Abstract
This study determined the physical, compressional, and binding properties of neem gum (NMG) obtained from the trunk of Azadirachta indica (A Juss) in a paracetamol tablet formulation in comparison with official Acacia gum BP (ACA). The physical and flow properties were evaluated using density parameters: porosity, Carr’s index, Hausner’s ratio, and flow rate. Compressional properties were analyzed using Heckel and Kawakita equations. The tensile strength, brittle fracture index, and crushing strength–friability/disintegration time ratio were used to evaluate the mechanical properties of paracetamol tablets while the drug release properties of the tablets were assessed using disintegration time and dissolution times. Tablet formulations containing NMG exhibited faster onset and higher amount of plastic deformation during compression than those containing ACA. Neem gum produced paracetamol tablets with lower mechanical strength; however, the tendency of the tablets to cap or laminate was lower when compared to those containing ACA. Inclusion of NMG improved the balance between binding and disintegration properties of paracetamol tablets produced than those containing ACA. Neem gum produced paracetamol tablets with lower disintegration and dissolution times than those containing ACA.
KEY WORDS: brittle fracture index, crushing strength–friability/disintegration time ratio, in vitro release profile, neem gum, tensile strength
INTRODUCTION
Binders are employed in pharmaceutical tablet formulations to provide adequate mechanical properties by promoting the bonding existing between the different components of a powder mix in a formulation thereby enhancing the strength of the eventual tablet produced (2,16,25). Various natural, synthetic, and semi-synthetic substances such as starches, celluloses, and gums have been employed in pharmaceutical tablet formulations as binders (2,4,25). Gum is an example of hydrophilic substances employed in pharmaceutical solid dose formulations mainly as binders and directly compressible excipients (26).
Although previous studies have evaluated neem gum in one form or the other as a tablet binder (9,25,30), suspending and film coating agent (19,30), or as a directly compressible excipient (26), the present study assessed the activity of neem gum as a binder by evaluating various properties of the granules and tablets prepared with the gum. The flow and compressional properties were used in evaluating the granules while the tablets were assessed by the mechanical property, crushing strength–friability/disintegration time ratio (CSFR/DT) and in vitro drug release profile.
The flow property of the granules was determined using the Hausner’s ratio, Carr’s index, and flow rate. In evaluating the compression data, Heckel and Kawakita and Ludde equations (11,17) were used, while tensile strength and brittle fracture index (BFI) were used to analyze the mechanical properties of the paracetamol tablets. The CSFR/DT was used to evaluate the tablet quality by assessing the balance between the strength and weakness of the paracetamol tablets.
The mechanical properties of tablets can be evaluated by measuring the bond strength using tensile strength (T) and lamination tendency using the BFI values. The BFI, which is calculated from the tensile strength of tablets with (the hole acting as a stress concentrator within the tablet) and without holes according to Eq. 7, measures the localized stress relief within tablets, the tendency of a tablet to cap or laminate. The BFI value ranges from 0 to 1, with values approaching unity indicating the propensity of the tablet to cap or laminate while values tending toward zero indicate the propensity of the material to relief localized stress (6,8,12,14,15,24,27).
The CSFR/DT or disintegration efficiency ratio of a tablet has been suggested and used as a better index of measuring tablet quality through the simultaneous evaluation of the tablet strength (crushing strength) and weakness (friability). It also evaluates the negative effects of these parameters (tensile strength and friability) on DT (6), while also assessing the usefulness of a binder in a formulation. A higher CSFR/DT value suggests a better balance between binding and disintegration properties (31). Paracetamol powder was chosen for this study because of its poor compressibility properties which therefore requires a binding agent in order to form good quality tablets.
MATERIALS AND METHODS
Materials
The materials used were paracetamol BP (BDH Ltd., Poole, England), rice starch BP and lactose BP (AB Knight and Co., London, UK), sodium chloride (BDH Ltd., Poole, England), acetone and 99.8% ethanol (Sigma-Aldrich Laborchemikalien GMBH, 30926 Seelze, Germany), Acacia gum BP (Hopkin and Williams Chadwell, Heath Essex, England), and neem gum obtained from the incised trunk of Azadirachta indica (A. Juss) tree at the Obafemi Awolowo University, Ile-Ife, Nigeria.
Methods
Collection and Purification of Neem Gum
Crude neem gum was collected from the incised bark of A. indica trees at the Obafemi Awolowo University premises. The collected neem gum was hydrated in sufficient amount of distilled water for 5 days with intermittent stirring, and extraneous materials were removed by filtering using a Buchner funnel under negative pressure. The gum from the filtered slurry was precipitated with 99.8% ethanol; the precipitated gum was filtered, washed several times with acetone, and dried in a hot air oven at 30°C for 96 h before milling and sieving with a mesh no. 60 (250 μm) and then stored in an amber-colored bottle until needed.
Preparation of Paracetamol Granules
The wet granulation method of massing and screening was employed. Batches (200 g) of a basic formulation of paracetamol (90%, w/w), rice starch (4%, w/w), and lactose (6%, w/w) were dry mixed for 5 min in a Hobart planetary mixer (Hobart Canada Inc., Don Mills, ON, Canada) and then moistened with appropriate amounts of binding solution to produce granules containing different concentrations of neem gum or Acacia gum according to the formulae in Table I. Massing was continued for 5 min after which the wet masses were passed manually through a no. 12 mesh sieve (1,400 μm) to granulate the wet masses. The granules were dried in a hot air oven for 24 h at 50°C and re-sieved through a no. 16 mesh (1,000 μm). The degree of granule mixing was then determined by spectrophotometric assay of paracetamol at 249 nm and was found to be >0.95. Particle densities were determined with acetone as the displacement fluid.
Table I.
Formulae of Paracetamol Batches
| Ingredients | Concentration (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | |
| Paracetamol | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| Lactose | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Rice starch | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Neem gum | 5 | 4 | 3 | 2 | 1 | – | – | – | – | – |
| Acacia gum | – | – | – | – | – | 5 | 4 | 3 | 2 | 1 |
Determination of Moisture Content
The moisture content of the formulation, determined with an Ohaus moisture balance (Ohaus Scale Corporation, Pine Brook, NJ, USA), was between 0.6 and 2.0% (w/w).
Flow Characterization of Paracetamol Granules
The Hausner’s ratio of the granules determined as the ratio of the initial bulk volume to the tapped volume was obtained by applying 100 taps to 30 g of each paracetamol granule batches in a graduated cylinder at a standardized rate of 38 taps per minute (26). The Carr’s index was obtained from the relationship [(tapped density − bulk density)/tapped density] × 100. The flow rate of the granule was determined from the time t; it took 30 g of the granule to pass through the orifice of an Erweka flow rate meter (Erweka Apparatebau GmbH, Heusenstamm, Germany). The determinations were done in quadruplicate.
Determination of Compressional Properties
Heckel equation, which is generally used for relating the relative density, D, of a powder bed during compression to the applied pressure, P, is written as
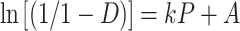 |
1 |
where the slope of the straight-line portion, k, is the reciprocal of the mean yield pressure, Py, of the material. The Py is inversely related to the ability of a material to deform plastically when compressed. The relative density, DA, can be calculated from the intercept, A, according to Eq. 2 (13).
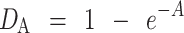 |
2 |
The Do describes the initial rearrangement phase of densification as a result of die filling at applied pressure zero while DB, which describes the phase of rearrangement at low pressures, is shown in Eq. 3.
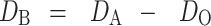 |
3 |
The degree of powder volume reduction, C, as described by Kawakita equation is shown in Eq. 4.
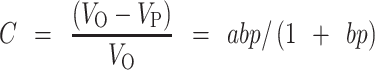 |
4 |
The equation can be rearranged to give P/C = P/a + 1/ab, where a and b are constants which describes the initial porosity of the powder material before compression and the plasticity of the material, respectively. The initial relative density of the material, Di, is calculated from the expression (1 − a). The Di explains the packed initial relative density of tablets upon application of small pressures like tapping. The load needed to reduce the powder bed by 50%, Pk, is calculated from the reciprocal of b (7,20,29). The Pk is an inverse measure of the amount of plastic deformation occurring during compression.
Preparation of Paracetamol Tablets
Tablets weighing 555 mg each were prepared from the 250 to 710 μm size fractions of granules by compressing them for 30 s with predetermined loads on a Carver hydraulic hand press (Model C, Carver Inc., Menomonee Falls, WI, US). Before each compression, the die (12.5 mm diameter) and the flat-faced punches were lubricated with a 2% (w/w) dispersion of magnesium stearate/talc (1:1) in acetone. After ejection, the tablets were stored over silica gel for 24 h to allow for elastic recovery and hardening and to prevent falsely low-yield values. Their weights (w) and dimensions were then determined within ±1 mg and 0.01 mm, respectively, and their relative densities (D) were calculated by using the following equation:
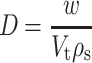 |
5 |
where Vt is the volume (in cubic centimeters) of tablet and ρs is the particle density (in grams per cubic centimeter) of the granules. The porosity of the granules was determined from the expression 1 − D.
Determination of Crushing Strength, Friability, and Tensile Strength of Paracetamol Tablets
A digital Erweka hardness tester (G.B. Caleva, Dorset, England, Typ: THT10) was used at room temperature to determine the load (F) required to diametrically break the tablet (crushing strength) into two equal halves. Tablets with signs of lamination and capping were not used. The friability of the tablets was determined with a Roche friabilator (Erweka Apparatebau, Germany) operated at 25 rev/min for 4 min. The mean of four determinations was taken for the crushing strength and friability values.
The tensile strength (T) of the normal tablets and apparent tensile strength (To) of tablets containing a hole were calculated using Eq. 6.
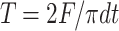 |
6 |
where T (or To) is the tensile strength of the tablet (in meganewtons per square meter), F is the load (in meganewtons) needed to cause fracture, d is the tablet diameter (in meters), and t is tablet thickness (in meters). Results were taken from tablets which split cleanly into two halves without any sign of lamination. All measurements were made in quadruplicate. The BFI of the tablets were then calculated using Eq. 7:
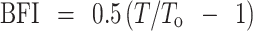 |
7 |
Determination of Disintegration Time of Paracetamol Tablets
The DT of the paracetamol tablets were determined in distilled water at 37 ± 0.5°C in a BP Manesty six station disintegration test unit (Manesty Machines Limited, Liverpool, UK). Tablets were placed on the wire mesh just above the surface of the distilled water in the disintegration unit tube. The time taken for each tablet to disintegrate and all the granules to go through the wire mesh was recorded. Results were expressed as an average of six determinations.
In Vitro Dissolution Test
The dissolution rate of the paracetamol tablets was determined according to the Rotating Basket (USP Apparatus I) method using 900 mL of distilled water as the dissolution medium. The dissolution test was performed at 100 rpm at a temperature of 37 ± 0.5°C. Five-milliliter samples of dissolution medium were removed at predetermined time intervals and replaced with equal volume of fresh dissolution medium at the same temperature. Sampling was done for 1 h. The absorbances of the samples withdrawn were determined spectrophotometrically at 249 nm, and the concentration of drug in each sample was determined from the Beer–Lambert plot of pure paracetamol powder. The determination was carried out in triplicate.
Statistical Analysis
The results obtained were subjected to a one-way analysis of variance to test if any significant effect exists between binder type, binder concentration, compression pressure, and the test parameters.
RESULTS AND DISCUSSION
Table II shows the results of density and flow parameters of paracetamol granules. The granule density values were generally higher than the bulk and tapped density for all the paracetamol granule formulations. This result is expected as the granule density determination does not include the intra- and inter-granular voids present in the bulk volume. The bulk density of the paracetamol granules decreased with increase in the concentration of NMG while there was an increase for paracetamol granules containing ACA with increase in concentration. This could be due to the mean granular size of the paracetamol formulations and increase in concentration of NMG which led to the formation of large irregularly shaped granules forming more arches and bridges, thus a decrease in packing per unit space while the increase in concentration of ACA led to the formation of smaller granules causing an increase in packing per unit space (1,29). The result also shows a decrease in porosity with increase in binder concentration for both paracetamol tablets containing NMG and ACA. The reduction observed suggests that increase in binder concentration led to a reduction in pockets of unwanted air vacuoles present in the granulation (34).
Table II.
Physical and Flow Properties of Paracetamol Granules Containing Neem and Acacia Gum
| Binder | Binder concentration (%, w/w) | Bulk density (g/cm3) | Granule density (g/cm3) | Mean granule size (μm) | Porosity (%) | HR | CI | FR (g/s) |
|---|---|---|---|---|---|---|---|---|
| NMG | 1.0 | 0.349 ± 0.002a | 1.593 ± 0.015 | 1,098 | 78.092 | 1.152 | 13.184 | 3.15 ± 0.12 |
| 2.0 | 0.341 ± 0.020 | 1.554 ± 0.050 | 1,109 | 78.057 | 1.150 | 13.010 | 3.32 ± 0.09 | |
| 3.0 | 0.339 ± 0.012 | 1.539 ± 0.105 | 1,118 | 77.973 | 1.147 | 12.853 | 3.52 ± 0.11 | |
| 4.0 | 0.335 ± 0.005 | 1.496 ± 0.010 | 1,127 | 77.607 | 1.116 | 10.428 | 4.15 ± 0.20 | |
| 5.0 | 0.333 ± 0.002 | 1.478 ± 0.025 | 1,144 | 77.470 | 1.108 | 9.756 | 4.36 ± 0.08 | |
| ACA | 1.0 | 0.338 ± 0.005 | 1.439 ± 0.020 | 1,040 | 76.511 | 1.172 | 14.646 | 3.52 ± 0.09 |
| 2.0 | 0.341 ± 0.002 | 1.448 ± 0.020 | 1,078 | 76.450 | 1.167 | 14.322 | 3.62 ± 0.09 | |
| 3.0 | 0.355 ± 0.010 | 1.486 ± 0.015 | 1,089 | 76.110 | 1.138 | 12.129 | 3.84 ± 0.13 | |
| 4.0 | 0.375 ± 0.014 | 1.490 ± 0.050 | 1,094 | 74.832 | 1.109 | 9.856 | 4.23 ± 0.10 | |
| 5.0 | 0.408 ± 0.002 | 1.498 ± 0.030 | 1,101 | 72.764 | 1.069 | 6.422 | 4.39 ± 0.08 |
HR Hausner’s ratio, CI Carr’s index, FR friability, NMG neem gum, ACA Acacia gum BP
aMean ± SD
The flow descriptors HR, CI, and FR values of the paracetamol granules (Table II) suggest that the two binders, i.e., NMG and ACA, have comparable activities in enhancing the flow of the granules. The observed results also showed that the flow properties of the granules were dependent on binder concentration, with higher binder concentration producing lower values of the parameters determined. The HR and CI values calculated for all the formulations are below the 1.2 and 20%, standards, respectively.
Figure 1 shows representative plots for paracetamol granule formulations containing 3.0% (w/w) binder. The mean yield pressure, Py, was calculated from the regions of the plots showing linearity with the highest correlation coefficient for all formulations (generally between 79.905 and 159.81 MN m−2). The intercept, A, was obtained from the extrapolation of the region for the determination of Py; the values of DA and DB were obtained from Eqs. 2 and 3, respectively. The values of Py, Do, DA, and DB for the formulations are presented in Table III.
Fig. 1.

Heckel plots for paracetamol granulation containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
Table III.
Parameters Obtained from Density Measurements: Heckel and Kawakita Plots for Paracetamol Tablet Formulation
| Gum binder | Binder concentration (%, w/w) | Heckel analysis | Kawakita analysis | ||||
|---|---|---|---|---|---|---|---|
| P y (MN m−2) | D o | D A | D B | D i | P k (MN m−2) | ||
| NMG | 1.0 | 314.615 | 0.243 | 0.640 | 0.397 | 0.290 | 6.667 |
| 2.0 | 270.270 | 0.236 | 0.617 | 0.381 | 0.275 | 6.289 | |
| 3.0 | 238.095 | 0.232 | 0.657 | 0.425 | 0.252 | 5.882 | |
| 4.0 | 181.818 | 0.228 | 0.674 | 0.446 | 0.254 | 5.747 | |
| 5.0 | 175.439 | 0.225 | 0.666 | 0.441 | 0.262 | 5.618 | |
| ACA | 1.0 | 467.778 | 0.244 | 0.628 | 0.384 | 0.270 | 9.091 |
| 2.0 | 406.190 | 0.228 | 0.693 | 0.465 | 0.271 | 8.621 | |
| 3.0 | 370.370 | 0.237 | 0.658 | 0.421 | 0.273 | 8.065 | |
| 4.0 | 285.714 | 0.252 | 0.624 | 0.372 | 0.284 | 7.143 | |
| 5.0 | 196.078 | 0.293 | 0.651 | 0.358 | 0.291 | 5.650 | |
NMG neem gum, ACA Acacia gum BP
The Do values of paracetamol granules containing NMG were lower at all concentrations than those containing ACA at all concentrations. This suggests that ACA facilitated a higher initial packing of the granules in the die at all concentrations. The DB represents the phase of rearrangement of particles at low pressure; the DB value of paracetamol granule formulations containing NMG increased with increase in binder concentration while it decreased with increasing binder concentration for paracetamol granules containing ACA. This result could be due to high porosity exhibited by granules containing NMG at all concentrations compared to granules containing ACA; this high porosity gives room for extensive rearrangement at low pressures. The Py values decreased with increase in binder concentration for all paracetamol formulations, with formulations containing NMG having lower values compared to ACA. This implies that the onset of plastic deformation of the paracetamol granule formulations containing NMG was faster than those containing ACA.
Representative Kawakita plots for paracetamol granules containing 3.0% (w/w) of binders are as shown in Fig. 2. The plots show a linear relationship at all compression pressures with correlation coefficient of 0.999. Kawakita constants, a and b, were calculated from the slope and intercept of the linear plots. The Di values obtained from the expression 1 − a decreased with increase in binder concentration for paracetamol granules containing NMG while it increased with increase in binder concentration for paracetamol granules containing ACA. The Di values were higher than the corresponding values of Do because Do describes the loose initial relative density of the batches due to die filling while Di measures the packed initial relative density of the granules on application of small pressure such as tapping (24,29).
Fig. 2.

Kawakita plots for paracetamol granulation containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
The Pk values generally decreased with increase in binder concentration for all paracetamol formulations. This suggests that the increase in binder concentration made the granules softer and also improved plastic deformation under pressure; materials that are soft and readily deform under pressure generally have low values of Pk (3,5). However, the Pk values of paracetamol granule formulation containing NMG are lower than those containing ACA at all concentrations. This suggests that paracetamol granules containing NMG are softer and readily deformed plastically under pressure when compared to granules containing ACA. Although flow indicators HR and CI of the formulations are below the 1.2 and 20% standards, respectively, formulations containing ACA as binder have lower HI and CI values and higher FR values than those containing NMG at higher concentrations. This result could be explained by the Di and Pk values of paracetamol granule formulations containing NMG which are considerably lower than those containing ACA; studies have suggested that higher values of Di and small values of Pk indicate good flowability and small cohesiveness, respectively (28). The Py, which is different from Pk, explains the onset of plastic deformation during compression, while Pk explains the total amount of plastic deformation occurring during the compression process. Thus, the present result suggests that the use of NMG as a binder facilitated a fast onset of plastic flow and enhanced the total amount of plastic deformation occurring in the paracetamol granules when compared to ACA (5).
The tensile strength results of the diametrical compression test on the paracetamol tablets with (To) or without (T) a hole at their center generally fit the equation:
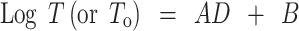 |
8 |
with correlation coefficient >0.947. A and B are constants, which depended on the nature and concentration of binder present in the formulation and on whether the tablet had a hole in it or not.
Representative plots of tensile strength against relative density for paracetamol tablet formulations containing 3.0% (w/w) of NMG or ACA as binder are shown in Fig. 3. It can be observed that, at all relative densities, the tensile strength of tablets without a hole at their center was generally higher than those with a hole in their center (7,12). The values of T for paracetamol formulation batches at relative density 0.90 are as presented in Table IV. The result shows that the T (To) generally increases with increase in binder concentration for all paracetamol tablet formulations containing NMG or ACA. Previous studies have shown that the presence of high concentration of plasto-elastic binding agent leads to an increase in plastic deformation of the formulation and consequently leads to the formation of more solid bonds with consequent increase in tablet strength and resistance to fracture (6) and that the degree of bonding depends on the amount of the binding agent present (21). The effect of gum binder type on T at all concentrations shows that paracetamol tablet formulation containing ACA generally had higher T than those containing NMG. This suggests that inclusion of NMG in a paracetamol tablet formulation would produce weaker tablets when compared to those containing ACA.
Fig. 3.

Plots of tensile strength versus relative density for paracetamol tablets containing 3.0% (w/w) binder: (NMG ●, ACA ○, without holes ———, with holes - - - - - - - - -)
Table IV.
Tensile Strength and Brittle Fracture Index Values of Paracetamol Tablet Formulations Containing Different Concentrations of NMG and ACA at Relative Density 0.90
| Gum binder | Binder concentration (%, w/w) | T (MN m−2) | T o (MN m−2) | BFI |
|---|---|---|---|---|
| NMG | 1.0 | 15.573 ± 1.025a | 12.319 ± 0.225 | 0.132 |
| 2.0 | 18.156 ± 0.920 | 14.925 ± 1.305 | 0.108 | |
| 3.0 | 19.119 ± 1.200 | 15.973 ± 1.450 | 0.098 | |
| 4.0 | 20.365 ± 1.025 | 17.837 ± 1.250 | 0.071 | |
| 5.0 | 21.113 ± 0.092 | 18.944 ± 0.085 | 0.057 | |
| ACA | 1.0 | 10.614 ± 0.220 | 7.094 ± 1.025 | 0.248 |
| 2.0 | 20.310 ± 1.005 | 15.291 ± 1.110 | 0.164 | |
| 3.0 | 22.341 ± 1.250 | 17.063 ± 1.215 | 0.155 | |
| 4.0 | 23.570 ± 0.255 | 18.747 ± 1.222 | 0.129 | |
| 5.0 | 28.560 ± 0.022 | 24.048 ± 1.002 | 0.094 |
BFI brittle fracture index, NMG neem gum, ACA Acacia gum BP
aMean ± SD
The BFI values (Table IV) at relative density 0.90 implies a general decrease as binder concentration increases for all paracetamol tablet formulation. This implies that increase in concentration of gum binder led to a reduction in the propensity of the paracetamol tablets to cap or laminate. This could be due to the presence of binder at the interparticulate junctions which facilitated plastic deformation for the relief of localized stresses (2,10). The result also shows that at all concentrations and relative density of 0.90, the BFI of paracetamol tablets containing NMG was lower than those containing ACA. This suggests that although the inclusion of NMG as a binder in a paracetamol tablet formulation may not necessarily enhance the strength of the tablet when compared to ACA, however, the tendency of the paracetamol tablets to cap or laminate would be reduced when compared to using ACA.
The crushing strength values of paracetamol tablets containing different concentrations of NMG or ACA at a relative density 0.90 are as presented in Table V. There was a general increase in crushing strength with increase in gum binder concentration. This result is expected because increase in binder concentration has been shown to increase particle–particle contact points resulting in the creation of more solid bonds; hence, higher crushing strength values were obtained. Increase in relative density also led to increase in crushing strength of all paracetamol tablet formulations as shown in Fig. 4. Increase in packing fraction (relative density) has also been shown to decrease intra-granular and inter-granular voids, causing breakage of granules and creating more contact points leading to an increase in the degree of bonding between the particles (10).
Table V.
Crushing Strength, Disintegration Time, Friability, and Crushing Strength–Friability/Disintegration Time Ratio Values of Paracetamol Tablet Formulations at Relative Density 0.90
| Gum binder | Binder concentration (%, w/w) | Crushing strength (N) | DT (min) | Friability (%) | CSFR/DT |
|---|---|---|---|---|---|
| NMG | 1.0 | 126.560 ± 1.950a | 15.985 ± 1.025 | 4.932 | 1.605 |
| 2.0 | 135.760 ± 1.250 | 47.312 ± 0.008 | 2.342 | 1.225 | |
| 3.0 | 151.930 ± 2.035 | 52.982 ± 1.002 | 1.480 | 1.938 | |
| 4.0 | 164.680 ± 2.220 | 61.946 ± 1.205 | 1.018 | 2.611 | |
| 5.0 | 167.610 ± 1.980 | 62.621 ± 1.030 | 0.495 | 5.407 | |
| ACA | 1.0 | 85.650 ± 2.225 | 18.973 ± 0.905 | 9.730 | 0.464 |
| 2.0 | 162.280 ± 2.230 | 62.350 ± 1.024 | 3.106 | 0.838 | |
| 3.0 | 165.040 ± 2.055 | 84.424 ± 0.550 | 1.802 | 1.085 | |
| 4.0 | 174.910 ± 2.048 | 95.627 ± 1.003 | 1.602 | 1.142 | |
| 5.0 | 193.800 ± 2.205 | 114.520 ± 1.001 | 0.641 | 2.640 |
DT disintegration time, CSFR/DT Friability and Crushing Strength–Friability/Disintegration Time Ratio, NMG neem gum, ACA Acacia gum BP
aMean ± SD
Fig. 4.

Effect of relative density on crushing strength of paracetamol tablets containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
The result in Table V and Fig. 5 shows that friability decreases with increase in crushing strength, binder concentration, and relative density. This could be due to the same reasons for the increase in crushing strength.
Fig. 5.

Effect of relative density on friability of paracetamol tablets containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
The result in Table V and Fig. 6 shows the effect of binder concentration and relative density on disintegration time of paracetamol tablets containing NMG or ACA. An increase in binder concentration and relative density led to a significant (F(2, 15) = 21.019, p < 0.005) increase in disintegration time. Natural gums, when used as binder in a tablet formulation, form a viscous mucilaginous film/barrier as tablet comes in contact with disintegrating fluid. This film/barrier reduces the penetration of disintegrating fluid into the tablet core consequently increasing the disintegration time of the tablets. In addition, the simultaneous increase in relative density which leads to a decrease in tablet porosity further slows down penetration of the disintegrating fluid into the tablets. This ultimately leads to retarded swelling and slow creation of active disintegration mechanisms, consequently leading to increased disintegration time (2,6,33). Also, there was a significant effect of binder type on DT at 1.0% (w/w) (F(1, 5) = 81.963, p = 0.001), 3.0% (w/w) (F(1, 5) = 0.129, p < 0.0005), and 5.0% (w/w) (F(1, 5) = 0.329, p < 0.0005) binder concentrations. The DT value of the paracetamol tablet formulations at all concentrations was higher than the official limit of 15 min for uncoated tablets suggesting a probable use of the gum in sustained release tablet dosage form.
Fig. 6.

Effect of relative density on disintegration time of paracetamol tablets containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
It is observed that the CSFR/DT values (Table V) generally increased with increase in binder concentration for all paracetamol tablet formulations. However, the CSFR/DT values of paracetamol tablets containing NMG were higher than those containing ACA, suggesting that the use of NMG as a binder would produce a better balance between binding and disintegration properties of a paracetamol tablet formulation than ACA binder.
Representative plots of cumulative percent paracetamol released against time for tablets containing 3.0% (w/w) NMG or ACA are as shown in Fig. 7. The values of t20 and t40 (time taken for 20% and 40% of paracetamol to be released) were calculated from the plots for formulations containing 1.0% (w/w), 3.0% (w/w), and 5.0% (w/w) of NMG or ACA at relative density of 0.90 and are presented in Table VI. The result shows that t20 (F(2, 15) = 24.743, p < 0.0005) and t40 (F(2, 15) = 92.405, p < 0.0005) values significantly increased with increase in binder concentration for all paracetamol tablet formulation batches. This result could also be due to the formation of thicker film/barrier around the tablets with increase in binder concentration which affected the disintegration of the tablet and consequently the release rate of paracetamol from the tablet. Although the t20 and t40 values are generally lower for paracetamol tablets containing NMG, suggesting a faster release of the specified amount of paracetamol from the tablets than those containing ACA, however, at 3.0% (w/w) binder concentration, there was a significant effect of binder type on t40 (F(1, 5) = 14.164, p = 0.020) while on t20 (F(2, 5) = 2.612, p > 0.05), there was no significant effect; at 1.0% (w/w) binder concentration, there exists a significant effect of binder type on t20 (F(1, 5) = 115.478, p < 0.0005) and t40 (F(1, 5) = 9.761, p = 0.035); at 5.0% (w/w), there was also a significant effect of binder type on t20 (F(1, 5) = 0.359, p < 0.0005) and t40 (F(1, 5) = 0.105, p < 0.0005).
Fig. 7.

Plots of cumulative percent paracetamol released against time for tablets containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
Table VI.
Disintegration and Dissolution Profile of Paracetamol Tablets at Relative Density 0.90
| Gum binder | Binder concentration (%, w/w) | DT (min) | t 20 (min) | t 40 (min) | t 1 (min) | k 1 | k 2 |
|---|---|---|---|---|---|---|---|
| NMG | 1.0 | 15.985 ± 1.025 | 9.471 ± 0.105a | 21.692 ± 0.090 | 16.113 | 0.0094 | 0.0509 |
| 3.0 | 52.982 ± 1.002 | 22.393 ± 0.130 | 50.225 ± 0.110 | 23.608 | 0.0045 | 0.0119 | |
| 5.0 | 62.621 ± 1.030 | 27.820 ± 1.020 | 60.402 ± 0.120 | 24.038 | 0.0053 | 0.0106 | |
| ACA | 1.0 | 18.973 ± 0.905 | 12.368 ± 0.120 | 23.034 ± 0.105 | 15.043 | 0.0074 | 0.0354 |
| 3.0 | 84.424 ± 0.550 | 23.719 ± 0.155 | 52.822 ± 0.112 | 21.617 | 0.0053 | 0.0100 | |
| 5.0 | 114.520 ± 1.001 | 48.492 + 0.110 | 79.343 ± 0.122 | 23.641 | 0.0018 | 0.0057 |
DT disintegration time, NMG neem gum, ACA Acacia gum BP
aMean ± SD
The data obtained were further subjected to Kitazawa analysis (18) which involves the integrated form of Noyes–Whitney equation (23) written as:
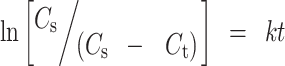 |
9 |
where Cs is the concentration of solute at saturation, Ct is the concentration of solute at time t, and k is the dissolution rate constant. Representative plots of ln[Cs/(Cs − Ct)] against t for tablets containing 3.0% (w/w) NMG or ACA are as shown in Fig. 8. The Kitazawa plots showed two straight regression lines of slopes k1 and k2. The time t1 (the time required for breakup of tablets into fragments), at which the two lines intersect, was calculated from the plot. The values of t1, k1, and k2 for paracetamol tablets containing 1.0% (w/w), 3.0% (w/w), and 5.0% (w/w) of NMG or ACA at relative density 0.90 are shown in Table VI.
Fig. 8.

Plot of ln(C
s/(C
s − C
t)) against time for paracetamol tablets containing 3.0% (w/w) binder: NMG  and ACA
and ACA 
The result shows that k1 values are lower than k2 values, implying that the dissolution rate of the drug was faster after the breakup of tablets, that is, after t1. This result could be due to changes in surface area of the dissolving particles caused by disintegration and de-aggregation of the tablets which manifested in the substantial increase in release rate of paracetamol after t1. Values of t1, k1, and k2 for paracetamol tablets containing NMG were higher at all concentrations than those containing ACA; however, the amount of paracetamol released after t1 in tablets containing NMG was higher than in ACA. This suggests that although de-aggregation or breakage of paracetamol tablets containing ACA was faster than those containing NMG, tablets containing NMG had higher dissolution rates. This result could be due to several factors including the crushing strength and disintegration time of the tablets containing NMG which were lower than those of ACA. The result could also be due to the chemical composition of the gums. Studies have shown that neem gum unlike other natural gums contains an appreciable amount of tightly linked polysaccharides and proteins (22,32), which might have enhanced the faster dissolution of tablets containing neem gum, particularly after de-aggregation. The result also shows that the dissolution rate constant before and after t1 was dependent on the binder concentration in the tablet formulation. There was a decrease in the release rate of paracetamol with increase in binder concentration before and after the breakup of the tablets. This result could be due to the same reason given above for the increase in disintegration time; it, however, led to a reduction in the release rate of paracetamol from the tablets.
CONCLUSION
From the present study, it can be concluded that:
Neem gum as a binder produced paracetamol granules with comparable flow characteristics with Acacia gum.
Neem gum as a binder in paracetamol tablet formulations would provide a faster onset and amount of plastic deformation under compression pressure than Acacia gum.
Although inclusion of neem gum in a paracetamol tablet formulations may not necessarily enhance the tablet strength, however, the tendency of the tablets to cap or laminate would be reduced when compared with Acacia gum.
Neem gum as a binder would provide a better balance between the binding and disintegration properties of a paracetamol tablet formulation.
Neem gum binder would produce paracetamol tablets with faster release properties when compared with Acacia gum.
ABBREVIATIONS
- NMG
Neem gum
- ACA
Acacia gum BP
- CSFR/DT
Crushing strength–friability/disintegration time ratio
- BFI
Brittle fracture index
- T
Tensile strength
- DT
Disintegration time
- HR
Hausner’s ratio
- CI
Carr’s index
- FR
Friability
Contributor Information
Abayomi Tolulope Ogunjimi, Email: ohtolu@gmail.com.
Gbenga Alebiowu, Phone: +234-80-69384107, Email: galebiowu@rediffmail.com, Email: gbengaalaba@gmail.com.
References
- 1.Adeagbo AA, Alebiowu G. Evaluation of cocoa butter as potential lubricant for coprocessing in pharmaceutical tablets. Pharm Dev Tech 2008;13:197–204. [DOI] [PubMed]
- 2.Adetogun EA, Alebiowu G. Properties of Delonix regia seed gum as a novel tablet binder. Acta Pol Pharm Drug Res. 2009;66(4):433–438. [PubMed] [Google Scholar]
- 3.Adetogun GE, Alebiowu G. Influence of Delonix regia seed gum on the compressional characteristics of paracetamol tablet formulation. J Drug Del Sci Tech 2007;17(6):443–5.
- 4.Akin-Ajani OD, Itiola OA, Odeku OA. Effect of plantain and corn starches on the mechanical and disintegration properties of paracetamol tablets. AAPS Pharm Sci Tech. 2005;6:E458–E463. doi: 10.1208/pt060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alebiowu G, Itiola OA. Compressional characteristics of native and pregelatinized forms of sorghum, plantain and corn starches and the mechanical properties of their tablets. Drug Dev Ind Pharm. 2002;28(6):663–672. doi: 10.1081/DDC-120003857. [DOI] [PubMed] [Google Scholar]
- 6.Alebiowu G, Itiola OA. The effects of starches on mechanical properties of paracetamol tablet formulations. I. Pregelatinization of starch binders. Acta Pharm. 2003;53:231–237. [PubMed] [Google Scholar]
- 7.Alebiowu G, Osinoiki KA. Assessment of tapioca starches obtained after different steeping periods as binders in a paracetamol tablet formulation. Farmacia. 2010;58(3):341–352. [Google Scholar]
- 8.Farahmand B, Nikbin K. Predicting fracture and fatigue crack growth properties using tensile properties. Eng Fract Mech. 2008;75:2144–2155. doi: 10.1016/j.engfracmech.2007.10.012. [DOI] [Google Scholar]
- 9.Gangurde AB, Malode SS, Bhambar RS. Preliminary evaluation of neem gum as tablet binder. Indian J Pharm Educ Res. 2008;42(4):344–347. [Google Scholar]
- 10.Hancock BC, Carlson GT, Ladipo DD, Langdon BA, Mullarney MP. The powder flow and compact mechanical properties of two recently developed matrix forming polymers. J Pharm Pharmacol. 2001;53:1193–1199. doi: 10.1211/0022357011776630. [DOI] [PubMed] [Google Scholar]
- 11.Heckel RW. Density–pressure relationships in powder compaction. Trans Metall Soc AIME. 1961;221:671–675. [Google Scholar]
- 12.Hiestand EN, Wells JF, Poet CB, Ochs JF. Physical processes of tableting. J Pharm Sci. 1977;66(3):510–519. doi: 10.1002/jps.2600660413. [DOI] [PubMed] [Google Scholar]
- 13.Humbert-Droz P, Gurny R, Mordier D, Doelker E. Densification behavior of drugs presenting availability problems. Int J Pharm Technol Prod Manuf. 1983;4:29–35. [Google Scholar]
- 14.Itiola OA, Pilpel N. Formulation effects on the mechanical properties of metronidazole tablets. J Pharm Pharmacol. 1991;43:145–147. doi: 10.1111/j.2042-7158.1991.tb06655.x. [DOI] [PubMed] [Google Scholar]
- 15.Itiola OA, Pilpel N. Tabletting characteristics of metronidazole formulations. Int J Pharm. 1986;31:99–105. doi: 10.1016/0378-5173(86)90218-8. [DOI] [Google Scholar]
- 16.Joneja SK, Harcum WW, Skinner G, Barnum PE, Guo JH. Investigating the fundamental effects of binders on pharmaceutical tablet performance. Drug Dev Ind Pharm. 1999;25:1129–1135. doi: 10.1081/DDC-100102279. [DOI] [PubMed] [Google Scholar]
- 17.Kawakita K, Ludde KH. Some considerations in powder compression equations. Powder Technol. 1970/71;4:61–8.
- 18.Kitazawa S, Johno I, Ito Y, Teramura S, Okada J. Effects of hardness on the disintegration and dissolution rate of uncoated caffeine tablets. J Pharm Pharmacol. 1975;27:765–770. doi: 10.1111/j.2042-7158.1975.tb09397.x. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni AP, Yunus RS, Dehghan MHD. Application of neem gum for aqueous film coating of ciprofloxacin tablets. Int J Appl Res Nat Prod. 2013;6(3):11–19. [Google Scholar]
- 20.Lin C, Cham T. Compression behaviour and tensile strength of heat-treated polyethylene glycols. Int J Pharm. 1995;118:169–179. doi: 10.1016/0378-5173(94)00343-4. [DOI] [Google Scholar]
- 21.Mattsson S, Bredenberg S, Nystrom C. Formulation of high tensile strength rapidly disintegrating tablets—evaluation of the effect of some binder properties. STP Pharma Sci. 2001;11:211–220. [Google Scholar]
- 22.Nayak BR, Rao R, Pattabiraman TN. Studies on plant gums. Isolation and characterisation of the major polysaccharide from neem (Azadirachta indica) gum. Proc Indian Acad Sci B Exp Biol. 1978;87(10):261–269. [Google Scholar]
- 23.Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–934. doi: 10.1021/ja02086a003. [DOI] [Google Scholar]
- 24.Odeku OA, Itiola OA. Evaluation of the effects of khaya gum on the mechanical and release properties of paracetamol tablets. Drug Dev Ind Pharm. 2003;29(3):311–20. [DOI] [PubMed]
- 25.Ogunjimi AT, Alebiowu G. A quantitative study of the influence of coprocessing of binders on the mechanical properties of paracetamol tablets. Braz J Pharm Sci. 2010;46(2):205–212. doi: 10.1590/S1984-82502010000200006. [DOI] [Google Scholar]
- 26.Ogunjimi AT, Alebiowu G. Flow and consolidation properties of neem gum coprocessed with two pharmaceutical excipients. Powder Technol. 2013;246:187–192. doi: 10.1016/j.powtec.2013.04.051. [DOI] [Google Scholar]
- 27.Ogunjimi AT, Alebiowu G. Material and tableting properties of Azadirachta indica gum with reference to official Acacia gum. Acta. Pol. Pharm. 2014; in press. [PubMed]
- 28.Pesonen T, Paronen P. Evaluation of a new cellulose material as a binding agent for the direct compression of tablets. Drug Dev Ind Pharm. 1986;12:2091–2111. doi: 10.3109/03639048609042625. [DOI] [Google Scholar]
- 29.Podczeck F, Sharma M. The influence of particle size and shape of components of binary powder mixtures on the maximum volume reduction due to packing. Int J Pharm. 1996;137:41–47. doi: 10.1016/0378-5173(95)04420-5. [DOI] [Google Scholar]
- 30.Prajapati VD, Jani GK, Moradiya NG, Randeria NP. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr Polym. 2013;92(2):1685–1699. doi: 10.1016/j.carbpol.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Upadrashta SM, Katikaneni PR, Nuessle NO. Chitosan as a tablet binder. Drug Dev Ind Pharm. 1992;18(15):1701–1708. doi: 10.3109/03639049209040896. [DOI] [Google Scholar]
- 32.Usha LS, Pattabiraman TN. Studies on plant gums. I. Identification of nitrogenous compounds in neem (Azadirachta indica) gum and isolation of d-glucosamine. Indian J Biochem. 1967;4(3):181–183. [PubMed] [Google Scholar]
- 33.Washburn EW. The dynamics of capillary flow. Phys Rev. 1992;17:273–281. doi: 10.1103/PhysRev.17.273. [DOI] [Google Scholar]
- 34.Wu CY, Ruddy O, Bentham AC, Best SM, Elliot JA. Modelling the mechanical behavior of pharmaceutical powders during compaction. Powder Technol. 2005;152:107–117. doi: 10.1016/j.powtec.2005.01.010. [DOI] [Google Scholar]


