Abstract
Perfluoroalkyl substances (PFAS) are persistent and ubiquitous environmental contaminants, and human exposure to these substances may be related to preeclampsia, a common pregnancy complication. Previous studies have found serum concentrations of PFAS to be positively associated with pregnancy-induced hypertension and preeclampsia in a population with high levels of exposure to perfluorooctanoate. Whether this association exists among pregnant women with background levels of PFAS exposure is unknown. Using data from the Norwegian Mother and Child Cohort Study conducted by the Norwegian Institute of Public Health, we carried out a study of nulliparous pregnant women enrolled in 2003–2007 (466 cases, 510 noncases) to estimate associations between PFAS concentrations and an independently validated diagnosis of preeclampsia. We measured levels of 9 PFAS in maternal plasma extracted midpregnancy; statistical analyses were restricted to 7 PFAS that were quantifiable in more than 50% of samples. In proportional hazards models adjusted for maternal age, prepregnancy body mass index (weight (kg)/height (m)2), educational level, and smoking status, we observed no strongly positive associations between PFAS levels and preeclampsia. We found an inverse association between preeclampsia and the highest quartile of perfluoroundecanoic acid concentration relative to the lowest quartile (hazard ratio = 0.55, 95% confidence interval: 0.38, 0.81). Overall, our findings do not support an increased risk of preeclampsia among nulliparous Norwegian women with background levels of PFAS exposure.
Keywords: Norwegian Mother and Child Cohort Study, perfluoroalkyl substances, perfluorooctanoic acid, perfluorooctane sulfonate, perfluoroundecanoic acid, preeclampsia
Preeclampsia is a serious complication of pregnancy that consists of new-onset hypertension combined with kidney dysfunction, which frequently leads to preterm delivery. Preeclampsia affects approximately 3% of pregnancies in the United States (1) and in Norway (2), and it is a leading cause of maternal morbidity and mortality. The cause of preeclampsia remains obscure, but it is likely multifactorial, with both genetic and environmental contributions. Risk factors for preeclampsia include nulliparity, personal or family history of preeclampsia, hypertension, diabetes, autoimmune disorders, kidney disease, obesity, multiple gestation, history of subfertility, long interval between pregnancies (3), and advanced maternal age (4, 5). Cigarette smoking during pregnancy is associated with an estimated 30% reduction in risk (6). Exposure to certain environmental contaminants may increase a woman's risk of developing preeclampsia (7, 8).
Perfluoroalkyl substances (PFAS) are persistent chemicals that have been detected in blood samples from numerous human populations worldwide (9–12). The most commonly measured PFAS, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), have been used in industrial and consumer products, such as surface treatments for carpets and fabrics, food packaging, and fire-fighting foam (13). In non-occupationally exposed populations, ingestion of PFAS in food is believed to be the principal source of exposure (14–16). Other pathways of PFAS exposure may include drinking water (17), breast milk (18), and house dust (16). PFAS are highly stable and resist metabolism or degradation (9, 19). In human serum, the elimination half-lives are estimated to be 4.8 years for PFOS and 2.3 years for PFOA (20, 21).
Despite the ubiquitous presence of PFAS in humans, the potential for adverse health effects resulting from chronic, low-level exposure has not been adequately studied, particularly among pregnant women. A recent study of non-occupationally exposed women living in an area of high PFOA contamination in drinking water found PFOA and PFOS to be positively associated with preeclampsia (22). The possibility that exposure to these widespread environmental contaminants during pregnancy may increase the risk of preeclampsia is of substantial public health concern and merits further examination. We explored this question in a case-cohort study of women with background levels of exposure who were enrolled in the Norwegian Mother and Child Cohort Study (MoBa).
METHODS
Participants
This is a substudy within MoBa, a prospective pregnancy cohort study conducted by the Norwegian Institute of Public Health (23–25). The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate. Participants were recruited from throughout Norway in 1999–2008. Pregnant women who scheduled a routine ultrasound examination between 17 and 20 weeks of gestation were invited by mail to participate in the study, and 39% of invited women participated. Informed consent was obtained from each MoBa participant upon recruitment. Data were linked to the Medical Birth Registry of Norway (MBRN) (26). Further details may be found at www.fhi.no/morogbarn. The present study is based on version 4.301 of the quality-assured data files released for research.
Eligibility requirements for this analysis were pregnancy with a singleton infant, no previous live births or stillbirths, no chronic hypertension before pregnancy, a midpregnancy plasma sample preserved in ethylenediaminetetraacetic acid, and enrollment in MoBa in 2003–2007. We restricted the study to women with no previous live births or stillbirths because PFAS have been shown to decline with recent pregnancy and lactation (27) and because nulliparous women may have different risk factors for preeclampsia than women with previous pregnancies (3, 28). We restricted eligibility to women who enrolled in 2003 or later because the laboratory analysis of PFAS required ethylenediaminetetraacetic acid anticoagulation, a process initiated in MoBa in 2003.
From 549 eligible cases of validated preeclampsia in this time frame, 500 were randomly selected for the study. From approximately 21,500 eligible pregnancies, 567 were selected at random as the subcohort sample, 17 of whom were also validated cases. Three of the original subcohort members reported chronic hypertension to the MBRN and were excluded from the present analysis. One member of the subcohort had a gestational age at delivery reported to the MBRN of less than 20 weeks and thus was excluded because she was never at risk to develop preeclampsia. We were missing data for each modeled covariate for less than 6% of participants.
Exposure
Maternal nonfasting blood samples were collected at hospitals and maternity units across Norway at study enrollment. Samples were shipped at ambient temperature to the MoBa biorepository in Oslo, Norway, where plasma was separated, aliquoted, and stored at −80°C. The majority of the samples were received and processed the day after collection (25). PFAS are chemically stable (29), and therefore changes in plasma concentrations during shipping were expected to be minimal. A recent study found no change in concentrations of 4 PFAS in serum during 10 days of storage at room temperature (30).
Concentrations of 9 PFAS were measured in maternal plasma using high-performance liquid chromatography/tandem mass spectrometry at the Norwegian Institute of Public Health. Additional details of the analytic process have been published previously (31). Statistical analyses were limited to PFAS that were quantifiable in greater than 50% of the samples: PFOS, perfluoroheptane sulfonate (PFHpS), perfluorohexane sulfonate (PFHxS), PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), and perfluoroundecanoic acid (PFUnDA). The limit of quantification (LOQ) was 0.05 ng/mL for these 7 PFAS. The total area of linear and branched isomers was integrated for quantification of PFOS. A total of 25 blinded specimens from a single pool were analyzed in batches with the sample specimens for quality assurance and quality control purposes.
Outcome
At the time of delivery, birth attendants reported the presence of preeclampsia to the MBRN using a standard form. A validation study was conducted to quantify the performance of the MBRN in identifying preeclampsia (K.K., unpublished data, 2014). Antenatal medical records were reviewed for evidence of meeting the diagnostic criteria for preeclampsia published by the American College of Obstetricians and Gynecologists (32). Validated preeclampsia in our study required evidence of both of the following at the same antenatal clinic visit: 1) systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg that occurred after 20 completed weeks of gestation and 2) proteinuria, defined as a urine dipstick measurement of at least 1+.
Our definition differs from that of the American College of Obstetricians and Gynecologists in that we relied on a single urine dipstick measurement, whereas their definition recommends the use of a 24-hour urine collection to diagnose proteinuria. The results of 24-hour urine collection were generally not available to us in antenatal records. Additionally, our definition required that hypertension and proteinuria be documented at the same clinic visit. The gestational week of the clinic visit during which these diagnostic criteria were met was recorded. Gestational age was estimated by the attending midwife based on a combination of ultrasound and last menstrual period dates.
Although the MBRN validation study (K.K., unpublished data, 2014) also considered hospital discharge diagnoses of preeclampsia, our study excluded cases that could not be confirmed using the antenatal medical record. Antenatal medical records from a sample of MoBa participants with no preeclampsia reported to the MBRN were also examined in the independent validation study; however, in the random subcohort from the larger MoBa population, the noncase status was not independently validated. On the basis of the validation study, we estimated that the negative predictive value of having no preeclampsia reported to the MBRN was approximately 95%.
We excluded both cases and cohort members with evidence of chronic hypertension because diagnosis of preeclampsia in the presence of chronic hypertension is clinically complicated and often ambiguous (33). Chronic hypertension was defined as any of the following: 1) hypertension reported to the MBRN or on the MoBa questionnaire, 2) an International Classification of Diseases, 10th Revision code corresponding to chronic hypertension on the antenatal medical chart, or 3) systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg diastolic before 20 weeks of gestation documented on the antenatal medical chart.
Other variables
At the time of enrollment in MoBa, women provided information via questionnaire regarding a number of demographic and lifestyle characteristics and events in their reproductive and medical history. Covariate information was obtained as follows: maternal age at delivery was reported to the MBRN; prepregnancy body mass index (BMI) was calculated as the participant's self-reported prepregnancy weight in kilograms divided by height in meters squared; maternal educational level was self-reported in response to a questionnaire item requesting the highest level of education completed; and maternal smoking during pregnancy was self-reported in response to the question, “Do you smoke now (after you became pregnant)?” at a median of 18 weeks gestation.
Creatinine and cystatin C were measured in midpregnancy plasma samples to evaluate whether changes in kidney function and glomerular filtration rate could be detected. High-density lipoprotein (HDL) cholesterol was measured in the same nonfasting plasma sample. All 3 analytes were measured at the National Institute of Environmental Health Sciences using an Olympus AU400e chemistry immuno-analyzer (Olympus America, Center Valley, Pennsylvania). The procedure used to measure creatinine was a kinetic modification of the Jaffe procedure (34), in which creatinine reacts with picric acid at an alkaline pH. The cystatin C assay was based on the sol particle immunoassay (35), using colloidal gold particles coated with anti-cystatin C–specific polyclonal antibodies. HDL concentration was measured using reagents from Beckman Coulter (Brea, California).
Statistical analysis
We used weighted Cox proportional hazards models to estimate hazard ratios and 95% confidence intervals by quartile of plasma concentration of each PFAS. Weights were based on the inverse probability of selection into the case-cohort study (36). The outcome was validated preeclampsia, and the event time was the gestational week at which the participant met the diagnostic criteria. Modeled covariates were selected based on a directed acyclic graph representing associations reported in the literature. The minimally sufficient adjustment set was identified using DAGitty, version 1.0 (www.dagitty.net). The modeled covariates and their categorizations were: maternal age at delivery (continuous); prepregnancy BMI (continuous); maternal educational level (≤12, 13–16, >16 years); and smoking at midpregnancy (yes or no). Additional analyses were conducted with separate models adjusted for plasma creatinine, cystatin C, or HDL cholesterol, with each modeled as a continuous variable.
Concentrations of PFAS were analyzed in 2 ways: 1) in quartiles, with the lowest quartile serving as the reference category (except for PFDA, which was categorized as at or above versus below the median because more than 25% of values were below the LOQ) and 2) as natural-log–transformed continuous variables to assess linear trends. Values below the LOQ were replaced by a single value equal to the expected value of the log-normal distribution, given that the value was below the LOQ; this was calculated as the mean of values below the LOQ randomly drawn from the estimated log-normal distribution (37). The proportional hazards assumption was evaluated through regression and graphical inspection of the weighted Schoenfeld residuals plotted against time.
We examined the shape of the dose-response function using logistic regression models of the association between each continuous PFAS exposure, modeled as a restricted cubic spline function with 5 knots at the 5th, 25th, 50th, 75th, and 95th percentiles (or, in the case of PFDA, with 4 knots at the 25th, 50th, 75th, and 95th percentiles), and preeclampsia as a binary outcome. All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina). The logistic spline models were fitted using a SAS macro created by Desquilbet and Mariotti (38).
RESULTS
The present analysis included 976 nulliparous women (466 cases and 510 noncases) for whom we had complete information on modeled covariates. The participants ranged in age from 16 years to 44 years (Table 1). There were no notable differences in age distributions between cases and noncases. BMI ranged from 15.4 to 48.8, and there were more cases than noncases with a BMI of 30 or greater (16% of cases vs. 7% of noncases). The majority of participants had 13–16 years of education (75% of cases and 70% of noncases). Overall, 7% of participants reported current smoking during pregnancy, with a slightly lower prevalence among cases (6%). The median duration of pregnancy was shorter among cases (274 days) than noncases (282 days), and 21% of cases had pregnancies shorter than 37 weeks of gestation, compared with less than 5% of noncases.
Table 1.
Characteristics of Subjects in a Subcohort of the Norwegian Mother and Child Cohort Study, 2003–2007
| Characteristic | Cases, % (n = 466) | Noncases, % (n = 510) |
|---|---|---|
| Maternal age at delivery, years | ||
| 16–24 | 17 | 16 |
| 25–29 | 43 | 41 |
| 30–34 | 30 | 33 |
| 35–44 | 10 | 10 |
| Prepregnancy body mass indexa | ||
| 15.4–24.9 | 56 | 72 |
| 25.0–29.9 | 28 | 20 |
| 30.0–48.8 | 16 | 7 |
| Maternal education, years | ||
| ≤12 | 3 | 2 |
| 13–16 | 75 | 70 |
| >16 | 21 | 25 |
| Other education | 1 | 2 |
| Smoked during pregnancy | ||
| Yes | 6 | 7 |
| No | 94 | 93 |
| Duration of pregnancy, completed weeks | ||
| 24–27 | 1 | 0 |
| 28–31 | 4 | 0.4 |
| 32–36 | 16 | 4 |
| 37–41 | 77 | 87 |
| 42–43 | 3 | 9 |
a Weight (kg)/height (m)2.
Of the 7 PFAS quantifiable in plasma samples from more than 50% of participants (Table 2), PFOS had the highest median concentration (12.87 ng/mL), and it was quantifiable in 100% of samples. PFOA had the second highest median concentration (2.78 ng/mL), and it was also quantifiable in 100% of samples. The other measured PFAS in descending order of median concentration were PFHxS, PFNA, PFUnDA, PFHpS, and PFDA. PFDA was quantifiable in 71% of samples. Interassay coefficients of variation ranged from 9% for PFOA to 30% for PFHpS (Table 2). The PFAS were moderately to highly correlated with one another (Table 3). The lowest correlation observed was between PFDA and PFHpS (rs = 0.18), and the highest was between PFNA and PFDA (rs = 0.75). PFOS and PFOA were correlated at rs = 0.64.
Table 2.
Plasma Concentrations of 7 Perfluoroalkyl Substancesa in Subjects in a Subcohort of the Norwegian Mother and Child Cohort Study, 2003–2007
| Perfluoroalkyl Substance | Abbreviated Name | % > LOQ | Chain Lengthb | Plasma Concentration (ng/mL) by Percentile |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | CVc | ||||
| Perfluorooctanoic acid | PFOA | 100.0 | 7 | 1.43 | 2.14 | 2.78 | 3.57 | 5.15 | 8.6 |
| Perfluorononanoic acid | PFNA | 99.7 | 8 | 0.24 | 0.39 | 0.54 | 0.74 | 1.12 | 13.3 |
| Perfluorodecanoic acid | PFDA | 71.1 | 9 | <LOQ | <LOQ | 0.10 | 0.18 | 0.34 | 27.0 |
| Perfluoroundecanoic acid | PFUnDA | 83.7 | 10 | <LOQ | 0.08 | 0.17 | 0.27 | 0.47 | 22.2 |
| Perfluorohexane sulfonate | PFHxS | 99.5 | 6 | 0.27 | 0.49 | 0.69 | 0.95 | 1.78 | 14.6 |
| Perfluoroheptane sulfonate | PFHpS | 83.6 | 7 | <LOQ | 0.09 | 0.15 | 0.23 | 0.37 | 29.8 |
| Perfluorooctane sulfonate | PFOS | 100.0 | 8 | 6.07 | 9.69 | 12.87 | 17.03 | 25.53 | 11.4 |
Abbreviations: CV, coefficient of variation; LOQ, limit of quantification.
a The 7 perfluoroalkyl substances were those with concentrations that could be quantitated in more than 50% of samples.
b Refers to the number of carbons in the fully-fluorinated alkyl chain.
c Interassay coefficient of variation was calculated as (standard deviation/mean) × 100 for 25 pooled samples.
Table 3.
Spearman Correlation Coefficients Between Plasma Concentrations of Perfluoroalkyl Substancesa in Subjects in a Subcohort of the Norwegian Mother and Child Cohort Study, 2003–2007
| PFOA | PFNA | PFDA | PFUnDA | PFHxS | PFHpS | PFOS | |
|---|---|---|---|---|---|---|---|
| PFOA | 0.61 | 0.45 | 0.31 | 0.54 | 0.53 | 0.64 | |
| PFNA | 0.75 | 0.51 | 0.45 | 0.31 | 0.42 | ||
| PFDA | 0.54 | 0.27 | 0.18 | 0.25 | |||
| PFUnDA | 0.40 | 0.24 | 0.33 | ||||
| PFHxS | 0.50 | 0.58 | |||||
| PFHpS | 0.74 | ||||||
| PFOS |
Abbreviations: PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid.
a The 7 perfluoroalkyl substances were those with concentrations that could be quantitated in more than 50% of samples.
None of the 7 PFAS had strongly positive associations with preeclampsia, either in quartile analyses or when PFAS were entered as continuous natural-log–transformed variables (Table 4). Certain quartiles of PFHpS and PFOS had weakly positive associations with preeclampsia. Interestingly, there was an inverse association between PFUnDA and preeclampsia; the highest quartile of PFUnDA had an adjusted hazard ratio of 0.55 (95% confidence interval: 0.38, 0.81) relative to the lowest quartile, and each increase of 1 ln-ng/mL of PFUnDA was associated with an adjusted hazard ratio of 0.78 (95% confidence interval: 0.66, 0.92). Additional adjustment for plasma creatinine or plasma cystatin C did not produce meaningful changes in any effect estimates (results not shown). Additional adjustment of the PFUnDA models for HDL also did not produce meaningful changes in the effect estimates (results not shown).
Table 4.
Hazard Ratios for Preeclampsia Onset Associated With Plasma Perfluoroalkyl Substance Concentrations in Subjects in a Subcohorta of the Norwegian Mother and Child Cohort Study, 2003–2007
| Perfluoroalkyl Substance | Plasma Concentration, ng/mL, Range | Unadjusted HR | 95% CI | Adjustedb HR | 95% CI |
|---|---|---|---|---|---|
| PFOA | |||||
| Quartile 1 | 0.32–2.11 | 1.00 | Referent | 1.00 | Referent |
| Quartile 2 | 2.12–2.77 | 0.99 | 0.70, 1.42 | 1.03 | 0.70, 1.50 |
| Quartile 3 | 2.77–3.56 | 0.93 | 0.65, 1.32 | 0.92 | 0.63, 1.35 |
| Quartile 4 | 3.56–11.28 | 0.88 | 0.61, 1.25 | 1.01 | 0.69, 1.48 |
| Per ln-unit | 0.84 | 0.63, 1.13 | 0.89 | 0.65, 1.22 | |
| PFNA | |||||
| Quartile 1 | <LOQ–0.39 | 1.00 | Referent | 1.00 | Referent |
| Quartile 2 | 0.39–0.54 | 0.85 | 0.60, 1.22 | 0.88 | 0.60, 1.30 |
| Quartile 3 | 0.54–0.74 | 0.92 | 0.64, 1.31 | 1.04 | 0.71, 1.53 |
| Quartile 4 | 0.74–3.54 | 0.80 | 0.56, 1.15 | 0.88 | 0.60, 1.29 |
| Per ln-unit | 0.84 | 0.66, 1.07 | 0.90 | 0.70, 1.16 | |
| PFDA | |||||
| Below median | <LOQ–0.10 | 1.00 | Referent | 1.00 | Referent |
| At or above median | 0.10–1.74 | 0.81 | 0.63, 1.05 | 0.88 | 0.67, 1.16 |
| Per ln-unit | 0.86 | 0.74, 1.00 | 0.88 | 0.75, 1.04 | |
| PFUnDA | |||||
| Quartile 1 | <LOQ–0.08 | 1.00 | Referent | 1.00 | Referent |
| Quartile 2 | 0.08–0.17 | 0.55 | 0.38, 0.79 | 0.51 | 0.35, 0.76 |
| Quartile 3 | 0.17–0.27 | 0.57 | 0.40, 0.82 | 0.60 | 0.41, 0.88 |
| Quartile 4 | 0.27–1.01 | 0.49 | 0.34, 0.71 | 0.55 | 0.38, 0.81 |
| Per ln-unit | 0.76 | 0.65, 0.89 | 0.78 | 0.66, 0.92 | |
| PFHxS | |||||
| Quartile 1 | <LOQ–0.49 | 1.00 | Referent | 1.00 | Referent |
| Quartile 2 | 0.49–0.69 | 0.94 | 0.66, 1.34 | 0.86 | 0.59, 1.26 |
| Quartile 3 | 0.69–0.95 | 1.00 | 0.70, 1.42 | 1.01 | 0.69, 1.49 |
| Quartile 4 | 0.95–11.47 | 0.84 | 0.59, 1.19 | 0.93 | 0.64, 1.36 |
| Per ln-unit | 0.86 | 0.70, 1.06 | 0.91 | 0.72, 1.14 | |
| PFHpS | |||||
| Quartile 1 | <LOQ–0.09 | 1.00 | Referent | 1.00 | Referent |
| Quartile 2 | 0.09–0.15 | 1.22 | 0.85, 1.74 | 1.30 | 0.88, 1.92 |
| Quartile 3 | 0.15–0.22 | 1.01 | 0.71, 1.45 | 1.01 | 0.69, 1.48 |
| Quartile 4 | 0.22–1.19 | 1.04 | 0.73, 1.48 | 1.12 | 0.77, 1.63 |
| Per ln-unit | 1.01 | 0.85, 1.20 | 1.03 | 0.86, 1.24 | |
| PFOS | |||||
| Quartile 1 | 1.44–9.66 | 1.00 | Referent | 1.00 | Referent |
| Quartile 2 | 9.67–12.79 | 1.18 | 0.82, 1.68 | 1.12 | 0.76, 1.65 |
| Quartile 3 | 12.80–16.91 | 0.85 | 0.59, 1.21 | 0.88 | 0.60, 1.29 |
| Quartile 4 | 16.91–56.61 | 1.08 | 0.75, 1.53 | 1.09 | 0.75, 1.58 |
| Per ln-unit | 1.08 | 0.82, 1.42 | 1.13 | 0.84, 1.52 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LOQ, limit of quantification; PFDA, perfluorodecanoic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFUnDA, perfluoroundecanoic acid.
a A total of 466 women developed preeclampsia.
b Adjusted for maternal age, prepregnancy body mass index (weight (kg)/height (m)2), education completed, and smoking during pregnancy.
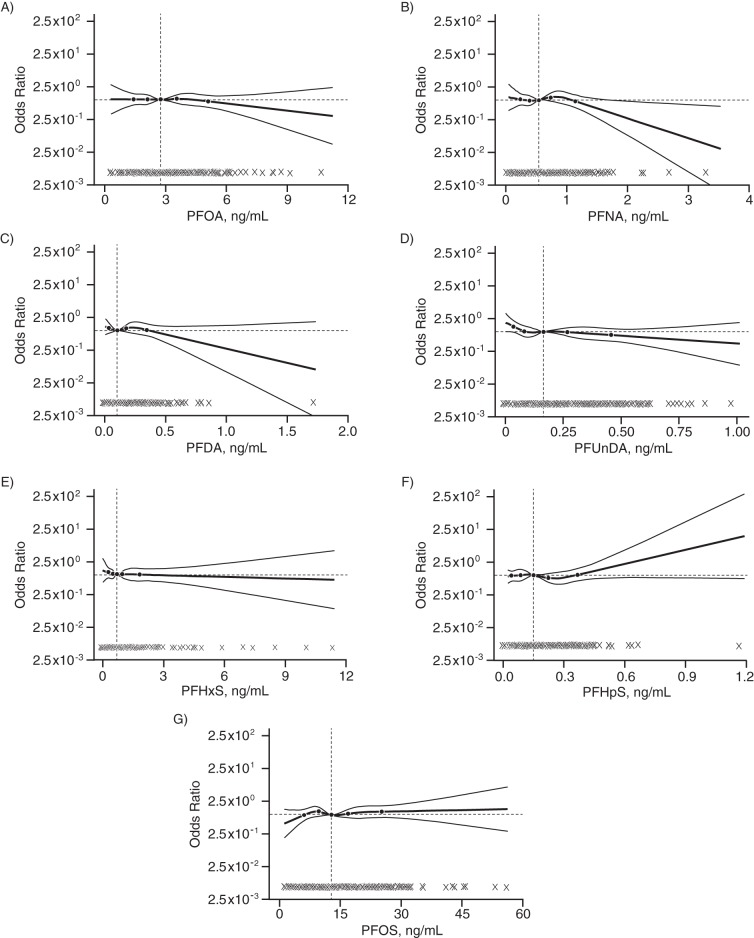
The shape of the dose-response function was characterized using restricted cubic spline functions for the association between each continuous PFAS exposure variable and preeclampsia (Figure 1). The spline graphs suggested weakly inverse associations of PFNA (Figure 1B), PFDA (Figure 1C), and PFUnDA (Figure 1D) with preeclampsia. There was some indication in the spline graphs of an increase in preeclampsia risk at the higher concentrations of PFHpS (Figure 1F) and perhaps PFOS (Figure 1G). Overall, the results of the logistic regression analysis using restricted cubic spline functions appeared to parallel the results of the Cox proportional hazards models (Table 4).
Figure 1.
Adjusted dose-response relationships between plasma concentrations of perfluoroalkyl substances at midpregnancy and the natural-log–transformed odds ratio for preeclampsia using restricted cubic spline functions among 976 pregnant women enrolled in the Norwegian Mother and Child Cohort Study, 2003–2007. Odds ratios were adjusted for maternal age, prepregnancy body mass index (weight (kg)/height (m)2), education completed, and smoking during pregnancy. Restricted cubic spline functions have 5 knots at the 5th, 25th, 50th, 75th, and 95th percentiles, with the exception of PFDA, which has only 4 knots at the 25th, 50th, 75th, and 95th percentiles. The horizontal line denotes an odds ratio of 1, the vertical line denotes the median of the observed distribution, and observed values are indicated by dots near the x-axis. PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid.
DISCUSSION
In the present study of plasma PFAS concentrations and preeclampsia among 976 nulliparous Norwegian women with background levels of exposure, we observed 1 inverse association (PFUnDA) and 2 weakly positive associations (PFHpS and PFOS). A series of related studies on pregnancy-induced hypertension and preeclampsia conducted in a population exposed to PFOA-contaminated drinking water reported positive associations with both PFOA and PFOS (22, 39). The difference between the results of our study and those of previous studies may be partly attributed to differences in exposure assessment, differences in preeclampsia case definition, or the restriction of our study to nulliparous women.
Our study directly measured PFAS at midpregnancy, before the onset of preeclampsia, whereas previous studies used indirect methods to assess PFAS during pregnancy. Specifically, one study used predictive modeling to assign exposure at the time of pregnancy (39); another measured exposure concentration in serum up to 5 years after the pregnancy of interest (22). Exposures assessed differently could be biased by different unmeasured factors.
The PFOA concentrations measured or predicted in previous studies were substantially higher than the concentrations measured in our study. The shape of the dose-response relationship of PFOA concentration with preeclampsia may be nonlinear, with effects only observable at higher exposure concentrations than those present in our study. However, differences in exposure magnitude cannot explain the lack of statistically significant association between PFOS and preeclampsia found in our study, because the median PFOS concentration in the previous study was approximately equal to ours (22). The plasma concentrations of PFOA, PFNA, PFDA, PFUnDA, PFHxS, and PFOS measured in our study were similar to serum concentrations measured in women in Norway in 2007 (12) and women in California in 2009 (40). PFOS and PFOA concentrations were similar to those reported in a sample of US adult female blood donors in 2006, whereas PFNA and PFHxS concentrations in our study were somewhat lower (41).
Our determination of preeclampsia was validated through review of antenatal medical records, whereas previous studies used self-reported preeclampsia (22, 39). The positive predictive value of self-reported preeclampsia in a recent systematic review was 50%–60% (42). The use of medical records to validate the diagnosis may have reduced outcome misclassification in our study, possibly through the exclusion of other hypertensive disorders of pregnancy.
Our study was restricted to nulliparous women, who have a higher risk of preeclampsia than do parous women (43). Women who develop preeclampsia in their second or later pregnancies may have a different underlying set of risk factors than nulliparous women. Approximately half of the women in the previous studies (22, 39) were nulliparous. The association between midpregnancy PFAS plasma concentrations and preeclampsia may differ between nulliparous and parous women. Our restriction to nulliparous women also ensured that measured PFAS concentrations did not reflect recent declines in body burden due to previous pregnancy and lactation (27, 44, 45).
We observed an inverse association between PFUnDA and preeclampsia. We are not aware of any previous studies that have evaluated this association. There are several possible explanations for this finding, which may result from chance, causality, confounding, or pharmacokinetics. One possible explanation for the inverse association between PFUnDA and preeclampsia may be the mediation of HDL cholesterol. In a separate study within the MoBa cohort, all 7 PFAS studied were positively associated with HDL cholesterol levels at midpregnancy, and PFUnDA showed the strongest association with HDL (46). As higher levels of HDL are considered protective against cardiovascular disease (47) and low HDL cholesterol levels during pregnancy have been associated with increased risk of preeclampsia (48), it is possible that an environmental factor leading to increased HDL levels could in fact reduce the risk of preeclampsia. Adjustment for HDL produced no meaningful change in the estimated association between PFUnDA and preeclampsia. However, if the association between PFUnDA and preeclampsia were mediated by HDL, then adjustment for HDL might produce estimates that were biased or difficult to interpret (49, 50). Moreover, it is unknown whether the previously observed association between PFAS and HDL in pregnant women is causal or the result of unmeasured confounding or pharmacokinetics.
Strengths of this study include the use of a validated diagnosis of preeclampsia, which serves to reduce outcome misclassification, and the use of a highly sensitive assay for PFAS, which allowed for the quantification of several PFAS that have not been studied extensively. However, our ability to interpret the lack of association between concentrations of these infrequently studied compounds and preeclampsia was restricted by the fact that we found limited variation in exposure concentration of these compounds.
In contrast to previous studies (22, 39), we were able to directly measure PFAS in plasma samples taken midpregnancy, before the onset of preeclampsia. However, the possibility remains that an asymptomatic stage of the disease may already be present at midpregnancy. If this hypothetical presymptomatic stage were to alter kidney function, it could also influence excretion and plasma concentrations of PFAS. Adjustment for plasma creatinine or cystatin C levels did not affect our results, which suggests that the estimates were not confounded by measurably impaired kidney function. We cannot rule out the possibility of bias due to confounding by an unmeasured variable.
As previously noted, the participation rate in MoBa was 39% of eligible women. Selection bias may have influenced the results of our study in unpredictable ways. For example, if unmeasured variables were related to both exposure and selection into the study or to both selection and the outcome, bias could result. However, we are unaware of any probable strong confounders that were unmeasured in this study.
Our preeclampsia case definition excluded pregnancies without available antenatal medical records or for whom the absence of hypertension before 20 weeks of gestation could not be established. Preeclampsia superimposed on chronic hypertension may have different associations with PFAS than new-onset preeclampsia, but our study did not evaluate superimposed preeclampsia. Our study did not capture women who only had recorded signs of preeclampsia in the hospital (approximately 20% of all validated cases); therefore, our study results may not be generalizable to all cases. Another potential limitation of our study may be the lower median BMI in this population as compared with US populations. Only 11% of participants had a BMI of 30 or greater, whereas 32% of US women aged 20–39 years have a BMI in this range (51). This difference may limit generalizability of these findings to US populations.
Overall, we did not observe any strongly positive associations between plasma PFAS concentration at midpregnancy and preeclampsia among nulliparous women. Our results suggest that examining the potential health effects of a longer list of PFAS may be worthwhile. Further research should examine whether the observed inverse association between PFUnDA and preeclampsia is present in other populations, and if so, investigate mechanisms that may underlie this association.
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Research Triangle Park, North Carolina (Anne P. Starling, Donna D. Baird, Quaker Harmon, Jane A. Hoppin, Matthew P. Longnecker); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Anne P. Starling, Stephanie M. Engel, David B. Richardson); Division of Environmental Medicine, Department of Exposure and Risk Assessment, Norwegian Institute of Public Health, Oslo, Norway (Line S. Haug, Georg Becher, Cathrine Thomsen, Azemira Sabaredzovic); Department of Obstetrics and Gynecology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Alison M. Stuebe); Department of Maternal and Child Health, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Alison M. Stuebe); Medical Birth Registry of Norway, Norwegian Institute of Public Health, Bergen, Norway (Kari Klungsøyr); Department of Global Public Health and Primary Health Care, University of Bergen, Bergen, Norway (Kari Klungsøyr); Department of Chemistry, University of Oslo, Oslo, Norway (Georg Becher); Department of Genes and Environment, Division of Epidemiology, Norwegian Institute of Public Health, Oslo, Norway (Merete Eggesbø); Cellular and Molecular Pathology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Research Triangle Park, North Carolina (Gregory S. Travlos, Ralph E. Wilson); and Division of Epidemiology, Norwegian Institute of Public Health, Oslo, Norway (Lill I. Trogstad, Per Magnus).
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS). A.P.S. was supported by an extramural award (1-F30-ES022126-01) from the National Institute of Environmental Health Sciences. The validation of preeclampsia diagnosis was funded by the National Institute of Child Health and Human Development (R01HD058008; principal investigator, S.M.E.). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract N01-ES-75558), NIH/National Institute of Neurological Disorders and Stroke (grants UO1 NS 047537-01 and UO1 NS 047537-06A1), and the Norwegian Research Council/FUGE (grant 151918/S10).
We thank Dr. Sue Edelstein of NIEHS Multi-Media Services for her graphic design contributions.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: None declared.
REFERENCES
- 1.Wallis AB, Saftlas AF, Hsia J, et al. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 2.Klungsøyr K, Morken NH, Irgens L, et al. Secular trends in the epidemiology of pre-eclampsia throughout 40 years in Norway: prevalence, risk factors and perinatal survival. Paediatr Perinat Epidemiol. 2012;26(3):190–198. doi: 10.1111/j.1365-3016.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- 3.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346(1):33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 4.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Trogstad L, Magnus P, Stoltenberg C. Pre-eclampsia: risk factors and causal models. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):329–342. doi: 10.1016/j.bpobgyn.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Conde-Agudelo A, Althabe F, Belizán JM, et al. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181(4):1026–1035. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 7.Saldana TM, Basso O, Baird DD, et al. Pesticide exposure and hypertensive disorders during pregnancy. Environ Health Perspect. 2009;117(9):1393–1396. doi: 10.1289/ehp.0900672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Ren C, Delfino RJ, et al. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;117(11):1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromme H, Tittlemier SA, Völkel W, et al. Perfluorinated compounds–exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212(3):239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Kannan K, Corsolini S, Falandysz J, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38(17):4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, Kuklenyik Z, Reidy JA, et al. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) Environ Sci Technol. 2007;41(7):2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 12.Haug LS, Thomsen C, Becher G. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ Sci Technol. 2009;43(6):2131–2136. doi: 10.1021/es802827u. [DOI] [PubMed] [Google Scholar]
- 13.D'Eon JC, Mabury SA. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol. 2011;45(19):7974–7984. doi: 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- 14.Houde M, Martin JW, Letcher RJ, et al. Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol. 2006;40(11):3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 15.Haug LS, Huber S, Becher G, et al. Characterisation of human exposure pathways to perfluorinated compounds–comparing exposure estimates with biomarkers of exposure. Environ Int. 2011;37(4):687–693. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Egeghy PP, Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Environ Epidemiol. 2011;21(2):150–168. doi: 10.1038/jes.2009.73. [DOI] [PubMed] [Google Scholar]
- 17.Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol. 2009;43(15):5565–5575. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen C, Haug LS, Stigum H, et al. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ Sci Technol. 2010;44(24):9550–9556. doi: 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- 19.Lau C, Anitole K, Hodes C, et al. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 20.Bartell SM, Calafat AM, Lyu C, et al. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118(2):222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein CR, Savitz DA, Dougan M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol. 2009;170(7):837–846. doi: 10.1093/aje/kwp212. [DOI] [PubMed] [Google Scholar]
- 23.Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 24.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 25.Rønningen KS, Paltiel L, Meltzer HM, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21(8):619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79(6):435–439. [PubMed] [Google Scholar]
- 27.Brantsæter AL, Whitworth KW, Ydersbond TA, et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int. 2013;54:74–84. doi: 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostello D, Catlin TK, Roman L, et al. Preeclampsia in the parous woman: who is at risk? Am J Obstet Gynecol. 2002;187(2):425–429. doi: 10.1067/mob.2002.123608. [DOI] [PubMed] [Google Scholar]
- 29.Frömel T, Knepper TP. Biodegradation of fluorinated alkyl substances. Rev Environ Contam Toxicol. 2010;208:161–177. doi: 10.1007/978-1-4419-6880-7_3. [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Wong LY, Basden BJ, et al. Effect of temperature and duration of storage on the stability of polyfluoroalkyl chemicals in human serum. Chemosphere. 2013;91(2):115–117. doi: 10.1016/j.chemosphere.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009;1216(3):385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 32.ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 33.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 34.Jaffe M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Zeitschrift für Physiologische Chemie. 1886;10(5):391–400. [Google Scholar]
- 35.Tanaka M, Matsuo K, Enomoto M, et al. A sol particle homogeneous immunoassay for measuring serum cystatin C. Clin Biochem. 2004;37(1):27–35. doi: 10.1016/j.clinbiochem.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 37.Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol. 2003;157(4):355–363. doi: 10.1093/aje/kwf217. [DOI] [PubMed] [Google Scholar]
- 38.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 39.Savitz DA, Stein CR, Bartell SM, et al. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology. 2012;23(3):386–392. doi: 10.1097/EDE.0b013e31824cb93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Park JS, Petreas M. Temporal changes in the levels of perfluorinated compounds in California women's serum over the past 50 years. Environ Sci Technol. 2011;45(17):7510–7516. doi: 10.1021/es2012275. [DOI] [PubMed] [Google Scholar]
- 41.Olsen GW, Lange CC, Ellefson ME, et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol. 2012;46(11):6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- 42.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt) 2013;22(1):37–47. doi: 10.1089/jwh.2012.3740. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fromme H, Mosch C, Morovitz M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44(18):7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- 45.Fei C, McLaughlin JK, Tarone RE, et al. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starling AP, Engel SM, Whitworth KW, et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int. 2014;62:104–112. doi: 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stampfer MJ, Sacks FM, Salvini S, et al. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325(6):373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 48.Enquobahrie DA, Williams MA, Butler CL, et al. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17(7):574–581. doi: 10.1016/j.amjhyper.2004.03.666. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1(1):4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 51.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]