Abstract
Second-generation assays for anti-cyclic citrullinated peptide (anti-CCP), a highly sensitive and specific marker for rheumatoid arthritis (RA), have redefined the epidemiology of RA. In the Women's Health Initiative (WHI) RA study (2009–2011), we evaluated the prevalence of anti-CCP positivity among 15,691 (10.2% of 161,808) WHI participants aged 50–79 years who reported RA. Using stored baseline specimens, we measured serum anti-CCP, rheumatoid factor (RF), and antinuclear antibody in a defined sample of 9,988 of black, white, and Hispanic women. In a subset of women, we measured plasma cytokine levels and number of copies of the human leukocyte antigen (HLA)-DRB1 (HLA-DRB1) shared epitope in DNA by means of Luminex polymerase chain reaction typing (Luminex Corporation, Austin, Texas). We validated classification of probable clinical RA in 2 clinics as anti-CCP positivity or self-reported validated use of disease-modifying antirheumatic drugs (DMARDs). The prevalence of anti-CCP positivity was 8.1%, and the prevalence of RF positivity was approximately 16.0%. DMARD use including prednisone was reported by 1,140 (11.4%) participants (841 excluding prednisone) but by 57.5% of anti-CCP-positive women. The prevalence of 2 shared epitopes was also much higher for anti-CCP-positive women (18.2%, as opposed to only 5.5% for women with anti-CCP-negative DMARD-positive RA and 6.6% for anti-CCP-negative, RF-negative DMARD nonusers). Median cytokine levels were much higher for anti-CCP-positive/RF-positive women. Women with anti-CCP-positive RA and anti-CCP-negative RA had different characteristics with regard to HLA shared epitope, cigarette smoking, and inflammation (cytokines).
Keywords: cytokines, epidemiologic methods, genetics, rheumatoid arthritis
Rheumatoid arthritis (RA) is a common inflammatory joint disease that affects about 1% of the adult US population (1–3). RA is much more common in women, approximately 2-fold higher than in men. The specific etiology of RA is not known. The study of the epidemiology of RA has been limited because RA diagnosis previously required clinical evaluation of signs and symptoms, radiographic studies, and the presence of nonspecific markers, including rheumatoid factor (RF), along with an elevated erythrocyte sedimentation rate or C-reactive protein level. A major advance for the study of RA epidemiology came in the 1990s with the identification and development of second-generation assays for autoantibodies designated anti-cyclic citrullinated peptide (anti-CCP) that are both sensitive (60%–70%) and very specific (90%–98%) for the diagnosis of RA (4–10).
Citrullinated peptides and proteins are produced through posttranslational modification of arginine and occur in many proteins related to both inflammation and cigarette smoking, but antibodies to citrullinated proteins are almost exclusively identified in persons with RA (9, 10). A very important question is whether anti-CCP-negative RA is a unique subcategory of RA or whether, with further advances in the sensitivity of citrullinated peptide antibody assays, more RA cases will be included within the anti-CCP-positive category.
Several alleles of the human leukocyte antigen (HLA)-DRB1 gene (HLA-DRB1) are associated with susceptibility to RA. These alleles encode specific amino acid sequences in the hypervariable region of the HLA-DRB1 molecule referred to as the “shared epitope” (11–13). RA patients who are anti-CCP-positive have a much higher prevalence of the shared epitope. Similarly, cigarette smoking is a risk factor for anti-CCP-positive RA. Higher levels of proinflammatory cytokines are also more prevalent among persons with anti-CCP-positive RA (14–16).
The Women's Health Initiative's (WHI) included 161,808 women between the ages of 50 and 79 years at baseline (1993–1998) (17), providing a valuable opportunity to evaluate the ability of the combination of serological tests, anti-CCP positivity, and use of disease-modifying antirheumatic drugs (DMARDs) to identify probable clinical RA cases among women from the community rather than from a clinical series. A chart-review validation study carried out in 2 of the WHI centers found a low specificity of self-reported RA, which was improved by coupling it with self-reported use of DMARDs and anti-CCP status (18). Based on this validation study and previous studies of anti-CCP in the literature, we classified women who reported RA and were anti-CCP-positive, or were anti-CCP-negative but reported DMARD use, as likely to have clinical RA. Probable clinical RA cases were then evaluated in relation to the HLA-DRB1 shared epitope and cytokine levels within the WHI sample. The relationship of anti-CCP status, DMARD use, RF status, antinuclear antibody level, and other risk factors to total mortality over 9–10 years of follow-up has been reported separately (19).
METHODS
Participants and data collected in the WHI
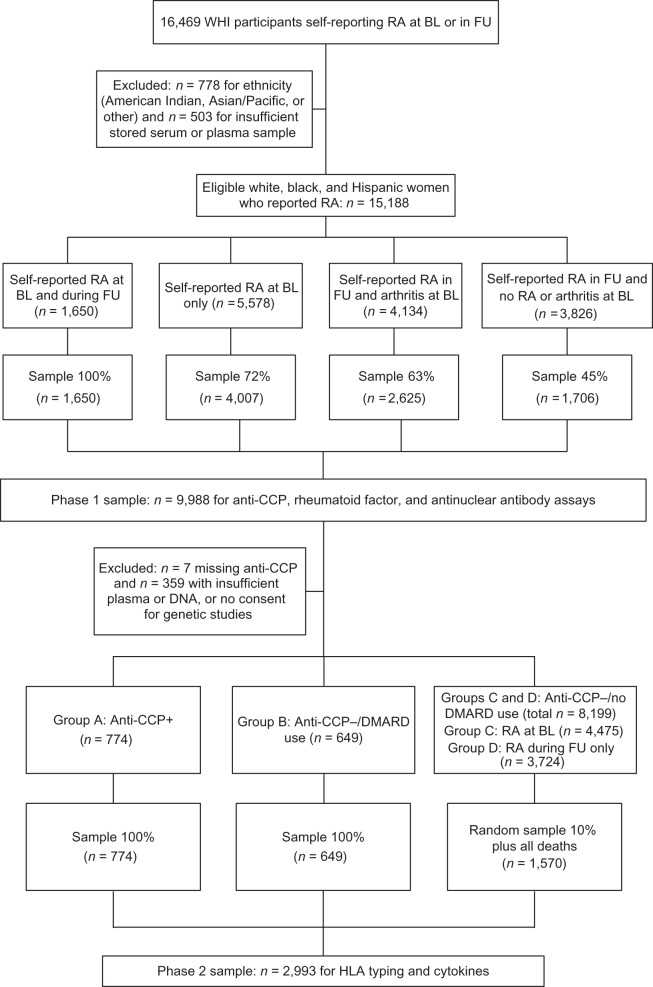
Many previous papers have described the WHI. Briefly, between 1993 and 1998, 40 US clinical centers enrolled 161,808 women aged 50–79 years (mean age = 62.8 years) into either a randomized clinical trial (n = 68,132) or an observational study (n = 93,676) (17). At baseline, 76,192 (47.1%) WHI participants reported a history of arthritis and 7,872 (4.9%) specifically reported RA. At yearly follow-up visits, women were asked whether they had developed any new arthritis, RA, or osteoarthritis. A total of 10,426 women reported a diagnosis of RA during follow-up, of whom 1,829 had previously reported RA at baseline; in addition, 5,783 women had reported other arthritis, not RA, at baseline. Women were not required to re-report previously reported RA. The present study was limited to white, black, and Hispanic women in the WHI with available blood samples (18), leaving 15,188 women who reported RA at baseline or follow-up and were eligible for this study. Of these, 9,998 (66%) were sampled for the current study (Figure 1). A detailed description of phase 1 sampling has been published elsewhere (18).
Figure 1.
Sampling frame for the Women's Health Initiative (WHI) rheumatoid arthritis (RA) study, 2009–2011. The phase 2 sample of the WHI (2010–2011) included 4 groups—group A: anti-cyclic citrullinated peptide (anti-CCP)-positive women (100% sample (n = 774, excluding 28 with insufficient plasma or DNA samples)); group B: anti-CCP-negative women using disease-modifying antirheumatic drugs (DMARDs) (100% sample (n = 649)); group C: anti-CCP-negative women with no DMARD use who reported RA at baseline (BL) (10% random sample plus all deaths in this subgroup (n = 921)); and group D: anti-CCP-negative women with no DMARD use who reported RA at follow-up (FU) only (10% random sample plus all deaths in this subgroup (n = 649)). HLA, human leukocyte antigen.
Based on the anti-CCP results from phase 1 (2009–2010), a phase 2 (2010–2011) sample of 2,993 participants was selected (Figure 1) for measurement of cytokines and HLA-DR typing for shared epitopes. The phase 2 sample included 4 groups—group A: anti-CCP-positive women (100% sample (n = 774, excluding 28 with insufficient plasma or DNA samples)); group B: anti-CCP-negative women with DMARD use (100% sample (n = 649)); group C: anti-CCP-negative women with no DMARD use who reported RA at baseline (10% random sample plus all deaths in this subgroup (n = 921)); and group D: anti-CCP-negative women with no DMARD use who reported RA at follow-up only (10% random sample plus all deaths in this subgroup (n = 649)) (Figure 1).
DMARD use was defined as current use of hydroxychloroquine, sulfasalazine, minocycline, methotrexate, leflunomide, azathioprine, cyclosporine, gold, cyclophosphamide, antirheumatic biological agents, or oral steroids (19). DMARD use excluding prednisone was also addressed in the analyses. DMARD use was based on recall of current use by participants and further review by WHI staff of medication bottles brought to the clinic (a point prevalence). Assessment of medication use at baseline was repeated in the WHI observational study arm at year 3 of follow-up and in the clinical trial arm at years 1, 3, 6, and 9 of follow-up. Therefore, DMARD use was defined as reported current DMARD use at baseline or at 1, 3, 6, or 9 years of follow-up. Among women who reported RA at baseline, 634 used DMARDs (excluding prednisone) in the study; 461 (73%) reported DMARD use at baseline, 29 (4%) reported DMARD use for the first time at year 1, 114 (18%) first reported DMARD use at year 3, and 31 (5%) first reported use after year 3. Among women without a history of RA at baseline, 207 used DMARDs during this study, including 31 with first reported use of DMARDs at baseline, 12 with first use at year 1, 101 at year 3, and 63 after year 3. These data excluded the use of prednisone.
Serum biomarkers and HLA-DRB1 typing
Using baseline serum samples stored at −70°F and not previously thawed, anti-CCP and RF assays were performed in the Rheumatology Clinical Research Laboratory at the University of Colorado, as previously described, with anti-CCP positivity defined as ≥5 U/L (19). HLA-DRB1 typing was carried out in the laboratory of Dr. Massimo Trucco at the University of Pittsburgh (20). Additional details on these methods are presented in the Appendix.
Multiplex cytokine profiling
Multiplex cytokine profiling was performed in the Robinson Laboratory at Stanford University using baseline plasma samples stored at −70°F. The human 22-cytokine Beadlyte kit (Upstate Group Inc., Charlottesville, Virginia) and the Luminex xMAP 100IS platform (Luminex Corporation, Austin, Texas) were used according to the manufacturers' protocols, except that we used 50% of the recommended serum and buffer volumes. The laboratory ran 100 samples in duplicate at 100% and 50% dilution, and there was little difference in the cytokine levels. All cytokine profiling analyses were performed using 3 μg/mL HeteroBlock (Omega Biologicals Inc., Bozeman, Montana) in order to minimize nonspecific false elevations in cytokine readouts due to RF (21).
Many of the cytokine levels were very low, resulting in variability of laboratory measurements. In 40% of samples, interleukin-17 was not measurable (i.e., the level was 0). Levels of interleukin-4 were very low, such that the 25th percentile was only 1 pg/mL. Given the skewed values for most cytokines, the median value and interquartile range (25th–75th percentiles) were better estimates than means. There were 153 blind duplicate samples for cytokine measurements. With high variability classified as >30% variation between blind samples, most cytokine measurements had at least 70% of blind duplicates with <30% variability; the exceptions were interleukin-12 (69%), interleukin-4 (63%), and interleukin-2 (65%).
Statistical analysis
As described above, the study sample consisted of 9,988 women who were sampled from 15,188 eligible WHI participants who reported RA at baseline or during follow-up (Figure 1). “RA at baseline” includes women who reported RA at both baseline and follow-up. Sampling fractions also varied by coronary heart disease status at baseline and by racial/ethnic group. To account for the stratified sampling and to correctly represent the entire population of black, white, and Hispanic women in the WHI who reported RA, we determined sampling weights, defined as 1/sampling fraction, for each woman and incorporated them into the analyses. Analyses were performed with SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina). All P values were 2-sided at α = 0.05.
RESULTS
Characteristics of WHI women reporting RA
The 9,988 participants in the current study included 5,712 (57.2%) from the observational study arm of WHI and 4,276 (42.8%) from the clinical trial arm—1,058 (10.6%) Hispanic women, 6,487 (65.0%) white women, and 2,443 (24.4%) black women. In terms of age, 1,167 participants were aged 50–54 years, 1,822 were aged 55–59 years, 4,488 were aged 60–69 years, and 2,511 were aged 70–79 years. A higher percentage of black women as compared with white women reported a history of RA at both baseline and follow-up. Women who reported RA at baseline were older, had less education, and had much higher prevalences of joint pain and poor health, lower levels of physical activity, and greater disability on the Short Form 36 health survey; all differences were significant (P < 0.0001) in comparison with no reported RA (not shown).
Anti-CCP levels were successfully measured in 9,981 women. Seven women were missing anti-CCP data, and 22 were missing RF data. One person was missing DMARD information at baseline only. Thus, the total numbers shown in the tables vary because of missing data. There were 812 women (8.1%, 95% confidence interval (CI): 7.6, 8.7) who were anti-CCP-positive (Table 1). The mean anti-CCP level for anti-CCP-positive women was 68 U/mL, and approximately 70% had levels over 30 U/mL (not shown). Among women who were anti-CCP-negative (n = 9,169), 94% had levels of <1 U/mL, 4% had levels of 1–2 U/mL, 0.9% had levels of 2–3 U/mL, 0.5% had levels of 3–4 U/mL, and 0.3% (n = 15) had levels of 4–5 U/mL. These titers did not vary by DMARD use at baseline or by RF status. The prevalence of anti-CCP positivity was higher for black women (9.6%) than for white (7.9%) or Hispanic (6.2%) women (Table 1). Anti-CCP positivity was much higher for participants who reported RA at baseline (612 of 5,654; 10.8%, 95% CI: 10.0, 11.6) than for those who reported RA during follow-up only (200 of 4,327; 4.6%, 95% CI: 4.0, 5.3). In weighted analyses, the prevalence of anti-CCP positivity was higher among women reporting RA at baseline in all age groups (<60 years: 11.7%, 95% CI: 10.4, 13.1 (262 of 2,236 women); 61–69 years: 10.3%, 95% CI: 9.2, 11.4 (313 of 3,032); and ≥70 years: 9.1%, 95% CI: 7.8, 10.4 (178 of 1,960)), for a total prevalence of 10.4% (753 of 7,228 women) (data not shown).
Table 1.
Anti-CCP Positivity and Use of DMARDs at Baseline, by Race and Ethnicity, in a Subsample (n = 9,988) of Participants With Self-Reported Rheumatoid Arthritis (n = 15,188a), Women's Health Initiative Rheumatoid Arthritis Study, 2009–2011
| Reported History of RA and Ethnicity |
Anti-CCP Positivity |
DMARD Useb at Baseline |
DMARD Useb at Any Time |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. | No. | % | Total No. | No. | % | Total No. | No. | % | |
| RA reported at baseline | |||||||||
| Black | 1,160 | 149 | 12.8 | 1,159 | 77 | 6.6 | 1,160 | 106 | 9.1 |
| Hispanic | 367 | 41 | 11.2 | 367 | 21 | 5.7 | 367 | 30 | 8.2 |
| White | 4,127 | 422 | 10.2 | 4,130 | 363 | 8.8 | 4,130 | 498 | 12.1 |
| Total | 5,654c | 612 | 10.8 | 5,656d | 461 | 8.1 | 5,657 | 634 | 11.2 |
| P value | 0.039 | 0.013 | 0.003 | ||||||
| RA reported at follow-up only | |||||||||
| Black | 1,282 | 86 | 6.7 | 1,283 | 11 | 0.9 | 1,283 | 51 | 4.0 |
| Hispanic | 691 | 25 | 3.6 | 691 | 2 | 0.3 | 691 | 18 | 2.6 |
| White | 2,354 | 89 | 3.8 | 2,357 | 18 | 0.8 | 2,357 | 138 | 5.9 |
| Total | 4,327c | 200 | 4.6 | 4,331 | 31 | 0.7 | 4,331 | 207 | 4.8 |
| P value | 0.0001 | 0.332 | 0.001 | ||||||
| Grand total | |||||||||
| Black | 2,442 | 235 | 9.6 | 2,442 | 88 | 3.6 | 2,443 | 157 | 6.4 |
| Hispanic | 1,058 | 66 | 6.2 | 1,058 | 23 | 2.2 | 1,058 | 48 | 4.5 |
| White | 6,481 | 511 | 7.9 | 6,487 | 381 | 5.9 | 6,487 | 636 | 9.8 |
| Total | 9,981c | 812 | 8.1 | 9,987d | 492 | 4.9 | 9,988 | 841 | 8.4 |
| P value | 0.002 | <0.0001 | <0.0001 | ||||||
Abbreviations: anti-CCP, anti-cyclic citrullinated peptide; DMARDs, disease-modifying antirheumatic drugs [excluding prednisone]; RA, rheumatoid arthritis.
a Includes white, black, and Hispanic Women's Health Initiative participants who reported RA at baseline or follow-up and had available blood samples.
b Excluding prednisone.
c Information on anti-CCP status at baseline was missing for 7 women.
d Information on DMARD use at baseline was missing for 1 woman.
DMARD use
Among the 9,988 participants, 9,147 (91.6%, 95% CI: 91.0, 92.1) never reported DMARD use, 492 (4.9%, 95% CI: 4.5, 5.4) reported use of DMARDs at both baseline and follow-up, and 349 (3.5%, 95% CI: 3.1, 3.9) reported DMARD use at follow-up only, excluding prednisone. With the addition of prednisone as a DMARD, the prevalence of DMARD use at any time would increase from 8.4% to 11.4%. A much higher proportion of anti-CCP-positive women—57.5% (n = 467)—were on DMARDs. Excluding prednisone, approximately 51% (n = 417) of anti-CCP-positive women were on DMARDs, of whom 275 (33.9%) reported DMARD use at baseline and 142 (17.5%) reported DMARD use at a follow-up visit only. Only 4.6% (n = 423) of women who were anti-CCP-negative were on DMARDs (excluding prednisone) at any time: 2.4% (n = 217) at baseline and 2.2% (n = 206) at follow-up only (not shown). Methotrexate was the most commonly used drug; 36.3% (n = 295) of the anti-CCP-positive women were on methotrexate, including 23.5% (n = 191) at baseline. Approximately 2.0% (n = 185) of anti-CCP-negative women were on methotrexate, including 78 at baseline.
In weighted analysis (Table 2), 1,079 women were anti-CCP-positive and 647 women were anti-CCP-negative and using DMARDs (excluding prednisone) at any time—a total of 1,726 probable RA cases, with a prevalence of 1.1% among 154,673 black, white, and Hispanic women in the WHI. Restricting the analysis to women who reported RA at baseline, 752 were anti-CCP-positive and 344 were anti-CCP-negative and using DMARDs—a total of 1,096 women with probable clinical RA, with a prevalence of 0.71%. The prevalence was consistent with the estimate in the literature of approximately 1%.
Table 2.
Relationship of Rheumatoid Arthritis at Baseline or Follow-up to Anti-CCP Status,a DMARD Use, and RF Status in a Subsample of Participants With Self-Reported Rheumatoid Arthritis (Weighted Analysesb), Women's Health Initiative Rheumatoid Arthritis Study, 2009–2011
| Anti-CCP Status | DMARD Use at Any Time |
RF Status | History of RA at Baseline |
RA at Follow-up Only |
Total |
|||
|---|---|---|---|---|---|---|---|---|
| Weighted n | % | Weighted n | % | No. | % | |||
| + | + | + | 424 | 5.9 | 71 | 0.9 | 495 | 3.3 |
| + | + | − | 37 | 0.5 | 20 | 0.3 | 57 | 0.4 |
| + | − | + | 218 | 3.0 | 114 | 1.4 | 332 | 2.2 |
| + | − | − | 73 | 1.0 | 122 | 1.5 | 195 | 1.3 |
| − | + | + | 74 | 1.0 | 42 | 0.5 | 116 | 0.8 |
| − | + | − | 270 | 3.8 | 261 | 3.3 | 531 | 3.5 |
| − | − | + | 718 | 10.0 | 766 | 9.6 | 1,484 | 9.8 |
| − | − | − | 5,396 | 74.8 | 6,552 | 82.4 | 11,948 | 78.8 |
| Total | 7,210 | 100.0 | 7,948 | 100.0 | 15,158 | 100.0 | ||
| All anti-CCP+ | 752 | 10.4 | 327 | 4.1 | 1,079 | 7.1 | ||
| All DMARD+ | 805 | 11.2 | 394 | 5.0 | 1,199 | 7.9 | ||
| All RF+ | 1,434 | 19.9 | 993 | 12.5 | 2,427 | 16.0 | ||
Abbreviations: anti-CCP, anti-cyclic citrullinated peptide; DMARDs, disease-modifying anti-rheumatic drugs [excluding prednisone]; RA, rheumatoid arthritis; RF, rheumatoid factor.
a +, positive; –, negative.
b Weighted numbers of participants are slightly lower than those in the original sample because of missing data on anti-CCP and RF status.
Rheumatoid factor
RF was measured successfully in 9,966 women (data were missing for 22 women). In weighted analyses, RF was positive for 2,427 (16.0%) of the 15,158 participants in the weighted sample, including 19.9% (1,434 of 7,210) who reported RA at baseline and 12.5% (993 of 7,948) who reported RA at follow-up only. RF was positive for 76.6% (827 of 1,079) of anti-CCP-positive women and 89.7% (495 of 552) of women who were both anti-CCP-positive and using DMARDs. In contrast, only 11.4% (1,600 of 14,079) of anti-CCP-negative women were RF-positive, including 116 (17.9%) of 647 women who were anti-CCP-negative and using DMARDs at any time (Table 2).
HLA-DRB1 shared epitope
HLA-DRB1 typing was completed for 2,281 of the 2,993 participants in the phase 2 sample. In weighted analyses restricted to women who reported RA at baseline (n = 6,737), 3,736 women (55.4%, 95% CI: 54.3, 56.7) had no shared epitope, 2,598 (38.6%, 95% CI: 37.4, 39.7) had 1 shared epitope, and 403 (6.0%, 95% CI: 5.4, 6.5) had 2 shared epitopes (Table 3). The prevalence of the shared epitope was strongly associated with anti-CCP positivity but not with anti-CCP negativity/DMARD use; that is, the prevalences of 2 and 1 copies of the shared epitope were 18.2% and 50.6%, respectively, for anti-CCP-positive women and only 4.6% and 37.2% for anti-CCP-negative women. Similarly, among women in the anti-CCP-negative/no DMARD use/RF-negative group, only 4.8% had 2 shared epitopes and 36.5% had 1 shared epitope (Table 3).
Table 3.
Number of Shared HLA-DRB1 Epitopes, by Anti-CCP Status,a DMARD Use at Baseline, and RF Status (Weighted), in a Subsample of Participants With Self-Reported Rheumatoid Arthritis at Baseline (n = 6,737b), Women's Health Initiative Rheumatoid Arthritis Study, 2009–2011
| Anti-CCP, DMARD, and RF Status | No. of Shared Epitopes |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 |
1 |
2 |
||||||
| No. | Row % | No. | Row % | No. | Row % | No. | Row % | |
| Anti-CCP+ | ||||||||
| DMARD+ | 83 | 26.8 | 155 | 50.2 | 71 | 23.0 | 309 | 100.0 |
| DMARD− | 131 | 34.8 | 191 | 50.8 | 54 | 14.4 | 376 | 100.0 |
| Total | 214 | 31.2 | 346 | 50.5 | 125 | 18.3 | 685 | 100.0 |
| Anti-CCP− | ||||||||
| DMARD+/RF− | 96 | 52.7 | 74 | 40.7 | 12 | 6.6 | 182 | 100.0 |
| DMARD+/RF+ | 35 | 66.0 | 17 | 32.1 | 1 | 1.9 | 53 | 100.0 |
| DMARD−/RF− | 3,058 | 58.7 | 1,900 | 36.5 | 251 | 4.8 | 5,209 | 100.0 |
| DMARD−/RF+ | 333 | 54.8 | 261 | 42.9 | 14 | 2.3 | 608 | 100.0 |
| Total | 3,522 | 58.2 | 2,252 | 37.2 | 278 | 4.6 | 6,052 | 100.0 |
| Grand total | 3,736 | 55.4 | 2,598 | 38.6 | 403 | 6.0 | 6,737 | 100.0 |
Abbreviations: anti-CCP, anti-cyclic citrullinated peptide; DMARDs, disease-modifying antirheumatic drugs [excluding prednisone]; HLA, human leukocyte antigen; RF, rheumatoid factor.
a +, positive; –, negative.
b Excludes women who reported rheumatoid arthritis at follow-up only.
Cytokines
Cytokine levels were measured in the phase 2 sample. Overall, median levels of most cytokines were significantly higher for anti-CCP-positive women than for anti-CCP-negative women, whether they used DMARDs or not (data not shown). When results were stratified by RF status (Table 4), cytokine levels were higher for anti-CCP-positive women than for anti-CCP-negative women only for those who were also RF-positive, and were highest for women who were both anti-CCP-positive and RF-positive (Table 4). Current smoking did not explain these results; median cytokine levels were significantly higher for anti-CCP-positive/RF-positive women than for anti-CCP-negative/RF-positive or RF-negative women among both smokers and nonsmokers (not shown). There were no differences in median cytokine levels for smokers as compared with nonsmokers. For example, for never smokers versus smokers, the median interleukin-6 level was 6.2 pg/mL versus 6.1 pg/mL for anti-CCP-negative/RF-negative women and 14.9 pg/mL versus 16.9 pg/mL (P = 0.24) for anti-CCP-positive/RF-positive women. Results for other cytokine measures were similar to the results for interleukin-6. Additionally, we previously reported that cytokine levels were higher for anti-CCP-positive participants with 1 or 2 copies of the shared epitope than for those with none (22) (not shown).
Table 4.
Mediana Cytokine Levels at Baseline, by Anti-CCP and RF Status,b Among Participants in the Women's Health Initiative Rheumatoid Arthritis Study, 2009–2011
| Cytokine | Anti-CCP and RF Status and Baseline Cytokine Level, pg/mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Anti-CCP+ (n = 770)c |
Anti-CCP− (n = 2,214)d |
P Value (Anti-CCP+ vs. Anti-CCP−) |
||||||
| RF− (n = 157) | RF+ (n = 613) | P Value | RF− (n = 1,912) | RF+ (n = 302) | P Value | RF+ | RF− | |
| IL-1b | 1.6 | 2.4 | <0.0001 | 1.5 | 1.8 | <0.0001 | <0.0001 | 0.661 |
| IL-2 | 3.3 | 12.6 | <0.0001 | 3.2 | 5.3 | <0.0001 | <0.0001 | 0.587 |
| IL-4 | 1.6 | 2.3 | <0.0001 | 1.6 | 1.8 | 0.001 | <0.0001 | 0.916 |
| IL-5 | 3.0 | 4.1 | <0.0001 | 3.0 | 3.3 | <0.0001 | <0.0001 | 0.641 |
| IL-6 | 6.3 | 15.2 | <0.0001 | 6.1 | 7.5 | <0.0001 | <0.0001 | 0.092 |
| IL-7 | 6.9 | 8.5 | <0.0001 | 6.9 | 7.6 | <0.0001 | 0.001 | 0.908 |
| IL-8 | 8.9 | 9.8 | 0.001 | 8.6 | 9.3 | 0.001 | 0.303 | 0.247 |
| IL-10 | 2.5 | 3.2 | <0.0001 | 2.4 | 2.7 | <0.0001 | <0.0001 | 0.292 |
| IL-12 | 12.0 | 22.9 | <0.0001 | 12.6 | 16.0 | <0.0001 | <0.0001 | 0.756 |
| IL-13 | 2.4 | 4.3 | <0.0001 | 2.4 | 3.0 | <0.0001 | <0.0001 | 0.796 |
| Granulocyte-macrophage colony-stimulating factor | 144.0 | 170.2 | 0.001 | 147.1 | 141.9 | 0.758 | 0.001 | 0.800 |
| Monocyte chemotactic protein 1 | 15.3 | 20.9 | <0.0001 | 14.5 | 16.2 | <0.0001 | <0.0001 | 0.265 |
| Macrophage inflammatory protein 1β | 45.4 | 45.9 | 0.877 | 41.9 | 46.5 | 0.0003 | 0.530 | 0.043 |
| Tumor necrosis factor α | 21.7 | 37.7 | <0.0001 | 21.6 | 25.1 | <0.0001 | <0.0001 | 0.605 |
| Interferon γ | 40.8 | 74.8 | <0.0001 | 41.1 | 50.6 | <0.0001 | <0.0001 | 0.749 |
| IL-17 | 0.8 | 4.2 | <0.0001 | 0.4 | 2.3 | <0.0001 | 0.007 | 0.820 |
| White blood cell count (× 109 cells/L) | 5.5 | 6.2 | <0.0001 | 5.8 | 5.7 | 0.088 | <0.0001 | 0.041 |
Abbreviations: anti-CCP, anti-cyclic citrullinated peptide; DMARDs, disease-modifying antirheumatic drugs [excluding prednisone]; IL, interleukin; RF, rheumatoid factor.
a Wilcoxon test of median values.
b +, positive; –, negative.
c Information on RF status was missing for 2 women; information on cytokine levels was missing for 2 women.
d Information on RF status was missing for 2 women; information on cytokine levels was missing for 3 women.
Cigarette smoking
Women who were anti-CCP-positive with no DMARD use reported a higher prevalence of current cigarette smoking, about twice as high as the other groups. Women in the anti-CCP-positive/DMARD-use and anti-CCP-negative/DMARD-use groups had prevalences of cigarette smoking similar to those in the remaining WHI sample or the anti-CCP-negative/no-DMARD-use group (Table 5). There was no association between number of copies of the shared epitope and current or former cigarette smoking for either anti-CCP-positive women or anti-CCP-negative women. For example, among anti-CCP-positive women, 40% with 0 copies of the shared epitope were never smokers as compared with 37% with 1 copy of the shared epitope and 44% with 2 copies.
Table 5.
Cigarette Smoking Status, by Self-Reported Rheumatoid Arthritis, DMARD Use at Baseline, and Anti-CCP Status,a Among Participants in the Women's Health Initiative Rheumatoid Arthritis Study, 2009–2011b
| Smoking Status |
Anti-CCP+/DMARD Use (n = 275) |
Anti-CCP+/No DMARD Use (n = 537) |
Anti-CCP−/DMARD Use (n = 217) |
Anti-CCP−/No DMARD Use (n = 8,951) |
No Reported History of RA (n = 133,741) |
Age- Adjusted P Value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Past smoker | 138 | 50.2 | 245 | 45.6 | 104 | 48.0 | 3,557 | 39.7 | 56,458 | 42.2 | <0.0001 |
| Current smoker | 20 | 7.3 | 75 | 14.0 | 15 | 6.9 | 751 | 8.4 | 9,095 | 6.8 | <0.0001 |
Abbreviations: anti-CCP, anti-cyclic citrullinated peptide; DMARDs, disease-modifying antirheumatic drugs [excluding prednisone]; RA, rheumatoid arthritis.
a +, positive; –, negative.
b Totals reflect the fact that information on anti-CCP status was missing for 7 women and information on DMARD use was missing for 1 woman.
DISCUSSION
In the current study, we used anti-CCP serological testing to evaluate participant characteristics and classify women reporting RA in the WHI. This study documented that in the WHI, a reported history of RA included a very large percentage of women who probably did not have clinical RA. Second, we found that anti-CCP-positive RA and anti-CCP-negative RA with DMARD use have different epidemiologic and laboratory characteristics, including the shared epitope, cytokine levels, and cigarette smoking, consistent with the findings of other recent clinical studies. Future clinical, genetic, and epidemiologic studies of RA must separate anti-CCP-positive and -negative cases (14, 15, 21).
The prevalence of anti-CCP positivity in the community would be too low for this measure to be a useful screening test for possible RA. The addition of reported new joint symptomatology with anti-CCP measurements may be useful in identifying incident or evolving RA in epidemiologic studies (23).
RA patients are more likely to have 1 or 2 shared HLA-DRB1 epitopes, but the majority of persons with 2 shared epitopes in the community do not have RA. Therefore, identification of shared epitopes is probably not a useful approach for identifying persons at risk of RA. Possibly the addition of other genetic markers (24) plus the shared epitope would improve the measurement of host susceptibility and aid in the identification of a higher-risk cohort for incident RA.
Inflammation is associated with citrullination of proteins, but antibodies to citrullinated proteins in blood are almost entirely restricted to persons with RA and, to a lesser extent, connective tissue diseases. The association of smoking and possibly other environmental determinants of lung disease with RA risk suggests that inflammation in the lungs may be a contributing cause in the etiology and natural history of RA, especially anti-CCP-positive RA in a genetically susceptible host (25, 26). The relationship between cigarette smoking and the HLA-DRB1 shared epitope is inconsistent among RA studies (27). Cigarette smoking remains the strongest risk factor for RA, and smoking cessation could possibly prevent incident RA (28).
The results of the WHI should be interpreted carefully considering the limitations of our study. Current DMARD use, as noted, was measured at baseline and at years 1, 3, 6, and 9 for women in the clinical trial and at baseline and year 3 for women in the observational study, and we had no information on history of DMARD use prior to entry into the WHI. It is possible that some anti-CCP-negative women could have been treated with DMARDs for RA in the past and then have become asymptomatic and stopped all DMARD therapy, and yet had clinical RA. However, this should have been rare. In the validation study (19), the prevalence of clinical RA among women who reported RA but were not using DMARDs and were anti-CCP-negative was only 6%.
Another potential source of misclassification is that our assays were conducted only at baseline. It is possible that some anti-CCP-negative participants may have converted to anti-CCP positivity during follow-up, particularly among women who only reported RA at follow-up. However, the literature indicates that seropositivity often predates diagnosis by years (1). Furthermore, of women who reported RA during follow-up, only 4.8% ever reported DMARD use at any visit. It is possible that the development of new antibody tests for citrullinated proteins may result in some of the women who are anti-CCP-negative converting to a positive antibody test (29). Additional studies are needed to clarify the nature of anti-CCP-negative RA and its clinical characteristics and course, in addition to its potential to convert to anti-CCP-positive RA. Finally, the variability of cytokine laboratory measurements limits interpretation of our results. However, the very large differences in cytokine levels by anti-CCP positivity and RF status are unlikely to have been due to laboratory variability.
In conclusion, there are substantial differences in the characteristics of persons with anti-CCP-positive RA as compared with anti-CCP-negative RA (defined by DMARD use)—for example, a much higher proportion with the shared epitope and higher cytokine levels. Clearly, just obtaining a history of RA is of little value. Ascertainment of RA cases through medical records may be biased by severity of disease, availability of rheumatology services, and other comorbid conditions that identify the patient in the clinical system.
A major question for epidemiologists is whether there are unique agent(s) in the causal pathway of autoimmune diseases such as RA (30, 31). Future epidemiologic studies could probe different populations that have unique lifestyles, environmental exposures, and genetic polymorphisms. Combining such studies with improved techniques for measuring immune function, including both innate and adaptive immune responses at the earliest stage of symptoms or initial conversion to anti-CCP positivity, may be possible in population epidemiologic studies.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Lewis H. Kuller, Rachel H. Mackey); Section of Rheumatology, Department of Medicine, Washington Hospital Center, Washington, DC (Brian T. Walitt); Department of Medicine, School of Medicine, University of Colorado, Aurora, Colorado (Kevin D. Deane, V. Michael Holers); Department of Medicine, School of Medicine, Stanford University, Stanford, California (William H. Robinson, Jeremy Sokolove); Department of Neurology, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania (Yuefang Chang); and Department of Medicine, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania (Larry W. Moreland).
This work was supported by BAA NHLBI-WH-09-01 contract HHSN268200960006C from the National Heart, Lung, and Blood Institute. The Women's Health Initiative (WHI) was funded by the National Heart, Lung, and Blood Institute through contracts N01WH22110, N01WH24152, N01WH32100–N01WH32102, N01WH32105, N01WH32106, N01WH32108, N01WH32109, N01WH32111–N01WH32113, N01WH32115, N01WH32118, N01WH32119, N01WH32122, N01WH42107–N01WH42126, N01WH42129–N01WH42132, and N01WH44221.
The WHI Investigators—Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, Maryland); Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, Washington); Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts), Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC), Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, California), Rebecca Jackson (The Ohio State University, Columbus, Ohio), Cynthia A. Thomson (University of Arizona, Tucson, Arizona), Jean Wactawski-Wende (University at Buffalo, State University of New York, Buffalo, New York), Marian Limacher (University of Florida, Gainesville, Florida), Robert Wallace (University of Iowa, Iowa City, Iowa), Lewis Kuller (University of Pittsburgh, Pittsburgh, Pennsylvania), and Sally Shumaker (School of Medicine, Wake Forest University, Winston-Salem, North Carolina); WHI Memory Study: Sally Shumaker (School of Medicine, Wake Forest University, Winston-Salem, North Carolina).
For a list of all of the investigators who have contributed to the science of the WHI, please visit https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx.
Conflict of interest: none declared.
APPENDIX
Expanded Study Methods
Serum biomarkers
Using baseline serum samples stored at −70°F and not previously thawed, second-generation anti-cyclic citrullinated peptide (anti-CCP), rheumatoid factor (RF), and antinuclear antibody assays were carried out in the Rheumatology Clinical Research Laboratory at the University of Colorado. Briefly, anti-CCP (immunoglobulin G) antibodies were measured using commercially available anti-CCP enzyme-linked immunosorbent assay kits (Diastat; Axis-Shield Diagnostics Ltd., Dundee, United Kingdom). Anti-CCP antibodies were measured in arbitrary units (AU) per mL and were considered positive at a cutoff value ≥5 AU/mL, which has been demonstrated to be more than 98% specific for rheumatoid arthritis (RA). RF was measured quantitatively by the reactivity of diluted test serum with heterologous immunoglobulin G in solution via nephelometry, which provided continuously variable quantitative results in international units (IU) (Dade Behring, Newark, Delaware). Per the 1987 American College of Rheumatology RA classification criteria, the positive cutoff value for these tests is set so that 5% of a population of 490 randomly selected healthy anonymous blood donors are positive. Quality control was routinely assessed by means of a procedure in which all autoantibody-positive serum samples (anti-CCP and/or RF) were retested in a blinded fashion, along with 5% of the negative sera. There was greater than 97.5% agreement in repeat testing (18, 32–34).
Human leukocyte antigen (HLA)-DRB1 typing
HLA-DRB1 typing was performed in the laboratory of Dr. Massimo Trucco at the University of Pittsburgh (20). Luminex microspheres (Luminex Corporation, Austin, Texas) were incorporated with infrared and red dyes. These dyes give each microsphere a unique spectral address, allowing for 100 different classifications of beads. Each classification of bead is then bound with a particular oligonucleotide probe. DNA samples are amplified by means of polymerase chain reaction (PCR) using primers that are biotinylated. PCR products are hybridized with a mixture of microspheres that have a predetermined set of oligonucleotide probes bound to them. The PCR product will find complimentary sequences and bind with the probe (bead) they recognize. Individual sample reactions are then washed to remove any unbound PCR product. Samples are then stained with R-phycoerythrin-conjugated streptavidin that binds to the biotinylated primer. Samples are then processed through a Luminex flow analyzer. The fluorescent intensity of phycoerythrin on each classification of microsphere is translated into a positive or negative reaction. The assignment of HLA typing is based on the reaction pattern and analyzed using the current HLA sequence data.
REFERENCES
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.de Hair MJH, Lehmann KA, van de Sande MGH, et al. The clinical picture of rheumatoid arthritis according to the 2010 American College of Rheumatology/European League Against Rheumatism criteria: is this still the same disease? Arthritis Rheum. 2012;64(2):389–393. doi: 10.1002/art.33348. [DOI] [PubMed] [Google Scholar]
- 3.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373(9664):659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 4.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 5.van Venrooij WJ, Pruijn GJM. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res. 2000;2(4):249–251. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Venrooij WJ, Zendman AJW, Pruijn GJM. Autoantibodies to citrullinated antigens in (early) rheumatoid arthritis. Autoimmun Rev. 2006;6(1):37–41. doi: 10.1016/j.autrev.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43(8):1831–1835. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Coenen D, Verschueren P, Westhovens R, et al. Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin Chem. 2007;53(3):498–504. doi: 10.1373/clinchem.2006.078063. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal R, Liao K, Nair R, et al. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1472–1483. doi: 10.1002/art.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiik AS, van Venrooij WJ, Pruijn GJM. All you wanted to know about anti-CCP but were afraid to ask. Autoimmun Rev. 2010;10(2):90–93. doi: 10.1016/j.autrev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Nepom GT, Byers P, Seyfried C, et al. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- 12.Wordsworth BP, Lanchbury JS, Sakkas LI, et al. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rønningen KS, Spurkland A, Egeland T, et al. Rheumatoid arthritis may be primarily associated with HLA-DR4 molecules sharing a particular sequence at residues 67–74. Tissue Antigens. 1990;36(5):235–240. doi: 10.1111/j.1399-0039.1990.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 14.Willemze A, van der Woude D, Ghidey W, et al. The interaction between HLA shared epitope alleles and smoking and its contribution to autoimmunity against several citrullinated antigens. Arthritis Rheum. 2011;63(7):1823–1832. doi: 10.1002/art.30409. [DOI] [PubMed] [Google Scholar]
- 15.Meyer PW, Hodkinson B, Ally M, et al. HLA-DRB1 shared epitope genotyping using the revised classification and its association with circulating autoantibodies, acute phase reactants, cytokines and clinical indices of disease activity in a cohort of South African rheumatoid arthritis patients. Arthritis Res Ther. 2011;13(5):R160. doi: 10.1186/ar3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laki J, Lundstrom E, Snir O, et al. Very high levels of anti-citrullinated protein antibodies are associated with HLA-DRB1*15 non-shared epitope allele in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(7):2078–2084. doi: 10.1002/art.34421. [DOI] [PubMed] [Google Scholar]
- 17.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 18.Walitt B, Mackey R, Kuller L, et al. Predictive value of autoantibody testing for validating self-reported diagnoses of rheumatoid arthritis in the Women's Health Initiative. Am J Epidemiol. 2013;177(9):887–893. doi: 10.1093/aje/kws310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuller LH, Mackey R, Walitt BT, et al. Determinants of mortality among postmenopausal women who report rheumatoid arthritis in the Women's Health Initiative [published online ahead of print December 23, 2013] Arthritis Rheum. doi: 10.1002/art.38268. ( doi:10.1002/art38286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng J, Hurley CK, Baxter-Lowe LA, et al. Large-scale oligonucleotide typing for HLA-DRB1/3/4 and HLA-DQB1 is highly accurate, specific, and reliable. Tissue Antigens. 1993;42(5):473–479. doi: 10.1111/j.1399-0039.1993.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 21.Chandra PE, Sokolove J, Hipp BG, et al. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R102. doi: 10.1186/ar3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talabi MB, Mackey R, Moreland LW, et al. The associations of HLA-DR shared epitope alleles and serum cytokines among postmenopausal women with rheumatoid factor and anti-citrullinated protein antibody-positive rheumatoid arthritis [abstract] Arthritis Rheum. 2012;64:S895. [Google Scholar]
- 23.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhernakova A, Stahl EA, Trynka G, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7(2):e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles JT, Danoff SK, Sokolove J, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease [published online ahead of print May 28, 2013] Ann Rheum Dis. doi: 10.1136/annrheumdis-2012-203160. ( doi:10.1136/annrheumdis-2012-203160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H-S, Irigoyen P, Kern M, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56(6):1745–1753. doi: 10.1002/art.22703. [DOI] [PubMed] [Google Scholar]
- 28.Källberg H, Ding B, Padyukov L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–511. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki A, Yamada R, Yamamoto K. Citrullination by peptidylarginine deiminase in rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108(1):323–339. doi: 10.1196/annals.1422.034. [DOI] [PubMed] [Google Scholar]
- 30.Thewissen M, Somers V, Venken K, et al. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007;123(2):209–218. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Scher JU, Ubeda C, Equinda M, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64(10):3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deane KD, O'Donnell CI, Hueber W, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62(11):3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes-Austin JM, Deane KD, Derber LA, et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA) Ann Rheum Dis. 2013;72(6):901–907. doi: 10.1136/annrheumdis-2012-201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolfenbach JR, Deane KD, Derber LA, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–1742. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]