Abstract
We conducted a systematic review and meta-analysis to characterize the relationship between smoking and miscarriage. We searched the PubMed database (1956–August 31, 2011) using keywords and conducted manual reference searches of included articles and reports of the US Surgeon General. The full text of 1,706 articles was reviewed, and 98 articles that examined the association between active or passive smoking and miscarriage were included in the meta-analysis. Data were abstracted by 2 reviewers. Any active smoking was associated with increased risk of miscarriage (summary relative risk ratio = 1.23, 95% confidence interval (CI): 1.16, 1.30; n = 50 studies), and this risk was greater when the smoking exposure was specifically defined as during the pregnancy in which miscarriage risk was measured (summary relative risk ratio = 1.32, 95% CI: 1.21, 1.44; n = 25 studies). The risk of miscarriage increased with the amount smoked (1% increase in relative risk per cigarette smoked per day). Secondhand smoke exposure during pregnancy increased the risk of miscarriage by 11% (95% CI: 0.95, 1.31; n = 17 studies). Biases in study publication, design, and analysis did not significantly affect the results. This finding strengthens the evidence that women should not smoke while pregnant, and all women of reproductive age should be warned that smoking increases the risk of miscarriage.
Keywords: abortion, miscarriage, pregnancy, smoking, tobacco
Smoking during pregnancy causes low birthweight, placental abruption, and sudden infant death syndrome (1, 2). Nonetheless, 14% of pregnant women and 23% of women of reproductive age report being smokers, with secondhand smoke exposure even more prevalent, at 37% of pregnant women (3–5). Miscarriage, or loss of the fetus before it is viable, is the most common complication of pregnancy, occurring in 12%–26% of recognized pregnancies. Although many studies have addressed the association between miscarriage and smoking, the evidence has been considered inconclusive. The US Surgeon General's most recent conclusion, from the 2004 report (2), classified the evidence as suggestive but not sufficient to infer causation, and the most recent edition of the authoritative textbook, Williams Obstetrics, also describes the lack of consistency (6). Most miscarriages end early in pregnancy, during an interval over which a woman might not yet have learned of being pregnant or begun prenatal care. The benefits of quitting smoking early in pregnancy include increased birthweight and a lower risk of preterm birth, but these effects would never manifest for a woman who quits smoking but also miscarries in the first trimester (6). Thus, a more complete understanding of the relationship between smoking and miscarriage is important for preconception counseling and public health programs for women of reproductive age. This systematic review and meta-analysis focuses on the association of smoking (active and passive) with miscarriage (7).
METHODS
We conducted a systematic review and meta-analysis using the guidelines of the Meta-Analysis of Observational Studies in Epidemiology consensus statement and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (8, 9).
Inclusion criteria
Studies eligible for inclusion in the meta-analysis were original observational or experimental studies. Eligible studies compared the risks of miscarriage between women who were exposed and those who were not exposed to tobacco smoke from cigarettes. Relevant exposures were smoking of cigarettes by the mother and secondhand smoke exposure in pregnant women. Articles in any language were eligible and were translated as necessary using Google Translate (http://translate.google.com/). We excluded duplicate publications and publications with duplicated data (e.g., studies conducted on the same registry with overlapping years). No quality measures were used to select studies for inclusion.
Literature search strategy
Two reviewers independently searched the PubMed database for articles published between 1956 and August 31, 2011, relevant to smoking and risk of adverse pregnancy outcomes. The search terms were (“smoking” OR “tobacco”) AND “pregnancy.” From the chosen articles, those relevant to miscarriage or perinatal death were selected for inclusion and/or review of references. We then conducted manual searches by checking references of the articles identified in the PubMed searches. The articles referenced by all relevant articles (original articles, reviews, and letters) were searched by at least 1 reviewer, and the articles referenced by included articles and all Surgeon General reports regarding tobacco and health were searched by 2 reviewers. Disagreements on final inclusion status were resolved by discussion.
Data abstraction
Study data were collected and managed using REDCap electronic data capture tools (10). Two reviewers independently extracted data from all articles on study type, country, calendar years of the pregnancies in the studies, population characteristics, participant inclusion and exclusion criteria, recruitment methods, participation and follow-up rates, exposure and outcome definitions, features of data collection, numbers of participants, effect sizes, and statistical significance tests. Differences in item coding were resolved through discussion between the reviewers, and the κ statistic was computed to assess agreement between reviewers. The median Strout-Fleiss reliability statistic for continuous variables was 0.99, and the median κ statistic for covariates analyzed was 0.52.
Definition of outcomes
Miscarriage was defined in different ways, including by gestational age ranges, karyotype, and fetal weight. Common upper thresholds for gestational age were between 12 and 28 weeks. Many authors did not provide a definition of miscarriage, and for these studies, any outcome described as “miscarriage,” “spontaneous abortion,” or “pregnancy loss” without other description was used and combined with other miscarriage outcomes. Reproductive lifetime history of miscarriage (ever had at least 1 miscarriage over the lifetime) was analyzed separately from miscarriage in an individual pregnancy.
Grouping of exposures
Any study that used as its exposure indicator “smoking,” “smoker,” “secondhand smoke,” “environmental tobacco smoke,” “lives with a smoker,” or “partner/husband is a smoker” was considered eligible. Studies were included that described the amount of smoking exposure. Exposures were categorized by type (active/passive), timing in relation to pregnancy, amount of exposure, and the source of exposure if given. Reference exposures included “0 cigarettes per day,” “nonsmoker,” “never smoker,” “no secondhand smoke exposure,” and others indicating no exposure to tobacco smoke. Studies that collected smoking exposure data before or during pregnancy were categorized as prospective, and case-control studies or others that collected smoking exposure data after pregnancy were categorized as retrospective. The window of smoking exposure was categorized as follows: 1) before pregnancy (including those who continued and those who quit during pregnancy), 2) during pregnancy, 3) lifetime exposure or after all studied pregnancies, 4) ex-smoker at the time of pregnancy, 5) quit at conception or in early pregnancy, and 6) not specified. “Any active smoking” refers to definitions of smoking as at least 1 cigarette per day and to the undefined terms of “smoker” and “smoking.”
Analysis
The random-effects model of DerSimonian and Laird (11) was chosen to account for heterogeneity of study populations and designs. The estimate of relative risk ratio used was the odds ratio, risk ratio, or hazard ratio, as given in the original article. For studies without an estimated relative risk ratio, risk ratios or odds ratios were calculated from available data as appropriate. A continuity correction (12) of 0.5 was applied to studies (13–15) with counts of 0 in 1 or more cells of the 2 × 2 table.
Relative risk ratios for “any active smoking” were combined, as were those for categories of 1–10, 11–20, and 21 or more cigarettes smoked per day (16). Because too few studies that examined secondhand smoke or history of miscarriage gave results by the amount of exposure, no dose-response analyses were conducted for this exposure and outcome, respectively. Instead, all studies that included relative risk ratios for various categories of exposure were included in “any exposure” analyses after combining the multiple relative risk ratios into a single estimate for each study (17). Analyses were conducted using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
Dose-response analysis
All relative risk ratios given for the risk of stillbirth, neonatal death, or perinatal death based on the number of cigarettes smoked per day, regardless of categorization scheme, were analyzed in a dose-response meta-regression separately for each outcome. We used a SAS macro for meta-analysis of linear and nonlinear dose-response relationships that combines studies of the same relationship that have different exposure levels (18, 19). For closed-ended categories, the midpoints were taken as the dose. For open-ended categories (those specified with minimum but not maximum numbers of cigarettes per day, such as “20 or more”), we imputed a maximum number of cigarettes per day based on the category minimum, then took the midpoint of the minimum and the imputed maximum as the dose. The imputed maximum was based on the approximate mean number and categorical distribution of cigarettes smoked per day among women in the National Health Interview Survey (20). For categories with minima of 40 or more cigarettes per day, the maximum was 45; for categories with minima of 30–39 cigarettes per day, the maximum was 40; for categories with minima of 25–29 cigarettes per day, the maximum was 35; for categories with minima of 20–24 cigarettes per day, the maximum was 30; for categories with minima of 15–19 cigarettes per day, the maximum was 25; and for categories with minima of 2–14 cigarettes per day, the maximum was 20.
Heterogeneity and study quality
Heterogeneity between studies was assessed using the I2 statistic, which represents the percent of total variation that is true between-studies heterogeneity (21). Statistical significance of the heterogeneity was analyzed with the Q statistic. When heterogeneity was statistically significant and high, reasons for the heterogeneity were examined using random-effects meta-regression for both continuous and dichotomous variables (22, 23). Subgroups were contrasted on the basis of exposure timing in relation to pregnancy (specified as during pregnancy vs. not specified or other). Many studies likely collected data on smoking and miscarriage but did not include this information in the title, abstract, or indexed keywords and, consequently, studies that focused specifically on smoking are likely overrepresented in the included articles compared with the universe of eligible articles on the outcome. To estimate the potential impact of including only studies focused on smoking, we compared the results from subgroups on the basis of keywords in the article titles. We searched for the terms, “smok,” “tobac,” and “cigar” in the titles of the articles and considered these to be articles that focused on smoking. Articles without any of these terms in the titles we considered not to be focused on smoking. Because miscarriage is relatively common (occurring in 10%–20% of pregnancies), odds ratios were converted to risk ratios, and the results from using these data were compared with those obtained with odds ratios, risk ratios, and hazard ratios combined (24). If unexplained heterogeneity remained, post hoc meta-regression and subgroup analyses were conducted on other variables collected. Study quality assessment was undertaken using indicators to address information bias, confounding, selection bias, and violation of statistical assumptions. Adjusted estimates were compared with crude estimates, and estimates based on the best analytical model (best and most appropriate control of confounding) were compared with other estimates. Potential confounders considered important were maternal age, education, and socioeconomic status. Adjusting for prior pregnancy loss is inappropriate for estimation of the causal effect of smoking on miscarriage, so studies that adjusted for this were excluded from the “best analytical model” analysis (25).
Publication bias
Publication bias was analyzed for each comparison by visual analysis of funnel plots, Egger regression, Begg rank correlation, funnel plot regression, and trim-and-fill tests using the PUB_BIAS macro (26). Sensitivity analyses were conducted by comparing results obtained after excluding studies with very high relative risk ratios.
Imputation
One study required the use of imputed data for variance estimation because the numbers provided were miscarriage rates by categories of number of cigarettes per day, and only the total number of smokers was given (27). The distribution of cigarettes smoked per day was assumed to be similar to that reported by other studies; 50% of the smoking subjects were assigned to the category of 11–20 cigarettes per day, and 25% each were assigned to the categories of 1–10 and 21 or more cigarettes per day. Imputation of the covariates of national smoking prevalence and national cigarettes per capita was also performed for each study on the basis of year and country. Both the year of publication and the midpoint year of study pregnancies were used for the year value. Smoking prevalence data were primarily obtained from the World Health Organization Global Infobase (complete reference list available upon request) (28). Cigarettes per capita data were primarily obtained from 1 report (29), with values for the United States from the Centers for Disease Control and Prevention (30) and a few other data points from another source (31).
RESULTS
Studies included in the systematic review and meta-analysis
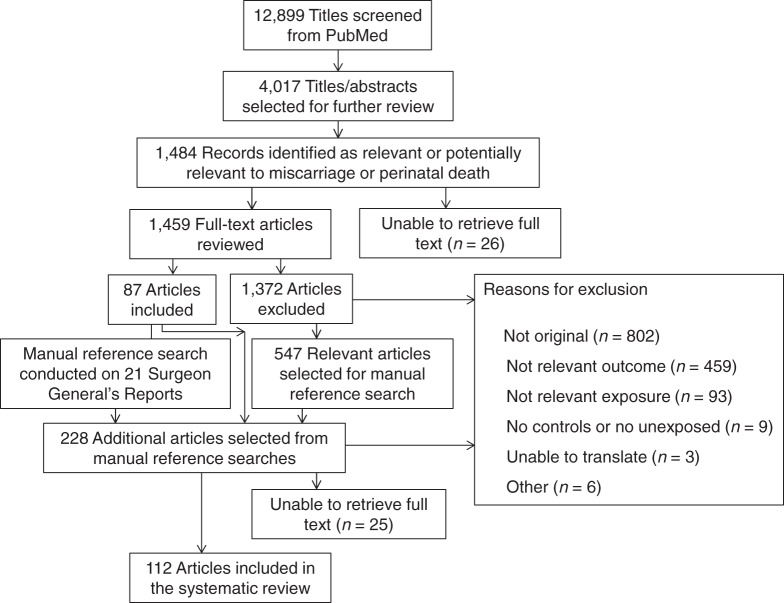
From the initial 12,899 articles identified by keywords for tobacco and pregnancy, 112 articles were selected for inclusion in the systematic review, and 98 were selected for the meta-analyses (Figure 1, Table 1). Articles were excluded if they did not study miscarriage or miscarriage alone (n = 459), they did not analyze smoking in relation to miscarriage (n = 93), they lacked a control group or unexposed group (n = 9), translation was not possible (n = 3), or for other reasons (n = 6). Twenty-eight studies were placed into 8 sets of overlapping data, with between 2 and 9 studies per set. These sets were identified on the basis of the studies being conducted in the same country, during the same years, and using the same registry or population. Often, these were duplicate publications by the same authors, though a few were registry studies with overlapping years. The largest study appropriate for each analysis was chosen from each set. Of the 112 studies, 21 examined secondhand smoke exposure of the mother, and 107 examined active maternal smoking. Thirty-one percent of the studies were conducted in the United States, and the remainder were conducted in 25 other countries or sets of countries. Sixteen were published in languages other than English. Twenty-three studies used history of miscarriage as the outcome, and 93 used miscarriage in an individual pregnancy. All results refer to miscarriage in an individual pregnancy unless otherwise specified. A list of the studies that were included in each analysis is provided in Web Table 1, available at http://aje.oxfordjournals.org/. Twelve duplicate studies and 2 studies without enough data given were not used in any analyses.
Figure 1.
Selection of studies included in the systematic review and meta-analysis of smoking and risk of miscarriage.
Table 1.
Characteristics of Included Studies
| First Author, Year (Reference No.) | Study Design | Exposure | Exposed, % | Outcome | Location | Study Years | No. of Subjects |
|---|---|---|---|---|---|---|---|
| Adolfsson, 2006 (47)a | Cross-sectional | Active | 19.6 | Miscarriage | Sweden | 1983–2003 | 2,503,605 |
| Agnesi, 1997 (15)b | Case-control | Active | 9.7 | Miscarriage | Italy | 1987–1988 | 216 |
| Agnesi, 2011 (48)b | Case-control | Active | 6.7 | Miscarriage | Italy | 1987–1999 | 462 |
| Ahlborg, 1991 (49) | Prospective cohort | Active/passive | 36/15 | Miscarriage to 13 weeks | Sweden | 1980–1983 | 3,261 |
| Ancel, 2000 (50) | Case-control | Active | 12 | Miscarriage at 14–21 weeks | Multiple European | 1994–1997 | 4,507 |
| Armstrong, 1992 (51) | Retrospective cohort | Active | 33 | Miscarriage to 28 weeks | Canada | 1953–1984 | 47,146 |
| Axelsson, 1984 (52) | Cross-sectional | Active | 27 | Miscarriage | Sweden | 1950–1982 | 1,131 |
| Baba, 2011 (53) | Case-control | Active/passive | 17/26 | Miscarriage to 12 weeks | Japan | 2001–2005 | 1,290 |
| Baird, 1985 (54) | Cross-sectional | Active | 19 | History of miscarriage | United States | 1954–1983 | 468 |
| Baste, 2008 (55) | Cross-sectional | Active | 63 | History of miscarriage | Norway | 1965–1999 | 10,512 |
| Bech, 2005 (56) | Prospective cohort | Active | 17 | Miscarriage to 28 weeks | Denmark | 1996–2002 | 88,482 |
| Bernhard, 1948 (57) | Cross-sectional | Active | 8 | History of miscarriage | Germany | 1916–1947 | 5,548 |
| Bernhard, 1964 (58) | Retrospective cohort | Active | 8 | Miscarriage to 25.5 weeks | Germany | 1943–1949 | 10,803 |
| Bhattacharya, 2010 (59)c | Retrospective cohort | Active | 34 | Miscarriage to 28 weeks | United Kingdom | 1950–2005 | 49,272 |
| Blanco-Muñoz, 2009 (60) | Nested case-control | Active/passive | 42/46 | Miscarriage to 20 weeks | Mexico | 2001–2004 | 107 |
| Blohm, 2008 (61) | Cross-sectional | Active | 25 | History of miscarriage | Sweden | 1977–2001 | 733 |
| Boyles, 2000 (62)d | Case-control/prospective cohort | Activee | 26 | Miscarriage to 22 weeks | United States | 1995–1997 | 970 |
| Campbell, 2011 (63) | Prospective cohort | Active | 70 | Miscarriage to 20 weeks | Australia | 1999–2008 | 279 |
| Cavedon, 1987 (64) | Retrospective cohort | Active | 5 | Miscarriage to 28 weeks | Italy | 1930–1982 | 3,332 |
| Chatenoud, 1998 (65) | Case-control | Active/passive | 16/45 | Miscarriage 4–12 weeks | Italy | 1990–1995 | 2,325 |
| Cnattingius, 2000 (66)a | Case-control | Activee | 16 | Miscarriage 6–12 weeks | Sweden | 1996–1998 | 1,515 |
| Cone, 1998 (67) | Retrospective cohort | Active | 13 | Miscarriage 6–28 weeks | United States | 1990–1991 | 418 |
| Cope, 1973 (68) | Cross-sectional | Active | 25 | History of miscarriage to 20 weeks | Australia | 1941–1971 | 4,992 |
| Coste, 1991 (69) | Case-control | Active | 28 | Miscarriage | France | 1988–1988 | 558 |
| Danish Health Board, 1995 (70) | Cross-sectional | Active | 33 | History of miscarriage | Denmark | 1963–1992 | 68,065 |
| de Weerd, 2003 (71) | Prospective cohort | Active | 25 | Miscarriage to 16 weeks | Netherlands | 1987–1990 | 240 |
| Domínguez-Rojas, 1994 (72) | Retrospective cohort | Active | 38 | Miscarriage to 20 weeks | Spain | 1963–1991 | 691 |
| Donovan, 1977 (73) | Prospective cohort | Active | 65 | Miscarriage to 28 weeks | United Kingdom | 1972–1973 | 1,274 |
| Downing, 1966 (74) | Prospective cohort | Active | 47 | Miscarriage | United States | 1952–1958 | 5,659 |
| Ericson, 1986 (75) | Nested case-control | Active/passive | 17/55 | Miscarriage | Sweden | 1980–1981 | 1,142 |
| Eskenazi, 1995 (14) | Prospective cohort | Active | 15 | Miscarriage | United States | 1965–1994 | 52 |
| Fabiani, 1988 (76) | Case-control | Active | 30 | Miscarriage | Italy | 1983–1984 | 227 |
| Fergusson, 1979 (27) | Cross-sectional | Active | 40 | History of miscarriage | New Zealand | 1948–1977 | 1,248 |
| Fuentes, 2010 (77) | Prospective cohort | Passive | 18 | Miscarriage 2–8.5 weeks | Chile | 2004–2005 | 57 |
| Gallicchio, 2009 (78) | Cross-sectional | Active | 19 | Miscarriage to 20 weeks | United States | 1965–2008 | 1,882 |
| Farioli, 2010 (79)a | Case-control | Active/passive | 15/21 | Miscarriage 6–12 weeks | Sweden | 1996–1998 | 1,327 |
| George, 2006 (43)a | Case-control | Active/passivee | 15/21 | Miscarriage 6–12 weeks | Sweden | 1996–1998 | 1,327 |
| Guerra-Shinohara, 2010 (80) | Prospective cohort | Active | 13 | Miscarriage to 20 weeks | Brazil | 2004–2005 | 100 |
| Habek, 2011 (81) | Retrospective cohort | Active | 64 | Miscarriage | Croatia | 1994–1994 | 53 |
| Hafez, 2001 (33) | Cross-sectional | Passive | 52 | History of miscarriage | Egypt | 1963–2000 | 1,934 |
| Hall, 1992 (82)c | Prospective cohort | Active | 28 | Miscarriage | United Kingdom | 1980–1989 | 1,261 |
| Halmesmaki, 1989 (83) | Case-control | Active/passive | 26/36 | Miscarriage 6–23 weeks | Finland | 1988–1988 | 161 |
| Hansteen, 1990 (42) | Case-control | Active | 39 | Miscarriage to 26 weeks | Norway | 1985–1987 | 610 |
| Hardy, 1972 (84) | Prospective cohort | Active | 44 | Miscarriage | United States | 1962–1963 | 1,329 |
| Harlap, 1980 (85) | Prospective cohort | Active | 25 | Miscarriage 5–27 weeks | United States | 1974–1977 | 32,019 |
| Harrison, 1990 (86) | Prospective cohort | Active | 1 | Miscarriage | Australia | 1988–1988 | 650 |
| Hemminki, 1983 (87) | Cross-sectional | Active | 15 | Miscarriage | Finland | 1931–1981 | 2,714 |
| Himmelberger, 1978 (88) | Retrospective cohort | Active | Not stated | Miscarriage to 20 weeks | United States | 1963–1972 | 12,914 |
| Hrubá, 1997 (89) | Cross-sectional | Active/passive | 45 | Miscarriage/history of miscarriage | Czech Republic | 1911–1993 | 7,397 |
| Hudson, 1945 (90) | Retrospective cohort | Active | 38 | Miscarriage | United States | 1943–1945 | 645 |
| Hughes, 1994 (13) | Cross-sectional | Active | 48 | Miscarriage | Canada | 1990–1992 | 48 |
| Kharazmi, 2010 (91) | Cross-sectional | Active | 21 | History of miscarriage | Finland | 1916–2001 | 3,636 |
| Khoury, 2004 (92) | Prospective cohort | Active | 30 | Miscarriage to 20 weeks/ history of miscarriage | United States | 1978–1993 | 191 |
| Kizer, 1967 (93) | Cross-sectional | Active | 39 | Miscarriage | Venezuela | 1966–1967 | 6,566 |
| Kline, 1977 (94)f | Case-control | Active | 36 | Miscarriage | United States | 1974–1976 | 883 |
| Kline, 1980 (95)f | Case-control | Active | 34 | Miscarriage | United States | 1974–1978 | 1,293 |
| Kline, 1980 (96)f | Case-control | Active | 34 | Miscarriage | United States | 1974–1978 | 1,295 |
| Kline, 1983 (97)f | Case-control | Active | 34 | Miscarriage of trisomic fetus | United States | 1974–1979 | 1,603 |
| Kline, 1995 (41)f | Case-control | Active | 34 | Miscarriage to 28 weeks | United States | 1974–1986 | 3,911 |
| Koller, 1983 (98) | Prospective cohort | Active | 19 | Miscarriage | Germany | 1964–1971 | 6,533 |
| Kullander, 1971 (99) | Prospective cohort | Active | 44 | Miscarriage 8–30 weeks | Sweden | 1963–1964 | 6,195 |
| Kyyronen, 1989 (100) | Case-control | Active | 37 | Miscarriage | Finland | 1973–1983 | 419 |
| Lacuska, 1968 (101) | Retrospective cohort | Active | 4 | Miscarriage | Czech Republic | 1964–1967 | 3,670 |
| Lemasters, 1989 (102) | Retrospective cohort | Active | 20 | Miscarriage to 20 weeks | United States | 1963–1985 | 2,909 |
| Maconochie, 2007 (103) | Case-control | Active/passive | 24 | Miscarriage to 13 weeks | United Kingdom | 1980–2001 | 6,709 |
| Makay, 1968 (104) | Cross-sectional | Active | 9 | History of miscarriage | Hungary | 1935–1966 | 2,341 |
| Martin, 2000 (105)c | Retrospective cohort | Active | 34 | Miscarriage to 27 weeks | United Kingdom | 1969–1997 | 3,150 |
| Maximovich, 1995 (106) | Retrospective cohort | Active | 19 | Miscarriage | United States | 1992–1993 | 80 |
| Medina, 1990 (107) | Cross-sectional | Active | 55 | History of miscarriage | Chile | 1943–1988 | 100 |
| McKean, 1978 (108)f | Case-control | Active | 36 | Miscarriage | United States | 1974–1976 | 883 |
| Meeker, 2007 (109)g | Prospective cohort | Passive | 11 | Miscarriage to 20 weeks | United States | 1994–2003 | 460 |
| Meeker, 2007 (110)g | Prospective cohort | Passivee | 50 | Miscarriage to 20 weeks | United States | 1994–1998 | 339 |
| Mey, 1967 (111) | Cross-sectional | Active | 14 | History of miscarriage | Germany | 1906–1965 | 1,981 |
| Mishra, 2000 (112) | Cross-sectional | Active | 44 | History of miscarriage | Australia | 1988–1996 | 2,617 |
| Morales, 1997 (113) | Cross-sectional | Active | 26 | History of miscarriage | United Kingdom | 1950–1984 | 119 |
| Murphy, 1974 (114)h | Retrospective cohort | Active | 46 | Miscarriage | United Kingdom | 1969–1973 | 12,013 |
| Murphy, 1978 (115)h | Retrospective cohort | Active | 46 | Miscarriage | United Kingdom | 1969–1973 | 12,013 |
| Nakamura, 2004 (116) | Cross-sectional | Active/passive | 24/45 | Miscarriage/history of miscarriage | Brazil | 2001–2001 | 596 |
| Ness, 1999 (117)d | Case-control/prospective cohort | Activee | 26 | Miscarriage to 22 weeks | United States | 1995–1997 | 970 |
| Nielsen, 2006 (118)i | Nested case-control | Active | 45 | Miscarriage to 28 weeks | Denmark | 1991–1995 | 1,921 |
| O'Lane, 1963 (119) | Cross-sectional | Active | 46 | Miscarriage | United States | 1961–1962 | 1,914 |
| Padrón Garcia, 1990 (120) | Retrospective cohort | Active | 44 | Miscarriage | Cuba | 1986–1987 | 1,018 |
| Palmgren, 1971 (121)j | Prospective cohort | Active | 48 | Miscarriage 8–30 weeks | Sweden | 1964–1967 | 4,312 |
| Palmgren, 1973 (122)j | Prospective cohort | Active | 48 | Miscarriage | Sweden | 1964–1967 | 4,312 |
| Pandya, 1996 (123) | Cross-sectional | Active | 3 | Miscarriage 10–13 weeks | United Kingdom | 1992–1995 | 16,806 |
| Pattinson, 1991 (124) | Prospective cohort | Active/passive | 28/22 | Miscarriage | Canada | 1984–1989 | 69 |
| Raatikainen, 2007 (125) | Cross-sectional | Active | 27 | History of miscarriage | Finland | 1960–2001 | 25,591 |
| Rasch, 2003 (126) | Case-control | Active | 22 | Miscarriage 6–16 weeks | Denmark | 1994–1996 | 1,486 |
| Risch, 1988 (127) | Cross-sectional | Active | 20 | Miscarriage | United States and Canada | 1914–1981 | 6,282 |
| Rumeau-Rouquette, 1972 (128) | Cross-sectional | Active | 10 | History of miscarriage | France | 1934–1967 | 3,984 |
| Sandahl, 1989 (129)a | Case-control | Active | 49 | Miscarriage 5–28 weeks | Sweden | 1980–1985 | 2,747 |
| Scholl, 1986 (130) | Prospective cohort | Active | 34 | Miscarriage | United States | 1982–1984 | 775 |
| Schwartz, 1972 (131) | Prospective cohort | Active | 17 | Miscarriage | France | 1963–1969 | 6,989 |
| Selevan, 1985 (132) | Case-control | Active | 13 | Miscarriage | Finland | 1972–1980 | 445 |
| Simoes, 1985 (133) | Cross-sectional | Active | 25 | History of miscarriage | Brazil | 1978–1979 | 6,179 |
| Stein, 1981 (134)f | Case-control | Active | 30 | Miscarriage | United States | 1974–1980 | 4,088 |
| Stein, 1981 (135)f | Case-control | Active | 30 | Miscarriage | United States | 1974–1980 | 4,088 |
| Triopon, 2006 (136) | Prospective cohort | Active/passive | 21/43 | Miscarriage | France | 2002–2005 | 63 |
| Underwood, 1965 (137) | Retrospective cohort | Active | 27 | Miscarriage | United States | 1931–1961 | 16,158 |
| van Ravenswaaij, 2011 (138) | Retrospective cohort | Active | 7 | Miscarriage to 16 weeks | Netherlands | 2002–2006 | 28,566 |
| Venners, 2004 (139) | Prospective cohort | Passive | 59 | Miscarriage to 6 weeks | China | 1996–1998 | 526 |
| Wallander, 1970 (140)j | Prospective cohort | Active | 50 | Miscarriage | Sweden | 1964–1967 | 4,478 |
| Warburton, 1979 (141)f | Case-control | Active | 30 | Miscarriage to 28 weeks | United States | 1974–1978 | 966 |
| Wilcox, 1990 (142) | Prospective cohort | Active/passive | 5/8 | Miscarriage 1–6 weeks | United States | 1982–1986 | 171 |
| Windham, 1992 (143) | Case-control | Active/passive | 20/28 | Miscarriage to 20 weeks | United States | 1986–1987 | 1,926 |
| Windham, 1999 (144) | Prospective cohort | Active/passive | 18/28 | Miscarriage to 20 weeks | United States | 1990–1991 | 5,142 |
| Windham, 1999 (145) | Cross-sectional | Active | 16 | History of miscarriage | United States | 1966–1990 | 362 |
| Winter, 2002 (146) | Retrospective cohort | Active | 11 | Miscarriage to 7 weeks | Australia | 1994–1999 | 1,196 |
| Wisborg, 2003 (147)i | Prospective cohort | Active | 29 | Miscarriage 12–27 weeks | Denmark | 1989–1996 | 17,497 |
| Wu, 1998 (148) | Cross-sectional | Active | 24 | History of miscarriage | United States | 1959–1988 | 12,465 |
| Yuan, 1994 (149) | Cross-sectional | Active | 19 | History of miscarriage | Japan | 1961–1992 | 261 |
| Zabriskie, 1963 (46) | Cross-sectional | Active | 48 | Miscarriage | United States | 1931–1961 | 5,619 |
a Only 1 article with this footnote was used in each analysis because all used data from the Swedish Medical Birth Register with overlapping time periods between 1980 and 2003.
b Only 1 article with this footnote was used in each analysis because these 2 case-control studies used 216 of the same pregnancies from 1987 to 1988.
c Only 1 article with this footnote was used in each analysis because all used data from the Aberdeen Maternity and Neonatal Databank in Scotland with overlapping time periods between 1950 and 2000.
d Only the article by Ness et al. (115) was used because the article by Boyles et al. (61) is a duplicate publication of the same data.
e Study used a biochemical measure to ascertain smoking or secondhand smoke exposure.
f Only 1 article with this footnote was used in each analysis because all used data from the same ongoing case-control study in Manhattan (New York, New York) with recruitment from 1974 to 1986.
g Only 1 article with this footnote was used in each analysis because both used data from 3 Boston, Massachusetts, clinics with overlapping time periods between 1994 and 2003.
h Only 1 article by Murphy et al. (112) was used because the second article by Murphy et al. (113) is a duplicate publication of the same data in a letter.
i Only 1 article with this footnote was used in each analysis because both used data from the Danish Hospital Discharge Register with overlapping time periods between 1989 and 1996.
j Only 1 article with this footnote was used in each analysis because all used data from the same subjects between 1964 and 1967.
Active maternal smoking
Results of the meta-analysis
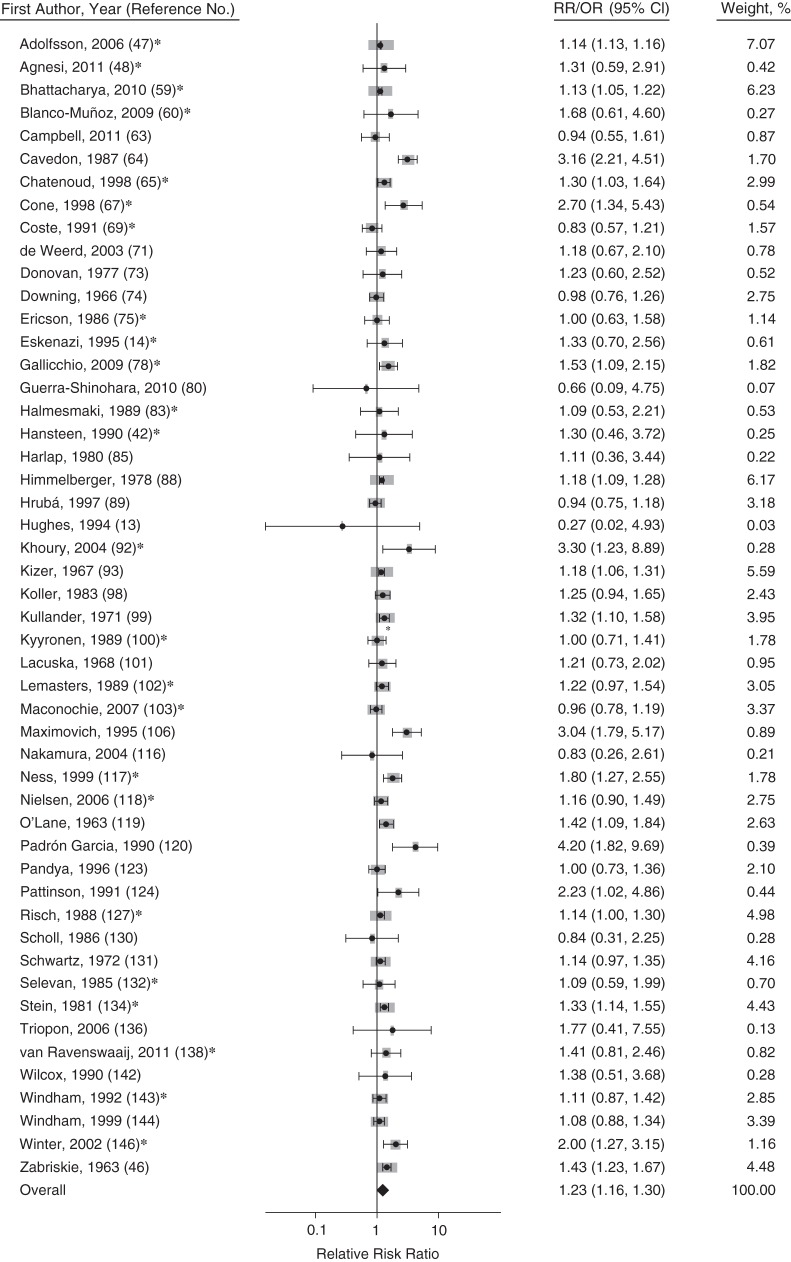
The summary relative risk for miscarriage among female smokers using only crude relative risk ratios was 1.27 (95% confidence interval (CI): 1.18, 1.37; n = 35 studies), and that using only adjusted relative risk ratios was 1.17 (95% CI: 1.04, 1.31; n = 19 studies). All further analyses used adjusted and crude relative risks together because of the similarity of these estimates, which were not significantly different (P = 0.45). The summary relative risk of miscarriage with any active smoking was 1.23 (95% CI: 1.16, 1.30; n = 50 studies, Figure 2). Former smokers were no more likely to have a miscarriage than were never smokers (summary relative risk ratio (RRR) = 0.90, 95% CI: 0.69, 1.16, n = 7 studies). Women who quit smoking at conception or early in pregnancy had a 25% lower risk of miscarriage than those who did not smoke around the time of pregnancy at all, but the difference was not statistically significant (summary RRR = 0.75, 95% CI: 0.55, 1.02; n = 4 studies).
Figure 2.
Forest plot for the association of any active maternal smoking and risk of miscarriage (150). Gray boxes represent the weight of the study in the meta-analysis. Studies with asterisks have odds ratios (ORs) presented; studies without asterisks have relative risks (RRs) presented. Bars, 95% confidence intervals (CIs).
History of miscarriage
Any active smoking was associated with an increased risk of having a history of miscarriage (at least 1 miscarriage in the woman's lifetime; summary RRR = 1.47, 95% CI: 1.26, 1.70; n = 22 studies). Former smokers had nonsignificantly increased risk of having a history of miscarriage (summary RRR = 1.33, 95% CI: 0.96, 1.85; n = 2 studies).
Assessment of heterogeneity
Heterogeneity of the summary relative risk of miscarriage with any active smoking was statistically significant and moderate (I2 = 60%, P < 0.0001; see Web Table 2 for detailed results). None of the following factors significantly affected the summary relative risk: year of publication, midpoint year of the study pregnancies, the exposure prevalence in the study population, or per-capita cigarette consumption (P values > 0.25). Studies that defined miscarriage as pregnancy loss with a maximum gestational age of 20 weeks or greater had a higher summary relative risk of miscarriage than those that defined it with a maximum gestational age of less than 20 weeks (1.49 vs. 1.24, respectively, P < 0.05). Cohort studies had higher summary relative risks than studies with other designs (e.g., case-control or cross-sectional studies; 1.39 vs. 1.17, respectively, P < 0.05). Studies that defined smoking as during the pregnancy in which miscarriage risk was measured gave a summary relative risk that was higher than those with exposures without a specified time or specified as smoking before pregnancy, lifetime smoker, smoking during subsequent pregnancy, or at another time (summary RRRs = 1.32 vs. 1.21, P for difference < 0.01). Studies conducted in the United States had a nonsignificantly higher summary relative risk than studies conducted in other countries (P for difference = 0.08). The summary relative risk ratio after converting odds ratios from 24 studies to risk ratios was 1.22 (95% CI: 1.15, 1.29; n = 50 studies).
Dose-response analysis
The relative risk increased with increasing numbers of cigarettes smoked per day. For 1–10, 11–19, and 20 or more cigarettes per day, the summary relative risks were 1.08 (95% CI: 0.96, 1.21; n = 16 studies), 1.25 (95% CI: 1.17, 1.34; n = 9 studies), and 1.42 (95% CI: 1.19, 1.70; n = 11 studies), respectively. Figure 3 shows the dose-response relationship with an estimated increment per cigarette of relative risk by 1.01 (95% CI: 1.01, 1.02; n = 31 studies). The relationship did not depart significantly from linearity based on the comparison of the log-likelihood for a model with cubic spline variables to that for a model with only a linear term (P = 0.59) (32).
Figure 3.
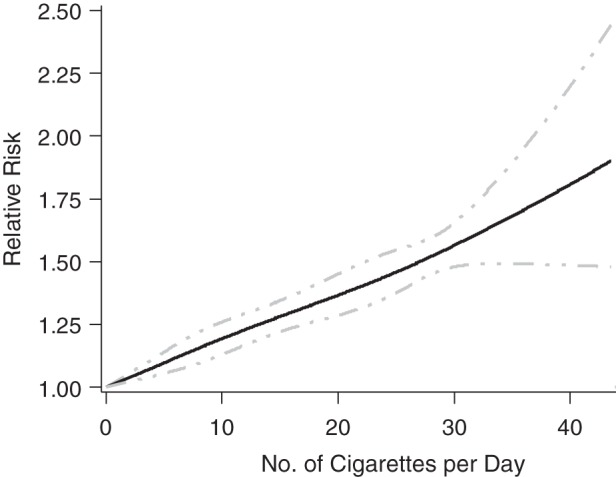
Relative risk of miscarriage versus number of cigarettes smoked per day.
Study quality
Studies that specified a gestational age range in their definitions of miscarriage had higher relative risk ratios than studies that did not (summary RRRs = 1.33 vs. 1.15, P for difference = 0.02, Web Table 2). Only 2 studies adjusted at least for maternal age in addition to education or socioeconomic status while not adjusting for prior pregnancy loss. Consequently, studies that adjusted at least for maternal age and did not adjust for prior pregnancy loss were considered to have used the best analytical model. Factors that did not affect the summary relative risk ratios were prospective or biochemical measurement of smoking exposure, adjustment for confounders, having the best analytical model, analysis of only 1 pregnancy per woman, and high (>80%) participation and follow-up rates (all P values > 0.35, Web Table 2).
Assessment of publication bias
Formal statistical tests for publication bias (Egger ordinary least squares regression, the Begg rank correlation, funnel plot weighted least squares regression, and the trim and fill tests) did not demonstrate bias among studies that reported “any active smoking” analyses (all P values > 0.35). The funnel plot was fairly symmetrical, suggesting no major impact of publication bias (Figure 4).
Figure 4.
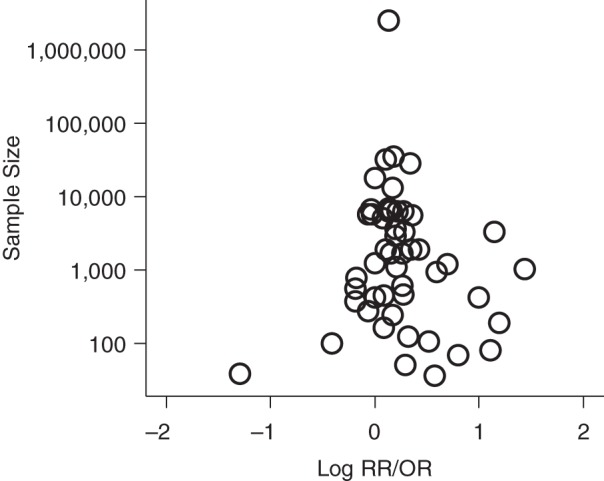
Funnel plot for studies that analyzed any active smoking and the risk of miscarriage. OR, odds ratio; RR, relative risk.
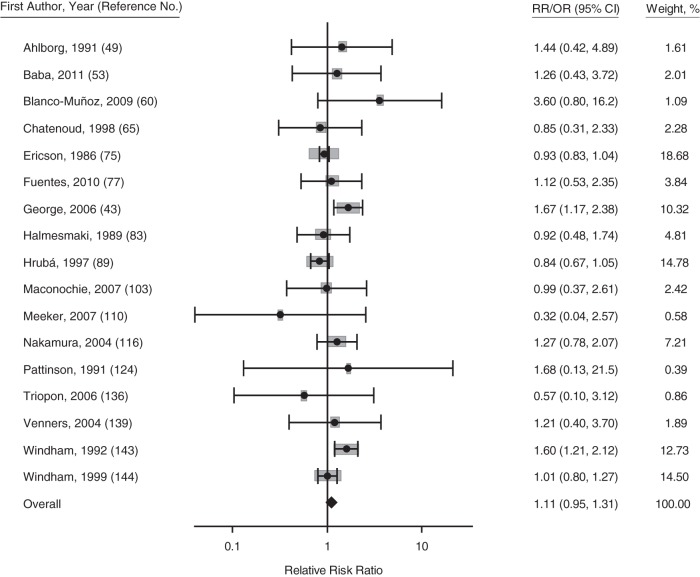
Maternal secondhand smoke exposure
Secondhand smoke exposure was not significantly associated with miscarriage, (Figure 5) (summary RRR = 1.11, 95% CI: 0.95, 1.31; I2 = 45%; n = 17 studies). Using studies that included nonsmokers only or those that adjusted for active smoking did not change the result (summary RRR = 1.13, 95% CI: 0.91, 1.42; n = 13 studies). The only study that examined history of miscarriage found that secondhand smoke exposure was associated with increased risk of having a history of miscarriage by 21% (P < 0.05) (33). None of the following publication bias tests was statistically significant: the Egger test, Begg rank correlation, funnel plot weighted least squares regression, and the trim and fill test (all P values > 0.5).
Figure 5.
Forest plot for the association between maternal secondhand smoke exposure and risk of miscarriage (150). Gray boxes represent the weight of the study in the meta-analysis. Studies with asterisks have odds ratios (ORs) presented; studies without asterisks have relative risks (RRs) presented. Bars, 95% confidence intervals (CIs).
DISCUSSION
Most literature reviews published since the 1970s have emphasized inconsistency of the evidence on smoking as a cause of miscarriage (see representative articles (34, 35)), although several have concluded that smoking causes miscarriage (see representative articles (36–38)). Two meta-analyses published in 1984 (39) and 1995 (40) included only 6 and 13 studies, respectively, but found pooled relative risks very similar to the results in the present report (1.24 and 1.32, respectively). This systematic review and meta-analysis confirmed an association of maternal smoking during pregnancy with risk for miscarriage and also showed that risk increases with the amount smoked. The results are consistent with either no effect or a modest effect of secondhand smoke exposure on risk of miscarriage (95% CI: 0.95, 1.31).
Like other meta-analyses of observational data, our review has several limitations reflecting the reliance on secondary data gathered from publications. First, we were unable to fully investigate the effect of timing of smoke exposure across pregnancy on miscarriage risk. Although we found that women who had quit smoking were not at increased risk of miscarriage compared with never smokers, whether a woman needs to quit months before pregnancy, weeks into pregnancy, or at some other time to lower risk is currently unknown. Women who quit smoking upon recognition of their pregnancies had a lower risk of miscarriage than did never smokers. However, this result was based on only 4 studies and was not statistically significant. However, if true, this finding may reflect a selection bias, because women who quit upon learning of pregnancy are likely to be particularly health conscious. Lower risks of miscarriage in quitters could also be due to reverse causation, because death of the fetus often precedes clinical miscarriage. Viable pregnancies cause nausea and food aversions and may affect the mother's desire to smoke. Thus, a mother with a viable pregnancy may be more likely to quit than a mother with a nonviable pregnancy, leading to the apparent reduced risk of miscarriage in quitters. Another problem with exposure classification in some studies was the inclusion of occasional or social smokers with nonsmokers for estimation of risks. Such misclassification would bias the total effect of smoking toward the null, leading this meta-analysis to underestimate the true relative risk.
Miscarriage is a heterogeneous clinical entity that is typically classified by the gestational age of the loss and the karyotype of the conceptus. The relative risk of miscarriage for smokers was greater when miscarriage was defined as pregnancy loss with a maximum gestational age of 20 weeks or greater. However, the definition of miscarriage is only a proxy for the gestational ages at which women actually miscarried, and more precise data on gestational age–specific risks would be useful. Three papers examined differences in the effects of smoking on miscarriages classified by karytyope of the conceptus. These data were not meta-analyzed because the largest such study had data on 10 times more cases than the next largest study. Using 2,305 cases, this study found a case-case odds ratio of 1.2 (95% CI: 0.8, 1.8) for the effect of smoking on miscarriage in normal versus chromosomally aberrant (nontrisomic) losses (41). The results of 2 smaller studies also had small and nonsignificant differences among the effects of smoking on karyotype groups (42, 43).
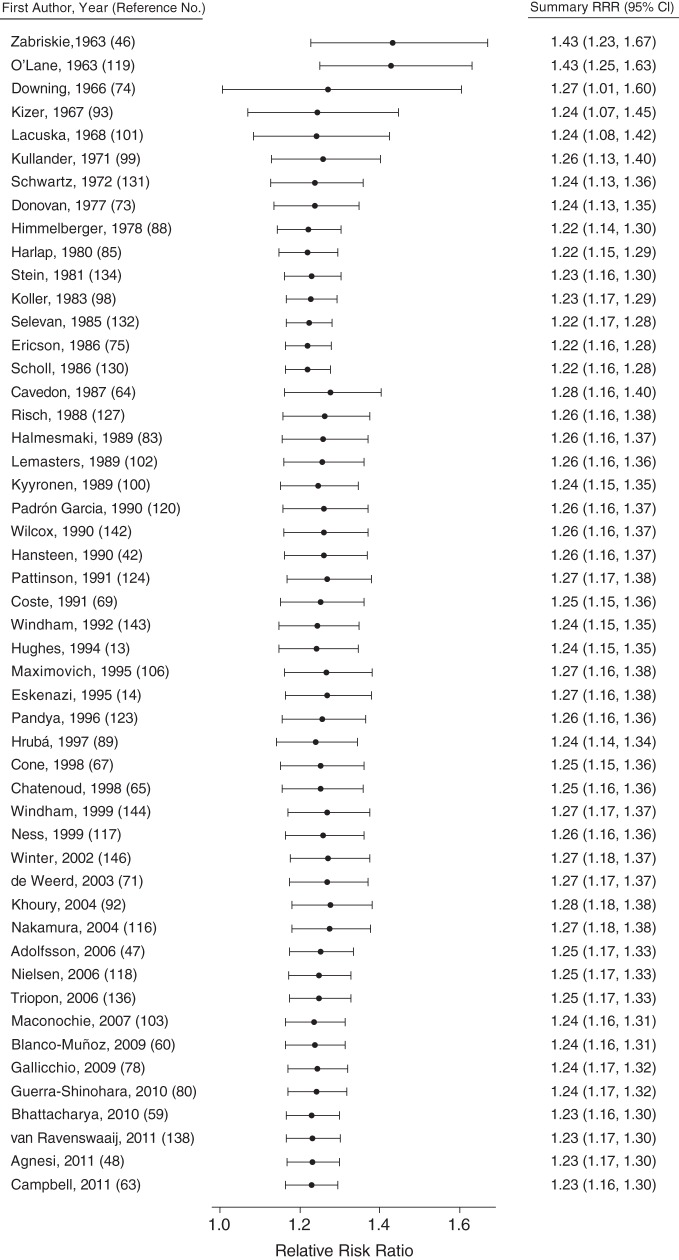
To examine why previous reviews did not generally conclude that the association between smoking and miscarriage was consistent, we conducted cumulative meta-analyses of the 50 studies that assessed the risk of miscarriage with a report of “any active smoking” (Figure 6). We iteratively combined the estimates from the 50 studies, plotting the cumulative estimate from the first 2 studies through the entire group. This analysis demonstrated that an increased risk of miscarriage has been consistently documented since the publication of the first 2 studies in 1963. The relative risk estimates in the cumulative meta-analysis were all statistically significant and ranged from 1.22 to 1.43.
Figure 6.
Forest plot for the cumulative meta-analysis of any active maternal smoking and risk of miscarriage (150). Studies are sorted chronologically in order of publication date, and each point represents the summary relative risk ratio (RRR) including all studies published before and including the study listed on the corresponding line. Bars, 95% confidence intervals (CIs).
Thus, the consistency of the pooled estimate has not changed significantly over the last 50 years. Why has consensus not yet been reached on smoking and miscarriage? Comparison of this systematic review with other reviews demonstrates that much of the relevant literature was not included in these earlier reports. For example, only 34 of the 112 articles included in this study were referenced by at least 1 of the US Surgeon General reports on the health effects of smoking published from 1964 to 2010. Many reviews of smoking and pregnancy, environmental exposures and reproduction, and causes of miscarriage have stated that the evidence is inconsistent without conducting systematic reviews. Multiple recent literature reviews that we found cited only 1 or 2 original articles on smoking and miscarriage (44, 45), and some limited their references to prior reviews (36). Despite the reviews that found the evidence to be unconvincing, we demonstrated that the association between smoking and miscarriage, first documented by Zabriskie (46) in 1963, has endured half a century of research.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Beth L. Pineles, Edward Park, Jonathan M. Samet).
The project was supported by the National Institute on Drug Abuse (grant 5F30DA032190 to B.L.P.).
We thank Tiffany Chen for her help in obtaining the articles and study data.
This paper was presented at the American Public Health Association's 140th Annual Meeting and Exposition, San Francisco, California, October 27–31, 2012.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Drug Abuse. The funding sources played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 2.US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 3.US Department of Health and Human Services. National Health and Nutrition Examination Survey Data. Vol 2010. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2010. [Google Scholar]
- 4.US Department of Health and Human Services. BRFSS Annual Survey Data: Survey Data and Documentation. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Surveillance, Epidemiology, and Laboratory Services, Public Health Surveillance Program Office; 2011. [Google Scholar]
- 5.Tong VT, Jones JR, Dietz PM, et al. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29. [PubMed] [Google Scholar]
- 6.Cunningham FG, Leveno KJ, Bloom SL, et al. Chapter 9. Abortion. In: Cunningham FG, Leveno KJ, Bloom SL, et al., editors. Williams Obstetrics. Vol 23. New York, NY: The McGraw-Hill Companies, Inc.; 2010. pp. 215–237. [Google Scholar]
- 7.US Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 13.Hughes EG, Yeo J, Claman P, et al. Cigarette smoking and the outcomes of in vitro fertilization: measurement of effect size and levels of action. Fertil Steril. 1994;62(4):807–814. doi: 10.1016/s0015-0282(16)57009-5. [DOI] [PubMed] [Google Scholar]
- 14.Eskenazi B, Gold EB, Lasley BL, et al. Prospective monitoring of early fetal loss and clinical spontaneous abortion among female semiconductor workers. Am J Ind Med. 1995;28(6):833–846. doi: 10.1002/ajim.4700280615. [DOI] [PubMed] [Google Scholar]
- 15.Agnesi R, Valentini F, Mastrangelo G. Risk of spontaneous abortion and maternal exposure to organic solvents in the shoe industry. Int Arch Occup Environ Health. 1997;69(5):311–316. doi: 10.1007/s004200050153. [DOI] [PubMed] [Google Scholar]
- 16.Hertzmark E, Spiegelman D. The SAS METAANAL Macro. http://www.hsph.harvard.edu/donna-spiegelman/software/metaanal/ Accessed May 31, 2012.
- 17.Borenstein M, Hedges LV, Higgins JPT, et al. Vol 1. Chichester, West Sussex, United Kingdom: John Wiley & Sons, Ltd.; 2009. Multiple Outcomes or Time-points Within a Study. Introduction to Meta-Analysis (Statistics in Practice) p. 225. [Google Scholar]
- 18.Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Spiegelman D. The SAS %METADOSE Macro. http://www.hsph.harvard.edu/donna-spiegelman/software/metadose/ Accessed May 31, 2012.
- 20.Burns DM, Major JM, Shanks TJ. Changes in number of cigarettes smoked per day: cross-sectional and birth cohort analyses using NHIS. In: Amarcher RH, Marcus SE, editors. Those Who Continue to Smoke: Is Achieving Abstinence From Smoking Harder and Do We Need to Change Our Interventions? Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2003. pp. 83–99. [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis (Statistics in Practice) Vol 1. Chichester, West Sussex, United Kingdom: John Wiley & Sons, Ltd.; 2009. Identifying and quantifying heterogeneity; p. 107. [Google Scholar]
- 22.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. http://mason.gmu.edu/~dwilsonb/ma.html. Accessed June 30, 2012.
- 23.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 24.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 25.Howards PP, Schisterman EF, Heagerty PJ. Potential confounding by exposure history and prior outcomes: an example from perinatal epidemiology. Epidemiology. 2007;18(5):544–551. doi: 10.1097/ede.0b013e31812001e6. [DOI] [PubMed] [Google Scholar]
- 26.Rendina-Gobioff G, Kromrey JD. PUB_BIAS: A SAS Macro for Detecting Publication Bias in Meta-analysis. http://analytics.ncsu.edu/sesug/2006/PO04_06.PDF. Accessed June 13, 2012.
- 27.Fergusson DM, Horwood LJ, Shannon FT. Smoking during pregnancy. N Z Med J. 1979;89(628):41–43. [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Global Infobase: NCD Indicators: Tobacco use prevalence. http://www.who.int/ncd_surveillance/infobase/web/InfoBaseCommon/ Accessed August 16, 2012.
- 29.Guindon GE, Boisclair D. Economics of tobacco control paper no. 6: past, current, and future trends in tobacco use. Health, Nutrition, and Population (HNP) Discussion Paper Series. http://siteresources.worldbank.org/HEALTHNUTRITIONANDPOPULATION/Resources/281627-1095698140167/Guindon-PastCurrent-whole.pdf . Accessed August 24, 2012. [Google Scholar]
- 30.Giovino GA, Schooley MW, Zhu BP, et al. Surveillance for selected tobacco-use behaviors–United States, 1900–1994. MMWR CDC Surveill Summ. 1994;43(3):1–43. [PubMed] [Google Scholar]
- 31.La Vecchia C, Harris RE, Wynder EL. Comparative epidemiology of cancer between the United States and Italy. Cancer Res. 1988;48(24 Pt 1):7285–7293. [PubMed] [Google Scholar]
- 32.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 33.Hafez AS, Fahim HI, Badawy HA. Socioenvironmental predictors of abortion and stillbirths in an industrial community in Egypt. J Egypt Public Health Assoc. 2001;76(1-2):1–16. [PubMed] [Google Scholar]
- 34.Pirani BB. Smoking during pregnancy. Obstet Gynecol Surv. 1978;33(1):1–13. doi: 10.1097/00006254-197801000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C Embryo Today. 2008;84(1):1–15. doi: 10.1002/bdrc.20119. [DOI] [PubMed] [Google Scholar]
- 36.Delpisheh A, Brabin L, Brabin BJ. Pregnancy, smoking and birth outcomes. Womens Health (Lond Engl) 2006;2(3):389–403. doi: 10.2217/17455057.2.3.389. [DOI] [PubMed] [Google Scholar]
- 37.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10(2):267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 38.Walsh RA. Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Hum Biol. 1994;66(6):1059–1092. [PubMed] [Google Scholar]
- 39.McIntosh ID. Smoking and pregnancy: I. Maternal and placental risks. Public Health Rev. 1984;12(1):1–28. [PubMed] [Google Scholar]
- 40.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40(4):385–394. [PubMed] [Google Scholar]
- 41.Kline J, Levin B, Kinney A, et al. Cigarette smoking and spontaneous abortion of known karyotype. Precise data but uncertain inferences. Am J Epidemiol. 1995;141(5):417–427. doi: 10.1093/oxfordjournals.aje.a117444. [DOI] [PubMed] [Google Scholar]
- 42.Hansteen IL. Occupational and lifestyle factors and chromosomal aberrations of spontaneous abortions. Prog Clin Biol Res. 1990;340B:467–475. [PubMed] [Google Scholar]
- 43.George L, Granath F, Johansson AL, et al. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology. 2006;17(5):500–505. doi: 10.1097/01.ede.0000229984.53726.33. [DOI] [PubMed] [Google Scholar]
- 44.Triche EW, Hossain N. Environmental factors implicated in the causation of adverse pregnancy outcome. Semin Perinatol. 2007;31(4):240–242. doi: 10.1053/j.semperi.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins S. Smoking in pregnancy. Curr Opin Obstet Gynecol. 2002;14(2):145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Zabriskie JR. Effect of cigaret smoking during pregnancy. Study of 2000 cases. Obstet Gynecol. 1963;21(4):405–411. [PubMed] [Google Scholar]
- 47.Adolfsson A, Larsson PG. Cumulative incidence of previous spontaneous abortion in Sweden in 1983–2003: a register study. Acta Obstet Gynecol Scand. 2006;85(6):741–747. doi: 10.1080/00016340600627022. [DOI] [PubMed] [Google Scholar]
- 48.Agnesi R, Valentini F, Fedeli U, et al. Maternal exposures and risk of spontaneous abortion before and after a community oriented health education campaign. Eur J Public Health. 2011;21(3):282–285. doi: 10.1093/eurpub/ckq073. [DOI] [PubMed] [Google Scholar]
- 49.Ahlborg G., Jr, Bodin L. Tobacco smoke exposure and pregnancy outcome among working women. A prospective study at prenatal care centers in Orebro County, Sweden. Am J Epidemiol. 1991;133(4):338–347. doi: 10.1093/oxfordjournals.aje.a115886. [DOI] [PubMed] [Google Scholar]
- 50.Ancel PY, Saurel-Cubizolles MJ, Di Renzo GC, et al. Risk factors for 14–21 week abortions: a case-control study in Europe. The Europop Group. Hum Reprod. 2000;15(11):2426–2432. doi: 10.1093/humrep/15.11.2426. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82(1):85–87. doi: 10.2105/ajph.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Axelsson G, Lütz C, Rylander R. Exposure to solvents and outcome of pregnancy in university laboratory employees. Br J Ind Med. 1984;41(3):305–312. doi: 10.1136/oem.41.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baba S, Noda H, Nakayama M, et al. Risk factors of early spontaneous abortions among Japanese: a matched case-control study. Hum Reprod. 2011;26(2):466–472. doi: 10.1093/humrep/deq343. [DOI] [PubMed] [Google Scholar]
- 54.Baird DD, Wilcox AJ. Cigarette smoking associated with delayed conception. JAMA. 1985;253(20):2979–2983. [PubMed] [Google Scholar]
- 55.Baste V, Moen BE, Riise T, et al. Infertility and spontaneous abortion among female hairdressers: the Hordaland Health Study. J Occup Environ Med. 2008;50(12):1371–1377. doi: 10.1097/JOM.0b013e3181858723. [DOI] [PubMed] [Google Scholar]
- 56.Bech BH, Nohr EA, Vaeth M, et al. Coffee and fetal death: a cohort study with prospective data. Am J Epidemiol. 2005;162(10):983–990. doi: 10.1093/aje/kwi317. [DOI] [PubMed] [Google Scholar]
- 57.Bernhard P. The influence of cigarette smoking on pregnant women [in German] Zentralbl Gynakol. 1948;70(1):18–31. [PubMed] [Google Scholar]
- 58.Bernhard P. Effects on the mother [in German] MMW Fortschr Med. 1964;82:95–100. [Google Scholar]
- 59.Bhattacharya S, Townend J, Bhattacharya S. Recurrent miscarriage: Are three miscarriages one too many? Analysis of a Scottish population-based database of 151,021 pregnancies. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):24–27. doi: 10.1016/j.ejogrb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Blanco-Muñoz J, Torres-Sánchez L, López-Carrillo L. Exposure to maternal and paternal tobacco consumption and risk of spontaneous abortion. Public Health Rep. 2009;124(2):317–322. doi: 10.1177/003335490912400220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blohm F, Friden B, Milsom I. A prospective longitudinal population-based study of clinical miscarriage in an urban Swedish population. BJOG. 2008;115(2):176–182. doi: 10.1111/j.1471-0528.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 62.Boyles SH, Ness RB, Grisso JA, et al. Life event stress and the association with spontaneous abortion in gravid women at an urban emergency department. Health Psychol. 2000;19(6):510–514. doi: 10.1037//0278-6133.19.6.510. [DOI] [PubMed] [Google Scholar]
- 63.Campbell S, Lynch J, Esterman A, et al. Pre-pregnancy predictors linked to miscarriage among Aboriginal and Torres Strait Islander women in North Queensland. Aust N Z J Public Health. 2011;35(4):343–351. doi: 10.1111/j.1753-6405.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- 64.Cavedon G, Figà-Talamanca I. Correlates of early fetal death among women working in industry. Am J Ind Med. 1987;11(5):497–504. doi: 10.1002/ajim.4700110503. [DOI] [PubMed] [Google Scholar]
- 65.Chatenoud L, Parazzini F, di Cintio E, et al. Paternal and maternal smoking habits before conception and during the first trimester: relation to spontaneous abortion. Ann Epidemiol. 1998;8(8):520–526. doi: 10.1016/s1047-2797(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 66.Cnattingius S, Signorello LB, Annerén G, et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med. 2000;343(25):1839–1845. doi: 10.1056/NEJM200012213432503. [DOI] [PubMed] [Google Scholar]
- 67.Cone JE, Vaughan LM, Huete A, et al. Reproductive health outcomes among female flight attendants: an exploratory study. J Occup Environ Med. 1998;40(3):210–216. doi: 10.1097/00043764-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Cope I, Lancaster P, Stevens L. Smoking in pregnancy. Med J Aust. 1973;1(14):673–677. doi: 10.5694/j.1326-5377.1973.tb110621.x. [DOI] [PubMed] [Google Scholar]
- 69.Coste J, Job-Spira N, Fernandez H. Risk factors for spontaneous abortion: a case-control study in France. Hum Reprod. 1991;6(9):1332–1337. doi: 10.1093/oxfordjournals.humrep.a137535. [DOI] [PubMed] [Google Scholar]
- 70.Danish Health Board . Pregnancy and Smoking: Documentation and Intervention. Vol 5. Kùbenhavn, Denmark: Danish Health Board; 1995. [Google Scholar]
- 71.de Weerd S, Steegers-Theunissen RP, de Boo TM, et al. Maternal periconceptional biochemical and hematological parameters, vitamin profiles and pregnancy outcome. Eur J Clin Nutr. 2003;57(9):1128–1134. doi: 10.1038/sj.ejcn.1601654. [DOI] [PubMed] [Google Scholar]
- 72.Domínguez-Rojas V, de Juanes-Pardo JR, Astasio-Arbiza P, et al. Spontaneous abortion in a hospital population: Are tobacco and coffee intake risk factors? Eur J Epidemiol. 1994;10(6):665–668. doi: 10.1007/BF01719278. [DOI] [PubMed] [Google Scholar]
- 73.Donovan JW. Randomised controlled trial of anti-smoking advice in pregnancy. Br J Prev Soc Med. 1977;31(1):6–12. doi: 10.1136/jech.31.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Downing GC, Chapman WE. Smoking and pregnancy. A statistical study of 5,659 patients. Calif Med. 1966; 104(3):187. [PMC free article] [PubMed] [Google Scholar]
- 75.Ericson A, Källén B. An epidemiological study of work with video screens and pregnancy outcome: II. A case-control study. Am J Ind Med. 1986;9(5):459–475. doi: 10.1002/ajim.4700090507. [DOI] [PubMed] [Google Scholar]
- 76.Fabiani L, Pupi M, Leoni V. Social and health variables in relation to spontaneous abortion [in Italian] Ann Ostet Ginecol Med Perinat. 1988;109(1):18–27. [PubMed] [Google Scholar]
- 77.Fuentes A, Muñoz A, Barnhart K, et al. Recent cigarette smoking and assisted reproductive technologies outcome. Fertil Steril. 2010;93(1):89–95. doi: 10.1016/j.fertnstert.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 78.Gallicchio L, Miller S, Greene T, et al. Cosmetologists and reproductive outcomes. Obstet Gynecol. 2009;113(5):1018–1026. doi: 10.1097/AOG.0b013e3181a1f906. [DOI] [PubMed] [Google Scholar]
- 79.Farioli A, Curti S, Violante FS, et al. Re: “Smoking and miscarriage risk” [letter] Epidemiology. 2010;21(6):918. doi: 10.1097/EDE.0b013e3181e57008. [DOI] [PubMed] [Google Scholar]
- 80.Guerra-Shinohara EM, Pereira PM, Kubota AM, et al. Increased MMA concentration and body mass index are associated with spontaneous abortion in Brazilian women: a pilot study. Clin Chim Acta. 2010;411(5-6):423–427. doi: 10.1016/j.cca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 81.Habek D, Kovačević M. Adverse pregnancy outcomes and long-term morbidity after early fetal hypokinesia in maternal smoking pregnancies. Arch Gynecol Obstet. 2011;283(3):491–495. doi: 10.1007/s00404-010-1395-3. [DOI] [PubMed] [Google Scholar]
- 82.Hall MH, Harper V. Smoking and pre-eclampsia. In: Poswillo D, Alberman E, editors. Effects of Smoking on the Fetus, Neonate and Child. Bath, United Kingdom: Oxford University Press; 1992. pp. 81–88. [Google Scholar]
- 83.Halmesmaki E, Valimaki M, Roine R, et al. Maternal and paternal alcohol consumption and miscarriage. Br J Obstet Gynaecol. 1989;96(2):188–191. doi: 10.1111/j.1471-0528.1989.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 84.Hardy JB, Mellits ED. Does maternal smoking during pregnancy have a long-term effect on the child? Lancet. 1972;300(7791):1332–1336. doi: 10.1016/s0140-6736(72)92777-8. [DOI] [PubMed] [Google Scholar]
- 85.Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2(8187):173–176. doi: 10.1016/s0140-6736(80)90061-6. [DOI] [PubMed] [Google Scholar]
- 86.Harrison KL, Breen TM, Hennessey JF. The effect of patient smoking habit on the outcome of IVF and GIFT treatment. Aust N Z J Obstet Gynaecol. 1990;30(4):340–342. doi: 10.1111/j.1479-828x.1990.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 87.Hemminki K, Mutanen P, Saloniemi I. Smoking and the occurrence of congenital malformations and spontaneous abortions: multivariate analysis. Am J Obstet Gynecol. 1983;145(1):61–66. doi: 10.1016/0002-9378(83)90340-x. [DOI] [PubMed] [Google Scholar]
- 88.Himmelberger DU, Brown BW, Jr, Cohen EN. Cigarette smoking during pregnancy and the occurrence of spontaneous abortion and congenital abnormality. Am J Epidemiol. 1978;108(6):470–479. doi: 10.1093/oxfordjournals.aje.a112645. [DOI] [PubMed] [Google Scholar]
- 89.Hrubá D, Kachlik P. Relation between smoking in reproductive-age women and disorders in reproduction [in Czech] Ceska Gynekol. 1997;62(4):191–196. [PubMed] [Google Scholar]
- 90.Hudson GS, Rucker MP. Spontaneous abortion. JAMA. 1945;129(8):542–544. [Google Scholar]
- 91.Kharazmi E, Fallah M, Luoto R. Miscarriage and risk of cardiovascular disease. Acta Obstet Gynecol Scand. 2010;89(2):284–288. doi: 10.3109/00016340903380758. [DOI] [PubMed] [Google Scholar]
- 92.Khoury JC, Miodovnik M, Buncher CR, et al. Consequences of smoking and caffeine consumption during pregnancy in women with type 1 diabetes. J Matern Fetal Neonatal Med. 2004;15(1):44–50. doi: 10.1080/14767050310001650716. [DOI] [PubMed] [Google Scholar]
- 93.Kizer S. Effect of the smoking habit on pregnancy, labor and the newborn [in Spanish] Rev Obstet Ginecol Venez. 1967;27(4):595–643. [PubMed] [Google Scholar]
- 94.Kline J, Stein ZA, Susser M, et al. Smoking: a risk factor for spontaneous abortion. N Engl J Med. 1977;297(15):793–796. doi: 10.1056/NEJM197710132971501. [DOI] [PubMed] [Google Scholar]
- 95.Kline J, Shrout P, Stein Z, et al. Drinking during pregnancy and spontaneous abortion. Lancet. 1980;2(8187):176–180. doi: 10.1016/s0140-6736(80)90062-8. [DOI] [PubMed] [Google Scholar]
- 96.Kline J, Stein Z, Susser M, et al. Environmental influences on early reproductive loss in a current New York City study. In: Porter IH, Hook EB, editors. Human Embryonic and Fetal Death. New York, NY: Academic Press; 1980. pp. 225–240. [Google Scholar]
- 97.Kline J, Levin B, Shrout P, et al. Maternal smoking and trisomy among spontaneously aborted conceptions. Am J Hum Genet. 1983;35(3):421–431. [PMC free article] [PubMed] [Google Scholar]
- 98.Koller S. Risk Factors During Pregnancy. Berlin, Germany: Springer; 1983. [Google Scholar]
- 99.Kullander S, Kallen B. A prospective study of smoking and pregnancy. Acta Obstet Gynecol Scand. 1971;50(1):83–94. doi: 10.3109/00016347609156779. [DOI] [PubMed] [Google Scholar]
- 100.Kyyronen P, Taskinen H, Lindbohm ML, et al. Spontaneous abortions and congenital malformations among women exposed to tetrachloroethylene in dry cleaning. J Epidemiol Community Health. 1989;43(4):346–351. doi: 10.1136/jech.43.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lacuska A, Bohunicky F, Filo S. Smoking and pregnancy. Cesk Gynekol. 1968;33(3):197–200. [PubMed] [Google Scholar]
- 102.Lemasters GK, Pinney SM. Employment status as a confounder when assessing occupational exposures and spontaneous abortion. J Clin Epidemiol. 1989;42(10):975–981. doi: 10.1016/0895-4356(89)90162-5. [DOI] [PubMed] [Google Scholar]
- 103.Maconochie N, Doyle P, Prior S, et al. Risk factors for first trimester miscarriage–results from a UK-population-based case-control study. BJOG. 2007;114(2):170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 104.Makay L, Vincze J. Smoking and pregnancy. Orv Hetil. 1968;109(34):1867–1869. [PubMed] [Google Scholar]
- 105.Martin CL, Hall MH, Campbell DM. The effect of smoking on pre-eclampsia in twin pregnancy. BJOG. 2000;107(6):745–749. doi: 10.1111/j.1471-0528.2000.tb13335.x. [DOI] [PubMed] [Google Scholar]
- 106.Maximovich A, Beyler SA. Cigarette smoking at time of in vitro fertilization cycle initiation has negative effect on in vitro fertilization-embryo transfer success rate. J Assist Reprod Genet. 1995;12(2):75–77. doi: 10.1007/BF02211373. [DOI] [PubMed] [Google Scholar]
- 107.Medina E, Arteaga P, Pizarro L, et al. Effects of cigarette smoking in women [in Spanish] Rev Med Chil. 1990;118(3):253–258. [PubMed] [Google Scholar]
- 108.McKean HE. Smoking and abortion. N Engl J Med. 1978;298(2):113–114. [PubMed] [Google Scholar]
- 109.Meeker JD, Missmer SA, Vitonis AF, et al. Risk of spontaneous abortion in women with childhood exposure to parental cigarette smoke. Am J Epidemiol. 2007;166(5):571–575. doi: 10.1093/aje/kwm128. [DOI] [PubMed] [Google Scholar]
- 110.Meeker JD, Missmer SA, Cramer DW, et al. Maternal exposure to second-hand tobacco smoke and pregnancy outcome among couples undergoing assisted reproduction. Hum Reprod. 2007;22(2):337–345. doi: 10.1093/humrep/del406. [DOI] [PubMed] [Google Scholar]
- 111.Mey R, Gorg I. Smoking and pregnancy. Med Klin. 1967;62(1):5–10. [PubMed] [Google Scholar]
- 112.Mishra GD, Dobson AJ, Schofield MJ. Cigarette smoking, menstrual symptoms and miscarriage among young women. Aust N Z J Public Health. 2000;24(4):413–420. doi: 10.1111/j.1467-842x.2000.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 113.Morales AW, Marks MN, Kumar R. Smoking in pregnancy: a study of psychosocial and reproductive risk factors. J Psychosom Obstet Gynaecol. 1997;18(4):247–254. doi: 10.3109/01674829709080695. [DOI] [PubMed] [Google Scholar]
- 114.Murphy JF, Mulcahy R. The effects of cigarette smoking, maternal age and parity on the outcome of pregnancy. J Ir Med Assoc. 1974;67(11):309–313. [PubMed] [Google Scholar]
- 115.Murphy J, Mulcahy R. Cigarette smoking and spontaneous abortion. Br Med J. 1978;1(6118):988. doi: 10.1136/bmj.1.6118.988-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura MU, Alexandre SM, Kuhn dos Santos JF, et al. Obstetric and perinatal effects of active and/or passive smoking during pregnancy. Sao Paulo Med J. 2004;122(3):94–98. doi: 10.1590/S1516-31802004000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340(5):333–339. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]
- 118.Nielsen A, Hannibal CG, Lindekilde BE, et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet Gynecol Scand. 2006;85(9):1057–1065. doi: 10.1080/00016340600589560. [DOI] [PubMed] [Google Scholar]
- 119.O'Lane JM. Some fetal effects of maternal cigaret smoking. Obstet Gynecol. 1963;22(2):181–184. [PubMed] [Google Scholar]
- 120.Padrón Garcia DM, Sanchez Pérez BB. Tobacco use and pregnancy [in Spanish] Rev Cubana Enferm. 1990;6(1):62–68. [PubMed] [Google Scholar]
- 121.Palmgren B, Wallander B. Cigarette smoking and abortion. Consecutive prospective study of 4,312 pregnancies. Lakartidningen. 1971;68(22):2611–2616. [PubMed] [Google Scholar]
- 122.Palmgren B, Wahlen T, Wallander B. Toxaemia and cigarette smoking during pregnancy. Prospective consecutive investigation of 3,927 pregnancies. Acta Obstet Gynecol Scand. 1973;52(2):183–185. doi: 10.3109/00016347309158310. [DOI] [PubMed] [Google Scholar]
- 123.Pandya PP, Snijders RJ, Psara N, et al. The prevalence of non-viable pregnancy at 10–13 weeks of gestation. Ultrasound Obstet Gynecol. 1996;7(3):170–173. doi: 10.1046/j.1469-0705.1996.07030170.x. [DOI] [PubMed] [Google Scholar]
- 124.Pattinson HA, Taylor PJ, Pattinson MH. The effect of cigarette smoking on ovarian function and early pregnancy outcome of in vitro fertilization treatment. Fertil Steril. 1991;55(4):780–783. doi: 10.1016/s0015-0282(16)54248-4. [DOI] [PubMed] [Google Scholar]
- 125.Raatikainen K, Huurinainen P, Heinonen S. Smoking in early gestation or through pregnancy: a decision crucial to pregnancy outcome. Prev Med. 2007;44(1):59–63. doi: 10.1016/j.ypmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 126.Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82(2):182–188. doi: 10.1034/j.1600-0412.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 127.Risch HA, Weiss NS, Clarke EA, et al. Risk factors for spontaneous abortion and its recurrence. Am J Epidemiol. 1988;128(2):420–430. doi: 10.1093/oxfordjournals.aje.a114982. [DOI] [PubMed] [Google Scholar]
- 128.Rumeau-Rouquette C, Goujard J, Kaminski M, et al. Perinatal mortality, previous obstetric history and use of tobacco. J Gynecol Obstet Biol Reprod (Paris) 1972;1(7):723–729. [PubMed] [Google Scholar]
- 129.Sandahl B. Smoking habits and spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 1989;31(1):23–31. doi: 10.1016/0028-2243(89)90023-3. [DOI] [PubMed] [Google Scholar]
- 130.Scholl TO, Salmon RW, Miller LK. Smoking and adolescent pregnancy outcome. J Adolesc Health Care. 1986;7(6):390–394. doi: 10.1016/s0197-0070(86)80240-6. [DOI] [PubMed] [Google Scholar]
- 131.Schwartz D, Goujard J, Kaminski M, et al. Smoking and pregnancy. Results of a prospective study of 6,989 women. Rev Eur Etud Clin Biol. 1972;17(9):867–879. [PubMed] [Google Scholar]
- 132.Selevan SG, Lindbohm ML, Hornung RW, et al. A study of occupational exposure to antineoplastic drugs and fetal loss in nurses. N Engl J Med. 1985;313(19):1173–1178. doi: 10.1056/NEJM198511073131901. [DOI] [PubMed] [Google Scholar]
- 133.Simoes MJ. Study of frequency of smoking during pregnancy, Ribeirao Preto, Sao Paulo [in Portuguese] Rev Cinc Biomed. 1985;6:61–69. [PubMed] [Google Scholar]
- 134.Stein Z, Kline J, Levin B, et al. Epidemiologic studies of environmental exposures in human reproduction. In: Berg GG, Maillie HD, editors. Measurement of Risks. New York, NY: Plenum Press; 1981. pp. 163–183. [Google Scholar]
- 135.Stein Z. Early fetal loss. Birth Defects Orig Artic Ser. 1981;17(1):95–111. [PubMed] [Google Scholar]
- 136.Triopon G, Tailland ML, Faillie JL, et al. In vitro fertilization and smoking: use of urinary cotinine and expired air carbon monoxide measurements [in French] Gynecol Obstet Fertil. 2006;34(11):1043–1050. doi: 10.1016/j.gyobfe.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 137.Underwood P, Hester LL, Laffitte T., Jr, et al. The relationship of smoking to the outcome of pregnancy. Am J Obstet Gynecol. 1965;91(2):270–276. doi: 10.1016/0002-9378(65)90211-5. [DOI] [PubMed] [Google Scholar]
- 138.van Ravenswaaij R, Tesselaar-van der Goot M, de Wolf S, et al. First-trimester serum PAPP-A and fβ-hCG concentrations and other maternal characteristics to establish logistic regression-based predictive rules for adverse pregnancy outcome. Prenat Diagn. 2011;31(1):50–57. doi: 10.1002/pd.2610. [DOI] [PubMed] [Google Scholar]
- 139.Venners SA, Wang X, Chen C, et al. Paternal smoking and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol. 2004;159(10):993–1001. doi: 10.1093/aje/kwh128. [DOI] [PubMed] [Google Scholar]
- 140.Wallander BE, Hall FL, Palmgren BA. Re: “Smoking in pregnancy” [letter] Nord Med. 1970;84(50):1602. [PubMed] [Google Scholar]
- 141.Warburton D, Susser M, Stein Z, et al. Genetic and epidemiologic investigation of spontaneous abortion: relevance to clinical practice. Birth Defects Orig Artic Ser. 1979;15(5A):127–136. [PubMed] [Google Scholar]
- 142.Wilcox AJ, Weinberg CR, Baird DD. Risk factors for early pregnancy loss. Epidemiology. 1990;1(5):382–385. doi: 10.1097/00001648-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 143.Windham GC, Swan SH, Fenster L. Parental cigarette smoking and the risk of spontaneous abortion. Am J Epidemiol. 1992;135(12):1394–1403. doi: 10.1093/oxfordjournals.aje.a116250. [DOI] [PubMed] [Google Scholar]
- 144.Windham GC, Von Behren J, Waller K, et al. Exposure to environmental and mainstream tobacco smoke and risk of spontaneous abortion. Am J Epidemiol. 1999;149(3):243–247. doi: 10.1093/oxfordjournals.aje.a009798. [DOI] [PubMed] [Google Scholar]
- 145.Windham GC, Elkin EP, Swan SH, et al. Cigarette smoking and effects on menstrual function. Obstet Gynecol. 1999;93(1):59–65. doi: 10.1016/s0029-7844(98)00317-2. [DOI] [PubMed] [Google Scholar]
- 146.Winter E, Wang J, Davies MJ, et al. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223. doi: 10.1093/humrep/17.12.3220. [DOI] [PubMed] [Google Scholar]
- 147.Wisborg K, Kesmodel U, Henriksen TB, et al. A prospective study of maternal smoking and spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82(10):936–941. doi: 10.1034/j.1600-0412.2003.00244.x. [DOI] [PubMed] [Google Scholar]
- 148.Wu T, Buck G, Mendola P. Maternal cigarette smoking, regular use of multivitamin/mineral supplements, and risk of fetal death: the 1988 National Maternal and Infant Health Survey. Am J Epidemiol. 1998;148(2):215–221. doi: 10.1093/oxfordjournals.aje.a009626. [DOI] [PubMed] [Google Scholar]
- 149.Yuan P, Wada N, Arai M, et al. Maternal drinking and smoking and the risk of birth defects [in Japanese] Nihon Koshu Eisei Zasshi. 1994;41(8):751–758. [PubMed] [Google Scholar]
- 150.Foster GA. Problems commonly associated with forest plots addressed using high resolution graphics in SAS. Proceedings of the Thirty-first Annual SAS Users Group International Conference; San Francisco, CA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.