Abstract
A simple but novel mixed surfactant system was designed to fabricate a self-nanoemulsifying drug delivery system (SNEDDS) based on hydrophilic–lipophilic balance (HLB) value. The impacts of HLB and molecular structure of surfactants on the formation of SNEDDS were investigated. After screening various oils and surfactants, nifedipine (NDP)-loaded liquid SNEDDS was formulated with Imwitor® 742 as oil and Tween®/Span® or Cremophor®/Span® as mixed surfactant. Droplet size of the emulsions obtained after dispersing SNEDDS containing Tween®/Span® in aqueous medium was independent of the HLB of a mixed surfactant. The use of the Cremophor®/Span® blend gave nanosized emulsion at higher HLB. The structure of the surfactant was found to influence the emulsion droplet size. Solid SNEDDS was then prepared by adsorbing NDP-loaded liquid SNEDDS comprising Cremophor® RH40/Span® 80 onto Aerosil® 200 or Aerosil® R972 as inert solid carrier. Solid SNEDDS formulations using higher amounts (30–50% w/w) of Aerosil® 200 exhibited good flow properties with smooth surface and preserved the self-emulsifying properties of liquid SNEDDS. Differential scanning calorimetry and X-ray diffraction studies of solid SNEDDS revealed the transformation of the crystalline structure of NDP due to its molecular dispersion state. In vitro dissolution study demonstrated higher dissolution of NDP from solid SNEDDS compared with NDP powder.
KEY WORDS: HLB, molecular structure, nifedipine, poorly water-soluble drug, self-emulsifying drug delivery system
INTRODUCTION
A major problem in oral drug formulations is the low bioavailability of active drugs. This may lead to high intersubject and intra-subject variabilities and therapeutic failure. The improvement of drug bioavailability presents one of the most challenges in drug formulations. One of the most interesting approaches is the use of a self-nanoemulsifying drug delivery system (SNEDDS) (1,2). SNEDDS is anhydrous form of nanoemulsions or preconcentrated nanoemulsions. It is an isotropic mixture of oil, surfactant(s), and drug, which spontaneously forms thermodynamically stable oil-in-water nanoemulsions (usually with globule size <200 nm) when introduced into the aqueous phase under gentle agitation conditions (3). SNEDDS can also contain a co-emulsifier or cosurfactant and/or solubilizer to facilitate nanoemulsification or improve drug incorporation. The advantages of SNEDDS include the possibility of filling them into unit dosage forms (e.g., soft/hard gelatin capsule), maintaining physical and/or chemical stability upon long-term storage, improving the bioavailability of poorly water-soluble drug, and reducing the blood profile variation in the patients confronted with gastrointestinal (GI) problem (4,5). However, the SNEDDS as liquid dosage forms has limitations such as low drug loading capacity and excipient–capsule incompatibility (2,6). To overcome these complications, the liquid SNEDDS is adsorbed onto inert carrier, such as silicon dioxide, to produce solid SNEDDS.
In the formulation of a SNEDDS, the following points should be considered: (1) solubility of the drug in different oils, surfactants, and cosurfactant/cosolvents and (2) selection of oil, surfactant, and cosurfactant/cosolvent based on the solubility of the drug. The optimum concentrations of oil, surfactant, and cosurfactant/cosolvent necessary to promote self-emulsification are determined by the construction of a ternary phase diagram. However, the experimental determination of a phase diagram is a time-consuming process, requiring careful synthesis and characterization of all phases in a system. Wang et al. (7) proposed an alternative method to prepare SNEDDS containing a mixture of surfactants with similar structure (i.e., ethoxylated sorbitan monoester-type hydrophilic surfactant (Tween®) and sorbitan monoester-type lipophilic surfactant (Span®)) by calculating hydrophilic–lipophilic balance (HLB). Although the HLB system is not absolute in predicting the formulation behavior, it is a very good starting point for achieving emulsification (8).
The HLB of the surfactant offers essential information on its potential use in the formulation of SNEDDS. The SNEDDS formulation with high HLB surfactant can form oil-in-water nanoemulsions that immediately and rapidly spread in the aqueous medium. It would keep the drug solubilized for a prolonged period of time at the absorption site for effective absorption and prevent drug precipitation within the GI lumen (9). More than one surfactant may be blended together to achieve the desired HLB. A mixture of different surfactant types often exhibit synergism in their effects in the properties of a system. This synergism can be attributed to nonideal mixing effects in the aggregates, resulting in critical micellization concentration and interfacial tension that are substantially lower than that expected on the basis of the properties of the unmixed surfactants (10). In addition to the appropriate HLB value, the constitution and molecular structure of mixed surfactants on the water/oil interface is also an important factor affecting the formation of nanoemulsions after dispersion in a medium. Recently, Wang et al. (7) reported that different pairs of surfactants with similar structure (i.e., Tween® and Span®) at the optimum HLB value corresponding oil phases provide different droplet sizes depending on the molecular structure of the surfactant. To date, there are a limited number of published reports available in the literature that have prepared the SNEDDS by using mixed surfactants of polysorbates based on the HLB of the mixture (e.g., 7,11). The pharmaceutical nanoemulsions are often composed of other surfactants with different structures, for example, polyoxyls, which has not been reported in the literature. Therefore, it is interesting to investigate the effect of the HLB of different blends of surfactants.
For a poorly water-soluble drug, drug dissolution was the slowest step during the drug absorption process, causing low drug bioavailability. In this study, nifedipine (NDP), a well-known and most widely used coronary vasodilator from the group of dihydropyridine derivatives, was chosen as a model drug. NDP is practically insoluble in water, with a solubility of 5.8 mg/L in water, pKa <1, and log P of 2.50 (12). Numerous attempts have been made to improve its therapeutic efficacy and patient compliance, for example, pellets, granules, microparticles, and solid dispersions using different carriers (e.g., urea, lactose, polyethylene glycol, polyvinylpyrrolidone, phospholipids, polycaprolactone, acrylic polymers, etc.).
In the present study, the solid SNEDDS containing NDP was developed based on the HLB of mixed surfactants. To select the oil and surfactant in the formulation, the solubility of NDP in various vehicles including oils and surfactant was determined. Different surfactants and their combinations, depending on the HLB value, were used for preparing SNEDDS. Solid SNEDDS was prepared by mixing the SNEDDS with inert solid carriers. The SNEDDS and solid SNEDDS were then characterized for their size after dispersion, morphology, physicochemical properties, and drug dissolution behavior.
MATERIALS AND METHODS
Materials
NDP was purchased from Xilin Pharmaceutical Raw Material Co., Ltd. (Jintan, China). As NDP was light-sensitive, all samples were kept in an amber-colored container or wrapped in aluminum foil during the whole experimental process. Olive oil, castor oil, sunflower oil, almond oil, apricot oil, and coconut oil were purchased from P.C. Drug Center (Bangkok, Thailand). Caprylic/capric triglycerides (Miglyol® 812 and Miglyol® 810) and caprylic/capric glyceride (Imwitor® 742) were purchased form Sasol (Brunsbüttel, Germany). Miglyol® 812 and Miglyol® 810 differ only in the C8/C10 ratio. Miglyol® 810 has lower C10 content than Miglyol® 812. Hexanoic acid, octanoic acid, decanoic acid, oleic acid, and ricinoleic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Polyoxyethylene (20) sorbitan monolaurate (Tween® 20; HLB, 16.7), polyoxyethylene (20) sorbitan monooleate (Tween® 80; HLB, 15.0), sorbitan monolaurate (Span® 20; HLB, 8.6), and sorbitan monooleate (Span® 80; HLB, 4.3) were purchased from Fluka (St. Louis, MO, USA). Polyoxyl 40 hydrogenated castor oil (Cremophor® RH40; HLB, 14–16) and polyoxyl 35 castor oil (Cremophor® EL; HLB, 12–14) were a gift from BASF (Thai) Co., Ltd. (Khlong Toei, Thailand). The chemical structure of the investigated surfactants is shown in Fig. 1. Fumed silica hydrophilic grade (Aerosil® 200) and hydrophobic grade (Aerosil® R972) were supplied by Evonik Industries (Essen, Germany).
Fig. 1.
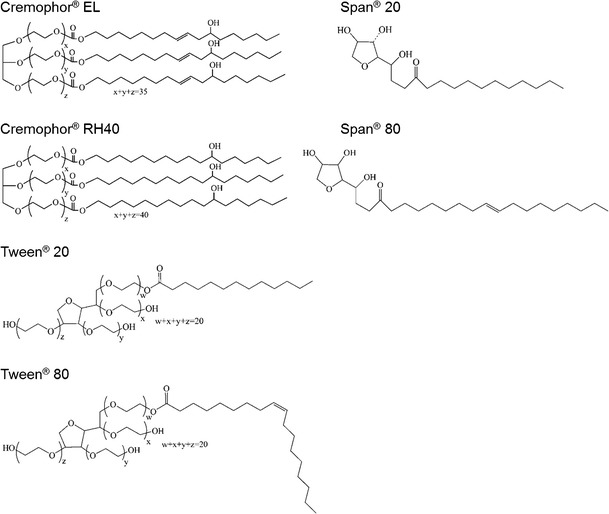
Chemical structure of the surfactants investigated in this study
Determination of Drug Solubility in Various Vehicles
The solubility of NDP was determined in various vehicles by adding an excess amount of NDP (500 mg) in 1 mL of pure vehicle in glass tubes. The drug suspension was equilibrated at 25°C in a thermostatically controlled bath for 72 h. After equilibration, the tubes were centrifuged at 3,500 rpm for 15 min and the clear supernatants were analyzed for NDP with a high-performance liquid chromatography (HPLC; model JASCO PU-2089plus quaternary gradient inert pump and JASCO UV-2070plus multiwavelength UV–Vis detector; Jasco, Tokyo, Japan) using Luna 5u C18 column (5 μm, 4.6 nm × 25 cm) (Phenomenex, Torrance, CA, USA). The mobile phase composing of water, acetonitrile, and methanol (50:25:25) was filtered through a 0.22-μm membrane filter and degassed in a sonicator bath before use. The flow rate of the mobile phase was 1 mL/min, and the UV detection wavelength was 235 nm.
Preparation of Mixed Surfactant System
A frequently used method for the selection of surfactants is known as the HLB method. The hydrophilic surfactant (Tween® 20, Tween® 80, Cremophor® RH40, or Cremophor® EL) was mixed with a hydrophobic surfactant (Span® 20 or Span® 80). There were eight binary mixed surfactant systems obtained as follows: Tween® 20/Span® 20, Tween® 20/Span® 80, Tween® 80/Span® 20, Tween® 80/Span® 80, Cremophor® RH40/Span® 20, Cremophor® RH40/Span® 80, Cremophor® EL/Span® 20, and Cremophor® EL/Span® 80. The HLB number of each mixed surfactant system (HLBmix) was calculated by the following equation:
 |
1 |
where HLBA and HLBB are HLB values and fA and fB are the weight fractions of surfactant A and surfactant B, respectively. The HLBmix required in this study ranged from 8 to 15 (13). The surfactant mixing ratio calculated from the previously mentioned equation was defined as the weight percent of the corresponding surfactants, as shown in Table I.
Table I.
Ratio of Mixed Surfactants Used in the Formulations Prepared at Various HLB Values
| HLB | Surfactant mixing ratio (weight percent) | |||||||
|---|---|---|---|---|---|---|---|---|
| Tween® 20/Span® 20 | Tween® 20/Span® 80 | Tween® 80/Spa® 20 | Tween® 80/Span® 80 | Cremophor® EL/Span® 20 | Cremophor® EL/Span® 80 | Cremophor® RH40/Span® 20 | Cremophor® RH40/Span® 80 | |
| 8 | N/A | 29.8/70.2 | N/A | 34.6/65.4 | N/A | 42.5/57.5 | N/A | 34.6/65.4 |
| 8.5 | N/A | 33.9/66.1 | N/A | 39.3/60.7 | N/A | 48.3/51.7 | N/A | 39.3/60.9 |
| 8.6 | 0/100 | 34.7/65.3 | 0/100 | 40.2/59.8 | 0/100 | 49.4/50.6 | 0/100 | 40.2/59.8 |
| 9 | 4.9/95.1 | 37.9/62.1 | 6.3/93.2 | 43.9/56.1 | 9.1/90.9 | 54.0/46.0 | 6.3/93.7 | 43.9/56.1 |
| 9.5 | 11.2/88.9 | 41.9/58.1 | 14.1/85.9 | 48.6/51.4 | 20.5/79.5 | 59.8/40.2 | 14.1/85.9 | 48.6/51.4 |
| 10 | 17.3/82.7 | 46.0/54.0 | 21.9/78.1 | 53.3/46.7 | 31.8/68.2 | 65.5/34.5 | 21.9/78.1 | 53.3/46.7 |
| 10.5 | 23.5/76.5 | 50.0/50.0 | 29.7/70.3 | 57.9/42.1 | 43.2/56.8 | 71.3/28.7 | 29.7/70.3 | 57.9/42.1 |
| 11 | 29.6/70.4 | 54.0/46.0 | 37.5/62.5 | 62.6/37.4 | 54.5/45.5 | 77.0/23.0 | 37.5/62.5 | 62.5/37.4 |
| 11.5 | 35.8/64.2 | 58.1/41.9 | 45.3/54.7 | 67.3/32.7 | 65.9/34.1 | 82.8/17.2 | 45.3/54.7 | 67.3/32.7 |
| 12 | 42.0/58.0 | 62.1/37.9 | 53.1/46.9 | 72.0/28.0 | 77.3/22.7 | 88.5/11.5 | 53.1/46.9 | 72.0/28.0 |
| 12.5 | 48.1/51.9 | 66.1/33.9 | 60.9/39.1 | 76.6/23.4 | 88.6/11.4 | 94.3/5.7 | 60.9/39.1 | 76.6/23.4 |
| 13 | 54.3/45.7 | 70.2/29.8 | 68.8/31.3 | 81.3/18.7 | 100/0 | 100/0 | 68.8/31.2 | 81.3/18.7 |
| 13.5 | 60.5/39.5 | 74.2/25.8 | 76.6/23.4 | 86.0/14.0 | N/A | N/A | 76.6/23.4 | 86.0/14.0 |
| 14 | 66.7/33.3 | 78.2/21.8 | 84.4/15.6 | 90.7/9.3 | N/A | N/A | 84.4/15.6 | 90.7/9.3 |
| 14.5 | 72.8/27.2 | 82.3/17.7 | 92.2/7.8 | 95.3/4.7 | N/A | N/A | 92.2/7.8 | 95.3/4.7 |
| 15 | 79.0/21.0 | 86.3/13.7 | 100/0 | 100/0 | N/A | N/A | 100/0 | 100/0 |
N/A not applicable
Preparation of SNEDDS Formulations
Preparation of Liquid SNEDDS
The formulations were prepared by mixing oil (Imwitor® 742) with mixed surfactant systems at a ratio of 1:1. The formulations were stored at ambient temperature (25°C) until further use. Selected formulations that corresponded to an HLBmix of 10 were loaded with NDP at the concentration of 30 mg/mL. Drug-loaded formulations were stored in the amber glass container, at 25°C, for 3 days to observe immediate stability. Unstable formulations (e.g., precipitation of drug crystals and/or phase separation) were excluded from the study.
Preparation of Solid SNEDDS
Selected SNEDDS formulations were simply adsorbed onto two types of silicon dioxide, i.e., Aerosil® 200 (hydrophilic grade) and Aerosil® R972 (hydrophobic grade), by trituration using 20%, 30%, 40%, and 50% (w/w) silicon dioxide in the formulation.
Characterization of SNEDDS
The formulations were reconstituted by gentle mixing in distilled water at a dilution ratio of 1:100. The mixtures were gently folded and stored at ambient temperature (25°C) for 2 h before further characterization. In case of solid SNEDDS, the mixtures were also centrifuged at a speed of 2,000 rpm for 10 min to separate the solid carriers.
Visual Observation of Self-emulsification
Evaluation of the self-emulsifying properties of SNEDDS was visually observed (i.e., until a clear homogenous system was obtained).
Droplet Size Analysis
The droplet size of the emulsion formed after reconstitution of SNEDDS was determined by static laser light scattering (model LA-950; Horiba, Kyoto, Japan). Reconstituted samples were withdrawn and diluted to a final droplet concentration of approximately 0.05% (w/w) with distilled water. The instrument measured the angular dependence of the intensity of the laser beam scattered by the dilute emulsions and then used the Mie theory to calculate the droplet size distributions that gave the best fit between theoretical predictions and empirical measurements. A relative refractive index of 1.2 (ratio of the indices between the oil and water phases) was used. All measurements were repeated three times, and the values of mean diameter were reported.
Morphology Examination of Solid SNEDDS
The external structure of the solid SNEDDS was investigated by a scanning electron microscope (SEM; model Maxim-2000; CamScan Analytical, London, England) with an accelerating voltage of 15 keV. The samples were fixed on a stub using double-sided adhesive tape and coated in a vacuum with thin gold layer before investigation.
Solid State Characterization of Solid SNEDDS
The physical state of NDP in solid SEDDS was characterized by differential scanning calorimetry (DSC; model Sapphire DSC; Perkin Elmer, Waltham, MA, USA). The samples were placed in standard aluminum pans and scanned at a temperature ramp speed of 10°C/min and heat flow from 20°C to 200°C. Furthermore, powder X-ray scattering measurements were carried out with a powder X-ray diffractometer (PXRD; model MiniFlex II; Rigaku, Tokyo, Japan) at room temperature using monochromatic CuKα radiation at 15 mA and at 30 kV over a range of 2θ angles from 5° to 40° with an angular increment of 4°/min.
In Vitro Dissolution Study
The dissolution test was carried out using a USP dissolution apparatus II (Pharma Test, Hainburg, Germany) with 900 mL of simulated gastric fluid (SGF) USP without pepsin (pH 1.2) as a dissolution medium at 37 ± 0.5°C. The paddle speed was adjusted to 50 rpm. Different formulations (NDP powder, liquid SNEDDS, and solid SEDDS (A40 and A50)) filled in hard capsules (equivalent to 10 mg of NDP) were put into a sinker before placing in a dissolution vessel, which was protected from light. At predetermined time intervals (i.e., 5, 10, 15, 30, 60, 90, and 120 min), 5-mL aliquots of the medium were collected, filtered through 0.45-μm nylon membrane filters to remove the agglomerated silicon dioxide, and analyzed for the NDP concentration by HPLC analysis as mentioned previously. The same volume (5 mL) of fresh medium was added to compensate for the loss due to sampling. The dissolution experiments were carried out in triplicate.
Statistical Analysis
Analysis of variance and Levene’s test for homogeneity of variance were carried out using SPSS version 10.0 for Windows (SPSS Inc., Chicago, IL, USA). Post hoc testing (p < 0.05) of the multiple comparisons was performed by either the Scheffé or Games–Howell test depending on whether Levene’s test was insignificant or significant, respectively.
RESULTS AND DISCUSSION
Solubility of NDP in Various Vehicles
In the formulation of SNEDDS, the selection of suitable oil, surfactant, and cosurfactant has an important role in enhancing the solubility of drug and drug loading. The components in SNEDDS formulations should be selected to have maximum drug solubility along with good miscibility with each other to produce a stable formulation (14). The solubility of NDP in various vehicles is presented in Table II. Higher solubility of NDP in the oil phase was an important criterion, as it would help the nanoemulsion to maintain the drug in solubilized form. Among the oils and fatty acids tested in this study, Imwitor® 742 (caprylic/capric glyceride), which is an amphiphilic compound with surface active property, showed the highest solubility of NDP and was then selected as an oil component. Imwitor® 742 can promote water penetration and self-dispersibility of lipid formulations and had good solvent capacity for NDP. Some hydroxyl groups within the glycerol ester of Imwitor® 742 are free, contributing to its polarity and excellent solvent properties for many drugs. NDP solubility in fatty acids was comparable to the vegetable oils and fatty acid esters (Miglyol®) but much lower than Imwitor® 742.
Table II.
Solubility of Nifedipine in Various Vehicles (n = 3)
| Vehicle | Solubility of nifedipine (μg/mL) |
|---|---|
| Oils | |
| Almond oils | 1,322.6 ± 207.4 |
| Apricot oils | 1,209.6 ± 22.3 |
| Caster oils | 7,656.5 ± 2,003.2 |
| Coconut oils | 1,869.6 ± 206.3 |
| Imwitor® 742 | 11,828.4 ± 1,655.3 |
| Miglyol® 810 | 4,027.0 ± 258.2 |
| Miglyol® 812 | 3,867.8 ± 152.0 |
| Olive oils | 1,244.9 ± 208.8 |
| Sunflower oils | 1,511.7 ± 174.9 |
| Fatty acids | |
| Decanoic acid | 1,590.5 ± 166.3 |
| Hexanoic acid | 4,020.3 ± 247.0 |
| Octanoic acid | 2,579.3 ± 199.8 |
| Oleic acid | 452.8 ± 66.8 |
| Ricinoleic acid | 3,271.5 ± 309.4 |
| Surfactants | |
| Cremophor® EL (HLB, 12–14) | 61,270.3 ± 6,150.7 |
| Cremophor® RH40 (HLB, 14–16) | 67,214.5 ± 9,823.5 |
| Span® 20 (HLB, 8.6) | 3,073.3 ± 69.2 |
| Span® 80 (HLB, 4.3) | 2,822.1 ± 535.1 |
| Tween® 20 (HLB, 16.7) | 76,066.3 ± 22,768.9 |
| Tween® 80 (HLB, 15) | 66,988.4 ± 2,479.5 |
| Solvents | |
| Acetonitrile | 8,461.4 ± 433.2 |
| Ethanol | 25,038.4 ± 6,540.4 |
| Isopropanol | 20,458.0 ± 6,596.8 |
| Water | 5.8 ± 0.1 |
Various hydrophilic nonionic surfactants with a relatively high HLB, such as polysorbates (Tween®) and polyoxyls (Cremophor®), have been widely used due to their relatively low toxicity (15). The results shown in Table II suggest that NDP was highly soluble in Tween® and Cremophor®. Tween® 20 (HLB, 16.7) was found to have the maximum solubilizing capacity, while Span® 80 (HLB, 4.3) demonstrated the minimum solubilizing capacity for NDP. It could be seen that the solubility of NDP was dependent on the HLB of the surfactant; that is, the higher the HLB, the higher the solubility of NDP. The solubility of NDP in some organic solvents has also been tested as shown in Table II. This information can be used for selecting a suitable system for HPLC analysis.
Preparation and Characterization of SNEDDS
A surfactant dissolved in liquid can either adsorb at the interface or self-assemble to form micelles, resulting from the hydrophobic effect. The lyophobic group of the surfactant tends to be expulsed from the liquid in which the surfactant is dissolved. The adsorption of surfactants at the interface induces a structural change in the interfacial area and, in many cases, a decrease of the interfacial tension. It seems obvious that, by changing the surfactant, the interfacial tension decreases to a different degree which affects the final droplet size (16). The HLB method has been demonstrated to be a useful tool in selecting the optimal type of surfactants for a certain oil phase (11). SNEDDS was prepared with 50% (w/w) oil phase and 50% (w/w) mixed surfactants at HLB values ranging from 8 to 15. Eight series of mixed surfactants, consisting of four different types of hydrophilic surfactants (i.e., Tween® 20, Tween® 80, Cremophor® RH40, and Cremophor® EL) and two types of lipophilic surfactants (i.e., Span® 20 and Span® 80), were examined to determine the suitable HLB to obtain SNEDDS. Blends of surfactants at various ratios were used to prepare mixed surfactants with a range of HLB values (Table I).
The effect of the HLB of different mixed surfactants on the droplet size of emulsions obtained after dispersion in aqueous medium is shown in Figs. 2 and 3. The results suggested that, even though the HLB values were the same, there is a wide difference in the emulsion droplet size. It is obvious that the droplet size depended primarily on surfactant molecular structure. Nanoemulsions could be formed from mixtures of Cremophor® and Span® at suitable HLB (Fig. 2). It could be seen that the droplet size decreased when the HLB of mixed surfactants (Cremophor®/Span®) was >9 for those using Span® 80 and 11.5 for those using Span® 20. At an HLB of 10, for example, mixtures of Cremophor® (polyethoxylated castor oil, which is a blend of ricinoleic acid, polyglycol ester, glycerol polyglycol esters, and polyglycols) and lipophilic surfactant with longer CH chain length (C18, Span® 80) produced the nano-sized emulsion, while those with shorter CH chain length (C12, Span® 20) provided only micron-sized emulsion. From Fig. 2, it is observed that the increase in HLB exhibited a decreasing trend; that is, the emulsion droplet size decreases with a higher HLB. Based on the HLB calculation, higher values indicate that surfactants have higher hydrophilicity, which facilitates reduction in the curvature of the interface for the oil that own relatively high solubility, leading to smaller droplet size (17). This is the possible reason for the decreasing trend in emulsion droplet size.
Fig. 2.
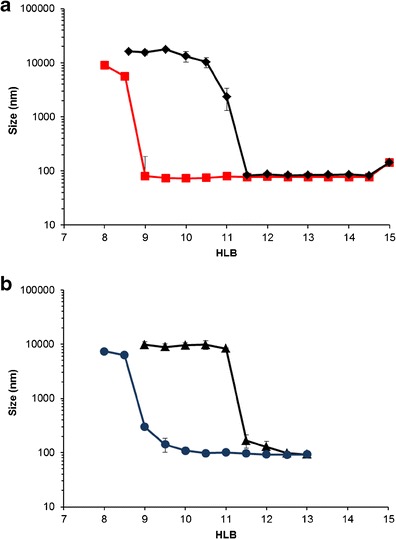
a, b Droplet size of emulsions containing Imwitor® 742 and Cremophor®/Span® system (ratio of 1:1) as a function of HLB. Diamond Cremophor® RH40/Span® 20, square Cremophor® RH40/Span® 80, triangle Cremophor® EL/Span® 20, and circle Cremophor® EL/Span® 80
Fig. 3.
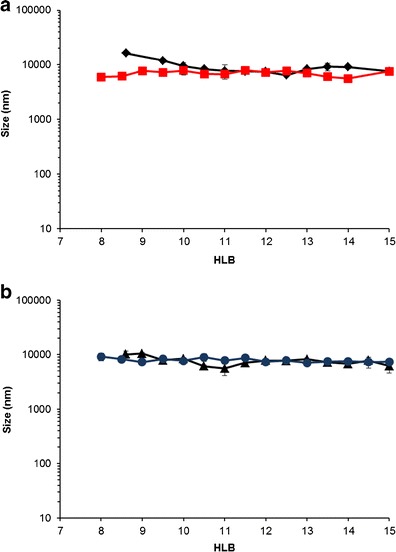
a, b Droplet size of emulsions containing Imwitor® 742 and Tween®/Span® system (ratio of 1:1) as a function of HLB. Diamond Tween® 80/Span® 20, square Tween® 80/Span® 80, triangle Tween® 20/Span® 20, and circle Tween® 20/Span® 80
In contrast, nanosized emulsions could not be obtained in the system containing Tween® and Span® (Fig. 3). In any combination of Tween® and Span®, the emulsion droplet size was not significantly different (approximately 10 μm). The correlation between HLB and mean droplet size demonstrates the nearly linear line rather than the sigmoid curve reported previously (7). This is probably attributable to the absence of cosurfactant in our experiments. In their formulations, however, the cosurfactant (i.e., 1,2-octanediol) is added in the formulations containing mixtures of Tween® 80 and Span® 20. The addition of 1,2-octanediol decreases the surfactant content necessary to produce nanoemulsions and significantly affects the droplet size (7). Without the cosurfactant, it is difficult to reduce the effective HLB to a value within the range required for nanoemulsion formation.
Figure 4 shows the schematic representation of the configuration into oil-in-water (nano)emulsion. The results are in agreement with Dai et al. (18) who reported that the molecular structure of the surfactant has a significant effect on the final emulsion droplet size. However, the change in hydrophilic surfactant (Cremophor® EL and Cremophor® RH40) in mixed surfactants, when the lipophilic surfactant remained unchanged, did not influence the emulsion droplet size. This means that the structure of Cremophor® has a greater effect on the droplet size than that of Span®. These results suggested that the difference in CH chain length between Cremophor® and Span® assisted the formation of nanoemulsions. However, at HLB = 12–13, nanoemulsions can be produced from all Cremophor® and Span® blends. It seems that a branch alkyl structure of both Cremophor® EL and Cremophor® RH40 had an effect on the penetration of oil onto the curved film interface, thus resulting in the self-formation of nanoemulsion (19).
Fig. 4.
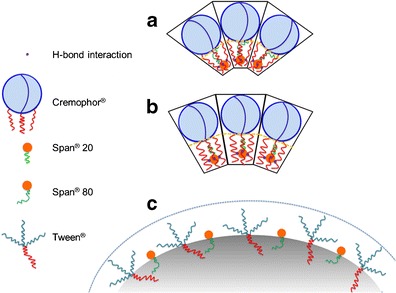
Schematic representation of the micellar configuration into oil-in-water (nano)emulsion containing a Cremophor®/Span® 80, b Cremophor®/Span® 20, and c Tween®/Span®80
Molecular modeling and docking studies of Cremophor® EL and γ-tocotrienol were reported by Alayoubi et al. (20). The low-energy docked structures clearly suggested that γ-tocotrienol binds to Cremophor® EL deep inside the hydrophobic pocket. For Cremophor® EL, it was observed that most of the low-energy structures are formed when the isoprenyl group of γ-tocotrienol is docked near the hydrophobic acyl chains, forming a hydrogen bond with the hydroxyl group of Cremophor® EL. In this study, the molecule of Span®, which is structurally similar to γ-tocotrienol, may be docked in the hydrophobic pocket size in Cremophor®. Chemically, Span® 80, featuring unsaturated fatty acid side chains, may repulse the hydrophobic chain in Cremophor®, attributing to smaller droplet size of emulsion than Span® 20 (saturated fatty acid), as presented in Fig. 4.
From the results obtained, the SNEDDS formulation containing a mixture of Cremophor® RH40 and Span® 80 at a ratio that corresponded to an HLB of 10 was selected for loading a poorly water-soluble drug, NDP (about 30 mg/mL). The NDP-loaded SNEDDS was incubated at 25°C for 3 days. A yellow and clear solution was apparently observed without drug precipitation and phase separation. The SNEDDS formulation was diluted (100-fold) with distilled water and then kept for 2 h before droplet size measurements. Droplet size of both blank and NDP-loaded SNEDDS after dispersing in aqueous medium was found to be similar (approximately 73–74 nm), as shown in Table III. This confirmed the self-nanoemulsifying nature of SNEDDS.
Table III.
Droplet Size of SNEDDS Using a Mixed Surfactant of Cremophor® RH40/Span® 80, Prepared at an HLB of 10, After Dispersion in Water (n = 3)
| Formulation | Size (nm) ± SD |
|---|---|
| Blank SNEDDS | 73.1 ± 1.0 |
| Nifedipine-loaded SNEDDS | 74.0 ± 0.8 |
| Nifedipine-loaded solid SNEDDS | 75.0 ± 2.0 |
Preparation and Characterization of Solid SNEDDS
The selected NDP-loaded SNEDDS formulation (using Imwitor® 742 and mixed surfactant of Cremophor® RH40 and Span® 80, at an HLB value of 10) was adsorbed onto fumed silica (amorphous anhydrous colloidal silicon dioxide), i.e., Aerosil® 200 or Aerosil® R972. When the amount of hydrophilic adsorbent, Aerosil® 200, in the formulation was 20%, a paste-like, semisolid mass was obtained after incorporating liquid SNEDDS to the adsorbent. However, free-flowing powders were obtained when the amount of Aerosil® 200 was 30% or more based on its large surface area (200 m2/g). Jannin et al. (21) stated that up to 70% w/w of SEDDS is possible to be adsorbed onto suitable solid carriers. By using hydrophobic adsorbent, Aerosil® R972 (surface area of 110 m2/g), liquid SNEDDS was readily transformed to highly viscous oleogels regardless of the amount of adsorbent (i.e., 20–50% in the formulation). Figure 5 shows the SEM images of Aerosil® 200, Aerosil® R972, and solid SNEDDS containing 40% of Aerosil® 200 or Aerosil® R972. Both Aerosil® 200 and Aerosil® R972 appeared with a rough surface with porous particles (Fig. 5a, b). However, the solid SNEDDS appeared as smooth surface particles agglomerated to form larger particles (Fig. 5c, d). This indicated that the liquid SNEDDS is adsorbed or coated on the surface of Aerosil®. Moreover, the solid SNEDDS containing Aerosil® R972 showed a smoother surface than that containing Aerosil® 200, resulting in its appearance as gel. No distinct crystal was evident on the surface of the particles after sorbing the liquid SNEDDS on the surface of Aerosil®.
Fig. 5.
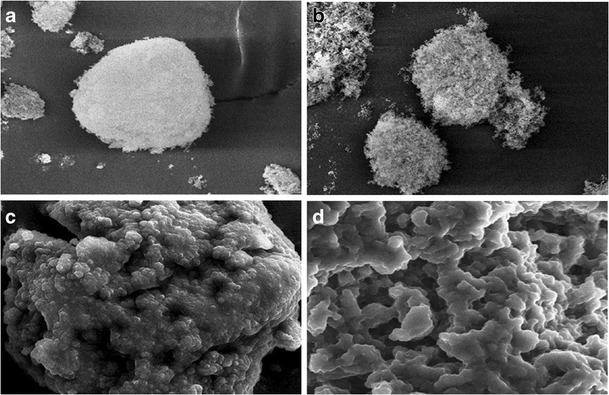
SEM images of a Aerosil® 200, b Aerosil® R972, c solid SNEDDS containing 40% Aerosil® 200, and d solid SNEDDS containing 40% Aerosil® R972
The solid state properties of NDP in the solid SNEDDS were investigated because it would have an important influence on the in vitro dissolution and in vivo release characteristics. The DSC curves of NDP, physical mixture of NDP and Aerosil®, SNEDDS, and solid SNEDDS formulations containing a mixture of Cremophor® RH40 and Span® 80 are shown in Fig. 6. Pure NDP showed a sharp endothermic peak at approximately 174°C. Aerosil® 200 and Aerosil® R972 did not show any peak over the whole range of temperatures tested. The physical mixture showed a small endothermic peak for NDP. No representative peak of NDP was observed for solid SNEDDS formulations, indicating that the drug was present in molecularly dissolved state in solid SNEDDS (22). The PXRD patterns of NDP, physical mixture of NDP and Aerosil®, SNEDDS, and solid SNEDDS formulations containing a mixture of Cremophor® RH40 and Span® 80 are shown in Fig. 7. The NDP raw material is crystalline as demonstrated by sharp and high-intensity peaks. Both Aerosil® powders are amorphous, having no crystalline structure. The same characteristic peaks of NDP but with low intensity were observed in the physical mixture of NDP and Aerosil®. All the SNEDDS and solid SNEDDS formulations did not show the characteristic peaks of NDP. These findings suggest that the NDP crystals are converted into the amorphous form in the SNEDDS.
Fig. 6.
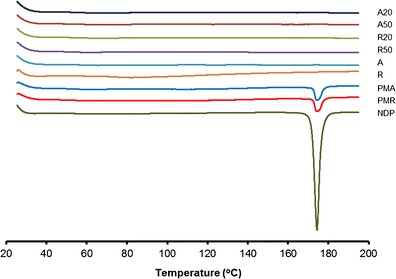
DSC thermograms of NDP, physical mixture, SNEDDS, and solid SNEDDS formulations containing Cremophor® RH40 and Span® 80. NDP nifedipine, A Aerosil® 200, R Aerosil® R972, PMA physical mixture of NDP and Aerosil® 200, PMR physical mixture of NDP and Aerosil® R972, A20 solid SNEDDS containing 20% Aerosil® 200, A50 solid SNEDDS containing 50% Aerosil® 200, R20 solid SNEDDS containing 20% Aerosil® R972, R50 solid SNEDDS containing 50% Aerosil® R972
Fig. 7.
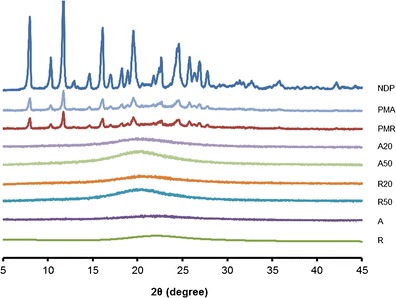
Powder X-ray diffractograms of NDP, physical mixture, SNEDDS, and solid SNEDDS formulations containing Cremophor® RH40 and Span® 80. NDP nifedipine, A Aerosil® 200, R Aerosil® R972, PMA physical mixture of NDP and Aerosil® 200, PMR physical mixture of NDP and Aerosil® R972, A20 solid SNEDDS containing 20% Aerosil® 200, A50 solid SNEDDS containing 50% Aerosil® 200, R20 solid SNEDDS containing 20% Aerosil® R972, R50 solid SNEDDS containing 50% Aerosil® R972
In Vitro Dissolution Test
In vitro dissolution experiments were conducted to evaluate the effect of different types and amounts of adsorbent on the dissolution of NDP from the solid SNEDDS formulations. Figure 8 shows the percentage of NDP dissolved from different formulations in SGF. NDP dissolution in SGF was incomplete, i.e., approximately 10% of NDP dissolved, and precipitation of NDP was observed. In the self-emulsifying system, a mixture of oil, surfactant, and drug forms oil-in-water emulsions when introduced into an aqueous phase. It is suggested that the oil/surfactant and water phases effectively swell, decrease the oil droplet size, and eventually increase the dissolution rate. As shown in Fig. 8, the dissolution of NDP from SNEDDS and solid SNEDDS was significantly improved, compared with the NDP powder, and no precipitation was noticed until the end of the experiment. This might be due to the increased effective surface area and alteration in the native crystalline form of the drug, as discussed previously.
Fig. 8.
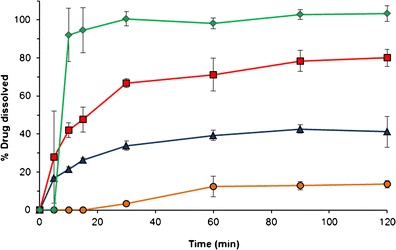
Percentage of NDP released from different formulations, in SGF, at 37°C. Diamond liquid SNEDDS filled in hard capsule, square A50, triangle A40, and circle NDP powder. NDP nifedipine, A50 solid SNEDDS containing 50% Aerosil® 200, A40 solid SNEDDS containing 40% Aerosil® 200
The liquid SNEDDS formulation gave dissolution of approximately 90% within 10 min, after the dissolution of hard gelatin capsule, as a result of fast spontaneous emulsion formation. The drug dissolution from solid SNEDDS formulations was lower than that of liquid SNEDDS. It is possible that the desorption process from the adsorbent may delay the first step of drug dissolution. Moreover, excipients such as Aerosil® may have a relatively strong interaction with the adsorbed SNEDDS, impairing the dissolution and extent of NDP. The percentage of NDP dissolved in SGF at 20 and 120 min is shown in Table IV. The NDP dissolved from most of the formulations containing Aerosil® R972 was lower than that containing Aerosil® 200 at the same amount. It is likely that the viscous oleogels of the formulations containing Aerosil® R972 retarded drug dissolution from the solid SNEDDS. Furthermore, drug dissolution was improved when the amount of Aerosil® 200 was increased. This may result from the free-flowing characteristics of the solid SNEDDS obtained from the higher amount of Aerosil® 200.
Table IV.
Dissolution of Nifedipine in SGF After 20 and 120 min (n = 3)
| Sample | % Drug dissolved | |
|---|---|---|
| At 20 min | At 120 min | |
| A20 | 11.23 | 19.88 |
| A30 | 11.96 | 19.82 |
| A40 | 28.79 | 41.20 |
| A50 | 54.00 | 80.05 |
| R20 | 15.50 | 30.51 |
| R30 | 11.87 | 15.34 |
| R40 | 16.20 | 20.62 |
| R50 | 20.51 | 32.80 |
| Nifedipine powder | 1.15 | 13.58 |
| Liquid SNEDDS filled in hard capsules | 92.89 | 103.35 |
CONCLUSION
The present study has demonstrated the development of the SNEDDS based on the HLB of mixed surfactants. Depending on the molecular structure of the surfactants used, the change in HLB affected the size of emulsion droplets after dispersing in the aqueous medium. The use of Cremophor®/Span® blends resulted in SNEDDS that can produce nano-sized emulsions after dispersion. The selected formulation was adsorbed onto Aerosil® 200 or Aerosil® R972 to produce solid SNEDDS. The formulations using higher amounts (30–50% w/w) of Aerosil® 200 exhibited good flow properties with smooth surface and preserved the self-emulsifying properties of liquid SNEDDS. The DSC and PXRD analyses indicated that NDP in solid SNEDDS may be in the molecular dispersion state. In vitro dissolution study demonstrated greater drug dissolution profiles of solid SNEDDS compared with NDP. It is concluded that the NDP-loaded solid SNEDDS containing mixed surfactants for oral administration is a promising dosage form with good in vitro pharmaceutical results.
ACKNOWLEDGMENTS
The authors wish to acknowledge The Thailand Research Fund for the financial support (grant number BRG5480013). YW is financially supported by the Royal Golden Jubilee Ph.D. Program (grant number PHD/0346/2550). The authors thank BASF (Thai) Co., Ltd. (Thailand) and Gattefosse (France) who kindly donated the samples.
REFERENCES
- 1.Neslihan GR, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Mu H, Holm R, Mullertz A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 2013;453(1):215–24. doi: 10.1016/j.ijpharm.2013.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomed. 2010;5(10):1595–616. [DOI] [PubMed]
- 4.Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25(1):47–58. doi: 10.1016/S0169-409X(96)00490-5. [DOI] [Google Scholar]
- 5.Cole ET, Cade D, Benameur H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv Drug Deliv Rev. 2008;60(6):747–56. doi: 10.1016/j.addr.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Kallakunta VR, Bandari S, Jukanti R, Veerareddy PR. Oral self emulsifying powder of lercanidipine hydrochloride: formulation and evaluation. Powd Technol. 2012;221:375–82. doi: 10.1016/j.powtec.2012.01.032. [DOI] [Google Scholar]
- 7.Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci. 2009;330(2):443–8. doi: 10.1016/j.jcis.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan CD, Rice DA. Predicting o/w emulsion stability by the “required HLB equation”. J Disper Sci Technol. 1990;11(1):83–91. doi: 10.1080/01932699008943238. [DOI] [Google Scholar]
- 9.Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010;15(21–22):958–65. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Razavizadeh BM, Mousavi-Khoshdel M, Gharibi H, Behjatmanesh-Ardakani R, Javadian S, Sohrabi B. Thermodynamic studies of mixed ionic/nonionic surfactant systems. J Colloid Interface Sci. 2004;276(1):197–207. [DOI] [PubMed]
- 11.Gullapalli RP, Sheth BB. Influence of an optimized non-ionic emulsifier blend on properties of oil-in-water emulsions. Eur J Pharm Biopharm. 1999;48(3):233–8. doi: 10.1016/S0939-6411(99)00048-X. [DOI] [PubMed] [Google Scholar]
- 12.Yalkowsky S, He Y, Jain P. Handbook of aqueous solubility data. Tucson: CRC; 2010. [Google Scholar]
- 13.Buyukozturk F, Benneyan JC, Carrier RL. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J Control Rel. 2010;142(1):22–30. doi: 10.1016/j.jconrel.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Balakrishnan P, Lee BJ, Oh DH, Kim JO, Hong MJ, Jee JP, et al. Enhanced oral bioavailability of dexibuprofen by a novel solid self-emulsifying drug delivery system (SEDDS) Eur J Pharm Biopharm. 2009;72(3):539–45. doi: 10.1016/j.ejpb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Hauss DJ. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59(7):667–76. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Myers D. Surfaces, Interfaces, and Colloids. New York: Wiley; 2002. Surface activity and surfactant structures; pp. 21–39. [Google Scholar]
- 17.Kong M, Chen X, Park H. Design and investigation of nanoemulsified carrier based on amphiphile-modified hyaluronic acid. Carbohydr Polym. 2011;83(2):462–9.
- 18.Dai L, Li W, Hou X. Effect of the molecular structure of mixed nonionic surfactants on the temperature of miniemulsion formation. Colloids Surf A Physicochem Eng Asp. 1997;125(1):27–32. doi: 10.1016/S0927-7757(96)03859-9. [DOI] [Google Scholar]
- 19.Rao SV, Shao J. Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs: I. Formulation development. Int J Pharm. 2008;362(1–2):2–9. doi: 10.1016/j.ijpharm.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Alayoubi A, Satyanarayanajois SD, Sylvester PW, Nazzal S. Molecular modelling and multisimplex optimization of tocotrienol-rich self emulsified drug delivery systems. Int J Pharm. 2012;426(1–2):153–61. doi: 10.1016/j.ijpharm.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev. 2008;60(6):734–46. doi: 10.1016/j.addr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi H, Nagira S, Yamamoto H, Kawashima Y. Solid dispersion particles of amorphous indomethacin with fine porous silica particles by using spray-drying method. Int J Pharm. 2005;293(1–2):155–64. doi: 10.1016/j.ijpharm.2004.12.019. [DOI] [PubMed] [Google Scholar]


