Abstract
Paracellular route is a natural pathway for the transport of many hydrophilic drugs and macromolecules. The purpose of this study was to prospectively evaluate the ability of novel co-processed non-ionic surfactants to enhance the paracellular permeability of a model hydrophilic drug metformin using Caco-2 (human colonic adenocarcinoma) cell model. A three-tier screen was undertaken to evaluate the co-processed blends based on cytotoxicity, cellular integrity, and permeability coefficient. The relative contribution of the paracellular and the transcellular route in overall transport of metformin by co-processed blends was determined. Immunocytochemistry was conducted to determine the distribution of tight-junction protein claudin-1 after incubation with the co-processed blends. It was found that novel blends of Labrasol and Transcutol-P enhanced metformin permeability by approximately twofold with transient reduction in the transepithelia electrical resistance (TEER) and minimal cytotoxicity compared with the control, with the paracellular pathway as the major route of metformin transport. Maximum permeability of metformin (∼10-fold) was mediated by Tween-20 blends along with >75% reduction in the TEER which was irreversible over 24-h period. A shift in metformin transport from the paracellular to the transcellular route was observed with some Tween-20 blends. Immunocytochemical analysis revealed rearrangement of the cellular borders and fragmentation on treatment with Tween-20 blends. In conclusion, cytotoxicity, cellular integrity, and permeability of the hydrophilic drugs can be greatly influenced by the polyoxyethylene residues and medium chain fatty acids in the non-ionic surfactants at clinically relevant concentrations and therefore should be thoroughly investigated prior to their inclusion in formulations.
KEY WORDS: caco-2 cells, co-processed non-ionic surfactants, metformin, paracellular transport, permeability coefficient
INTRODUCTION
The paracellular pathway represents an effective mechanism for the transport and delivery of highly water-soluble, poorly lipid-soluble, cationic drugs, and macromolecules (15). Many marketed products including cimetidine and metformin are transported across the intestinal epithelia via the paracellular pathway. The paracellular route is defined by the aqueous pathway between adjacent cells of the gastrointestinal (git) epithelia and is restricted at the apical side by the tight junction (TJ) or zonula occludens (ZO) proteins, occludin, claudin, ZO-1, ZO-2, cingulin, and 7H6 (4). The rate-limiting step in the absorption of drugs via the paracellular route are the TJs, which form narrow pores and act as gatekeepers to the passage of low-molecular-weight compounds. Therefore, hydrophilic drugs such as metformin exhibit saturable kinetics through the paracellular pathway due to the narrow pores of the TJs (31). To date, numerous approaches, including the use of surfactants, ZO toxin, delta G, and clostridium perfringens enterotoxin have been explored to make TJs reversibly permeable to poorly bioavailable drugs and macromolecules in order to enhance the paracellular permeability of these molecules (12,37).
Formulation excipients, such as non-ionic surfactants, are extensively used as absorption enhancers to improve absorption of poorly soluble and permeable drugs belonging to the BDDCS (proposed by (5)) classes II–IV. These absorption enhancers have been shown to increase the permeability of drugs across epithelial barriers in a concentration-dependent manner (11). Even though, it is widely recognized that majority of non-ionic surfactants increase the permeability of drugs through the transcellular pathway, studies using human colonic adenocarcinoma cells (Caco-2) have shown that several surfactants, such as sodium dodecyl sulphate, sodium caprate, and long chain acylcarnitines can increase the permeability of drugs through the paracellular pathway (15). Labrasol, a non-ionic surfactant, has been shown to increase the paracellular permeability of mannitol in Caco-2 cells by opening the TJ proteins, F-actin, and ZO-1 (40). In another study, Tween-20 was found to enhance the paracellular permeability of metformin, but compromised the viability of Caco-2 cell monolayer (10,11). Because, individual non-ionic surfactants have been shown to concurrently enhance the paracellular permeability of hydrophilic drugs and produce cytotoxicity in Caco-2 cells, we wanted to prepare co-processed non-ionic surfactants that retain the property of enhancing paracellular permeability and exhibit reduced cytotoxicity. To the best of our knowledge, there are no reports in the literature evaluating the effects of co-processed non-ionic surfactants on the paracellular permeability of hydrophilic drugs in Caco-2 cells. Hence, the objectives of this study were (1) to evaluate the role of the co-processed non-ionic surfactants Labrasol, Tween-20, Transcutol-P, and Lauroglycol-90 and the triglycerides Maisine 35-1 and Peceol in the enhancement of the paracellular transport of a model hydrophilic drug, namely, metformin, in Caco-2 cells; (2) to determine the contribution of the paracellular and/or transcellular route in the transport of metformin across Caco-2 cells in the presence of novel co-processed excipients; and (3) to evaluate the effect of novel co-processed excipients on the TJ protein, claudin-1, by immunocytochemistry.
MATERIALS AND METHODS
Materials
Caco-2 cells at passage number 18 were obtained from the American Type Culture Collection (VA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), 3-(4,5-dimethylthiazoyl)-2,5-diphenyltetrazolium bromide (MTT), heat-inactivated fetal bovine serum (FBS), penicillin–streptomycin, Lucifer yellow, Trypsin-EDTA, paraformaldehyde, sodium azide, DMSO, phosphate-buffered saline (PBS), Hank’s Balanced Salt Solution (HBSS), and T-75 flasks were purchased from VWR (NJ, USA). Labrasol, Transcutol-P, Lauroglycol-90, Peceol, and Maisine-35-1 were kind gifts from Gattefosse (NJ, USA). Tween-20 was purchased from VWR (NJ, USA). Metformin was purchased from Fisher Scientific (PA, USA). [14C]-Metformin (110.2 mCi/mmol) was obtained from Moravek Biochemicals, Inc. (CA, USA). Nonspecific organic cation transporter (OCT), multidrug and toxic compound extrusion (MATE), and plasma membrane monoamine transporter (PMAT) inhibitor, 1-methyl-4-phenylpyridinium (MPP+) was purchased from Sigma Aldrich (MO, USA). The polycarbonate transwell inserts for the permeability experiments were obtained from Corning (NY, USA). Greiner Bio-One 96 well culture plates were purchased from Fisher Scientific (PA, USA). The EVOMTM epithelial voltmeter was purchased from World Precision Instruments (FL, USA). The CytoScintTM-ES Liquid Scintillation Cocktail (MP Biomedicals) was purchased from VWR (NJ, USA). For the immunocytochemistry experiments, the primary antibody (rabbit polyclonal antibody against claudin-1) and the anti-rabbit IgG Alexa fluor-594 secondary antibody were purchased from Fisher Scientific (PA, USA). All of the other chemicals were of high purity or analytical grade and used as received.
Cell Culture
Caco-2 cells were cultured in T-75 flasks in DMEM, supplemented with 4.5 g/L glucose, 4 mM l-glutamine, sodium pyruvate, 20% heat-inactivated FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in an atmosphere of 5% CO2. Aseptic conditions were maintained at all times. The cells were routinely maintained by replacing the culture media with fresh media every 72 h and by regular passage. For the cytotoxicity study and transport experiments, the cells were used between passage numbers 20 and 40. Transwell membranes were used for the transport studies after the cells were grown for 21–28 days and when the transepithelia electrical resistance (TEER) value reached ≥300 Ω cm2.
Determination of Cell Viability
To evaluate the effect of surfactants on the viability of the Caco-2 cells, an MTT assay was developed based on a previous method (25). Briefly, the cells were seeded on 96-well tissue culture plates at a density of 1 × 104 cells per well and allowed to grow in CO2 incubator at 37°C for 3 days until confluent monolayers were obtained. For the cytotoxicty studies, the supplemented media was removed, and individual excipients, co-processed formulation blends (Table I), and/or metformin (10 mM) solution (in sterile supplemented media) were added; the cells were incubated for 4 h. After the 4-h incubation, 20 μL of MTT reagent (5 mg/mL in PBS) was added to the wells, and the cells were incubated for an additional 4 h. The complete medium was removed, and the formazan crystals were dissolved by the addition of 100 μL DMSO to each well. The absorbance was measured using Molecular Devices SpectraMax M5e (CA, USA) at 560 nm, and the background at 670 nm was subtracted. All of the experiments were conducted in triplicate. The data are represented as the means ± standard deviation (SD).
Table I.
Composition and HLB of Novel Co-Processed Blends of Labrasol, Transcutol-P and Tween-20
| Composition | 1(a) 0.05% Labrasol | 1 (b) 0.05% Labrasol | 1(c) 0.05% Labrasol | 1(d) 0.05% Labrasol |
| 1% Lauroglycol | 0.5% Transcutol | 0.5% Transcutol | ||
| 1% Maisine | 1% Peceol | |||
| HLB = 14 | HLB = 5.22a | HLB = 4.23a | HLB = 3.45a | |
| Composition | 2(a) 0.5% Transcutol | 2(b) 0.5% Transcutol | 2(c) 0.5% Transcutol | 2(d) 0.5% Transcutol |
| 1% Lauroglycol | 1% Maisine | 1% Peceol | ||
| HLB = 4.2 | HLB = 4.84a | HLB = 4.04a | HLB = 3.24a | |
| Composition | 3(a) 0.5% Tween-20 | 3(b) 0.5% Tween-20 | (c) 0.5% Tween-20 | 3(d) 0.5% Tween-20 |
| 1% Lauroglycol | 0.5% Transcutol | 0.5% Transcutol | ||
| 1% Maisine | 1% Peceol | |||
| HLB = 16.7 | HLB = 7.34a | HLB = 6.15a | HLB = 5.48a |
a
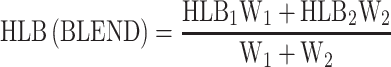
W 1 = weight1, W 2 = weight2
Determination of Cellular Integrity
Preparation of Transwell Plates for TEER Measurements
To determine the integrity of the Caco-2 monolayers, the TEER was monitored before and after treatment with novel co-processed excipients. Briefly, 12-well Transwell inserts (Corning transwell polycarbonate plates; diameter, 6.5 mm; pore size, 3.0 μm) were used for seeding Caco-2 cells at a density of 2 × 105 cells/cm2, and the cells were grown for 21–28 days until confluent monolayers were obtained. The transwell plate supplemented media was changed every other day, and membranes were used in the experiments when the TEER value reached ≥300 Ω cm2. The TEER measurement studies were initiated by replacing the supplemented media in the donor (apical) and receiver (basolateral) compartments with the transport solution HBSS. The TEER was monitored using World Precision Instruments EVOMTM epithelial voltmeter.
Recovery of Caco-2 Cell Monolayer TEER Value
The recovery of the TJ integrity after treatment with novel co-processed formulation excipients was monitored by measuring the TEER values at defined time points for up to 24 h and before and after the Lucifer yellow permeability assay. Transwell membranes were prepared based on the criteria described in the “Preparation of Transwell Plates for TEER Measurements” section. The Caco-2 monolayers were incubated with Lucifer yellow and/or the transport solution containing co-processed excipients (Table I) for 4 h. At the end of the incubation period, the transport solution was discarded, and the cell monolayers were three times washed with PBS. Fresh HBSS was added, and the TEER was measured for up to 24 h. The TEER values obtained after treatment with the formulation blends were expressed as relative percentages compared with the baseline values obtained for the vehicle control. All of the experiments were conducted in triplicate. The data are represented as the means ± SD.
Effect of Novel Co-Processed Excipients on Lucifer Yellow Permeability
Any chemical that enhances Lucifer yellow permeability, a marker of paracellular TJ integrity, can be considered to disrupt the TJs and the Caco-2 monolayer integrity. The paracellular permeability after the exposure of Caco-2 cells to co-processed excipients was measured using Lucifer yellow. Transwell membranes were prepared based on the criteria described in the “Preparation of Transwell Plates for TEER Measurements” section. After preincubation with HBSS, the transport solution containing Lucifer yellow (100 μg/mL in DMSO) with and without the co-processed excipients (in sterile transport solution) was added to the donor (apical) chamber, and the Caco-2 cells were incubated for 4 h. The co-processed excipients tested were based on the IC50 values obtained from the MTT cytotoxicity assay, and the concentrations selected were well below the cytotoxic value. Samples were collected from the receiver (basolateral) compartment every 30 min for up to 4 h and replaced with fresh HBSS. The fluorescence intensity of the samples due to alterations in the Lucifer yellow permeability was measured at an excitation wavelength (Ex) of 450 nm and an emission wavelength (Em) of 520 nm using a Molecular Devices SpectraMax M5e and the Lucifer yellow apparent permeability (Papp, in centimeter per second) was determined. All of the experiments were conducted in triplicate. The data are represented as the means ± SD.
Effect of Novel Co-Processed Excipients on Metformin Permeability
Metformin was chosen as the model drug in order to determine the effect of novel co-processed excipients on the paracellular permeability (uptake) of a hydrophilic drug. As previously mentioned in “Effect of Novel Co-Processed Excipients on Lucifer Yellow Permeability” section, transwell membranes were pre-incubated in HBSS at 37°C for 30 min before initiating the transport studies. The transport solution containing co-processed excipients (Table I), 10 mM metformin, and 0.5 μCi 14C-metformin (a mix of cold and 14C-metformin in sterile transport solution was added to monitor the transport of metformin) was added to the donor (apical) chamber to evaluate the uptake of metformin in the presence of co-processed formulation blends, and the plates were further incubated for 4 h. At the end of the 4-h incubation period, samples were collected from the receiver (basolateral) compartment in a scintillation vial containing a scintillation cocktail. The radioactivity in the samples was measured using a Packard Tri-Carb Liquid Scintillation Analyzer (CT, USA). To determine the integrity of the Caco-2 monolayers, the TEER was monitored before and after treatment with the co-processed excipient blends and metformin. The recovery of TJ integrity after treatment with the co-processed formulation blends and metformin was monitored by measuring the TEER values at defined time points before and for up to 24 h after the metformin permeability assay. The TEER values obtained for the co-processed formulation blends are expressed as relative percentages compared with the baseline values obtained for the vehicle control. All of the experiments were conducted in triplicate. The data are represented as the means ± SD.
Contribution of the Transcellular and Paracellular Pathways to Metformin Transport in the Presence of Novel Co-Processed Excipients
Because non-ionic surfactants can enhance the absorption of drugs through both the transcellular and the paracellular pathway, the contribution of each pathway to the transport of metformin was evaluated. Briefly, transport studies were performed in the presence of MPP+, a nonspecific inhibitor of OCT, MATE, and PMAT transporters. Cell monolayers were pre-incubated with 0.05 mM MPP+ for 30 min, the media was then replaced with transport solution containing co-processed excipients (Table I), 10 mM metformin, and 0.5 μCi 14C-metformin in sterile transport solution; plates were further incubated for 4 h. Following 4-h incubation, samples were collected from the receiver (basolateral) compartment in a scintillation vial containing scintillation cocktail. The radioactivity in the samples was measured using a Packard Tri-Carb Liquid Scintillation Analyzer (CT, USA) and the apparent permeability (Papp, in centimeter per second) of metformin and the percentage contribution of the paracellular and transcellular pathways to transport of metformin across Caco-2 cell monolayer were determined. All of the experiments were conducted in triplicate. The data are represented as the means ± SD.
Effect of Novel Co-Processed Excipients on the Tight Junction Protein Claudin-1 by Immunocytochemistry
To evaluate the effect of novel co-processed excipients on the expression of the tight junction protein claudin-1, immunocytochemical techniques were employed. Transwell membranes were prepared as previously described in “Effect of Novel Co-Processed Excipients on Lucifer Yellow Permeability” section. Cell monolayers were incubated with various co-processed non-ionic surfactants (Table I) for 4 h. The medium was removed and the monolayers were washed with PBS three times at room temperature. The cells were fixed with 4% paraformaldehyde for 15 min, rinsed three times with PBS, and then permeabilized with 0.2% Tween-20 for 10 min. After further rinsing with PBS, the cells were blocked with 1% bovine serum albumin (BSA) for 1 h. The monolayers were washed again with PBS and then incubated with prediluted (in TBS, pH 7.6, 1% BSA in buffer, and less than 0.1% sodium azide) primary antibody (rabbit polyclonal antibody against claudin-1) overnight at 4°C. After three washes with PBS, the cells were incubated for 1 h with TRITC-labeled goat anti-rabbit IgG antibody (Alexa Fluor® 594 Goat Anti-Rabbit IgG) diluted at 1:200 in PBS. After several washes, the filter was cut out, mounted on glass slides, and examined under Nikon Eclipse TE2000-S fluorescent microscope.
Data Analysis
All of the experiments were conducted in triplicates, and the means calculated. The results are reported as the means ± SD. A two-tailed Student’s t test was performed at p < 0.05. The significant differences between groups are marked by asterisks in the figures. The Lucifer yellow and metformin apparent permeability (Papp, in centimeter per second) were determined using the following formula:
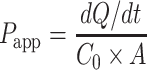 |
where dQ/dt is the flux across the monolayer, A is the surface area of the transwell membrane, and C0 is the initial concentration of Lucifer yellow or 14C-metformin in the apical chamber. The percentage contribution of the paracellular and transcellular pathways to the transport of metformin across the Caco-2 cell monolayer was calculated using the following equations:
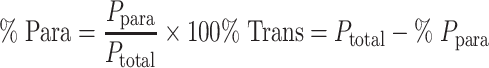 |
where Ptotal = Papp in the Caco-2 monolayer, Ppara is the Papp of the paracellular pathway,% Para is the contribution of the paracellular pathway, and % Trans refers to the contribution of the transcellular pathway. The maximum absorbable dose (MAD) of metformin in grams was predicted from the apparent permeability using the formula (41), MAD = Peffhuman × S × A × Tsi, where Peff is the effective human jejunal permeability (obtained from Papp data), S is the solubility of the drug, A is the absorption surface area, and Tsi is the transit time to the absorption site.
RESULTS
Cell Viability Studies of Non-Ionic Surfactants
One of the objectives of this study was to determine the viability of Caco-2 cells incubated with known and novel co-processed non-ionic surfactants at varying concentrations with or without metformin using the MTT assay. Because the transit time of an oral dose of drug through the intestine is 3–4 h (9), the cell viability studies were conducted for 4 h (6,9,11,46). The non-ionic surfactants under consideration in this study (Tables I and II) showed significant differences in the viability of Caco-2 cells incubated at 37°C for 4 h, and their effects were concentration-dependent (Table III). Transcutol-P showed an IC50 value of 2.0 ± 0.35% (v/v), whereas Lauroglycol-90, Peceol and Maisine-35-1 (Table III) were not cytotoxic over the concentration range tested (1–10% (v/v)). The positive control polyethylene glycol-based surfactant, Labrasol, proved to be the most cytotoxic in Caco-2 cells (Table III) with an IC50 value of 0.22 ± 0.01% (v/v) and this result is in close agreement with the IC50 value of 0.23 ± 0.025% (v/v) reported by Ujhelyi et al. (45). Another polyethylene glycol-based positive control, Tween-20, produced an IC50 value of 4.9 ± 0.02% (v/v), which is comparable to that reported in the literature (IC50 value of ∼3% (v/v) reported by Dimitrijevic et al. (11)) and IC50 value of 0.004 ± 0.0001% (v/v) reported by Ujhelyi et al. (45). Tween-20 can be susceptible to auto-oxidation due to the storage conditions, elevated temperature, and light. Auto-oxidation may lead to the formation of significant levels of hydroperoxides in the product (20), which can contribute to higher cytotoxicity, as observed by Ujhelyi et al. (45), as against that reported by Dimitrijevic et al. (11) and in the present study. The co-incubation of the surfactants with metformin (10 mM) did not significantly alter the viability of Caco-2 cells as compared to the viability obtained in the absence of metformin (Table III), except for Tween-20, which indicates that metformin has minimal effect on the cytotoxicity observed with individual surfactants in Caco-2 cells. The co-incubation of Tween-20 with 10 mM metformin caused an approximately twofold lowered IC50 value of Tween-20 (2.50 ± 0.32% (v/v)) as compared to the viability observed in the absence of metformin (IC50 value of 4.9 ± 0.02% (v/v)) which indicates that metformin did not had a cytoprotective effect on the Tween-20 mediated cytotoxicity in Caco-2 cells. Based on the cell viability results, non-ionic surfactants were blended with triglycerides in different ratios such that (1) the IC50 value of the co-processed formulation excipients was a nontoxic concentration (below the IC50 of the individual excipients) and (2) the hydrophilic–lipophilic balance (HLB) of the co-processed surfactant blends was less than 7.0 for formulating water-in-oil (w/o) type of emulsion of hydrophilic drugs and macromolecules. The cell viability results for the co-processed blends of Labrasol, Transcutol-P, and Tween-20 indicated that the blends were nontoxic at the fabricated ratios and concentrations (Fig. 1).
Table II.
Chemical and Functional Characteristics of Non-Ionic Surfactants
| Excipient | Chemical name | HLB | Function |
|---|---|---|---|
| Peceol | Glyceryl monooleate type 40NF | 3 | Oily vehicle; solubilizer; bioavailability enhancer |
| Maisine 35-1 | Glyceryl monolinoleate NF | 4 | Oily vehicle; bioavailability enhancer |
| Transcutol-P | Diethylene glycol monoethylether | 4.2 | Solubilizer; co-solvent; penetration enhancer |
| Lauroglycol-90 | Propylene glycol monolaurate | 5 | Water insoluble surfactant; bioavailability enhancer |
| Labrasol | Caprylocaproyl polyoxyl-8 glycerides NF | 14 | Water dispersible surfactant; bioavailabilty enhancer |
| Tween-20 | Polyoxyethylene sorbiton monolaurate | 16.7 | Oily vehicle; non-ionic surfactant; bioavailability enhancer |
HLB hydrophilic and lipophilic balance refers to the hydrophilic and lipophilic parts of surfactants
Table III.
Cytotoxicity Profile (IC50 values) of Non-Ionic Surfactants in Caco-2 cells after incubation for 4 h at 37°C
| Excipient | IC50 values of Excipients (%, v/v) | IC50 values of Excipients and Metformin HCL (10 mM) (%, v/v) |
|---|---|---|
| Tween-20 | 4.90 ± 0.02 | 2.50 ± 0.32 |
| Labrasol | 0.22 ± 0.01 | 0.24 ± 0.05 |
| Transcutol-P | 2.04 ± 0.35 | 1.84 ± 0.36 |
| Lauroglycol-90 | >1 | >1 |
| Maisine 35-1 | >1 | >1 |
| Peceol | >1 | >1 |
Fig. 1.
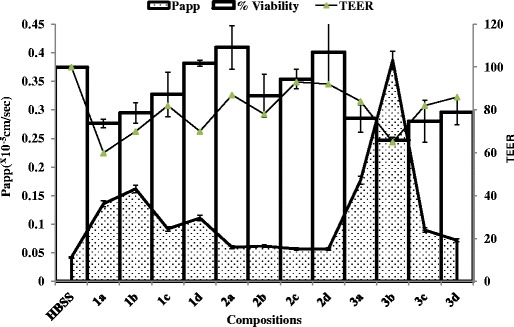
Cumulative effect of novel co-processed Labrasol, Transcutol-P, and Tween-20 blends on cytotoxicity, TEER, and Lucifer yellow permeability across Caco-2 cell monolayers after 4 h incubation at 37°C. The data are represented as means ± SD (n = 3)
Cellular Integrity Studies
TEER Measurements
Transepithelial electrical resistance measurements were conducted to determine the TJ integrity after incubation of the Caco-2 cell monolayers with the surfactants on the donor (apical) chamber of transwell plates (the TJs proteins are located on the apical side of the monolayers). Because the transit time of an oral dose of drug through the intestine is 3–4 h (9), the TEER was determined up to 4 h after treatment with the co-processed excipients, whereas the recovery of the cells was monitored for up to 24 h. Although, 0.05% (v/v) Labrasol had minimal effect on cell viability as indicated by the MTT assay (Fig. 1), the TEER value of the Caco-2 monolayers exposed to 0.05% (v/v) Labrasol alone (Fig. 2a, blend 1a) was reduced significantly (<70% of the baseline values, p < 0.05) compared with the other co-processed blends of Labrasol (Fig. 2a): co-processed Labrasol blend 1b (0.05% (v/v) Labrasol and 1% (v/v) Lauroglycol-90) and blend 1d (0.05% (v/v) Labrasol, 0.5% (v/v) Transcutol-P, and 1% (v/v) Peceol). A minimal effect on the TEER value was observed with co-processed formulation excipients containing 0.5% (v/v) Transcutol-P compared with the media control (Fig. 2b, blends 2a–d). Similar to the effect observed with 0.05% (v/v) Labrasol, Tween-20 in a blend containing 1% (v/v) Lauroglycol-90 showed a reduced TEER value (Fig. 2c, blend 3b, ∼60% of baseline values; p < 0.05) following incubation with Caco-2 cell monolayer at 37°C for 4 h compared with all other co-processed blends under evaluation in this study. These results indicate that the decreased TEER will lead to increased paracellular permeability, but the extent to which an excipient will reduce TEER will depend on the concentration used in the formulation and on the type and nature of the functional groups attached to the excipient.
Fig. 2.
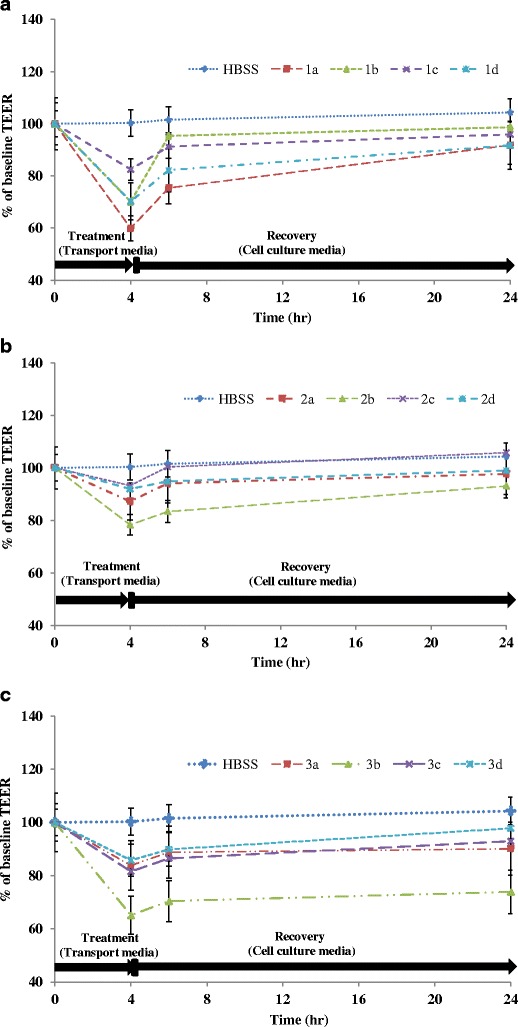
Effect of novel co-processed blends of Labrasol (1A), Transcutol (1B), and Tween-20 (1C) on the TEER of Caco-2 cell monolayers after 4 h incubation at 37°C. The data are represented as means ± SD (n = 3)
Recovery of Caco-2 Cell Monolayer TEER Value
Because changes in the TEER value have been shown to modulate paracellular permeability of xenobiotics and endogenous inflammatory cytokines (precursors of cellular viability events), we wanted to determine whether Caco-2 cell monolayers incubated with known non-ionic surfactants or co-processed blends disrupt the TJ integrity in a reversible or irreversible manner. Therefore, the transport medium was replaced with fresh HBSS 4 h after incubation with individual and co-processed excipient blends, and the TEER was monitored over 24 h. The TEER value obtained after treatment with 0.05% (v/v) Labrasol, which was markedly decreased at 4 h (Fig. 2a, blend 1a) compared to that obtained with the other surfactants, recovered slowly and reached approximately 70% of the baseline values at 6 h and recovered to ∼90% of the baseline values at 24 h. Transcutol-P at 0.5% (v/v) and Tween-20 at 0.5% (v/v) did not show any appreciable reduction in the TEER values (Fig. 2b, c, blends 2a and 3a), which was corroborated by their minimal effects on cell viability (Fig. 1).
A significant reduction in the TEER value (<70% of baseline, p < 0.05) was observed with the co-processed Labrasol blends, 1b (0.05% (v/v) Labrasol and 1% (v/v) Lauroglycol-90) and blend 1d (0.05% (v/v) Labrasol, 0.5% (v/v) Transcutol, and 1% (v/v) Peceol) as shown in Fig. 2a. However, the TEER values had returned to the control values by 24 h after removal of the surfactants. The co-processed blend composed of 0.5% (v/v) Tween-20 with 1% (v/v) Lauroglycol-90 showed the maximum reduction in the TEER value after the 4-h incubation period (approximately 60% of baseline; p < 0.05; Fig. 2c, blend 3b) compared with the media control (HBSS) and other blends of Tween-20. The TEER value of Caco-2 cell monolayers after treatment with formulation blend 3b (0.5% (v/v) Tween-20 with 1% (v/v) Lauroglycol-90) failed to recover to the baseline values over the 24-h period, indicating irreversible disruption of the paracellular TJ integrity (Fig.2c, blend 3b). Collectively, these results indicate that irreversibility of TEER on incubation with Tween-20 blend 3b would lead to permanent opening of the TJ that would allow the passage of extracellular components along with the drug molecules. Irreversibility of TEER indicates markedly higher damage to the TJ proteins than transient TEER alterations as seen with Labrasol blends, 1b and 1d.
Effect of Co-processed Blends of Non-Ionic Surfactants on Lucifer Yellow Permeability
Alterations in TEER can have a direct consequence on the paracellular permeability in Caco-2 cell monolayers due to the effects on TJ proteins (18). Hence, to confirm our TEER findings, we evaluated the paracellular transport of Lucifer yellow, which is predominantly transported via the paracellular route. After the cell viability experiments, the co-processed blends of surfactants were evaluated to determine their ability to affect the Lucifer yellow permeability and alter the TEER value in Caco-2 cell monolayers. Labrasol blend 1a at 0.05% (v/v) and co-processed blend with 1% (v/v) Lauroglycol-90 blend 1b showed enhanced Lucifer yellow permeability (approximately three- and fourfold, respectively) and a reduced TEER value in Caco-2 cell monolayers after incubation at 37°C for 4 h compared with the media control (HBSS; Fig. 3a). The apparent Lucifer yellow permeability in the presence of 0.5% (v/v) Transcutol-P blend 2a or co-processed with non-ionic surfactants, blends 2b–2d was low (p < 0.05), compared with that obtained with the Labrasol containing blends (Fig. 3b, blends 2a–2d). Tween-20 at 0.5% (v/v) alone, showed enhanced Lucifer yellow permeability across Caco-2 cell monolayers (approximately threefold), and this result was supported by a concomitant decrease in the TEER values (Fig. 3c, blend 3a). The apparent Lucifer yellow permeability after incubation with the co-processed blend of 0.5% (v/v) Tween-20 and 1% (v/v) Lauroglycol-90 was highest (∼tenfold, Fig. 1c, blend 3b; p < 0.05) compared with all other individual and co-processed blends of non-ionic surfactants (Fig. 3a, b, c), and a significant reduction in the TEER value was obtained after the 4-h incubation period.
Fig. 3.
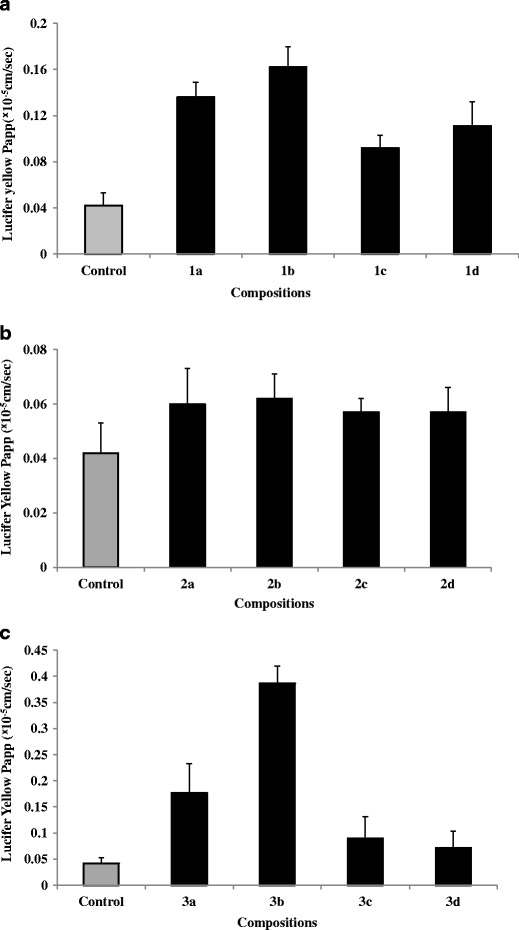
Lucifer yellow permeability in presence of novel co-processed blends of Labrasol (2A), Transcutol-P (2B), and Tween-20 (2C) across Caco-2 cell monolayers after 4 h incubation at 37°C. HBSS containing 0.5% DMSO was used as a vehicle control. All measurements are expressed as means ± SD (n = 3)
Of all of the fabricated novel co-processed non-ionic surfactants, the blend of 0.05% (v/v) Labrasol and 0.5% (v/v) Tween-20 containing 1% (v/v) Lauroglycol-90 showed maximal enhancement of the Lucifer yellow permeability and maximal reduction of the TEER value (Fig. 1, blends 1b and 3b). The enhanced Lucifer yellow permeability and reduced TEER obtained through the incorporation of 1% (v/v) Lauroglycol-90 with Labrasol and Tween-20 in formulation blends (blends 1b and 3b, respectively) may be associated with the fact that 1% (v/v) Lauroglycol 90 does not significantly lower the polarity of Labrasol and Tween-20 in the blends (HLB > 5) compared to the other Labrasol blends which leads to an increased paracellular permeability of Lucifer yellow.
Effect of Co-Processed Blends of Non-Ionic Surfactants on the Distribution of the Tight Junction Protein Claudin-1
Claudin-1, a member of the transmembrane protein family claudin, plays an important role in the maintenance of the polarity, structure, and paracellular transport function of the tight junctions. Alterations in the expression of claudin-1 can decrease the TJ resistance, thereby causing “leakiness” of the TJ and increased paracellular permeability (46). Therefore, Caco-2 cells were exposed to co-processed formulation blends to evaluate the effect of non-ionic surfactants on the expression and localization of claudin-1, which were determined by immunostaining followed by fluorescence microscopy. The incubation of Caco-2 cells with the formulation blends for 4 h altered the distribution of claudin-1 significantly. Compared with the continuous bands of claudin-1 observed in the cellular borders of the immunolabeled media control (Fig. 4, control), the blend containing 0.05% (v/v) Labrasol and 1% (v/v) Lauroglycol (Fig. 4b, blend 1b) exhibited diminished immunostaining of the cell borders, and this decrease was supported by a ∼25% reduction in TEER values (Fig. 2a, blend 1b) and an approximately fourfold increase in Lucifer yellow permeability (Fig. 3a, blend 1b). The formulation blends containing 0.5% (v/v) Tween-20 resulted in a rearrangement of the cellular borders with significant fragmentation and the presence of “holes”, as observed by the diminished immunostaining of the cell borders (Fig. 4i–l). Such rearrangement of cellular borders may explain the enhanced overall permeability of Lucifer yellow (Fig. 3c, blends 3a–3d).
Fig. 4.
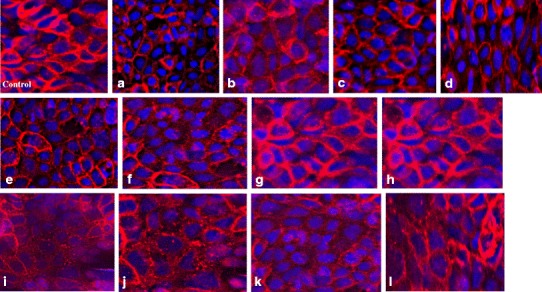
Immunostaining of tight junction protein claudin-1 in Caco-2 cell monolayers after 4 h incubation with novel co-processed blends of Labrasol (a–d), Transcutol-P (e–h), and Tween-20 (i–l)
Effect of Co-Processed Blends of Non-Ionic Surfactants on Metformin Permeability
One of the objectives of this study was to evaluate whether certain co-processed blends of non-ionic surfactants can enhance the uptake of metformin through the paracellular route because the transport of metformin has been shown to be variable, incomplete, and preferentially through the paracellular pathway (31). The apparent permeability of 10 mM metformin and 0.5 μCi 14C-metformin (Papp = 3.710 ± 0.050 × 10−7 cm/sec, Table IV) was found to be in agreement with that reported by (31). Formulation blends containing 0.05% (v/v) Labrasol alone or co-processed with other surfactants showed approximately twofold-enhanced metformin permeabilities (Papp = 4.912 ± 0.058–6.632 ± 0.089 × 10−7 cm/sec, blends 1a–1d, respectively; Table IV) in Caco-2 monolayer after co-incubation with metformin at 37°C for 4 h compared with control incubations with 10 mM metformin (Papp = 3.710 ± 0.050 × 10−7 cm/sec; Table IV). TEER measurements conducted at 4 h indicated an approximately 20% reduction from the baseline values for all Labrasol-containing co-processed formulation blends in the presence of metformin, but these recovered to the baseline values over a 24-h time period (Fig. 5a). These results confirm our findings that, although some Labrasol-containing co-processed blends decrease the TEER values and increase the Lucifer yellow permeability (see “Cellular Integrity Studies” and “Effect of Co-processed Blends of Non-Ionic Surfactants on Lucifer Yellow Permeability” sections), their co-incubation with 10 mM metformin had minimal effect on the TEER value and an approximately twofold increase in the metformin permeability. Transcutol-P at 0.5% (v/v) alone or in blends with other surfactants showed approximately similar or close to 1.5-fold increase in the apparent metformin permeability value (Papp = 3.816 × 1.088–5.650 ± 0.531 × 10−7 cm/sec, blends 2b–2d, respectively). There was close to a 20% reduction in the TEER value from the baseline values for all Transcutol-P-containing co-processed formulation blends in the presence of metformin, but these recovered to the baseline values over a 24-h period (Fig. 5b). Formulation blends containing 0.5% (v/v) Tween-20 with other non-ionic surfactants showed the maximum permeability of metformin (approximately four- to tenfold increase) across Caco-2 cell monolayers (Papp = 1.305 ± 0.120–3.998 ± 0.642 × 10−6 cm/sec, blends 3a–3d, respectively; Table IV) and a 75% reduction in the TEER values at 4 h compared with control incubations containing 10 mM metformin (Papp = 3.710 ± 0.050 × 10−7 cm/sec; Table IV and Fig. 5c). In addition, the monolayers incubated with the Tween-20-containing co-processed formulation blends in the presence of metformin failed to recover to the baseline TEER values over a 24-h period.
Table IV.
Metformin Apparent Permeability (P app) in the Presence of Novel Co-Processed Blends of Labrasol, Transcutol-P and Tween-20
| Compositions | Contribution | |||
|---|---|---|---|---|
| P app (×10−7 cm/sec)a | %Parab | %Transc | MAD(g)(para) d | |
| Metformin 10 mM | 3.710 ± 0.050 | 69 | 31 | 0.086 |
| 1(a) 0.05% Labrasol + Metformin (10 mM) | 5.943 ± 0.096* | 67 | 33 | 0.139 |
| 1(b) 0.05% Labrasol + 1% Lauroglycol + Metformin (10 mM) | 6.632 ± 0.089* | 68 | 32 | 0.155 |
| 1(c) 0.05% Labrasol + 0.5% Transcutol + 1% Maisine + Metformin (10 mM) | 5.306 ± 0.047* | 69 | 31 | 0.124 |
| 1(d) 0.05% Labrasol + 0.5% Transcutol + 1% Peceol +Metformin (10 mM) | 4.912 ± 0.058 | 73* | 27 | 0.115 |
| Metformin 10 mM | 3.710 ± 0.050 | 69 | 31 | 0.086 |
| 2(a) 0.5% Transcuto + Metformin (10 mM) | 5.650 ± 0.531* | 63 | 37 | 0.132 |
| 2(b) 0.5% Transcutol + 1% Lauroglycol + Metformin (10 mM) | 4.074 ± 0.022* | 75 | 25 | 0.095 |
| 2(c) 0.5% Transcutol + 1% Maisine + Metformin (10 mM) | 3.816 ± 1.088 | 64 | 36 | 0.089 |
| 2(d) 0.5% Transcutol + 1% Peceol + Metformin (10 mM) | 3.893 ± 0.054* | 78* | 22 | 0.091 |
| Metformin 10 mM | 0.3710 ± 0.050 | 69 | 31 | 0.086 |
| 3(a) 0.5% Tween-20 + Metformin (10 mM) | 3.998 ± 0.642* | 42 | 58 | 0.936 |
| 3(b) 0.5% Tween-20 + 1% Lauroglycol + Metformin (10 mM) | 4.107 ± 0.267* | 36* | 64 | 0.962 |
| 3(c) 0.5% Tween-20 + 0.5% Transcutol + 1% Maisine + Metformin (10 mM) | 3.703 ± 0.879* | 29* | 71 | 0.867 |
| 3(d) 0.5% Tween-20 + 0.5% Transcutol + 1% Peceol + Metformin (10 mM) | 1.305 ± 0.120* | 74* | 26 | 0.305 |
MAD Peff, human × S × A × Tsi where, Peff effective human jejunal permeability, S solubility of drug, A absorption surface area, Tsi transit time for the absorption site
aApparent permeability of 10 mM metformin across Caco-2 monolayers following 4-h incubation period (n = 3 ± S.D.)
bPercentage contribution of paracellular route
cPercentage contribution of transcellular route
dMaximum absorbable dose (MAD) of metformin in gram predicted from Peff, human. MAD values were calculated using metformin Peff from paracellular route
*p < 0.05
Fig. 5.
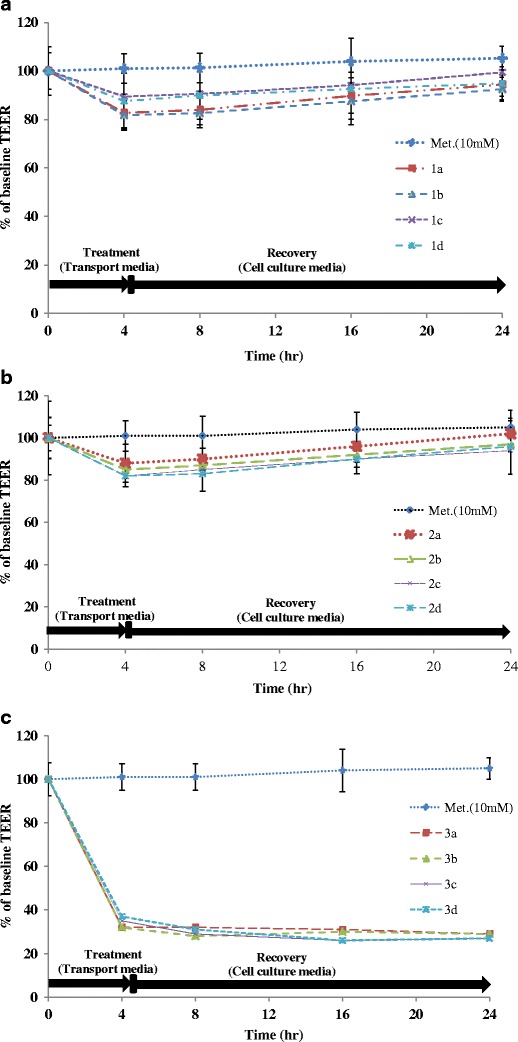
Effect of novel co-processed Labrasol (a), Transcutol (b), and Tween-20 (c) blends on the TEER following co-incubation with 10 mM metformin across Caco-2 cell monolayers after 4 h incubation at 37°C. The data are represented as means ± SD (n = 3)
Contribution of the Transcellular and Paracellular Pathway to Metformin Transport in the Presence of Co-Processed Blends of Non-Ionic Surfactants
The absorption of metformin has been found to be mediated via a wide range of apical uptake transporters, including OCT-1, SERT, and PMAT, which play a minor role (21,24,49), and the paracellular route which is the predominant pathway (90–95%) for metformin transport across Caco-2 cells (31). Therefore, to evaluate the contribution of the transcellular and paracellular pathways to metformin transport in the presence of co-processed blends of nonionic surfactants, Caco-2 cells were pre-incubated with 0.05 mM MPP+, a nonspecific OCT-1, MATE1, and PMAT inhibitor (uptake transporters that have been implicated in the transport of metformin in the git and liver) (21,24,31,49), and the metformin transport was determined. In the presence of 0.05 mM MPP+, the transcellular and paracellular contributions to metformin transport (Papp = 3.710 ± 0.050 × 10−7 cm/sec) were found to be approximately 31% and 69%, respectively (Table IV). When co-incubated with formulation blends containing 0.05% (v/v) Labrasol, 0.5% (v/v) Transcutol-P, and 1% (v/v) Peceol, it was found that the contribution of the paracellular pathway to metformin transport was most significant for blends 1d and 2d (approximately 78%, p < 0.05; Table IV). Co-processed excipients containing 0.5% (v/v) Tween-20 and/or 1% (v/v) Lauroglycol, 0.5% (v/v) Transcutol-P, and 1% (v/v) Maisine reversed metformin transport process from the paracellular (∼31% in the absence of formulation blends) to the transcellular route (∼78% in the presence of formulation blends 3a–3c, Table IV; p < 0.05), compared with blend 3d, which contains 0.5% (v/v) Tween-20, 0.5% (v/v) Transcutol-P, and 1% (v/v) Peceol. Because Caco-2 cells were pre-incubated with MPP+, we expected a complete inhibition of the transcellular-mediated transport of metformin. However, our results indicate that transporters other than OCT-1, MATE1, and PMAT may be involved in the transcellular transport of metformin in the gastrointestinal tract. In contrast, 1% (v/v) Peceol in formulation blend 3d did not alter the metformin transport via the paracellular route. It is possible that metformin was initially transported through the transcellular route in the presence of formulation blends 3a–3d causing a ∼ten-to elevenfold increase in the total metformin permeability compared with metformin alone (Table IV). Once the transcellular route was saturated, metformin was transported across Caco-2 monolayers through the paracellular route in blends 3b–3c which indicates a reversal in the metformin permeability pathway. These results reinforce our conclusions from “Effect of Co-Processed Blends of Non-Ionic Surfactants on the Distribution of the Tight Junction Protein Claudin-1” section, that incubation of Caco-2 cell monolayers with the Tween-20 blends causes rearrangement of the TJ protein claudin-1 and a shift in the metformin transport from the transcellular to the paracellular pathway.
Effect of Co-Processed Blends of Non-Ionic Surfactants on MAD of Metformin by In Vitro In Vivo Extrapolation
Because many of the fabricated formulation blends were found to be significant contributors to the paracellular transport of metformin, the maximum dose of metformin that could be absorbed if the blends under investigation were to be utilized for formulation development was predicted from the apparent permeability values using the equation developed by Sun et al. (41), which uses the effective human jejunal permeability Peff value to predict the oral absorption. As metformin has been shown to be extensively absorbed from the upper intestine (duodenum and jejunum) the apparent permeability data for metformin (Papp) obtained in the presence and absence of co-processed blends of non-ionic surfactants was utilized to predict the maximum absorbable dose (MAD). Based on the solubility profile of metformin (25 mg/mL), it was found that approximately 86 mg of metformin can be absorbed through the paracellular route in the absence of any surfactant from an oral dose of 1 g (Table IV). In the presence of Labrasol- and Transcutol-P-containing formulation blends, the maximum dose of metformin that could be absorbed was predicted to be approximately 100–150 mg. The formulations containing Tween-20 and/or other surfactants such as Lauroglycol-90 and Transcutol-P could significantly enhance the absorption of metformin through the paracellular route by a factor of two- to tenfold (∼960 mg; Table IV).
DISCUSSION
The paracellular pathway is a promising route for the delivery of hydrophilic drugs and macromolecules (3,43,49). Non-ionic surfactants have been shown to enhance the permeability of hydrophilic drugs through the paracellular pathway but compromised the cellular viability (19,31,34,35,49). Through this study, we found that co-processed blends of Labrasol and Transcutol could transiently disrupt transepithelial resistance and enhance the paracellular permeability of a hydrophilic model drug metformin by approximately twofold at nontoxic concentrations compared to the individual excipients.
The paracellular pathway forms a highly hydrated pore that can accommodate ions of various sizes (∼8 Ǻ radii) and charges depending upon cell–cell interactions and interaction of the ions with the TJ proteins (2). Several studies have shown enhanced permeability of various cations, inulin, and non-ionic surfactant polyethylene glycol 4000 (PEG 4000) across the rat intestinal epithelia, indicating an inverse relationship between molecular radii (∼8 Ǻ radii) and TJ permeability (1,28). Therefore, the extent to which a non-ionic surfactant can decrease TEER and increase paracellular permeability will depend on the flexibility or conformation of the surfactant and on the HLB.
Labrasol and Tween-20 are hydrophilic polyoxyethylene glycol-based non-ionic surfactants with a polyoxyethylene substructure that serves as the hydrophilic part of the molecule, and caprylic acid and lauric acid (medium chain fatty acids) as the hydrophobic region (Table II). In contrast, Transcutol-P and Lauroglycol-90 are hydrophobic diethylene glycol- and propylene glycol-based non-ionic surfactants, respectively, and diethylene glycol and propylene glycol serve as the hydrophilic regions of these molecules. Lauric acid forms the hydrophobic region in Lauroglycol-90. Because the number of ethylene oxide residues and alkyl chains determine the aqueous solubility and the ability of the surfactants to diffuse through the aqueous TJ pore, Labrasol and Tween-20, which are highly hydrophilic, may have a higher propensity to diffuse through the TJ pore compared to Transcutol-P and Lauroglycol-90. However, it is not known how the hydrophilic or hydrophobic regions in a non-ionic surfactant orient themselves when they diffuse through the gradient aqueous TJ pore. Based on the literature evidence, we know that medium chain fatty acids (MCFA) caprylic acid and lauric acid, can enhance the paracellular permeability of hydrophilic drugs such as dextran by increasing the intracellular Ca+2 levels via the phospholipase (PLC)-dependent pathway (8,22). These studies indicate that MCFA in the non-ionic surfactants probably interact with the TJ proteins and contribute to their toxicity as reflected by the activation of pro-inflammatory cytokines interleukin (IL-8) and tumor necrosis factor alpha (TNF-α) in Caco-2 cells (8,23,36). In the current study, due to the presence of polyoxyethylene glycol residues and MCFA, Labrasol and Tween-20 (HLB >14) were found to be more cytotoxic than Lauroglycol-90 (HLB <5). Therefore, when a higher and a lower HLB value surfactant was combined at nontoxic concentrations, the blends fabricated were found to be hydrophobic with HLB ∼7.0 with significantly reduced cytotoxicity as seen in Fig. 1.
Transepithelial resistance and Lucifer yellow permeability studies indicated that the modulation of the TJ permeability was dependent on the HLB and the presence of MCFA in the blends. Labrasol and Transcutol-P blends with HLB <5 (Table I) transiently reduced TEER and enhanced Lucifer yellow permeability by approximately two- to fourfold (Fig. 1). The greatest enhancement in Lucifer yellow permeability (∼tenfold) was observed with the blend of Tween-20 and Lauroglycol-90 (HLB >5) that caused irreversible lowering of TEER as a consequence of significant fragmentation and diminished immunostaining of the cellular borders. In studies conducted by Narai et al. (26), the authors found that irreversible changes in TEER value preceded cellular viability events when Caco-2 cells were incubated with cytotoxicants at lower concentrations, whereas at higher cytotoxicant concentrations, the cellular viability events paralleled the alterations in the TEER values; this observation was also evident in our study from diminished immunostaining of claudin-1 (47), a protein involved in maintaining TJ structure, and rearrangement of cellular borders following exposure to Tween-20 blends (Fig. 4i–l). These results highlight the fact that non-ionic surfactant blends with HLB <5.0 (hydrophobic in nature), function as paracellular permeability enhancers with minimal cytotoxicity. Blends with HLB >5.0 (less hydrophobic) retain the ability to enhance paracellular permeability, but irreversibly open the TJ and cause increased cytotoxicity as evident from dimished immunostaining of claudin-1, rearrangement of cellular borders, and presence of holes following exposure to Tween-20 blends (Fig. 4i–l).
Numerous mechanisms by which surfactants can modulate the TJ opening including the activation of PLC-mediated signaling pathway, ATP depletion, tyrosine kinase–phosphatase pathway, and the depletion of extracellular calcium and disruption of cell–cell adhesion has been proposed (33,43). The non-ionic surfactants can alter TJ permeability through the Na+, K+-ATPase/Na+-glucose transport system, which has been implicated in the organization and regulation of the TJ integrity by “solvent drag” mechanism (28,32). PEG a non-ionic surfactant has been shown to inhibit the Na+, K+-ATPase system (29,44). Inhibition of the Na+, K+-ATPase system in the intestinal enterocytes can cause increased glucose uptake by the intestinal Na+-glucose transporter, namely the sodium–glucose linked transporter (SGLT-1), with a concomitant increase in the intracellular sodium transport, a decrease in TEER value and an increase in the paracellular permeability. Because polyoxyethylene glycol- and MCFA-based Tween-20 blends have HLB >5, the blends may have a higher propensity to inhibit the Na+, K+-ATPase system, which was evident from more than 40% decrease in TEER by 4-h incubation period and ∼tenfold increase in Lucifer yellow permeability (Fig. 2c). Moreover, the TEER failed to return to the baseline values over the 24-h period after removal of Tween-20 blends, which was not the case with Labrasol blends. Labrasol blends probably have a lower propensity to inhibit the Na+, K+-ATPase system seen from the transient decrease in the TEER that returned to the control values by 24-h period with approximately two- to fourfold increase in Lucifer yellow permeability. Transcutol-P, a flexible diethylene glycol monoethyl ether is hydrophobic in nature (HLB = 4.2) because it lacks polyethylene oxide chains in its structure. In addition, Transcutol-P does not contain medium chain fatty acids, therefore the Transcutol blends (HLB <5) most likely have the least interactions with the TJ proteins which was evident from minimal or no decrease in the TEER and approximately twofold increase in Lucifer yellow permeability.
Metformin, an oral antihyperglycemic agent, has been shown to be transported through the paracellular space, which are located on the apical side of human enterocytes, through an active and saturable process (47). It is a BCS class III drug with high solubility and poor permeability profile. At higher doses metformin exhibits nonlinear and saturable pharmacokinetics (13,28,39,40,45) that increases the residence time of metformin in the intestinal lumen causing gastrointestinal upset, nausea, and diarrhea in patients. The mechanism through which metformin elicits its effect on glucose transport remains debatable. In type 2 diabetes, the functional activities of SGLT-1 (transports glucose and galactose from the lumen of the intestine into the enterocytes) and GLUT2 (glucose transporter 2, involved in the transport of glucose from the intestinal lumen through the TJ by the paracellular route) are increased (36). Metformin has been shown to lower glucose transport by decreasing SGLT-1 and increasing GLUT2 activities in intestinal enterocytes. This inhibition of SGLT-1 by metformin leads to decreased glucose uptake with a concomitant decrease in sodium and water transport through the paracellular route. Since inhibition of SGLT-1 causes increased GLUT2 activities, glucose transport can be switched from sodium-dependent to passive paracellular transport, along with increased water transport (36). In the current study, the co-incubation of non-ionic surfactant blends with 10 mM metformin most likely causes complimentary and complex interplay between metformin and the surfactants with the transcellular and paracellular route transporters. As a result, the activation of the Na+, K+-ATPase system by non-ionic surfactant blends (28) and the inhibitory effects of metformin on SGLT-1/GLUT2 (36) causes increased sodium and glucose transport, minimal reduction in TEER, and an enhancement of the paracellular permeability of metformin by approximately two- to fourfold on co-incubation with Labrasol and Transcutol-P blends (Table IV). Tween-20 blends produced >tenfold enhancement in metformin permeability but caused more than 70% decrease in TEER by 4 h which was irreversible even at 24 h. The irreversibility of TEER following incubation with Tween-20 blends could be due to pronounced effect of Tween-20 blends on Na+, K+-ATPase system. The results from our study parallel those obtained by Dimitrijevic et al. (11), who evaluated the effect of solulans and polysorbates on metformin permeability. These researchers found that 5% (v/v) Tween-20 enhanced metformin permeability by approximately 20-fold in Caco-2 cell model but caused extensive cellular damage.
Because non-ionic surfactants have the ability to enhance the permeability of xenobiotics through both, the transcellular (solubilizing, disorganizing, and penetrating the intercellular lipid bilayer) and the paracellular pathway (14,17,47,49), we explored the role of transcellular and paracellular route in the transport of metformin using MPP+, a non-specific inhibitor of uptake transporters involved in the metformin transport. Metformin was found to be predominantly (∼70%) transported through the paracellular route in the presence and absence of Labrasol and Transcutol-P blends (Table IV). We observed a shift in the metformin transport from the paracellular to the transcellular route on co-incubation with Tween-20 blends, 3a-3c (Table IV). The shift in metformin transport was not observed with Tween-20 blend 3d containing 0.5% (v/v) Transcutol and 1% (v/v) Peceol. Similar transport mechanism exists for glucose in the small intestine (28). The authors have shown that following the transcellular absorption of glucose mediated by the Na+, K+-ATPase system, the paracellular absorption of glucose is triggered by the activation of SGLT-1 due to the presence of luminal glucose, leading to increase in water and Na+ absorption, facilitating the opening of paracellular pathway and increase in the TJ permeability (29,44). The increase in TJ permeability has been found to trigger flux of several nutrients through the paracellular pathway. In our study, Tween-20 blends, 3a–3d, most likely modulates metformin transport differentially. First, the blends probably initiated the transcellular transport of metformin by solubilizing and permeating the lipid bilayer, which caused approximately three- to fourfold increase in metformin permeability. Due to the inhibitory effects of metformin on the glucose uptake, the concentration of glucose in the transwell apical chamber increased, which triggered the activation of SGLT-1 and an increase in Na+ absorption. The passage of high amounts of Na+ through the TJ produced more than 70% drop in TEER within 4 h and opened the paracellular pathway causing enhanced metformin permeability, as observed with Tween-20 blends, 3a–3d. Hydrophilic Tween-20 blends, 3a–3c (HLB >6) significantly contributed to the enhanced transcellular transport of metformin, compared to blend 3d containing Transcutol-P and Peceol. It should be noted that the non-ionic surfactants can regulate the TJ opening through other competing pathways including the inhibition of Na+, K+-ATPase system (44), and the depletion of extracellular Ca+2 levels. Saturated fatty acids such as lauric acid (component of Tween-20) have a greater propensity to complex with and deplete extracellular Ca+2 levels, opening the TJ compared to oleic and linoleic unsaturated fatty acids, which are the components of Peceol and Maisine 35-1. Therefore, Tween-20 blends may exhibit enhanced ability to modulate the transport of hydrophilic drugs through competing transcellular and paracellular pathways, but at the expense of the TJ irreversibility.
Among the blends evaluated in this study, Labrasol and Transcutol blends retained the ability to enhance paracellular permeability of metformin with minimal cytotoxicity compared to Tween-20 blends. Therefore, we employed a prospective approach to predict the metformin dose that could be absorbed if Labrasol or Transcutol blends would be used for formulation development. In the past three decades, the Caco-2 cell model has been extensively used as an in vitro system to evaluate the intestinal permeability (jejunal and duodenal) and predict the oral absorption of new chemical entities. Although Caco-2 cells are derived from human colorectal (large intestine) adenocarcinoma, when cultured in the presence of supplemented media and experimental conditions mentioned in the manuscript, the cells, upon reaching confluency as a monolayer, differentiate and polarize, such that they morphologically (form microvilli in apical side) and functionally (express efflux and uptake transporters) mimic enterocytes of the small intestine (1,7,16,30). The use of such predictive models aids the screening of formulations that exhibit the best absorption profile for in vivo testing. Approximately 150–960 mg of metformin could be absorbed, if a dose of 1 g were to be formulated with the co-processed blends evaluated in this study. The predicted absorbed dose is much higher than the ∼2 μg/mL Cmax that is achieved following ingestion of an 850 mg oral dose of metformin (13). The predicted dose does not take into account the barrier effect of the mucus in the intestinal lumen that can significantly reduce the permeability of a drug molecule nor does it take into account the effect of gastric motility and the transit time that can alter the absorption profile of metformin significantly since metformin is slowly absorbed from the duodenum and jejunum. Because non-ionic surfactants can have an osmotic effect in the small intestine, these surfactants can alter the gastric motility and the transit time of a dosage form contributing to significantly lower absorption profile of the drug molecules. Hence, the results from this study reinforce the use of in vitro cell models for screening and selection of optimal formulation excipients to determine the effect of the excipients on the permeability of a drug molecule prior to performing exhaustive and resource-intensive animal studies to evaluate the bioavailability of a drug molecule.
CONCLUSIONS
Non-ionic surfactants are widely used as solubility and permeability enhancers in solid and liquid dosage forms. In the past decade, the propensity of formulation excipients to modulate xenobiotic transporters has lead to prospective screening of the surfactants in Caco-2 cell models prior to their inclusion in the formulations. Through this study, we have made an attempt to fabricate novel co-processed blends of known non-ionic surfactants with the goal of enhancing paracellular permeability of a model hydrophilic drug, metformin, without compromising cellular viability. We have demonstrated that at lower concentrations, formulation blends of Labrasol and Transcutol-P retain the ability to enhance paracellular permeability of a hydrophilic drug, metformin, with minimal effect on TEER and cellular viability. Such formulation blends would therefore be suitable for development of a dosage form for pharmacokinetic studies in animal models.
References
- 1.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2012;64:280–9. doi: 10.1016/j.addr.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:1–16. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aungst BJ, Saitoh H, Burcham DL, Huang S, Mousa SA, Hussain MA. Enhancement of the intestinal absorption of peptides and nonpeptides. J Contr Rel. 1996;41(1–2):19–31. doi: 10.1016/0168-3659(96)01353-3. [DOI] [Google Scholar]
- 4.Balda M, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134(4):1031–49. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benet LZ, Broccatelli F, Opera TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–47. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buyukozturk F, Benneyan JC, Carrier RL. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J Control Release. 2010;142(1):22–30. doi: 10.1016/j.jconrel.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988;48:1936–42. [PubMed] [Google Scholar]
- 8.Coyne CB, Ribeiro CMP, Boucher RC, Johnson LG. Acute mechanism of medium chain fatty acid-induced enhancement of airway epithelial permeability. JPET. 2003;305:440–50. doi: 10.1124/jpet.102.047654. [DOI] [PubMed] [Google Scholar]
- 9.Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986;27:886–92. doi: 10.1136/gut.27.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788(4):892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrijevic D, Shaw JA, Florence AT. Effect of some non ionic surfactants on transepithelial permeability in Caco-2 cells. J Pharm Pharmacol. 1999;52:157–62. doi: 10.1211/0022357001773805. [DOI] [PubMed] [Google Scholar]
- 12.Fasano A, Uzzao S. Modulation of intestinal tight junctions by zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99(6):1158–64. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Gershanik T, Haltner E, Leh C, Benita S. Charge-dependent interaction of self-emulsifying oil formulations with Caco-2 cells monolayers: binding, effects on barrier function and cytotoxicity. Int J Pharm. 2000;211(1–2):29–36. doi: 10.1016/S0378-5173(00)00591-3. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Sakai T, Hasegawa Y, Nishikawahara T, Tomioka H, Iida A, et al. Physiological mechanism for enhancement of paracellular drug transport. J. Contr. Rel. 1999;62:141–8. doi: 10.1016/S0168-3659(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–9. [PubMed] [Google Scholar]
- 17.Hochman J, Artursson P. Mechanism of absorption enhancement and tight junction regulation. J. Contr. Rel. 1994;29:253–67. doi: 10.1016/0168-3659(94)90072-8. [DOI] [Google Scholar]
- 18.Hubatsch I, Ragnarsson EGE, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2:2111–9. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
- 19.Hu Z, Tawa R, Konishi T, Shibata N, Takada K. A novel emulsifier, labrasol, enhances gastrointestinal absorption of gentamicin. Life Sci. 2001;69(24):2899–910. doi: 10.1016/S0024-3205(01)01375-3. [DOI] [PubMed] [Google Scholar]
- 20.Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008;97(8):2924–35. doi: 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- 21.Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20(5):379–86. doi: 10.2133/dmpk.20.379. [DOI] [PubMed] [Google Scholar]
- 22.Lindmark T, Kimura Y, Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. JPET. 1998;284(1):362–9. [PubMed] [Google Scholar]
- 23.Matter K, Balda MS. Biogenesis of tight junctions: the C-terminal domain of occludin mediates basolateral targeting. J Cell Sci. 1998;111(Pt 4):511–9. doi: 10.1242/jcs.111.4.511. [DOI] [PubMed] [Google Scholar]
- 24.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephol. 2006;17(8):2127–35. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Narai A, Arai S, Shimizu M. Rapid decrease in transepithelial electrical resistance of human intestinal Caco-2 cell monolayers by cytotoxic membrane perturbents. Toxicol In Vitro. 1997;11(4):347–54. doi: 10.1016/S0887-2333(97)00026-X. [DOI] [PubMed] [Google Scholar]
- 27.Noel M. Kinetic study of normal and sustained release dosage forms of metformin in normal subjects. Res Clin Forums. 1979;1:33–44. [Google Scholar]
- 28.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–36. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 29.Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biology Cell. 1983;47:323–30. [Google Scholar]
- 30.Prasad YV, Puthli SP, Eaimtralarn S. Enhanced intestinal absorption of vancomycin with labrasol and d-alpha-tocopheryl PEG 1000 succinate in rats. Int J Pharm. 2003;250(1):181–90. doi: 10.1016/S0378-5173(02)00544-6. [DOI] [PubMed] [Google Scholar]
- 31.Proctor WR, Bourdet DL, Thakker DR. Mechanisms underlying saturable intestinal absorption of metformin. Drug Met Dispos. 2008;36(8):1650–8. doi: 10.1124/dmd.107.020180. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of toght junctions. Am. J. Physiol Renal Physiol. 2003;285:F388–96. doi: 10.1152/ajprenal.00439.2002. [DOI] [PubMed] [Google Scholar]
- 33.Rege BD, Yu XL, Hussain AS, Polli JE. Effect of common excipients on Caco-2 transport of low-permeability drugs. J Pharm Sci. 2001;90(11):98–107. doi: 10.1002/jps.1127. [DOI] [PubMed] [Google Scholar]
- 34.Rege BG, Kao JPY, Polli JE. Effects of non-ionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16:237–46. doi: 10.1016/S0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 35.Sakai M, Imai T, Ohtake H, Azuma H, Otagiri M. Effects of absorption enhancers on cytoskeletal actin filaments in Caco-2 cell monolayers. Life Sci. 1998;63(1):45–54. doi: 10.1016/S0024-3205(98)00235-5. [DOI] [PubMed] [Google Scholar]
- 36.Sakar Y, Meddah B, Faouzi MA, Cherrah Y, Bado A, Ducroc R. Metformin-induced regulation of intestinal-glucose transporters. J Physio Pharmacol. 2010;61(3):301–7. [PubMed] [Google Scholar]
- 37.Salama NN, Fasano A, Thakar M, Eddington N. The impact of ΔG on the oral bioavailability of low bioavailable therapeutic agents. JPET. 2005;312:199–205. doi: 10.1124/jpet.104.073205. [DOI] [PubMed] [Google Scholar]
- 38.Sambol NC, Chiang JO, Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with non-insulin dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–21. doi: 10.1177/009127009603601105. [DOI] [PubMed] [Google Scholar]
- 39.Scheen AJ. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 1996;30:359–71. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 40.Sha X, Yan G, Wu Y, Li J, Fang X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur J Pharm Sci. 2005;477–486. [DOI] [PubMed]
- 41.Sun D, Yu LX, Hussain MA, Wall DA, Smith RL, Amidon GL. In vitro testing of drug absorption for drug developability assessment. Cur Opin Drug Discov Dev. 2004;7:75–85. [PubMed] [Google Scholar]
- 42.Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm Res. 1994;11(8):1132–42. doi: 10.1023/A:1018984731584. [DOI] [PubMed] [Google Scholar]
- 43.Turner J. Show we the pathway! Regulation of paracellular permeability by Na+-glucose cotransport. Adv Drug Deliv Rev. 2000;41:265–81. doi: 10.1016/S0169-409X(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 44.Tucker GT, Casay C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–46. doi: 10.1111/j.1365-2125.1981.tb01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ujhelyi Z, Fenyvesi F, Varadi J, Feher P, Kiss T, Veszelka S, et al. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur J Pharm Sci. 2012;47:564–73. doi: 10.1016/j.ejps.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Will C, Fromm M, Müller D. Claudin tight junction proteins: novel aspects in paracellular transport. Perit Dial Int. 2008;28(6):577–84. [PubMed] [Google Scholar]
- 47.Wright E, Loo D, Hirayama B, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology. 2004;19:370–6. doi: 10.1152/physiol.00026.2004. [DOI] [PubMed] [Google Scholar]
- 48.Yu Q, Wang Z, Li P, Yang Q. The effect of various absorption enhancers on tight junction in the human intestinal Caco-2 cell line. Drug Dev Ind Pharm. 2012;1–6. [DOI] [PubMed]
- 49.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Met Dispos. 2007;35:1956–62. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]


