Abstract
The objective of this study was to study the effect of formulation compositions on physicochemical properties and anti-Propionibacterium acnes activity of film-forming solutions containing alpha-mangostin-rich extract (AM). Film-forming solution bases and film-forming solutions containing AM were prepared by using Eudragit RL PO or Klucel LF or combinations of them as film-forming polymers. Rheological properties, pH values of the solutions, and mechanical properties of the dry films were investigated. An optimized formulation was selected and evaluated for the film surface, in vitro AM release, an anti-P. acnes activity, and potential for being a skin irritant. It was found that mechanical properties of the dry films were affected by total polymer contents, ratios of Klucel LF/Eudragit RL PO, AM, and contents of triethyl citrate. The film-forming solutions containing AM had pH values around 7.0. Their flow curves exhibited Newtonian flow behaviors. The optimized formulation provided films possessing smooth and nonporous surfaces. These films showed greater anti-P. acnes activity than their base films without toxicity to skin fibroblasts. Furthermore, AM released from the film matrix obeyed Higuchi's equation. In conclusion, the film-forming solutions containing AM had potential for treatment of acne vulgaris caused by P. acnes. However, further in vivo study is necessary to determine their efficacy and safety for using in patients suffering from acne vulgaris.
KEY WORDS: acne vulgaris, alpha-mangostin, film-forming solutions, physicochemical properties, Propionibacterium acnes
INTRODUCTION
Acne vulgaris is one of the most common skin diseases affecting people 13–45 years of age. It was reported that the prevalence of acne in teenagers was around 94.4% for males and 92% for females. Among them, 14% suffered from moderate to severe acne. The prevalence tended to lower with age. It was around 10% after 25 years and 6% after 40 years of age (1). Acne vulgaris is not a life-threatening disease but it can lead to physical problems such as inflammatory lesions, permanent scars, and keloids. More importantly, it can affect psychosocial development with reduced self-esteem, emotional distress and social inhibition, and thus be considered as one of the risk factors for suicide (2). Acne appears in skin areas rich in pilosebaceous units, i.e., face, back, and trunk (3). It is attributed to many factors including androgen activity, increase in sebum production, follicular hyperkeratinization, change in sebum lipid quality, and proliferation of Propionibacterium acnes within the follicles (4).
P. acnes is a gram-positive anaerobic bacillus recognized as the most important cutaneous microflora contributing to an inflammatory acne response. It secretes biologically active molecules such as lipases, proteases, hyaluronidases, and chemotactic factors that induce production of proinflammatory cytokines. Furthermore, these chemotactic factors could induce migration of neutrophils to the acne lesions to initiate the inflammatory reaction (5,6). Nowadays, selection of agents for acne treatment is usually tailored to the individual patient depending on causative factors and severity of the acne. However, treatment is mostly based on antibiotics in either oral forms or topical forms of erythromycin, clindamycin, minocycline, and other such medications (1,2). Due to the fact that incidences of antibiotic-resistant P. acnes have been increasingly reported and becoming a critical problem throughout the world (5), the use of natural compounds from medicinal plants possessing anti-P. acnes activity and anti-inflammatory effect has been currently considered as an alternative to antibiotics. One of such compounds is alpha-mangostin, a xanthone derivative which is a major constituent in the rinds of the Mangosteen fruit (Garcinia mangostana L.; family of Clusiaceae). It has various biological activities, for example, anticancer activity on human hepatoma, breast cancer, and colon preneoplastic lesions; antioxidant properties; and an activity on P. acnes and reactive oxygen species causing inflammatory reactions in acne (3,5,6). In Southeast Asia, a crude extract from mangosteen fruit rinds has been used as a traditional medicine for treatment of skin infections and acne (7,8). The products containing this crude extract are available as transdermal patches and semisolid products as either gels or creams. Although these products could be administered by simply attaching to or applying on skin lesions, they still possess some drawbacks. Transdermal patches cause observable marks on patients' faces and can even cause the skin to become inflexible. Consequently, they have not been accepted by some patients. As regards the use of gels and creams, they can be worn off from the skin easily, especially when they are diluted with sweat, and can thus lead to failure of the treatment (9). Moreover, because of the brownish black color of the crude extract, these products also possess unpleasant color. Therefore, the use of purified mangosteen fruit rind extract (alpha-mangostin-rich extract, AM) as an active ingredient instead of the crude extract should be more appropriate. Due to a high anti-P. acnes activity of AM, it could avoid a high concentration of the active ingredient and unpleasant color of products.
A film-forming solution is a mixture of polymers dispersed homogenously in volatile solvents. It was used previously as glue for joining damaged tissues without the use of threads and needles in eye surgery (10), cutaneous wounds treatment (11), and also skin protection during radiotherapy (12). It could form films when in contact with tissue surfaces and bond the apposed wound edges. Nowadays, a film-forming solution is employed as a novel approach for transdermal drug delivery. It could be applied on the skin easily like a liquid and then form an almost invisible film controlling drug release into the skin following evaporation of the solvent (13). Because of its favorable characteristics and advantages over the conventional transdermal drug delivery systems—for example, wearing off resistance, providing an invisible film on the skin, and causing less inflexible skin—it is currently being accepted as an attractive system for transdermal drug delivery (13–15). Consequently, this study would formulate a novel topical product for anti-P. acnes by using films forming solutions as a delivery system of AM.
The objectives of this study were to study the effect of formulation compositions on physicochemical properties and anti-P. acnes activity of film-forming solutions containing AM, to study in vitro release of AM from the film matrix and to evaluate its potential for being a skin irritant.
MATERIALS AND METHODS
Materials
Standard alpha-mangostin was purchased from Chengdu Biopurify Phytochemicals Ltd. (China). Ammonio methacrylate copolymer type A (Eudragit RL PO), a water-insoluble polymer, and hydroxypropylcellulose (Klucel LF), a water-soluble polymer, were kindly provided by Darmstadt Ltd. (Germany) and Wilmington Ltd. (USA), respectively. Triethyl citrate was purchased from Sigma-Aldrich Inc. (USA). Absolute ethanol, chloroform, dichloromethane, and hexane were supplied by Mallinckrodt Baker Inc. (USA). Ethyl acetate and methanol were purchased from Labscan Asia Co., Ltd. (Thailand). All of these solvents were of analytical grade. Brain heart infusion broth and agar were purchased from Merck Ltd. (Germany). Cellulose dialysis tube with a molecular weight cutoff of 12,000 was obtained from Sigma-Aldrich (USA). Normal human foreskin fibroblast (NHFF) cells isolated from human foreskin biopsies were kindly provided by Biotec, NSTDA (Thailand). DMEM, fetal bovine serum, l-glutamine, trypsin-EDTA solution, and phosphate buffer were purchased from Invitrogen (USA). Methylthiazolydiphenyl-tetrazolium bromide (MTT) was purchased from Sigma-Aldrich (USA).
Preparation of Mangosteen Fruit Rind Extract and Purification
Mangosteen fruits were purchased from a local market in Pathumthani province. They were cleaned with tap water three times. The calyxes were removed and the fruits were cut along their circumferences into two parts. The flesh was discarded and the fruit rinds were cleaned again with tap water and then dried in a hot air oven (Contherm, New Zealand) at 60°C until a constant weight was obtained. The dried rinds were ground to powder and extracted with dichloromethane for three times. The crude extract was concentrated by using a vacuum rotary evaporator (Büchi, Switzerland) and then purified by using column chromatography containing Silica gel 60, 0.063–0.200 mm mesh (Merck, Germany) with a mixture of hexane and ethyl acetate at the ratio of 7:3 as an eluent. Each 50 ml fraction was identified for alpha-mangostin by using a thin layer chromatography (TLC) technique. Briefly, a silica gel GF254 plate (Merck, Germany) was developed with hexane and ethyl acetate at the ratio of 6:4. The components were identified under UV light at a wavelength of 254 nm by an Rf value compared with a standard solution of alpha-mangostin. The fractions containing alpha-mangostin were collected and the solvent was removed by using the vacuum rotary evaporator. The AM in a solid form was kept in a desiccator for further use. Alpha-mangostin content in AM was determined by a high-performance liquid chromatography (HPLC) technique. Briefly, an HPLC instrument (PerkinElmer, USA) with a UV detector for UV detection at 319 nm was used in this study. A column (Inertsil, USA) for separation was a reversed-phase octadecyl column (25 cm × 4.5 mm) with a particle size of 5 μm. A mobile phase was 94% methanol. It was pumped at a flow rate of 1.0 ml/min at a temperature of 30°C. Samples were dissolved in the mobile phase at a concentration of 12 μg/ml and analyzed in triplicate for the content of alpha-mangostin. Calculation for alpha-mangostin content was based on a linear regression equation for a standard curve of alpha-mangostin.
Determination of a Minimum Inhibitory Concentration of AM for P. acnes
A minimum inhibitory concentration (MIC) of AM for anti-P. acnes was determined in triplicate by a microdilution assay (3). P. acnes (DMST no. 14914, Thailand) was incubated in brain–heart infusion (BHI) broth under anaerobic conditions for 72 h and adjusted with sterile water to yield about 1.0 × 108 CFU/ml. The P. acnes suspension was then diluted with BHI broth to a concentration of 5% v/v and dispensed into 96-well plates with a volume of 100 μl to each well. AM was dissolved in 2% dimethylsulfoxide (DMSO) to make a concentration of 32 μg/ml. Then, serial twofold dilutions were made in a concentration range from 32 to 0.5 μg/ml. One hundred microliter of each AM solution was dispensed into wells containing the P. acnes suspension. Subsequently, these 96-well plates were incubated in anaerobic conditions at 37°C for 72 h. Inhibition of microbial growth was identified by the clear solution obtained after incubation. MIC of AM for P. acnes is the lowest concentration that will give clear solutions in the wells. In this study, both nutrient broth and 2% DMSO without AM were used as negative controls, and amoxicillin solution (1 mg/ml) was used as a positive control.
Preparation of Film-Forming Solutions
Formulations of film-forming solution bases are shown in Table I. They were prepared on a weight basis. Briefly, Eudragit RL PO for the formulations containing only Eudragit RL PO as a film-forming polymer was dissolved in absolute ethanol. Klucel LF for the formulations containing only Klucel LF or mixtures of Eudragit RL PO and Klucel LF were dissolved in 80% v/v ethanol. These were stirred continuously until clear solutions were obtained. Triethyl citrate used as a plasticizer was then added (if specified in Table I) and stirred thoroughly. The obtained solutions were stored in screwed test tubes and sealed tightly with Parafilm® M (Brand GmbH, Germany) for using in further experiments. For the case of a film-forming solution containing AM (later called AM film-forming solution), the AM was added into the solutions to give a final concentration equaling to 1,000 times of MIC. The pH values of representative preparations were determined in triplicate by using a pH meter (Hanna instruments 8417, USA). Skin sensations following application of film-forming solutions on the skin with a volume of 10 μl/cm2 were assessed by five research members. Times for achieving dry film on skin were measured for three times.
Table I.
Formulation Compositions of Film-Forming Solution Bases and Mechanical Properties of the Films Obtained from them (n = 3, mean ± SD)
| Formulations | Content (% w/w) | Klucel LF/Eudragit RL PO ratios | Mechanical properties | |||
|---|---|---|---|---|---|---|
| Klucel LF | Eudragit RL PO | Triethyl citrate | Tensile strength (Newton/mm2) | % Elongation at break | ||
| 5%E-T1 | 0 | 5 | 1 | –a | 1.57 ± 0.35 | 31.03 ± 7.58 |
| 8%E-T1 | 0 | 8 | 1 | –a | 3.72 ± 0.44 | 134.23 ± 13.33 |
| 11%E-T1 | 0 | 11 | 1 | –a | 5.23 ± 0.32 | 197.58 ± 22.76 |
| 5%K-T1 | 5 | 0 | 1 | –a | 0.65 ± 0.02 | 17.54 ± 1.22 |
| 8%K-T1 | 8 | 0 | 1 | –a | 2.89 ± 0.30 | 50.34 ± 6.59 |
| 11%K-T1 | 11 | 0 | 1 | –a | 3.98 ± 0.15 | 65.83 ± 5.76 |
| 8%K1-E1-T1 | 4 | 4 | 1 | 1:1 | 2.43 ± 0.21 | 19.24 ± 4.48 |
| 8%K3-E1-T1 | 6 | 2 | 1 | 3:1 | 3.89 ± 0.40 | 53.10 ± 5.48 |
| 8%K5-E1-T1 | 6.67 | 1.33 | 1 | 5:1 | 8.84 ± 0.18 | 72.09 ± 2.30 |
| 8%K5-E1-T0 | 6.67 | 1.33 | 0 | 5:1 | 20.24 ± 1.85 | 8.76 ± 4.91 |
| 8%K5-E1-T2 | 6.67 | 1.33 | 2 | 5:1 | 2.17 ± 0.34 | 124.54 ± 27.46 |
| 11%K1-E1-T1 | 5.5 | 5.5 | 1 | 1:1 | 4.49 ± 0.09 | 17.10 ± 2.97 |
| 11%K3-E1-T1 | 8.25 | 2.75 | 1 | 3:1 | 7.79 ± 0.89 | 27.65 ± 3.16 |
| 11%K5-E1-T1 | 9.17 | 1.93 | 1 | 5:1 | 11.47 ± 0.32 | 47.81 ± 1.27 |
aFormulation contained only one polymer
Rheological Property Measurement of AM Film-Forming Solutions
The rheological measurement was performed in triplicate in a steady shear sweep mode using a controlled-stress rheometer (Bohlin Gemini HR Nano, Malvern Instrument, UK) equipped with a cone and plate geometry (2° cone and 55 mm diameter). The initial and final shear rates were set at 0.1 and 100 s−1, respectively. The temperature was set at 25 ± 0.1°C.
Determination of Mechanical Properties of Films
Dry films were prepared as follows: film-forming solutions with a volume of 15 ml were poured on Teflon molds (6 × 10 cm) and then placed in the hot air oven at temperature of 40°C (Contherm, New Zealand) for 48 h. The dry films of film-forming solution bases and AM film-forming solutions (called “base film” and “AM film”, respectively) were kept in a desiccator for mechanical testing. The specimens were prepared by cutting the films into rectangular shapes (1.5 × 10 cm) and were then investigated for their mechanical properties, i.e., tensile strength and percent elongation at break by using a Universal Testing Machine (Instron 8801, USA). Briefly, the test device was equipped with a 20 N load sensor. The films were placed between two vertical grips. Initial distance between these vertical grips was set at 40 mm. The movable grip was then driven upward with a speed of 500 mm/min until the films were ruptured. Tensile strength and percent elongation at break of each film represents the maximum stress and elongation that the films could withstand, while being pulled before break, were determined. The reported values are the values of mean and standard deviations (SD) of the data as taken from three specimens.
Morphology of Dry Films
Surface morphology of a representative base film and an AM film were observed on their gold-coated surfaces using a scanning electron microscope (JSM-5410LV, JEOL, Japan) at 10 kV.
Determination of Tack of Films
Tack of films (2 × 2 cm) was determined by a probe tack test using a controlled-strain rheometer (model ARES, TA Instrument, USA) having a normal force measuring unit and equipped with a probe tack fixture. It was expressed as the maximum force for pulling a tacking probe (diameter, 5 mm) from the samples' surface with a speed of 10 mm/s after pressing the sample with 100 gf for 1 s. Each sample was tested in triplicate and the data was reported in terms of mean ± SD.
FT-IR Spectroscopy for AM/Base Film Interaction Study
In order to investigate the interactions between AM and base film, Fourier transform infrared (FT-IR) spectra of AM, base film, and AM film were determined individually with an FT-IR Spectometer (PerkinElmer model Spectrum One, USA). The test samples were ground and mixed with KBr powder at a ratio of 1:100 and pressed to a pellet for this study. Signal averages were obtained for 32 scans at a resolution of 4 cm−1.
In Vitro Release Study
Release of AM from a representative AM film through cellulose dialysis membrane was performed by using modified Franz diffusion cells in triplicate. The dry film was cut into the size of a 2 × 2 cm square covering of the diffusion area (1.78 cm2) and was then placed on the cellulose dialysis membrane. The membrane with an AM film on one side was placed between the donor and receptor units, which was filled with 1% Tween 80 in pH 7.4 phosphate buffer solution to increase solubility of AM in the buffer solution for the precise analysis of released alpha-mangostin content. Precise amounts of the receiving solution were withdrawn at 5, 15, 30, 60, 120, 180, 240, 300, 360, 420, and 480 min, respectively. The volume of the receiving solution was maintained by replacing the amount withdrawn with an equal volume of 1% Tween 80 in pH 7.4 phosphate buffer solution. Throughout the time of a diffusion study, the receiving solution was kept well stirred with a magnetic stirrer, and the temperature was maintained at 37 ± 1°C. The withdrawn receiving solution was analyzed by using an HPLC technique as previously described.
In Vitro Skin Irritation Test
The in vitro skin irritation test was performed to investigate the potential of AM film-forming solution and AM film as a skin irritant. NHFF cells were cultured in DMEM containing 10% v/v fetal bovine serum and 2 mM l-glutamine at 37°C in 5% CO2 atmosphere and subcultured every 3–4 days using trypsin-EDTA solution. The cells were seeded in 24-well plates with a density of 5.0 × 104 cells/well. After incubation for 5 days, the cells reached confluence and were exposed to 200 μl of either 5% w/v AM film-forming solution in normal saline or 5% w/v AM film dispersed in normal saline for 10 min and 24 h, respectively. After exposure, the cells were washed twice with phosphate buffer. Then, 200 μl of 0.5 mg/ml MTT solution in medium was added and incubated for 2 h. The MTT formazan was extracted with 0.04 Normality HCl–isopropanol for 30 min and the absorbance of the extract was measured at 570 nm with a microplate reader (Molecular Devices, USA). In this study, a film-forming solution base and a base film were also tested. Wells containing medium and MTT solution without NHFF cells were used as blanks. The 100% cell viability was calculated from the results of wells containing NHFF cells with no exposure of the test solutions. The assay was performed in six replicates and reported as a mean of percent cell viability ± SD.
Anti-P. acnes Activity of AM Films
This experiment was performed by using a disk diffusion method (3). Briefly, P. acnes was incubated in BHI medium with 1% glucose for 72 h under anaerobic conditions. It was adjusted with sterile water to yield about 1.0 × 108 CFU/ml. An inoculum was added to molten BHI with glucose agar and mixed thoroughly. Then, it was poured over the BHI with glucose agar base set in a sterile Petri dish and left solidity. A representative base film and an AM films were prepared and cut into circular shapes with a diameter of 7 mm and placed on the agar. The plates were incubated at 37°C for 72 h under anaerobic conditions. Each film was tested three times. Their anti-P. acnes activity was reported as a mean of inhibition zone diameter (in millimeter) ± SD.
RESULTS AND DISCUSSION
Preparation of AM and Determination of a MIC of AM for P. acnes
Dichloromethane crude extract of mangosteen fruit rinds was separated into six fractions by using a column chromatography technique and analyzed with TLC for phytochemical screening. TLC fingerprints of each fraction observed under UV light (254 nm) are shown in Fig. 1. They show that standard alpha-mangstin (lane A) could move up the TLC plate with an Rf value of 0.49. In lane 1–6, components consisting in the fraction 1–6 were separated in a TLC plate. One of the components consisting the fraction 2–6 possessed an Rf value consistent with the Rf value of the standard alpha-mangostin in lane A implying that these fractions contained alpha-mangostin. The TLC fingerprints also show that the number of components consisting in the fraction 5–6 was less than that of the fraction 2–4. It could be concluded that the fraction 5 and 6 contained mainly alpha-mangostin.
Fig. 1.
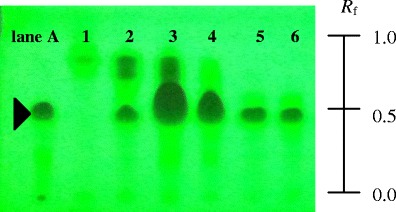
TLC fingerprints with hexane and ethyl acetate at a ratio of 6:4. Lane A standard alpha-mangostin, lane 1–6 fraction 1–6, respectively
Alpha-mangostin content in extracts was determined by using an HPLC technique. It was found that alpha-mangostin in extracts of the fraction 5 and 6 were 60.93% and 33.47% w/w, respectively. Due to having the highest content of alpha-mangostin, the extract from fraction 5 was used in the entire experiment. This particular extract will be referred to as AM.
The results of MIC determination of AM for P. acnes shown in Table II suggest that 0.5 μg/ml of AM could not inhibit P. acnes growth. Anti-P. acnes activity increased when the concentration was increased to a level greater than or equal to 1.0 μg/ml. This finding agreed with Koh et al. (16) in that alpha-mangostin was a potent antibacterial agent against Gram-positive pathogens with MIC of 0.78–1.56 μg/mL; moreover, its major antibacterial mechanism was rapid disruption of cytoplasmic membrane integrity leading to breakdown and increased permeability of cell membrane in a concentration-dependent manner. To achieve highly effective P. acnes, inhibition activity of AM in the film-forming solution formulations, an AM concentration of 1,000 μg/g or 1 mg/g, i.e., 1,000 times of MIC, was used in this study.
Table II.
Determination of MIC of AM for P. acnes
| Test solutions | P. acnes growth |
|---|---|
| 0.5 μg/ml AM | + |
| 1.0 μg/ml AM | − |
| 2.0 μg/ml AM | − |
| 4.0 μg/ml AM | − |
| 8.0 μg/ml AM | − |
| 16.0 μg/ml AM | − |
| 32.0 μg/ml AM | − |
| DMSO (negative control) | + |
| Brain–heart infusion broth (negative control) | + |
| Amoxicillin 1 mg/ml (positive control) | − |
+ growth, − no growth
Preparation of Film-Forming Solutions
Film-forming solution bases containing ingredients shown in Table I were prepared and kept in screwed test tubes. All of them were transparent and colorless. Their ability to flow, examined roughly by turning down the test tubes containing these preparations, seemed to depend on polymer content. It was found that the more the polymer content, the more viscous the solution. The formulations containing polymer content at the concentration of 5% w/w (5%E-T1 and 5%K-T1) could flow freely like water. They could not be retained when applied on the skin, but tended to run off, instead. On the other hand, the formulations containing 8% (8%E-T1 and 8%K-T1) and 11% (11%E-T1 and 11%K-T1) of the polymer content could be held on the skin without running off after they were applied on the skin with a volume of 10 μl/cm2. This result implies that 5% w/w of the polymer content could not provide enough consistency of the solutions for used in the film-forming solution formulation.
Determination of Physical Properties of Dry Films Obtained from Film-Forming Solutions
Dry films obtained from film-forming solutions were transparent and colorless. No visible macrophase separation was found in any of them. This result implies that there was good compatibility between the ingredients which consists the formulations. The results of mechanical properties determination of the films as shown in Table I suggest that their tensile strength and percent elongation at break that represented their hardness and elasticity, respectively, were dependent on the formulation compositions as detailed in the following sections.
Effect of Polymer Content
Mechanical properties of dry films obtained from film-forming solution bases using only Eudragit RL PO or Klucel LF at various concentrations are shown in Table I. The 11%E-T1 containing more content of Eudragit RL PO than 8%E-T1 and 5%E-T1 provided films possessing higher tensile strength and percent elongation at break than films obtained from 8%E-T1 and 5%E-T1. This finding followed a similar trend of tensile strength and percent elongation at break of base films using Klucel LF as a film-forming polymer. It implies that film-forming solutions consisting of more total polymer content would provide stronger and tougher films than the preparations containing less polymer content. This result follows from the greater polymer content, the larger intermolecular forces that exist between polymer chains (14). When comparing between the mechanical properties of the films from Eudragit RL PO formulations and the films from Klucel LF formulations containing the same polymer content, the Eudragit RL PO films were stronger and tougher than the Klucel LF films. This suggests that intermolecular forces between polymer chains of Eudragit RL PO such as hydrogen bonds and hydrophobic interactions were stronger than those of Klucel LF. In addition, Eudragit RL PO polymer chains might be more flexible and easier to fold than those of Klucel LF leading to the films possessing more percent elongation at break (15).
Effect of Klucel LF/Eudragit RL PO Ratios
Even though Eudragit RL PO could provide films that were much stiffer and tougher than Klucel LF, this chemical has a vast disadvantage that its water insolubility (17). Therefore, absolute ethanol must be used as a vehicle for Eudragit RL PO formulations. Due to the fact that the absolute ethanol can cause skin irritation, Klucel LF which is a water-soluble polymer was used as a major component in the formulations in this study. Consequently, the formulations containing both Klucel LF and Eudragit RL PO at various ratios were studied; 80% v/v ethanol was used as a solvent of these formulations. Table I shows that when the ratio of Klucel LF/Eudragit RL PO increased (that is K1-E1-T1 to K5-E1-T1), both tensile strength and percent elongation at break of the films obtained from these preparations increased. It also shows that the formulations containing both Klucel LF and Eudragit RL PO gave the films with higher tensile strength than the formulation containing only Klucel LF at the same total polymer content. However, only films obtained from 8%K5-E1-T1 had significantly higher percent elongation at break than pure Klucel LF formulations. According to the rule of mixtures (18), both tensile strength and percent elongation at break of the films obtained from these formulations should fall in between the values of pure Klucel LF and pure Eudragit RL PO. Furthermore, they should increase when the content of the tough component, i.e., Eudragit RL PO increased, or, in the other words, when the ratio of Klucel LF/Eudragit RL PO decreased. However, in this present case, there might be a microphase separation between water-soluble component (Klucel LF) and water-insoluble component (Eudragit RL PO) that led to the behaviors opposite to the mixing rule. Unfortunately, an evidence of phase separation could not be observed optically because both polymers possessed similar refractive indices. Generally, the mechanical properties of a polymer can be improved by the toughening effect of microdomains (18). Accordingly, the presence of a small amount of Eudragit RL PO may act as toughening microdispersed phase of the system. Therefore, both tensile strength and percent elongation at break of the films were improved if only a small amount of Eudragit RL PO was presented in the formulations. From the obtained results, 8%K5-E1-T1 provided films possessing optimized mechanical properties. This formulation was then selected for further studies.
Effect of Triethyl Citrate as a Plasticizer
In previous sections, triethyl citrate with a concentration of 1% w/v was used as a plasticizer in the formulations. It is interesting to carry out further investigation on the effect of a plasticizer on the mechanical properties of the films. Here, triethyl citrate concentrations were varied at 0%, 1%, and 2% w/w to achieve 8%K5-E1-T0, 8%K5-E1-T1, and 8%K5-E1-T2, respectively. Tensile strength and percent elongation at break of the films obtained from these formulations were compared. Table I shows that the films obtained from 8%K5-E1-T0, 8%K5-E1-T1, and 8%K5-E1-T2 possessed different mechanical properties. These results are in agreement with Lin et al. (19), who found that the films containing more plasticizer possessed lower tensile strength with higher percent elongation at break than the films containing less plasticizer. It is possible that triethyl citrate acting as a plasticizer could reduce the intermolecular forces between the polymer chains and reduce the strength of a film. Moreover, it could increase the flexibility of polymer chains resulting in tougher films. Therefore, the films containing more triethyl citrate were weaker and more extendable than the films containing less triethyl citrate. From the results of this study, 8%K5-E1-T1 still provided the films possessing optimized mechanical properties and was therefore chosen as a representative for the next experiment.
Preparation and Determination of Physical Properties of AM Film-Forming Solution and AM Film
After AM was added into a representative film-forming solution base of 8% K5-E1-T1, at a concentration of 1 mg/g, AM-8% K5-E1-T1 was obtained. In addition, AM was added to 11% K5-E1-T1 to obtain AM-11% K5-E1-T1 for comparison. Both solutions were transparent with a slightly yellowish color. Their pH values were 7.0 ± 0.0 and 6.9 ± 0.0, respectively. The flow behavior and viscosity of AM-8%K5-E1-T1 and AM-11%K5-E1-T1 were investigated using a steady shear sweep test at 25 ± 0.1°C. The flow curves and the viscosity profiles obtained from this study are shown in Figs. 2 and 3, respectively, indicating that both AM-8%K5-E1-T1 and AM-11%K5-E1-T1 exhibited Newtonian flow behaviors. This indicates that the polymers consisting in these formulations could not set up a three-dimensional network throughout the system properly because they were dispersed in a poor solvent. Therefore, polymer chain–chain interactions were favored over the polymer–solvent interactions resulting in a more contracted polymer chain (20). From the viscosity profile (Fig. 3), it was found that AM-11%K5-E1-T1 had a higher viscosity than AM-8%K5-E1-T1 due to a greater polymer content. This finding was confirmed by Eqs. (1) and (2), which define the flow curves of AM-11%K5-E1-T1 and AM-8%K5-E1-T1, respectively. The coefficients of linear regression equations for AM-11%K5-E1-T1 and AM-8%K5-E1-T1 representing their viscosities were approximately 1.27 and 0.48 Pas, respectively, suggesting that AM-11%K5-E1-T1 was approximately 2.6 times more viscous than AM-8%K5-E1-T1.
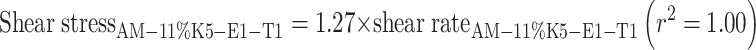 |
1 |
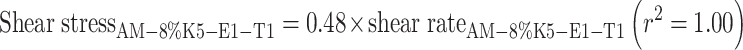 |
2 |
Fig. 2.
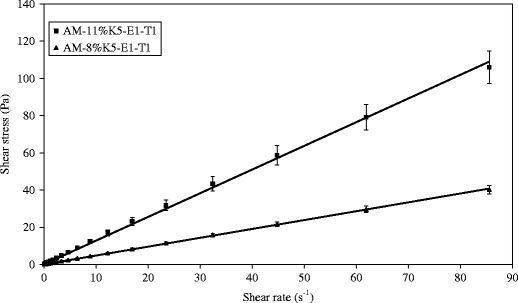
Flow curves of AM-8%K5-E1-T1 and AM-11%K5-E1-T1
Fig. 3.
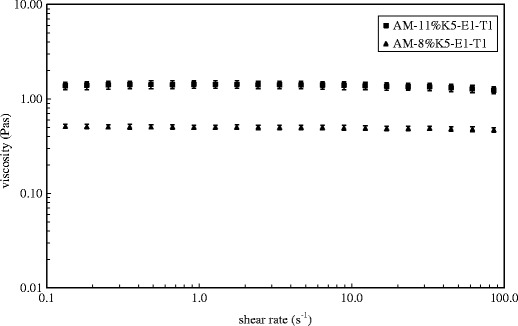
Logarithmic plots of viscosities against shear rates of AM-8%K5-E1-T1 and AM-11%K5-E1-T1
Skin sensations following application of AM-8% K5-E1-T1 and AM-11% K5-E1-T1 on the skin was evaluated by research members. It was found that both AM-8% K5-E1-T1 and AM-11% K5-E1-T1 could be applied on the skin surface easily and could in situ form thin films within 2.5 ± 0.1 and 3.3 ± 0.1 min, respectively, at an ambient temperature after applying on the back hand skin with a volume of 10 μl/cm2. AM-8% K5-E1-T1 provided almost invisible film and did not leave any obvious marks on the applied skin after evaporation of solvent as shown in Fig. 4. On the contrary, the dry films from AM-11% K5-E1-T1 was obviously observed on the applied skin and led to more skin fixation sensations than that of AM-8% K5-E1-T1. This suggests that the preparations containing more polymer content would be more viscous and would leave obvious marks on the skin. Consequently, AM-8% K5-E1-T1 was accepted as a suitable formulation.
Fig. 4.
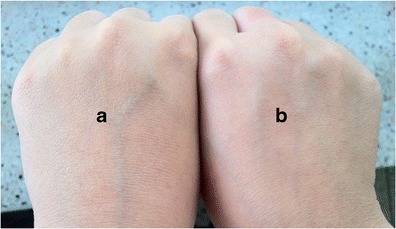
A photograph of a AM-8%K5-E1-T1 film on back hand skin after application on the skin with a volume of 10 μl/cm2 and b back hand skin without a film
The surface morphology of 8% K5-E1-T1 film and AM-8% K5-E1-T1 film as observed by an SEM technique shown in Fig. 5 indicate that the surface of the base film and the film containing AM were similar in that they were smooth and nonporous. Moreover, these SEM micrographs insisted that there was no macrophase separation found in these films suggesting that AM was molecularly dispersed in the film base (17). Tensile strength and percent elongation at break of the AM-8%K5-E1-T1 film were investigated and compared to those of the 8%K5-E1-T1 films. Tack of the films representing the force required to separate a probe from the films' surfaces was additionally determined. Figure 6 shows that the mechanical properties of the films slightly decreased after addition of AM. It was found that the difference between tensile strength, percent elongation at break, and tack of 8%K5-E1-T1 films and AM-8%K5-E1-T1 films were statistically significant at p values of 0.039, 0.026, and 0.019, respectively, as analyzed by using paired-sample t test. This indicates that AM incorporated into the formulation could interrupt intermolecular forces of film-forming polymer chains. In addition, AM–polymer interactions might occur instead leading to decreases in tensile strength, percent elongation at break, and tack (17). This observation was confirmed by FT-IR spectra shown in Fig. 7. The FT-IR spectrum of AM (Fig. 7(a)) shows the stretching of hydroxyl (OH) and carbonyl groups at 3,421–3,265 and 1,643–1,584 cm−1, respectively (21). When it was added into 8%K5-E1-T1 film, a characteristic AM band could be found in the FT-IR spectrum of AM-8%K5-E1-T1 film (Fig. 7(c)) as a shoulder at 1,584 cm−1. In addition, it led to some differences between the FT-IR spectrum of 8%K5-E1-T1 (Fig. 7(b)) and the AM-8%K5-E1-T1 film. It could be observed that the FT-IR spectrum of 8%K5-E1-T1 film at 3,450 and 1,631 cm−1 shifted to 3,447 and 1,640 cm−1, respectively. This might be due to intermolecular interactions, probably H bonding, between the hydroxyl (−OH) group in 8%K5-E1-T1 film and AM, and interactions between the carbonyl (C=O) group of AM and 8%K5-E1-T1 film (19,22), respectively.
Fig. 5.
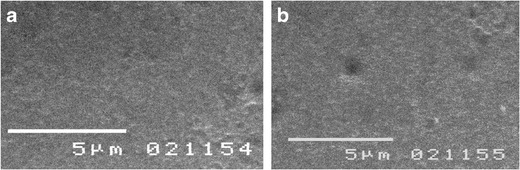
SEM micrographs: a 8% K5-E1-T1 film and b AM-8% K5-E1-T1 film (10 kV with a magnification of 10,000)
Fig. 6.
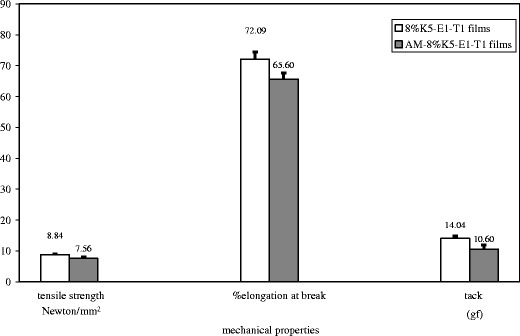
Mechanical properties of 8% K5-E1-T1 film compared to AM-8% K5-E1-T1 film
Fig. 7.
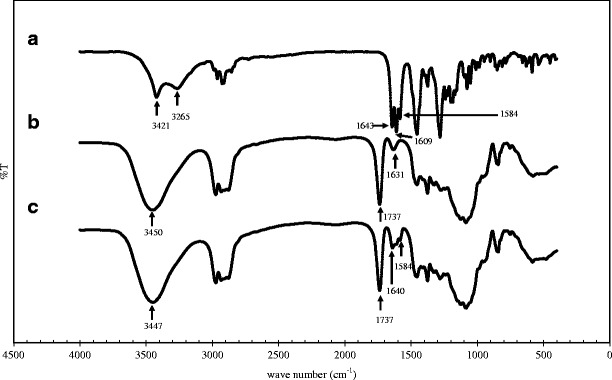
FT-IR spectra: a AM, b 8%K5-E1-T1 film, and c AM-8%K5-E1-T1 film
In Vitro Release Study
Release study of AM from AM-8%K5-E1-T1 film via a cellulose dialysis membrane with molecular weight cutoff of 12,000 was performed by using modified Franz diffusion cells. The release profile in Fig. 8(a) shows that AM was released from AM-8%K5-E1-T1 film continuously without burst release. This confirmed that AM was molecularly dispersed in the film matrix and could form interactions with a film matrix properly (14). AM-8%K5-E1-T1 film was almost completely exhausted within 300 min (5 h) as seen in the release profile showing the beginning of a plateau at 300 min. Figure 8(b) shows a trend of an AM release profile before reaching a plateau obeying Higuchi's equation (r2 = 0.9957) with a release rate of 6.01% release·minute−1/2. This implies that the rate-limiting step of AM release was predominantly determined by a diffusion process of AM within the film matrix rather than permeation through the membrane (19). This might be the polymers consisting in the film matrix-impeded movement and formed interactions with AM, and thus resulted in a controlled release of AM. However, because the in vitro release study showed how AM was released from the film matrix but did not show how AM permeated through the skin, permeation of AM through animal skin and in vivo pharmacodynamics of the film-forming solutions containing AM should be investigated further.
Fig. 8.
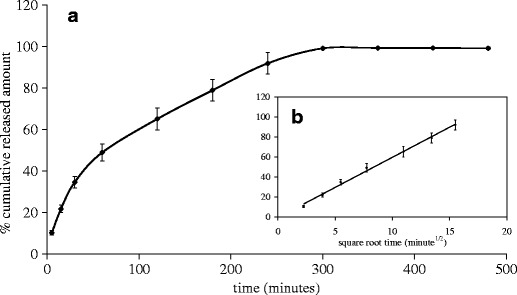
Release profiles of AM from AM-8%K5-E1-T1 film: a a plot of percent cumulative release amount of AM against time and b a plot of percent cumulative release amount of AM against square root of time
In Vitro Skin Irritation Test
The in vitro skin irritation test of AM-8%K5-E1-T1 and AM-8%K5-E1-T1 films was performed by using the MTT assay and was reported as %cell viability (%CV) of NHFF cells. For this experiment, 8%K5-E1-T1 and 8%K5-E1-T1 films were also investigated. The NHFF cells exposed to AM-8%K5-E1-T1 and AM-8%K5-E1-T1 films could survive with %CV of 101.6 ± 3.7% and 100.8 ± 2.9%, respectively. This suggests that both AM-8%K5-E1-T1 and AM-8%K5-E1-T1 films were not toxic to NHFF cells and did not have a potential for being a skin irritant. Figure 9 shows the comparable %CV of NHFF cells exposed to all test products. In addition, the differences in %CV for each pair of these preparations, i.e., (1) 8%K5-E1-T1 (%CV = 101.0 ± 4.4%) and AM-8%K5-E1-T1, and (2) 8%K5-E1-T1 films (%CV = 101.7 ± 3.8%) and AM-8%K5-E1-T1 films, were analyzed by using paired-sample t test. It was found that the %CV of the cells exposed to 8%K5-E1-T1 and AM-8%K5-E1-T1, and to 8%K5-E1-T1 films and AM-8%K5-E1-T1 films were not statistically different at p values of 0.823 and 0.287, respectively. This implies that AM added into the preparations at the concentration of 1 mg/g did not lead to more toxicity of the preparations. Therefore, AM-8%K5-E1-T1 and AM-8%K5-E1-T1 film could be considered as safe for skin use.
Fig. 9.
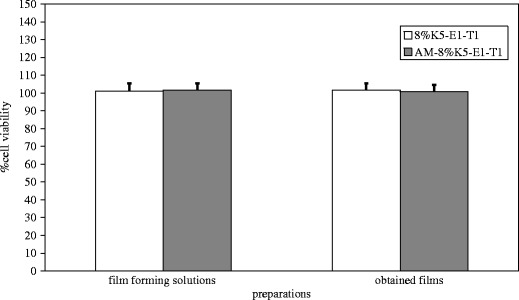
Percentage cell viability of NHFF cells exposed to 8%K5-E1-T1, 8%K5-E1-T1 film, AM-8%K5-E1-T1, and AM-8%K5-E1-T1 film
Anti-P. acnes activity of AM films
8%K5-E1-T1 film and AM-8%K5-E1-T1 film were investigated for their anti-P. acnes activities by using a disk diffusion method. The result shown in Fig. 10 indicates that AM-8%K5-E1-T1 film (area a) could inhibit P. acnes growth with a clear zone possessing a diameter of 23.5 ± 1.5 mm. However, 8%K5-E1-T1 film, which was a film base of AM-8%K5-E1-T1, did not show anti-P. acnes activity (area b). This suggests that AM added into the film-forming solution at concentration of 1 mg/g was enough for inhibiting P. acnes growth.
Fig. 10.
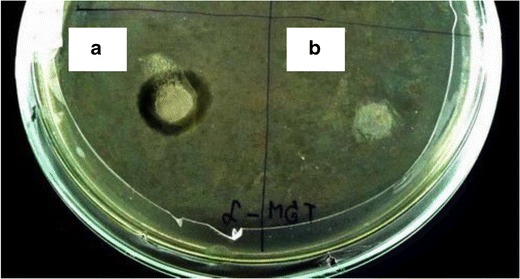
Inhibition zone: a AM-8%K5-E1-T1 film and b 8%K5-E1-T1 film
CONCLUSIONS
Physicochemical properties of either film-forming solutions or dry films obtained from film-forming solutions were markedly affected by formulation compositions. It was found that flowability of film-forming solution bases was dependent on polymer content in the formulations. When AM was added, it changed the color of film-forming solution bases from colorless to a slightly yellowish color. The rheological measurement suggested that the representative formulations, AM-8%K5-E1-T1 and AM-11%K5-E1-T1, exhibited the same flow behavior, i.e., a Newtonian flow. The viscosity profiles pointed out that their viscosities tended to increase with increase in total polymer content. Moreover, the skin sensations following application of AM film-forming solutions suggested that the formulation containing more total polymer content would lead to obvious marks on the skin and a skin fixation sensation. On the other hand, the formulation containing an excessively low total polymer content could not be retained on the applied skin and tended to run off. The film-forming solutions containing more total polymers content provided the films with more tensile strength and percent elongation at break. When ratios of Klucel LF/Eudragit RL PO in film-forming solution bases were increased, both tensile strength and percent elongation at break of the films obtained from these preparations also increased. Due to a plasticizer action of triethyl citrate, the films containing more triethyl citrate were weaker and more extendable than the films containing less triethyl citrate. AM incorporated into the film-forming solution bases could affect not only the color but also the mechanical properties of the obtained films. It slightly decreased tensile strength, percent elongation at break, and tack of the films by forming interactions with the polymers consisting in the film matrix. From this study, the optimized AM film-forming solution was AM-8%K5-E1-T1 containing 1 mg/g of AM, 8% w/w of mixture of Klucel LF and Eudragit RL PO at the ratio of 5:1, and 1% w/w triethyl citrate. It could be applied on the skin easily and did not leave any obvious marks on the skin. Its release profile obeying Higuchi's equation suggested that AM release was predominantly controlled by the diffusion process of AM in the film matrix. In vitro skin irritation tests showed that AM-8%K5-E1-T1 could be accepted as safe for skin use. More importantly, it showed more excellent anti-P. acnes activity than its base film. This suggested that AM-8%K5-E1-T1 had potential for treatment of acne vulgaris caused by P. acnes. However, further in vivo study and clinical trials will be necessary to determine its efficacy and safety for using in patients suffering from acne vulgaris.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the National Science and Technology Development Agency (NSTDA) for financial support under the Young Scientist and Technologist Programme (YSTP) grant no. SP54-MT06 (academic year 2011) and Assistant Professor Sarin Tadtong and Associate Professor Suwanna Vorarat for their technical assistance and helpful suggestions.
Abbreviations
- AM
Alpha-mangostin-rich extract
- BHI
Brain–heart infusion
- CFU
Colony-forming unit
- CO2
Carbon dioxide
- cm
Centimeter
- °C
Degree Celsius
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
Dimethylsulfoxide
- FBS
Fetal bovine serum
- FT-IR
Fourier transform infrared
- g
Gram
- gf
Gram force
- HCl
Hydrochloric acid
- HPLC
High-performance liquid chromatography
- i.e.
id est
- kg
Kilogram
- kV
Kilovolt
- mM
Millimolar
- MIC
Minimum inhibitory concentration
- min
Minutes
- mg
Milligram
- ml
Milliliter
- mm
Millimeter
- MTT
Methylthiazolydiphenyl-tetrazolium bromide
- N
Newton
- N
Normality
- NHFF
Normal human foreskin fibroblast
- nm
Nanometer
- Pa
Pascal
- PBS
Phosphate buffer saline
- pH
Potential of hydrogen
- r2
Coefficient of determination
- rpm
Round per minute
- s
Second
- SD
Standard deviation
- SEM
Scanning electron microscopy
- UV
Ultraviolet
- v/v
Volume by volume
- w/v
Weight by volume
- w/w
Weight by weight
- μg
Microgram
- μl
Microliter
- μm
Micrometer
- %
Percent
REFERENCES
- 1.Nguyen R, Su J. Treatment of acne vulgaris. Paediatr Child Health. 2011;21:119–125. doi: 10.1016/j.paed.2010.09.012. [DOI] [Google Scholar]
- 2.Grange PA, Weill B, Dupin N, Batteux F. Does inflammatory acne result from imbalance in the keratinocyte innate immune response? Microbes Infect. 2010;12:1085–1090. doi: 10.1016/j.micinf.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol. 2005;101:330–333. doi: 10.1016/j.jep.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Adalatkhah H, Pourfarzi F, Sadeghi-Bazargani H. Flutamide versus a cyproterone acetate-ethinyl estradiol combination in moderate acne: a pilot randomized clinical trial. Clin Cosmet Investig Dermatol. 2011;4:117–121. doi: 10.2147/CCID.S20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007;78:401–408. doi: 10.1016/j.fitote.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Sampath PD, Vijayaragavan K. Ameliorative prospective of alpha-mangostin, a xanthone derivative from Garcinia mangostana against β-adrenergic cathecolamine-induced myocardial toxicity and anomalous cardiac TNF-α and COX-2 expressions in rats. Exp Toxicol Pathol. 2008;60:357–364. doi: 10.1016/j.etp.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y. Alpha-mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci. 2004;95:33–40. doi: 10.1254/jphs.95.33. [DOI] [PubMed] [Google Scholar]
- 8.Azimi H, Fallah-Tafti M, Khakshur AA, Abdollahi M. A review of phytotherapy of acne vulgaris: perspective of new pharmacological treatments. Fitoterapia. 2012;83:1306–1317. doi: 10.1016/j.fitote.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder IZ, Franke P, Schaefer UF, Lehr C-M. Delivery of ethinylestradiol from film forming polymeric solutions across human epidermis in vitro and in vivo in pigs. J Control Release. 2007;118:196–203. doi: 10.1016/j.jconrel.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Ritterband DC, Meskin SW, Shapiro DE, Kusmierczyk J, Seedor JA, Koplin RS. Laboratory model of tissue adhesive (2-octyl cyanoacrylate) in sealing clear corneal cataract wounds. Am J Ophthalmol. 2005;140:1039–1043. doi: 10.1016/j.ajo.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 11.Singer AJ, Thode HC. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238–248. doi: 10.1016/j.amjsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Graham P, Browne L, Capp A, Fox C, Graham J, Hollis J, et al. Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys. 2004;58:241–246. doi: 10.1016/S0360-3016(03)01431-7. [DOI] [PubMed] [Google Scholar]
- 13.Zurdo Schroeder I, Franke P, Schaefer UF, Lehr C-M. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur J Pharm Biopharm. 2007;65:111–121. doi: 10.1016/j.ejpb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Guo R, Du X, Zhang R, Deng L, Dong A, Zhang J. Bioadhesive film formed from a novel organic–inorganic hybrid gel for transdermal drug delivery system. Eur J Pharm Biopharm. 2011;79:574–583. doi: 10.1016/j.ejpb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Silva-Weiss A, Bifani V, Ihl M, Sobral PJA, Gómez-Guillén MC. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013;31:458–466. doi: 10.1016/j.foodhyd.2012.11.028. [DOI] [Google Scholar]
- 16.Koh J-J, Qiu S, Zou H, Lakshminarayanan R, Li J, Zhou X, et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. BBA Biomembr. 1828;2013:834–844. doi: 10.1016/j.bbamem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Apu AS, Pathan AH, Shrestha D, Kibria G, Jalil R. Investigation of in vitro release kinetics of carbamazepine from Eudragit® RS PO and RL PO matrix tablets. Trop J Pharm Res. 2009;8:145–152. doi: 10.4314/tjpr.v8i2.44523. [DOI] [Google Scholar]
- 18.Horak Z, Fortelny I, Kolarik J, Hlavati D, Sikora A. (2005) Polymer blends. Encyclopedia of polymer science and technology, 4th ed. Wiley: New York. p. 1–59.
- 19.Lin S-Y, Lee C-J, Lin Y-Y. Drug–polymer interaction affecting the mechanical properties, adhesion strength and release kinetics of piroxicam-loaded Eudragit E films plasticized with different plasticizers. J Control Release. 1995;33:375–381. doi: 10.1016/0168-3659(94)00109-8. [DOI] [Google Scholar]
- 20.A-sasutjarit R, Sirivat A, Vayumhasuwan P. Viscoelastic properties of carbopol 940 gels and their relationships to piroxicam diffusion coefficients in gel bases. Pharm Res. 2005;22:2134–2140. doi: 10.1007/s11095-005-8244-2. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad M, Yamin BM, Mat Lazim A. A study on dispersion and characterisation of alpha-mangostin loaded pH sensitive microgel systems. Chem Cent J. 2013;7:85–90. doi: 10.1186/1752-153X-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawant PD, Luu D, Ye R, Buchta R. Drug release from hydroethanolic gels. Effect of drug's lipophilicity (logP), polymer–drug interactions and solvent lipophilicity. Int J Pharm. 2010;396:45–52. doi: 10.1016/j.ijpharm.2010.06.008. [DOI] [PubMed] [Google Scholar]


