Abstract
As the spermatogenesis- and oogenesis-specific basic helix-loop-helix 1 (SOHLH1) transcription factor has been shown to be essential for spermatogonial differentiation in mice, we examined the immunoexpression of this protein in the testis of the rhesus monkey (Macaca mulatta) during puberty, the stage of development when spermatogonial differentiation is initiated in higher primates. Immunopositive SOHLH1 cells were observed only on the basement membrane of the seminiferous cords and tubules. Prior to puberty, essentially 100% of SOHLH1-positive spermatogonia co-expressed the glial cell line-derived neurotrophic factor family receptor alpha 1 (GFRα1), a marker for undifferentiated spermatogonia, and >80% of the immunopositive SOHLH1 cells exhibited only cytoplasmic staining of this transcription factor. Nuclear-only SOHLH1 was found in <10% of spermatogonia in testes from pre-pubertal animals. Puberty was associated with a dramatic and progressive increase in the percentage of immunopositive SOHLH1 cells with nuclear-only staining, and this was associated with (i) a marked reduction in the fraction (∼100–20%) of SOHLH1-positive germ cells co-expressing GFRα1 and (ii) a significant increase in the proportion of SOHLH1-positive spermatogonia that co-expressed the tyrosine kinase receptor (cKIT). Spermatogonia exhibiting nuclear SOHLH1 staining were found to be cKIT positive, but not all cKIT-positive spermatogonia exhibited nuclear SOHLH1 staining. Taken together, these results suggest that, in the monkey, nuclear location of SOHLH1 is closely associated with spermatogonial differentiation.
Keywords: spermatogenesis, SOHLH1, spermatogonial differentiation, rhesus monkey
Introduction
In the rodent, the spermatogenesis- and oogenesis-specific basic helix-loop-helix 1 and 2 (SOHLH1 and SOHLH2) transcription factors, which are expressed only in the gonads (Ballow et al., 2006), have been shown to be essential for spermatogenesis (Ballow et al., 2006; Hao et al., 2008; Toyoda et al., 2009; Barrios et al., 2012; Suzuki et al., 2012). Mice deficient in either SOHLH1 or SOHLH2 are infertile due to a block to spermatogonial differentiation (Ballow et al., 2006; Hao et al., 2008; Toyoda et al., 2009; Suzuki et al., 2012), and mutation of SOHLH1 in humans is associated with azoospermia (Choi et al., 2010).
In light of the role for these transcription factors in spermatogenesis, we examined the association between immunoexpression of SOHLH1 and spermatogonial differentiation at the onset of puberty in the rhesus monkey (Macaca mulatta), a representative higher primate. The seminiferous cords of the pre-pubertal monkey testis contain only Sertoli cells and undifferentiated Type A spermatogonia (Marshall and Plant, 1996; Ramaswamy et al., 2000; Simorangkir et al., 2005, 2012), and therefore the juvenile stage of primate development provides a stable baseline state for examining the cell biology of spermatogonial differentiation, which in the monkey is initiated by the increase in gonadotrophin secretion that is observed in association with the onset of puberty at ∼3.5 years of age (Plant et al., 2005).
In order to relate the pattern of immunoexpression of SOHLH1 in the peripubertal monkey testis to spermatogonial differentiation, established markers of proliferating undifferentiated spermatogonia and differentiating spermatogonia were simultaneously examined using dual immunoflorescence. For this purpose, the expression of glial cell line-derived neurotrophic factor family receptor alpha 1 (GFRα1) and the tyrosine kinase receptor (cKIT) was used as a marker for undifferentiated and differentiating spermatogonia, respectively (Mauduit et al., 1999, Aponte et al., 2005, Hermann et al., 2009, 2010, Gassei et al., 2010). In primates, two types of undifferentiated spermatogonia are generally recognized, namely, type A dark (Ad) and A pale (Ap) (Clermont, 1972; Luetjens et al., 2005; Simorangkir et al., 2005; Amann, 2008; Muciaccia et al., 2013). Ad spermatogonia are characterized by homogenous and dense chromatin as revealed by the nuclear stain, hematoxylin (Simorangkir et al., 2005) and express GFRα1 (Hermann et al., 2009), while Ap spermatogonia exhibit a granular pattern of chromatin (Simorangkir et al., 2005) and may express GFRα1 and/or cKIT (Hermann et al., 2009).
Interestingly, we observed that the initiation of spermatogonial differentiation at the time of puberty in the monkey was associated with a translocation of SOHLH1 into the nucleus in association with the upregulation of cKIT. These findings providing the first description of the cellular localization of SOHLH1 in the primate testis are presented and their translational significance is discussed.
Materials and Methods
Animals and testicular tissue
Testicular tissue from 15 male rhesus monkeys (M. mulatta) was used. The majority of these animals had been castrated at various ages for earlier studies (Simorangkir et al., 2005, 2012). The monkeys had been maintained under a controlled photoperiod (lights on 07:00–19:00 hours) and an ambient temperature of 21°C. The original experiments were conducted in accordance with NIH Guidelines for the Care and Use of Experimental Animals, and were approved by the University of Pittsburgh Institutional Animal Committee on Use and Care.
Experimental design
At the time of castration, the 15 animals were classified into 5 developmental groups (n=3/group): mid-juvenile (MJ, 17–19 months of age), late juvenile (LJ, 33–36 months of age), early pubertal (EP, 38–47 months of age), mid-pubertal (MP, 45–51 months of age) and adult (AD, 71–144 months of age). For animals in the LJ, EP and MP groups, testicular development had been tracked by monitoring testicular volume and location (inguinal versus scrotal) and morning (∼08:30 hours) and nighttime (∼19:00 hours) circulating testosterone (T) concentrations on a weekly basis as reported previously (Simorangkir et al., 2012). The initiation of the onset of puberty in the male rhesus monkey may be recognized by an increase in nighttime circulating T concentrations, which, in our laboratory, typically occurs at ∼36 months of age (Plant, 1985). Animals classified as LJ had testicular volume of <5 ml and showed no evidence of increased circulating T concentrations. Animals were considered EP when combined testicular volume reached 5 ml and nighttime T concentration was maintained in excess of 1 ng/ml for several weeks. MP animals were classified by a combined testicular volume of at least 15 ml and maintenance of nighttime T concentrations in excess of 1 ng/ml for >10 weeks. Of the three adults, testicular tissue was obtained from two animals that had been used in an earlier study by our laboratory (Ramaswamy et al., 2007) or provided from one monkey by Dr Kyle Orwig, Magee-Womens Research Institute.
Processing of testicular tissue for immunohistochemistry
In all cases, a fragment of testis that had been fixed in 10% neutral buffered formalin or 4% paraformaldehyde and paraffin-embedded was cut at 5 µm thickness and used for dual fluorescence immunohistochemical staining for SOHLH1-GFRα1 or SOHLH1-cKIT.
Immunohistochemistry
Testis sections (5 µm thick) were de-paraffinized in xylenes and rehydrated through a series of decreasing concentrations of ethyl alcohol and washed in PBS (0.1 M, pH 7.2). The sections were then subjected to antigen retrieval for 1 h at 97.5°C in ethylenediaminetetraacetic acid buffer (1 mM EDTA solution, pH 8.0, with 0.05% Tween-20), allowed to cool at room temperature for 30 min and washed in PBST buffer (PBS with 0.1% Tween-20, pH 7.2). This was followed by incubations in blocking buffer (PBS with 5–10% normal donkey serum, 3% BSA and 1% Triton X-100) for 30 min at room temperature in a humidified box and then in a cocktail of primary antibodies as follows: For SOHLH1-GFRα1 combination, the primary antibody cocktail consisted of anti-SOHLH1 guinea pig polyclonal antibody (1:250) generated against macaque SOHLH1 amino acids 126–300, using the pET-23 system (Novagen, Merck KGaA, Darmstadt, Germany) and immunoaffinity purified over Affi-Gel 10 (Bio-Rad Laboratories, Inc., Hercules CA, USA) and a mouse monoclonal against recombinant human GFRα1 (1:200; R&D Systems, Inc., Minneapolis, MN, USA). For the SOHLH1-cKIT combination, the cocktail of primary antibodies consisted of the SOHLH1 antibody noted above and a rabbit polyclonal against human cKIT (1:400; DAKO North America, Inc., Carpinteria, CA, USA). The sections were incubated in the respective primary antibody combinations overnight at 4°C in a humidified box. Sections were then rinsed in PBST, incubated in a mixture of secondary fluorescent antibodies (1:200) as follows: for the detection of SOHLH1-GFRα1 signals, a combination of Cy3-conjugated AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch Laboratories, Inc., West Groove, PA, USA) and Alexa Flour 488 donkey anti-mouse IgG (Invitrogen Corporation, Carlsbad, CA, USA), respectively, was used. For the detection of SOHLH1-cKIT signals, a combination of Alexa Fluor 488-conjugated AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch Laboratories, Inc.) and Cy3-conjugated AffiniPure donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.) was used. Sections were incubated in these secondary antibody combinations for 45 min at room temperature in the dark in a humidified box. Finally, sections were washed in PBST, briefly cleared in xylenes and cover slipped with Fluoromount-G mounting medium (Southern Biotech, Birmingham, AL, USA).
For controls, testis sections from an adult monkey were processed for the double immunoflourescence procedures described above, but with selective substitution of the primary antibodies for pre-immune serum (SOHLH1), normal rabbit serum (cKIT) or a normal mouse IgG-1 fraction (GFRα1) (Fig. 1).
Figure 1.
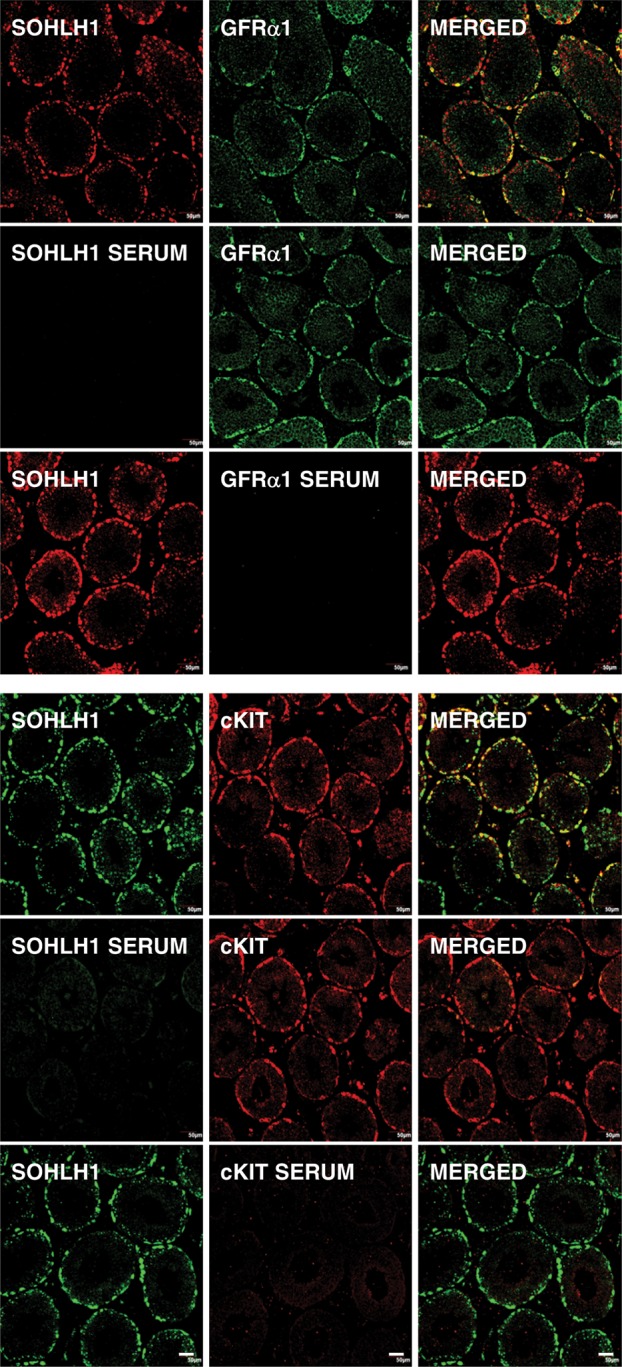
Control patterns of immunostaining observed during dual immunofluorescence for SOHLH1 and GFRα1 (top 9 panels) and SOHLH1 and cKIT (bottom 9 panels) revealed in confocal projections (20×; 1 µm optical sections). The top triplet of projections in each set of panels show the single label for each of the ligands and the merged image on the right-hand side. In the middle triplets of projections, the SOHLH1 primary antibody has been substituted with pre-immune sera and in the lower triplets, the primary antibody for GFRα1 or cKIT was replaced with normal mouse IgG-1 fraction or normal rabbit serum, respectively. Scale bar, 50 µm.
Confocal imaging of dual fluorescence was performed as described previously (Ramaswamy et al., 2008), using an Olympus FV1000 confocal microscope equipped with a four-laser system with transmitted light, differential interference contrast and complete integrated image analysis software system (Olympus America, Inc., Melville, NY). Optical images along the z-axis were collected at 1-µm intervals. Composite digital images were then converted to TIFF format and imported into Adobe Photoshop (Adobe Photoshop CS5 Extended, Version 12.1x64; Adobe Systems, Inc., San Jose, CA, USA) for presentation.
Enumeration of SOHH1-GFRα1 and SOHLH1-cKIT cells
SOHLH1-positive spermatogonia, residing on the basement membrane of the seminiferous cord/tubule, were counted for each animal. They were classified, based on the intracellular location of SOHLH1 staining, as cytoplasmic-only and nuclear-only types. During this counting, dual SOHLH1-GFRα1 and SOHLH1-cKIT stained spermatogonia were also enumerated. The total number of SOHLH1-positive spermatogonia counted in each group ranged from 494 to 500 (MJ), 457 to 505 (LJ), 499 to 550 (EP), 490 to 600 (MP) and 498 to 510 (AD) for the SOHLH1-GFRα1 combination. For the SOHLH1-cKIT combination, these numbers were 253–257 (MJ), 252–261 (LJ), 250–255 (EP), 258–262 (MP) and 246–261 (AD). In both cases, the respective total number of SOHLH1 cells was considered 100% and the percent of each type, i.e. cytoplasmic-only and nuclear-only and SOHLH1-GFRα1 or SOHLH1-cKIT dual positive, was then calculated. Finally, the mean and SEM of each type were calculated for each group and data presented as histogram.
Statistical analysis
All numerical data are presented as mean ± SEM. Categories of SOHLH1-positive cells were expressed as percentages of the total population of SOHLH1 cells for a given stage of development, and the significance of differences between categories across development was determined using one-way ANOVA, followed by the Neuman–Keuls multiple comparison test (Prism 5, GraphPad Software, Inc., La Jolla, CA, USA). Significance was assigned at P ≤ 0.05.
Results
SOHLH1 and GFRα1 expression in spermatogonia during peripubertal development
In the testes of MJ and LJ groups, all germ cells appeared to be immunopositive for SOHLH1, and the transcription factor was predominantly located in the cytoplasm of spermatogonia (Fig. 2A). With the onset of puberty (EP group), a significant population of spermatogonia displayed SOHLH1 immunostaining in the nucleus, while those with cytoplasmic staining decreased significantly (Fig. 2A). A further increase in nuclear SOHLH1 in association with a decrease in cytoplasmic staining for this transcription factor was apparent in spermatogonia in the MP and AD groups (Fig. 2A). In contrast, GFRα1 staining was exclusively localized in the cytoplasm of spermatogonia at all stages of development, and coincident with the reduction in cytoplasmic SOHLH1 staining with the progression of puberty, dual SOHLH1-GFRα1 staining of spermatogonia also declined (Fig. 2A). In the AD, <25% of nuclear-only SOHLH1-positive spermatogonia co-stained with GFRα1.
Figure 2.
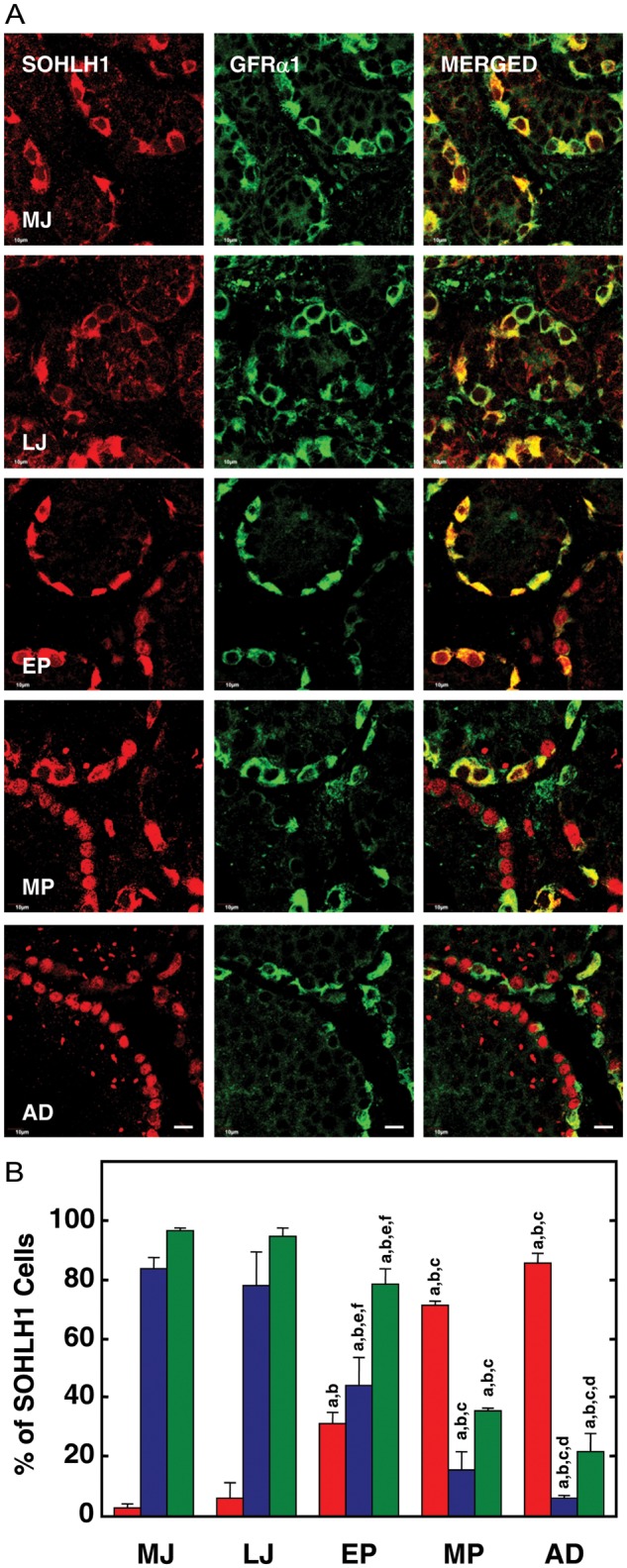
(A) Confocal projections (100×; 1 µm optical sections) illustrating the distribution of SOHLH1 (left-hand panels) and GFRα1 (middle panels) immunostaining in 5 µm sections of testis from a MJ, LJ, EP, MP and AD rhesus monkey. The merged image of the two signals is shown in the right-hand panel. Note the predominantly cytoplasmic staining for SOHLH1 in the juvenile testis and that it is co-localized with that for GFRα1. With the initiation of puberty, SOHLH1 begins to exhibit a nuclear location which is exemplified in the adult. Scale bar, 10 µm. (B) The percentage (mean ± SEM) of immunopositive SOHLH1 spermatogonia exhibiting nuclear-only staining (red bars), cytoplasmic-only staining (blue bars) and co-expressing GFRα1 (green bars) in testes from MJ, LJ, EP, MP and AD rhesus monkeys. Note the progressive and dramatic increase in nuclear location of SOHLH1 that is initiated with the onset of puberty and is associated with a reduction in GFRα1 staining. n=3 for each developmental group. Letters on top of the bars denote significant differences from MJ (a), LJ (b), EP (c), MP (d and e) and AD (f).
The percentages of SOHLH1-positive germ cells exhibiting (i) nuclear SOHLH1 staining only, (ii) cytoplasmic SOHLH1 staining only and (iii) co-staining with GFRα1 are shown in Fig. 2B.
Representative high-power confocal profiles of the intracellular patterns of SOHLH1 and GFRα1 immunostaining in spermatogonia are shown in Fig. 3.
Figure 3.
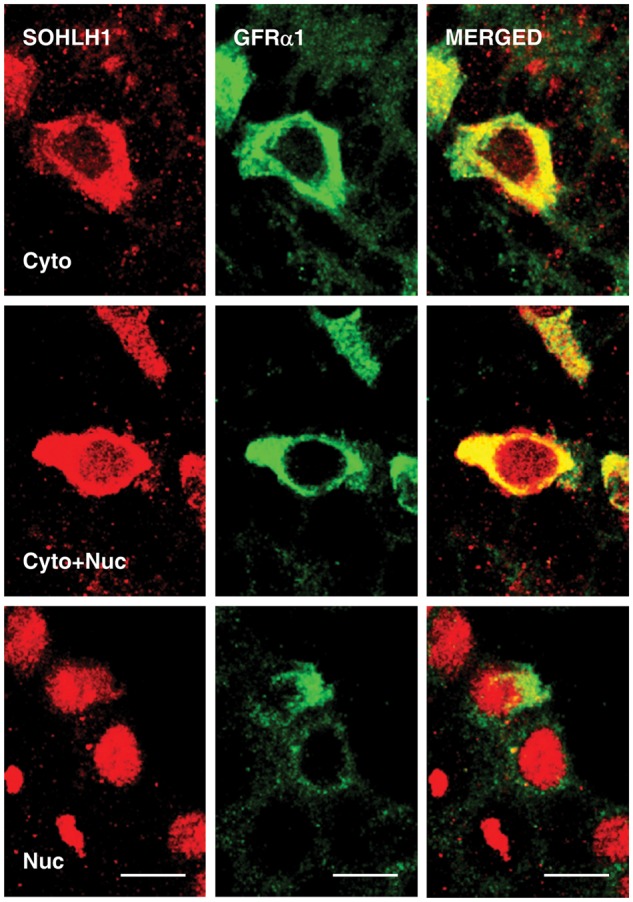
High-power confocal projections of dual immunofluorescence labeling of SOHLH1 (left hand panels) and GFRα1 (middle panels) in 5 µm sections of monkey testis. The right-hand panels show the merged signal. Three patterns of SOHLH1 staining of spermatogonia staining were observed: cytoplasmic-only (top panels), cytoplasmic and nuclear (middle panels) and nuclear-only (lower panels). Scale bar, 10 µm.
SOHLH1 and cKIT expression in spermatogonia during peripubertal development
The same developmental shift in the intracellular location of SOHLH1 immunostaining of spermatogonia described above was also evident in this dual immunoflouresence analysis (Fig. 4A). cKIT immunostaining of germ cells, on the other hand, was absent in testes of the MJ group, and only very weak staining for this marker of differentiation was observed in an occasional spermatogonia in the LJ group (Fig. 4A). A dramatic increase in cKIT-labeled spermatogonia, however, was evident with the onset of puberty (EP group), and further increases were noted in the MP and AD groups (Fig. 4A). In all cases, cKIT staining was exclusively cytoplasmic (Fig. 4A). Moreover, co-localization of SOHLH1 and cKIT immunostaining in spermatogonia began to appear during EP, continued to increase during MP and remained elevated in the AD stage (Fig. 4A). The enumeration of the foregoing observations is presented in Fig. 4B, and confocal profiles of intracellular patterns of SOHLH1 and cKIT immunostaining in spermatogonia are shown in Fig. 5.
Figure 4.
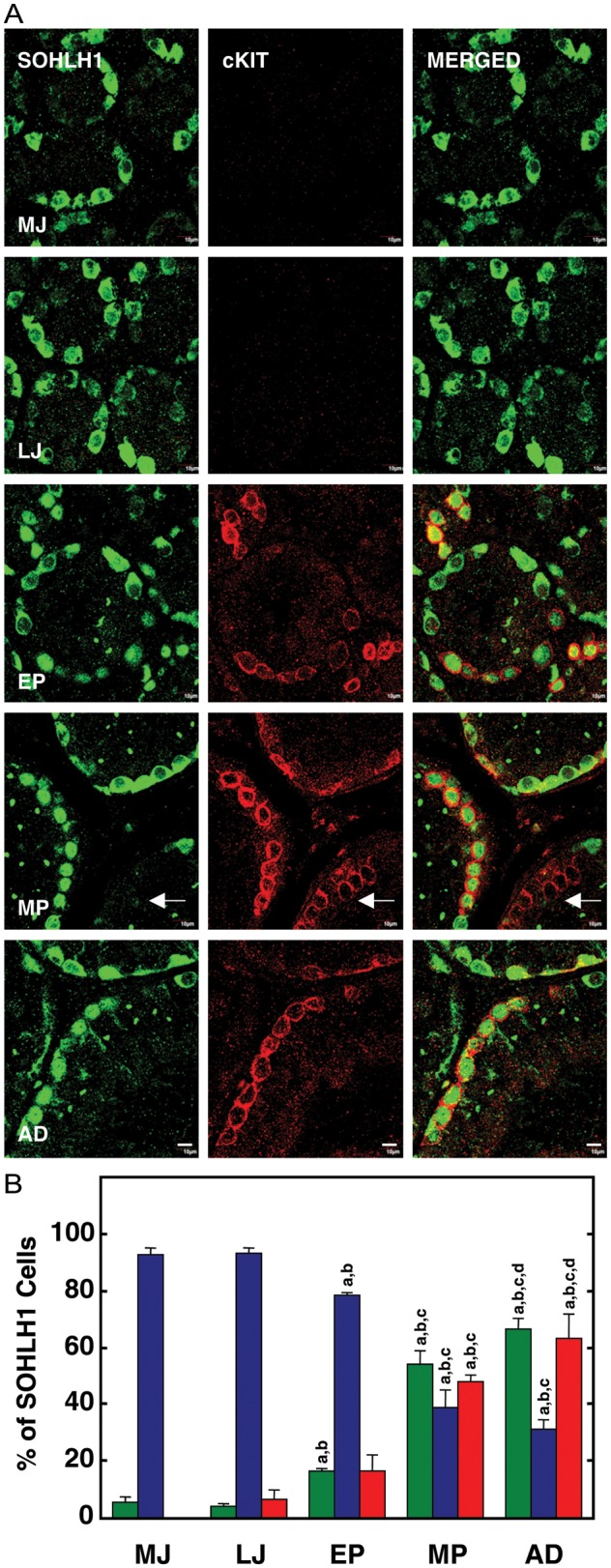
(A) Confocal projections (100×; 1 µm optical sections) illustrating the distribution of SOHLH1 (left hand panels) and cKIT (middle panels) immunostaining in 5 µm sections of testis from a MJ, LJ, EP, MP and AD rhesus monkey. The merged image of the two signals is shown in the right-hand panel. Note again the predominantly cytoplasmic staining for SOHLH1 in the juvenile testis and the absence of cKIT staining in these groups. With the initiation of puberty, SOHLH1 begins to exhibit a nuclear location in association with the appearance of cKIT immunostaining. Arrows in the MP group indicate a chain of cKIT-only spermatogonia. Scale bar, 10 µm. (B) The percentage of immunopositive SOHLH1 spermatogonia exhibiting nuclear-only staining (green bars), cytoplasmic-only staining (blue bars) and co-expressing cKIT (red bars) in testes from MJ, LJ, EP, MP and AD rhesus monkeys. Note again the progressive and dramatic increase in nuclear location of SOHLH1 that is initiated with the onset of puberty and is associated with an increase in cKIT staining. n=3 for each developmental group. Letters on top of the bars denote significant differences from MJ (a), LJ (b), EP (c) and MP (d).
Figure 5.
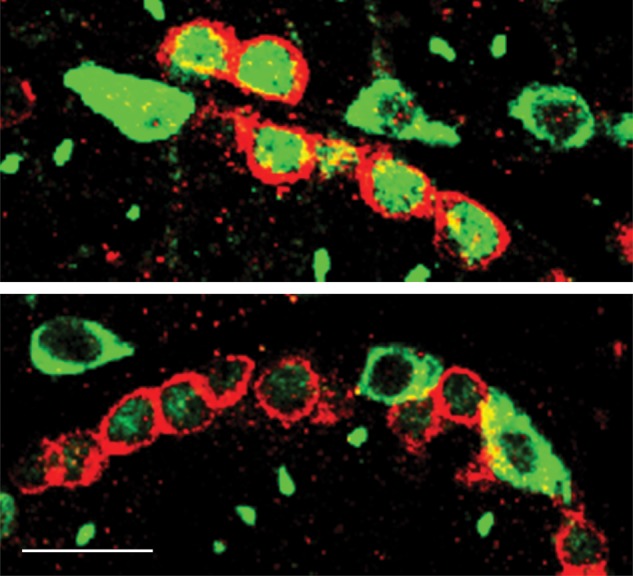
Confocal profiles of intracellular patterns of SOHLH1 (green) and cKIT (red) immunostaining in spermatogonia from an AD (top panel) and a MP (bottom panel) monkey. Note that cKIT-positive spermatogonia may or may not co-express nuclear SOHLH1 immunostaining. Scale bar, 20 µm.
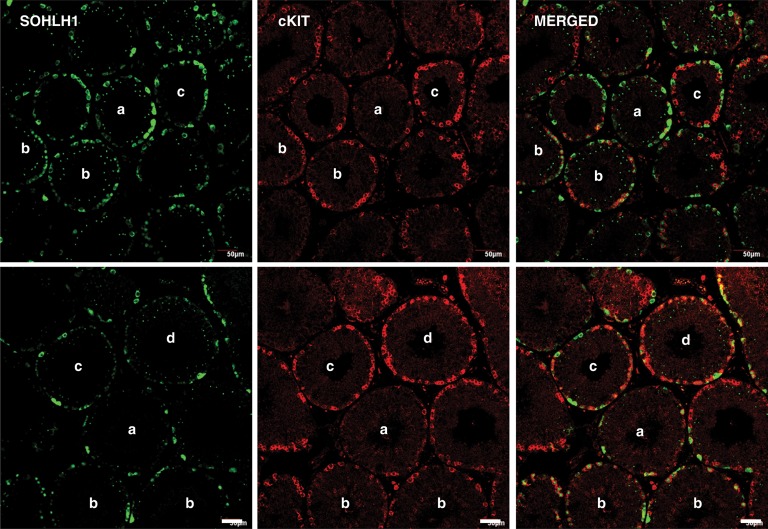
Interestingly, nuclear SOHLH1 staining of spermatogonia was not always associated with cKIT expression in the cytoplasm, and in the developmental comparison this was particularly noticeable in the MP group (Fig. 4A, arrow). Further examination of seminiferous tubules in AD testes dual labeled for SOHLH1 and cKIT confirmed both cKIT protein-containing spermatogonia with or without nuclear SOHLH1 (Fig. 6, Tubules b and Tubule d, respectively). In the latter case, ‘chains’ of spermatogonia with cKIT-only staining were observed (Fig. 6, Tubule d). Two additional patterns of SOHLH1 and cKIT immunostaining were found in AD seminiferous tubules. In the first of these, several nuclear/cytoplasmic SOHLH1-positive spermatogonia were observed with an occasional cKIT-positive cell (Fig. 6, Tubules a) and in the second, spermatogonia with cytoplasm labeled with one, but not both, of the proteins were observed (Fig. 6, Tubules c).
Figure 6.
Confocal projections (20×; 1 µm optical sections) illustrating the variations in the association of SOHLH1 (left-hand panels) and cKIT (middle panels) staining in 5 µm sections of testis from two AD rhesus monkeys. The merged image of the two signals is shown in the right-hand panel. Note cytoplasmic SOHLH1-positive but cKIT-negative staining (Tubules a), dual nuclear SOHLH1 cytoplasmic cKIT staining (Tubules b), cytoplasmic SOHLH1 or cKIT staining (Tubules c) and cKIT-only staining (Tubule d) patterns. Scale bar, 50 µm.
Discussion
Studies on SOHLH1 are very limited, and to date none have examined the primate testis (neither monkey nor human). Here, we report on the cellular location of this transcription factor at the time spermatogonial differentiation is spontaneously initiated in the rhesus monkey, i.e. at the time of puberty. As in boys, puberty in the male monkey is delayed for several years after birth and unfolds over a protracted period of time (Plant and Witchel, 2006). This developmental time frame is vastly different from the rodent where spermatogonial differentiation is initiated a few days after birth (de Rooij and Russell, 2000), questioning whether rats and mice provide the best models to fully recapitulate the developmental biology of the human testis during the critical developmental phase of puberty. Moreover, the tissue set we have generated from normal monkeys throughout puberty extends over a period of 24 months or more from the LJ stage until the MP stage. In our view, it is unlikely that such a repository of normal tissue from animals in which the endocrine status was documented (Simorangkir et al., 2012) will ever be generated again, and the possibility of systematically collecting such normal tissue from boys is simply out of the question.
This study of the rhesus monkey is the first to examine the expression of SOHLH1 in germ cells in the primate testis. At all stages of post-natal development from the MJ (monkeys between 17 and 19 months of age) through the peripubertal phase (animals between 33 and 51 months of age) and in adults, immunopositive SOHLH1 cells were observed on the basement membrane of the seminiferous cords and tubules. Prior to puberty, which in the rhesus monkey is typically initiated at around 3 years of age (Plant, 1985), spermatogonia are the only germ cells present in the testis, and these are almost exclusively undifferentiated (Marshall and Plant, 1996; Ramaswamy et al., 2000; Simorangkir et al., 2005, 2012). The latter view is consistent with the finding in the present study that nearly 100% of SOHLH1-immunopositive spermatogonia at these stages of juvenile development co-expressed GFRα1, a marker for undifferentiated spermatogonia in primates (Hermann et al., 2009; Gassei et al., 2010; Simorangkir et al., 2012), as in rodents (Hermann et al., 2010). Two major types of undifferentiated spermatogonia have been described in primates (type Ad and Ap) (see Luetjens et al., 2005) and, in the rhesus monkey, these are present in approximately equal numbers in the testis at all stages of post-natal development (Marshall and Plant, 1996, Simorangkir et al., 2005). Since all germ cells in the testis of MJ and LJ animals appeared to be SOHLH1 positive, it may be concluded that both Ad and Ap spermatogonia express this transcription factor.
In two independent analyses, >80% of the immunopositive SOHLH1 cells in the two juvenile groups (MJ and LJ) exhibited only cytoplasmic staining, which produced an unambiguous signal resulting in a characteristic doughnut-like appearance of spermatogonia at these pre-pubertal stages of development. Nuclear-only SOHLH1 staining was found in <10% of spermatogonia in testes from juvenile animals. The initiation of puberty in the monkey, which as in other primates is triggered by an increase of gonadotrophin secretion after a protracted period of hypogonadotropism since infancy (Plant and Witchel, 2006), was associated with a dramatic and progressive increase in the percentage of immunopositive SOHLH1 cells with nuclear-only staining for the protein, and in the adult ∼75% of SOHLH1-positive spermatogonia exhibited only nuclear staining (Figs 2B and 4B). The increase in nuclear SOHLH1 with the progression of puberty was associated with a dramatic reduction in the fraction (∼100–20%) of SOHLH1-positive germ cells which co-expressed GFRα1, suggesting that nuclear location of the transcription factor was associated with spermatogonial differentiation in the monkey, as in the mouse (Suzuki et al., 2012). This view is consistent with the finding that the pubertal increase in nuclear SOHLH1 staining was directly correlated with a significant increase in the proportion of SOHLH1-positive spermatogonia that co-expressed cKIT. The progressive and inverse changes in immunostaining for GFRα1 and cKIT during puberty have been previously reported for the animals used in this study (Simorangkir et al., 2012).
SOHLH1 is primarily cytoplasmic in the embryonic mouse gonad and post-natal Day 4 testis, prior to initiation of spermatogonial differentiation (Ballow et al., 2006). In the adult mouse, SOHLH1 localization was nuclear, and SOHLH1-positive spermatogonia were invariable GFRα1 negative (Suzuki et al., 2012), and this observation would seem to be consistent with the present finding that, in the adult monkey, nuclear-only staining for SOHLH1 was seldom found in association with GFRα1 expression.
In the 7-day-old mouse, SOHLH1 was found to be expressed in both undifferentiated (cKIT−) and differentiating (cKIT+) fractions of germ cells separated by magnetic-activated cell sorting (Barrios et al., 2012), and this was shown to also be the case in the adult mouse, as demonstrated by Suzuki et al. (2012) using immunohistochemistry. In the latter study, SOHLH1 was expressed in less primitive generations of undifferentiated spermatogonia (presumably Aal) and preceded the expression of cKIT, which was first observed at stages VI–VII of the seminiferous epithelial cycle (stages that precede the transformation of undifferentiated spermatogonia (Aal) to the first generation of differentiating spermatogonia (A1) (Ahmed and de Rooij, 2009; Plant, 2010). SOHLH1 expression was maintained in cKIT-positive spermatogonia (differentiating) until stages IV–VI, which coincides with the appearance of the most mature premeoitic cells (intermediate and B spermatogonia) (Suzuki et al., 2012).
In the adult monkey, four generations of differentiating spermatogonia are recognized (B1, B2, B3 and B4) (Clermont, 1972; Ehmcke et al., 2005; Marshall et al., 2005; Simorangkir et al., 2009), and in the adult, ‘chains’ of spermatogonia exhibiting intense nuclear SOHLH1 staining were found to be either cKIT positive or cKIT negative. Similarly, cKIT-positive spermatogonia did not always exhibit nuclear SOHLH1 staining. Based on the mouse studies, it is likely that SOHLH1+/cKIT− cells represent a less differentiated subset of spermatogonia, while SOHLH1−/cKIT+ cells represent a more differentiated subset. These results underline the need for a systematic study of the expression of SOHLH1 and cKIT with respect to the 12 stages of the seminiferous epithelial cycle of the monkey (Clermont, 1972) in order to gain a more precise insight into the role that SOHLH1 plays in the lineage of differentiating spermatogonia.
Interestingly, nucleocytoplasmic shuttling of another basic helix-loop-helix transcription factor (transcription factor E3) has been recently implicated in the regulation of differentiation in embryonic stem cells (see Tsuneyoshi and Dunn, 2013). Although the genes that are targeted following import of SOHLH1 into the nucleus of spermatogonia in the monkey testis remain to be studied, it is anticipated that they will likely be the same as those established from studies of transgenic mice (Ballow et al., 2006; Barrios et al., 2012; Suzuki et al., 2012), where SOHLH1 directly repressed the expression of GFRα1 while up-regulating that of cKIT.
In summary, we have shown that SOHLH1 is expressed exclusively in pre-meiotic germ cells of the primate testis, and that at the time of puberty when spermatogonial differentiation is initiated, there is a major redistribution of the intracellular localization of this transcription factor. Before puberty when type A undifferentiated spermatogonia are the only germ cells present in the monkey testis, SOHLH1 is located predominantly in the cytoplasm. With the initiation of spermatogonial differentiation at the onset of puberty and the appearance of differentiating B spermatogonia, nuclear location of SOHLH1 becomes apparent and this increases as puberty progresses. In the adult testis, ∼75% of SOHLH1-positive spermatogonia exhibit only nuclear staining for the transcription factor. This finding suggests that, in the monkey, as in the mouse, SOHLH1 (and presumable SOHLH2—not studied) plays a role in regulating spermatogonial differentiation.
Authors’ roles
S.R.; contributed to the design of the study, supervised the immunoflourescence procedures, analysed data, prepared figures and wrote the manuscript with T.M.P.: B.S.R. and R.M.R.; performed the dual immunoflourescence: H.S.; generated the antibody to SOHLH1 and contributed conceptually; G.R.M., mentored B.S.R. and together contributed conceptually; A.R.; contributed to the design of the study, evaluation of data and to writing of the manuscript, T.M.P., led the design of the study and the writing of the manuscript, supervised analysis of data and preparation of figures.
Funding
This work was supported by National Institutes of Health (U54HD08610, R01HD013254 and R01HD072189 to T.M.P. and HD044858 to A.R.).
Conflict of interest
None declared.
Acknowledgements
We thank Dr. Robert B. Gibbs, Department of Pharmaceutical Sciences, University of Pittsburgh School of Pharmacy for the use of the Institution's Confocal Microscopy Facility and Dr. Kyle E. Orwig, Department of Obstetrics, Gynecology and Reproductive Sciences for the donation of tissue. Seyed Nourashrafeddin and Carolyn Phalin assisted with the immunoflourescence analyses.
References
- Ahmed EA, de Rooij DG. Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol. 2009;558:263–277. doi: 10.1007/978-1-60761-103-5_16. [DOI] [PubMed] [Google Scholar]
- Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–487. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- Aponte PM, van Bragt MPA, de Rooij DG, van Pelt AMM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113:727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- Ballow D, Meistrich ML, Matzuk MM, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006;294:161–167. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, Pellegrini M, Ottolenghi S, Rossi P, Jannini EA, Dolci S. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci. 2012;125:1455–1464. doi: 10.1242/jcs.092593. [DOI] [PubMed] [Google Scholar]
- Choi Y, Jeon S, Choi M, Lee MH, Park M, Lee DR, Jun KY, Kwon Y, Lee OH, Song SH, et al. Mutations in SOHLH1 gene associate with nonobstructive azoospermia. Hum Mutat. 2010;31:788–793. doi: 10.1002/humu.21264. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- De Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Ehmcke J, Luetjens CM, Schlatt S. Clonal organization of proliferating spermatogonial cells in adult males of two species of non-human primates, Macaca mulattta and Callithrix jacchus. Biol Reprod. 2005;72:293–300. doi: 10.1095/biolreprod.104.033092. [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Dhir R, Schlatt S. Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRa1-positive subpopulation in men. J Med Primatol. 2010;39:83–91. doi: 10.1111/j.1600-0684.2009.00397.x. [DOI] [PubMed] [Google Scholar]
- Hao J, Yamamoto M, Richardson TE, Chapman KM, Denard BS, Hammer RE, Zhao GQ, Hamra FK. Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells. 2008;26:1587–1597. doi: 10.1634/stemcells.2007-0502. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetjens CM, Weinbauer GF, Wistuba J. Primate spermatogenesis: new insights into comparative testicular organization, spermatogenic efficiency and endocrine control. Biol Rev. 2005;80:475–488. doi: 10.1017/s1464793105006755. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Ramaswamy S, Plant TM. Gonadotropin-independent proliferation of the pale type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Biol Reprod. 2005;73:222–229. doi: 10.1095/biolreprod.104.038968. [DOI] [PubMed] [Google Scholar]
- Mauduit C, Hamamah S, Benahmed M. Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update. 1999;5:535–545. doi: 10.1093/humupd/5.5.535. [DOI] [PubMed] [Google Scholar]
- Muciaccia B, Boitani C, Berloco P, Nudo F, Spadetta G, Stefanini M, de Rooij DG, Vicini E. Novel stage classification of human spermatogenesis based on acrosome development. Biol Repro. 2013 doi: 10.1095/biolreprod.113.111682. doi:10.1095/biolreprod.113.111682. [DOI] [PubMed] [Google Scholar]
- Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- Plant TM. Undifferentiated primate spermatogonia and their endocrine control. Trends Endocrinol and Metab. 2010;21:488–495. doi: 10.1016/j.tem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in non-human primates and humans. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wasserman PM, editors. Knobil and Neill's Physiology of Reproduction. San Diego, CA, USA: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- Plant TM, Ramaswamy S, Simorangkir DR, Marshall GR. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann NY Acad Sci. 2005;1061:149–162. doi: 10.1196/annals.1336.016. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Plant TM, Marshall GR. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious Sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta) Biol Reprod. 2000;63:82–88. doi: 10.1095/biolreprod63.1.82. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr., Plant TM. Effect of continuous intravenous administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Ehmcke J, Schlatt S, Plant TM. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Plant TM. A re-examination of proliferation and differentiation of type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Human Reprod. 2009;24:1596–1604. doi: 10.1093/humrep/dep051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simorangkir DR, Ramaswamy S, Marshall GR, Roslund R, Plant TM. Sertoli cell differentiation in rhesus monkey (Macaca mulatta) is an early event in puberty and precedes attainment of the adult complement of undifferentiated spermatogonia. Reproduction. 2012;143:513–522. doi: 10.1530/REP-11-0411. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, Rajkovic A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol. 2012;361:301–312. doi: 10.1016/j.ydbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda S, Miyazaki T, Miyazaki S, Yoshimura T, Yamamoto M, Tashiro F, Yamato E, Miyazaki J. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol. 2009;325:238–248. doi: 10.1016/j.ydbio.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Tsuneyoshi N, Dunn NR. Guards at the gate to embryonic stem cell differentiation. Cell. 2013;153:281–283. doi: 10.1016/j.cell.2013.03.037. [DOI] [PubMed] [Google Scholar]