegetal pole on the opposite site [5–8]. The first meridional division occurs according to the animal-vegetal axis of the zygote [9, 10]. The two daughter blastomeres each inherit similar proportions of animal and vegetal ooplasm. During the second division, the animal and vegetal gradient is distributed differently to the four blastomeres due to the different orientations of the cleavage planes [1, 2]. The meridional division generates daughter blastomeres containing animal and vegetal ooplasm. The equatorial division generates one blastomere with mostly animal ooplasm and one with mostly vegetal ooplasm. Partitioning of the ooplasm may introduce developmentally significant asymmetries between the blastomeres at the 4-cell stage. In 1997, Edwards and Beard introduced a model of early cell determination [5]. They proposed that the two blastomeres inheriting animal and vegetal ooplasm are precursors of the inner cell mass (ICM), the blastomere inheriting mainly animal ooplasm is the precursor of the trophectoderm (TE) and the blastomere inheriting mainly vegetal ooplasm is the precursor of the germ line [5, 8, 11]. This model of early asymmetry was extrapolated to the human and supported by the polarized distribution of proteins such as Leptin and STAT3 in the oocyte, their differential distribution between the blastomeres at the 4-cell stage and later on between the ICM and TE [6] and by the reciprocal expression of OCT-4 and βhCG transcripts at the 4-cell stage [11, 12]. Indirect evidence of oocyte polarity in the human was given by the correlation between the site of sperm deposition in the oocyte and the embryo development rate [13]. The early interpretation of Edwards’s model that the generated differences would be deterministic proved wrong as early cells can change their fate after repositioning [14]. However, the altered orientation of the cleavage planes in non-tetrahedral-shaped embryos may cause aberrant distributions of animal and vegetal ooplasm, including cell organelles and maternally inherited transcripts and proteins, between the blastomeres [5, 6]. Whether this affects the development and the implantation potential of non-tetrahedral-shaped 4-cell stage human embryos is unknown.
Selecting the most competent embryo within a cohort constitutes a major challenge [15, 16]. Generally, embryo assessment is limited to static microscopic evaluations [17–19]. Alternative selection methods include genetic screening [20–22], morphokinetics [23], metabolomics [24, 25], proteomics, oxygen consumption measurement and birefringence imaging [26]. The daily application of these innovating techniques is limited as they are invasive, complex, time-consuming, expensive and/or not yet proven to be superior to the static morphological evaluations, in particular compared to blastocyst culture [27]. Hence, embryo selection based on morphology remains the most common and generally accepted method to date. The spatial arrangement of the blastomeres within the embryo is currently not considered as a potential criterion for embryo selection [18, 28].
We examined whether the arrangement of the blastomeres at the 4-cell stage, as a result of the previous cleavage planes, has a value in the assessment of the developmental potential towards day 3 and day 5 of human preimplantation development and/or in the assessment of pregnancy.
Materials and methods
This study is an observational retrospective cohort study. The treatment of the patients in this study did not differ from routine assisted reproductive procedures. The study was conducted at the Centre for Reproductive Medicine at the UZ Brussel during a 3 year period.
Ovarian stimulation and oocyte retrieval
The patients underwent either natural or stimulated cycles. Ovarian stimulation was performed using urinary (Menopur, Ferring Pharmaceuticals A/S, Copenhagen, Denmark) or recombinant FSH (Puregon, NV Organon, Oss, The Netherlands; or Gonal F, Merck-Serono, Geneva, Switzerland) in combination with a GnRH antagonist (Orgalutran, NV Organon) or agonist (Suprefact, Aventis Pharma, Frankfurt, Germany) protocol. Final oocyte maturation was achieved by injection of hCG (Pregnyl, Schering-Plough, Oss, The Netherlands, or Profasi, Merck-Serono). Oocyte retrieval was carried out using vaginal ultrasound-guided puncture of ovarian follicles 36 h after hCG administration. Intravaginally administered progesterone (Utrogestan, Besins, Brussels, Belgium) was used for luteal phase supplementation.
Data collection and embryo culture
The study included 901 ICSI cycles (86.6 %), 86 IVF cycles (8.3 %) and 53 IVF vs ICSI cycles (5.1 %). Ejaculated semen was used in the majority of the cycles (93.3 %), testicular sperm was used in 6.7 % of the cycles. PGD cycles and cycles using vitrified oocytes or in vitro matured oocytes were excluded. Oocyte denudation, ICSI and IVF were performed according to standard procedures. Embryos were cultured in vitro at 37 °C in an atmosphere of 6 % CO2 and 5 % O2 in individual 25 μl droplets of sequential media formulations under paraffin oil. In total, 71 cycles were performed using Universal IVF Medium, EmbryoAssistTM and BlastAssist® (Medicult, De Pinte, Belgium), 357 cycles were performed using Vitrolife sequential media (G5 SeriesTM, Vitrolife, Göteborg, Sweden) and 612 cycles were performed using Quinn’s Advantage® protein plus sequential media (SAGE, Rochford Medical Ltd, Coventry, England). All systems were used according to manufacturer’s instructions. Sixteen to 18 h post-insemination, fertilization was assessed by the presence of two pronuclei. On day 2 of preimplantation development, 4-cell stage human embryos developing from normally fertilized oocytes were examined once using inverted microscopy (magnification 400×) for inspection of the arrangement of the blastomeres within the embryo according to the previous cleavage planes. Hereby, tetrahedral-shaped 4-cell embryos were distinguished from non-tetrahedral-shaped embryos. Embryos that did not display a perfect tetrahedron and embryos displaying a non-tetrahedral arrangement were rolled in the culture dish in order to verify their arrangement. Fragmentation did not hinder the evaluation of blastomere arrangement. Annotations regarding blastomere arrangement were confined to two persons in order to limit inter-observer variability. These annotations were blinded for further embryo evaluations and embryo selection for transfer. Further development of the embryos was evaluated on a daily basis. Blastocyst evaluation relied on the scoring system described by Gardner and Schoolcraft [29]. Embryo transfer was performed on day 3 or day 5 using a soft catheter (K-Soft 5100, Cook, Bloomington, USA).
Outcome parameters
The embryological outcome parameters were embryo quality on day 3 and on day 5. Embryo quality on day 3 was based on cell stage and fragmentation rate. High-quality embryos had at least seven blastomeres and maximum 10 % of fragmentation. Good-quality embryos had at least seven blastomeres with more than 10 % but not more than 50 % of fragmentation or six blastomeres with maximum 20 % of fragmentation. Poor-quality embryos were defined as having more than 50 % of fragmentation, six blastomeres with more than 20 % of fragmentation or less than six blastomeres. Embryo quality on day 5 was based on blastulation stage and appearance of ICM and TE. High-quality embryos on day 5 were defined as blastocysts with at least a fully developed blastocoel (Gardner’s Scale type 3 to 6) with a tightly packed ICM with many cells (Gardner’s Scale A) and a good or average TE layer forming an epithelium with sufficient cells (Gardner’s Scale A to B) [30]. Good-quality embryos were defined as blastocysts with a loosely packed ICM (Gardner’s Scale B) and a good or average TE (Gardner’s Scale A to B) or as early blastocysts (Gardner’s Scale type 1 to 2). Poor-quality blastocysts were defined as ICM/TE combinations in which at least one of both structures contained very few cells (Gardner’s Scale C) or was absent (score D). Poor-quality embryos on day 5 included also embryos that failed to blastulate i.e. compacted embryos, cleavage stage embryos and degenerated embryos.
The clinical outcome measurements were positive βhCG, clinical pregnancy, and clinical pregnancy with fetal heart beat. A clinical pregnancy was defined as a pregnancy with an intra-uterine gestational sac observed at a transvaginal ultrasound scan performed at 7 weeks of gestation. A biochemical pregnancy was evidenced by the detection of βhCG without developing into a clinical pregnancy and with the exclusion of ectopic pregnancies. Definitions were adopted from Zegers-Hochschild et al. [31]. The clinical data were analysed for single embryo transfers (SETs) that were performed on either day 3 or day 5 and of which the transferred embryo was annotated as being a tetrahedral or a non-tetrahedral 4-cell stage embryo on day 2. SETs using embryos that did not expose four cells on day 2 and double embryo transfers were not considered for analysis.
Statistical analyses
The Chi-square test with Yates’ correction was used to analyze the embryological data. The clinical data and the quality of the embryos transferred were analysed with the two-tailed Fisher’s exact test. Female age, a predictive parameter for pregnancy, was compared between both groups with the independent Student’s t-test. All tests were interpreted with a significance level of 95 % (P <0.05).
Results
In total, 1,200 cycles were evaluated on day 2 of preimplantation development. In 1,040 cycles, at least one normally fertilized 4-cell stage embryo was present. In total, 3,546 4-cell stage embryos were examined for their arrangement of the blastomeres. Of these, 2,803 embryos (79 %) displayed a tetrahedral (T) arrangement, 743 embryos (21 %) displayed a non-tetrahedral (nT) arrangement. There was no significant difference in the percentage of T and nT embryos that originated from the different stimulation protocols (Chi-square, p > 0.05). 9.2 % of T embryos were created by conventional IVF compared to 12.5 % of the nT embryos (Chi-square, p = 0.01). There was no difference between the T and nT group in the percentage of embryos created with ejaculated sperm (92 % vs 91.8 %, respectively) and testicular sperm (8 % vs 8.2 %, respectively) (Chi-square, p > 0.05).
Embryological data
The embryological data are summarized in Tables 1 and 2. Embryo quality on day 3 was examined for 2,792 former T-shaped embryos and 742 former nT-shaped embryos (Table 1). T-shaped 4-cell stage embryos gave rise to significantly more high-quality embryos on day 3 (p < 0.001). nT-shaped embryos developed significantly more into poor-quality embryos (p < 0.001). The developmental potential towards the blastocyst stage on day 5 was available for 1,418 embryos, i.e. 1,147 T- and 271 nT-shaped 4-cell stage embryos (Table 2). Significantly more former T-shaped embryos developed into high-quality blastocysts (p = 0.036). nT-shaped embryos developed significantly more into poor-quality embryos on day 5 (p = 0.002). Poor embryo quality on day 5 included blastocysts with poor ICM and/or TE as well as non-blastulated embryos (compacted, cleavage stage and degenerated embryos). A Significant difference was found between T- and nT-shaped embryos regarding blastulation. T-shaped embryos had a higher chance to blastulate (p = 0.009). nT-shaped embryos arrested more at the cleavage stages (p < 0.001). The maternal age on day 3 and day 5 was not significantly different between the T and the nT embryos in the different quality groups (p > 0.05).
Table 1.
Embryo development on day 3
| T (n = 2,792) | nT (n = 742) | P-value | ||||
|---|---|---|---|---|---|---|
| High-quality | ≥7c F ≤10 % | 1,801 | 64,5 % | 398 | 53,6 % | <0,001 |
| Good-quality | ≥7c F >10- ≤ 50 % or 6c F ≤20 % | 770 | 27,6 % | 217 | 29,3 % | 0,394 |
| Poor-quality | ≥7c F >50 % or 6c F >20 % or <6c | 221 | 7,9 % | 127 | 17,1 % | <0,001 |
The table shows the quality of the transferred and non-transferred embryos on day 3
T tetrahedral, nT non-tetrahedral, n number, c cell stage, F fragmentation
Statistics: Chi square test with Yates’ correction
Table 2.
Embryo development on day 5
| T (n = 1,147) | nT (n = 271) | P-value | ||||
|---|---|---|---|---|---|---|
| High-quality | At least full BL, ICM grade A, TE grade A or B | 222 | 19,4 % | 37 | 13,7 % | 0,036 |
| Good-quality | Early BL or BL with ICM grade B, TE grade A or B | 406 | 35,4 % | 83 | 30,6 % | 0,157 |
| Poor-quality | ICM and/or TE grade C or D | 519 | 45,2 % | 151 | 55,7 % | 0,002 |
| Compacted, cleavage stage or degenerated embryos | ||||||
| Blastulation | At least early blastocyst | 812 | 70,8 % | 169 | 62,4 % | 0,009 |
| No blastulation | Compacted embryos | 238 | 20,7 % | 62 | 22,9 % | 0,491 |
| Cleavage stage embryos | 66 | 5,8 % | 35 | 12,9 % | 0,001 | |
| Degenerated embryos | 31 | 2,7 % | 5 | 1,8 % | 0,554 | |
The table shows the quality of the transferred and non-transferred embryos on day 5
T tetrahedral, nT non-tetrahedral, n number, BL blastocyst, ICM inner cell mass, TE trophectoderm
Blastocyst scoring according to Gardner and Schoolcraft (1999). Statistics: Chi square test with Yates’ correction
Clinical data
In total, 330 SETs were performed on day 3 and on day 5 using embryos that exposed four cells on day 2. The results are summarized in Tables 3 and 4. For day 3 as well as for day 5 embryo transfers, the female age was not significantly different between the two groups. Out of the 195 SETs on day 3, 157 (81 %) came from the T group and 38 (19 %) from the nT group. On day 5, 135 SETs were performed, of which 111 (82 %) came from the T group and 24 (18 %) from the nT group. Pregnancy rates at the level of positive βhCG, biochemical pregnancy, clinical pregnancy and clinical pregnancy with fetal heart beat were comparable between the two groups for embryo transfers on day 3 and on day 5.
Table 3.
Clinical outcome per single embryo transfer on day 3
| T (n = 157) | nT (n = 38) | P-value | |||
|---|---|---|---|---|---|
| Female age | 33,5 ± 5,14 | 34,3 ± 5,07 | 0,349 | ||
| Positive βhCG | 45 | 28,7 % | 7 | 18,4 % | 0,226 |
| Biochemical pregnancy | 3 | 1,9 % | 1 | 2,6 % | 0,583 |
| Clinical pregnancy | 42 | 26,8 % | 6 | 15,8 % | 0,209 |
| Clinical pregnancy with fetal heart beat | 36 | 22,9 % | 5 | 13,2 % | 0,267 |
T tetrahedral, nT non-tetrahedral, n number
Statistics: Age: independent Student’s t-test, Clinical outcome: two-tailed Fisher’s exact test
Table 4.
Clinical outcome per single embryo transfer on day 5
| T (n = 111) | nT (n = 24) | P-value | |||
|---|---|---|---|---|---|
| Female age | 31 ± 3,9 | 30,5 ± 4,19 | 0,529 | ||
| Positive βhCG | 60 | 54,1 % | 15 | 62,5 % | 0,503 |
| Biochemical pregnancy | 3 | 2,7 % | 1 | 4,2 % | 0,547 |
| Clinical pregnancy | 57 | 51,4 % | 14 | 58,3 % | 0,653 |
| Clinical pregnancy with fetal heart beat | 53 | 47,8 % | 13 | 54,2 % | 0,655 |
T tetrahedral, nT non-tetrahedral, n number
Statistics: Age: independent Student’s t-test, Clinical outcome: two-tailed Fisher’s exact test
The quality of the embryos selected for SET on day 3 and on day 5 is summarized in Table 5. In the T group, 72.6 % of the embryos selected for SET on day 3 were of high-quality. In the nT group this was 60.5 % (p = 0.167). More poor-quality embryos have been transferred on day 3 in the nT group (p = 0.024). On day 5, 70.3 % of the embryos from the T group were of high-quality versus 70.8 % of the embryos from the nT group (p ~ 1). Cleavage stage embryos and degenerated embryos were not transferred on day 5.
Table 5.
Quality of the embryos selected for single embryo transfer
| Day 3 transfer | T (n = 157) | nT (n = 38) | P-value | |||
| High-quality | ≥7c F ≤10 % | 114 | 72,6 % | 23 | 60,5 % | 0,167 |
| Good-quality | ≥7c F >10- ≤ 50 % or 6c F ≤20 % | 42 | 26,8 % | 12 | 31,6 % | 0,550 |
| Poor-quality | ≥7c F >50 % or 6c F >20 % or <6c | 1 | 0,6 % | 3 | 7,9 % | 0,024 |
| Day 5 transfer | T (n = 111) | nT (n = 24) | ||||
| High-quality | At least full BL, ICM grade A, TE grade A or B | 78 | 70,3 % | 17 | 70,8 % | 1,000 |
| Good-quality | Early BL or BL with ICM grade B, TE grade A or B | 25 | 22,5 % | 5 | 20,8 % | 1,000 |
| Poor-quality | Compacted embryos or BL with ICM and/or TE grade C | 8 | 7,2 % | 2 | 8,4 % | 1,000 |
T tetrahedral, nT non-tetrahedral, n number, c cell stage, F fragmentation, BL blastocyst, ICM inner cell mass, TE trophectoderm
Blastocyst scoring according to Gardner and Schoolcraft (1999). Statistics: two-tailed Fisher’s exact test
Discussion
About one out of five (21 %) 4-cell stage human embryos did not display the expected tetrahedral arrangement of the blastomeres on day 2 of preimplantation development. This percentage of aberrant arrangement is similar to the proportions observed in mice [4, 32].
Our results showed significant differences in the developmental potential of tetrahedral- and non-tetrahedral-shaped 4-cell stage human embryos. Tetrahedral-shaped embryos had a higher chance to develop into high-quality embryos on day 3 and on day 5. In particular, non-tetrahedral-shaped embryos showed a reduced chance to form blastocysts and an increased risk to arrest at the cleavage stages. This finding is in line with Ebner et al. (2008) who observed that an ovoid zona pellucida (ZP) favors the generation of atypical cleavage patterns resulting in aberrant blastomere arrangements with a reduced number of contact points between them [33]. The low number of contact points was associated with delayed compaction and blastocyst formation. In our study, the examined embryos originated in general from round non-ovoid oocytes and ZP and so, no correlation could be made between the cleavage pattern and oocyte or ZP shape. The number of contact points between the blastomeres in our non-tetrahedral-shaped embryos was four. In the tetrahedral group, the number of contact points varied from four to six. Compaction normally occurs during the fourth cleavage division [34] and is mediated through E-cadherin [35, 36]. Cleavage abnormalities or loss of physical contact between blastomeres at an early developmental stage can lead to disturbances in the distribution of E-cadherin which may induce compaction failure [37]. Nevertheless, neither the mean contact surface nor the number of contact surfaces of a day 3 embryo seems to have an additional value in embryo viability when performing day 3 transfer [38]. No correlation has been found between the “roundness” of a day 3 embryo and live birth following day 3 SET [39].
The observed developmental differences between the tetrahedral and non-tetrahedral embryos were not correlated to the different stimulation protocols. Contradictory to the results of Ebner et al. 2012, we did not find more non-tetrahedral embryos after ICSI and after testicular sperm extraction [40]. Hence, we could not confirm the assumption that the non-tetrahedral shape is under paternal influence. Authors also postulated that the non-tetrahedral arrangement could be the result of a mitotic spindle defect which in turn may cause aneuploidy [40]. Despite the small sample size and the use of arrested embryos that are known to carry a higher rate of aneuploidy [41], it might be an interesting path to explore.
In the present study, embryo selection for transfer was based on classical embryo morphology parameters. The tetrahedral or non-tetrahedral status of the embryo was blinded. Pregnancy rates on day 3 and on day 5 seemed independent of the tetrahedral/non-tetrahedral status on day 2. The percentage of high- and good-quality embryos selected for SET on day 3 and on day 5 was comparable. Though not significant, it is noteworthy that there was a benefit for the tetrahedral group of 11 % in terms of clinical pregnancy after SET on day 3. It can be hypothesized that compaction might also be a bottleneck for the non-tetrahedral embryos in vivo. However, once the former non-tetrahedral embryo went through the process of compaction and developed into a blastocyst of adequate quality on day 5, this embryo had equal chances for success compared to a former tetrahedral embryo.
Tetrahedral and non-tetrahedral embryos originate from differently orientated cleavage planes. Hereby, the polarized ooplasm may be distributed in another way to the blastomeres of tetrahedral and non-tetrahedral 4-cell stage embryos [5, 8, 11]. Although at a lower level, non-tetrahedral-shaped embryos were able to form high-quality blastocysts and transfer of such blastocysts led to normal pregnancy rates. Hence, the assumed altered distribution of ooplasm in non-tetrahedral-shaped embryos did not seem to have a clear effect on the formation of ICM and TE or on pregnancy outcome. The question whether early blastomeres are predestined to become either ICM or TE because of polarization in the oocyte has been challenged by Van de Velde et al. [42]. The four dissociated blastomeres of a tetrahedral-shaped 4-cell stage human embryo were individually capable to develop into blastocysts with ICM and TE. Therefore, the four blastomeres are equivalent regarding developmental potential. Whether the four blastomeres are also equivalent within the structure of the non-manipulated embryo is not known. However, randomly labeling one out of four blastomeres showed that within the structure of the embryo this blastomere participates in both cell types of the blastocyst, i.e. ICM and TE [43]. Some murine studies showed that, depending on the orientation of the second cleavage planes, the four blastomeres expose different developmental capabilities [32, 44] and different epigenetic modifications [45, 46]. In tetrahedral-shaped embryos, the blastomeres have predictable fates. One of the blastomeres of the former 2-cell stage embryo is directed predominantly to the embryonic part and the other one to the abembryonic part of the blastocyst. When the orientation of the earlier of the second cleavage divisions is meridional, the polarity of this embryonic–abembryonic axis can be predicted [32]. In this formation, the blastomere that inherits only vegetal components contributes more to the mural TE. This blastomere also exposes decreased levels of methylation of arginine 17 and 26 on histone H3 [32, 45]. On the contrary, in non-tetrahedral-shaped embryos, no tendency for allocation was observed to specific parts of the blastocyst and no epigenetic variations were found [32, 44, 45]. Other studies in mice suggested no prelocalization of developmental determinants in the early blastomeres and showed that the second polar body is not a reliable morphogenetic determinant for the first cleavage [47, 48]. Some claim that there is no rotation of the spindle itself but a movement of the blastomeres [49].
From our study, it can be concluded that a non-tetrahedral arrangement of the blastomeres within the 4-cell stage human embryo may affect its subsequent development. These embryos fail more often to compact compared to their tetrahedral counterparts. This may be due to the reduced number of contact points between the blastomeres in a non-tetrahedral formation. Still, non-tetrahedral-shaped embryos are capable to form blastocysts with ICM and TE. Therefore, we do not expect a predisposition of the blastomeres that is linked to the inherited ooplasm. If the non-tetrahedral-shaped 4-cell stage embryos develop into adequate embryos at the moment of transfer, their pregnancy rates do not differ from embryos originating from a tetrahedral arrangement. Therefore, the present data do not support a benefit for the tetrahedral arrangement of blastomeres as a new selection criterion when the transfers are performed on day 3 and especially on day 5.
Limitations of this study are its retrospective and non-randomized design. Also, we could not distinguish between embryos that came from two meridional divisions and from two equatorial divisions within the non-tetrahedral group. The two types of non-tetrahedral embryos should be distinguishable from each other by the location of the second polar body [4, 32]. However, in practice, this is difficult because of polar body movement, polar body fragmentation and/or cellular fragmentation. Culturing in a time-lapse system could allow a more in dept analysis of the blastomere arrangement and could record possible movements or rotations of the blastomeres. It might be interesting to conduct a randomized controlled trial with transfer on day 2 after time-lapse culture in order to study the clinical significance of the blastomere arrangement at the 4-cell stage or the orientation of the second cleavage planes.
Acknowledgments
The authors wish to thank the laboratory staff of the Centre for Reproductive Medicine, UZ Brussel.
Footnotes
Capsule Tetrahedral-shaped 4-cell stage human embryos have a better developmental capacity up to the blastocyst stage compared to non-tetrahedral-shaped embryos.
References
- 1.Gulyas BJ. A reexamination of cleavage patterns in eutherian mammalian eggs: rotation of blastomere pairs during second cleavage in the rabbit. J Exp Zool. 1975;193:235–48. doi: 10.1002/jez.1401930212. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert SF. Developmental biology. 5. Sunderland: Sinauer Associates; 1997. [Google Scholar]
- 3.Edwards RG, Steptoe PC, Purdy JM. Fertilization and cleavage in vitro of preovulatory human oocytes. Nature. 1970;227:1307–9. doi: 10.1038/2271307a0. [DOI] [PubMed] [Google Scholar]
- 4.Gardner RL. Experimental analysis of second cleavage in the mouse. Hum Reprod. 2002;12:3178–89. doi: 10.1093/humrep/17.12.3178. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RG, Beard HK. Oocyte polarity and cell determination in early mammalian embryos. Mol Hum Reprod. 1997;3:863–905. doi: 10.1093/molehr/3.10.863. [DOI] [PubMed] [Google Scholar]
- 6.Antczak M, Van Blerkom J. Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol Hum Reprod. 1997;3:1067–86. doi: 10.1093/molehr/3.12.1067. [DOI] [PubMed] [Google Scholar]
- 7.Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod. 1999;14:429–47. doi: 10.1093/humrep/14.2.429. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RG. Genetics of polarity in mammalian embryos. Reprod Biomed Online. 2005;11:104–14. doi: 10.1016/S1472-6483(10)61305-3. [DOI] [PubMed] [Google Scholar]
- 9.Howlett SK, Bolton VN. Sequence and regulation of morphological and molecular events during the first cycle of mouse embryogenesis. J Embryol Exp Morphol. 1985;87:175–206. [PubMed] [Google Scholar]
- 10.Payne D, Adachi D, Kato Y, Ueno Y, Mio Y, Flaherty S. Polarity and early human embryo development. Fertil Steril. 2004;82:S7. doi: 10.1016/j.fertnstert.2004.07.023. [DOI] [Google Scholar]
- 11.Edwards RG, Hansis C. Initial differentiation of blastomeres in 4-cell human embryos and its significance for early embryogenesis and implantation. Reprod Biomed Online. 2005;11:206–18. doi: 10.1016/S1472-6483(10)60960-1. [DOI] [PubMed] [Google Scholar]
- 12.Hansis C, Grifo JA, Krey LC. Candidate lineage marker genes in human preimplantation embryos. Reprod Biomed Online. 2004;8:577–83. doi: 10.1016/S1472-6483(10)61106-6. [DOI] [PubMed] [Google Scholar]
- 13.Nagy ZP, Liu J, Joris H, Bocken G, Desmet B, Van Ranst H, et al. The influence of the site of sperm deposition and mode of oolemma breakage at intracytoplasmic sperm injection on fertilization and embryo development rates. Hum Reprod. 1995;10:3171–7. doi: 10.1093/oxfordjournals.humrep.a135881. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MH. From mouse egg to mouse embryo: polarities, axes, and tissues. Annu Rev Cell Dev Biol. 2009;25:483–512. doi: 10.1146/annurev.cellbio.042308.113348. [DOI] [PubMed] [Google Scholar]
- 15.Mastenbroek S, van der Veen F, Aflatoonian A, Shapiro B, Bossuyt P, Repping S. Embryo selection in IVF. Hum Reprod. 2011;26:964–6. doi: 10.1093/humrep/der050. [DOI] [PubMed] [Google Scholar]
- 16.Ajduk A, Zernicka-Goetz M. Advances in embryo selection methods. F1000 Biol Rep. 2012;4:11. doi: 10.3410/B4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–40. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]
- 18.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 19.Prados F, Debrock S, Lemmen J, Agerholm A. The cleavage stage embryo. Hum Reprod. 2012;27:i50–71. doi: 10.1093/humrep/des224. [DOI] [PubMed] [Google Scholar]
- 20.Wilton L. Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22:512–8. doi: 10.1002/pd.388. [DOI] [PubMed] [Google Scholar]
- 21.Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Mol Hum Reprod. 2008;14:703–10. doi: 10.1093/molehr/gan062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–66. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 23.Wong C, Chen AA, Behr B, Shen S. Time-lapse microscopy and image analysis in basic and clinical embryo development research. Reprod Biomed Online. 2013;26:120–9. doi: 10.1016/j.rbmo.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Hardarson T, Ahlström A, Rogberg L, Botros L, Hillensjö T, Westlander G, et al. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27:89–96. doi: 10.1093/humrep/der373. [DOI] [PubMed] [Google Scholar]
- 25.Vergouw CG, Kieslinger DC, Kostelijk EH, Botros LL, Schats R, Hompes PG, et al. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012;27:2304–11. doi: 10.1093/humrep/des175. [DOI] [PubMed] [Google Scholar]
- 26.Nel-Themaat L, Nagy ZP. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta. 2011;32:S257–63. doi: 10.1016/j.placenta.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Papanikolaou EG, D’haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Hum Reprod. 2005;20:3198–203. doi: 10.1093/humrep/dei217. [DOI] [PubMed] [Google Scholar]
- 28.Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update. 2003;9:251–62. doi: 10.1093/humupd/dmg021. [DOI] [PubMed] [Google Scholar]
- 29.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. London: Parthenon Publishing; 1999. pp. 378–88. [Google Scholar]
- 30.Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirtheghem A. Blastocyst formation in in vitro versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83:1397–403. doi: 10.1016/j.fertnstert.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod. 2009;24:2683–7. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 32.Piotrowska-Nitsche K, Zernicka-Goetz M. Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev. 2005;122:487–500. doi: 10.1016/j.mod.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008;23:62–6. doi: 10.1093/humrep/dem280. [DOI] [PubMed] [Google Scholar]
- 34.Nikas G, Ao A, Winston RM, Handyside AH. Compaction and surface polarity in the human embryo in vitro. Biol Reprod. 1996;55:32–7. doi: 10.1095/biolreprod55.1.32. [DOI] [PubMed] [Google Scholar]
- 35.Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD. Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995;10:1571–8. doi: 10.1093/HUMREP/10.6.1571. [DOI] [PubMed] [Google Scholar]
- 36.Bloor DJ, Metcalfe AD, Rutherford A, Brison DR, Kimber SJ. Expression of cell adhesion molecules during human preimplantation embryo development. Mol Hum Reprod. 2002;8:237–45. doi: 10.1093/molehr/8.3.237. [DOI] [PubMed] [Google Scholar]
- 37.Alikani M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum Reprod. 2005;20:3369–75. doi: 10.1093/humrep/dei242. [DOI] [PubMed] [Google Scholar]
- 38.Paternot G, Spiessens M, Verstreken D, Van Bauwel J, Debrock S, D’Hooghe T, et al. Is there a link between blastomere contact surfaces of day 3 embryos and live birth rate? Reprod Biol Endocrinol. 2012;10:78. doi: 10.1186/1477-7827-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamran SC, Reichman DE, Missmer SA, Correia KF, Karaca N, Romano A, et al. Day 3 embryo shape as a morphologic selection parameter in in vitro fertilization. J Assist Reprod Genet. 2012;29:1135–9. doi: 10.1007/s10815-012-9842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebner T, Maurer M, Shebl O, Moser M, Mayer RB, Duba HC, et al. Planar embryos have poor prognosis in terms of blastocyst formation and implantation. Reprod Biomed Online. 2012;25:267–72. doi: 10.1016/j.rbmo.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Munné S. Chromosome abnormalities and their relationship to morphology and development of human embryos. Reprod Biomed Online. 2005;12:234–53. doi: 10.1016/S1472-6483(10)60866-8. [DOI] [PubMed] [Google Scholar]
- 42.Van de Velde H, Cauffman G, Tournaye H, Devroey P, Liebaers I. The four blastomeres of a 4-cell stage human embryo are able to develop individually into blastocysts with inner cell mass and trophectoderm. Hum Reprod. 2008;23:1742–7. doi: 10.1093/humrep/den190. [DOI] [PubMed] [Google Scholar]
- 43.Mottla GL, Adelman MR, Hall JL, Gindoff PR, Stillman RJ, Johnson KE. Lineage tracing demonstrates that blastomeres of early cleavage-stage human pre-embryos contribute to both trophectoderm and inner cell mass. Hum Reprod. 1995;10:384–91. doi: 10.1093/oxfordjournals.humrep.a135949. [DOI] [PubMed] [Google Scholar]
- 44.Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005;132:479–90. doi: 10.1242/dev.01602. [DOI] [PubMed] [Google Scholar]
- 45.Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007;445:214–8. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres-Padilla ME. Cell identity in the preimplantation mammalian embryo: an epigenetic perspective from the mouse. Hum Reprod. 2008;23:1246–52. doi: 10.1093/humrep/dem434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiiragi T, Solter D. First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature. 2004;430:360–4. doi: 10.1038/nature02595. [DOI] [PubMed] [Google Scholar]
- 48.Zheng JG, Huo T, Chen T, Wang C, Zhang N, Tian N, et al. Understanding three-dimensional spatial relationship between the mouse second polar body and first cleavage plane with full-field optical coherence tomography. J Biomed Opt. 2013;18:10503. doi: 10.1117/1.JBO.18.1.010503. [DOI] [PubMed] [Google Scholar]
- 49.Louvet-Vallée S, Vinot S, Maro B. Mitotic spindles and cleavage planes are oriented randomly in the two-cell mouse embryo. Curr Biol. 2005;15:464–9. doi: 10.1016/j.cub.2004.12.078. [DOI] [PubMed] [Google Scholar]