Abstract
The objective of the present study is to investigate the effect of hydrocarbon chain length in 1,2-alkanediols on percutaneous absorption of metronidazole (MTZ). Twelve formulations (1,2-propanediol, 1,2-butanediol, 1,2-pentanediol, 1,2-hexanediol in 4% concentration, 1,2-hexanediol, and 1,2-heptanediol in 1% concentration, in the absence and presence of 1,4-cyclohexanediol, respectively) were studied in an in vitro hairless mouse skin model using Franz diffusion cell. Based on the flux values and retardation ratios (RR), a penetration retardation effect on percutaneous absorption of MTZ was observed for the formulations containing 1,2-diols having six- to seven-carbon chain in the presence of 1,4-cyclohexanediol (1,2-hexanediol with chain length of six hydrocarbons, RRs are 0.69 and 0.76 in the concentration of 4% and 1%, respectively; 1,2-heptanediol with chain length of seven hydrocarbons, RR is 0.78 in the concentration of 1%). On the other hand, no retardation effect was observed in formulations containing short alkyl chains (RRs of 1,2-propanediol, 1,2-butanediol, and 1,2-pentanediol are 0.99, 1.61, and 0.96, respectively). Instead, a penetration enhancement effect was observed for 1,2-diols having four and five carbons. In other words, effect of 1,2-alkanediols on percutaneous absorption of MTZ can be systematically modulated by simply varying number of –CH2 groups in the hydrocarbon chain—from being a penetration enhancer to retardant. These observations shed light on mechanism of the penetration enhancement and retardation effect and provide insight into rational design of penetration enhancers and retardants. Furthermore, the combination of 1,2-alkanediols and 1,4-cyclohexanediol could become a general vehicle for controlled release of pharmaceutical and cosmetic active ingredients.
Figure.

ᅟ
KEY WORDS: 1,2-alkanediols; controlled release; hydrocarbon chain length; skin penetration
INTRODUCTION
Skin penetration retardants prevent absorption and penetration of active ingredients or excipients into deeper skin layer and decrease the diffusivity and thermodynamic activity of the active ingredient in skin (1). Penetration enhancers and retardants are both referred to as penetration modifiers since they act by modifying the structure of stratum corneum (2,3). Despite receiving less attention in comparison to penetration enhancement (4–7), study of penetration retardation effect has significant theoretical and practical implications. For example, understanding of penetration retardation could provide invaluable insight in understanding of penetration enhancement from an entirely different prospective and shed light on interactions between molecules and skin layer in general. Discovery of safe and effective penetration retardants could lead to development of superior topical products with few side effects, for example, such as sunscreens, insect repellents, and protection creams for chemical warfare agents.
Since stratum corneum (SC) layer is the primary barrier to skin penetration, interaction among molecules in the formulations and their interactions with molecules comprised of the SC layer are most likely responsible for observed penetration enhancement and retardation effect. Hadgraft et al. have proposed that ceramide 6 in the SC layer plays an important role (8). Wertz (9) through modeling study suggests that ceramide 6 possesses the highest hydrogen bonding capability among the various ceramides present in stratum corneum. Hadgraft et al. proposes several theories to explain the mechanism of penetration retardation. It is believed that the action of retardants could be explained by their interaction with ceramide 6 that form the largest group of lipids in the stratum corneum. The one-sided H-bonding of permeation modifiers with ceramide 6 suggests its activity as an enhancer, whereas two-sided interactions imply its role as a retardant. For example, 3-dodecanoyloxazolidin-2-one (N-0915), S,S-dimethyl-N-(4-bromobenzoyl) iminosulfurane, and tert-butyl 1-dodecyl-2-oxoazepan-3-yl-carbamate are capable of forming multiple two-sided H-bonds with ceramide 6 suggesting retardation behavior (3,8,10). Our own study (11) suggests that possible hydrogen-bonding interaction between 1,4-cyclohexanediol/1,2-hexanediol complex and ceramide 6 might be responsible for the observed penetration retardation effect on percutaneous absorption of azelaic acid. More recently, we also found that the duo action of skin lipid disruption and two-sided H-bonding is most likely responsible for the synergistic retardation effect on percutaneous absorption of metronidazole (MTZ) (12).
Several authors have reported on the structure–activity relationships in series of chemical modifiers (13–17). Moreover, based on the mechanisms of the action of penetration modifier, a number of related factors affecting on the course of enhancement/retardation have been reported, e.g., the chain length effect (18). Bouwstra et al. (19) demonstrate that alkyl-azones having alkyl chains six to 12 carbons in the hydrophobic chain are intercalated in the bilayer in SC layer, but the hexyl analog causes no disordering of the lipid structure. Interestingly, Li et al. (12) show that 1,2-hexanediol (six-carbon chain) together with 1,4-cyclohexanediol acts synergistically to exert penetration retardation effect on percutaneous absorption of MTZ.
The present study is designed to systematically investigate the effect of hydrocarbon chain length (from three to seven) in 1,2-alkanediols on percutaneous absorption of MTZ to develop a general vehicle for controlled release of a wide variety of active compounds. MTZ is selected as a model drug, which is a member of nitroimidazole family of compounds (logP value is −0.18, pKa value is 2.6, and solubility is 8.22 ± 0.12 and 6.27 ± 0.09 mg/ml in water and pH 7.4 PBS, respectively). It is an active agent for the treatment of skin diseases, such as rosacea and acne. Percutaneous absorption of MTZ was investigated using an in vitro hairless mouse skin model.
MATERIALS AND METHODS
Materials
Chemicals
MTZ was purchased from ZhongAn Pharmaceutical, Tianjin, China. 1,4-Cyclohexanediol was obtained from Sigma-Aldrich (St. Louis, MO, USA). 1,2-Propanediol, 1,2-butanediol, 1,2-pentanediol, and 1,2-hexanediol are readily available from various commercial sources. Klucel® MF was obtained from Hercules, Inc. (Wilmington, DE, USA). All other chemicals are of analytical grade.
Synthesis of 1,2-Heptanediol
1,2-Heptanediol used in the present study was synthesized in our laboratories. The synthetic scheme was depicted in Fig. 1.
Fig. 1.
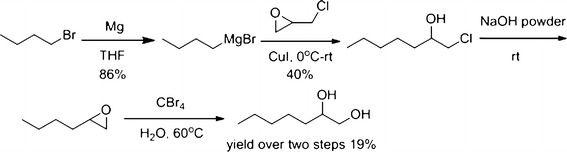
The synthetic scheme of 1,2-heptanediol
Butylmagnesium bromide was prepared from the reaction of n-butyl bromide and magnesium in THF. Then, the Grignard reagent reacted with 2-(chloromethyl)oxirane to give 1-chloroheptan-2-ol in 40% yield. Treating this intermediate with sodium hydroxide powder, followed by hydrolysis, afforded the target compound, 1,2-heptanediol, in 19% yield (two steps).
Skin Membranes
Male hairless mice (30–40 days old) were purchased from Radiation Medicine Institute for Laboratory Animal Research, Chinese Academy of Medical Sciences (Tianjin, China). The abdominal skin was removed from hairless mice, and subcutaneous fat carefully cleaned in normal saline. The skin samples were stored at −20°C and used promptly (in 3 days). Before each experiment, the skin samples were thawed to room temperature and hydrated for 1 h in phosphate-buffered saline (1× PBS), pH 7.4 by mounting on jacketed Franz diffusion cells, which were maintained at 37°C.
Methods
Preparation of Formulations
Twelve formulations were prepared (see Table I for details). Klucel® MF was used as the gelling agent. The general procedure is as follows. For example, to prepare formulation F2, 1% of 1,4-cyclohexanediol was dissolved in a solution of 1,2-propanediol (4%) in water, and MTZ (0.75%) was dispersed in the above solution with a stirrer until MTZ was dissolved. Then Klucel® MF (0.75%) was added to the solution while stirring until the solution was gelled.
Table I.
Formulations Containing Metronidazole (F1–F12)
| Formulation | Ingredient (%) | |||
|---|---|---|---|---|
| Metronidazole | Klucel® MF | 1,2-Propanediol | 1,4-Cyclohexanediol | |
| F1 | 0.75 | 0.75 | 4.0 | – |
| F2 | 0.75 | 0.75 | 4.0 | 1.0 |
| Metronidazole | Klucel® MF | 1,2-Butanediol | 1,4-Cyclohexanediol | |
| F3 | 0.75 | 0.75 | 4.0 | – |
| F4 | 0.75 | 0.75 | 4.0 | 1.0 |
| Metronidazole | Klucel® MF | 1,2-Pentanediol | 1,4-Cyclohexanediol | |
| F5 | 0.75 | 0.75 | 4.0 | – |
| F6 | 0.75 | 0.75 | 4.0 | 1.0 |
| Metronidazole | Klucel® MF | 1,2-Hexanediol | 1,4-Cyclohexanediol | |
| F7 | 0.75 | 0.75 | 4.0 | – |
| F8 | 0.75 | 0.75 | 4.0 | 1.0 |
| F9 | 0.75 | 0.75 | 1.0 | – |
| F10 | 0.75 | 0.75 | 1.0 | 1.0 |
| Metronidazole | Klucel® MF | 1,2-Heptanediol | 1,4-Cyclohexanediol | |
| F11 | 0.75 | 0.75 | 1.0 | – |
| F12 | 0.75 | 0.75 | 1.0 | 1.0 |
In Vitro Skin Permeation Studies
Hairless mouse skin prehydrated with 1× PBS, pH 7.4, was mounted on Franz diffusion cell (Pharmacopoeia Standard Instrument Factory, Tianjin, China) with SC side facing the donor chamber. The receptor chamber (17 ml in volume) was filled with 1× PBS, pH 7.4, and continuously stirred at 500 r.p.m. using a magnetic stirrer. The temperature was maintained at 37 ± 0.1°C. After incubation for 1 h at 37°C, the formulations were applied in an infinite dose approach (100 mg of each formulation, which corresponds to 750 μg of MTZ) evenly over a surface area of 1.77 cm2. The donor chamber was covered with Parafilm® to minimize evaporation of the formulations. Each set of experiments was run in six replicates. At the end of each time interval (1, 2, 4, 8, 12, 16, 20, and 24 h), the skin surface was wiped with cotton ball soaked with 1× PBS. The tape-stripping method (average ten strips) was used to remove the SC layer (20). MTZ retained in the epidermis and dermis layer was collected by methanol extraction. After tape stripping, the remaining skin was minced, vortexed with 1 ml methanol, and centrifuged; the supernatant was removed. The extraction step was repeated three times. The supernatants were combined, filtered, and ready for analysis. The receptor medium was withdrawn from the receptor and ready for analysis.
HPLC Analytical Method
The analysis of MTZ was performed using HPLC (HP 1100, Agilent Technologies, Inc.) equipped with a 250× 4.6 mm stainless steel C18 column (5 μm, Thermo, USA). The column was eluted with a mobile phase of a degassed and filtered (0.45 μm; Millipore) mixture of double distilled water–methanol (80:20, v/v) at a flow rate of 1.0 ml/min. Injection volumes were 20 μl. The UV detector wavelength was set at 310 nm for detection of MTZ. The concentration of MTZ in pH 7.4 PBS was determined at 0, 2, 4, 6, 8, 12, and 24 h; the data were shown in Table II.
Table II.
The Data of Stability Study of MTZ in pH 7.4 PBS (n = 3)
| Time point | 0 h | 2 h | 4 h | 6 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|---|
| Concentration (mg/ml) | 1.02 ± 0.04 | 1.03 ± 0.05 | 1.01 ± 0.08 | 1.05 ± 0.07 | 1.10 ± 0.10 | 1.04 ± 0.05 | 1.01 ± 0.07 |
The analytical method was validated for linearity, precision, accuracy, lowest limit of detection (LOD), and lowest limit of quantitation (LOQ). The plot showed good linearity with correlation coefficient of 0.9997. LOD and LOQ were determined to be 100 and 150 ng/ml, respectively. Intraday variability was less than 0.2%, and interday variability was also calculated to be less than 2.0%.
Data and Statistical Analysis
The most basic diffusion equation is Fick’s first law which describes steady-state flux per unit area (J) in terms of the partition of the permeant between the skin and the applied formulation (K), its diffusion coefficient (D) in the intercellular channels of diffusional path length (h), the applied concentration of the permeant in the vehicle (capp), and the concentration of the permeant in the receptor phase (crec)
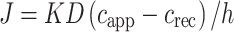 |
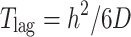 |
The amount of active present in the samples was determined using the validated assay method. For data presentation, mean flux, lag time, and cumulative amount after 24 h were calculated. The flux values of MTZ permeated through the skin membranes into the receptor medium were determined from slopes of plots of concentration in the receptor phase as a function of time and expressed as micrograms per square centimeter per hour using linear regression (Microsoft Excel) (21). The degree of penetration retardation is defined as the retardant ratio (RR), which is calculated from the following equation (22):
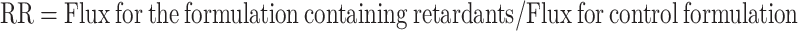 |
The values greater than 1.00 represent enhancement effect, and values smaller 1.00 represent retardation effect. Paired two-tailed Student’s t test is performed to calculate the statistical significance (*p < 0.05, **p < 0.01). Values are given as means ± SD.
RESULTS
The formulations containing 1,2-alkanediols having three- to seven-carbon alkyl chain were prepared to study effect of chain length of the 1,2-diols on percutaneous absorption of MTZ. Due to the lower water solubility of 1,2-heptanediol than the other 1,2-diols used in this study, the concentration of 1,2-heptanediol in the formulation F11 and F12 was 1%, as opposed to 4% for rest of the 1,2-diols. To make a fair comparison with other formulations, the formulations containing 1,2-hexanediol at the concentration of 1% were also prepared (F9, F10).
In Vitro Skin Permeation Study
The permeation data and profiles for all of the formulations in the presence and absence of 1,4-cyclohexanediol were summarized in Table III and Fig. 2. The RR values of F2 and F6 were about 1.00, which indicates that there were no retardation effect on the penetration of MTZ in the formulations containing 1,2-propanediol and 1,2-pentanediol. Interestingly, the flux of F4 was 5.43 ± 0.42 μg/cm2/h, in comparison with 3.37 ± 0.32 μg/cm2/h for F3 (RR value is 1.61), showing an enhancement effect of MTZ in the presence of 1,2-butanediol and 1,4-cyclohexanediol. However, 1,2-diols having alkyl chain length of six and seven carbons show a retardation effect on the penetration of MTZ by approximately 30% (RR values are 0.69, 0.76, and 0.78 for F8, F10, and F12 compared with controls, respectively). Even at two different concentrations of 1,2-hexanediol (F8 at 4% and F10 at 1%), the synergistic retardation effect was observed in both cases. For the formulations containing 1,2-heptanediol and 1,4-cyclohexanediol, the flux value is 4.56 ± 0.74 μg/cm2/h, which is significantly lower than that of control (F11, the flux value is 5.83 ± 0.47 μg/cm2/h) (p < 0.01).
Table III.
Skin Permeation Parameters of MTZ at 750 μg Dose
| Formulation | T lag (h) | Flux (μg/cm2/h) | Amount in collection medium at 24 h (μg) | RR |
|---|---|---|---|---|
| F1 | 0.90 ± 0.10 | 3.80 ± 0.38 | 628.76 ± 23.28 | – |
| F2 | 0.88 ± 0.12 | 3.76 ± 0.33 | 627.96 ± 26.79 | 0.99 |
| F3 | 1.06 ± 0.31 | 3.37 ± 0.32 | 631.36 ± 14.79 | – |
| F4 | 0.14 ± 0.04 | 5.43 ± 0.42 | 683.37 ± 16.20* | 1.61 |
| F5 | 0.98 ± 0.31 | 4.81 ± 0.21 | 521.04 ± 9.45 | – |
| F6 | 0.74 ± 0.04 | 4.61 ± 0.31 | 564.20 ± 23.13** | 0.96 |
| F7 | 0.98 ± 0.06 | 3.82 ± 0.25 | 574.30 ± 12.19 | – |
| F8 | 1.20 ± 0.37 | 2.63 ± 0.03 | 508.62 ± 18.43* | 0.69 |
| F9 | 0.93 ± 0.21 | 2.89 ± 0.42 | 552.31 ± 15.53 | – |
| F10 | 1.38 ± 0.24 | 2.20 ± 0.31 | 511.10 ± 22.28* | 0.76 |
| F11 | 0.27 ± 0.10 | 5.83 ± 0.47 | 580.23 ± 18.21 | – |
| F12 | 0.51 ± 0.16 | 4.56 ± 0.74 | 539.15 ± 11.76* | 0.78 |
Each value represented the mean ± SD (n = 6)
RR retardant ratio (RR flux for the formulation containing retardation/flux for control formulation)
*p < 0.01; **p < 0.05
Fig. 2.

a Profiles of skin permeation of MTZ: comparison of F1 and F2, n = 6. b Profiles of skin permeation of MTZ: comparison of F3 and F4, n = 6. c Profiles of skin permeation of MTZ: comparison of F5 and F6, n = 6. d Profiles of skin permeation of MTZ: comparison of F7 and F8, n = 6. e Profiles of skin permeation of MTZ: comparison of F9 and F10, n = 6. f Profiles of skin permeation of MTZ: comparison of F11 and F12, n = 6
All formulations containing 1,4-cyclohexanediol were significantly different from controls (without 1,4-cyclohexanediol) in terms of the cumulative amounts after 24 h (Q24) (p < 0.05) with exception of F2 (containing 1,2-propanediol) (p > 0.05). The Q24 value of F4 (683.37 ± 16.20 μg) and F6 (564.20 ± 23.13 μg) was higher than F3 (631.36 ± 14.79 μg) and F5 (521.04 ± 9.45 μg), respectively (p < 0.01). These data show an interesting observation that 1,2-butanediol and 1,2-pentanediol actually act as penetration enhancers to enhance the permeation of MTZ as measured by Q24 values. The amounts collected at 24 h for the formulations containing 1,2-hexanediol and 1,2-heptanediol in the presence of 1,4-cyclohexanediol, however, were significantly lower than those in the absence of 1,4-cyclohexanediol (p < 0.01), which indicates a retardation effect on the penetration of MTZ.
Furthermore, similar lag time (Tlag) values were observed from the formulation pair F1 and F2 (1,2-alkanediol = 1,2-propanediol), indicating that 1,2-propanediol (three-carbon chain) has neither enhancement nor retardation effect. However, the Tlag value of F4 (0.14 ± 0.04 h) was markedly shorter than that of the control (1.06 ± 0.31 h), which indicates that a combination of 1,2-butanediol and 1,4-cyclohexanediol leads to a more rapid diffusion of MTZ across the stratum corneum, an indication of a penetration enhancement effect. Same findings, though in smaller scale, were also observed for the formulation pair F5 and F6 (1,2-alkanediol = 1,2-pentanediol), Tlag = 0.74 (F6, 1,2-pentanediol and 1,4-cyclohexanediol) vs. Tlag = 0.98 (F5, the control).
On the other hands, the formulations having 1,2-hexanediol and 1,2-heptanediol in the presence of 1,4-cyclohexanediol have longer lag times (Tlag) than the corresponding controls, suggesting a penetration retardation effect. Thus, in the presence of 1,4-cyclohexanediol, effect of 1,2-alkanediols on percutaneous absorption of MTZ is strongly chain length dependent—from being no effect whatsoever (three carbons) to enhancement effect (four and five carbons) to retardation effect (six and seven carbons). Percutaneous absorption of MTZ can be systematically modulated by selecting 1,2-alkanediols of different chain length.
In Vitro Epidermis/Dermis Retention Study
Treatment of many dermatological disorders relies on the ability of active agents to effectively penetrate the SC layer from applied formulations and reach epidermis and dermis layer (23). In the present study, epidermis/dermis retention of MTZ from all formulations was determined and listed in Fig. 3.
Fig. 3.

a Percentage of epidermal retention of MTZ: comparison of F1 and F2. Mean ± SD, n = 6. b Percentage of epidermal retention of MTZ: comparison of F3 and F4. Mean ± SD, n = 6. c Percentage of epidermal retention of MTZ: comparison of F5 and F6. Mean ± SD, n = 6. d Percentage of epidermal retention of MTZ: comparison of F7 and F8. Mean ± SD, n = 6. e Percentage of epidermal retention of MTZ: comparison of F9 and F10. Mean ± SD, n = 6. f Percentage of epidermal retention of MTZ: comparison of F11 and F12. Mean ± SD, n = 6. SD standard deviation
Similarly to the values of flux, Q24 and Tlag, the epidermis/dermis retention of MTZ from the formulations containing 1,2-propanediol (F1 vs. F2) showed no statistical difference (p > 0.05). The formulations of F4 and F6, however, revealed significantly lower retention in epidermis/dermis comparing with their controls (F3 and F5) (p < 0.05). The percentage of MTZ retention for formulation F4 dropped to 2.8% at 24 h after administration, in comparison to 8.8% for F3. These results suggested 1,4-cyclohexanediol and 1,2-diols with four and five hydrocarbons serve as enhancers to enhance penetration of MTZ while decreasing its skin retention.
Interestingly, percentage of MTZ retained in the epidermis and dermis layer for the formulations containing 1,2-hexanediol or 1,2-heptanediol was nearly 15%, showing similar results with comparison to their controls (p > 0.05). These data indicate that formulations containing 1,4-cyclohexanediol and 1,2-diols having six to seven carbons give lower flux values and accumulated amounts in the collection medium at 24 h, while maintaining a steady absorption of MTZ in epidermis and dermis layer when compared to their controls (F7, F9, and F11). It was an interesting observation that a combination of 1,4-cyclohexanediol and 1,2-diols with six- to seven-carbon chain length synergistically decreases systematic penetration of MTZ without reducing its retention in the epidermis and dermis layer. This is an ideal scenario for development of dermatological formulations—maintain high concentration at dermatologically viable epidermis/dermis layer to enhance therapeutic efficacy while decrease penetration into system circulation to minimize any potential side effect.
DISCUSSIONS
Skin penetration retardation effect in the presence of 1,2-hexanediol and 1,4-cyclohexanediol was surprisingly discovered through a high-throughput screening study in our laboratories (Zyleris PharmaTech, unpublished data). Our objective has been to understand mechanistic aspect of this discovery. In our previous study (12), a proposed retardation mechanism is as follows: 1,2-Hexanediol forms a complex with 1,4-cyclohexanediol. This complex penetrates into the bilayer in SC layer and undergoes a two-sided H-bonding with ceramide 6 in the bilayer. The two-sided H-bonding interaction retards penetration of MTZ. The mechanism provides support for the argument by Hadgraft (8) that H-bonding between modifiers and ceramide 6 in the SC layer is responsible for the mechanism of penetration retardation effect. Kaushik et al. (18) investigated the penetration modifier interaction with SC ceramide using chemical modeling. Although the percutaneous retardation effect is known, the detailed mechanistic investigations are few and far in between.
The position of a modifier in the lamellar structure would depend on the balance between its hydrophilic and lipophilic properties (8). Nevertheless, how long of the lipophilic chain is enough to disrupt the SC bilayer and condense the SC lipids to make the skin more (or less) permeable is still an open question. Bouwstra et al. (19) demonstrated that alkyl-azones having alkyl chains six to 12 carbons in the hydrophobic chain were intercalated in the bilayer, but the hexyl analog caused no disordering of the lipid structure. Compounds with shorter hydrocarbon chains (two to four) might be situated well into the polar region. In our earlier study (12), we have shown that 1,2-hexanediol (six-carbon chain) is able to disrupt the SC bilayer and carry 1,4-cyclohexanediol into SC layer, resulting in the observed penetration retardation effect.
In the present study, effect of chain length in 1,2-alkanediols percutaneous absorption of MTZ was systematically investigated. There are two significant findings in the present study. The first one is that hydrocarbon chain length in 1,2-alkanediols has a profound effect on percutaneous absorption of MTZ. The second, interestingly, is that by varying the chain length, 1,2-alkanediol can be switched from being no effect to a penetration enhancer to retardant—a rather surprising discovery.
The observed penetration retardation effect for the formulation containing 1,2-heptanediol (seven carbons) and 1,4-cyclohexanediol offers further evidence to support our proposed two-sided H-bonding mechanism (Fig. 4a). Besides, three-dimensional structures were built using Molecular Operating Environment. Figure 4b showed the possible H-bond between the penetration retarders (1,2-hexanediol and 1,4-cyclohexanediol) and ceramide 6. The structures of ceramide 6 were kept rigid, and the penetration retarders were brought in close proximity of the ceramide 6 molecules to mimic H-bond formations. The maximum distance between donor–acceptor pairs is less than 3.5 Å to make the H-bond formation (18). 1,2-Alkanediols having six- and seven-carbon atoms seem able to form a strong complex with 1,4-cyclohexanediol. This complex is, in turn, able to intercalate into bilayer of SC layer and form two-sided H-boning interactions with ceramide 6 molecules in the bilayer, leading to the observed penetration retardation effect. It is our hypothesis that the six- and seven-carbon chain is lipophilic enough to intercalate into the bilayer. In addition, the whole complex between 1,2-hexanediol or 1,2-heptanediol and 1,4-cyclohexaneiol is strong enough to be pulled into the bilayer in SC layer.
Fig. 4.

a Proposed hydrogen bonds interactions between ceramides and modifier molecules (1,2-hexanediol/1,2-heptanediol and 1,4-cyclohexanediol). - - - H-bonding. b Computer-generated potential interactions of ceramide 6 molecules with penetration retarders
For the formulations containing 1,2-propanediol, the interaction between the diol and 1,4-cyclohexanediol is simply too weak. The presence of 1,4-cyclohexanediol has no effect whatsoever, indicating no complex formation in this case. Neither enhancement nor retardation effect was observed in this case. The same results were obtained for the formulations in the presence and absence of 1,4-cyclohexanediol.
Difference in the effect on penetration of MTZ in the presence and absence of 1,4-cyclohexanediol strongly suggests that a complex (intimate interaction) between 1,2-alkanediols (having four to seven carbons) and 1,4-cyclohexanediol indeed is formed. The interaction of this complex with the bilayer in the SC layer is most likely responsible for modulation effect on penetration of MTZ.
For the formulations containing 1,2-alkanediols having four- and five-carbon chains, the interaction between 1,2-alkanediols and 1,4-cyclohexanediol might be strong enough to form complexes. However, these 1,2-alkanediols, having relatively shorter carbon chains, are not lipophilic enough to pull entire complexes into the bilayer. Since the two –OH groups, being polar, are situated at opposite ends of 1,4-cyclohexanediol, it is reasonable to assume that only portion of the complex that could intercalate into the bilayer is carbon chain in the 1,2-diols (Fig. 5). Due to the weaker lipophilicity of these shorter 1,2-alkanediols, the intercalation might only cause partial disturbance of the bilayer. The fact that penetration enhancement effect was observed for the formulations containing 1,2-alkanediols and 1,4-cyclohexanediol having four- and five-carbon chains indicates that 1,2-butanediol or 1,2-pentanediol in the formulations did disturb packing (lessening) of the bilayer in SC layer. This lessening effect causes disordering and opening up of the bilayer in the SC layer, but not strong enough to pull 1,4-cyclohexanediol with it (Fig. 5). The reasons are: (a) weaker lipophilicity of these 1,2-alkanediols and (b) two OH– groups in 1,4-cyclohexanediol at both ends—it would need a strong lipophilic 1,2-alkanediol to pull 1,4-cyclohexanediol into the bilayer. The proposed two-sided H-bonding would not be possible in this case. Only one-sided H-bonding between –OH groups in 1,2-alkanediols and in ceramide 6 would be possible. Thus, a penetration enhancement effect was observed. Furthermore, the results for 1,2-propanediol lends additional experimental support to the proposed mechanism.
Fig. 5.

Schematic representation between ceramides and modifier molecules (1,2-butanediol/1,2-pentanediol)
As the chain length increases (six and seven carbons), 1,2-alkanediols become more lipophilic, thereby stronger intercalators. The strong intercalation into the bilayer would pull 1,4-cyclohexanediol with it, leading to inclusion of the complex into the bilayer. The complex would then form two-sided H-bonding with ceramide 6 molecules on both sides, resulting in the observed penetration retardation effect.
Thus, the present study suggests that for 1,2-alkanediols having shorter hydrocarbon chain (four and five), only the 1,2-alkanediol side of the complex intercalates into the bilayer—i.e., the complex intercalates vertically into the bilayer. The presence of 1,4-cyclohexanediol could make opening up of packed bilayer more significantly, thereby making 1,2-alkanediols stronger penetration enhancers when compared to the control (i.e., no 1,4-cyclohexanediol). For the same 1,2-diol, difference in the results for the formulations in the presence and absence of 1,4-cyclohexanediol clearly supports the role of 1,4-cyclohexanediol and the complex formation. As the hydrocarbon chain grows longer, the whole complex intercalates into the bilayer, 1,2-alkanediols become penetration retardants. The observed penetration retardation and enhancement effect provides strong and complimentary experimental support for our proposed two-sided H-bonding mechanism.
Additional support for the proposed mechanism can be drawn from comparison between the data for 1,2-butanediol (four carbons) and 1,2-pentanediol (five carbons). 1,2-Pentanediol, due to its longer carbon chain, is more lipophilic than 1,2-butanediol, but less lipophilic than 1,2-hexanediol. Therefore, it is a stronger intercalator than 1,2-butanediol and but weaker one than 1,2-hexanediol. Thus, 1,2-pentanediol is in an overlap zone with a strong penetration enhancer (1,2-butanediol) in one end and a penetration retardant in the other end (1,2-hexanediol). The cross-over point from being a penetration enhancer to retardant is 1,2-pentanediol. It is postulated that in the case of 1,2-pentanediol, overall interaction with the bilayer is a contribution from both whole complex interaction (Fig. 4, retardation effect) and vertical intercalation (Fig. 5, enhancement effect). Thus, the overall penetration enhancement effect of 1,2-pentanediol was partially canceled out by contribution of whole complex intercalation, which is responsible for the retardation effect. The observed results are sum of these two opposite effects. Thus, 1,2-pentanediol is a weaker penetration enhancer than 1,2-butanediol, which has been shown experimentally in the present study. The fact that 1,2-pentanediol is still a penetration enhancer indicates that vertical intercalation is more dominant than whole complex intercalation.
Interestingly, the observed enhancement or retardation effect seems to be independent of chemical structure of the active used in the study. For example, the effect has been observed in two actives with completely different chemical structures (11,12): azelaic acid (alkyl dicarboxylic acid family) and MTZ (nitroimidazole family). This again supports our hypothesis that the observed effect is due to interactions between 1,2-alkanediols and 1,4-cyclohexanediol and their interactions with the skin bilayer. Structure of an active has nothing to do with it. Notice that chemical structures of azelaic acid and metronidazole could not be more different—one being a straight chain dicarboxylic acid and the other having a five-atom heterocycle ring structure. It is expected that the modulation effect could be observed in many pharmaceutical and cosmetic actives. Study is currently underway in our laboratories to test other actives in order to validate the hypothesis. To the best of our knowledge, the data from the present study offer one of the first examples that an excipient system can modulate in a systematic and predictable fashion not only degree of its skin penetration effect (strong vs. weak enhancement) but also nature of skin penetration effect (enhancement vs. retardation).
The present study also suggests a central role played by 1,4-cyclohexanediol. In the presence of this molecule, 1,2-alkanediol can be switched from being no effect to a penetration enhancer to retardant. As discussed above, the observed enhancement or retardation effect seems to be independent of chemical structure of an active molecule. Mouse skin is known to be much more permeable than human or pig skin. One of reasons we chose mouse skin model is that if we were able to demonstrate the modulation effect in more permeable mouse skin, the effect would likely be found in the less permeable human or pig skin. That is because as molecules are easier to get caught in smaller holes (figuratively speaking) in skin tissues than in bigger holes. Preliminary data from our laboratories seem to indicate that similar modulation effect was also found in in vitro pig skin model as well. Thus, this system (1,4-cyclohexanediol and 1,2-alkanediols) could become a general vehicle for controlled release of a wide variety of pharmaceutical and cosmetic active compounds. Studies are currently underway in our laboratories to further develop and fine-tune the system as a general delivery vehicle.
CONCLUSION
Percutaneous absorption of MTZ from topical formulations containing 1,2-alkanediols and 1,4-cyclohexanediol shows a strong dependence on the chain length in 1,2-alkanediols. The effect can be switched from penetration enhancement (four and five carbons) to retardation (six and seven carbons). Release of the active molecule could be modulated from being strong enhancement to weak enhancement and to retardation by simply varying chain length of 1,2-alkanediols. A two-sided H-bonding mechanism is proposed to explain the observations.
The present study provides a mechanistic insight into effect of interactions among molecules in topical formulations and their interactions with the skin bilayer on percutaneous absorption. A thorough understanding of these interactions could lead to rational design of penetration enhancers and retardants, resulting in superior topical products for pharmaceutical and personal care applications. Furthermore, optimization of the system (1,4-cyclohexanediol and 1,2-alkanediols) would make significant advance in development of a general vehicle for controlled release of a wide variety of active compounds.
Contributor Information
Fengping Tan, Email: jerryzhang@zyleris.com, Email: tangfengping@163.com.
Jerry Zhang, Email: jerryzhang@zyleris.com.
References
- 1.Asbill CS, Michniak BB. Percutaneous penetration enhancers: local versus transdermal activity. Pharm Sci Technol Today. 2000;3:36–41. doi: 10.1016/S1461-5347(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 2.Kaushik D, Batheja P, Kifoyle B, Rai V, Michniak-Kohn B. Percutaneous permeation modifiers: enhancement versus retardation. Expert Opin Drug Deliv. 2008;5:517–529. doi: 10.1517/17425247.5.5.517. [DOI] [PubMed] [Google Scholar]
- 3.Purdon C. Synthesis and evaluation of aminocaprolactam derivatives as novel skin penetration retarders. Ph.D. thesis. College of Pharmacy, University of South Carolina, Columbia; 2005.
- 4.Fang JY, Hwang TL, Leu YL. Effect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogels. Int J Pharm. 2003;250:313–325. doi: 10.1016/S0378-5173(02)00540-9. [DOI] [PubMed] [Google Scholar]
- 5.Rajadhyaksha V, Pfister WR. Oxazolidinones. Drug Cosmet Ind. 1996;1:36–47. [Google Scholar]
- 6.Seth B. Transdermal delivery using decycyloxazolidin-2-one. Arzneim Forsch Drug Res. 1999;42:120–122. [Google Scholar]
- 7.Kim N, El-Khalili M, Henary MM, Strekowski L, Michniak BB. Percutaneous penetration enhancement activity of aromatic S,S-dimethyliminosulfuranes. Int J Pharm. 1999;187:219–229. doi: 10.1016/S0378-5173(99)00194-5. [DOI] [PubMed] [Google Scholar]
- 8.Hadgraft J, Peck J, Williams DG, Pugh WJ, Allan G. Mechanisms of action of skin penetration enhancers/retarders: azone and analogues. Int J Pharm. 1996;141:17–25. doi: 10.1016/0378-5173(96)04609-1. [DOI] [Google Scholar]
- 9.Wertz PW. Epidermal lipids. In: Seminars in dermatology 11, 1992, pp. 106–13. [PubMed]
- 10.Song Y, Xiao C, Mendelsohn R, Zheng T, Strekowski L, Michniak B. Investigation of iminosulfuranes as novel transdermal penetration enhancers: enhancement activity and cytotoxicity. Pharm Res. 2005;22:1918–1925. doi: 10.1007/s11095-005-7416-4. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Su Q, Tan FP, Zhang J. Effect of 1,4-cyclohexanediol on percutaneous absorption and penetration of azelaic acid. Int J Pharm. 2010;387:167–171. doi: 10.1016/j.ijpharm.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Jia WB, Zhang Y, Tan FP, Zhang J. Synergistic effect of 1,4-cyclohexanediol and 1,2-hexanediol on percutaneous absorption and penetration of metronidazole. Int J Pharm. 2011;415:169–174. doi: 10.1016/j.ijpharm.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto H, Hashida M, Sezaki H. Effect of 1-alkyl or 1-alkenylazacycloalkanone derivatives on the penetration of drugs with different lipophilicities. J Pharm Sci. 1991;80:39–45. doi: 10.1002/jps.2600800111. [DOI] [PubMed] [Google Scholar]
- 14.Barry BW, Williams AC. Terpenes as skin penetration enhancers. In: Walters KA, Hadgraft J, editors. Pharmaceutical skin penetration enhancement. New York: Marcel Dekker; 1993. pp. 95–111. [Google Scholar]
- 15.Takayama K. O-Alkyl and O-acylmenthol derivatives as potential skin penetration promoters. Proc Intern Symp Control Release Bioact Mater. 1995;22:60–61. [Google Scholar]
- 16.Potts RO, Guy RH. A predictive algorithm for skin permeability: the effects of molecular size and hydrogen bond activity. Pharm Res. 1995;12:1628–33. [DOI] [PubMed]
- 17.Michniak BB, Player MR, Chapman JM, Jr, Sowell JW., Sr In vitro evaluation of a series of azone analogs as dermal penetration enhancers: IV amines. Int J Pharm. 1995;116:201–209. doi: 10.1016/0378-5173(94)00294-F. [DOI] [Google Scholar]
- 18.Kaushik D, Costache A, Michniak-Kohn B. Percutaneous penetration modifiers and formulation effects. Int J Pharm. 2010;386:42–51. doi: 10.1016/j.ijpharm.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Bouwstra JA, Gooris GS, Brussee J, Salomons-de Vries MA, Bras W. The influence of alkyl-azones on the ordering of the lamellae in human stratum corneum. Int J Pharm. 1992;79:141–148. doi: 10.1016/0378-5173(92)90105-B. [DOI] [Google Scholar]
- 20.Howes D, Guy R, Hadgraft J. Methods for assessing percutaneous absorption. The report and recommendations of ECVAM Workshop 13. Altern Lab Anim. 1996;24:81–106. [Google Scholar]
- 21.Batheja P, Song Y, Wertz P, Michniak-Kohn B. Effects of growth conditions on the barrier properties of a human skin equivalent. Pharm Res. 2009;26:1689–1700. doi: 10.1007/s11095-009-9879-1. [DOI] [PubMed] [Google Scholar]
- 22.Goodman M, Barry BW. Action of penetration enhancers on human skin as assessed by the permeation of model drugs 5-fluorouracil and estradiol. I. Infinite dose technique. J Invest Dermatol. 1988;91:323–327. doi: 10.1111/1523-1747.ep12475655. [DOI] [PubMed] [Google Scholar]
- 23.Cross SE, Roberts MS. The effect of occlusion on the epidermal penetration of parabens from a commercial test ointment, acetone and ethanol vehicles. J Invest Dermatol. 2000;115:914–918. doi: 10.1046/j.1523-1747.2000.00151.x. [DOI] [PubMed] [Google Scholar]


