Abstract
Hyperosmotic stresses represent one of the major constraints that adversely affect plants growth, development, and productivity. In this study, the focus was on early responses to hyperosmotic stress- (NaCl and sorbitol) induced reactive oxygen species (ROS) generation, cytosolic Ca2+ concentration ([Ca2+]cyt) increase, ion fluxes, and mitochondrial potential variations, and on their links in pathways leading to programmed cell death (PCD). By using BY-2 tobacco cells, it was shown that both NaCl- and sorbitol-induced PCD seemed to be dependent on superoxide anion (O2·–) generation by NADPH-oxidase. In the case of NaCl, an early influx of sodium through non-selective cation channels participates in the development of PCD through mitochondrial dysfunction and NADPH-oxidase-dependent O2·– generation. This supports the hypothesis of different pathways in NaCl- and sorbitol-induced cell death. Surprisingly, other shared early responses, such as [Ca2+]cyt increase and singlet oxygen production, do not seem to be involved in PCD.
Key words: Calcium, hyperosmotic stress, mitochondria, NaCl, Nicotiana tabacum, non-selective cation channels, programmed cell death, reactive oxygen species.
Introduction
Salt stress is known to have severe effects on plant growth and development (Tester and Davenport, 2003). High salinity leads to ionic, osmotic, and oxidative stress in plants (Zhu, 2001) that may result in the induction of signalling events that lead to programmed cell death (PCD) in higher plants (Huh et al., 2002; Lin et al., 2006; Shabala, 2009; Wang et al., 2010) and algae (Affenzeller et al., 2009). Such PCD could be regarded as a salt adaptation mechanism (Huh et al., 2002). Drought, which consists at least in part of a hyperosmotic stress, was also shown to induce PCD in plants (Duan et al., 2010). PCD is an active cellular process that facilitates the removal of unwanted or damaged cells and is essential for cellular differentiation and tissue homeostasis (van Doorn et al., 2011). PCD has effectively been proved to occur in response to various abiotic stresses (Kadono et al., 2010; van Doorn et al., 2011). Different types of PCD with overlapping morphological and physiological hallmarks have been described in plants, which has led to a call for a detailed classification of cell death events (van Doorn, 2011; van Doorn et al., 2011). Although the delineation between the different PCD types of sometimes remains difficult (van Doorn et al., 2011), typical hallmarks of PCD in plants frequently include the fragmentation of the DNA by specific nucleases (DNA laddering), condensation and shrinkage of the cytoplasm, release of cytochrome c from mitochondria, elevation of the cytosolic calcium concentration ([Ca2+]cyt), generation of reactive oxygen species (ROS), and an activity increase of caspase-like enzymes (van Doorn, 2011; Tsiatsiani et al., 2011).
Although early events reported in responses to ionic and non-ionic hyperosmotic stress such as a transient [Ca2+]cyt increase (Xiong et al., 2002; Donaldson et al., 2004; Lin et al., 2006; Kim et al., 2007; Parre et al., 2007; Ranf et al., 2008), generation of ROS (Zhu, 2001; Xiong et al., 2002; Lin et al., 2006; Zhang et al. 2013), or up-regulation of protein kinases (Zhang et al., 2013) seemed to be shared in plants, some other responses are specific to one of these stresses. Under saline conditions, Na+ enters the cells through non-selective cation channels (NSCCs; Demidchik and Tester, 2002), depolarizing the plasma membrane (Chen et al., 2007; Hua et al., 2008; Shabala and Cuin, 2008; Pandolfi et al., 2010; Wegner et al., 2011). In contrast, isotonic mannitol or sorbitol solutions cause significant membrane hyperpolarization (Li and Delrot, 1987; Zingarelli et al., 1999; Shabala et al., 2000; Shabala and Lew, 2002). The DNA laddering, due to endonuclease release through permeable transition pores (PTPs) leading to mitochondria depolarization (Huh et al., 2002; Lin et al., 2006) occurred in NaCl- but not in sorbitol-stressed cells (Affenzeller et al., 2009). Thus, although some common events are induced upon osmotic stress, multiple signal transduction pathways are involved in the response to ionic and non-ionic hyperosmotic treatments (Donaldson et al., 2004; Parré et al., 2007).
In this study, it was shown using Bright Yellow 2 (BY-2) cells that both ionic and non-ionic hyperosmotic stresses effectively induced early singlet oxygen (1O2) generation and an 1O2-dependent influx of Ca2+, which are not involved in PCD processes. The PCD observed in response to NaCl and sorbitol seemed to be dependent on delayed superoxide anion (O2·–) generation by NADPH-oxidase, this last being linked to Na+ influx through NSCCs and mitochondrial dysfunction only in the case of NaCl hyperosmotic stress.
Materials and methods
Cell culture conditions
Nicotiana tabacum L. BY-2 suspension cells were grown in Murashige and Skoog (MS) medium, pH 5.8 augmented with 30g l–1 sucrose and 0.2mg l–1 2,4 D (Pauly et al., 2001). Cells were maintained at 22±2 °C, under continuous darkness and continuous shaking (gyratory shaker) at 120rpm. Cell suspensions were subcultured weekly using a 1:15 dilution. All experiments were performed at 22±2 °C using log-phase cells (6 d after subculture) maintained in their culture medium. Cell density was ~4×105 cells ml–1.
Osmolality changes
The osmolality changes were systematically obtained by addition of 50 μl of sorbitol or NaCl from various stock solutions. For the measurement of extracellular medium osmolality changes after NaCl or sorbitol treatment, 100 μl of supernatant of cell suspensions treated with NaCl or sorbitol, were determined by the freezing depression method using an Automatic Micro-Osmometer Type 15 (Löser Messtechnik, Berlin, Germany).
Cell viability assays
Hyperosmosis-induced cell death in the cell suspension culture was determined by staining the dead cells with the vital dye Evans blue (0.005%, w/v) by mixing and incubating the cells and the dye for 10min. Then stained cells were observed under a microscope. When appropriate, a 15min pre-treatment with pharmacological effectors was done prior to NaCl or sorbitol exposure. Cells were counted under a microscope and cells that accumulated Evans blue were considered dead. At least 500 cells were counted for each independent treatment, and the procedure was repeated at least three times for each condition.
Monitoring of ROS production
The production of ROS was monitored by the chemiluminescence of the Cypridina luciferin analogue (CLA) as previously described (Kadono et al., 2006, 2010). CLA is known to react mainly with O2·– and 1O2 with light emission (Nakano, 1986), and allows measurement of extracellular ROS in plant cells (Tran et al., 2013). Chemiluminescence from CLA was monitored using an FB12-Berthold luminometer (with a signal integrating time of 0.2 s). For data analysis, the luminescence ratio (L/Lbasal) was calculated by dividing the intensity of CLA luminescence (L) by the luminescence intensity before treatment (Lbasal). The ROS scavengers 1,2-dihydroxybenzene-3,5-disulphonic acid disodium salt (Tiron), 1,4-diazabicyclo[2.2.2]octane (DABCO), and salicylhydroxamic acid (SHAM) were added 5min before NaCl and sorbitol treatment. Other inhibitors were added to the cell suspension 30min before NaCl and sorbitol treatment.
Aequorin luminescence measurements
The [Ca2+]cyt variations were recorded in a BY-2 cell suspension expressing the aequorin gene. Aequorin was reconstituted by overnight incubation in MS medium supplemented with 30g l–1 sucrose and 2.5 μM native coelenterazine. Cell culture aliquots (500 μl) were transferred carefully into a luminometer tube, and the luminescence counts were recorded continuously at 0.2 s intervals with a luminometer. Treatments were performed by pipette injection of 50 μl of the effectors (NaCl or sorbitol). The residual aequorin was discharged by addition of 500 μl of a 1M CaCl2 solution dissolved in 100% methanol. The resulting luminescence was used to estimate the total amount of aequorin in each experiment. Calibration of calcium measurement was performed by using the equation: pCa=0.332588(–logk)+5.5593, where k is a rate constant equal to luminescence counts per second divided by the total remaining counts (Knight et al., 1996). The results are expressed in micromolar Ca2+ and correspond to the mean±SD of 3–5 independent experiments.
Voltage clamp measurements
Experiments were conducted on 6-day-old cells maintained in their culture medium to limit stress (main ions in MS medium 28mM NO3 –, 16mM K+). Individual cells were immobilized by a microfunnel (~50–80 μm outer diameter) and controlled by a micromanipulator (WR6-1, Narishige, Japan). Impalements were carried out with a piezoelectric micromanipulator (PCS-5000, Burleigh Inst., USA) in a chamber (500 μl) made of Perspex. Voltage clamp measurements of whole-cell currents from intact cultured cells presenting a stable running membrane potential were carried out using the technique of the discontinuous single voltage clamp microelectrode (dSEVC; Finkel and Redman, 1984). In this technique, both current passing and voltage recording use the same microelectrode. Interactions between the two tasks are prevented by time-sharing techniques (sampling frequency 1.5–3kHz). Microelectrodes were made from borosilicate capillary glass (Clark GC 150F, Clark Electromedical, Pangbourne Reading, UK) pulled on a vertical puller (Narishige PEII, Japan). Their tips were <1 μm diameter; they were filled with 600mM KCl, and had electrical resistances between 20 MΩ and 50 MΩ with the culture medium. The capacity compensation of the microelectrode amplifier (Axoclamp 2A, Molecular Devices, Sunnyvale, CA, USA) was set to a subcritical level to produce the fastest electrode response. The relatively large size of the cells ensured a sufficiently high membrane time constant despite a relatively low input resistance (~40 MΩ). Specific software (pCLAMP 8) drives the voltage clamp amplifier. Voltage and current were simultaneously displayed on a dual input oscilloscope (Gould 1425, Gould Instruments Ltd, Hainault, UK), digitalized with a Digidata 1322A (Molecular Devices). In whole-cell current measurements, the membrane potential was held to the value of the resting membrane potential. Current recordings were obtained by hyperpolarizing pulses from –200 mV to +80 mV (20 mV, 2 s steps of current injection, 6 s of settling time). It was systematically checked that cells were correctly clamped by comparing the protocol voltage values with those really imposed. Only microelectrodes presenting a linear relationship were used.
Confocal microscopy
Confocal imaging was performed using an upright Leica Laser Scanning Confocal Microscope SP5 (Leica Microsystems, Germany) equipped with a ×63 oil immersion objective. To analyse the NaCl influx, the sodium indicator Sodium Green was used (Molecular Probes, USA). The BY-2 tobacco cells were pre-incubated for 15min with an NSCC inhibitor and then incubated with 200mM NaCl for 1h. Sodium Green indicator (10 μM) was added to the solution 30min after the beginning of the salt treatment. After incubation with NaCl and Sodium Green indicator, the BY-2 cells were washed with phosphate-buffered saline (PBS) buffer. The excitation wavelength was set at 514nm, and the emission was detected at 530±20nm.
Mitochondrial membrane potential measurement
Six-day-old BY-2 suspension cells were collected and washed by filtration in a suspension buffer containing 50mM HEPES, 0.5mM CaCl2, 0.5mM K2SO4, and 10mM glucose, pH 7.0 (Errakhi et al., 2008). After treatment, cells were stained with the mitochondrial membrane potential probe JC-1 by incubating 2ml of cell suspensions for 15min (24 °C in the dark) with 2 μg ml–1 JC-1 (3 μM). JC-1 was dissolved and stored according to the manufacturer’s instructions. Treated cells without prior washing were subjected to analysis using a Hitachi F-2000 fluorescence spectrophotometer. The excitation wavelength used was 500nm. Fluorescence signals were collected using a band pass filter centred at 530nm and 590nm.
Chemicals
All chemical products were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France), except JC1 and Sodium Green indicator which were from Molecular Probes (Saint Aubin, France). Stock solution of diphenyleneiodonium chloride (DPI; 10mM) was dissolved in dimethylsulphoxide (DMSO) in order to obtain a 0.01% final concentration of DMSO. This DMSO concentration did not induce any change in ROS or [Ca2+]cyt levels (not shown). All other chemicals were dissolved in water.
Statistical analysis
Data were analysed by analysis of variance (ANOVA), and the mean separation was achieved by Newman and Keuls multiple range test. All numerical differences in the data were considered significantly different at the probability level of P ≤ 0.05.
Results
Hyperosmotic changes induce cell death in N. tabacum BY-2 suspension-cultured cells
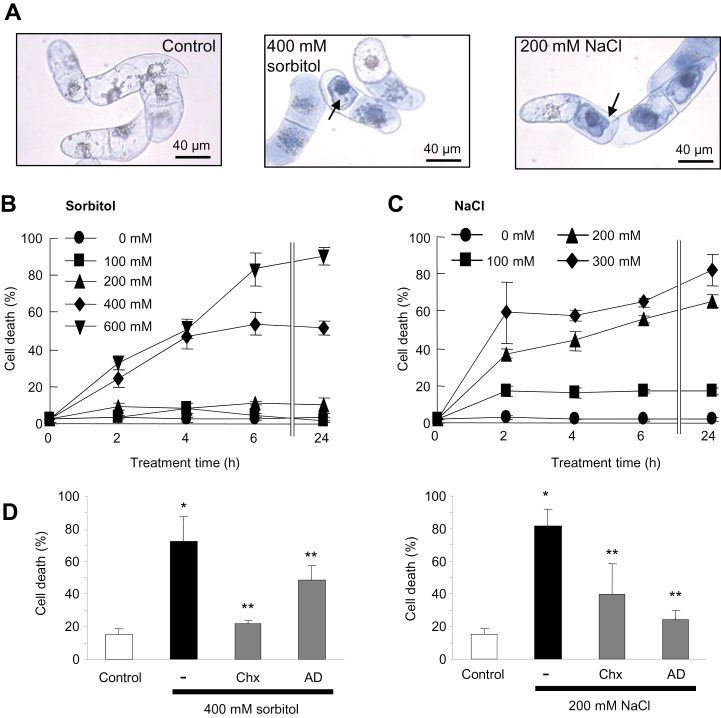
The impact of NaCl and sorbitol additions on osmolality changes in BY-2 medium was first evaluated and it was found that the concentrations of NaCl (200mM) and sorbitol (400mM) most frequently used in this study showed almost the same osmolality shifts (Table 1). These shifts in osmolality induced by 400mM sorbitol or 200mM NaCl led to the death of a part of the cell population, dead cells displaying large cell shrinkage (Fig. 1A), the hallmark of the PCD process (van Doorn, 2011). Cell death scoring at various concentrations of sorbitol and NaCl showed the time- and dose-dependent progression of death (Fig. 1B, C), half of the cells being dead after 4h at 400mM sorbitol and 200mM NaCl. In order to confirm whether this cell death was due to an active process requiring active gene expression and cellular metabolism, BY-2 cell suspensions were treated with actinomycin D (AD), an inhibitor of RNA synthesis, or with cycloheximide (Chx), an inhibitor of protein synthesis, each at 20mg ml–1, 15min prior to 200mM NaCl or 400mM sorbitol exposure. In both cases, AD and Chx significantly reduced cell death (Fig. 1D). These results indicated that this cell death required active cell metabolism, namely gene transcription and de novo protein synthesis. Taken together, these data showed that saline or non-saline hyperosmotic stress induced a rapid PCD of a part of the N. tabacum BY-2 suspension cell population.
Table 1.
Osmolality changes in the medium after treatment with NaCl and sorbitol
| Medium | NaCl (mM) | Sorbitol (mM) | |||||
|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 200 | 400 | 600 | ||
| Osmolality (mosmol) | 182 | 369 | 558 | 944 | 375 | 605 | 952 |
Fig. 1.
NaCl- and sorbitol-induced cell death in tobacco BY-2 cells. (A) Light micrographs of BY-2 cultured cells stained with Evans blue 2h after incubation with 400mM sorbitol (centre) or 200mM NaCl (right) compared with control cells maintained in their medium (left). Arrows indicate the cell shrinkage. (B) Effect of incubation time and concentration of sorbitol or NaCl on the extent of cell death. (C) Effect of pre-treatment with actinomycin D (AD; 20 μg ml–1) or cycloheximide (Chx; 20 μg ml–1) on sorbitol- and NaCl-induced cell death. Each data point and error bar reflect the mean and SD, respectively, of at least three independent replicates. *Significantly different from controls, P < 0.05; **significantly different from the NaC-l or sorbitol-treated cells, P < 0.05. (This figure is available in colour at JXB online.)
The kinetics of some early events classically detected upon saline stress or drought, namely an increase in cytosolic Ca2+, ion flux variations, ROS production, and mitochondrial membrane depolarization, were then followed, and it was checked how they could be involved in PCD induced by hyperosmotic stress.
Sorbitol- and NaCl-induced ROS generation
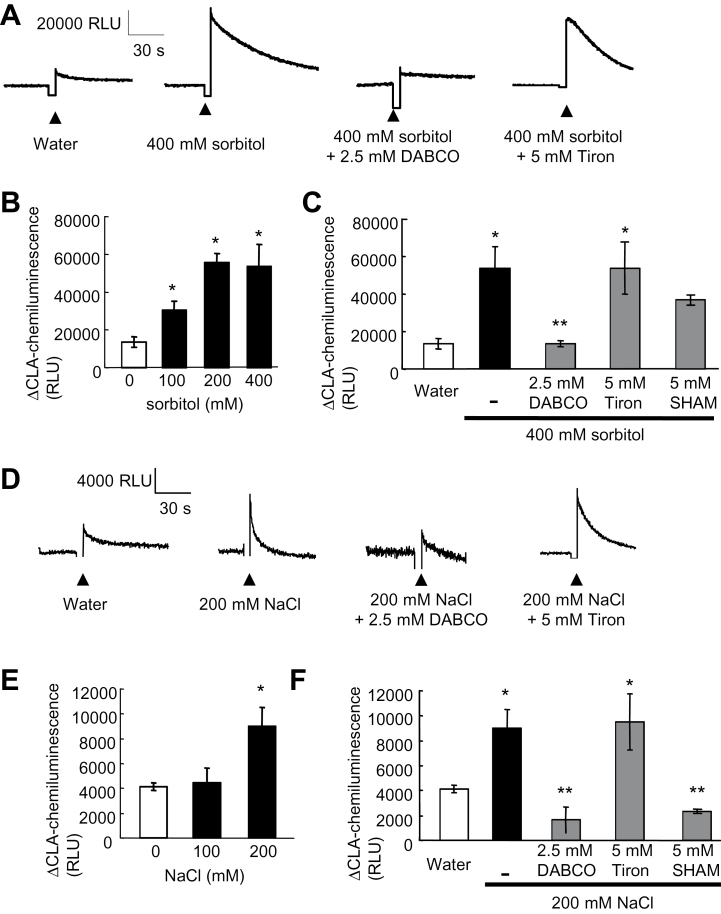
To study the effect of sorbitol on production of ROS in BY-2 cell suspension culture, the chemiluminescence of CLA, which indicates the generation of O2·– and 1O2, was used. Addition of 400mM sorbitol to BY-2 cell suspension culture resulted in transient production of ROS that reaches the maximal level immediately after treatment (Fig. 2A). This sorbitol-induced ROS generation was dose dependent (Fig. 2B) and could be blocked using DABCO, an 1O2 scavenger, but not Tiron, an O2·– scavenger (Fig. 2A, C). Addition of 200mM NaCl to BY-2 cell suspension culture also resulted in transient production of ROS that reaches the maximal level immediately after NaCl treatment (Fig. 2D, E). In the case of sorbitol, only DABCO was able to decrease the NaCl-induced CLA chemiluminescence (Fig. 2D, F). Thus, in both cases the early increase in CLA chemiluminescence seemed to be dependent on 1O2 generation but not on O2·– generation. SHAM, an inhibitor of peroxidase (POX) (Kawano et al., 1998; Hossain et al., 2013), which could be responsible for extracellular 1O2 generation (Kawano et al., 1998; Kanofsky, 2000; Guo et al., 2009), was thus used. Pre-treatment of the BY-2 cell suspension culture with 5mM SHAM only slightly reduced the increase in CLA chemiluminescence induced by 400mM sorbitol (Fig. 2C) but significantly reduced that induced by 200mM NaCl (Fig. 2F). This suggests the involvement of POX, at least in NaCl-induced 1O2 generation.
Fig. 2.
Induction of rapid ROS generation in tobacco BY-2 cells by sorbitol or NaCl. (A) Typical kinetics of the sorbitol-induced increase in CLA chemiluminescence reflecting the production ROS and modulation by ROS scavengers. (B) Effect of the concentration of sorbitol on ROS generation. (C) Modulation of sorbitol-induced ROS generation by DABCO, a scavenger of singlet oxygen, Tiron, a scavenger of the superoxide anion, or salicylhydroxamic acid (SHAM), an inhibitor of peroxidase. (D) Typical kinetics of the NaCl-induced increase in CLA chemiluminescence and modulation by ROS scavengers. (E) Effect of the concentration of NaCl on ROS generation. (F) Modulation of NaCl-induced ROS generation by DABCO, Tiron, or SHAM. Each data point and error bar reflect the mean and SD, respectively (n=5). *Significantly different from controls, P < 0.05; **significantly different from the NaCl- or sorbitol-treated cells, P < 0.05.
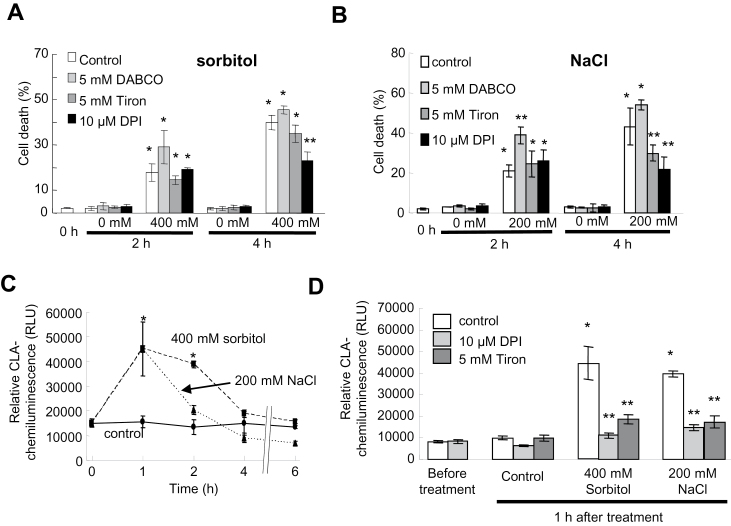
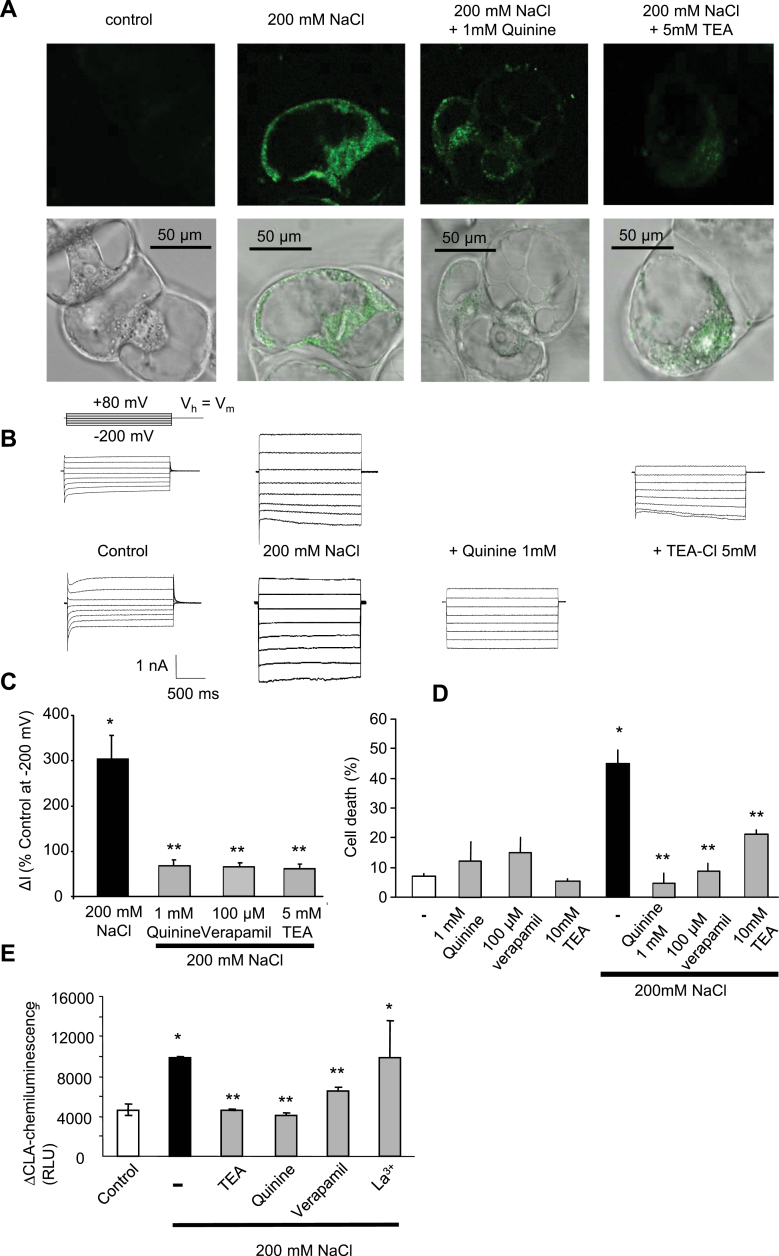
The impact of ROS pharmacology on NaCl- and sorbitol-induced PCD (Fig. 1) was further checked. DABCO, the 1O2 scavenger, failed to decrease sorbitol- (400mM) and NaCl- (200mM) induced cell death and even increased NaCl-induced cell death after 2h of treatment (Fig. 3A, B). For Tiron, the O2·– scavenger, there was no effect after 2h but a decrease in cell death could be observed after 4h treatment with NaCl (Fig. 3B). Thus, the hyperosmotic stress-induced cell death seemed not to be dependent on 1O2 generation but on O2·– generation. Thus a possible delayed O2·– generation after treatment with 400mM sorbitol or 200mM NaCl was searched for. After such hyperosmotic stress, increases in CLA chemiluminescence could be detected (Fig. 3C). In both cases of CLA chemiluminescence increases, the maximum chemiluminescence levels were reached after 1h and then decreased to the control level after 4h, the decrease being more rapid upon NaCl treatement (Fig. 3C). It is noteworthy that these increases occurred before cell death reached the plateau level (Fig. 1B, C). These increases in CLA chemiluminescence could be inhibited by pre-treatment with Tiron, but also with 10 μM DPI, an inhibitor of NADPH-oxidase (Fig. 3D), suggesting that the generation O2·– through enhancement of NADPH-oxidase activity was involved in the delayed ROS generation after treatment with sorbitol and NaCl (Fig. 3C). In agreement with this, 10 μM DPI could also significantly reduce both sorbitol- and NaCl-induced cell death after 4h (Fig. 3A, B).
Fig. 3.
Effects of ROS scavengers on NaCl- or sorbitol-induced cell death in BY-2 cells. (A) Effect of Tiron, DABCO, or DPI, an NADPH-oxidase inhibitor, on cell death induced by 400mM sorbitol after 2h or 4h treatment. (B) Effect of Tiron, DABCO, or DPI on cell death induced by 200mM NaCl after 2h or 4h treatment. (C) Time course of CLA chemiluminescence during 6h treatment with 400mM sorbitol or 200mM NaCl. (D) Inhibition of sorbitol and NaCl-induced delayed ROS generation by Tiron or DPI. Each data point and error bar reflect the mean and SD, respectively (n=3). *Significantly different from controls, P < 0.05; **significantly different from the NaCl- or sorbitol-treated cells, P < 0.05.
Sorbitol and NaCl induce a rapid change in [Ca2+]cyt
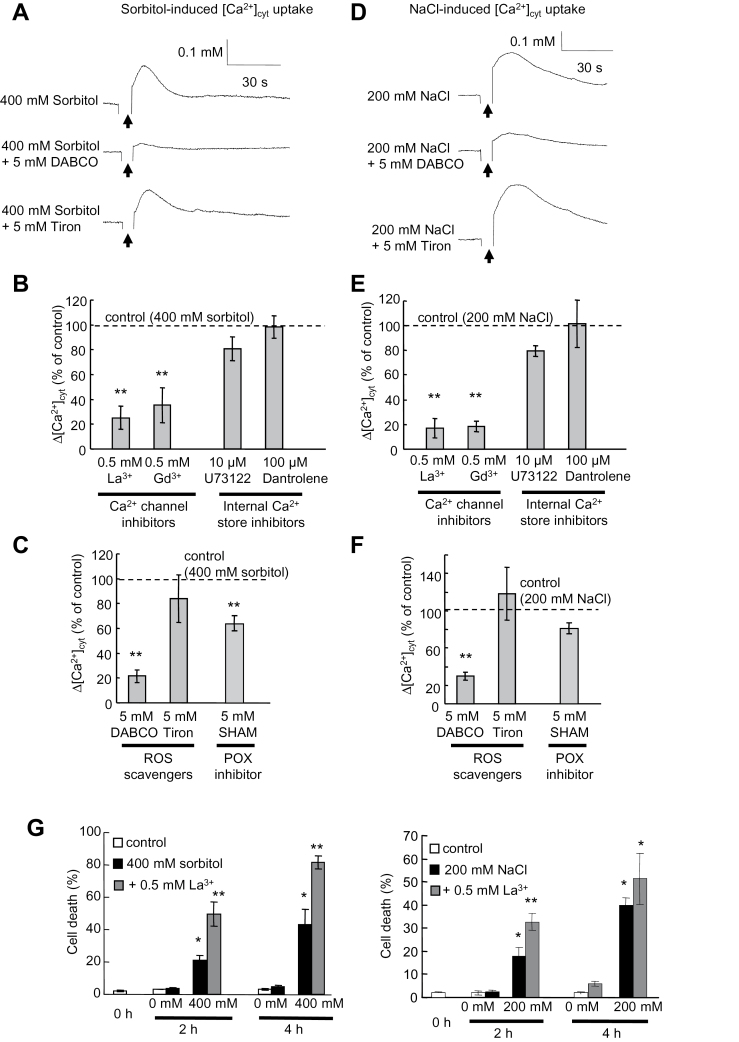
The changes in [Ca2+]cyt were monitored by the Ca2+-dependent emission of blue light from aequorin (Knight et al., 1996). Treatment of BY-2 cells with sorbitol (400mM) resulted in a rapid transient increase in aequorin luminescence (Fig. 4A) reflecting an increase in [Ca2+]cyt of 0.145±0.035 μM (n=23). This increase could be inhibited by the presence of Ca2+ channel blockers, LaCl3 and GdCl3, when Ca2+ internal store inhibitors (U73122 and dantrolene, Meimoun et al., 2009) showed no significant inhibitory effects (Fig. 4B), indicating that the sorbitol-induced increase in [Ca2+]cyt is mainly due to influx of Ca2+ across the plasma membrane through Ca2+ channels. As the generation of 1O2 by sorbitol occurred immediately upon hyperosmotic stress (Fig. 2A), the effect of ROS pharmacology on the sorbitol-induced increase in [Ca2+]cyt was further tested. The 1O2 scavenger DABCO strongly reduced the [Ca2+]cyt increase compared with that in control cells; the POX inhibitor SHAM showed lower efficiency, while no differences were seen with the O2·– scavenger Tiron (Fig. 4A, C). These results strongly suggested that sorbitol-induced Ca2+ uptake through Ca2+ channels occurs downstream of the sorbitol-induced 1O2 generation.
Fig. 4.
Induction of [Ca2+]cyt increase in aequorin-expressing tobacco BY-2 cells by sorbitol or NaCl. (A) Typical kinetics of sorbitol-induced increase in [Ca2+]cyt and modulation by ROS scavengers. (B) Modulation of sorbitol-induced [Ca2+]cyt increase by the calcium channel blockers La3+ or Gd3+ (500 μM each) and the internal stock release inhibitors U73122 (100 μM) and dantrolene (10 μM). (C) Modulation of sorbitol-induced [Ca2+]cyt increase by DABCO, a scavenger of singlet oxygen, Tiron, a scavenger of anion superoxide, or salicylhydroxamic acid (SHAM), an inhibitor of peroxidase. (D) Typical kinetics of the NaCl-induced increase in [Ca2+]cyt and modulation by ROS scavengers. (E) Modulation of the NaCl-induced [Ca2+]cyt increase by La3+, Gd3+ (500 μM each), U73122 (100 μM), or dantrolene (10 μM). (F) Modulation of NaCl-induced [Ca2+]cyt increase by DABCO, Tiron, or SHAM. (G) Effect of La3+ on cell death induced by 200mM NaCl (left) or 400mM sorbitol (right) after 2h or 4h treatment. Each data point and error bar reflect the mean and SD, respectively (n=5). *Significantly different from controls, P < 0.05; **significantly different from the NaCl- or sorbitol-treated cells, P < 0.05.
In the same way, treatment of BY-2 cells with NaCl (200mM) resulted in a transient increase in aequorin luminescence (Fig. 4D), reflecting an increase in [Ca2+]cyt of 0.217±0.059 μM (n=23). As for sorbitol, inhibitors of Ca2+ release from intracellular organelles (U73122 and dantrolene) failed to suppress the NaCl-induced increase in [Ca2+]cyt whereas Ca2+ channel blockers LaCl3 and GdCl3 were efficient at reducing the NaCl-induced increase in [Ca2+]cyt (Fig. 4E). In a similar manner to what was observed for sorbitol, this increase was shown to be inhibited by the presence of DABCO (Fig. 4F) but less efficiently by SHAM, whereas Tiron seemed even to increase this Ca2+ influx (Fig. 4D, F). The effect of the Ca2+ channel inhibitor La3+ on NaCl- and sorbitol-induced cell death in BY-2 suspension-cultured cells was then studied. Lanthanum (500 μM) failed to decrease sorbitol- (400mM) and NaCl- (200mM) induced cell death and even increases this cell death after 2h of treatment (Fig. 4G), as observed for DABCO with NaCl (Fig. 3B). These data are in agreement with the link observed between the immediate 1O2 generation inducing an influx of Ca2+ upon sorbitol or NaCl stress and further suggest that these early induced events are not involved in a pathway leading to PCD.
Hyperosmotic constraints induce change in membrane potential and ion channel activities
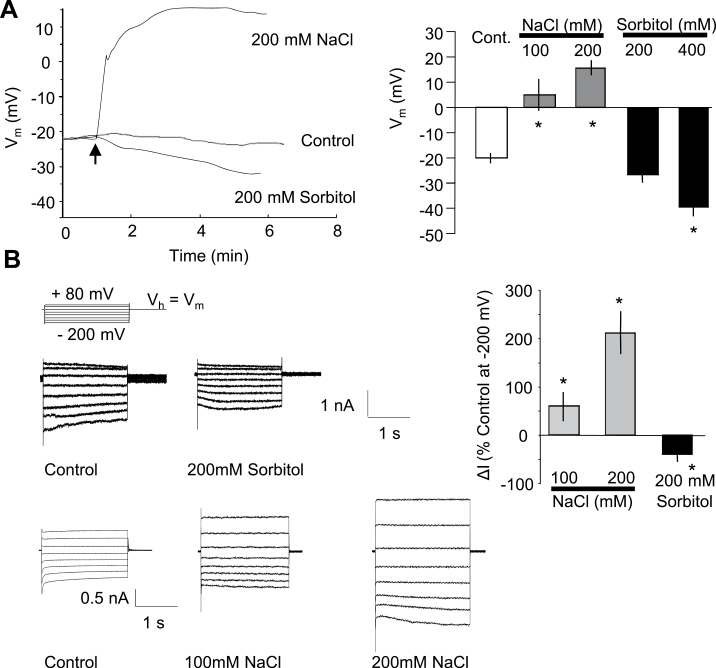
Saline and non-saline hyperosmotic stresses are well known to modify the plasma membrane potential (V m) of cells (Teodoro et al., 1998; Zingarelli et al., 1999; Shabala and Cuin, 2008; Wegner et al., 2011). By using an electrophysiological technique (dSEVC), the impact of NaCl and sorbitol on BY-2 cultured cell membrane potential was investigated. In control conditions in culture medium, the V m of BY-2 cells was –21.1±2.2 mV (n=15). In MS medium, the main ions are 16mM K+ and 28mM NO3 –; thus the equilibrium potential estimated for K+, E K, is about –46 mV ([K+]out=16mM with [K+]in estimated at 100mM). The equilibrium potential estimated for NO3 – is about –25 mV ([NO3 –]out=28mM with [NO3 –]in estimated at 5mM). As previously observed with cultured cells of Arabidopsis thaliana (Kadono et al., 2010; Tran et al., 2013) or tobacco (Gauthier et al., 2007), the occurrence of anion currents in most of the BY-2 cells in their culture medium could explain the mean polarization of around –20 mV recorded in control and non-stressed conditions. The mean control value of these currents at –200 mV and after 1.8 s of voltage pulse was –1.12±0.2 nA (n=11). These currents were shown to be sensitive to structurally unrelated anion channel inhibitors, 9-anthracene carboxylic acid (9-AC) and glibenclamide (gli) (Supplementary Fig. S1 available at JXB online), reinforcing the hypothesis of an anionic nature for these currents. Addition of NaCl to suspension cultures resulted in a significant membrane depolarization (Fig. 5A) when sorbitol induced a hyperpolarization of the cells (Fig. 5A), clearly indicating a difference between saline and non-saline hyperosmotic stress. The sorbitol-induced hyperpolarization was correlated with a decrease in anion current (Fig. 5B) when the NaCl-induced depolarization was correlated with a large increase in whole-cell ion current (Fig. 5B). The positive shifts of the reversal potential of the current upon addition of NaCl are in accordance with a current carried by Na+.
Fig. 5.
(A) Typical modulation of BY-2 cultured cell plasma membrane (PM) potential variations observed in response to NaCl or sorbitol. (B) Mean values of PM potentials recorded a few minutes after treatment with NaCl (100mM or 200mM) or sorbitol (200mM or 400mM). (C) Typical changes in whole-cell current profiles after treatments with sorbitol (up) or NaCl (down). The protocol was as illustrated; the holding potential (Vh) was Vm. (D) Mean values of whole-cell current variations (recorded at –200 mV and 1.8 s) after treatment with NaCl or sorbitol. Current variations are given as a percentage of the control level before treatments. The data correspond to means of at least five independent replicates, and error bars correspond to the SD. *Significantly different from controls, P < 0.05.
The influx of Na+ through the plasma membrane by NSCCs was the most probable reason for the cell depolarization (Demidchick et al., 2002a , b ), this Na+ uptake was further checked using the Na+-sensitive fluorescent probe Sodium Green (Wegner et al., 2011). An accumulation of fluorescence in the cytoplasm of the cells could be observed after 1h treatment with 200mM NaCl (Fig. 6A). This fluorescence could be reduced by using pre-treatments with inhibitors of NSCC, 5mM tetraethylammonium chloride (TEA+) or 1mM quinine (Demidchick et al., 2002a ) (Fig. 6A). Addition of 1mM quinine or 5mM TEA+ also allowed reduction of the NaCl-induced increase in currents (Fig. 6B, C) in BY-2 cells, as did verapamil (100 μM), another potent inhibitor of NSCCs (Demidchick et al., 2002a ). The impact of these NSCC blockers on the extent of NaCl-induced cell death was thus further tested. The NSCC blockers were efficient at reducing the cell death induced by 200mM NaCl (Fig. 6D), suggesting that NSCC activation is related to the NaCl-induced cell death. As this cell death was also dependent on a delayed O2·– generation by NADPH-oxidase (Fig. 3), the effect of the earliest activation of NSCCs by NaCl on this delayed ROS production was also checked. As for cell death, the NSCC blockers were efficient at reducing this NaCl-dependent delayed ROS production (Fig. 6E), suggesting that NaCl influx participates in the O2·– generation. Interestingly, the calcium channel blocker La3+ did not allow the delayed ROS generation to be decreased, confirming the hypothesis of the induction of different pathways in response to NaCl stress.
Fig. 6.
(A) Confocal imaging of NaCl accumulation in BY-2 cells after 1h treatment with 200mM NaCl using the Sodium Green fluorescent probe (left). Decrease in fluorescence in cells pre-treated with quinine (1mM) or TEA+ (5mM), blockers of non-selective cation channels (NSCCs), prior to NaCl treatment (right). Corresponding bright field images are shown on the line below. Each image is representative of symptoms observed in at least three independent experiments. (B) Variations of whole-cell currents recorded before and after addition of 200mM NaCl and subsequent addition of 1mM quinine or 5mM TEA+. The protocol was as illustrated; the holding potential (Vh) was Vm. (C) Mean values of whole-cell current variations (recorded at –200 mV and 1.8 s) after treatment with 200mM NaCl with or without the NSCC blockers quinine (1mM), TEA+ (5mM), or verapamil (200 μM). Current variations are given as a percentage of the control level before treatments. The data correspond to means of at least five independent replicates, and error bars correspond to the SD. (D) Effect of pre-treatments with the NSCC blockers on NaCl-induced cell death. The data correspond to means of at least four independent replicates, and error bars correspond to the SD. (E) Effect of pre-treatments with the NSCC blockers or with La3+ (500 μM) on NaCl-induced delayed ROS generation and cell death. The data correspond to means of at least five independent replicates, and error bars correspond to the SD. *Significantly different from controls, P < 0.05; **significantly different from the NaCl- or sorbitol-treated cells, P < 0.05. (This figure is available in colour at JXB online.)
Mitochondrial depolarization is involved in NaCl- but not sorbitol-induced cell death
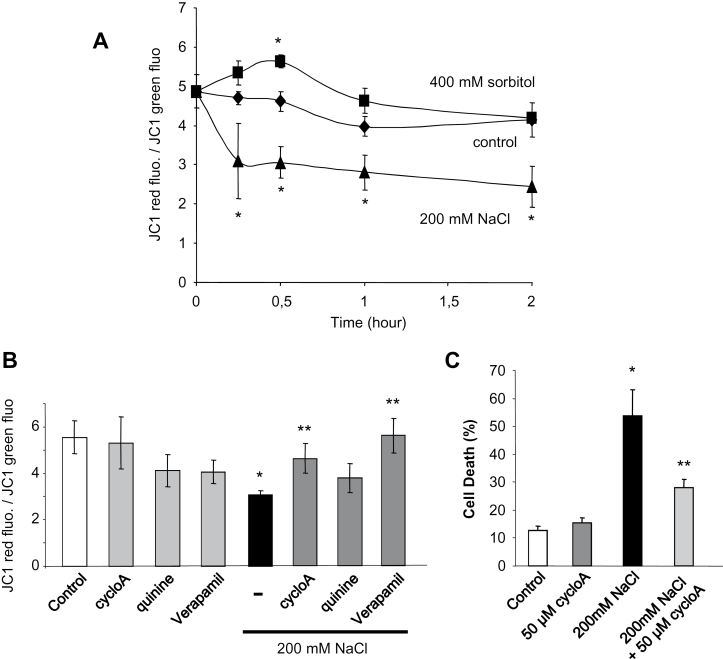
The role of mitochondria is well recognized in salinity tolerance (Jacoby et al., 2011), and dysfunction of mitochondria, leading to cytochrome c release upon salt stress, was shown to induce PCD in Thellungiella halophila suspension-cultured cells (Wang et al., 2010). Mitochondria are effectively pivotal in controlling cell life and PCD, through complex mechanisms that culminate in opening of PTPs leading to mitochondrial membrane potential (ΔΨm) loss (Vianello et al., 2007). It was thus checked whether NaCl and sorbitol lead to a decrease in ΔΨm in the present model. In untreated cells, the JC-1 fluorescence ratio of mitochondria displaying a high ΔΨm versus mitochondria presenting a low ΔΨm was greatly superior to 1 (Fig. 7A). This ratio decreased in a time-dependent manner upon addition of NaCl (200mM), indicating that NaCl induced a significant decrease of ΔΨm in some mitochondria when sorbitol (400mM) induced an increase of ΔΨm during the first 30min, the ratio reaching the control value after 2h (Fig. 7A). Since a mitochondrial ΔΨm decrease during cell death was reported to be due to the formation of the mitochondrial PTPs (Vianello et al., 2007), the effect of cyclosporin A (CsA), an inhibitor of PTPs, was tested on NaCl-induced mitochondrial depolarization. A pre-treatment with CsA significantly reduced the NaCl-induced mitochondrial depolarization after 15min (Fig. 7B), indicating that mitochondrial PTPs were involved in NaCl-induced mitochondrial depolarization. Moreover, pre-treatment with CsA significantly inhibited NaCl-induced cell death (Fig. 7C), indicating that PTP formation could participate in NaCl-induced cell death. As the activation of NSCCs occurs rapidly upon NaCl stress (Fig. 5A) and is involved in ROS generation (Fig. 6E), the impact of sodium influx on ΔΨm was investigated by using NSCC inhibitors. A significant reduction of NaCl-induced mitochondrial depolarization was observed after pre-treatment with verapamil (Fig. 7B). These data show that (i) two different pathways could be involved in the hyperosmotic stress-induced cell death; and (ii) the ROS generation dependent on Na+ influx could participate in the decrease in ΔΨm during NaCl-induced cell death.
Fig. 7.
(A) Variations of mitochondrial membrane potential (ΔΨm) of BY-2 tobacco cells after treatments with 200mM NaCl or 400mM sorbitol. (B) Effect of 50 μM cyclosporin A (CsA), an inhibitor of the permeability transition pore, 1mM quinine, or 200 μM verapamil, inhibitors of NSCCs, on ΔΨm variation induced by 200mM NaCl after 15min. The data reflect the means±SE of at least four independent experiments. (C) Effect of 50 μM CsA, 1mM quinine, or 200 μM verapamil on cell death induced by 200mM NaCl. The data reflect the means±SE of at least three independent replicates. *Significantly different from controls, P < 0.05; **significantly different from the NaCl-treated cells, P < 0.05.
Discussion
As expected from previous studies (Huh, 2002; Wang, 2010), the death of a part of the BY-2 cell population characterized by large cell shrinkage, a hallmark of the PCD process (van Doorn, 2011), was observed in response to NaCl-induced hyperosmotic stress. The extent of cell death was time and dose dependent, reaching about half of the population in 4h with 200mM NaCl. In order to check whether this cell death was due to an active mechanism requiring active gene expression and cellular metabolism, BY-2 cell suspensions were treated with AD, an inhibitor of RNA synthesis, or with Chx, an inhibitor of protein synthesis, prior to NaCl exposure. AD and Chx significantly reduced the NaCl-induced cell death. These results indicated that this cell death required active cell metabolism, namely gene transcription and de novo protein synthesis. The same behavior, namely time- and dose-dependent cell death characterized by cell shrinkage and requiring active metabolism using iso-osmotic concentrations of sorbitol, was observed here. This suggests that non-ionic hyperosmotic stress, like ionic hyperosmotic stress, could induce PCD in BY-2 cells as previously observed in various animal cell lines (Murata et al., 2002; Galvez et al., 2003; Niswander and Dokas, 2007). It was then checked whether some early events classically detected during hyperosmotic stress responses and PCD in plant could be involved in this cell death, namely ROS production, an increase in cytosolic Ca2+, ion flux variations, and mitochondrial membrane depolarization.
The earliest response observed after exposure of BY-2 cells to NaCl was an immediate peak of ROS. Addition of Tiron (a scavenger of O2·–) did not significantly reduce NaCl-induced ROS generation, whereas DABCO (a scavenger of 1O2) avoided this production. Although 1O2 formation is likely to occur during the exposure to high light intensities (Krieger-Liszkay, 2004), it was also found that different enzymes including POXs could produce extracellular 1O2 in animals (Kanofsky, 2000; Stief, 2003; Tarr and Valenzeno, 2003) and in plant cells (Kawano et al., 1998; Guo et al., 2009). As the studied cultured cells were non-photosynthetic, the POX inhibitor SHAM was further tested (Kawano et al., 1998; Hossain et al., 2013), and this could reduce the early NaCl-induced ROS generation effectively. Thus, it would appear that these early transient 1O2 generations are not specific to ionic or non-ionic hyperosmotic stress.
In the case of both NaCl and sorbitol treatments, rapid transient [Ca2+]cyt increases in BY-2 cells were observed, as previously described in various models (Knight et al., 1997; Donaldson et al., 2004; Lin et al., 2006; Parre et al., 2007; Ranf et al., 2008). In BY-2 cells, these [Ca2+]cyt increases were inhibited by the calcium channel blockers La3+ and Gd3+ but not by dantrolene and U73122, inhibitors of calcium-induced calcium release channels and of the inositol triphosphate receptor, respectively, known to be efficient in plants (Meimoun et al., 2009). This suggests that the [Ca2+]cyt increase was not due to Ca2+ release from intracellular Ca2+ stores, but to an influx through plasma membrane-permeable Ca2+ channels. Although rapid, the Ca2+ influxes happened after the 1O2 generation since DABCO pre-treatments strongly decreased these influxes, suggesting that they were dependent on the generation of 1O2. Accordingly, SHAM decreased the Ca2+ influxes to a lower extent and Tiron was unable to decrease them. This confirms the hypothesis that an 1O2-dependent Ca2+ influx was induced by sorbitol and NaCl and highlights the role of 1O2 as a signalling molecule (Fischer et al., 2013).
Perturbation of Ca2+ homeostasis in plant cells, as well as in animal cells, has been described as a prerequisite for PCD (Grant et al., 2000; Davis and Distelhorst, 2006; Lecourieux et al., 2006). It was not possible to ascertain that in the BY-2 population all cells respond with a Ca2+ increase in the face of hyperosmotic stress. However, if only non-dying cells respond with a Ca2+ increase, this Ca2+ increase has no role in inducing PCD. In other words, if only dying cells (or even all cells) respond with a Ca2+ increase, since La3+ was inefficient in decreasing NaCl- and sorbitol-induced cell death, and even enhanced this cell death, it could suggest that Ca2+ influx could participate in cell protection. After perception of salt stress, the Ca2+ spike generated in the cytoplasm of root cells is known to activate the Salt Overly Sensitive (SOS) signal transduction cascade to protect the cells from damage due to excessive ion accumulation (Ji et al., 2013). SOS3 encodes a myristoylated calcium-binding protein that appears to function as a primary calcium sensor to perceive the increase in cytosolic Ca2+ triggered by Na+ excess that has entered the cytoplasm. Upon binding to Ca2+, SOS3 is able to interact with and activate the protein kinase SOS2 which phosphorylates SOS3 proteins. SOS3–SOS2 interactions recruit SOS2 to the plasma membrane, leading to activation of the downstream target SOS1, an Na+/H+ antiporter allowing extrusion of excessive Na+ from the cytosol (Ji et al., 2013). However, in the present model, neither of these early linked events, Ca2+ increase and 1O2, seemed to be involved in PCD.
On the other hand, Tiron, a scavenger of O2·–, and DPI, an inhibitor of NADPH-oxidase, decreased the NaCl- and sorbitol-induced cell death and the delayed generation of ROS. This indicated that the delayed and more sustained O2·– generation from NADPH-oxidase activity could play a central role in the death of these cells. Several reports implicate NADPH-oxidase activity in production of ROS in salinity stress, with the ROS resulting in Ca2+ influx. In the present model, no effect of DPI on early stress-induced Ca2+ increase could be detected (Supplementary Fig. S2 available at JXB online), in accordance with the delayed DPI-sensitive ROS generation. However, from the present data, a delayed increase in Ca2+ linked to NADPH-oxidase activity cannot be excluded, but this increase in Ca2+ should be La3+ independent, since La3+ could not decrease cell death. It is obvious that sorbitol-induced ROS generation could not depend on Na+ influx and thus possibly other hyperosmotic-induced events (e.g. NO production) could participate in NADPH-oxidase-dependent ROS generation observed in response to sorbitol and NaCl. However, upon NaCl stress, the delayed ROS generation could be decreased by quinine and verapamil, putative inhibitors of NSCCs (Demidchik et al., 2002a , b). Even though no definitive molecular candidates have clearly emerged for NSCCs, it seems that various classes of NSCCs could be responsible for influx of Na+ under salt stress, especially a depolarization-activated class of NSCCs (Demidchik and Maathuis, 2007). A rapid and large depolarization of the BY-2 cells could be recorded upon NaCl addition due to an increase in a current sensitive to quinine and verapamil but also to TEA+, an inhibitor of K+ channels known to block some NSCCs (Demidchik et al., 2002a ). It could also be verified that the accumulation of Na+ in the cell was decreased upon pre-treatment with quinine or TEA+, strongly suggesting that NSCCs were responsible for Na+ influx into BY-2 cells. Moreover, these NSCC blockers were efficient in decreasing the NaCl-induced cell death, highlighting the toxic effect of Na+ as previously reported (Huh et al., 2002; Affenzeller et al., 2009; Wang et al., 2010). It is further noticeable that La3+, a potent inhibitor of some NSCCs (Demidchick et al., 2002a , b ), was unable to decrease NaCl-induced cell death as well as the delayed ROS generation, whereas the NSCC blockers quinine, verapamil, and TEA+ failed to decrease the NaCl-induced Ca2+ increase (Supplementary Fig. S3 available at JXB online). This indicates that the Ca2+ influx was completely dissociated from Na+ influx through NSCCs. Moreover, sorbitol- and NaCl-induced Ca2+ influxes presented the same characteristics and were induced upon hyperpolarization in the case of sorbitol and depolarization in the case of NaCl, suggesting the voltage independence of the transporter involved. Further studies will be needed to determine if putative ligand-activated calcium channels such as cyclic nucleotide-gated channels or glutamate receptors could be responsible for these Ca2+ influxes in BY-2 cells; however, a putative candidate could be the calcium regulatory protein annexin (Laohavisit et al., 2013).
Concerning ion flux regulation in response to sorbitol, a hyperpolarization of the BY-2 cells due to the decrease of anion currents was observed, as previously described in different models (Pennarun and Maillot, 1988; Teodoro et al., 1998; Shabala et al., 2000). Although such a regulation of anion current was shown to be involved during PCD induced by HrpNea, a hypersensitive elicitor from Erwinia amylovora (Reboutier et al. 2005), it did not seem to be involved in sorbitol-induced PCD since bromotetramisole, an activator of anion channels, failed to limit sorbitol-induced PCD (data not shown), in contrast to the previously observed effect of HrpNea (Reboutier et al., 2005).
Although ROS-activated outward-rectifying K+ channels (KORCs) were shown to be involved in salt-induced PCD (Demidchik et al., 2003, 2010), it was not possible to detect rapid activation of KORCs in response to NaCl or sorbitol. However, an activation of KORCs after addition of NaCl cannot be excluded, this current being masked in the large Na+ current recorded. On the other hand, KORC activation could also be delayed, as was recently reported in response to O3 (Tran et al., 2013).
Finally, one of the most important differences observed between sorbitol- and NaCl-induced PCD in BY-2 cells was the role of mitochondria. Sorbitol did not affect mitochondrial polarization and even slightly and transiently stimulated its polarization, whereas NaCl induced a large mitochondrial depolarization. This depolarization was probably involved in the formation of the PTP since CsA, an inhibitor of PTP, could reduce the NaCl-induced decrease in ΔΨm as previously described (Wang et al., 2010) and also the NaCl-induced cell death. This could be related to observation made on Micrasterias denticulate (Affenzeller et al., 2009) for which a typical laddering of the DNA was only observed for NaCl-stressed cells but not for sorbitol-treated cells. The endonuclease responsible for this laddering could be released from mitochondria through PTPs (Vianello et al., 2007). It is to be noted that the inhibitors of NSCCs, verapamil, quinine, and TEA+, were also efficient in limiting the loss in ΔΨm, suggesting a direct role for cytosolic Na+-induced mitochondrial dysfunction.
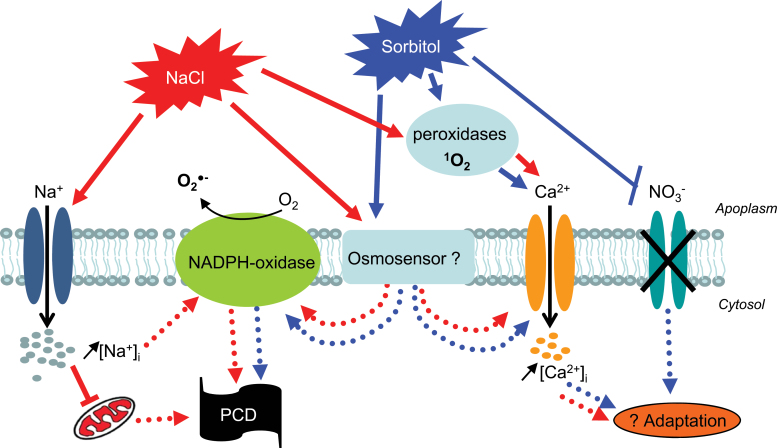
As already described in numerous models, in BY-2 cells overlapping responses exist in response to ionic and non-ionic hyperosmotic stress and, among them, a PCD process. However, some overlapping responses such as the early transient 1O2 generation responsible for an influx of Ca2+ did not seem to be involved in PCD progress, while other shared responses such as the delayed NADPH-oxidase stimulation were important for these processes (Fig. 8). Specific responses also seemed to be involved in the PCD processes since upon NaCl stress, NADPH-oxidase stimulation was at least partly due to Na+ influx through NSCCs, which also seemed to be responsible for mitochondrial depolarization not observed after the sorbitol challenge. Further studies will be needed to characterize fully the pathways leading to PCD induced by these hyperosmotic stresses.
Fig. 8.
Possible pathways induced by NaCl and sorbitol leading to cell death of tobacco cells. (This figure is available in colour at JXB online.)
Supplementary data
Supplementary data are available at JXB online.
Fgure S1. Typical anion current recorded in control conditions in BY-2 cells.
Figure S2. NaCl- and sorbitol-induced [Ca2+]cyt increase in aequorin-expressing-tobacco BY-2 cells after a pre-treatment with the inhibitor of NADPH-oxidase DPI (20 μM).
Figure S3. NaCl-induced [Ca2+]cyt increases in aequorin expressing-tobacco BY-2 cells after pre-treatments with the NSCC blockers quinine, verapamil, or TEA+.
Acknowledgements
This research was partially supported by the Japan Society for the Promotion of Science, Grant-in-Aid for JSPS Fellows to TK. EM was supported by Fondo Giovani provided by the Ministry of Education, Universities and Research (MUIR). SM and EA were supported by the Future and Emerging Technologies (FET) programme within the 7th Framework Programme for Research of the European Commission, under FET-Open grant number 293431. We thank Christian Mazars (LRSV UPS CNRS, Toulouse, France) for the kind gift of the BY-2 aequorin cell line.
References
- Affenzeller MJ, Darehshouri A, Andosch A, Lütz C, Lütz-Meindl U. 2009. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata Matthias. Journal of Experimental Botany 60, 939–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S. 2007. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. Journal of Experimental Botany 58, 4245–4255 [DOI] [PubMed] [Google Scholar]
- Davis MC, Distelhorst CW. 2006. Live free or die: an immature T cell decision encoded in distinct Bcl-2 sensitive and insensitive Ca2+ signals. Cell Cycle 5, 1171–1174 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Bowen HC, Maathuis FJ, Shabala SN, Tester MA, White PJ, Davies JM. 2002a. Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. The Plant Journal 32, 799–808 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. 2010. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. 2002b. Nonselective cation channels in plants. Annual Review of Plant Biology 53, 67–107 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJ. 2007. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175, 387–404 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. 2002. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology 128, 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson L, Ludidib N, Knight MR, Gehring C, Denby K. 2004. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Letters 569, 317–320 [DOI] [PubMed] [Google Scholar]
- Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen, Han C, Zhang Y, Li X. 2010. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis . New Phytologist 186, 681–695 [DOI] [PubMed] [Google Scholar]
- Errakhi R, Meimoun P, Lehner A, Vidal G, Briand J, Corbineau F, Rona JP, Bouteau F. 2008. Anion channel activity is necessary to induce ethylene synthesis and programmed cell death in response to oxalic acid. Journal of Experimental Botany 59, 3121–3129 [DOI] [PubMed] [Google Scholar]
- Finkel AS, Redman S. 1984. Theory and operation of a single microelectrode voltage clamp. Journal of Neuroscience Methods 11, 101–127 [DOI] [PubMed] [Google Scholar]
- Fischer BB, Hideg E, Krieger-Liszkay A. 2013. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxidant and Redox Signaling 18, 2145–2162 [DOI] [PubMed] [Google Scholar]
- Galvez AS, Ulloa JA, Chiong M, Criollo A, Eisner V, Barros LF, Lavandero S. 2003. Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effet of sorbitol. Journal of Biological Chemistry 278, 38484–38494 [DOI] [PubMed] [Google Scholar]
- Gauthier A, Lamotte O, Reboutier D, Bouteau F, Pugin A, Wendehenne D. 2007. Cryptogein-induced anion effluxes: electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitor-triggered cell death. Plant Signaling and Behavior 2, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J. 2000. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. The Plant Journal 23, 441–450 [DOI] [PubMed] [Google Scholar]
- Guo W, Ye Z, Wang G, Zhao X, Yuan J, Du Y. 2009. Measurement of oligochitosan–tobacco cell interaction by fluorometric method using europium complexes as fluorescence probes. Talanta 78, 977–982 [DOI] [PubMed] [Google Scholar]
- Hossain MS, Ye W, Hossain MA, Okuma E, Uraji M, Nakamura Y, Mori IC, Murata Y. 2013. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana . Bioscience Biotechnology Biochemistry 77, 977–983 [DOI] [PubMed] [Google Scholar]
- Hua JM, Wang XL, Zhai FQ, Yan F, Feng K. 2008. Effects of NaCl and Ca2+ on membrane potential of epidermal cells of maize roots. Agricultural Science in China 7, 291–296 [Google Scholar]
- Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM. 2002. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. The Plant Journal 29, 649–659 [DOI] [PubMed] [Google Scholar]
- Jacoby RP, Taylor NL, Millar AH. 2011. The role of mitochondrial respiration in salinity tolerance. Trends in Plant Science 16, 614–623 [DOI] [PubMed] [Google Scholar]
- Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X. 2013. The Salt Overly Sensitive (SOS) pathway: established and emerging roles. Molecular Plant 6, 275–286 [DOI] [PubMed] [Google Scholar]
- Kadono T, Tran D, Errakhi R, Hiramatsu T, Meimoun P, Briand J, Iwaya-Inoue M, Kawano T, Bouteau F. 2010. Increased anion channel activity is an unavoidable event in ozone-induced programmed cell death. PLoS One 5, e13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadono T, Yamaguchi Y, Furuichi T, Hirono M, Garrec JP, Kawano T. 2006. Ozone-induced cell death mediated with oxidative and calcium signaling pathways in tobacco Bel-W3 and Bel-B cell suspension cultures. Plant Signaling and Behavior 1, 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanofsky JR. 2000. Assay for singlet-oxygen generation by peroxidases using 1270-nm chemiluminescence. Methods in Enzymology 319, 59–67 [DOI] [PubMed] [Google Scholar]
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. 1998. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant and Cell Physiology 39, 721–730 [Google Scholar]
- Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schultke S, Lee SC, Kudla J, Luan S. 2007. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis . The Plant Journal 52, 473–484 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. 1996. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. The Plant Cell 8, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. 1997. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. The Plant Journal 12, 1067–1078 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. 2004. Singlet oxygen production in photosynthesis. Journal of Experimental Botany 56, 337–346 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Richards SL, Shabala L, et al. 2013. Salinity-induced calcium signaling and root adaptation in Arabidopsis require the calcium regulatory protein annexin1. Plant Physiology 163, 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence-signalling pathways. New Phytologist 171, 249–269 [DOI] [PubMed] [Google Scholar]
- Li ZS, Delrot S. 1987. Osmotic dependence of the transmembrane potential difference of broadbean mesocarp cells. Plant Physiology 84, 895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wang Y, Wang G. 2006. Salt stress-induced programmed cell death in tobacco protoplasts is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Journal of Plant Physiology 163, 731–739 [DOI] [PubMed] [Google Scholar]
- Meimoun P, Vidal G, Bohrer AS, Lehner A, Tran D, Briand J, Bouteau F, Rona JP. 2009. Intracellular Ca2+ stores could participate to abscisic acid-induced depolarization and stomatal closure in Arabidopsis thaliana . Plant Signaling and Behavior 4, 830–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Goshima F, Yamauchi Y, Koshizuka T, Takakuwa H, Nishiyama Y. 2002. Herpes simplex virus type 2 US3 blocks apoptosis induced by sorbitol treatment. Microbes and Infection 4, 707–712 [DOI] [PubMed] [Google Scholar]
- Nakano M, Sugioka K, Ushijima Y, Goto T. 1986. Chemiluminescence probe with Cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, for estimating the ability of human-granulocytes to generate O2– . Analytical Biochemistry 159, 363–369 [DOI] [PubMed] [Google Scholar]
- Niswander JM, Dokas LA. 2007. Hyperosmotic stress-induced caspase-3 activation is mediated by p38 MAPK in the hippocampus. Brain Research 1186, 1–11 [DOI] [PubMed] [Google Scholar]
- Pandolfi C, Potossin I, Cuin T, Mancuso S, Shabala S. 2010. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant and Cell Physiology 51, 422–434 [DOI] [PubMed] [Google Scholar]
- Pauly N, Knight MR, Thuleau P, Graziana A, Muto S, Ranjeva R, Mazars C. 2001. The nucleus together with the cytosol generates patterns of specific cellular calcium signatures in tobacco suspension culture cells. Cell Calcium 30, 413–421 [DOI] [PubMed] [Google Scholar]
- Parre E, Ghars MA, Leprince AS, Thiery L, Lefebvre D, Bordenave M, Richard L, Mazars C, Abdelly C, Savoure A. 2007. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis . Plant Physiology 144, 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarun AM, Maillot C. 1988. Cl– flux responding to a turgor drop in cells of Acer pseudoplatanus . Plant Physiology and Biochemistry 26, 117–124 [Google Scholar]
- Ranf S, Wunnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P. 2008. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. The Plant Journal 53, 287–299 [DOI] [PubMed] [Google Scholar]
- Reboutier D, Frankart C, Vedel R, Brault M, Duggleby RG, Rona JP, Barny MA, Bouteau F. 2005. A CFTR chloride channel activator prevents HrpN(ea)-induced cell death in Arabidopsis thaliana suspension cells. Plant Physiology and Biochemistry 43, 567–572 [DOI] [PubMed] [Google Scholar]
- Shabala S. 2009. Salinity and programmed cell death: unravelling mechanisms for ion specific signalling. Journal of Experimental Botany 60, 709–712 [DOI] [PubMed] [Google Scholar]
- Shabala S, Babourina O, Newman I. 2000. Ion-specific mechanisms of osmoregulation in bean mesophyll cells. Journal of Experimental Botany 51, 1243–1253 [PubMed] [Google Scholar]
- Shabala S, Cuin TA. 2008. Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669 [DOI] [PubMed] [Google Scholar]
- Shabala SN, Lew RR. 2002. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiology 129, 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief TW. 2003. The physiology and pharmacology of singlet oxygen. Medical Hypotheses 60, 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M, Valenzeno DP. 2003. Singlet oxygen: the relevance of extracellular production mechanisms to oxidative stress in vivo . Photochemical and Photobiological Sciences 2, 355–361 [DOI] [PubMed] [Google Scholar]
- Teodoro AE, Zingarelli L, Lado P. 1998. Early changes of Cl– efflux and H+ extrusion induced by osmotic stress in Arabidopsis thaliana cells. Physiologia Plantarum 102, 29–37 [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R. 2003. Na+ tolerance and Na+ transport in higher plants. Annals of Botany 91, 503–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, El-Maarouf-Bouteau H, Rossi M, Biligui B, Briand J, Kawano T, Mancuso S, Bouteau F. 2013. Post-transcriptional regulation of GORK channels by superoxide anion contributes to increases in outward-rectifying K+ currents. New Phytologist 198, 1039–1048 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. 2008. Singlet oxygen is the major reactive oxygen species involved in photo-oxidative damage to plants. Plant Physiology 148, 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiatsiani L, Van Breusegem F, Gallois P, Zavialov A, Lam E, Bozhkov PV. 2011. Metacaspases. Cell Death and Differentiation 18, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG. 2011. Classes of programmed cell death in plants, compared to those in animals. Journal of Experimental Botany 62, 4749–4761 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, et al. 2011. Morphological classification of plant cell deaths. Cell Death and Differentiation 18, 1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianello A, Zancani M, Peresson C, Petrussa E, Casolo V, Krajňáková J, Patui S, Braidot E, Macrì F. 2007. Plant mitochondrial pathway leading to programmed cell death. Physiologia Plantarum 129, 242–252 [Google Scholar]
- Wang J, Li X, Liu Y, Zhao X. 2010. Salt stress induces programmed cell death in Thellungiella halophila suspension-cultured cells. Journal of Plant Physiology 167, 1145–1151 [DOI] [PubMed] [Google Scholar]
- Wegner LH, Stefano G, Shabala L, Rossi M, Mancuso S, Shabala S. 2011. Sequential depolarization of root cortical and stelar cells induced by an acute salt shock—implications for Na(+) and K(+) transport into xylem vessels. Plant, Cell and Environment 34, 859–869 [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK. 2002. Cell signaling during cold, drought, and salt stress. The Plant Cell 14, S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Liu K, Zheng Y, Wang Y, Wang J, Liao H. 2013. Disruption of AtWNK8 enhances tolerance of Arabidopsis to salt and osmotic stresses via modulating proline content and activities of catalase and peroxidase. International Journal of Molecular Sciences 14, 7032–7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli L, Marre MT, Massardi F, Lado P. 1999. Effects of hyper-osmotic stress on K+ fluxes, H+ extrusion, transmembrane electric potential difference and comparison with the effects of fusicoccin. Physiologia Plantarum 106, 287–295 [Google Scholar]
- Zhu JK. 2001. Plant salt tolerance. Trends in Plant Science 6, 66–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.