Abstract
We demonstrate how direct, indirect, total, and overall effectiveness estimates and absolute benefits of rotavirus vaccines vary through the years following vaccine introduction. Privately insured US children in a large claims database were followed from age 8 months until they 1) experienced a hospitalization for rotavirus or acute gastroenteritis; 2) lost continuous health plan enrollment; 3) turned 20 months of age; or 4) reached the end of the study period. Vaccine effectiveness estimates in preventing rotavirus and acute gastroenteritis hospitalizations were estimated using Cox proportional hazards regression, stratified by calendar year and adjusted for birth month. Incidence rate differences were estimated to determine the absolute number of gastroenteritis hospitalizations prevented in the cohort. Among 905,718 children, 51%, 66%, 80%, and 86% received 1 or more doses of rotavirus vaccine in each year from 2007 to 2010. The direct vaccine effectiveness of 1 or more doses of rotavirus vaccine in preventing rotavirus gastroenteritis hospitalizations ranged from 87% to 92% each year. Accounting for indirect protection increased estimates of vaccine effectiveness by an additional 3%–8% among those vaccinated. Failing to account for population-level vaccine benefits in 2010, when circulation of rotavirus was low, could underestimate the sustained impact of the vaccine program.
Keywords: diarrhea; gastroenteritis; immunity, herd; pharmacoepidemiology; program effectiveness; rotavirus; rotavirus vaccines; use-effectiveness
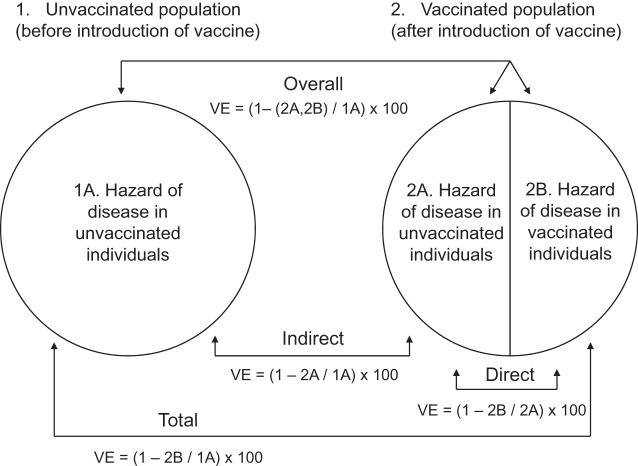
Most phase III vaccine efficacy trials determine the direct vaccine effectiveness (VE), generally measured as 1 minus the relative risk in the vaccinated group compared with the unvaccinated group. Some clinical trials and many postlicensure studies also measure herd protection, or indirect VE, defined as population-level effects of widespread vaccination on people not receiving the vaccine (1). Two additional population-level measures of VE, total and overall VE, account for both the direct and indirect effectiveness of a vaccine (Figure 1). Total VE combines the direct and indirect VE on individuals receiving the vaccine, whereas the overall VE weights the average of the total VE on individuals receiving the vaccine with the indirect VE on individuals not receiving the vaccine (1). Total VE can thus be interpreted as the complete benefit of vaccination in vaccine recipients, and overall VE can be interpreted as the public health benefit of vaccination. Despite challenges in estimating the 4 types of VE, they are essential to understanding the real-world impact of a vaccine (1–3).
Figure 1.
Types of vaccine effectiveness as described by Halloran et al. (1). A vaccinated population will still have some individuals within the population who are unvaccinated because 100% vaccination coverage is generally never achieved. VE, vaccine effectiveness.
Rotavirus was the leading cause of gastroenteritis in infants and young children, implicated in 55,000–70,000 hospitalizations in the United States prior to the availability of a rotavirus vaccine (4). Rotavirus vaccination is currently recommended by the Centers for Disease Control and Prevention (Atlanta, Georgia), and 2 vaccines are marketed in the United States (5). The pentavalent rotavirus vaccine (RV5), RotaTeq (Merck & Co., Inc., Whitehouse Station, New Jersey), administered orally in 3 doses at ages 2, 4, and 6 months, has been licensed since February 2006, and the monovalent rotavirus vaccine (RV1), Rotarix (GlaxoSmithKline Biologics, Research Triangle Park, North Carolina), administered orally in 2 doses at ages 2 months and 4 months, has been licensed since April 2008.
We compared direct, indirect, total, and overall rotavirus VE estimates for the prevention of rotavirus gastroenteritis (RGE) and acute gastroenteritis (AGE) hospitalizations from 2007 to 2010 to determine how these 4 VE estimates varied through the years after vaccine introduction. We also examined how the absolute number of gastroenteritis hospitalizations varied through the years.
METHODS
Data source
The MarketScan Research Databases (Truven Health Analytics, Inc., Ann Arbor, Michigan) contain data from more than 111 million individuals throughout the United States with commercial health insurance. In 2010, the database included approximately 920,000 infants, corresponding to approximately 25% of the US birth cohort and 50% of the US birth cohort with commercial insurance (6, 7).
Design and population
Data on infants with continuous insurance enrollment during infancy, at least 1 outpatient claim for any service or diagnosis, and an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), code for a livebirth between May 1, 2000, and April 30, 2005, or May 1, 2006, and April 30, 2010, were extracted from the databases (Table 1). If an infant or mother had a claim for a livebirth on multiple dates within a short period of time, the date of the first claim was used as the birth date. Follow-up for RGE began when infants turned 8 months of age and continued until a maximum age of 20 months. Infants younger than 8 months and infants receiving doses of rotavirus vaccine after 8 months were excluded so that rotavirus vaccine status could be treated as a single point exposure.
Table 1.
ICD-9-CM and CPT Codes Used in Analyses of US Commercially Insured Infants and Children 8–20 Months of Age (11–13)
| Description by Type of Code | Code |
|---|---|
| CPT code | |
| Rotavirus vaccine (RV5, RV1) | 90680, 90681 |
| DTaP vaccine or related vaccines, including combinations | 90696, 90698, 90700, 90701, 90702, 90714, 90715, 90718, 90720, 90721, 90723 |
| ICD-9-CM code | |
| Livebirth (singleton or multiple) | V30–V39 |
| Rotavirus gastroenteritis | 008.61 |
| Acute gastroenteritis | |
| Bacterial | 001–005 (excluding 003.2), 008.0–008.5 |
| Parasitic | 006–007 (excluding 006.3–006.6) |
| Viral | 008.6, 008.8 |
| Undetermined etiology (infectious) | 009.0–009.3 |
| Undetermined etiology (noninfectious) | 558.9 |
| Diarrhea not otherwise specified | 787.91 |
Abbreviations: CPT, Current Procedural Terminology; DTaP, diphtheria, tetanus, acellular pertussis vaccine; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
Infants with commercial insurance who failed to receive vaccines with high coverage rates (≥95%) may differ from infants receiving such vaccines with respect to unmeasured confounding factors, so we required all infants in our study to be vaccinated with at least 1 dose of diphtheria, tetanus, and acellular pertussis vaccine using Current Procedural Terminology codes (8).
Outcome, exposure, and covariate measurements
Outcomes of RGE and AGE were identified using ICD-9-CM codes. Any of the 15 diagnosis fields in the inpatient files of the databases was used to capture the ICD-9-CM code 008.61 for gastroenteritis due to rotavirus. Rotavirus-coded events underestimate the true burden of rotavirus disease because of lack of routine laboratory testing and coding; therefore, we performed sensitivity analyses, assuming 25% and 50% sensitivity of the 008.61 code (9, 10), and extracted and examined outcomes related to AGE (11–13). Emergency department and outpatient visits for RGE and AGE were not included in the analysis.
RV5 and RV1 vaccination status at 8 months of age was classified using the Current Procedural Terminology codes 90680 and 90681. To increase the sensitivity of vaccination status, we excluded infants living in states with state-funded rotavirus immunization programs (Alaska, Idaho, Massachusetts, Maine, North Dakota, New Hampshire, New Mexico, Oregon, Rhode Island, Vermont, Washington, Wisconsin, and Wyoming) (13).
To account for household-level variation in rotavirus vaccine coverage, disease, and mixing behaviors, we examined the number of other dependent children less than 10 years of age covered by the same insurance holder as the infant (considered to be older siblings). To account for geographical variation, we included the region and rurality of the child's residence, as defined by the US Department of Agriculture, Economic Research Service (14). To characterize general infant health and potential differences in susceptibility to rotavirus disease, we compared the percentage of infants who had overnight hospital stays unrelated to AGE prior to 2 months of age.
Data analysis
We used Cox proportional hazard regression models to estimate hazard ratios, comparing the hazard of RGE or AGE hospitalization among vaccinated infants to that among unvaccinated infants entering the cohort in 2007, 2008, 2009, or 2010 and subtracting the result from 1 to obtain estimates of direct VE by calendar year. We similarly estimated the indirect, total, and overall VE, varying the comparison cohorts accordingly. For indirect VE, we compared unvaccinated infants followed during each calendar year of the rotavirus vaccine period, 2007–2010, with (unvaccinated) infants followed during the baseline period, 2001–2005. For total VE, we compared vaccinated infants followed during each calendar year of the rotavirus vaccine period with (unvaccinated) infants followed during the baseline period. For overall or average VE, we compared all vaccinated and unvaccinated infants during each calendar year of the rotavirus vaccine period with (unvaccinated) infants followed during the baseline period.
In all regression analyses, age served as the underlying time scale, and infants were censored when they experienced a RGE or AGE hospitalization, lost continuous enrollment, reached 20 months of age, or reached the end of the study period on December 31, 2005, or December 31, 2010, whichever occurred first. Results were stratified by year to account for increasing vaccination coverage and possible variations in rotavirus transmission by year and adjusted for month of birth to account for the seasonality of rotavirus. Infants and children were allowed to contribute person-time during 2 calendar years. For example, an infant who turned 8 months of age on October 1, 2007, would contribute up to 3 person-months in 2007 and reenter the cohort on January 1, 2008, at age 11 months, to contribute up to 9 more calendar months of person-time in 2008. Infants followed during 2 calendar years in the baseline period were followed continuously.
We estimated incidence rate differences (IRDs) on the basis of the case count and person-years in the population and performed additional analyses assuming 25% and 50% sensitivity and 100% specificity of the RGE and AGE ICD-9-CM codes to determine the absolute number of RGE and AGE hospitalizations prevented by the rotavirus vaccine program in the cohort.
All analyses were conducted in SAS, version, 9.2, software (SAS Institute, Inc., Cary, North Carolina). This study was exempt from human subjects review by the institutional review board of the University of North Carolina at Chapel Hill (Chapel Hill, North Carolina).
RESULTS
Cohort
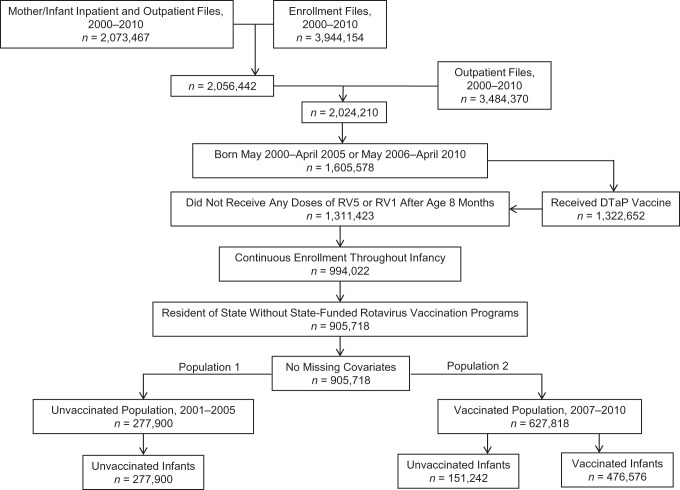
Approximately half (52%) of the 3.94 million infants identified in the enrollment files between January 2000 and December 2010 had a claim for a livebirth (Figure 2). After additional exclusions, 627,818 (78%) of the 905,718 children in the final cohort were born during the rotavirus vaccine period (476,576 were vaccinated with a rotavirus vaccine; 151,242 were unvaccinated). The other 277,900 children were born during the prevaccine baseline period. Among all 627,818 children followed during the rotavirus vaccine period, 379,262 (60%) were followed during parts of 2 calendar years.
Figure 2.
Derivation of the unvaccinated population (population 1) and the vaccinated population (population 2) in the rotavirus vaccine effectiveness cohort study of US commercially insured infants and children 8–20 months of age, 2001–2005 and 2007–2010. Births were identified using International Classification of Diseases, Ninth Revision, Clinical Modification, codes in inpatient and outpatient records. These records were restricted to infants 0 years of age and to females 10–50 years of age. Twenty-three of 905,718 infants were excluded from this cohort in the final rotavirus gastroenteritis analysis because their cohort entry date (8-month birthday) equaled their cohort exit date (rotavirus gastroenteritis hospitalization date (n = 4) or loss of health plan enrollment date (n = 19)); for the same reasons, 40 infants were excluded from the final acute gastroenteritis analysis. DTaP, diphtheria, tetanus, and acellular pertussis vaccine; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
Characteristics of the cohort
Almost 76% of the children born during the rotavirus vaccine period received at least 1 dose of RV5 or RV1, of which 79% completed the series. Vaccination rates varied by calendar year, ranging from 51% in 2007 to 86% in 2010. Most vaccinated children (91%) received RV5, and more than 3% received doses of RV5 and RV1. The vaccinated and unvaccinated children were generally comparable (Table 2); however, children residing in the Western United States were better represented during the baseline years than in the vaccine years (19% vs. 11%).
Table 2.
Characteristics of 905,718 Commercially Insured US Infants and Children Vaccinated or Unvaccinated With RV5 or RV1, 2001–2005 and 2007–2010
| Variable | Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2001–2005 | 2007a |
2008a |
2009a |
2010a |
|||||
| Prevaccine Period, % (n = 277,900) | Vaccinated, % (n = 68,380) | Not Vaccinated, % (n = 64,932) | Vaccinated, % (n = 175,768) | Not Vaccinated, % (n = 90,883) | Vaccinated, % (n = 249,840) | Not Vaccinated, % (n = 61,136) | Vaccinated, % (n = 254,249) | Not Vaccinated, % (n = 41,892) | |
| Male | 51.6 | 50.9 | 51.4 | 50.9 | 51.4 | 51.1 | 51.6 | 51.3 | 52.0 |
| Hospitalized overnight for non-AGE (before 2 months of age) | 3.9 | 4.0 | 4.0 | 3.9 | 4.0 | 3.6 | 4.0 | 3.5 | 4.0 |
| No. of siblings | 0.9 (1.0)b | 0.8 (1.0)b | 0.9 (1.0)b | 0.8 (1.0)b | 0.9 (1.0)b | 0.8 (1.0)b | 0.9 (1.1)b | 0.8 (1.0)b | 0.9 (1.1)b |
| US region of residence | |||||||||
| Northeast | 10.4 | 6.9 | 9.4 | 8.4 | 10.9 | 11.4 | 16.7 | 13.9 | 22.0 |
| North central | 24.4 | 31.1 | 34.6 | 30.0 | 34.4 | 28.4 | 31.1 | 27.9 | 26.8 |
| South | 46.0 | 53.5 | 43.9 | 52.3 | 41.9 | 50.3 | 39.3 | 46.2 | 37.2 |
| West | 19.1 | 8.5 | 12.1 | 9.3 | 12.8 | 10.0 | 13.0 | 12.0 | 14.1 |
| Population density of residence | |||||||||
| Metro with ≥1 million population | 57.7 | 56.5 | 58.7 | 58.1 | 57.3 | 60.3 | 58.0 | 61.4 | 59.8 |
| Metro with 250,000–1 million population | 19.6 | 19.3 | 16.6 | 19.1 | 16.8 | 18.2 | 15.8 | 18.6 | 16.2 |
| Metro with <250,000 population | 9.9 | 11.8 | 9.6 | 10.5 | 9.8 | 9.9 | 9.5 | 9.4 | 9.0 |
| Urban with ≥20,000 population, adjacent to metro area | 3.8 | 3.8 | 4.1 | 3.6 | 4.1 | 3.4 | 4.2 | 3.3 | 4.0 |
| Urban with ≥20,000 population, not adjacent to metro area | 1.9 | 1.8 | 2.5 | 1.8 | 2.6 | 1.7 | 2.4 | 1.5 | 2.1 |
| Urban with 2,500–19,999 population, adjacent to metro area | 4.3 | 4.0 | 4.9 | 4.1 | 5.3 | 3.9 | 5.4 | 3.6 | 4.9 |
| Urban with 2,500–19,999 population, not adjacent to metro area | 1.8 | 1.8 | 2.6 | 1.9 | 3.0 | 1.8 | 3.5 | 1.6 | 2.9 |
| Rural with <2,500 population, adjacent to metro area | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.5 | 0.4 |
| Rural with <2,500 population, not adjacent to metro area | 0.5 | 0.4 | 0.5 | 0.5 | 0.6 | 0.4 | 0.7 | 0.4 | 0.6 |
Abbreviations: AGE, acute gastroenteritis; metro, metropolitan; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
a A total of 379,262 (60%) infants and children were counted during 2 consecutive calendar years during the vaccine period, 2007–2010.
b Value is mean (standard deviation).
RGE hospitalizations
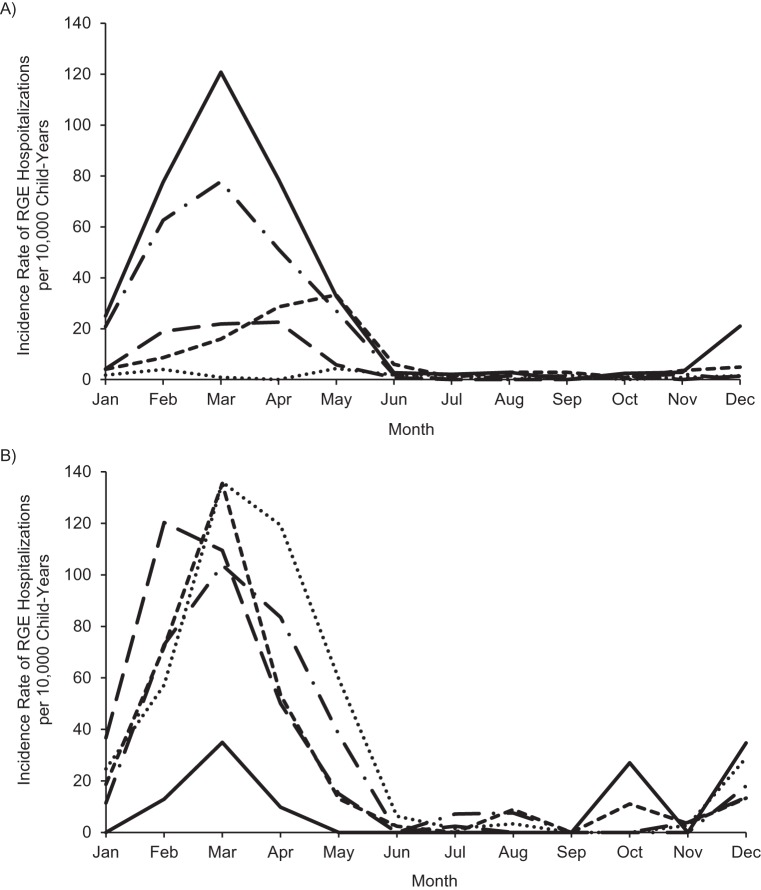
The number of infants and children in the cohort hospitalized with RGE during follow-up was 1,016 (0.11%). The percentage of infants and children hospitalized for RGE decreased during each calendar period as follows: for 2001–2005, 722/277,899 (0.26%); for 2007, 63/133,309 (0.05%); for 2008, 114/266,941 (0.04%); for 2009, 96/311,253 (0.03%); and for 2010, 21/296,323 (0.01%). The incidence rate of RGE hospitalization in March, the traditional peak of rotavirus activity, ranged from 121 per 10,000 child-years (95% confidence interval (CI): 106, 137) during the prevaccine period to 1 per 10,000 child-years (95% CI: 0, 5) in 2010 (Figure 3).
Figure 3.
A) Incidence of rotavirus gastroenteritis (RGE) hospitalizations per 10,000 child-years among commercially insured US infants and children 8–20 months of age, individual years following vaccine introduction (2007–2010) versus prevaccine years combined (2001–2005). Solid line, 2001–2005; dashed and dotted line, 2007; small dashed line, 2008; large dashed line, 2009; dotted line, 2010. B) Incidence of RGE hospitalizations per 10,000 child-years among commercially insured US infants and children 8–20 months of age, individual prevaccine years (2001–2005). Solid line, 2001; dashed and dotted line, 2002; small dashed line, 2003; large dashed line, 2004; dotted line, 2005.
AGE hospitalizations
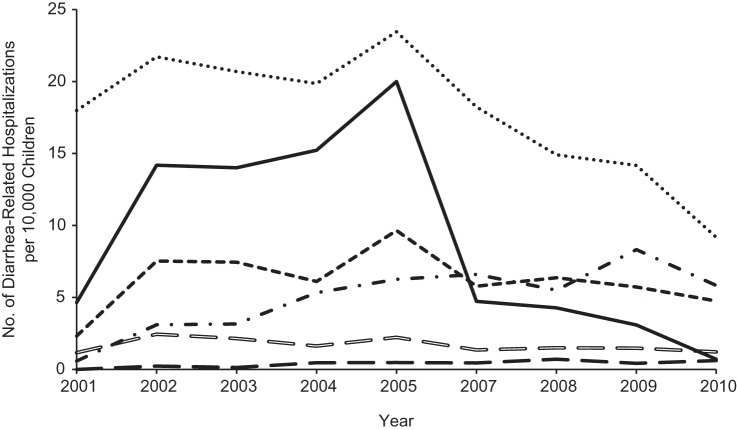
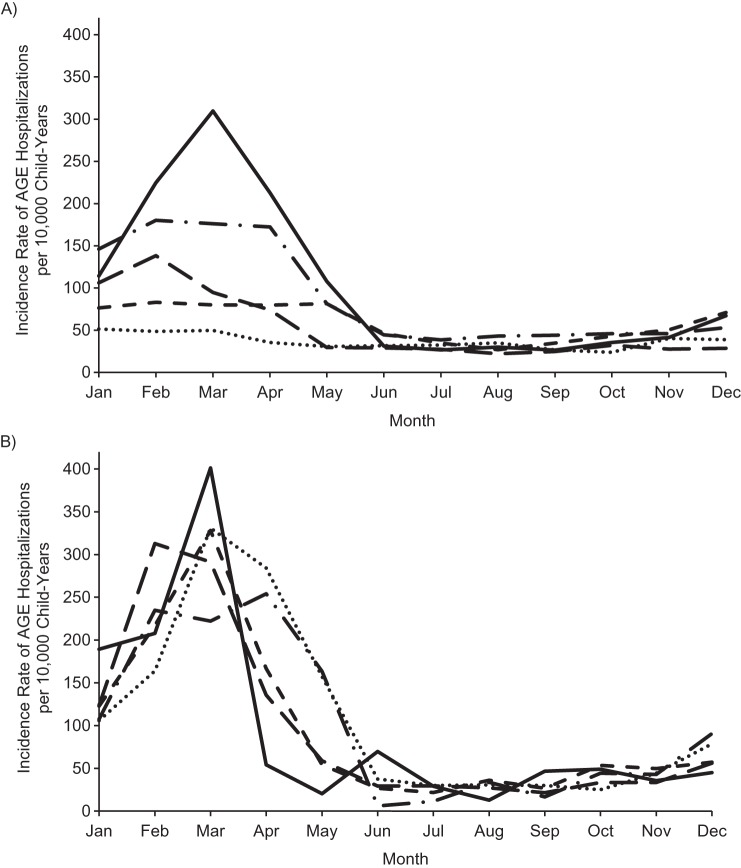
Compared with the prevaccine period, the proportions of children hospitalized annually for gastroenteritis during the rotavirus vaccine period were generally consistent for viral (excluding rotavirus), bacterial, and presumed infectious gastroenteritis, as well as for diarrhea from other causes, but decreased for rotavirus (as expected) and noninfectious diarrhea (Figure 4). Overall, the number of infants and children in the cohort with an AGE diagnosis was 4,483 (0.49%). The percentage of infants and children hospitalized for AGE during the prevaccine years was 0.73% (2,021/277,893). This percentage declined to 0.31% (413/133,306) in 2007 and continued to decline steadily during the vaccine years, reaching 0.17% (507/296,120) in 2010. Overall, nearly 1 quarter of the AGE diagnoses were coded as RGE. However, the proportion of children with AGE diagnostic codes that corresponded to RGE generally decreased with each successive calendar period (for 2001–2005, 36% (722/2,021); for 2007, 15% (63/413); for 2008, 16% (114/730); for 2009, 12% (96/812); and for 2010, 4% (21/507)). Despite the decline in the proportion of AGE diagnoses coded as RGE through the years, the monthly incidence rate of AGE by year followed a similar pattern as the monthly incidence rate of RGE by year (Figure 5).
Figure 4.
Diarrhea-related hospitalizations per 10,000 children among commercially insured US infants, 8–20 months of age, in the pre–rotavirus vaccine period (2001–2005) and rotavirus vaccine period (2007–2010). Solid black line, rotavirus (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), code 008.61); small black dashed line, viral excluding rotavirus (ICD-9-CM codes 008.6 and 008.8, excluding 008.61); large black dashed line, bacterial (ICD-9-CM codes 001–005 and 008.0–008.5, excluding 003.2); hollow dashed line, presumed infectious (ICD-9-CM codes 009.0–009.3); dotted line, presumed noninfectious (ICD-9-CM code 558.9); dashed and dotted line, diarrhea otherwise specified (ICD-9-CM code, 787.91).
Figure 5.
A) Incidence of acute gastroenteritis (AGE) hospitalizations per 10,000 child-years among commercially insured US infants and children, 8–20 months of age, individual years following vaccine introduction (2007–2010) versus prevaccine years combined (2001–2005). Solid line, 2001–2005; dashed and dotted line, 2007; small dashed line, 2008; large dashed line, 2009; dotted line, 2010. B) Incidence of AGE hospitalizations per 10,000 child-years among commercially insured US infants and children, 8–20 months of age, individual prevaccine years (2001–2005). Solid line, 2001; dashed and dotted line, 2002; small dashed line, 2003; large dashed line, 2004; dotted line, 2005.
Rotavirus VE
Direct VE of 1 or more doses of RV5 or RV1 in preventing RGE hospitalizations between ages 8–20 months ranged from 87% (95% CI: 58%, 92%) in 2007 and 87% (95% CI: 80%, 92%) in 2008 to 92% (95% CI: 87%, 95%) in 2009 (Table 3). The indirect VE varied more widely, from 14% (95% CI: −14%, 36%) in 2007 to 82% (95% CI: 70%, 90%) in 2010. Accounting for both direct and indirect VE among the rotavirus-vaccinated infants yielded a total VE estimate that increased from 91% (95% CI: 73%, 97%) in 2007 to 98% (95% CI: 96%, 99%) in 2010. The overall VE ranged from 40% (95% CI: 20%, 54%) in 2007 to 96% (95% CI: 93%, 97%) in 2010. The overall VE estimate was low in 2007 compared with that in 2008–2010, but the direct and total VE estimates were high (≥87%) across all 4 calendar years. The rotavirus VE estimates were substantially lower in the prevention of AGE hospitalization, but generally followed a similar pattern to the VE estimates in the prevention of RGE hospitalization (Table 4). Exceptions included the direct VE estimates, which increased through 2009 and then decreased in 2010, and the total VE estimates, which increased 4-fold from 2007 to 2008.
Table 3.
Rotavirus Vaccine Effectiveness Estimates Against Rotavirus Gastroenteritis Hospitalizationa in US Commercially Insured Infants and Children 8–20 Months of Age, 2007–2010
| Calendar Year by Effectiveness | Vaccinated With ≥1 Dose of RV5 or RV1, % | Vaccinated |
Unvaccinated |
Total (Vaccinated and Unvaccinated) |
Vaccine Effectivenessb | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | Total No. of Children | No. of Events | Total No. of Children | No. of Events | Total No. of Children | ||||
| Direct effectiveness | |||||||||
| 2007 | 51.3 | 3 | 68,380 | 60 | 64,929 | 87 | 58, 96 | ||
| 2008 | 65.9 | 23 | 175,890 | 91 | 91,051 | 87 | 80, 92 | ||
| 2009 | 80.3 | 22 | 250,035 | 74 | 61,218 | 92 | 87, 95 | ||
| 2010 | 85.9 | 8 | 254,377 | 13 | 41,946 | 90 | 75, 96 | ||
| Indirect effectiveness | |||||||||
| 2007 | 51.3 | 60 | 64,929 | 14 | −14, 36 | ||||
| 2008 | 65.9 | 91 | 91,051 | 44 | 30, 55 | ||||
| 2009 | 80.3 | 74 | 61,218 | 40 | 24, 53 | ||||
| 2010 | 85.9 | 13 | 41,946 | 82 | 70, 90 | ||||
| Total effectiveness | |||||||||
| 2007 | 51.3 | 3 | 68,380 | 91 | 73, 97 | ||||
| 2008 | 65.9 | 23 | 175,890 | 92 | 88, 95 | ||||
| 2009 | 80.3 | 22 | 250,035 | 95 | 92, 97 | ||||
| 2010 | 85.9 | 8 | 254,377 | 98 | 96, 99 | ||||
| Overall effectiveness | |||||||||
| 2007 | 51.3 | 63 | 133,309 | 40 | 20, 54 | ||||
| 2008 | 65.9 | 114 | 266,941 | 75 | 69, 79 | ||||
| 2009 | 80.3 | 96 | 311,253 | 83 | 79, 86 | ||||
| 2010 | 85.9 | 21 | 296,323 | 96 | 93, 97 | ||||
Abbreviations: CI, confidence interval; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
a During the prevaccine period (2001–2005), there were 722 events among 277,899 children.
b Adjusted for birth month and age using the formula, (1 – hazard ratio) × 100.
Table 4.
Rotavirus Vaccine Effectiveness Estimates Against Acute Gastroenteritis Hospitalizationa in US Commercially Insured Infants and Children 8–20 Months of Age, 2007–2010
| Calendar Year by Effectiveness | Vaccinated With ≥1 Dose of RV5 or RV1, % | Vaccinated |
Unvaccinated |
Total (Vaccinated and Unvaccinated) |
Vaccine Effectivenessb | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | Total No. of Children | No. of Events | Total No. of Children | No. of Events | Total No. of Children | ||||
| Direct effectiveness | |||||||||
| 2007 | 51.3 | 142 | 68,378 | 271 | 64,928 | 22 | 3, 37 | ||
| 2008 | 65.9 | 413 | 175,765 | 317 | 90,882 | 40 | 30, 48 | ||
| 2009 | 80.3 | 512 | 249,838 | 300 | 61,136 | 56 | 49, 62 | ||
| 2010 | 85.9 | 398 | 254,232 | 109 | 41,888 | 41 | 27, 53 | ||
| Indirect effectiveness | |||||||||
| 2007 | 51.3 | 271 | 64,928 | −8 | −24, 6 | ||||
| 2008 | 65.9 | 317 | 90,882 | 24 | 15, 33 | ||||
| 2009 | 80.3 | 300 | 61,136 | 9 | −3, 19 | ||||
| 2010 | 85.9 | 109 | 41,888 | 45 | 33, 54 | ||||
| Total effectiveness | |||||||||
| 2007 | 51.3 | 142 | 68,378 | 12 | −5, 27 | ||||
| 2008 | 65.9 | 413 | 175,765 | 48 | 43, 53 | ||||
| 2009 | 80.3 | 512 | 249,838 | 59 | 54, 62 | ||||
| 2010 | 85.9 | 398 | 254,232 | 65 | 62, 69 | ||||
| Overall effectiveness | |||||||||
| 2007 | 51.3 | 413 | 133,306 | 0 | −13, 11 | ||||
| 2008 | 65.9 | 730 | 266,647 | 40 | 35, 45 | ||||
| 2009 | 80.3 | 812 | 310,974 | 48 | 44, 52 | ||||
| 2010 | 85.9 | 507 | 296,120 | 62 | 58, 66 | ||||
Abbreviations: CI, confidence interval; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
a During the prevaccine period (2001–2005), there were 2,021 events among 277,893 children.
b Adjusted for birth month and age using the formula, (1 – hazard ratio) × 100.
Absolute benefits of rotavirus vaccination
Under the assumption of perfect sensitivity and specificity of the RGE ICD-9-CM code, 31–33 RGE hospitalizations per 10,000 child-years were prevented in vaccinated children, and 10–26 RGE hospitalizations per 10,000 child-years were prevented in unvaccinated children in the cohort during each calendar year from 2007–2010 (among vaccinated children, 6–21 hospitalizations were prevented by direct effects, and 10–26 hospitalizations were prevented by indirect effects) (Table 5). Considering the total effects in the vaccinated and indirect effects in the unvaccinated, to prevent 1 RGE hospitalization in our cohort, 315 (31.8/10,000)−1 to 421 (23.8/10,000)−1 children required a rotavirus vaccination. Assuming a more realistic scenario of 50% and 25% sensitivity of the RGE ICD-9-CM code, only 80 (31.8 × 4/10,000)−1 to 210 (23.8 × 2/10,000)−1 children may have required a rotavirus vaccination to prevent 1 RGE hospitalization. Compared with estimates relying on only RGE diagnostic codes, those using AGE diagnostic codes to estimate the number of RGE hospitalizations prevented in our cohort increased the number by 130%–180% among rotavirus-vaccinated children each calendar year (Table 6).
Table 5.
Absolute Reduction per 10,000 Child-Years of Rotavirus Gastroenteritis Hospitalizations Prevented by the Rotavirus Vaccination Program in Commercially Insured US Infants and Children 8–20 Months of Age, 2007–2010a
| Calendar Year by Effectiveness | Observed No. of RGE Hospitalizations in Minuend | No. of Child-Years in Minuend | Rate Per 10,000 Child-Years (IR1) | Observed No. of RGE Hospitalizations in Subtrahend | No. of Child-Years in Subtrahend | Rate Per 10,000 Child-Years (IR0) | Observed IRD Per 10,000 Child-Yearsb | 95% CI for IRD |
|---|---|---|---|---|---|---|---|---|
| Direct effectiveness: vaccinated versus unvaccinated in same year | ||||||||
| 2007 | 3 | 28,250 | 1.1 | 60 | 37,791 | 15.9 | −14.8 | −19.0, −10.6 |
| 2008 | 23 | 85,452 | 2.7 | 91 | 39,447 | 23.1 | −20.4 | −25.2, −15.5 |
| 2009 | 22 | 131,381 | 1.7 | 74 | 32,292 | 22.9 | −21.2 | −26.5, −16.0 |
| 2010 | 8 | 118,708 | 0.7 | 13 | 18,981 | 6.8 | −6.2 | −9.9, −2.4 |
| Indirect effectiveness: unvaccinated versus prevaccine periodc | ||||||||
| 2007 | 60 | 37,791 | 15.9 | 722 | 216,767 | 33.3 | −17.4 | −22.1, −12.7 |
| 2008 | 91 | 39,447 | 23.1 | 722 | 216,767 | 33.3 | −10.2 | −15.6, −4.9 |
| 2009 | 74 | 32,292 | 22.9 | 722 | 216,767 | 33.3 | −10.4 | −16.2, −4.6 |
| 2010 | 13 | 18,981 | 6.8 | 722 | 216,767 | 33.3 | −26.5 | −30.9, −22.0 |
| Total effectiveness: vaccinated versus prevaccine periodc | ||||||||
| 2007 | 3 | 28,250 | 1.1 | 722 | 216,767 | 33.3 | −32.3 | −35.0, −29.5 |
| 2008 | 23 | 85,452 | 2.7 | 722 | 216,767 | 33.3 | −30.6 | −33.3, −28.0 |
| 2009 | 22 | 131,381 | 1.7 | 722 | 216,767 | 33.3 | −31.6 | −34.2, −29.1 |
| 2010 | 8 | 118,708 | 0.7 | 722 | 216,767 | 33.3 | −32.6 | −35.1, −30.2 |
| Overall effectiveness: total in vaccine year versus prevaccine periodc | ||||||||
| 2007 | 63 | 66,041 | 9.5 | 722 | 216,767 | 33.3 | −23.8 | −27.2, −20.4 |
| 2008 | 114 | 124,899 | 9.1 | 722 | 216,767 | 33.3 | −24.2 | −27.1, −21.2 |
| 2009 | 96 | 163,673 | 5.9 | 722 | 216,767 | 33.3 | −27.4 | −30.1, −24.7 |
| 2010 | 21 | 137,689 | 1.5 | 722 | 216,767 | 33.3 | −31.8 | −34.3, −29.3 |
Abbreviations: CI, confidence interval; IRD, incidence rate difference.
a Assumes 100% sensitivity and specificity of the rotavirus gastroenteritis diagnostic code.
b Calculated using the formula IR1 − IR0.
c Prevaccine period, 2001–2005.
Table 6.
Absolute Reduction per 10,000 Child-Years of Acute Gastroenteritis Hospitalizations Prevented by the Rotavirus Vaccination Program in Commercially Insured US Infants and Children, 2007–2010a
| Calendar Year by Effectiveness | Observed No. of Acute Gastroenteritis Hospitalizations in Minuend | No. of Child-Years in Minuend | Rate Per 10,000 Child-Years (IR1) | Observed No. of AGE Hospitalizations in Subtrahend | No. of Child-Years in Subtrahend | Rate Per 10,000 Child-Years (IR0) | Observed IRD Per 10,000 Child-Yearsb | 95% CI for IRD |
|---|---|---|---|---|---|---|---|---|
| Direct effectiveness: vaccinated versus unvaccinated in same year | ||||||||
| 2007 | 142 | 28,209 | 50.3 | 271 | 37,696 | 71.9 | −21.6 | −33.5, −9.6 |
| 2008 | 413 | 85,259 | 48.4 | 317 | 39,319 | 80.6 | −32.2 | −42.2, −22.2 |
| 2009 | 512 | 131,096 | 39.1 | 300 | 32,165 | 93.3 | −54.2 | −65.3, −43.1 |
| 2010 | 398 | 118,520 | 33.6 | 109 | 18,932 | 57.6 | −24.0 | −35.3, −12.7 |
| Indirect effectiveness: unvaccinated versus prevaccine periodc | ||||||||
| 2007 | 271 | 37,696 | 71.9 | 2,021 | 216,117 | 93.5 | −21.6 | −31.1, −12.1 |
| 2008 | 317 | 39,319 | 80.6 | 2,021 | 216,117 | 93.5 | −12.9 | −22.7, −3.1 |
| 2009 | 300 | 32,165 | 93.3 | 2,021 | 216,117 | 93.5 | −0.3 | −11.6, 11.1 |
| 2010 | 109 | 18,932 | 57.6 | 2,021 | 216,117 | 93.5 | −35.9 | −47.5, −24.4 |
| Total effectiveness: vaccinated versus prevaccine periodc | ||||||||
| 2007 | 142 | 28,209 | 50.3 | 2,021 | 216,117 | 93.5 | −43.2 | −52.4, −34.0 |
| 2008 | 413 | 85,259 | 48.4 | 2,021 | 216,117 | 93.5 | −45.1 | −51.3, −38.9 |
| 2009 | 512 | 131,096 | 39.1 | 2,021 | 216,117 | 93.5 | −54.5 | −59.8, −49.2 |
| 2010 | 398 | 118,520 | 33.6 | 2,021 | 216,117 | 93.5 | −59.9 | −65.2, −54.7 |
| Overall effectiveness: total in vaccine year versus prevaccine periodc | ||||||||
| 2007 | 413 | 65,905 | 62.7 | 2,021 | 216,117 | 93.5 | −30.9 | −38.1, −23.6 |
| 2008 | 730 | 124,578 | 58.6 | 2,021 | 216,117 | 93.5 | −34.9 | −40.8, −29.0 |
| 2009 | 812 | 163,261 | 49.7 | 2,021 | 216,117 | 93.5 | −43.8 | −49.1, −38.5 |
| 2010 | 507 | 137,452 | 37.0 | 2,021 | 216,117 | 93.5 | −56.6 | −61.8, −51.4 |
Abbreviations: AGE, acute gastroenteritis; CI, confidence interval; IRD, incidence rate difference.
a Assumes 100% sensitivity and specificity of the AGE diagnostic codes.
b Calculated using the formula, IR1 − IR0.
c Prevaccine period, 2001–2005.
DISCUSSION
RV5 or RV1 was highly effective in preventing RGE hospitalizations in this population of commercially insured US infants and children aged 8–20 months. Direct VE was high across each calendar year, and indirect protection slightly increased the VE among rotavirus-vaccinated children. By comparison, in another study that examined RGE health care utilization in children under 5 years of age from January to June 2008 and January to June 2009 using the MarketScan Research Databases, high direct VE of 89% (95% CI: 79%, 94%) and 89% (95% CI: 84%, 93%) was also demonstrated (13). In clinical trials, a complete series (3 doses) of RV5 was 98% (95% CI: 88%, 100%) efficacious against severe RGE for the first full rotavirus season after vaccination among infants primarily in the United States and Finland, and a complete series (2 doses) of RV1 was 85% (95% CI: 70%, 94%) efficacious against hospitalizations for severe RGE from 2 weeks after the second dose until 1 year of age among infants in Latin America (15, 16). Our direct VE estimates were similar to those calculated in the aforementioned clinical trials, despite the fact that 21% of the infants in our postmarketing study did not complete a rotavirus vaccine series. In our view, this observation has 2 possible explanations.
First, partial completion of a rotavirus vaccine series may still result in high direct VE. This observation has been supported by other postmarketing studies, including an active, prospective, population-based case-control study of laboratory-confirmed RGE hospitalizations and emergency department visits in 3 US counties from January to June 2006 to January to June 2009. With rotavirus-negative AGE controls, the direct VEs of RV5 for 1-, 2-, and 3-dose rotavirus vaccine regimens were 74% (95% CI: 37%, 90%), 88% (95% CI: 66%, 96%), and 87% (95% CI: 71%, 94%) in children under 4 years of age (17). Another study that used a database from a large US health insurer to estimate 1- and 2-dose direct VE estimates in preventing RGE hospitalizations and emergency department visits for RV5 during the 2007 and 2008 rotavirus seasons found similarly high VE (for 1 dose, VE = 88%, 95% CI: 45%, 99%; for 2 doses, VE = 94%, 95% CI: 61%, 100%) (18).
An alternative explanation may be that our direct VE estimates are biased upward. A mathematical model showed that when a vaccine provides indirect protection, and the percent vaccinated in subpopulations is not equal (the likely scenario for most postmarketing studies), then direct VE estimates may be biased upward from clinical trial efficacy estimates because the vaccinated subpopulation will receive more indirect protection than the unvaccinated subpopulation (19). Thus, our direct VE estimates may in fact have included measures of indirect protection.
We expected indirect or “herd” protection against RGE hospitalizations to increase with each successive calendar year from 2007 to 2010, but this was not the case. The calendar year 2009 had a slightly lower indirect VE estimate than 2008 (44% vs. 40%). Although a lack of difference between these estimates cannot be ruled out given the overlapping confidence intervals, smaller or total absences of indirect protection in 1-year-olds in 2009 has been observed in other studies (11, 13, 20). Although some of these studies used external rates to estimate rotavirus vaccine coverage, and thus do not quantify measures of indirect VE, they have hypothesized that the low levels of rotavirus activity during the 2008 season allowed unvaccinated children to pass through the season without exposure to wild-type virus until 2009 (11, 20). However, because rotavirus activity in the United States was also curtailed in 2009, and the indirect VE estimate more than doubled to 82% in 2010 in our study, additional years of follow-up using the MarketScan Research Databases and other data sources may be needed to better establish time trends related to the indirect effectiveness of rotavirus vaccination in the United States. Rapid declines of rotavirus vaccine activity during the study period complicate the interpretation of the impact of indirect VE.
Compared with 2008 and 2009, in 2010, the more than 3-fold decrease in the direct IRD (despite high direct VE) and nearly 3-fold increase in the indirect IRD support our finding that rotavirus circulation was very limited in 2010 (Table 5, Figure 3). In such scenarios, reporting IRDs that incorporate impact at the population level (i.e., indirect, total, and overall) may be important if the objective is to measure the public health benefit rather than the clinical benefit. For instance, assuming perfect sensitivity and specificity, if the direct IRD in 2010 had been used rather than the overall IRD to calculate the number of children requiring a vaccine to prevent 1 RGE hospitalization, 1,613 children ((6.2/10,000)−1) or approximately 4–5 times as many would need to be vaccinated. From a clinical point of view, this estimate may be reasonable (i.e., physicians may be required to vaccinate a larger number of infants in 2010 compared with earlier years to prevent 1 RGE hospitalization); however, such use of the direct IRD in 2010 makes the rotavirus vaccine appear to be a less effective public health intervention when, in reality, the decreased circulation of rotavirus, which helped to substantially reduce the rate of hospitalizations in unvaccinated children, should be attributed to the vaccine, and in our view, accounted for in estimates when the public health perspective is of primary interest.
Our study has important strengths. First, across both time and vaccination status, the infants and children in the 5 calendar periods we examined were generally well balanced on selected covariates, which included proxies for health, potential sources of rotavirus infection, and population-level rotavirus vaccination coverage and mixing patterns. Because all cohort members were commercially insured and required to have at least 1 outpatient claim and at least 1 dose of diphtheria, tetanus, and acellular pertussis vaccine during infancy, such inclusion criteria may have led to the relatively good balance between the groups with regard to the measured, and hopefully, unmeasured, potential confounders. Second, because we used Cox proportional hazards regression, our analyses inherently adjusted for age. We also stratified by year to account for increasing vaccination coverage, and we adjusted for month of birth to account for the changing seasonality of rotavirus over the study period. Further adjustment using the covariates we described in Table 1 did not appreciably change our VE estimates (data not shown). This lack of change was not surprising because the groups seemed well balanced. Third, baseline rates (in 2001–2005) of RGE hospitalizations used to estimate indirect, total, and overall VE and corresponding IRDs in our study were the same as baseline rates from 2002 to 2006 in 1-year-olds in another study using MarketScan Research Databases (33 per 10,000 child-years) (13) and were similar to baseline rates from 2000 to 2006 in those aged 12–17 months in a State Inpatient Database study (32 per 10,000 child-years, 95% CI: 25, 40) (11). This finding was reassuring because 3 of the 4 VE and IRD measures relied on baseline estimates. Finally, our study provided some evidence that the decline of RGE hospitalizations from 2007 to 2010 was due to the rotavirus vaccines and not another extraneous cause. Figure 4 illustrates a sharp decline in the proportion of children hospitalized for RGE during the rotavirus vaccine period; however, proportions of children hospitalized from other infectious causes of gastroenteritis, including other viral and bacterial pathogens, remained consistent with pre–rotavirus vaccine years. This would be expected in the absence of time trends and when the rotavirus vaccine ICD-9-CM code is specific to RGE. Interestingly, the proportion of hospitalizations due to presumed noninfectious diarrhea declined steadily during the rotavirus vaccine period, for which reasons are currently unknown.
Our results should be interpreted with caution because of some limitations. First, the ICD-9-CM code for RGE likely had low sensitivity, and the sensitivity analyses made assumptions that may not have been entirely realistic, including that the sensitivity did not vary over time or between vaccinated and unvaccinated children, that estimates of 25% and 50% sensitivity were reasonable, and that the specificity of the RGE ICD-9-CM code was 100% (9, 10, 21). ICD-9-CM diagnostic codes for AGE were also subject to low sensitivity; a recent study conducted at 3 US children's hospitals found that only 52% of children hospitalized with AGE received a qualifying diagnostic code at discharge (22). Fortunately, low sensitivity of RGE or AGE ICD-9-CM codes would not bias VE estimates assuming 100% specificity. We can assume specificity close to 100% on the basis of a study conducted at a large US children's hospital that found the rotavirus ICD-9-CM code to be 97% specific (10), and another study showing RGE and AGE hospitalization patterns similar to those in our study (11).
Second, we limited follow-up of infants and children to 1 year (8–20 months of age) to minimize bias, because children enrolled for longer periods may differ with respect to unmeasured confounding factors from those enrolled for shorter periods of time. This restriction may have helped increase the generalizability of the results for those aged 8–20 months while decreasing the generalizability to other age groups. One modeling study and 1 study using a convenience sample of laboratories suggested that the US rotavirus vaccine program may have increased the mean age at which infants and children are first infected with rotavirus, and thus are potentially hospitalized with RGE (23, 24). Despite this potential shift, our study would still appropriately document rotavirus VE among those aged 8–20 months, and because RGE hospitalizations are generally most serious in very young children (e.g., <2 years), our study would still have captured many of the most clinically significant cases.
Third, our study considered infants receiving any number of doses of rotavirus vaccine as “vaccinated” and did not compare the direct VE of RV5 with that of RV1 because of the limited number of infants vaccinated with RV1. A few comparative effectiveness studies, as well as studies assessing partial rotavirus vaccine effectiveness, have been published, and ongoing monitoring should continue to assess these questions (17, 18, 25–28).
Fourth, our study could not confirm whether more children tested negative for rotavirus or were less frequently tested over time because we did not have access to laboratory results. Based on the US National Respiratory and Enteric Virus Surveillance System data, rotavirus testing may have decreased from July 2009 to June 2010 (29). In a study using these data, the numbers of antigen detection tests performed in 25 consistently reporting laboratories from the month of July to June of the following year during the periods 2000–2006, 2007–2008, and 2008–2009 were similar, but from July 2009 to June 2010, the number of tests declined by approximately 1 quarter to 9,909; however, the proportion of tests that were positive for rotavirus also declined by approximately half, from 9.0% and 10.7% in 2007–2008 and 2008–2009, respectively, to 4.6% in 2009–2010 (29). On the basis of these data, we assumed that, although testing may have decreased during the last rotavirus season in our study (in 2010), testing decreased because fewer children presented as inpatients with potential RGE. Thus, we do not think that changes in testing policies biased our results.
Finally, our study may have limited generalizability because it involved only US infants and children with commercial insurance and did not include those with Medicaid insurance (35% of the US population 0–18 years of age in 2012) or the uninsured population (9% of the US population 0–18 years of age in 2012) (7). Although data are limited, enrollment in Medicaid during childhood appears to have been steady over the last decade, alleviating some concern that fluctuations over time could further limit the generalizability and comparability of our cohorts (7, 30). Although direct VE is less likely to show relevant heterogeneity across US populations, all measures involving indirect VE would tend to be population specific.
If a vaccine has high direct VE, such measurements may only slightly underestimate the total VE because VE cannot exceed 100%. However, if low circulation of a pathogen is attributed to a vaccine program, failing to consider population-level VE measures on the absolute scale (e.g., indirect, total, and overall IRD) may substantially underestimate the sustained impact of the program on important public health outcomes.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Catherine A. Panozzo, Virginia Pate, David J. Weber, Michele Jonsson Funk, Til Stürmer, M. Alan Brookhart); Department of Family Medicine, School of Medicine, University of North Carolina, Chapel Hill, North Carolina (Sylvia Becker-Dreps); Division of Infectious Diseases, School of Medicine, University of North Carolina, Chapel Hill, North Carolina (David J. Weber); and Global Pharmacovigilance, Sanofi Pasteur, Swiftwater, Pennsylvania (Catherine A. Panozzo).
This project was supported by internal funds at the University of North Carolina, Chapel Hill, and by the National Institutes of Health (grant 1R21HD080214-01A1). C.A.P. received support from the University of North Carolina Center of Excellence in Pharmacoepidemiology and Public Health. S.B-D. is supported by the Fogarty International Center at the National Institutes of Health (grant 4K01TW008401-03). M.J.F. is supported by the Agency for Healthcare Research and Quality (grant K02HS017950). T.S. receives investigator-initiated research funding and support as principal investigator from the National Institute on Aging at the National Institutes of Health (grant R01 AG023178). T.S. also receives research funding as principal investigator of the University of North Carolina-DEcIDE Center from the Agency for Healthcare Research and Quality. M.A.B. receives investigator-initiated research funding from the National Institutes of Health (grants R01 AG042845, R21 HD080214, R01 AG023178) and through contracts with the Agency for Healthcare Research and Quality's DEcIDE program and the Patient Centered Outcomes Research Institute.
This work was presented at the 29th International Conference on Pharmacoepidemiology and Therapeutic Risk Management in Montreal, Canada, on August 27, 2013.
Conflict of interest: C.A.P. started working at Sanofi Pasteur while finishing this manuscript. S.B.-D. received investigator-initiated research grant funding from Merck. T.S. does not accept personal compensation of any kind from any pharmaceutical company, though he and V.P. receive salary support from the Center for Pharmacoepidemiology and from unrestricted research grants from pharmaceutical companies (GlaxoSmithKline, Merck, Sanofi) to the Department of Epidemiology, University of North Carolina at Chapel Hill. D.J.W. consults and is on the speaker's bureaus for Merck and Pfizer. M.A.B. has received research support Amgen and has sat on advisory boards for Amgen, Merck, and Pfizer (honoraria received by institution). He has received consulting fees from RxAnte, Inc., and World Health Information Science Consultants, LLC.
REFERENCES
- 1.Halloran ME, Struchiner CJ, Longini IM., Jr Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol. 1997;146(10):789–803. doi: 10.1093/oxfordjournals.aje.a009196. [DOI] [PubMed] [Google Scholar]
- 2.Orenstein WA, Bernier RH, Hinman AR. Assessing vaccine efficacy in the field. Further observations. Epidemiol Rev. 1988;10(1):212–241. doi: 10.1093/oxfordjournals.epirev.a036023. [DOI] [PubMed] [Google Scholar]
- 3.Schuchat A, Bell BP. Monitoring the impact of vaccines postlicensure: new challenges, new opportunities. Expert Rev Vaccines. 2008;7(4):437–456. doi: 10.1586/14760584.7.4.437. [DOI] [PubMed] [Google Scholar]
- 4.Charles MD, Holman RC, Curns AT, et al. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J. 2006;25(6):489–493. doi: 10.1097/01.inf.0000215234.91997.21. [DOI] [PubMed] [Google Scholar]
- 5.Cortese MM, Parashar UD Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58(RR-2):1–25. [PubMed] [Google Scholar]
- 6.United States Census Bureau, US Department of Commerce. The 2012 statistical abstract: births, deaths, marriages, & divorces. Updated June 27, 2012 http://www.census.gov/compendia/statab/cats/births_deaths_marriages_divorces.html. Accessed March 10, 2013.
- 7.The Henry J. Kaiser Family Foundation. State health facts: health insurance coverage of children 0–18. 2013. http://kff.org/other/state-indicator/children-0-18. Accessed December 12, 2013.
- 8.Centers for Disease Control and Prevention. Immunization information systems. CPT codes mapped to CVX codes. Updated September 11, 2013 http://www2a.cdc.gov/vaccines/iis/iisstandards/vaccines.asp?rpt=cpt. Accessed February 5, 2014.
- 9.Patel MM, Tate JE, Selvarangan R, et al. Routine laboratory testing data for surveillance of rotavirus hospitalizations to evaluate the impact of vaccination. Pediatr Infect Dis J. 2007;26(10):914–919. doi: 10.1097/INF.0b013e31812e52fd. [DOI] [PubMed] [Google Scholar]
- 10.Hsu VP, Staat MA, Roberts N, et al. Use of active surveillance to validate international classification of diseases code estimates of rotavirus hospitalizations in children. Pediatrics. 2005;115(1):78–82. doi: 10.1542/peds.2004-0860. [DOI] [PubMed] [Google Scholar]
- 11.Desai R, Curns AT, Steiner CA, et al. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012;55(4):e28–e34. doi: 10.1093/cid/cis443. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JE, Curns AT, Tate JE, et al. Trends in healthcare utilization for diarrhea and rotavirus disease in privately insured US children <5 years of age, 2001–2006. Pediatr Infect Dis J. 2009;28(10):874–878. doi: 10.1097/INF.0b013e3181a653cd. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Curns AT, Tate JE, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med. 2011;365(12):1108–1117. doi: 10.1056/NEJMoa1000446. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Agriculture, Economic Research Service. 2003 Rural-urban continuum codes. Updated May 10, 2013 http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. Accessed March 10, 2013.
- 15.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 17.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128(2):e267–e275. doi: 10.1542/peds.2010-3722. [DOI] [PubMed] [Google Scholar]
- 18.Wang FT, Mast TC, Glass RJ, et al. Effectiveness of an incomplete RotaTeq (RV5) vaccination regimen in preventing rotavirus gastroenteritis in the United States. Pediatr Infect Dis J. 2013;32(3):278–283. doi: 10.1097/INF.0b013e318275328f. [DOI] [PubMed] [Google Scholar]
- 19.Patel MM, Tate J, Cortese M, et al. The impact of indirect benefits of vaccination on postlicensure vaccine effectiveness estimates: a scenario analysis. Vaccine. 2010;28(50):7987–7992. doi: 10.1016/j.vaccine.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 20.Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis. 2011;53(3):245–253. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 21.Nelson EA, Tam JS, Bresee JS, et al. Estimates of rotavirus disease burden in Hong Kong: hospital-based surveillance. J Infect Dis. 2005;192(suppl 1):S71–S79. doi: 10.1086/431492. [DOI] [PubMed] [Google Scholar]
- 22.Matson DO, Staat MA, Azimi P, et al. Burden of rotavirus hospitalisations in young children in three paediatric hospitals in the United States determined by active surveillance compared to standard indirect methods. J Paediatr Child Health. 2012;48(8):698–704. doi: 10.1111/j.1440-1754.2012.02445.x. [DOI] [PubMed] [Google Scholar]
- 23.Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science. 2009;325(5938):290–294. doi: 10.1126/science.1172330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hull JJ, Teel EN, Kerin TK, et al. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J. 2011;30(1 suppl):S42–S47. doi: 10.1097/INF.0b013e3181fefd78. [DOI] [PubMed] [Google Scholar]
- 25.Patel MM, Steele D, Gentsch JR, et al. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30(1 suppl):S1–S5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 26.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States. Pediatr Infect Dis J. 2011;30(1 suppl):S56–S60. doi: 10.1097/INF.0b013e3181fefdc0. [DOI] [PubMed] [Google Scholar]
- 27.Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's national childhood vaccine schedule. Pediatr Infect Dis J. 2011;30(1 suppl):S25–S29. doi: 10.1097/INF.0b013e3181fefdee. [DOI] [PubMed] [Google Scholar]
- 28.Martinón-Torres F, Bouzón Alejandro M, Redondo Collazo L, et al. Effectiveness of rotavirus vaccination in Spain. Hum Vaccin. 2011;7(7):757–761. doi: 10.4161/hv.7.7.15576. [DOI] [PubMed] [Google Scholar]
- 29.Tate JE, Mutuc JD, Panozzo CA, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1 suppl):S30–S34. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, El Khoury AC, Itzler RF. The burden of rotavirus hospitalizations among Medicaid and non-Medicaid children younger than 5 years. Am J Public Health. 2009;99(suppl 2):S398–S404. doi: 10.2105/AJPH.2008.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]