Abstract
SN-38, an active metabolite of irinotecan, is up to 1,000-fold more potent than irinotecan. But the clinical use of SN-38 is limited by its extreme hydrophobicity and instability at physiological pH. To enhance solubility and stability, SN-38 was complexed with different cyclodextrins (CDs), namely, sodium sulfobutylether β-cyclodextrin (SBEβCD), hydroxypropyl β-cyclodextrin, randomly methylated β-cyclodextrin, and methyl β-cyclodextrin, and their influence on SN-38 solubility, stability, and in vitro cytotoxicity was studied against ovarian cancer cell lines (A2780 and 2008). Phase solubility studies were conducted to understand the pattern of SN-38 solubilization. SN-38-βCD complexes were characterized by differential scanning calorimetry (DSC), X-ray powder diffraction analysis (XRPD), and Fourier transform infrared (FTIR). Stability of SN-38-SBEβCD complex in pH 7.4 phosphate-buffered saline was evaluated and compared against free SN-38. Phase solubility studies revealed that SN-38 solubility increased linearly as a function of CD concentration and the linearity was characteristic of an AP-type system. Aqueous solubility of SN-38 was enhanced by about 30–1,400 times by CD complexation. DSC, XRPD, and FTIR studies confirmed the formation of inclusion complexes, and stability studies revealed that cyclodextrin complexation significantly increased the hydrolytic stability of SN-38 at physiological pH 7.4. Cytotoxicity of SN-38-SBEβCD complex was significantly higher than SN-38 and irinotecan in both A2780 and 2008 cell lines. Results suggest that SBEβCD encapsulated SN-38 deep into the cavity forming stable inclusion complex and as a result increased the solubility, stability, and cytotoxicity of SN-38. It may be concluded that preparation of inclusion complexes with SBEβCD is a suitable approach to overcome the solubility and stability problems of SN-38 for future clinical applications.
KEY WORDS: complexation, cyclodextrin, ovarian cancer, SN-38, solubility and stability enhancement
INTRODUCTION
SN-38 (7-ethyl-10-hydroxy-camptothecin), an active metabolite of irinotecan, is a topoisomerase I inhibitor and is up to 1,000-fold more potent than its parent drug. However, only about 2–8% of the prodrug is converted to SN-38 by carboxylesterases present in liver and cancer cells, and therefore, a high dose of irinotecan needs to be administered to get the desired therapeutic effect (1,2). For example Camptosar® has to be injected at a high dose of 150–180 mg/m2 intravenously over a period of 90 min to treat colorectal cancer. The conversion of irinotecan to SN-38 is highly variable among the patients. Direct administration of active metabolite SN-38 at very low dose could be the best alternative to irinotecan, but its clinical use is limited by its poor aqueous solubility and instability at physiological pH. The lactone ring of SN-38 hydrolyses at pH > 6 to give pharmacologically inactive carboxylate form (3).
Several approaches to overcome the problem of SN-38 solubility and stability have been proposed, including incorporation into nanoparticles (2,4,5), liposomes (6), and polymeric micelles (7–9), conjugating with water-soluble polymers like polyethylene glycol (10), PAMAM dendrimers (11), peptides (1), carbohydrates (12), etc. All these approaches involve tedious processing or synthetic steps and expert skills and industrialization or scale-up is also an important problem. To address this problem, we opted to develop SN-38-cyclodextrin complexes as they can be industrialized easily.
Cyclodextrins (CDs) are cyclic oligosaccharides with five to seven repeating α-d-glucopyranose units. There are more than 35 cyclodextrin-based formulations in the world market in about 14 different types of formulations including tablets, capsules, parenteral solutions, suppositories, nasal sprays, dermal products, ophthalmic products, etc. (13). Cyclodextrins have been extensively used to enhance the solubility, mask bitterness, improve stability, improve bioavailability, and decrease tissue irritation upon administration of a variety of poorly soluble drugs (13–15). Cyclodextrins have been shown to increase the cytotoxicity of several antineoplastic drugs like camptothecin (16,17), curcumin (18), doxorubicin, docetaxel (19), paclitaxel (20), etc. SN-38-βCD complex has been used to prepare in situ polymeric implants (21), but there was no detailed study reported on the effect of CDs on improving solubility, stability, and cytotoxicity of SN-38. In this study, we investigated the effects of four different types of modified β-cyclodextrins, namely, sodium sulfobutylether β-cyclodextrin (SBEβCD), hydroxypropyl β-cyclodextrin (HPβCD), randomly methylated β-cyclodextrin (RMβCD), and methylated β-cyclodextrin (MβCD) on the solubility, stability, and cytotoxicity of SN-38.
MATERIALS AND METHODS
Materials
SN-38 was purchased from AK Scientific, Union City, CA, USA. Sodium sulfobutyl ether β-cyclodextrin (Captisol®) was purchased from Cydex Pharmaceuticals, La Jolla, CA, USA. Randomly methylated β-cyclodextrin (Trappasol®) was purchased from Cyclodextrin Technologies Development Inc., Alachua, FL, USA. (2-Hydroxypropyl)-β-cyclodextrin, sodium carboxymethyl cellulose (NaCMC; average molecular weight Avg. Mw., 90 kDa), polyvinylpyrrolidone (PVP; Avg. Mw, 40 kDa), hydroxypropyl methylcellulose (HPMC; viscosity, 2,600–5,600 cp), methyl-β-cyclodextrin, and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich, St. Louis, MO, USA. Phosphate-buffered saline (PBS, pH 7.4) and fetal bovine serum (FBS) were purchased from Mediatech Inc., Manassas, VA, USA. All organic solvents used were of HPLC grade and purchased from VWR International.
Cell Culture
Human ovarian carcinoma cell line A2780 was kindly provided by Steve W. Johnson, Fox Chase Cancer Center, Philadelphia, PA and was cultured in RPMI-640 medium supplemented with 0.25 units/mL insulin. Human ovarian carcinoma cell line 2008 was provided by Dr. Qingxiu Zhang, Department of Molecular Therapeutics, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA and was cultured in RPMI-1640 medium. All the media were supplemented with 10% heat-inactivated FBS and 1% penicillin–streptomycin solution. Cells were incubated in a CO2 incubator at 37°C and 95% relative humidity. All cell lines used were of passage numbers 15–40.
HPLC Analysis
Quantification of SN-38 was performed on a reverse-phase HPLC system (Waters, Milford, MA, USA) with a UV–visible detector. A previously reported HPLC method that can analyze lactone and carboxylate forms of SN-38 simultaneously was used with modification of column temperature (22). In the reported method, column temperature was maintained at 40°C, whereas in this method, analysis was performed at room temperature (RT) without compromising on peak sharpness. The chromatographic separations were achieved using an Atlantis® 5 μm 4.6 × 250 mm C18 column at RT. An isocratic elution method of 1:1 acetonitrile/25 mM NaH2PO4 pH 3.1 buffer at a flow rate of 1 mL/min was used and absorbance was measured at λmax of 265 nm. Total run time was 10 min and elution time of SN-38 lactone form and carboxylate form were 3.92 and 2.58 min, respectively. The concentration of SN-38 from each sample was determined from the peak area observed, based on a calibration curve in the concentration range of 2–100 μg/mL (y = 79503x + 3907, R2 = 1).
Evaluation of Equilibration Time for Solubilization
Equilibration time for complexation and dissolution of SN-38 was evaluated by incubation of excess of SN-38 with cyclodextrin solution at RT in amber-colored vials on a laboratory shaker at 700 rpm for 5 days. To keep SN-38 in its active lactone form, cyclodextrin solutions were made with deionized (DI) water acidified with 0.02 N HCl to pH 3. After 24 h, 72 h, and 5 days of incubation, samples were removed, filtered through 0.45-μm Acrodisc® syringe filter (Pall Life Sciences, Port Washington, NY, USA), and analyzed for SN-38 concentration by HPLC (n = 3).
Phase Solubility Study
Phase solubility study was carried out by the method reported by Higuchi and Connors (23). Excess of SN-38 was added to 1 mL of different concentrations of cyclodextrin solution in amber-colored vials. The mixture was sonicated for 15 min (Branson 1510 ultrasonic cleaner, Ultrasonics) and rotated on a laboratory shaker at 700 rpm for 24 h. After equilibrium was reached, undissolved SN-38 was removed by filtering through a 0.45-μm filter. The clear solution after suitable dilution was analyzed by HPLC (n = 3). All the solutions and dilutions were made with DI water, and pH was adjusted to 3.0 using 0.02 N HCl to keep SN-38 in its active lactone form. The phase solubility diagram was constructed by plotting concentration of dissolved SN-38 against cyclodextrin concentration. The apparent stability constant (KS) of the complex was calculated from the linear portion of the phase solubility diagrams using the following equation:
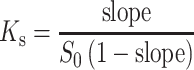 |
where S0 is the intrinsic solubility (or y-intercept) of the drug.
Differential Scanning Calorimetry Studies
Differential scanning calorimetry (DSC) analysis was performed using a Q200 DSC apparatus (TA Instruments, New Castle, DE, USA). Five to eight milligrams of sample was sealed in aluminum pans and scanned from 0°C to 400°C at a ramp rate of 10°C/min under a nitrogen purge at a flow rate of 50 mL/min. An empty aluminum pan was used as the reference.
X-ray Diffraction (XRD) Studies
X-ray powder diffraction patterns of samples were obtained using a diffractometer (Bruker X8, Billerica, MA, USA) equipped with an Apex II CCD detector. The X-ray source was Kα radiation from a copper target with graphite monochromater with a wavelength of 1.54 Å. The sample was prepared by pressing the powder into a pellet, which was fixed to a 0.3-mm cryo-loop (Hampton Research, Aliso Viejo, CA). Since the sample was not suspended in any matrix (oil, glass, etc.), there was no background to interfere with the signal. The range (2θ) of scans was from 5° to 60° at a speed of 2°/min.
Fourier Transform Infrared (FTIR) Spectroscopy
Infrared spectra of SN-38, SBEβCD, RMβCD, and their complexes were recorded on Shimadzu Fourier transform infrared spectrophotometer (IRAffinity-1) by potassium bromide disk method. Samples were mixed with dry powdered potassium bromide and compressed into transparent disk under high pressure and the spectra were recorded in the wave number region 4,000–600 cm−1.
Effect of Hydrophilic Polymers on Cyclodextrin Solubilization
Effect of three hydrophilic polymers on the SBEβCD solubilization of SN-38 was studied. Hydrophilic polymers and their concentrations were selected from the literature (24,25). Three different polymers PVP, NaCMC, and HPMC at concentrations of 0.25% and 0.5% w/v were used for this experiment. Briefly, excess of SN-38 was added to 1 mL of 20% or 40% w/v cyclodextrin solution with 0%, 0.25%, and 0.5% w/v hydrophilic polymer in amber-colored vials. The mixture was sonicated for 15 min and rotated on a laboratory shaker at 700 rpm. After 24 h, undissolved SN-38 was removed by filtering through a 0.45-μm filter. The clear solution after suitable dilution was analyzed by HPLC (n = 3) to determine the solubility of SN-38. All the solutions and dilutions were made with DI water, and pH was adjusted to 3.0 using 0.02 N HCl to keep SN-38 in its active lactone form.
Effect of Cyclodextrin Complexation on SN-38 Stability
The hydrolytic stability of SN-38 and SN-38-SBEβCD complex was assessed in pH 7.4 PBS at RT. To determine the stability of SN-38, 30 μL of SN-38 stock solution in DMSO (100 μg/mL) was added to 3 mL of PBS to get a final concentration of 1 μg/mL and incubated at RT. At different time points, samples were withdrawn and analyzed for the amount of lactone and carboxylate form by HPLC. The concentration of DMSO in the final solution was less than 1%. To determine the stability of SN-38-SBEβCD complex, lyophilized complex was dissolved in PBS to get a final cyclodextrin concentration of 1%, 2.5%, and 5% w/v. At various time points, samples were withdrawn and analyzed by HPLC. As reported earlier (26), all the stability samples were analyzed directly without any further dilutions. The percentage of SN-38 converted to inactive carboxylate form was plotted against time.
Molecular Modeling
Computer-aided modeling has been successfully applied in the study of host–guest interactions of cyclodextrin–drug inclusion complexes. To facilitate our study of the inclusion complex of SN-38 and SBEβCD, we carried out simulation of the interaction of SN-38 with SBEβCD. The 3-dimensional (3D) structure of SBEβCD was constructed by homology modeling from a reported β-cyclodextrin (βCD) X-ray crystal structure, which was obtained in a complex with α-amylase. The 3D structure of α-amylase-βCD was retrieved from the protein data bank (pdb id, 1j18). The 3D structure of βCD (Fig. 1a) was then extracted through Accelyrs Discovery Studio v3.5 [Accelrys Inc., San Diego, CA (2013)]. The resulted βCD 3D structure was thus employed as the template for homology modeling of SBEβCD. The sulfobutylether (SBE) groups of SBEβCD were grafted onto the hydroxyl groups of βCD. In construction of the SBEβCD homology model, four SBE groups were positioned on the C6-OH of the glucose subunit, and three SBE groups were placed on the C2-OH of the glucose subunit such that each glucose subunit has one SBE substitution (Fig. 1b). This was performed intentionally to reduce the steric hindrance or clashes from the addition of bulky sulfobutylether groups. The constructed SBEβCD was then optimized by Clean Geometry of the Discovery Studio 3.5 to further reduce the potential steric hindrance or clashes from those added SBE groups (Fig. 1c). The 3D structure of SN-38 was sketched through Chem3D Ultra 8.0 [Cambridge Soft Corporation, USA (2003)] and was energetically minimized by using MOPAC with 100 iterations and minimum RMS gradient of 0.10. Following the preparations of 3D structures of SBEβCD and SN-38, the drug molecule was docked into the SBEβCD model through Autodock 4.2, which screens for the proper binding mode of the guest (SN-38) in the host structure (SBEβCD), using pre-computed grids that was generated by Autogrid 4.0 and then a genetic algorithm modified with a local search to evaluate the guest-host binding free energy (ΔGb). The results from 50 randomly seeded runs were analyzed for possible conformations of the docked guest (SN-38) in the structure of host (SBEβCD). The closely related conformations were clustered together when their atomic coordinates exhibited a root mean square deviation less than 0.5 Å from each other. The conformation clusters were ranked by their averaged free binding energy (ΔGb) calculated from each member of the cluster. Only the top 10 clusters were analyzed through Accelyrs Discovery Studio for their binding interactions between the guest and the host, such as H-bonding and hydrophobic interactions. The conformation cluster with the lowest ΔGb was then taken as the most stable inclusion complex between the drug SN-38 and SBEβCD.
Fig. 6.
An overlay of FTIR spectra of a SN-38, SBEβCD, and SN-38-SBEβCD and b SN-38, RMβCD, and SN-38-RMβCD
In Vitro Cytotoxicity Studies
Cytotoxicity of SN-38-SBEβCD complex on ovarian cancer cell lines A2780 and 2008 was evaluated by MTT assay (27–29) and compared with the cytotoxicity of SN-38 and irinotecan. As SN-38 is not soluble in water, a stock solution was made in DMSO and suitable dilutions were made in growth medium, keeping the final DMSO concentration less than 0.1%. Briefly, 1–1.4 × 104 cells per well were plated in a 96-well pate. After 24 h of incubation, cells were treated with different concentrations (0.01 nM to 10 μM) of SN-38 or irinotecan or SN-38-SBEβCD complex and incubated for 48 and 72 h. Cells were then washed with PBS and 50 μL of 0.5 mg/mL MTT solution in serum-free medium was added. After 4 h of incubation, 150 μL of DMSO was added to dissolve the formazan crystals, and the optical density was measured using a microplate reader at 570 nm. The results are expressed as the percentage of cell viability as compared to the control cells (cells grown with media having no drug), and the IC50 was determined using GraphPad Prism 5 software (San Diego, CA, USA).
Statistical Analysis
Data are represented as mean ± SD from three separate experiments. Statistical analysis was performed using two-way ANOVA with general linear model (GraphPad Prism 6 software) followed by multiple comparison. A value of p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The primary goal of a formulation scientist is to develop a simple, economical, and easily industrializable formulation that can address the problems associated with an active pharmaceutical ingredient. Cyclodextrins have proved to be the best complexing agents and have been used in many marketed formulations to improve solubility, stability, and bioavailability. In comparison to natural CDs, modified β-CDs have been used widely in pharmaceutical formulations due to their improved solubility. In this study, we evaluated four different types of modified β-cyclodextrins, namely, SBEβCD, HPβCD, RMβCD, and MβCD, to enhance the solubility and stability of SN-38. The first two CDs, SBEβCD (Captisol®) and HPβCD, were chosen to develop intravenous formulation as they were approved by FDA for intravenous delivery and have been used in many marketed formulations. Other two methylated β-cyclodextrins, RMβCD and MβCD, were opted to develop a formulation for oral delivery, as they have better permeability in comparison to other cyclodextrins. RMβCDs have been used in nasal and ophthalmic formulations so far (13,30).
Evaluation of Equilibration Time for Solubilization
Amount of SN-38 dissolved per milliliter of cyclodextrin solution was plotted. As shown in Fig. 2, SN-38 solubility reached maximum after 24 h of incubation and was not increased even after 5 days of incubation, indicating that equilibration was achieved in 24 h. The same 24-h equilibration time was observed with all four types of cyclodextrins (SBEβCD, HPβCD, RMβCD, and MβCD) tested. Equilibration time of complexation/solubilization of SN-38 with cyclodextrins was not reported thus far. Equilibration time for SN-38 with four different CDs tested was identified as 24 h, and even after 5 days of incubation, the solubility was not enhanced further. This is consistent with the results obtained with a similar molecule, camptothecin, and was reported as <5 days (16,17). According to Saetern et al. (16), the equilibration between camptothecin and HPβCD was achieved in 3 days, but the equilibration was not tested before 3 days. So for each type of CD and the drug, the equilibration time has to be evaluated before proceeding to phase solubility studies to avoid a long incubation period.
Fig. 2.
Concentration of SN-38 in aqueous solutions of a SBEβCD, b HPβCD, c RMβCD, and d MβCD vs. incubation time (mean ± SD, n = 3)
Phase Solubility Study
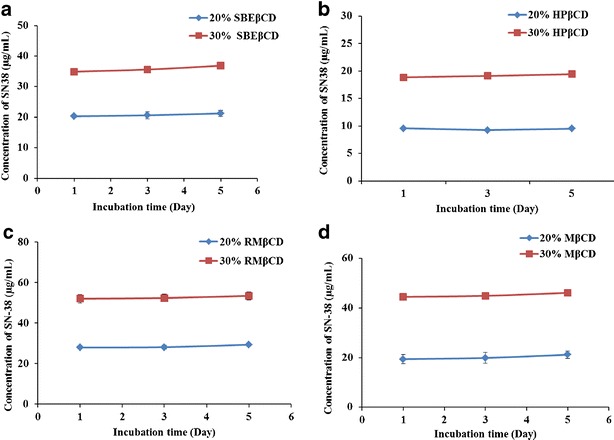
The phase solubility diagrams for the complex formation between different types of cyclodextrins and SN-38 are shown in Fig. 3. This plot shows that the aqueous solubility of the SN-38 increased linearly with cyclodextrin concentration with all the four types of cyclodextrins tested. According to Higuchi and Conner, the obtained phase solubility profiles can be classified as AP type (13), suggesting the formation of 1:1 complex formation at lower concentration (<20 μM) and 1:2 or higher drug/cyclodextrin complex formation at higher concentration, >20 μM. The apparent stability constant (KS) of the complexes was calculated from the initial linear portion of phase solubility plots. The KS values of the SN-38-SBEβCD, SN-38-HPβCD, SN-38-RMβCD, and SN-38-MβCD were 1,200.72, 500.05, 500.10, and 500.10 M−1, respectively.
Fig. 3.
Phase solubility diagram of SN-38 in water (pH adjusted to 3 with 0.02 N HCl) at room temperature (mean ± SD, n = 3)
From the solubility studies, the solubility of SN-38-CD complex was found in the order of SBEβCD > RMβCD > MβCD > HPβCD. The strength of the complex can be quantified as the apparent stability constants (KS), which can be calculated from the slope of intrinsic solubility of graph (24). The intrinsic solubility (S0) of SN-38 obtained from the y-intercept of phase solubility curves was ranged from 0.17 to 0.08 μg/mL. KS of the cyclodextrin-SN-38 complexes was found to be in the order of SBEβCD > RMβCD ≥ MβCD > HPβCD. The aqueous solubility of SN-38 was reported differently by various groups (3,31). But from solubility experiment, we found that the water solubility of pure SN-38 was 0.08 μg/mL, which is consistent with one of the previous reports (32). The experimental solubility was also in-line with the intrinsic solubility (S0) obtained from phase solubility curves. The solubility of SN-38 increased by 1,425, 1,038, 880, and 34.62 times with 40% solutions of RMβCD, MβCD, SBEβCD, and HPβCD, respectively.
DSC
Physicochemical properties of free drug molecules are different from those complexed with CDs, and they can be characterized by techniques like DSC, XRD, and FTIR. In differential scanning calorimetry, the alterations or shifts in the exo- and endothermic peaks of drug and drug–polymer inclusion complexes were studied, whereas X-ray diffraction analysis was used to analyze crystalline and amorphous nature of the same. IR spectroscopy analysis gives unique fingerprint pattern of peaks with each type of the molecule (18).
The DSC thermograms of SN-38, SBEβCD, RMβCD, their complexes SN-38-SBEβCD, and SN-38-RMβCD are shown in Fig. 4. SN-38 showed many characteristic sharp melting endothermic peaks at 282.5°C and 318°C and exothermic peaks at 235.12°C and 286.35°C. SBEβCD showed a broad endothermic peak followed by a broad exothermic peak between 267.5°C and 295.8°C. DSC thermogram of SN-38-SBEβCD was similar to the DSC thermogram of SBEβCD, and characteristic peaks of SN-38 completely disappeared, which indicates that SN-38 molecule probably included into the cavity of SBEβCD forming inclusion complex (Fig. 4a). Similar results were obtained with SN-38-RMβCD complex. RMβCD showed a broad endothermic melting peak between 340°C and 360°C, whereas SN-38-RMβCD complex showed no characteristic peaks of SN-38 indicating the formation of a stable inclusion complex (Fig. 4b).
Fig. 4.
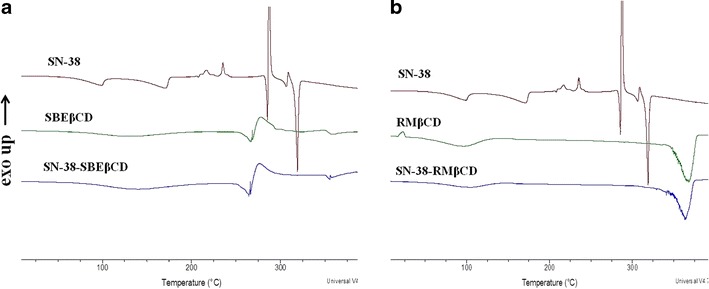
An overlay of differential scanning calorimetry thermograms of a SN-38, SBEβCD, and SN-38-SBEβCD and b SN-38, RMβCD, and SN-38-RMβCD
X-ray Diffraction Studies
X-ray diffraction patterns of SN-38, SBEβCD, RMβCD, their physical mixtures, and complexes are depicted in Fig. 5. X-ray diffractogram of SN-38 showed numerous sharp peaks indicating the crystalline nature of SN-38. Some of the intense peaks were at diffraction angles (2θ) of ∼10°, 13°, 24°, and 26° with approximate intensities of 968, 612, 887, and 895, respectively. As reported earlier, SBEβCD (33) and RMβCD (15) showed amorphous diffraction patterns. Diffractograms of SN-38-SBEβCD and SN-38-RMβCD complexes were very similar to their corresponding cyclodextrin diffractograms with no sharp peaks of SN-38 suggesting the formation of stable inclusion complexes, whereas their physical mixtures showed some characteristic peaks of SN-38 suggesting the presence of free drug. All the XRD results were in good agreement with those obtained by DSC studies confirming the complexation of SN-38 with cyclodextrins in the solid state.
Fig. 5.
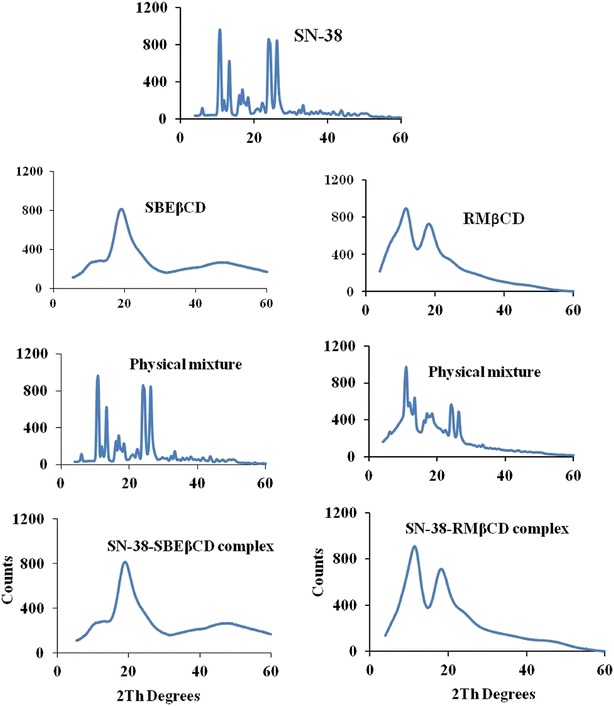
X-ray powder diffraction spectra of SN-38, SBEβCD, RMβCD, their physical mixtures, and complexes, SN-38-SBEβCD and SN-38-RMβCD
IR Spectroscopy
The IR spectra of SN-38 and the cyclodextrin complexes are shown in Fig. 6. SN-38 showed sharp absorption band at 1,732 cm−1 (stretching vibrations of C=O), multiple sharp peaks between 1,600 and 1,400 cm−1 (aromatic C=C stretching), strong absorption band at 1,168 cm−1 (C–O stretching), and multiple absorption bands between 750 and 900 cm−1 (=C–H stretching). Both SBEβCD and RMβCD showed characteristic absorption peaks for –OH (3,200–3,700 cm−1), C–O (1,050–1,150 cm−1), and C–H (1,350–1,480 cm−1) stretching vibrations. The FTIR spectra of SN-38-SBEβCD and SN-38-RMβCD complexes were almost similar to the spectra of the respective cyclodextrins with most of the SN-38 characteristic peaks which disappeared or appeared with reduced intensity, which reiterate the formation of inclusion complexes.
Fig. 1.
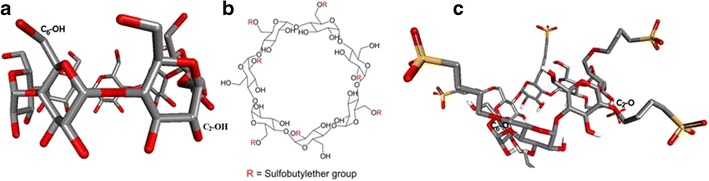
Construction of SBEβCD model: a 3D structure of βCD extracted from X-ray crystal structure of α-amylase-βCD complex; b 2D structure of the SBEβCD employed in the construction of 3D model of SBEβCD; c 3D model of SBEβCD
As motioned earlier, we selected methylated CDs for oral permeability of SN-38, whereas HPβCD and SBEβCD were selected for parenteral delivery of SN-38. Short-term toxicity studies (2 h incubation time) of methylated CDs, RMβCD, and MβCD on CaCo-2 cells revealed that they are very toxic at concentrations ≥50 μM and their toxicity was 10 times greater than pure SN-38 (data not shown). HPβCD and SBEβCD are generally considered as safe and approved by FDA. SBEβCD (Captisol®) has been approved by FDA for use in parenteral formulations up to 67.5% w/w (http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm). Based on the reported safety and solubility data, SN-38-SBEβCD complex was selected for further studies.
Molecular Modeling
The 3D structure of βCD extracted through Accelrys Discovery Studio v3.5 [Accelrys Inc., San Diego, CA (2013)] was employed as the template for homology modeling of SBEβCD. SBEβCD homology model was constructed with four SBE groups positioned on the C6-OH of the glucose subunit and three SBE groups placed on the C2-OH of the glucose subunit to reduce the steric hindrance from the addition of bulky sulfobutylether groups.
Our docking studies showed that SN-38 can interact with SBEβCD with a negative binding free energy (ΔGb = −8.83 kcal/mol), which indicated the formation of a stable inclusion complex. The inclusion complex is stabilized at least by three hydrogen bonds formed between (1) C20-OH and the O47 of SBEβCD, (2) C10-OH of SN-38 and the O=S from one of the SBE groups, and (3) C16a-C=O group of SN-38 and H56 of SBEβCD. These H-bonding interactions are shown in Fig. 7a. The H-bonding parameters are shown in Table I.
Fig. 7.
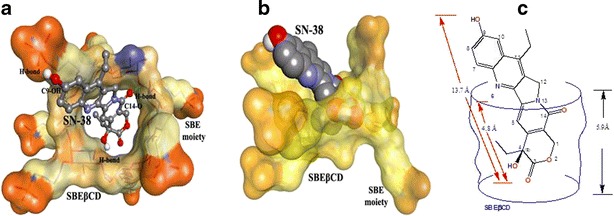
a Result of docking SN-38 (ball and stick; colored by element) into SBEβCD covered by solid surface). The docking revealed three H-bonds, which are illustrated by green dotted lines; b SN-38 (carbons: gray colored, oxygens: red colored, nitrogens: blue colored) enclosed in the SBEβCD cavity formed by cyclic oligoscaccharides. The sulfonic groups of sulfobutyl ether moieties are shown in orange color; c SN-38 sketched as shown in the cavity of SBEβCD (blue lines)
Table I.
The Result of SN-38 Docked into SBEβCD
| ΔG b a (kcal/mol) | Hydrogen bonds between SN-38 and SBEβCD | |||
|---|---|---|---|---|
| Atoms of SN-38 | Atoms of SBEβCD | H-bond distance (Å) | Angle (°) | |
| −8.83 | C4-OH | O47 | 1.94 | 126.6 |
| C9-OH | O11 | 1.84 | 158.9 | |
| C-14 C=O | H-56 | 2.59 | 90.9 | |
aBinding free energy
Our docking studies also revealed that the SN-38 interacts with SBEβCD in a way that its δ-lactone ring moiety is imbedded in the cavity of SBEβCD (Fig. 7b). The longest dimension of SN-38 is 13.7 Å, of which 4.8 Å portion is enclosed in the cavity of SBEβCD whose cavity has a 5.9-Å depth (Fig. 7c). The enclosure of the δ-lactone ring moiety of SN-38 in SBEβCD may contribute to the enhancement of stability of the complex of SN-38-SBEβCD in comparison with the free SN-38 molecule, which is vulnerable to degradation due to the hydrolysis of its δ-lactone ring. The δ-lactone ring of SN-38 in the complex of SN-38-SBEβCD is shielded from hydrolytic attacks in aqueous environment at physiological pH.
Our docking results are in agreement with the results from FTIR (Fig. 6) of the SN-38-SBEβCD complex. The formation of hydrogen bonding generally leads to significant band broadening and to lower mean absorption frequency (34) . The OH stretching band at 3,285 cm−1 is significantly reduced in the SN-38-SBEβCD complex in comparison with the free SN-38. The C=O stretching bands (1,657 and 1,636 cm−1) from C-16a C=O of the six-member ring lactam in SN-38 is also reduced and broadened in the SN-38-SBEβCD complex. These are likely due to the restriction in the vibrations from hydrogen bonding during complexation with SBEβCD.
Effect of Hydrophilic Polymers on Cyclodextrin Solubilization
Cyclodextrin solubilization values of SN-38 in presence of hydrophilic polymers are shown in Fig. 8. Forty percent SBEβCD solubilized 50 μg/mL of SN-38 without the presence of any hydrophilic polymer. Upon addition of hydrophilic polymers (PVP, NaCMC, and HPMC), the solubility was decreased significantly (p < 0.05). Twenty percent SBEβCD solubilized 19 μg/mL of SN-38 without the presence of any hydrophilic polymer. Upon addition of PVP and 0.25% NaCMC, the solubility was not altered, whereas addition of HPMC decreased SN-38 solubility significantly (p < 0.05). Furthermore, presence of 0.5% NaCMC increased the solubility of SN-38 (p < 0.05).
Fig. 8.
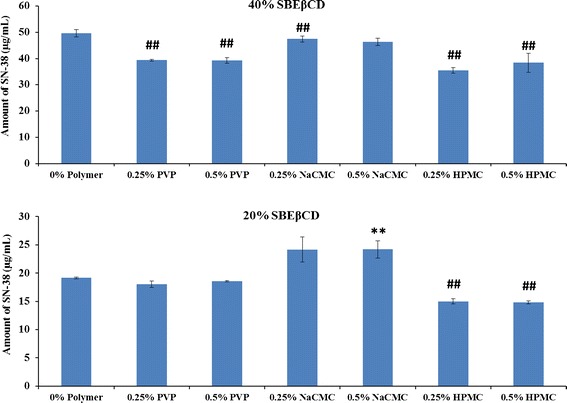
Effect of hydrophilic polymers on cyclodextrin solubilization of SN-38. The solubilization of SN-38 by SBEβCD in the presence of three different hydrophilic polymers PVP, NaCMC, and HPMC at concentration of 0% (with no polymer), 0.25%, and 0.5% w/v was plotted. **: Increased solubility in comparison to 0% polymer sample (p < 0.05); ##: Decreased solubility in comparison to 0% polymer sample (p < 0.05)
Hydrophilic polymers at lower concentration (0.1–1% w/v) have been reported to enhance the complexation efficiency of cyclodextrins. Both the hydrophilic polymers and CDs can form water-soluble complexes with hydrophobic drugs, but when used in combination, a synergistic solubilization effect can be observed (24). In this study, at 40% w/v concentration of SBEβCD, the solubility of SN-38 did not increase with any of the three polymers tested, but a decrease in SN-38 solubility was observed. At lower concentration (20% w/v) of SBEβCD, the solubility of SN-38 increased by NaCMC, but not by the other two polymers used. The exact reason for the decreased solubility of SN-38 in the presence of hydrophilic polymers has to be investigated yet, and for that, the intrinsic solubility of SN-38 in the presence of those polymers has to be assessed. Decrease or increase in solubility of SN-38 depends on the solubility of the formed complex as both water-soluble and insoluble complexes with polymers were reported (35,36).
Effect of Cyclodextrin Complexation on SN-38 Stability
Stability issues can limit the feasibility of a pharmaceutical formulation (37). Inclusion of chemically labile drug molecules into cyclodextrin cavity has shown to improve their stability (13). Kang et al. (17) showed that complexation of camptothecin with randomly methylated β-CDs increased its stability by 10 times. The effect of SN-38 stability on CD complexation has not been reported yet. In this study, the stability of SN-38-SBEβCD complex was studied and compared against free SN-38 in pH 7.4 PBS. Hydrolytic stability of SN-38-SBEβCD complex at physiological pH was assessed by incubating with pH 7.4 PBS and compared with the stability of free SN-38. As shown in Fig. 9, free SN-38 hydrolyzed rapidly to its inactive carboxylate form, whereas its hydrolysis rate was significantly reduced upon complexation with SBEβCD. Even at very low concentrations (1% to 5%), SBEβCD increased the stability of SN-38 by ∼5 times, after 2 h of incubation. Similarly, even after 24 h of incubation, about 70% of SN-38 complexed with SBEβCD was in its stable lactone form. This experiment clearly indicates that cyclodextrin complexation significantly increased the stability of SN-38.
Fig. 9.
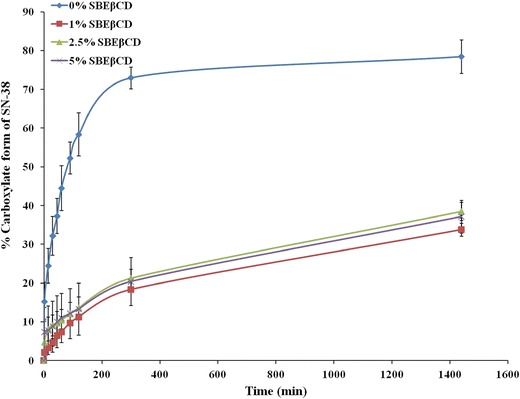
Stability profile of SN-38 complexed with SBEβCD at concentration of 1%, 2.5%, and 5% w/v in pH 7.4 PBS for a period of 24 h in comparison to free SN-38 (0% SBEβCD). Percentage of SN-38 converted to its inactive carboxylate form was plotted against incubation time in minutes
In Vitro Cytotoxicity
Concentration for inhibition of 50% cell (IC50) values of SN-38, irinotecan, and SN-38-SBEβCD complex in ovarian cancer cell lines A2780 and 2008 are shown in Table II. IC50 values of SN-38 following 48 and 72 h of incubation with A2780 cell line were 54 and 286 times lower, respectively, in comparison to irinotecan, whereas in 2008 cells, the IC50 values of SN-38 were about 188 and 480 times lower, respectively, in comparison to irinotecan at the same conditions (p < 0.05). As shown in Table II, the cytotoxicity of SN-38-SBEβCD complex was higher than SN-38 and irinotecan in both A2780 and 2008 cell lines after 48 and 72 h of incubation. In A2780 cells after 48 h of incubation, the IC50 value of SBEβCD is 21.42 ± 1.09 nM, which is about 54.5 and 3.5 times lesser than irinotecan (76.07 ± 0.50 nM) and SN-38 (4.15 ± 0.109 μM), respectively (p < 0.05). In 2008 cells after 48 h of incubation, the IC50 value of SBEβCD is 133.30 ± 2.82 nM, which is about 750 times lesser than that of irinotecan (26.38 ± 0.844 μM) and almost similar to that of SN-38 (140.27 ± 3.06 nM), respectively.
Table II.
Half Maximal Inhibitory Concentrations (IC50) of Irinotecan, SN-38, and SN-38-SBEβCD Against Ovarian Cancer Cell Lines, A2780 and 2008, After 48 and 72 h of Incubation (Mean ± SD, n = 3)
| 48 h incubation | 72 h incubation | |
|---|---|---|
| (IC50) | (IC50) | |
| A2780 cells | ||
| Irinotecan | 4.15 ± 0.109 μM | 2.21 ± 0.22 μM |
| SN-38 | 76.07 ± 0.50 nM* | 7.72 ± 0.50 nM* |
| SN-38-SBEβCD complex | 21.42 ± 1.09 nM** | 4.33 ± 0.26 nM** |
| 2008 cells | ||
| Irinotecan | 26.38 ± 0.844 μM | 12.88 ± 0.34 μM |
| SN-38 | 140.27 ± 3.06 nM* | 31.66 ± 0.79 nM* |
| SN-38-SBEβCD complex | 133.30 ± 2.82 nM*** | 29.69 ± 1.02 nM*** |
* p < 0.0001 in comparison to irinotecan, ** p < 0.001 in comparison to SN-38, *** p < 0.05 in comparison to SN-38
In vitro cytotoxicity studies revealed the greater cytotoxicity of SN-38 in comparison to irinotecan in both the ovarian cancer cell lines, A2780 and 2008. Though all the compounds found to inhibit the proliferation of cancer cells, the greater cytotoxicity of SN-38-SBEβCD complex in comparison to SN-38 may be attributed to the increased stability of SN-38 by complexation with CDs. This could be because the complexed form of SN-38, which is in equilibrium with the free SN-38, is less prone to hydrolysis and hence serves as a depot for continuous supply of SN-38 in its active lactone form (26).
CONCLUSION
In this study, we found that SN-38 forms stable inclusion complexes with cyclodextrins and its solubility increased 30–1,400-fold by complexation and solubility increased linearly with concentration of CDs. The SBEβCD increased the stability of SN-38 significantly and the cytotoxicity results suggest that the complexation between SN-38 and CDs also improves the antiproliferative activity of SN-38 on ovarian cancer cells. From the results presented above, it can be concluded SBEβCD could be the better complexing agent for the delivery of SN-38.
Acknowledgments
This work was partially supported by Texas A&M Health Science Center Cancer Research Council Seed Grant to SP.
Conflict of Interest
Authors declare no conflict of interest in this work.
References
- 1.Meyer-Losic F, Nicolazzi C, Quinonero J, Ribes F, Michel M, Dubois V, et al. DTS-108, a novel peptidic prodrug of SN38: in vivo efficacy and toxicokinetic studies. Clin Cancer Res. 2008;14(7):2145–53. doi: 10.1158/1078-0432.CCR-07-4580. [DOI] [PubMed] [Google Scholar]
- 2.Venkata K Yellepeddi, Kiran K Vangara, Srinath Palakurthi. In-vivo efficacy of PAMAM dendrimers-cisplatin complexes in SKOV-3 xenografted Balb/c nude mice. J Biotechnol Biomater. 2013, 3(2): S13:003.
- 3.Zhao H, Rubio B, Sapra P, Wu D, Reddy P, Sai P, et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem. 2008;19(4):849–59. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahimnejad P, Dinarvand R, Sajadi A, Jaafari MR, Nomani AR, Azizi E, et al. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomedicine. 2010;6(3):478–85. doi: 10.1016/j.nano.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Kiran KV, Jingbo LL, Srinath P. Hyaluronic Acid-decorated PLGA-PEG Nanoparticles for Targeted Delivery of SN-38 to Ovarian. Anticancer Res. 2013, 33(6): 2425–34. [PubMed]
- 6.Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, et al. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 2004;270(1–2):93–107. doi: 10.1016/j.ijpharm.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Gu Q, Xing JZ, Huang M, He C, Chen J. SN-38 loaded polymeric micelles to enhance cancer therapy. Nanotechnology. 2012;23(20):205101. doi: 10.1088/0957-4484/23/20/205101. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Ohkohchi N, Yasunaga M, Kuroda J, Koga Y, Kenmotsu H, et al. Detailed distribution of NK012, an SN-38-incorporating micelle, in the liver and its potent antitumor effects in mice bearing liver metastases. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(19):4822–31. doi: 10.1158/1078-0432.CCR-10-1467. [DOI] [PubMed] [Google Scholar]
- 9.Carie A, Rios-Doria J, Costich T, Burke B, Slama R, Skaff H, et al. IT-141, a polymer micelle encapsulating SN-38. Induces tumor regression in multiple colorectal cancer models. J Drug Deliv. 2011;2011:869027. doi: 10.1155/2011/869027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patnaik A, Papadopoulos KP, Tolcher AW, Beeram M, Urien S, Schaaf LJ, et al. Phase I dose-escalation study of EZN-2208 (PEG-SN38), a novel conjugate of poly(ethylene) glycol and SN38, administered weekly in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;71(6):1499–506. doi: 10.1007/s00280-013-2149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayalakshmi N, Ray A, Malugin A, Ghandehari H. Carboxyl-terminated PAMAM-SN38 conjugates: synthesis, characterization, and in vitro evaluation. Bioconjug Chem. 2010;21(10):1804–10. doi: 10.1021/bc100094z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafino A, Zonfrillo M, Andreola F, Psaila R, Mercuri L, Moroni N, et al. CD44-targeting for antitumor drug delivery: a new SN-38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosis. Curr Cancer Drug Targets. 2011;11(5):572–85. doi: 10.2174/156800911795655976. [DOI] [PubMed] [Google Scholar]
- 13.Loftsson T, Jarho P, Masson M, Jarvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2(2):335–51. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]
- 14.Battu SK, Repka MA, Maddineni S, Chittiboyina AG, Avery MA, Majumdar S. Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech. 2010;11(3):1466–75. doi: 10.1208/s12249-010-9520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sathigari S, Chadha G, Lee YH, Wright N, Parsons DL, Rangari VK, et al. Physicochemical characterization of efavirenz-cyclodextrin inclusion complexes. AAPS PharmSciTech. 2009;10(1):81–7. doi: 10.1208/s12249-008-9180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saetern AM, Nguyen NB, Bauer-Brandl A, Brandl M. Effect of hydroxypropyl-beta-cyclodextrin-complexation and pH on solubility of camptothecin. Int J Pharm. 2004;284(1–2):61–8. doi: 10.1016/j.ijpharm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Kang J, Kumar V, Yang D, Chowdhury PR, Hohl RJ. Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur J Pharm Sci. 2002;15(2):163–70. doi: 10.1016/S0928-0987(01)00214-7. [DOI] [PubMed] [Google Scholar]
- 18.Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV, et al. Inclusion complex of novel curcumin analogue CDF and beta-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012;29(7):1775–86. doi: 10.1007/s11095-012-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosse PY, Bressolle F, Pinguet F. In vitro modulation of doxorubicin and docetaxel antitumoral activity by methyl-beta-cyclodextrin. Eur J Cancer. 1998;34(1):168–74. doi: 10.1016/S0959-8049(97)00351-1. [DOI] [PubMed] [Google Scholar]
- 20.Bouquet W, Boterberg T, Ceelen W, Pattyn P, Peeters M, Bracke M, et al. In vitro cytotoxicity of paclitaxel/beta-cyclodextrin complexes for HIPEC. Int J Pharm. 2009;367(1–2):148–54. doi: 10.1016/j.ijpharm.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Manaspon C, Nittayacharn P, Vejjasilpa K, Fongsuk C, Nasongkla N. SN-38:beta-cyclodextrin inclusion complex for in situ solidifying injectable polymer implants. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3241–4. doi: 10.1109/IEMBS.2011.6090881. [DOI] [PubMed] [Google Scholar]
- 22.Xuan T, Zhang JA, Ahmad I. HPLC method for determination of SN-38 content and SN-38 entrapment efficiency in a novel liposome-based formulation, LE-SN38. J Pharm Biomed Anal. 2006;41(2):582–8. doi: 10.1016/j.jpba.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Ellison G, Klinowska T, Westwood RF, Docter E, French T, Fox JC. Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol. 2002;55(5):294–9. doi: 10.1136/mp.55.5.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loftsson T, Brewster ME. Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci. 2012;101(9):3019–32. doi: 10.1002/jps.23077. [DOI] [PubMed] [Google Scholar]
- 25.Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302(1–2):18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Kumar V, Yang D, Chowdhury PR, Hohl RJ. Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur J Pharm Sci Off J Eur Fed Pharma Sci. 2002;15(2):163–70. doi: 10.1016/s0928-0987(01)00214-7. [DOI] [PubMed] [Google Scholar]
- 27.Yellepeddi VK, Vangara KK, Kumar A, Palakurthi S. Comparative evaluation of small-molecule chemosensitizers in reversal of cisplatin resistance in ovarian cancer cells. Anticancer Res. 2012;32(9):3651–8. [PubMed] [Google Scholar]
- 28.Kumar A, Yellepeddi VK, Vangara KK, Strychar KB, Palakurthi S. Mechanism of gene transfection by polyamidoamine (PAMAM) dendrimers modified with ornithine residues. J Drug Target. 2011;19(9):770–80. doi: 10.3109/1061186X.2011.568061. [DOI] [PubMed] [Google Scholar]
- 29.Jwala J, Boddu SH, Shah S, Sirimulla S, Pal D, Mitra AK. Ocular sustained release nanoparticles containing stereoisomeric dipeptide prodrugs of acyclovir. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2011;27(2):163–72. doi: 10.1089/jop.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62(11):1607–21. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 31.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69(13):5292–3. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 32.Hillman EM, Moore A. All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast. Nat Photonics. 2007;1(9):526–30. doi: 10.1038/nphoton.2007.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain AS, Date AA, Pissurlenkar RR, Coutinho EC, Nagarsenker MS. Sulfobutyl ether(7) beta-cyclodextrin (SBE(7) beta-CD) carbamazepine complex: preparation, characterization, molecular modeling, and evaluation of in vivo anti-epileptic activity. AAPS PharmSciTech. 2011;12(4):1163–75. doi: 10.1208/s12249-011-9685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coates J (2000) Interpretation of infrared spectra, a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry, Wiley, New York
- 35.Cirri M, Maestrelli F, Corti G, Furlanetto S, Mura P. Simultaneous effect of cyclodextrin complexation, pH, and hydrophilic polymers on naproxen solubilization. J Pharm Biomed Anal. 2006;42(1):126–31. doi: 10.1016/j.jpba.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Zahm SH, Weisenburger DD, Babbitt PA, Saal RC, Vaught JB, Blair A. Use of hair coloring products and the risk of lymphoma, multiple myeloma, and chronic lymphocytic leukemia. Am J Public Health. 1992;82(7):990–7. doi: 10.2105/AJPH.82.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroju RK, Turner DC, Zhang H. Solubilizing efficiency and in vitro cytotoxicity of peptoad G. J Pharm Sci. 2010;99(4):2196–8. doi: 10.1002/jps.21921. [DOI] [PubMed] [Google Scholar]


