Abstract
Failing to remember whether we performed, or merely imagined performing, an everyday action can occasionally be inconvenient, but in some circumstances it can have potentially dangerous consequences. In this fMRI study, we investigated the brain activity patterns, and objective and subjective behavioral measures, associated with recollecting such everyday actions. We used an ecologically valid “reality-monitoring” paradigm in which participants performed, or imagined performing, specified actions with real objects drawn from one of two boxes. Lateral brain areas, including prefrontal cortex, were active when participants recollected both the actions that had been associated with objects and the locations from which they had been drawn, consistent with a general role in source recollection. By contrast, medial prefrontal and motor regions made more specific contributions, with supplementary motor cortex activity being associated with recollection decisions about actions but not locations, and medial prefrontal cortex exhibiting greater activity when remembering performed rather than imagined actions. These results support a theoretical interpretation of reality monitoring that entails the fine-grained discrimination between multiple forms of internally and externally generated information.
Keywords: Recollection, Prefrontal cortex
An example of a memory lapse that is familiar to many people is the experience of sitting in your car on the way to work and being unable to remember whether you locked your front door when you left home, or just thought about locking it. Similarly, as we age and typically become more aware of our apparently diminishing memory abilities, we may become preoccupied with concerns such as whether we really turned off the gas stove before going to bed, or just imagined doing so, with obvious consequences for psychological, and indeed physical, well-being. Indeed, forgetting whether or not a scheduled pill has already been taken may be a common reason that people fail to stick to prescribed medication regimes (Park & Kidder, 1996). Despite the importance of this memory ability to everyday life, and the fact that it has been the subject of a number of developmental and neurophysiological investigations (e.g., Foley & Johnson, 1985; McDaniel, Lyle, Butler, & Dornburg, 2008; Senkfor, Petten, & Kutas, 2002), few researchers have attempted to investigate the brain regions responsible for recollecting previous actions.
A number of different processes are likely to play a role in remembering whether or not planned actions were actually undertaken (McDaniel et al., 2008). For example, recollecting temporal information may be useful, particularly for actions that might be faced on multiple occasions. However, a potentially critical factor in discriminating actions that you might have performed from those you merely imagined may be “reality monitoring” (Johnson & Raye, 1981), an ability thought to involve an assessment of the qualitative characteristics of memory representations. These characteristics may reflect the kind of processing activity that occurred when a memory was encoded, perhaps perceptual or semantic details about an event, or the thoughts and feelings that one had at the time. Many studies of reality monitoring have contrasted memories of visually presented stimuli (e.g., words or pictures) with memories of stimuli that participants were instructed to imagine, with evidence suggesting that assessment of mnemonic characteristics such as perceptual detail can often serve as a useful basis for distinguishing perceived from imagined experiences (Johnson, Foley, Suengas, & Raye, 1988). An emerging body of functional neuroimaging research has indicated important roles for regions of prefrontal (PFC) and parietal cortices in such decision-making (Mitchell & Johnson, 2009). Lateral PFC regions are engaged during many tasks that involve recollecting the context in which previous events occurred (Dobbins, Foley, Schacter, & Wagner, 2002; Dobbins & Han, 2006; Ranganath, Johnson, & D’Esposito, 2000; Simons, Gilbert, Owen, Fletcher, & Burgess, 2005), with medial PFC appearing to make a more specific contribution during judgments that require differentiating between perceived and imagined information (Kensinger & Schacter, 2006; Simons, Davis, Gilbert, Frith, & Burgess, 2006; Simons, Owen, Fletcher, & Burgess, 2005; Vinogradov et al., 2006).
Recollecting whether we have performed, or merely imagined performing, everyday actions might involve the same kind of processing as distinguishing perceived from imagined words or objects, and might be associated with the engagement of similar brain regions. Memories of performed actions may comprise more sensory perceptual detail than do memories of imagined actions, as well as less information relating to the cognitive processes involved in generating vivid imagery-based scenarios. If so, reality monitoring of action memories would be predicted to elicit activity in medial PFC regions similar to those identified previously.
However, another view is that discriminating between performed and imagined actions might, phenomenologically, be very different from distinguishing perceived from imagined stimuli. Perceived/imagined judgments involve differentiating comparable visual imagery-based representations, albeit one kind that was generated internally, and one that was derived from the outside world. In contrast, reality monitoring of performed/imagined actions could be thought of as involving a much clearer distinction, between memories that include information relating to motor processes, such as visuomotor coordination, muscle control, and kinesthetic feedback, and memories with no overt motor component, instead predominantly comprising internally generated cognitive operations connected to covert action simulation. Participants could rely largely on monitoring retrieval for information relating to motor processes in order to make their recollection decisions (Nyberg et al., 2001; Senkfor et al., 2002; Zimmer, Helstrup, & Engelkamp, 2000), in which case it would be reasonable to expect activity differences in regions typically associated with motor planning and action execution, such as premotor cortex and supplementary motor area (Binkofski et al., 1999; for reviews, see Grèzes & Decety, 2001; Nachev, Kennard, & Husain, 2008).
To examine these issues, we asked participants to perform, or imagine performing, specified actions with everyday objects that the experimenter took from boxes located on either side of the participant. In a subsequent memory test, participants were presented with pictures of studied objects and were scanned using fMRI while recollecting either whether they had performed or imagined performing actions with the objects or whether the objects had been taken from the left or right box. A common nonmemory baseline condition was also included. We predicted that previous findings of lateral PFC and parietal involvement in general source recollection processes would be replicated in this paradigm, with activity being observed during recollection of both action and location. The main question concerned the involvement of medial PFC and motor regions: If the first account described above—that reality monitoring of action memories is analogous to differentiating perceived from imagined stimuli—is correct, we would predict that patterns of activity in medial PFC would be observed that would be similar to those reported by previous studies. On the other hand, if discriminating performed from imagined actions requires determining the presence amongst retrieved information of overt motor processes, activity differences would be expected in premotor and supplementary motor areas. To provide further insight into the kinds of mnemonic characteristics that might enable reality monitoring of action memories, immediately after leaving the scanner, participants rated their recollection of each studied object according to a number of qualitative factors that included the amounts of internally and externally generated information retrieved.
Method
Participants
The participants were 15 healthy adults (nine female, six male) between 18 and 38 years old. All of the participants were right-handed, with normal or corrected-to-normal vision. Before scanning, participants received written information about the scanning procedure and were screened using a comprehensive medical questionnaire. All participants provided written consent before taking part in the study in a manner approved by the Cambridge Local Research Ethics Committee. One additional participant had to be excluded due to presence of a “wrap around” artifact in the fMRI data owing to a larger than average head size.
Procedure
The stimuli consisted of 182 action statements involving small everyday objects (e.g., toy dog, stapler), developed from a list of 72 action statements created by McDaniel et al. (2008). In the study phase, which took place outside the fMRI scanner, the participant sat at a table facing the experimenter and two large, distinct-looking boxes (blue and red) located to the left and right of the participant (see Fig. 1), each of which contained 80 objects. At the beginning of each trial, the experimenter took an object out of one of the boxes and placed it on the table in front of the participant. The experimenter read aloud an action statement, and participants were asked either to perform the specified action with the object or to imagine performing the action without handling the object (e.g., “Perform. Open the book” or “Imagine. Open the book”). Half of the objects from each box were assigned to the perform or imagine conditions, with item order being pseudorandomized such that no more than six consecutive trials were of the same condition (left box, right box, perform, imagine). The order in which the 160 objects were presented was the same for each participant, but four versions of the task counterbalanced the allocation of each object to a location (left vs. right box) and task (perform vs. imagine) across subjects.
Fig. 1.
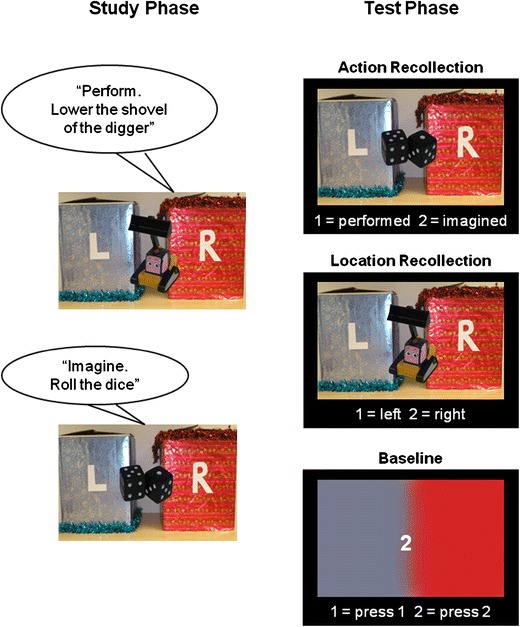
Schematic design of the study and test phases. In the study phase, real everyday objects were taken out of either the left or the right box and presented to the participant, who was asked to perform a specified action with the object or to imagine performing that action with the object. In the scanned test phase, photographs of the studied objects were presented, and participants were cued to recollect whether each object had been associated with a performed or imagined action (action recollection) or whether it had been taken out of the left or the right box (location recollection). In the perceptual baseline condition, participants had to press one of two buttons, according to the number on the screen
In the test phase, which took place some minutes later, once participants had been set up in the scanner, 160 photographs were presented of the studied objects, with the blue and red boxes in the background (see Fig. 1). A cue appeared at the bottom of the screen instructing the participant to make one of two possible judgments: “1 = performed 2 = imagined” indicated that participants should recollect whether they had performed or imagined an action with the object, or “1 = left 2 = right” indicated that participants should recollect whether the object had been taken out of the left or the right box. One second after the cue appeared, an object picture was presented in the center of the screen, and participants were given 3.5 s to make their judgments, which they indicated with a button response. In all, 80 trials were presented in each recollection condition. The test phase also included 80 trials of a perceptual baseline condition that attempted to control for properties of the task such as visual content, response selection, and so forth, which consisted of a half-blue, half-red image containing a “1” or a “2” in the center. In this case, the cue read “1 = press 1 2 = press 2,” and participants responded appropriately with the button box. The three conditions were pseudorandomly intermixed within the 240 memory test phase trials, such that no more than four consecutive trials were of the same condition, with the allocation of objects to the recollection of left/right versus performed/imagined conditions counterbalanced across subjects. The intertrial interval was jittered between 480 and 1,380 ms in order to achieve a higher effective sampling rate over trials.
On leaving the scanner, participants completed a computerized questionnaire assessing the qualitative characteristics of their memories for the studied everyday objects, on the basis of Johnson et al.’s (1988) Memory Characteristics Questionnaire. A picture of each object was presented, and participants were asked to rate their memory of the relevant study episode for four different characteristics. The first was the extent to which their memory for encountering that object involved specific visual information about the event—for example, clearly remembering the colors or shape of the object or of items in the surroundings, the light in the room, and so forth. The second characteristic related to specific tactile information about the event, such as the feeling of an object in the participant’s hand, the feeling of a participant’s arms against the table or of his or her legs against the chair, and so forth. The third memory question asked about the extent to which participants’ memory involved thinking about their internal feelings or reactions during the study trial. For example, they may have felt amused or surprised by an object, bored with the task, an object might have reminded them of other objects or events that they had experienced, and so forth. Finally, participants were asked to indicate the extent to which they had to actively try to remember the event in order to answer the memory test question. For example, they may have struggled to remember and tried very hard, or the memory might have just popped into their heads without any effort to remember. Ratings were made on a 5-point scale. Four versions of the questionnaire counterbalanced the order in which the object pictures were presented between participants. Each statistical analysis of the ratings was Bonferroni corrected for multiple comparisons.
Imaging acquisition and data analysis
A 3-T Siemens TIM Trio system was used to acquire structural and functional images (TR = 2.25 s; TE = 30 ms; 36 sequential axial slices oriented ~10º–20º to the AC–PC transverse plane, 2-mm thickness, 1-mm interslice skip; 3 mm × 3 mm in-plane resolution, 64 × 64 pixels; 78º flip angle; 550 volume acquisitions). The first six volumes were discarded to allow for T1 equilibration.
The data were analyzed using SPM 8 software (Wellcome Trust Centre for Neuroimaging, London). The volumes were corrected for motion by realigning all images with respect to the first and were corrected for differences in slice acquisition timing by resampling all slices in time to match the middle slice. The realigned volumes were normalized into 3-mm cubic voxels using the Montreal Neurological Institute (MNI) reference brain using fourth-degree B-spline interpolation, and smoothed with an isotropic 8-mm full-width-at-half-maximum Gaussian kernel. A high-pass filter of 1/128 Hz removed low-frequency noise, and an AR(1) model corrected for temporal autocorrelation. A random-effects statistical analysis was undertaken in two stages. First, variance in the blood oxygenation level dependent (BOLD) signal was decomposed with a set of regressors that coded the onsets of trials associated with correct responses in each condition, as well as a single error trial regressor, collapsed across all tasks. Each regressor was generated with delta functions corresponding to each trial onset, convolved with a canonical hemodynamic response function. Regressors representing residual movement related artifacts and the mean over scans completed the model.
Linear contrasts were used to obtain subject-specific estimates for each of the effects of interest. These estimates were entered into the second stage of analysis treating subjects as a random effect, using one-sample t tests across subjects. For all contrasts, a priori regions of interest for PFC, motor, and parietal regions were defined as 10-mm spheres centered on mean coordinates from our previous studies of reality monitoring (Simons et al., 2006; Simons, Henson, Gilbert, & Fletcher, 2008; Simons, Owen, et al., 2005) and from Binkofski et al.’s (1999) study of motor execution involving manipulable objects. Activations were reported if they exceeded the family-wise error threshold of p < .05, corrected for voxels within the region of interest. Activations occurring outside the regions of interest were reported if they exceeded the threshold of p < .05, corrected for multiple comparisons across the entire brain, and were greater than five voxels in extent. The peak locations of significant clusters were localized on an averaged structural scan, with approximate Brodmann areas obtained from the Talairach and Tournoux (1988) atlas, after using a nonlinear transform of MNI to Talairach coordinates (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
To further explore significant activations elicited in the regions of interest, mean-percentage-of-signal-change time courses were extracted from the subject-specific parameter estimates using a finite impulse response model (Henson, 2004) and subjected to repeated measures analyses of the area under the curve (auc) associated with each condition. These auc values were also used to explore correlations between brain activity and memory characteristic ratings. The Bonferroni method corrected for multiple comparisons.
Results
Behavioral results
Recollection accuracy (proportions of responses attributing items to the correct source) and reaction times for the scanned retrieval task are displayed in Table 1. Performance was high but below ceiling in all conditions. An analysis of accuracy using repeated measures analysis of variance (ANOVA) revealed no significant main effect of study condition (perceived vs. imagined), F(1, 14) = 0.14, n.s., or retrieval condition (reality monitoring of action memories vs. location recollection), F(1, 14) = 1.12, n.s., and no interaction, F(1, 14) = 1.12, n.s. Arcsine-transforming the accuracy data to control for possible skewing had no effect on the results: study condition, F(1, 14) = 0.07, n.s.; retrieval condition, F(1, 14) = 1.41, n.s.; interaction, F(1, 14) = 0.74, n.s. We observed a trend toward an effect of study condition on reaction times, F(1, 14) = 3.26, p = .09, but no effect of retrieval condition, F(1, 14) = 0.14, n.s. A significant Study Condition × Retrieval Condition interaction did emerge, F(1, 14) = 19.43, p < .01, driven by slower reaction times when participants were making reality-monitoring judgments for actions that had previously been imagined than for actions that had previously been performed, t(14) = 4.89, p < .001, but no corresponding difference between performed and imagined actions for location retrieval judgments, t(14) = 0.26, n.s.
Table 1.
Recollection accuracy and reaction time data as a function of study condition (performed vs. imagined actions) and test condition (action vs. location recollection)
| Condition | Accuracy | Reaction time (ms) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Action Recollection | ||||
| Performed | .93 | .07 | 1,630 | 178 |
| Imagined | .93 | .06 | 1,748 | 176 |
| Location Recollection | ||||
| Performed | .92 | .05 | 1,681 | 307 |
| Imagined | .91 | .07 | 1,671 | 258 |
Memory characteristics questionnaire
Examination of memory characteristic ratings (Table 2) revealed that memories of objects that had been involved in performed actions were rated as being significantly greater in internal, t(14) = 2.97, p = .01, and tactile, t(14) = 4.9, p < .001, detail than were objects that had been involved in imagined actions. We found no difference in terms of amount of visual detail, but participants did rate memories of performed actions as requiring significantly less effort to retrieve than did those of imagined actions, t(14) = 3.6, p < .01. Examining retrieval condition effects, reality monitoring of action memories was rated as requiring less effort than did location recollection, t(14) = 2.6, p < .05, although this effect did not survive Bonferroni correction; no other effects of the type of source recollection were apparent.
Table 2.
Postscan memory characteristics questionnaire: mean ratings of amount of internally generated thoughts/associations retrieved, amounts of tactile and visual detail, and effort required at retrieval
| Condition | Internal | Tactile | Visual | Effort |
|---|---|---|---|---|
| Action Recollection | ||||
| Performed | 2.99 | 3.87 | 4.02 | 1.57 |
| Imagined | 2.60 | 2.40 | 3.99 | 1.87 |
| Location Recollection | ||||
| Performed | 2.97 | 3.68 | 4.07 | 1.69 |
| Imagined | 2.56 | 2.51 | 3.93 | 1.95 |
Ratings were made on a 5-point scale, with higher numbers meaning greater amounts of richness/detail/effort.
Neuroimaging results
To determine the brain regions that were involved more during successful recollection than during the baseline condition, regardless of the kind of source detail retrieved, inclusive masking was used to identify common regions showing significant activity in both action and location recollection pairwise comparisons against baseline (Table 3). As is displayed in Fig. 2, we observed considerable overlap between the two kinds of recollection in a number of regions, including left ventrolateral PFC, anterior cingulate, and precuneus, as well as bilateral parietal, fusiform, and occipital cortices. Comparing the two recollection conditions against one another revealed that an inferior portion of left ventrolateral PFC (centered at −45, 32, 4; BA 45; Z = 3.8) and supplementary motor area (−12, 20, 58; BA 6/8; Z = 3.2) were significantly more active during reality monitoring of action memories (Fig. 3a), whereas location recollection was associated with greater activity in a region of the precuneus (−3, –70, 55; BA 7; Z = 3.6) (Fig. 3b). As we noted in the behavioral results, no significant differences in retrieval accuracy or reaction times emerged between the two source recollection conditions, precluding an explanation of the neuroimaging results in terms of task difficulty.
Table 3.
Regions showing significant activity common to both action and location recollection versus the baseline condition
| Brain Region | Coordinates | |||
|---|---|---|---|---|
| x | y | z | Z | |
| Regions of Interest From Previous Studies | ||||
| Left ventrolateral prefrontal cortex (BA 45) | −51 | 29 | 22 | 4.1 |
| Anterior cingulate cortex (BA 32) | −6 | 17 | 49 | 5.6 |
| Left inferior frontal gyrus (BA 44) | −42 | 8 | 31 | 3.8 |
| Left intraparietal sulcus (BA 40) | −30 | −55 | 49 | 4.6 |
| Right intraparietal sulcus (BA 40) | 36 | −46 | 46 | 4.0 |
| Precuneus (BA 7) | −9 | −70 | 43 | 3.5 |
| Additional Significant Regions | ||||
| Posterior cingulate cortex (BA 23) | 6 | −31 | 28 | 5.3 |
| Brainstem (BA 27) | 6 | −28 | −5 | 5.4 |
| Right fusiform cortex (BA 37) | 39 | −37 | −20 | 5.1 |
| Right fusiform cortex (BA 37) | 30 | −52 | −14 | 6.1 |
| Left fusiform cortex (BA 37) | −27 | −58 | −11 | 5.4 |
| Left occipital cortex (BA 18) | −33 | −88 | 10 | 5.8 |
| Right occipital cortex (BA 18) | 21 | −88 | −8 | 5.4 |
| Left occipital cortex (BA 18) | −6 | −94 | −11 | 5.4 |
Coordinates are in MNI atlas space (Cocosco, Kollokian, Kwan, & Evans, 1997), with brain regions and Brodmann areas (BAs) estimated from the Talairach and Tournoux (1988) atlas. Activations within regions of interest are reported that exceeded the threshold of p < .05, corrected for voxels within the region. Additional significant regions reported exceeded the threshold of p < .05, corrected for multiple comparisons across the entire brain.
Fig. 2.
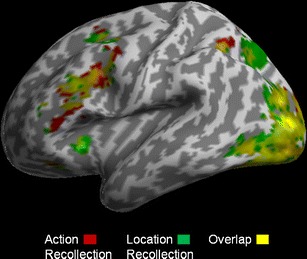
Brain regions activated during both action and location recollection, relative to the nonmemory baseline condition, displayed at an uncorrected threshold of p < .001. Considerable overlap between the two kinds of recollection is seen in regions that include lateral prefrontal, parietal, fusiform, and occipital cortices, all significant at the corrected thresholds (see the text for details)
Fig. 3.
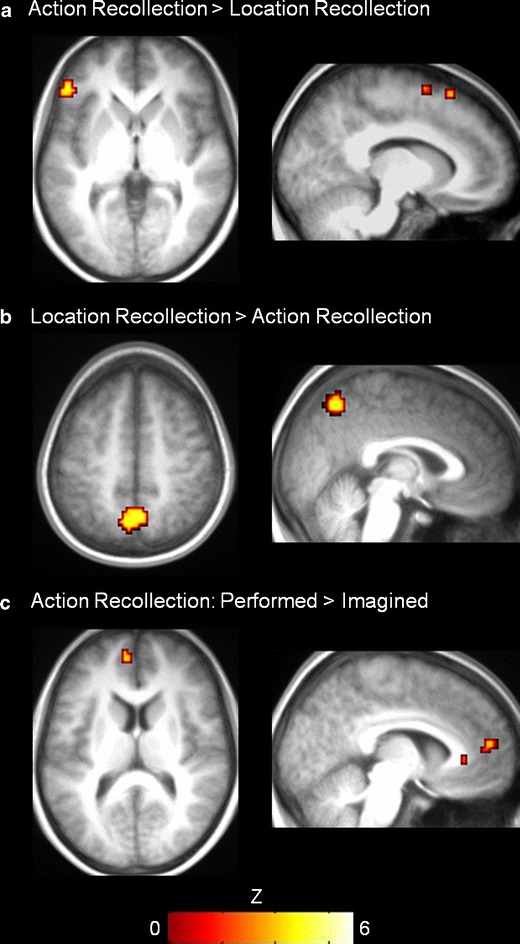
Brain regions active during the action memory task at the threshold of p < .05, corrected for voxels within regions of interest. (a) Action recollection was associated with significantly greater activity than was location recollection in left ventrolateral PFC (left panel) and in supplementary motor area (right panel). (b) Location recollection resulted in greater medial parietal activity, including the precuneus, as compared with recollection of actions. (c) Medial prefrontal cortex was the only area of the brain to show significantly greater activity specifically during action recollection of performed rather than imagined actions
In a subsequent analysis, we examined the brain areas associated specifically with recollecting whether actions were performed or imagined, with location recollection acting as a control. As can be seen in Fig. 3c, the only area of the brain to exhibit significant activity in the interaction contrast [action recollection (performed – imagined)] – [location recollection (performed – imagined)] was anterior medial PFC (centered on −6, 56, 13; BA 10; Z = 4.3). Inspecting the time course of activity in this region (Fig. 4a) confirmed the interaction, F(1, 14) = 11.57, p < .005, which was attributable to significantly greater activity during reality-monitoring judgments for actions that had previously been performed than for actions that had previously been imagined, t(14) = 3.49, p < .005, but no corresponding difference for location recollection, t(14) = 0.72, p = .48. Signal in this region during the reality monitoring of performed actions, as compared with the mean across all conditions, correlated significantly with the amount of internal detail that participants rated their action recollection judgments as involving (Fig. 5), r(13) = .64, p = .01. A significant nonparametric Spearman correlation verified that the strength of this association was not attributable solely to the participant who exhibited the highest activity, r s(13) = .56, p < .05. The correlation of anterior medial PFC signal with rated tactile detail, r(13) = .36, was not significant, given the small sample size, p = .19, and we found no correlations with rated visual detail, r(13) = −.04, or effort, r(13) = −.07. The apparent specificity of the link between activity in this region during reality monitoring of performed actions and rated internal detail was evidenced by the observation that the correlation was not significant when location recollection of performed actions was used as the dependent measure, r(13) = .37.
Fig. 4.

Time course analysis of activity during the action recollection and location recollection tasks in (a) anterior medial prefrontal cortex (amPFC), indicating greater activity specifically during reality-monitoring judgments about performed rather than imagined actions, and (b) precuneus, indicating greater activity specifically during location recollection judgments about performed rather than imagined actions
Fig. 5.

Scatterplots illustrating correlations between anterior medial prefrontal cortex (amPFC) activity during action recollection and postscan memory characteristics questionnaire ratings, identifying an association with ratings of retrieved internal detail (see the text for details; auc = area under curve)
The reverse interaction [action recollection (imagined – performed)] – [location recollection (imagined – performed)] elicited significant activity in left precuneus (−12, –67, 61; BA 7; Z = 3.4), located slightly more laterally than the cluster that emerged from the location-versus-action-recollection contrast. The significant interaction was driven by greater activity during location recollection judgments for performed than for imagined actions, t(14) = 3.13, p < .01, but no difference in the reality monitoring of action memories, t(14) = 1.15, p = .27 (Fig. 4b). Signal in this region did not correlate significantly with any of the memory characteristic ratings, and Williams’s (1959) test for nonindependent correlations revealed that anterior medial PFC activity was a significantly better predictor of rated internal detail than was precuneus signal, t(12) = 2.45, p < .05. No significant activity in either interaction contrast was observed in motor areas.
Note that the behavioral results included no significant study or test condition differences in terms of recollection accuracy, and significantly slower reaction times during reality monitoring of actions that had previously been imagined than for actions that had previously been performed. Additionally, overall, participants rated their reality-monitoring judgments for action memories as requiring significantly less effort than those for location recollection. Thus, a difference in task difficulty, however estimated, cannot provide a satisfactory explanation for the effects observed in the neuroimaging data.
Discussion
In this study, we sought to characterize the neurocognitive processes involved in reality monitoring for action memories—the ability to distinguish performed and imagined everyday actions. We investigated brain activity patterns, and objective and subjective behavioral measures, elicited by recollecting the actions that had been associated with everyday objects and the locations from which the objects had been drawn. Previous studies using paradigms that involved distinguishing perceived from imagined stimuli raised the possibility that regions of medial PFC might be important for distinguishing the “reality” of performed or imagined actions. However, generalization from studies involving the viewing of two-dimensional pictures of objects to the performing of actions with real three-dimensional objects cannot be assumed (Hintzman, 2011; Snow et al., 2011), and it may have been that reality monitoring of action memories can be accomplished simply by monitoring retrieval for records of motor processes, with associated activity in premotor or supplementary motor regions. The results revealed the involvement of both medial PFC and motor regions, with supplementary motor cortex activity being associated with decisions about actions but not about locations, and medial PFC activity reflecting the fine-grained discrimination between multiple forms of internally and externally generated information. We consider interpretations of these findings below.
Focusing first on brain regions playing a general role in recollection, contrasts of both action and location recollection against baseline were associated with common areas of activity in ventrolateral PFC, anterior cingulate, and precuneus, as well as in bilateral parietal cortex and extensive areas of the fusiform and occipital cortices. Many of these regions have previously been observed to be activated during a variety of source tasks, such as those that involve recollecting where (Gilbert, Henson, & Simons, 2010; Simons et al., 2006; Simons, Owen, et al., 2005) or when (Simons, Gilbert, et al., 2005; Turner, Simons, Gilbert, Frith, & Burgess, 2008) a stimulus was previously encountered, as well as details such as its size (Dobbins & Wagner, 2005; Ranganath et al., 2000), whether it was perceived or imagined by the participant (Simons et al., 2006; Simons et al., 2008; Turner et al., 2008), read aloud by the participant or the experimenter (Lagioia et al., 2011; Simons et al., 2008), studied as a word or a picture (Nolde, Johnson, & D’Esposito, 1998), or studied in the context of one or the other orienting task (Dobbins et al., 2002; Dobbins & Wagner, 2005; Kahn, Davachi, & Wagner, 2004; Simons, Owen, et al., 2005).
Although the present design did not allow for different stages of the reality-monitoring process to be distinguished, previous evidence suggests that some lateral PFC regions may play a preretrieval role during recollection, setting up strategies or verification criteria for retrieval—processes that have been termed retrieval orientation (Dobbins et al., 2002; Dobbins & Wagner, 2005; Ranganath et al., 2000; Simons, Gilbert, et al., 2005). For example, lateral PFC activity has been linked with cue specification and elaboration processes occurring prior to the instigation of a reality-monitoring retrieval search (Dobbins et al., 2002; Simons, Gilbert, et al., 2005), associated with the presentation of retrieval instructions rather than target stimuli (Simons et al., 2008). These regions may form part of a preretrieval network that includes more posterior brain areas such as fusiform cortex, as we observed in the present data, reflecting the manner in which retrieval may be controlled via attention to perceptual representations of presented cues and stimuli (Simons, Gilbert, et al., 2005).
Turning to brain regions with a particular role in memory for actions, greater activity during reality monitoring of action memories than for location recollection was observed in left ventrolateral PFC and supplementary motor area. This is consistent with an account in which retrieved information relating to the motor processes that were engaged during the study phase is maintained online and forms at least part of the basis for making action-related reality-monitoring decisions (Nyberg et al., 2001; Senkfor et al., 2002; Zimmer et al., 2000). It is notable that motor regions have not been among those reported by previous reality-monitoring studies as exhibiting activity that distinguished perceived from imagined information (e.g., Simons et al., 2006; Simons et al., 2008). However, future studies directly comparing performed–imagined and perceived–imagined tasks are needed to confirm that motor cortex activity is specific to reality monitoring for action memories.
One brain region that appears to play a key role during all kinds of reality-monitoring tasks is medial PFC, which in the present data was not sensitive to the non-reality-monitoring task of recollecting stimulus location, but exhibited significantly greater activity during reality monitoring of performed versus imagined actions. Insight into this result is provided by participants’ qualitative memory characteristic ratings, which indicated that greater retrieval of internally generated information occurred during recollection of performed than of imagined actions. Furthermore, signal in the peak anterior medial PFC voxel correlated significantly with ratings of internal detail retrieved during action recollection judgments. This rating pattern must be interpreted with caution, given the small sample size, but it can be considered in the context of Johnson and colleagues’ reality-monitoring framework (Johnson et al., 1988; Johnson & Raye, 1981) as suggesting that memory for performed actions might involve differentiating internally generated context details such as goal specification, movement planning, grasp point selection, and object weight estimation, as well as externally generated details such as tactile and other sensory perceptual processes. Furthermore, touching an everyday object and performing a familiar action with it may trigger additional internally generated memories and associations that might not be retrieved when imagining the action. Evidence suggests that participants regularly make use of fine-grained distinctions between such qualitative characteristics when making reality-monitoring judgments (Johnson et al., 1988; McDaniel et al., 2008), and the present data indicate that this principle may also apply when determining the reality of action memories.
The present findings add to previous indications that medial PFC regions are sensitive to subtle differences in the degrees of internally or externally generated information that are required when making recollection decisions. Activity in this region has been reported when memory for the study task previously undertaken with stimuli is contrasted against the location (Gilbert et al., 2010; Simons, Owen, et al., 2005), the time (Simons, Gilbert, et al., 2005), or the size (Dobbins & Wagner, 2005) of the stimuli. Other contrasts found to elicit differential medial PFC activity include recollecting whether stimuli were perceived or imagined (Simons et al., 2006; Simons et al., 2008; Turner et al., 2008) or whether they were generated by oneself or by an external agent (Lagioia et al., 2011; Simons et al., 2008). These functional imaging findings have recently been supplemented by evidence of a specific structural basis for reality monitoring in medial PFC. Individual differences in reality-monitoring ability in healthy volunteers were associated with morphological variability in the paracingulate sulcus, a tertiary sulcus in medial PFC that varies greatly in size between individuals (Buda, Fornito, Bergström, & Simons, 2011). Although attempting to locate sulcal landmarks on an averaged MRI scan is problematic, the medial PFC activity observed in the present data does appear to be in the vicinity of the participants’ average paracingulate sulcus location (most clearly illustrated in the right panel of Fig. 3c). In the present experiment, only correctly recollected trials were included in the fMRI analysis; future studies could contrast the activity associated with correct and incorrect recollection in order to distinguish whether activity is contingent on retrieval success.
To conclude, we used a reality-monitoring paradigm, in conjunction with both objective and subjective behavioral measures, to provide insights into the neurocognitive mechanisms involved in remembering everyday actions. A number of brain areas contributed to recollection generally, whether this involved retrieving the actions performed with real objects or the location from which the objects were drawn. Medial PFC and supplementary motor areas exhibited activity differences that were specific to the reality monitoring of action memories, consistent with memory for performed actions relying on monitoring of internally generated motoric processes and externally derived sensory perceptual details. This result converges with previous functional and structural MRI findings to support a theoretical interpretation of reality monitoring that involves the fine-grained qualitative discrimination between the multiple characteristics of event memory representations. Evaluating the relative extents of internally and externally generated detail that comprise our memories could allow us reliably to distinguish real experiences from those that we might have imagined, a decision-making ability that can have important implications for our well-being.
Acknowledgments
Author note
This research was supported by Medical Research Council (MRC; UK) Grant No. MC_US_A060_0046 to R.N.A.H., and by Biotechnology and Biological Sciences Research Council (UK) Grant No. BB/G014795/1 and a James S McDonnell Foundation Scholar Award to J.S.S. V.C.B. was supported by a scholarship from the German National Academic Foundation. We are grateful to the staff of the MRC Cognition and Brain Sciences Unit MRI Facility for scanning assistance, and to Matthew Apps for statistical advice.
References
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H-J. A fronto-parietal circuit for object manipulation in man: Evidence from an fMRI-study. European Journal of Neuroscience. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Buda M, Fornito A, Bergström ZM, Simons JS. A specific brain structural basis for individual differences in reality monitoring. Journal of Neuroscience. 2011;31:14308–14313. doi: 10.1523/JNEUROSCI.3595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RK-S, Evans AC. BrainWeb: Online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5(4(part 2/4)):S425. [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/S0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. Journal of Cognitive Neuroscience. 2006;18:1439–1452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Foley MA, Johnson MK. Confusions between memories for performed and imagined actions: a developmental comparison. Child Development. 1985;56:1145–1155. doi: 10.2307/1130229. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Henson RNA, Simons JS. The scale of functional specialization within human prefrontal cortex. Journal of Neuroscience. 2010;30:1233–1237. doi: 10.1523/JNEUROSCI.3220-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes, J., & Decety, J. (2001). Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapping, 12, 1–19. [DOI] [PMC free article] [PubMed]
- Henson, R. (2004). Analysis of fMRI time series: Linear time-invariant models, event-related fMRI and optimal experimental design. In R. S. J. Frackowiak (Ed.), Human brain function (2nd ed., pp. 793–822). San Diego, CA: Elsevier Academic Press.
- Hintzman, D. L. (2011). Research strategy in the study of memory: Fads, fallacies, and the search for the “coordinates of truth”. Perspectives on Psychological Science, 6, 253–271. [DOI] [PubMed]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology. General. 1988;117:371–376. doi: 10.1037/0096-3445.117.4.371. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981;88:67–85. doi: 10.1037/0033-295X.88.1.67. [DOI] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional–neuroanatomic correlates of recollection: Implications for models of recognition memory. Journal of Neuroscience. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes underlying memory attribution on a reality-monitoring task. Cerebral Cortex. 2006;16:1126–1133. doi: 10.1093/cercor/bhj054. [DOI] [PubMed] [Google Scholar]
- Lagioia A, Eliez S, Schneider M, Simons JS, Van der Linden M, Debbané M. Neural correlates of reality monitoring during adolescence. NeuroImage. 2011;55:1393–1400. doi: 10.1016/j.neuroimage.2010.12.058. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Lyle KB, Butler KM, Dornburg CC. Age-related deficits in reality monitoring of action memories. Psychology and Aging. 2008;23:646–656. doi: 10.1037/a0013083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D’Esposito M. Left prefrontal activation during episodic remembering: An event-related fMRI study. NeuroReport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Petersson KM, Nilsson LG, Sandblom J, Aberg C, Ingvar M. Reactivation of motor brain areas during explicit memory for actions. NeuroImage. 2001;14:521–528. doi: 10.1006/nimg.2001.0801. [DOI] [PubMed] [Google Scholar]
- Park DC, Kidder DP. Prospective memory and medication adherence. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Mahwah, NJ: Erlbaum; 1996. pp. 369–390. [Google Scholar]
- Ranganath, C., Johnson, M. K., & D’Esposito, M. (2000). Left anterior prefrontal activation increases with demands to recall specific perceptual information. Journal of Neuroscience, 20(22), RC108:1–5. [DOI] [PMC free article] [PubMed]
- Senkfor AJ, Petten CV, Kutas M. Episodic action memory for real objects: An ERP investigation with perform, watch, and imagine action encoding tasks versus a non-action encoding task. Journal of Cognitive Neuroscience. 2002;14:402–419. doi: 10.1162/089892902317361921. [DOI] [PubMed] [Google Scholar]
- Simons JS, Davis SW, Gilbert SJ, Frith CD, Burgess PW. Discriminating imagined from perceived information engages brain areas implicated in schizophrenia. NeuroImage. 2006;32:696–703. doi: 10.1016/j.neuroimage.2006.04.209. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW. Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. Journal of Neurophysiology. 2005;94:813–820. doi: 10.1152/jn.01200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Henson RNA, Gilbert SJ, Fletcher PC. Separable forms of reality monitoring supported by anterior prefrontal cortex. Journal of Cognitive Neuroscience. 2008;20:447–457. doi: 10.1162/jocn.2008.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Snow, J. C., Pettypiece, C. E., McAdam, T. D., McLean, A. D., Stroman, P. W., Goodale, M. A., & Culham, J. C. (2011). Bringing the real world into the fMRI scanner: Repetition effects for pictures versus real objects. Scientific Reports, 1. [DOI] [PMC free article] [PubMed]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system. An approach to cerebral imaging. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Turner MS, Simons JS, Gilbert SJ, Frith CD, Burgess PW. Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia. 2008;46:1442–1453. doi: 10.1016/j.neuropsychologia.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Simpson GV, Schulman BJ, Glenn S, Wong AE. Brain activation patterns during memory of cognitive agency. NeuroImage. 2006;31:896–905. doi: 10.1016/j.neuroimage.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Williams EJ. The comparison of regression variables. Journal of the Royal Statistical Society: Series B. 1959;21:396–399. [Google Scholar]
- Zimmer HD, Helstrup T, Engelkamp J. Pop-out into memory: A retrieval mechanism that is enhanced with the recall of subject-performed tasks. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2000;26:658–670. doi: 10.1037/0278-7393.26.3.658. [DOI] [PubMed] [Google Scholar]


