Abstract
Recent studies have shown the importance of monitoring microenvironmental conditions (temperature, relative humidity) experienced by the tablet bed during a pan coating process, thereby necessitating the need to understand how various process parameters influence these microenvironmental conditions. The process parameters studied in this work include exhaust air temperature, spray rate, inlet airflow rate, gun-to-bed distance, coating suspension percent solids, and atomization and pattern air pressure. Each of these process parameters was found to have an impact on the tablet bed relative humidity (RH), as measured using PyroButton data logging devices. A higher tablet bed RH was obtained with an increase in spray rate and atomization air pressure and with a decrease in exhaust air temperature, inlet airflow rate, gun-to-bed distance, suspension percent solids, and pattern air pressure. Based on this work, it can be concluded that the tablet bed thermodynamic conditions are a cumulative effect of the various process conditions. A strong correlation between the tablet bed RH and the frequency of tablet coating defect (logo bridging) was established, with increasing RH resulting in a higher percent of logo bridging events.
Key words: coating process parameters, logo bridging, microenvironment, PyroButtons, tablet bed humidity, tablet bed temperature
INTRODUCTION
Polymer films are applied to pharmaceutical solids for cosmetic, protective, or functional purposes (1–6). The application process is quite complex, with multiple variables related to the substrate characteristics, coating formulation, processing equipment, and processing conditions (7–11). Figure 1 shows a fishbone diagram of some of the variables that impact film quality and performance in a pan coating process. The current work focuses on monitoring the microenvironment (i.e., temperature and relative humidity) that tablets are subjected to during the coating to better understand the film formation process.
Fig. 1.
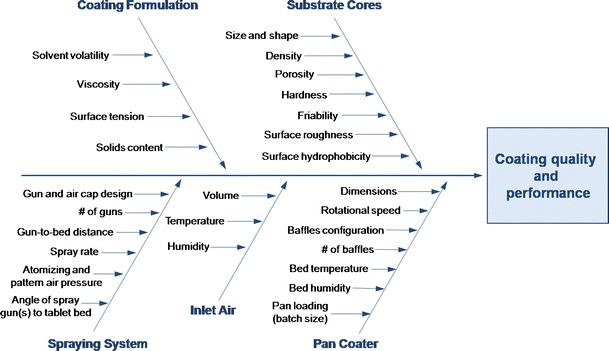
Diagram of variables that influence film formation and coating uniformity in pan coating processes
The thermodynamic conditions inside a coating pan have been shown to affect the quality of the film formed on the tablets. For example, it is well known that a relatively dry environment can result in tablet defects such as surface roughness, whereas a wet environment can cause tablets to stick or pick and, in extreme cases, actually dissolve during the coating process. The appearance and residual water content of coated tablets have been shown to be a direct function of coating conditions, specifically inlet air temperature and humidity, and spray rate (12). The humidity and temperature inside the pan are critical to solvent evaporation and film formation, especially with polymeric dispersions (13). Mathematical tools, such as the widely used TAAC (thermodynamic analysis of aqueous coating) program, based on equations described by Ebey (14), can be useful to make predictions on process adjustments, but such tools do not directly address the localized conditions experienced by the tablets (15). Additionally, the heat losses during the coating process are generally not accounted for, and therefore, the models tend to deviate from experimental results when used across different scales, where heat losses may be quite different. While the more recently revised models offer better predictions (16), only macroenvironmental changes are captured and the tablet bed microenvironment is not addressed.
While the regulatory emphasis on process analytical technologies and quality by design stress the importance of a thorough understanding of pharmaceutical processes, a fundamental knowledge of coating processes is still incomplete, especially in relation to the thermodynamic conditions in the coating process. Pandey et al. (17,18) have shown the utility of PyroButton data (temperature and humidity) logging devices in obtaining information regarding the thermodynamic microenvironment experienced by the tablets during the coating process. These tablet-sized devices (16-mm diameter) were secured at specific locations in the equipment and placed in the tablet bed and allowed to tumble freely along with the tablets (17–19). The tablet bed relative humidity (RH), as measured by PyroButtons moving freely with the tablets, was found to be a more sensitive response compared to conventionally monitored process variables. It was shown that controlling tablet bed humidity was critical for coated product quality for a tablet core/coating system combination with a narrow process design space. In separate studies, a strong correlation was established between the tablet bed RH and tablet defects such as logo bridging and bilayer tablet delamination rates on accelerated stability (18,20).
In a recent study, Pandey and Bindra (17) studied two process variables, inlet air RH and spray rate, and established their influence on tablet bed RH and logo bridging incidence rates. Expanding on these recent findings, the objective of the current research was to determine the impact of several other process variables not previously studied (i.e., inlet airflow, atomization and pattern air pressure, gun-to-bed distance, exhaust temperature, and suspension concentration) on the tablet bed microenvironment. In addition, a relationship between the tablet bed environmental conditions and coating defects (i.e., logo bridging) was also established.
MATERIAL AND METHODS
The placebo tablet formulation used in this study consisted of microcrystalline cellulose (FMC Co., Philadelphia, PA), anhydrous lactose (Kerry Bio-Sciences, Norwich, NJ), croscarmellose sodium (FMC Co.), and a relatively high amount (1.25%, w/w) of magnesium stearate (Mallinckrodt Chemicals, Saint Louis, MO). The formulation was compressed into debossed oval-shaped tablets at a target press weight of 200 mg. The tablets were coated with a HPMC-based Opadry coating system (Colorcon, PA) containing polyethylene glycol (PEG) 400 as a plasticizer. The use of this coating system for a tablet substrate with a high magnesium stearate level has been shown to result in a narrow process design space from the logo bridging view point (17). The tablets were coated in a 12-in. Vector pan coater, equipped with one spray gun, to a target theoretical coating weight gain of 3.0% (w/w). The pan speed was kept constant at 25 rpm. Aqueous coating suspensions at either 10% or 15% (w/w) solids were used. The coater was operated in “exhaust temperature control” mode, meaning that the unit self-adjusted the inlet air temperature to reach and maintain the exhaust temperature at the set target value. The other coating systems used for the last portion of the work were PVA-based Opadry II coating system and HPMC-based Opadry system containing triacetin as the plasticizer (both provided by Colorcon).
Several coating process parameters were studied by varying one parameter at a time to understand and quantify their impact on the coating pan thermodynamics and any effect on aesthetics of the coated tablet. The process parameters varied were spray rate, inlet airflow, exhaust temperature, gun-to-bed distance, atomization and pattern air pressure, and suspension percent solids. The effect of inlet air RH and spray rate on the incidence of coating defects was previously studied (17). Table I shows the processing parameters for the nine different batches manufactured during the current study.
Table I.
Experimental Batch Details and Corresponding Tablet Bed T and RH Results
| Run No. | Spray rate (g/min) | Inlet airflow (cfm) | Exhaust T (°C) | Atomization air (psi) | Pattern air (psi) | Gun-to-bed distance (in.) | Solids in suspension (%, w/w) | Tablet bed T (°C) | Tablet bed RH (%) |
|---|---|---|---|---|---|---|---|---|---|
| A | 5.5 | 45 | 44 | 12 | 7.5 | 5 | 15 | 40.3 | 26.3 |
| B | 7.5 | 45 | 44 | 12 | 7.5 | 5 | 15 | 38.8 | 33.5 |
| C | 5.5 | 40 | 44 | 12 | 7.5 | 5 | 15 | 40.5 | 28.1 |
| D | 5.5 | 35 | 44 | 12 | 7.5 | 5 | 15 | 39.4 | 31.8 |
| E | 5.5 | 45 | 40 | 12 | 7.5 | 5 | 15 | 36.8 | 33.1 |
| F | 5.5 | 45 | 44 | 12 | 7.5 | 3.5 | 15 | 38.5 | 38 |
| G | 5.5 | 45 | 44 | 7.5 | 7.5 | 5 | 15 | 42 | 23.5 |
| H | 5.5 | 45 | 44 | 12 | 0 | 5 | 15 | 38.6 | 34.1 |
| I | 5.5 | 45 | 44 | 12 | 7.5 | 5 | 10 | 38 | 39 |
PyroButton (Opulus, PA) data loggers (18) were used to record temperature and relative humidity during the coating runs. The devices were placed in the inlet and exhaust air plenums and also included in the tablet bed to freely tumble along with the tablets. A schematic diagram of the pan coater setup is shown in Fig. 2. The buttons were programmed to record data every 10 s, and the accuracy of the devices for temperature and RH was ±0.3°C and ±2%, respectively. A picture of PyroButton is shown in Fig. 3. A total of 100 tablets were randomly selected from each run and the tablets were visually inspected on both sides for the coating defects. The incidence of logo bridging was the main defect investigated in this study. Tablets were randomly selected from each batch and visually inspected for logo bridging, with observations made on both sides of the tablets. A tablet was defined to exhibit logo bridging when at least one of the four letters of the logo on a tablet face was significantly obscured such that the letter could not be read easily.
Fig. 2.
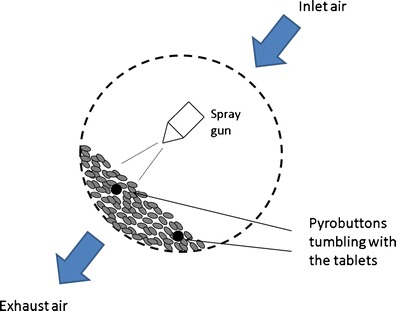
Pan coating process schematic utilizing Pyrobuttons to record the microenvironment experienced by the tablets during coating
Fig. 3.

Picture of PyroButton and USB adapter used to program it
RESULTS AND DISCUSSION
A coating run (run A, Table I) was conducted to serve as a reference batch for comparison when studying changes to the processing parameters. The temperature and RH data set obtained from PyroButtons is shown for run A in Fig. 4a, b for the PyroButtons that were moving freely with the tablets. Data from the two PyroButtons agree well with each other. As expected, the RH increased and the temperature (T) decreased upon initiation of the spray (t = 0 on x-axis). After a few minutes of spraying, the process reached a steady state, until the spray was stopped (at t = 33 min). The average tablet bed T for run A was 40.3°C, which is lower than the target exhaust temperature of 44°C. These data show that the temperature experienced by the tablets was cooler than the exhaust temperature and are in agreement with previous reports in the literature (18,21). The average tablet bed RH during the steady spraying phase for run A was 26.3%.
Fig. 4.
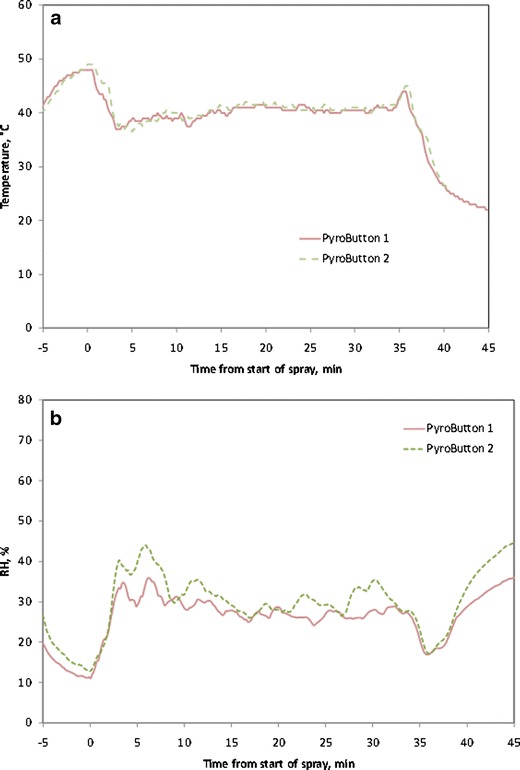
Processing conditions recorded by the PyroButtons during coating. a Temperature. b Percent relative humidity
Several processing parameters were varied, one at a time, as shown in Table I, and the results from those runs are discussed in the subsequent subsections.
Spray Rate
Spray rate is one of the most important coating process parameters. At one extreme, a high spray rate may cause coating defects such as twinning, picking and sticking, and logo bridging (18), whereas at the other extreme, a low spray rate may cause spray drying and a loss in coating efficiency. Spray rates are especially important if the tablet formulation or API is moisture-sensitive as residual water content in the coated tablets has been shown to be directly related to the spray rate (12). The rate at which the coating formulation is applied to the tablets influences the bed temperature and, thus, the rate of solvent evaporation. Spray rate, in conjunction with atomization air pressure, has been shown to affect spray characteristics, such as droplet size (22–24). Larger droplets are more likely to dissolve the outermost region of the tablet, potentially resulting in drug migration into the film coat (25). The spray rate per gun is usually limited by the diminished quality of the spray with increasing spray rate and not by the evaporative capacity of the pan.
In order to quantify the impact of spray rate on the coating microenvironment, the spray rate was increased from 5.5 g/min (for the reference run A) to 7.5 g/min (run B), with all other parameters held constant. The PyroButton data showed that the average tablet bed RH increased from 26.3% for run A to 33.5% for run B with a slight decrease in T (Table I), even though the same exhaust T was maintained for both conditions. Thus, the difference between the tablet bed and exhaust T is a function of the coating conditions (spray rate in this case). As the spray rate was increased, the amount of water sprayed onto the tablets at any given time also increased and resulted in the observed tablet bed RH increase. These observations are consistent with those reported previously by Pandey and Bindra (17) and demonstrate that the tablet bed microenvironment can be different between two runs with the same exhaust T.
Inlet Airflow Rate
The inlet air (flow rate, T, and RH) dictates the drying kinetics of the spray droplets as they travel from the spray nozzle to the tablet surface and thus is another critical processing parameter in film coating. A previous study by Pandey and Bindra (17) showed that inlet air RH can significantly impact both tablet bed and exhaust air RH. The consequences of inlet air relate predominately to the issues of drying. Insufficient drying can result in overly wet tablet surfaces, producing detrimental effects such as twinning, tablet agglomeration, and surface dissolution. At the other extreme, if drying occurs too rapidly, the polymer-containing droplets may dry prior to hitting the tablet surface (spray drying) or not sufficiently spread across the tablet surface, resulting in a rougher film surface. In addition to the flow rate, the humidity conditions of the inlet air also play a significant role in film formation. Maintaining a constant exhaust temperature does not compensate for the differences in inlet air RH, and adjusting other processing parameters (such as spray rate) to maintain a constant tablet bed RH produces coated products of similar quality (17).
In the current study, inlet airflow rate was varied at three levels—35, 40, and 45 cfm (runs A, C, and D, respectively)—with all other variables kept constant. As expected, as the inlet airflow increased, the tablet bed RH decreased, whereas the tablet bed T was not significantly affected by changes in inlet airflow rate within the range studied here (Table I). However, as can be observed by the magnitude of the changes, tablet bed RH is more sensitive to the variation in spray rate as compared to inlet airflow rate.
Researchers have often used the inlet airflow-to-spray rate ratio for process scale-up. It is believed that maintaining the inlet airflow-to-spray rate ratio constant allows for closer correlation between inlet and exhaust temperatures at different scales. This ratio, shown in Eq. 1, is defined as the drying capacity (26). A higher drying capacity would be expected to result in a drier environment and vice versa.
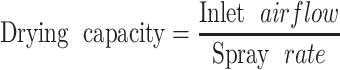 |
1 |
The drying capacity values were calculated and plotted against the tablet bed RH, as shown in Fig. 5. A good correlation between tablet bed RH and drying capacity was observed. The data indicate that drying capacity may be a good indicator of the RH in the tablet bed, although the relationship does not seem to be linear.
Fig. 5.
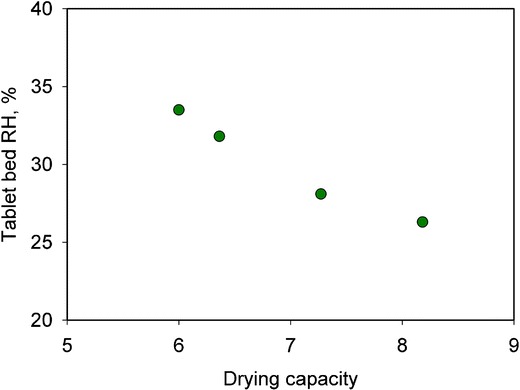
Relationship between drying capacity and the relative humidity in the table bed
Exhaust Temperature
Process control based upon adjustment of inlet air temperature to maintain a desired exhaust temperature is commonly used. The factors affecting the required inlet air temperature include spray rate, percent solids in coating solution, inlet airflow rate, and inlet air RH. The exhaust temperature is one of the process parameters that are generally kept constant during the pan coating process scale-up (15,26). In early literature, the exhaust temperature was assumed to be the same as the tablet bed temperature. Later studies using infrared gun measurements, however, showed that the tablet bed temperature is generally 2–3°C cooler than the exhaust air temperature. In more recent studies utilizing PyroButtons (17,18,21), the exhaust temperature was shown to be up to 10°C higher than the tablet bed temperature, and the magnitude of the difference was a function of the process conditions and batch size. In the absence of tablet bed temperature data, the exhaust temperature provides a rough estimate of the tablet bed temperature.
In the current work, exhaust temperature was varied from 44°C to 40°C (runs A and E, respectively) to study its impact on the tablet bed microenvironment. As expected, the tablet bed temperature decreased and RH increased when the exhaust temperature was lowered (Table I).
Gun-to-Bed Distance
The gun-to-bed distance is an important process parameter that oftentimes does not receive its due attention. It refers to the distance between the tip of the nozzle and an imaginary flat surface on the cascading bed of tablets. Therefore, this variable can be somewhat subjective and operator-dependent. As the droplets move in this region, the solvent can evaporate, leading to a decrease in size, or the droplets can coalesce, thus increasing in size. In general, a reduction in droplet size is observed. However, since the pattern air flattens the spray, there is also a significant droplet coalescence occurring (15), and the size of the droplet hitting the tablet surface is dependent on process parameters. If the gun-to-bed distance is too large, spray drying can be observed where smaller droplets dry completely before hitting the tablet surface, which in turn can lead to lower process efficiency and tablet defects such as rough surface or logo infilling. On the other hand, if the spray nozzle is placed too close to the tablet bed, relatively large droplets may reach the tablets, creating an over-wetted surface and thus increasing the chance for tablet defects, including twinning and surface dissolution. Another factor to consider is the viscosity of droplets hitting the tablets. If the droplets have the appropriate viscosity right before they hit the tablet surface, they coalesce to the tablet substrate and form a good quality film. The residual water is evaporated by conduction of heat from the tablet to the droplet at a rate dictated by the bed temperature.
In the current study, the gun-to-bed distance was changed from 5 to 3.5 in. (runs A and F) to study its impact on the coating microenvironment. As can be seen from the tablet bed RH data in Table I, this reduction in gun-to-bed distance resulted in a significant increase in tablet bed RH from 26.3% to 38.0% and a reduction in tablet bed temperature from 40.3°C to 38.5°C. This dramatic increase in tablet bed RH can be explained by larger-sized atomized droplets or lower-viscosity (more water) droplets reaching the tablet surface due to reduced drying. These data indicate that even though the conventionally monitored and controlled macroscopic variables (exhaust temperature, spray rate, inlet airflow, etc.) were kept constant between the two runs, the gun-to-bed distance significantly impacted the microenvironment that the tablets experienced.
Atomization and Pattern Air
The atomization air pressure used in coating processes dictates the droplet size and velocity of the solution or suspension exiting the spray nozzle. The pattern air on the other hand flattens out the spray cone and enables better and more uniform spray coverage across the tablet bed, which in turn improves coating uniformity between tablets (27). The pattern air can cause additional collision between droplets and result in an increase in droplet size. The size of the spray droplet impacts the drying kinetics as the droplet moves from the nozzle toward the tablet cores. A narrow droplet size distribution is desirable for good process control. Additionally, choosing the appropriate pattern air pressure is even more important when there are multiple spray nozzles being used. Pattern air pressure (or flow rate) should be chosen such that there is uniform spray coverage across the tablet bed without an overlap of the spray zones between the multiple guns. An overlap of spray regions would result in regions with much wetter conditions than desired, and tablets passing through those regions would receive additional spray, which could then lead to coating defects. Film roughness has been shown to be strongly affected by the balance between droplet spreading and drying, with a rougher film produced at a higher atomization air pressure (28). Excessive atomization air pressure can result in the production of smaller droplets that dry completely before reaching the tablet surface, leading to spray drying, loss of efficiency, and, in some cases, logo infilling or “orange peeling” coating defects. On the other hand, a low atomization air pressure can lead to ineffective atomization of the coating suspension, leading to bigger droplets that may not get spread and dried appropriately after they contact the tablet substrate.
For the current study, atomization and pattern air were varied one by one as separate runs and compared to the control run A. Atomization pressure was decreased from 12 psi (run A) to 7.5 psi (run G); for run H, no pattern air was used. As expected, with no pattern air pressure, an increase in tablet bed RH from 26.3% to 34.1% was observed, whereas a decrease in atomization air pressure resulted in a lower tablet bed RH (23.5%). The effect of atomization air pressure may seem counterintuitive at first, in that a reduced atomization air pressure should result in larger droplets and, hence, would likely result in a higher tablet bed RH. However, it should be kept in mind that as the atomization air pressure decreases, the droplet velocity also decreases, thereby increasing the transit time of the droplet and thus allowing for more solvent evaporation to occur. A slight increase in tablet bed temperature was also noted when the atomization air pressure was reduced, once again opposite to the case when pattern air pressure was set to zero.
Suspension or Polymer Concentration
The concentration of a polymer in solution significantly influences the viscosity of the liquid (2). From a practical standpoint, since coating materials (both polymer solutions and dispersions) must be atomized into a fine mist for coating processes, the viscosity of the material should generally be lower than 400 cP as the liquid can be delivered to the spray gun and atomized into droplets more readily. On the other hand, a higher polymer concentration can reduce the total coating time necessary to achieve the same coating weight gain, and therefore is more cost-effective. Additionally, shorter processing times are advantageous if the tablet cores are somewhat friable as more coating material deposited faster can enhance the mechanical strength of such tablets.
In order to understand the impact of solids concentration on the tablet bed conditions, a coating run was done using a 10% (w/w) total solids level (run I) and compared to the batch coated at 15% (w/w) total solids level (run A), with all other parameters held constant. Even though a decrease in solution/suspension viscosity will make the atomization more effective (at the same spray rate) and create smaller droplets (29), a significant increase in tablet bed RH from 26.3% to 39.0% was observed when the solids content of the suspension was decreased, similar to the effect of increasing the spray rate. This observation was likely due to the increase in the amount of water sprayed on the tablet bed with the coating suspension at the lower solids level.
Correlating Tablet Defects to Coating Conditions
Based on the data presented in the previous sections, PyroButtons clearly provide a more accurate view of the tablet bed microenvironment. Yet, it is important to understand the relevance of these microenvironmental conditions to the overall coating quality, or macroscopic characteristics. In this study, coating quality was assessed based on logo bridging, as described in “MATERIAL AND METHODS.” Figure 6 shows examples of coated tablets that were categorized as showing logo bridging versus ones that did not show any logo bridging.
Fig. 6.
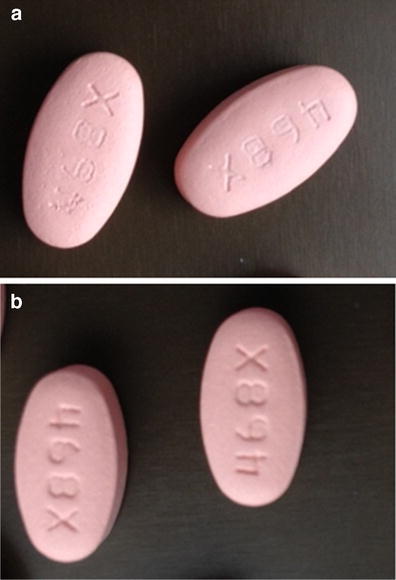
Examples of pictures considered showing logo bridging (a) versus no logo bridging (b) in this study
Figure 7 shows the incidence of logo bridging, expressed as a percent, as a function of tablet bed RH. Apart from the coating runs mentioned in Table I, Fig. 7 includes data from an additional coating run. This run was carried at a higher atomization pressure and spray rate (18 psi and 7.5 g/min, respectively) in comparison to the reference run (run A, Table I), with other factors kept the same as run A. This coating run was observed to have a tablet bed RH of 35%, and 25% of the tablets showed logo bridging. Figure 7 shows that regardless of which process parameter caused the change, an increase in tablet bed RH resulted in an increase in logo bridging events. A similar relationship between tablet bed RH and logo bridging incidence was recently reported by the authors (18) using spray rate, inlet air humidity, exhaust temperature, and pan speed, and it was found that out of those variables, only pan spray rate and inlet air humidity significantly affected the tablet bed microenvironment. The current work explores several other process variables that were not explored before but are generally known to have a role in the coating thermodynamics (Fig. 1).
Fig. 7.
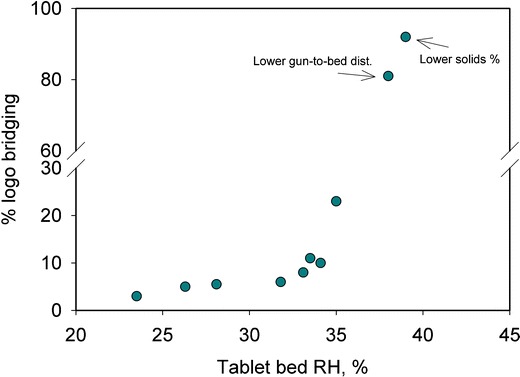
Relationship between tablet bed RH and percent of logo bridging
Batches that showed the most logo bridging events were the ones with lower suspension solids content (run I) and closer gun-to-bed distance (run F), followed by no pattern air (run H) and higher spray rate (run B) cases. Figure 8a, b provides a bar graph representation of the change in tablet bed microenvironment as the parameters were changed. Somewhat surprisingly, an increased atomization air pressure resulted in higher logo bridging events, suggesting that the logo bridging in this case was caused by droplets hitting the tablet surface at high velocities and not spreading and drying in a desired manner such that the film did not adhere well to the tablet substrate (Fig. 9).
Fig. 8.
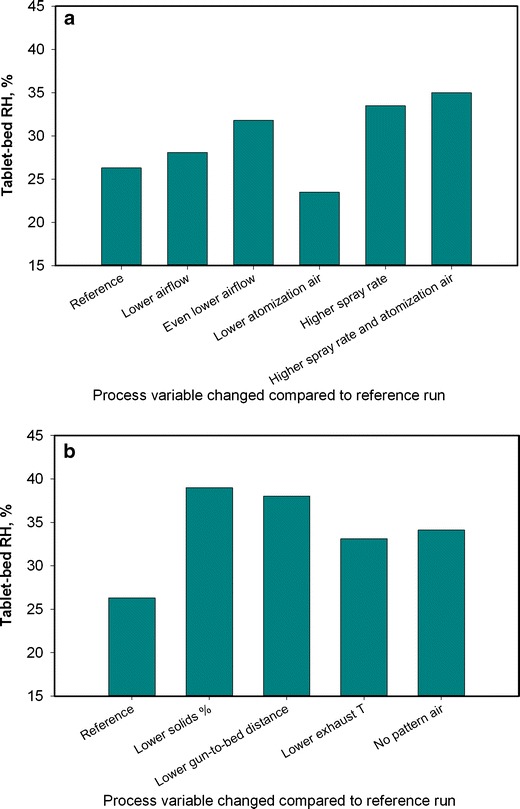
Bar graph representation of the effect of each process parameter on logo bridging response
Fig. 9.

Cross-section of a coated tablet showing that the underlying cause of logo bridging in this study was the film not adhering well to the tablet substrate
It should be noted that some degree of logo bridging was observed even at the lower end of the tablet bed humidity range evaluated (Fig. 7). This indicated that the choice of the coating formulation was not ideal for the tablet substrate used in this study. Therefore, two alternate coating formulations were tested to understand the role of coating formulation in logo bridging. Since the incidence of logo bridging is related to internal stresses in the film, a change was made to the plasticizer, where PEG 400 was replaced with triacetin at the same concentration. The second coating formulation tested used a completely different polymer system (PVA-based Opadry II coating system). The coating runs with both of the new formulations were performed at high tablet bed humidity condition (process conditions used were the same as run B with gun-to-bed distance at 4.5 in., resulting in close to 40% tablet bed RH), a condition known to produce high incidence of logo bridging for the HPMC/PEG 400 system. As expected, the PVA-based system did not show any logo bridging due to its known superior adhesion properties as compared to the HPMC-based systems (31). However, it was particularly interesting to note that the HPMC/triacetin system also did not show any logo bridging. This observation was not an obvious one, although there is limited existing literature that shows a relationship between plasticizer type (and concentration) and logo bridging observations (30); however, in that study, triacetin was not studied. It can be postulated that the HPMC/triacetin system offered better adhesion to the tablet substrate and/or reduced internal stresses within the film coat to mitigate the incidence of logo bridging.
CONCLUSIONS
The current work quantifies the impact of several process parameters (exhaust air temperature, spray rate, inlet airflow rate, gun-to-bed distance, coating suspension percent solids, and atomization and pattern air pressure) on the tablet bed microenvironment and logo bridging tablet coating defect. The results from this study demonstrate the utility of PyroButton data logging devices in measuring the tablet bed microenvironmental factors. A good correlation between the tablet bed RH and the number of logo bridging events was observed, with more logo bridging occurring at higher bed RH. Gun-to-bed distance and suspension solids level were found to be the most sensitive parameters in relation to the tablet bed RH and the number of observed logo bridging events. There are certain aspects of the process that are not addressed in this work, such as the nozzle type and design.
In the quality-by-design paradigm, a small-scale study such as this allows screening a range of process parameters that may be relevant to a particular tablet substrate and coating system with minimal resources. Once the key parameters are identified in relation to a particular response, a more detailed study (possibly a design of experiments to study interaction terms) can be conducted on those particular parameters. In addition, establishing a process operating space based on microenvironmental conditions at the small scale will facilitate a successful and more robust scale-up since it relates to the local environment that the tablets experience during coating.
It should also be pointed out that the findings from this work indicate that a complex multivariate process such as coating can be studied by a much more reduced set of parameters. In this study, it was shown that when studying logo bridging as a response, the main process parameters can be bundled into a single response, the tablet bed microenvironment, and a means for measuring that response was provided (PyroButtons).
ACKNOWLEDGEMENTS
The authors would like to thank Elena Zour, Shruti Gour, and Ganeshkumar Subramanian from Bristol-Myers Squibb and Bela Jancsik from OPULUS® Ltd. for their useful contributions to this work.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Porter SC, Felton LA. Techniques to assess film coatings and evaluate film-coated products. Drug Dev Ind Pharm. 2010;36(2):128–42. doi: 10.3109/03639040903433757. [DOI] [PubMed] [Google Scholar]
- 2.Bruce HF, Sheskey PJ, Garcia-Todd P, Felton LA. Novel low-molecular-weight hypromellose polymeric films for aqueous film coating applications. Drug Dev Ind Pharm. 2011;37:1439–45. doi: 10.3109/03639045.2011.584194. [DOI] [PubMed] [Google Scholar]
- 3.Siepmann F, Siepmann J, Walther M, MacRae R, Bodmeier R. Aqueous HPMCAS coatings: effects of formulation and processing parameters on drug release and mass transport mechanisms. Eur J Pharm Biopharm. 2006;63(Mar):262–9. doi: 10.1016/j.ejpb.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Siepmann F, Wahle C, Leclercq B, Carlin B, Siepmann J. pH-sensitive film coatings: towards a better understanding and facilitated optimization. Eur J Pharm Biopharm (Netherlands) 2008;68(Jan):2–10. doi: 10.1016/j.ejpb.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Felton LA, Timmins GS. A nondestructive technique to determine the rate of oxygen permeation into solid dosage forms. Pharm Dev Technol. 2006;11:1–7. doi: 10.1080/10837450600561208. [DOI] [PubMed] [Google Scholar]
- 6.Porter SC. Coating of pharmaceutical dosage forms. In: LVea A, editor. Remington: the science and practice of pharmacy. 22. London: Pharmaceutical Press; 2012. pp. 977–87. [Google Scholar]
- 7.Felton LA. Film coating of oral solid dosage forms. In: Swarbrick J, editor. Encyclopedia of pharmaceutical technology. 3. New York: Informa Healthcare; 2006. pp. 1729–47. [Google Scholar]
- 8.Porter SC, Verseput RP, Cunningham CR. Process optimization using design of experiments. Pharmaceut Technol (USA). 1997;21(Oct):60–70. [Google Scholar]
- 9.Pandey P, Turton R. Movement of different shaped particles in a pan-coating device using novel video-imaging techniques. AAPS PharmSci. 2005;6(2):Article 34. [DOI] [PMC free article] [PubMed]
- 10.Ketterhagen WR. Modeling the motion and orientation of various pharmaceutical tablet shapes in a film coating pan using DEM. Int J Pharm. 2011;409:137–49. doi: 10.1016/j.ijpharm.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Wilson KE, Crossman E. The influence of tablet shape and pan speed on intra-tablets film coating uniformity. Drug Dev Ind Pharm. 1997;23(12):1239–43. doi: 10.3109/03639049709146164. [DOI] [Google Scholar]
- 12.Ruotsalainen M, Heinamaki J, Taipale K, Yliruusi J. Influence of the aqueous film coating process on the properties and stability of tablets containing a moisture-labile drug. Pharm Dev Technol. 2003;8(4):443–51. doi: 10.1081/PDT-120024697. [DOI] [PubMed] [Google Scholar]
- 13.Felton LA. Mechanisms of polymeric film formation. Int J Pharm. 2013;457:423–7. [DOI] [PubMed]
- 14.Ebey GC. Thermodynamic model for aqueous film coating. Pharm Technol (USA) 1987;11(Apr):40–50. [Google Scholar]
- 15.Porter SC. Scale-up of film coating. In: Levin M, editor. Pharmaceutical process scale-up. 3. New York: Informa Healthcare; 2011. pp. 444–89. [Google Scholar]
- 16.Ende MT, Berchielli A. A thermodynamic model for organic and aqueous tablet film coating. Pharm Dev Technol (USA) 2005;10(Jan):47–58. doi: 10.1081/PDT-200035915. [DOI] [PubMed] [Google Scholar]
- 17.Pandey P, Bindra DS. Real-time monitoring of thermodynamic microenvironment in a pan coater. J Pharm Sci. 2013;102:336. doi: 10.1002/jps.23379. [DOI] [PubMed] [Google Scholar]
- 18.Pandey P, Ji J, Subramanian G, Gour S, Bindra DS. Understanding the thermodynamic micro-environment inside a pan coater using a data logging device. Drug Dev Ind Pharm. 2013. doi:10.3109/03639045.2013.772192. [DOI] [PubMed]
- 19.Staben Wobker ME, Mehrotra A, Carter BH. Use of commercial data loggers to develop process understanding in pharmaceutical unit operations. J Pharm Innov. 2010;5(4):169–80. doi: 10.1007/s12247-010-9092-0. [DOI] [Google Scholar]
- 20.Zacour BM, Pandey P, Subramanian G, Gao JZ, Nikfar F. Correlating bilayer tablet delamination tendencies to micro-environmental thermodynamic conditions during pan coating. Drug Dev Ind Pharm. 2013. doi:10.3109/03639045.2013.788014. [DOI] [PubMed]
- 21.Wobker MS, Mehrotra A, Carter B. Use of commercial data loggers to develop process understanding in pharmaceutical unit operations. J Pharm Innov. 2010;5(4):169–80.
- 22.Chen W, Chang S-H, Kiang S, Early W, Paruchuri S, Desai D. The measurement of spray quality for pan coating processes. J Pharm Innov. 2008;3:3–14. doi: 10.1007/s12247-008-9022-6. [DOI] [Google Scholar]
- 23.Wang J, Hemenway J, Chen W, Desai D, Varia S, et al. An evaluation of process parameters to improve coating efficiency of an active tablet film-coating process. Int J Pharm. 2012;427:163–9. doi: 10.1016/j.ijpharm.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Mueller R, Kleinebudde P. Comparison of a laboratory and a production coating spray gun with respect to scale-up. AAPS PharmSciTech. 2007;8(1):E21–E31. [DOI] [PMC free article] [PubMed]
- 25.Barbash D, Fulghum JE, Yang J, Felton LA. A novel imaging technique to investigate the influence of atomization air pressure on film–tablet interfacial thickness. Drug Dev Ind Pharm. 2009;35(4):480–6. doi: 10.1080/03639040802438381. [DOI] [PubMed] [Google Scholar]
- 26.Pandey P, Turton R, Joshi N, Hammerman E, Ergun J. Scale-up of a pan-coating process. AAPS PharmSciTech. 2006;7(4):E125–E132. [DOI] [PMC free article] [PubMed]
- 27.Rege BD, Gawel J, Kou JH. Identification of critical process variables for coating actives onto tablets via statistically designed experiments. Int J Pharm. 2002;237:87–94. doi: 10.1016/S0378-5173(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 28.Pandey P, Katakdaunde M, Turton R. Modeling weight variability in a pan coating process using Monte Carlo simulations. AAPS PharmSciTech. 2006;7(4):83. [DOI] [PubMed]
- 29.Chen W, Chang S-Y, Kiang S, Early W, Paruchuri S, Desai D. The measurement of spray quality for pan coating processes. J Pharm Innov. 2008;3(1):3–14.
- 30.Rowe RC, Forse SF. The effect of plasticizer type and concentration on the incidence of bridging of intagliations on film-coated tablets. J Pharm Pharmacol. 1981;33(3):174–5. doi: 10.1111/j.2042-7158.1981.tb13744.x. [DOI] [PubMed] [Google Scholar]
- 31.Rajabi-Siahboomi AR, Farrell TP. The applications of formulated systems for the aqueous coating of pharmaceutical oral solid dosage forms. In: McGinity JW, Felton LA editors. Aqueous polymeric coatings for pharmaceutical dosage forms, third edition. Informa Healthcare, 2008. p. 323–44.


