Abstract
Background. The complement system protects against extracellular pathogens and links innate and adaptive immunity. In this study, we investigated the anaphylatoxin C3a receptor (C3aR) in Chlamydia psittaci lung infection and elucidated C3a-dependent adaptive immune mechanisms.
Methods. Survival, body weight, and clinical score were monitored in primary mouse infection and after serum transfer. Bacterial load, histology, cellular distribution, cytokines, antibodies, and lymphocytes were analyzed.
Results. C3aR−/− mice showed prolonged pneumonia with decreased survival, lower weight, and higher clinical score. Compared to wild-type mice bacterial clearance was impaired, and inflammatory parameters were increased. In lung-draining lymph nodes of C3aR−/− mice the total number of B cells, CD4+ T cells, and Chlamydia-specific IFN-γ+ (CD4+ or CD8+) cells was reduced upon infection, and the mice were incapable of Chlamydia-specific immunoglobulin M or immunoglobulin G production. Performed before infection, transfer of hyperimmune serum prolonged survival of C3aR−/− mice.
Conclusions. C3a and its receptor are critical for defense against C. psittaci in mouse lung infection. In this model, C3a acts via its receptor as immune modulator. Enhancement of specific B and T cell responses upon infection with an intracellular bacterium were identified as hitherto unknown features of C3a/C3aR. These new functions might be of general immunological importance.
Keywords: Chlamydia psittaci, chlamydia, intracellular, bacterium, complement, C3a receptor, anaphylatoxin, adaptive immunity, defense
The intracellular gram-negative pathogen Chlamydia psittaci causes psittacosis in birds. Transmission of C. psittaci to humans can lead to life-threatening pneumonia with systemic dissemination [1, 2]. Infections of cattle and other domestic animals with non-avian strains can result in abortion, respiratory disorders, enteritis, and arthritis. Subclinical, protracted infections have impact on animal development and health [3–6].
All Chlamydia species including C. psittaci and other relevant human pathogens such as C. trachomatis and C. pneumoniae that cause genital, ocular, or respiratory infections, possess a virus-like biphasic developmental cycle characterized by extracellular, infectious “elementary bodies” and an intracellular, metabolically active form. These “reticulate bodies” reside within phagosome-like host cell inclusions [7, 8].
Toll-like receptors 2, 4, and Nod1 recognize chlamydial components. Neutrophils, macrophages, and T cells characterize the cellular response. Cytokines such as interleukin 1α/β (IL-1α/β), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 12 (IL-12), tumor necrosis factor α (TNF-α), granulocyte macrophage colony-stimulating factor (GM-CSF), and interferon γ (IFN-γ) orchestrate defense [9–11]. The humoral response ameliorates control of secondary infection with C. muridarum [12, 13]. Additionally, in genital reinfection caused by this mouse pathogen, Chlamydia-specific antiserum augments T cell immunity via Fc receptors on antigen-presenting cells (APCs), and enhances CD4+ T cell function [14, 15]. CD4+ and CD8+ T cells each are sufficient for effective defense against C. pneumoniae, with IFN-γ as key player [16].
The proteolytic complement cascade comprises essential defense against extracellular pathogens and links innate and adaptive immunity [17]. However, more recently there is evidence emerging that additionally, the complement system orchestrates adaptive immune responses and contributes to the control of intracellular pathogens [18–20]. Three main activation pathways lead to cleavage of complement factors C3 and C5 and release of the anaphylatoxins C3a and C5a. G protein-coupled receptors for C3a (C3aR) and C5a (C5aR, C5a1 receptor, CD88) mediate inflammation and play key roles in diseases including systemic lupus, arthritis, or colitis [17, 21, 22]. Although anaphylatoxin receptors are closely related, their functions overlap only partially; for example, in intestinal ischemia reperfusion injury C5a activates tissue-infiltrating neutrophils, whereas C3a limits neutrophil mobilization from bone marrow (BM) [23]. C5a serves as potent chemotaxin and activator of granulocytes and monocytes/macrophages [17]. In contrast, C3a only weakly affects these cells. The cleavage product C3b opsonizes pathogens promoting phagocytosis. Antigen-bound C3b (and C3d) augment antibody production and differentiation of B memory cells [24, 25]. Finally, the membrane attack complex downstream of C5 can lyse extracellular pathogens [26, 27].
Immune modulatory complement functions become increasingly relevant. APCs and activated T cells express anaphylatoxin receptors. Complement can affect cellular immunity by direct modulation of T cells or by enhancement of APC migration and antigen presentation [28–33].
There is little knowledge about complement during chlamydial infection: Elementary bodies activate complement in vitro, which reduces infectivity in cell culture [34–36]. Recently, our group has shown early, high, and long-lasting complement activation in C. psittaci mouse lung infection [19]. Experiments in C3−/− mice lacking all main effector functions revealed a protective role of complement in chlamydial infection. In contrast, the infection progressed similarly in wild type (WT), C5-deficient, and C5aR−/− animals, suggesting that biologically active C3 cleavage products play an important role.
In the present study, we show a critical role of the C3aR in protection against intracellular C. psittaci. Whereas most WT mice survived C. psittaci infection, C3aR−/− mice had impaired bacterial clearance, prolonged inflammation, and a higher death rate. Moreover, C3aR−/− mice failed to raise C. psittaci-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) and in comparison to WT mice developed weak B and C. psittaci-specific T cell responses in lung-draining lymph nodes (ldLNs). Our observations reveal a hitherto unknown role of the C3aR in adaptive immune responses towards an intracellular microorganism.
MATERIALS AND METHODS
Chlamydial Culture
The C. psittaci strain DC15 (kindly provided by K. Sachse; GenBank accession number CP002806.1) isolated from bovine abortion [37] was propagated in BHK-21 cells as described elsewhere [19]. Inclusion-forming units (IFU) were determined by titration onto HeLa-T cells [38]. For mock controls, BHK-21 cells were processed identically but without Chlamydia and diluted similarly as infected cells. Polymerase chain reaction (PCR) confirmed Mycoplasma-free preparations.
Mouse Strains
Ten to 12 weeks-old male C3aR−/− (B6.129X1-C3ar1tm1Raw– [39]), C3−/− (B6.129S4-C3tm1Crr/J– [40]) and WT (Charles River Laboratories) mice on C57BL/6J background were used. Only in experiments depicted in Supplementary Figure 1, BALB/c mice C3aR−/− (C.129S4-C3ar1tm1Cge/J–Jackson Laboratory) and corresponding WT (Janvier SAS) mice were analyzed. All animal experiments were approved by the Local District Government and carried out in adherence to German regulations for protection of animal life (permit: 33.12-42502-04-091624).
Chlamydia psittaci Lung Infection
For C. psittaci infection mice were anesthetized using 0.1 mL/10 g body weight of the following solution: Ketamine (Albrecht) 0.5 mL [100 mg/mL]; Xylazine (Bayer) 0.1 mL [2%]; NaCl 4.4 mL [0.9%]. Then, 30 µL of 0.9% NaCl solution containing 4 × 104 IFU (C57BL/6J) or 1.3 × 104 IFU (BALB/c) or mock material were intranasally applied [41].
Determination of the Bacterial Load in Lungs and Spleens
Homogenates from right lung or spleen were obtained as described elsewhere [19]. For titration of bacterial burden, thawed, diluted tissue homogenates were centrifuged onto monolayers of HeLa-T cells growing on cover slides. After 24 hours, the slides were washed and fixed with ice-cold methanol before being stained with Chlamydia-specific antibody (Pathfinder Chlamydia culture confirmation system; Bio-Rad). IFU per mL were determined by immunofluorescence microscopy (Zeiss).
Lung Histopathology and Cell Analyses in Broncho-alveolar Lavage Fluid (BALF)
Grading of lung histopathology by light microscopy (Zeiss) on Hematoxylin and Eosin (Merck) stained lung sections was performed as described elsewhere [19]. BALF and cells were obtained as described elsewhere [19]. Cells (4–18 µm) were counted (Scepter Cell Counter, Merck) and used for preparation of cytospin slides. After Diff-Quick staining (Medion Diagnostic) cellular distribution was determined by light microscopy based on 300 cells per slide. All preparations were graded by a blinded expert.
Determination of Myeloperoxidase and of Cytokine Levels in Lung Homogenates
Concentration of granulocyte marker myeloperoxidase (MPO) in lung homogenate was determined as described elsewhere [19] using the mouse MPO Enzyme-linked Immunosorbent Assay (ELISA) Kit (HyCult Biotechnology).
For the quantification of cytokines (IFN-γ, TNF, MCP-1, IL-6, and IL-10), the Mouse Inflammation Cytometric Bead Array (BD Biosciences) was used.
Cell Analyses, T Cell Stimulation, and Intracellular IFN-γ Staining in Lung-draining Lymph Nodes
A cell strainer (BD Biosciences) was used to obtain single cell suspensions of lymph nodes (LNs). Cells were washed with phosphate-buffered saline (PBS) + 1% fetal calf serum (FCS) and counted (Scepter). Fcγ-receptors were blocked for 15 minutes at room temperature with the anti-mouse CD16/32 antibody (101 302; BioLegend). Lymphocytes were stained for 30 minutes at 4 °C cells with anti-mouse CD3, CD4, CD8, and CD19 antibodies. After several wash steps with PBS, CellFIX solution (BD Biosciences) was applied.
To determine C. psittaci-specific T cell responses, BM cells were obtained from femur and tibia of WT mice and cultivated for 7 days in RPMI 1640, 10% FCS, glutamine, β-Mercaptoethanol (43 µM), 100 U/mL penicillin, 100 µg/mL streptomycin, and 5 ng/mL GM-CSF (Invitrogen). These semi-mature bone marrow-derived cells (BMDCs) were infected with C. psittaci (multiplicity of infection = 3) or mock-treated. Twenty-four hours later approximately 1:50 co-cultures of these APCs with T cells from ldLNs obtained from infected WT or C3aR−/− mice were started. Restimulated T cells were harvested after 24 hours and stained for surface markers (see above). For intracellular IFN-γ staining the Cytofix/Cytoperm Fixation/Permeabilization Solution Kit was used (BD Biosciences) according to manufacturer's instructions. Analyses were performed on a FACSCalibur (BD Biosciences).
ELISAs for IgM and IgG
Whole blood was collected by heart puncture, mixed with 100 µL of 200 mM EDTA (Sigma) and transferred to Microtainer SST vials (BD Biosciences). After centrifugation (10 000 × g, 10 minutes, 4 °C) the supernatant was frozen at −80 °C. Determination of anti-Chlamydia IgM and IgG levels was performed as described elsewhere [19] using crude C. psittaci homogenate (1 µg/mL) or mock-material as antigen. Depending on experimental group and ELISA, 1:25 to 1:2500 diluted plasma was added. Horseradish peroxidase-linked antibodies were used for detection of IgM (550 588; BD Biosciences) and IgG (115-036-062; Dianova). Standard curves defining arbitrary units were obtained using pooled plasma from C. psittaci-infected mice.
Serum Transfer Experiment
C57BL/6J WT mice were infected with C. psittaci on day 0 and, after their recovery, on day 28. Blood was collected by heart puncture on day 49. Sera from several mice were pooled, centrifuged, and heat-inactivated for functional decomplementation (56 °C, 1 hour). C3aR−/− mice, serving as serum recipients, were infected with C. psittaci (4 × 104 IFU per mouse). Control serum was transferred intravenously 1 day before infection. Hyperimmune serum was applied (in a volume of 200 µL, diluted in 0.9% NaCl) either 1 day before or 7 days after chlamydial infection.
Sample Sizes and Statistics
Survival of mice was statistically analyzed with the Log-rank (Mantel-Cox) test. Usually, infected and mock-treated mice were compared first using an unpaired t-test. If significant differences were found here, further statistical analyses were applied comparing WT with knockout animals or different time points (9 and 14 days postinfection). Most data sets were transformed to their logarithms to achieve Gaussian distribution. Parametric data with this distribution were analyzed by unpaired t-test (Supplementary Figure 2D), 1-way ANOVA followed by Tukey multiple comparison test, or by 2-way ANOVA followed by Bonferroni Posttest. For all other data Mann-Whitney test or Kruskal-Wallis test followed by Dunn multiple comparison test were applied. For details and group sizes of every experiment see the corresponding figure legends. For graphs and statistical evaluation, GraphPad Prism V5 (GraphPad Software Inc.) was used.
RESULTS
Our previous work indicated that the protective function of C3 in C. psittaci-induced mouse pneumonia must be located “between” C3 and C5 [19]. Following the hypothesis that C3a signaling mediates this defense and in order to elucidate the mechanism, lung infection experiments were performed on C3aR−/− mice.
C3aR Is Protective During Second Week of Chlamydia psittaci Lung Infection
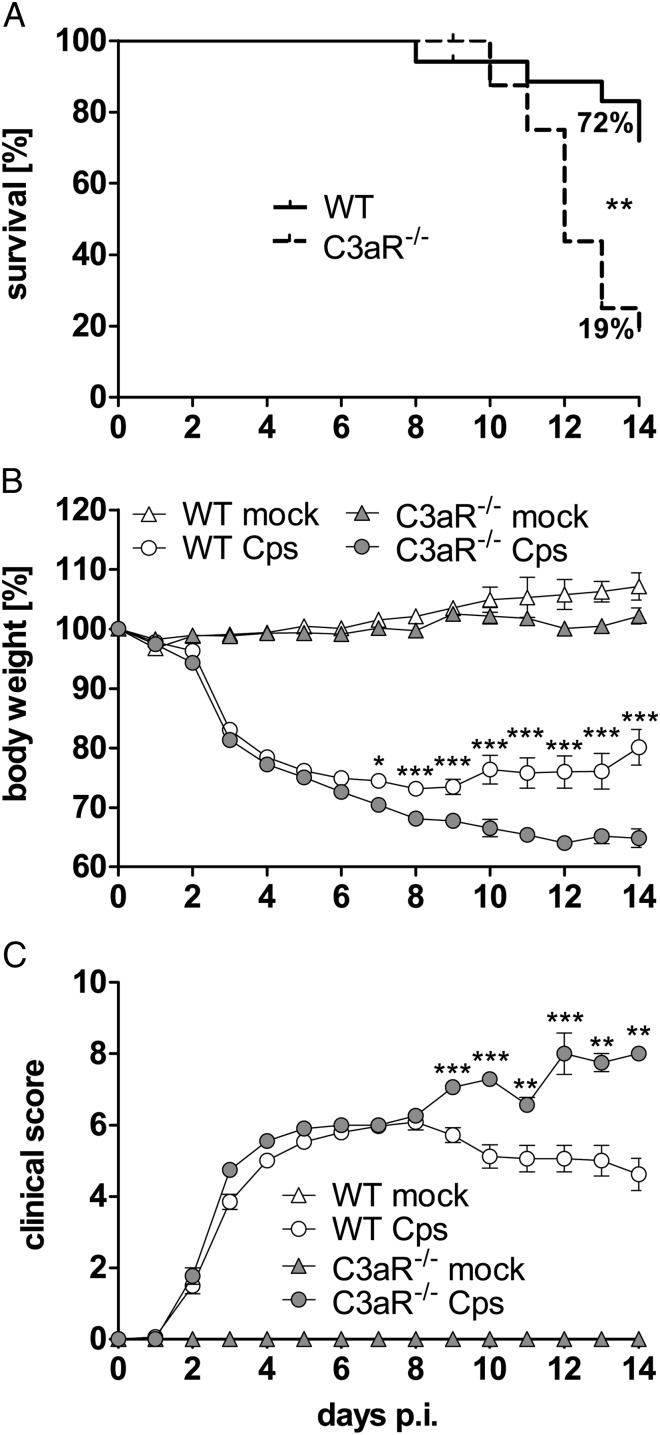
C3aR−/− (C57BL/6J) and corresponding WT mice were infected with 4 × 104 IFU of C. psittaci. During the first week, survival rate, weight loss, and clinical score were similar in WT and C3aR−/− mice. In contrast, at later time points, infected C3aR−/− animals showed higher weight loss and clinical score (Figure 1B and 1C); and eventually only 19% of the knockouts survived until day 14 postinfection (Figure 1A), whereas 72% of WT animals stayed alive. Differences between WT and C3aR−/− mice were preserved in the BALB/c background (Supplementary Figure 1), and the course of disease in C3aR−/− mice resembled the second phase (>8 days postinfection) of lung infection of C3−/− mice (C57BL/6J) (Supplementary Figure 2). These observations indicate that C3aR plays a role in anti-chlamydial immunity that extends to late phases of the immune response.
Figure 1.
C3aR is protective during the second week of C. psittaci lung infection. Thirty-one C3aR−/− or 34 of the corresponding C57BL/6J WT mice were infected intranasally with 4 × 104 IFU of C. psittaci DC15. Body weight and clinical score were recorded until day 14 p.i. Appearance of mice was assessed using the parameters vocalization, body posture, locomotion, breathing, piloerection, overall attention or curiosity, and secretion (eyes, nose, and anal region). Based on severity, dysfunction in each parameter was rated as 1 or 2 points. The overall physical condition was determined by adding all points resulting in a clinical score of untroubled (0 points) to moribund (≥10 points; in this case, mice were euthanized). Only 19% of infected C3aR−/− mice survived for 2 weeks. Fourteen mock-treated C3aR−/− and 12 WT mice did not exhibit any clinical symptoms. After daily monitoring, mice were sacrificed either 9 or 14 d p.i. for further analyses. Survival of mice (A) was statistically analyzed with the Log-rank (Mantel-Cox) test. Body weight (B) was analyzed with 2-way ANOVA and Bonferroni Posttest and clinical score (C) with Mann-Whitney test. Means ± standard errors of the means are depicted. Asterisks indicate significant differences between infected C3aR−/− and the corresponding infected WT mice (*P ≤ .05; **P ≤ .01; ***P ≤ .001). Abbreviations: ANOVA, analsyis of variance; C3aR, C3a receptor; d p.i., days postinfeciton; IFU, inclusion-forming unit; WT, wild type.
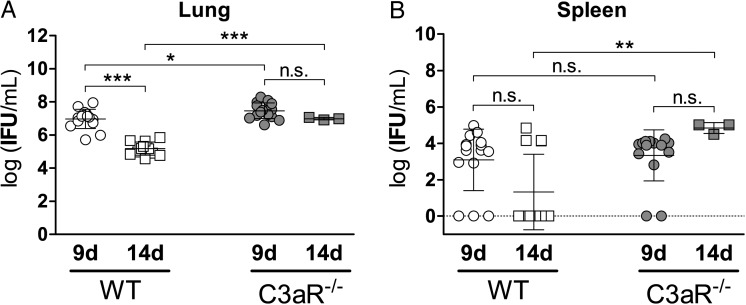
Impaired Clearance of Chlamydia From Lungs and Spleens Without C3aR
The chlamydial load in lung was higher in C3aR−/− compared to WT animals 9 and 14 days postinfection (Figure 2A). Moreover, in WT mice, the bacterial burden declined between these two time points, whereas IFU stayed elevated in C3aR−/− mice. In spleen, mean bacterial load and percentage of C. psittaci-positive organs in WT and knockout mice were similar on day 9 postinfection (>80%; Figure 2B). However, the bacterial burden was significantly higher on day 14 in spleens of the surviving C3aR−/− mice. The IFU/mL in spleen homogenates from both infected strains did not follow Gaussian distribution. Instead, the pattern suggests two subgroups (resulting in large standard deviations), either harboring no detectable viable bacteria indicating successful defense, or approximately 104 IFU/mL C. psittaci suggesting failed defense against dissemination. Although 69% of WT spleens were C. psittaci-negative on day 14 postinfection, all C3−/− survivors were C. psittaci-positive.
Figure 2.
Bacterial load in lungs and spleens of C. psittaci-infected WT and C3aR−/− mice 9 and 14 d p.i. The chlamydial burden was determined by titration of tissue homogenates from sacrificed mice onto HeLa-T cell monolayers and subsequent (24 hours) immunofluorescence analysis. No Chlamydia were detected in mock-treated animals (data not shown). Bacterial loads in lung (A) were normally distributed and analyzed with 1-way ANOVA and Tukey multiple comparison test. For values in spleen (B) without Gaussian distribution Kruskal-Wallis test followed by Dunn multiple comparison test was used. Means ± SD are depicted. Asterisks indicate significant differences (*P ≤ .05; **P ≤ .01; ***P ≤ .001; n.s. = not significant) between infected C3aR−/− (n = 15 for day 9 and n = 3 for day 14 p.i.) and the corresponding infected WT mice (n = 15 for day 9 and n = 13 for day 14 p.i.). Abbreviations: ANOVA, analysis of variance; C3aR, C3a receptor; d p.i., days postinfection; IFU, inclusion-forming unit; SD, standard deviation; WT, wild type.
Lung Histopathology, Granulocytosis, and Cytokine Responses in Lung Homogenate and BALF
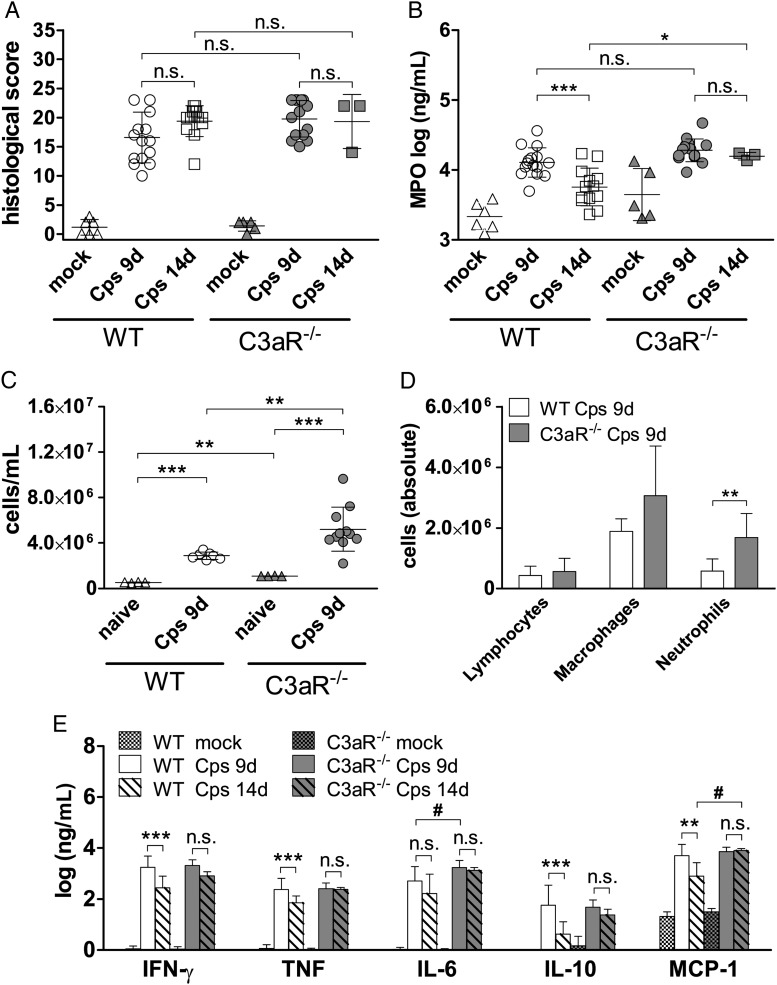
Summing up various parameters of lung histopathology there was no significant difference in “general” C. psittaci-induced inflammation (Figure 3A).
Figure 3.
Lung histopathology, granulocyte marker myeloperoxidase, cytokine levels in lung homogenates, and cell analyses in BALF. A, Histopathological score in lung. Mock-treated mice showed only a minimal inflammatory score. Degree of lung inflammation was scored in H&E-stained sections based on hemorrhaging, inflammatory cells, affected area, edema, peribronchiolar infiltrates, luminal exudates, and overall disease pathology score. Mouse lung pathology was determined by adding all of the points, resulting in a score of 0 (not affected) to 26 (highly affected) points. Since granulocytes can only influence 7 of 26 points of the general histological score, these cells were assessed in more detail applying measurements of their marker enzyme MPO (B). Levels of mock-treated mice were significantly lower than levels of corresponding C. psittaci-infected animals. C, Cell counts (diameter 4–18 µm) in BALF of naïve and infected mice. D, Cellular distribution in BALF of infected mice. The group size for data on WT mice depicted in (C) and (D) was: naïve n = 4; 9 d p.i. n = 7, and on C3aR−/− mice: naïve n = 4; 9 d p.i. n = 11. E, Cytokine levels of WT and C3aR−/− mice. Levels of mock-treated mice were significantly lower than levels of corresponding C. psittaci-infected animals. In addition to differences indicated by asterisks, levels of monocyte chemotactic protein-1 (MCP-1) on day 14 p.i. and of IL-6 on day 9 p.i. were significantly higher in C3aR−/− compared to WT mice (indicated by # P ≤ .05). IL12p70 was below the limit of detection and is not depicted. The group size for measurements on WT mice depicted in (A), (B), and (E) was: mock n = 6; 9 d p.i. n = 15; 14 d p.i. n = 13, and on C3aR−/− mice: mock n = 5; 9 d p.i. n ≥ 14; 14 d p.i. n = 3. Data depicted in panels (A) and (E) were statistically analyzed with Kruskal-Wallis test followed by Dunn multiple comparison test. Data in (B) and (C) were analyzed with 1-way ANOVA and Tukey multiple comparison test. Data depicted in (D) were analyzed with 2-way ANOVA and Bonferroni Posttest after logarithmic transformation. Means ± SD are depicted. Asterisks indicate significant differences between infected WT and C3aR−/−, the different time points, and between naïve and infected mice (*P ≤ .05; **P ≤ .01; ***P ≤ .001; n.s. = not significant). Abbreviations: ANOVA, analysis of variance; BALF, broncho-alveolar lavage fluid; C3aR, C3a receptor; d p.i., days postinfection; IL, interleukin; MPO, myeloperoxidase; SD, standard deviation; WT, wild type.
To assess granulocyte infiltration, we measured MPO highly expressed by these cells in lung homogenates (Figure 3B). Infection led to increased MPO activity in both mouse strains. Correlating with overall bacterial loads in lung, MPO levels in WT mice declined from day 9 to 14 in WT but stayed elevated in C3aR−/− mice.
In BALF, the total number of cells was higher in infected compared to non-infected animals. Moreover, total cell numbers and neutrophil counts were higher in C3aR−/− compared to WT mice (Figure 3C and 3D). In fact, on day 9 postinfection, the infection-triggered influx of granulocytes into the alveoli was even more pronounced in knockout mice than the smaller difference in MPO lung homogenate levels had suggested (Figure 3D). The overall unsuccessful attempt of the neutrophils to combat C. psittaci might participate in organ damage.
In lung homogenates of WT and knockout mice, IFN-γ, TNF, MCP-1, IL-6, and IL-10 were elevated compared to mock-treated animals (Figure 3E). On day 9 postinfection the C. psittaci-induced cytokine pattern was essentially identical in WT and C3aR−/− animals (with only slightly higher concentrations of IL-6 in C3aR−/− mice as exception). Most cytokine concentrations declined in WT mice between day 9 and 14 postinfection. Then, only MCP-1 levels were significantly higher in C3aR−/− mice (Figure 3E).
During the first week of infection, our results show similar primary infection and dissemination of C. psittaci in WT and C3aR−/− mice. However, thereafter only WT mice started to clear the infection. Consistently, C3aR−/− mice showed ongoing inflammation with prolonged elevated MPO and cytokine levels, and more inflammatory cells compared to WT mice. The late C3aR-dependent protection against C. psittaci (reflected by the increased death rate of C3aR−/− mice starting not before day 8 in C57BL/6J and day 13 postinfection in BALB/c mice) suggested an impaired adaptive immune response.
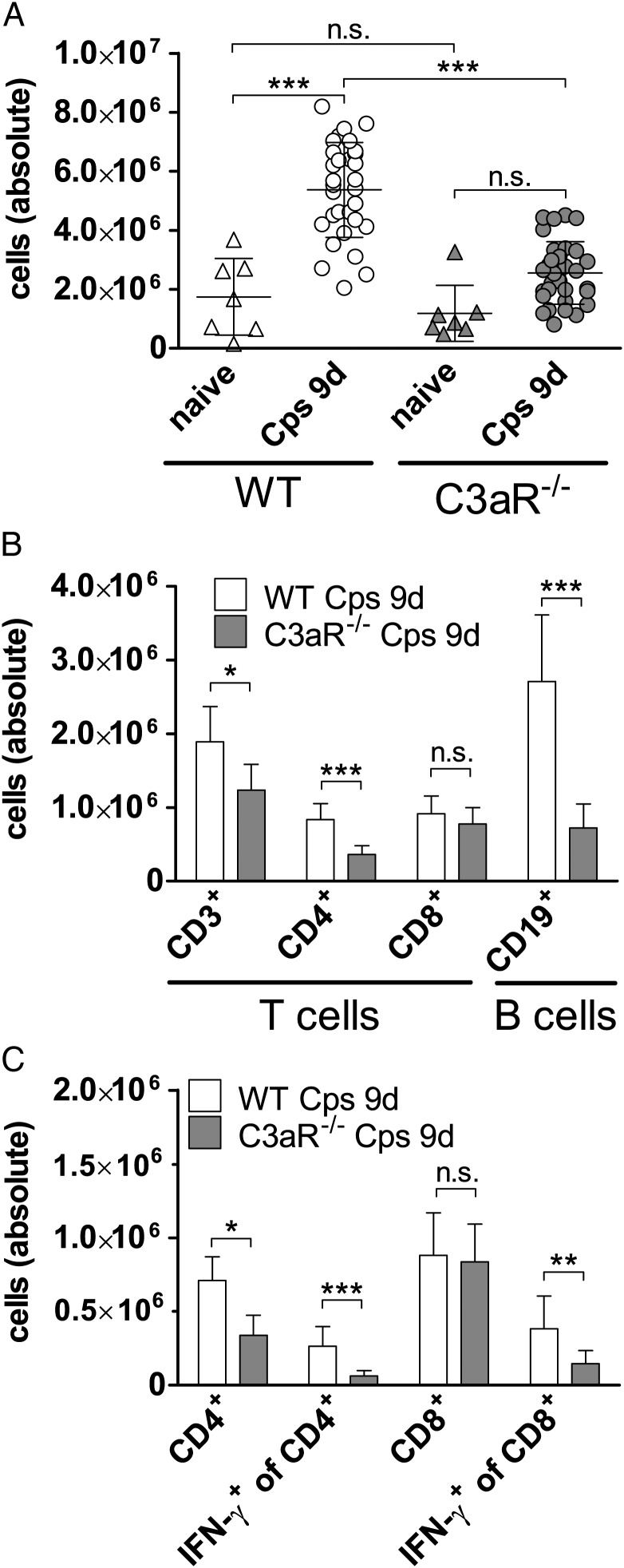
C3aR−/− Mice Show Fewer B and T cells in Lung-draining Lymph Nodes and Reduced IFN-γ Response After Chlamydial Infection
We determined absolute cell numbers (approximately 90% being CD3+ or CD19+ lymphocytes) in ldLNs of naive and infected mice (Figure 4A). Although total cell numbers increased in infected WT animals on day 9, the numbers in C3aR−/− remained nearly unchanged. Flow cytometry showed a lower rise of B and CD4+ T cells in ldLNs of infected C3aR−/− compared to WT mice (Figure 4B). Intracellular INF-γ production by T cells was measured after 24 hours of their restimulation by C. psittaci-infected WT BM-derived dendritic cells (DCs; Figure 4C). Intriguingly, without C3aR, Chlamydia-specific T cell responses were reduced in both CD4+ and CD8+ subsets.
Figure 4.
Cellular analyses in lung-draining lymph nodes of WT and C3aR−/− mice and IFN-γ production by T cells. A, Absolute cell numbers (>90% were B and T cells) were counted for each mouse in ldLNs of naïve and infected animals (9 d p.i.). Whenever an infected mouse was euthanized on day 9 p.i., these numbers were determined resulting in 31 infected WT and 31 infected C3aR−/− mice. In addition, 7 naïve mice of each strain were included. Data were statistically analyzed with 1-way ANOVA and Tukey multiple comparison test. B, Absolute cell numbers of CD3+, CD4+ and CD8+ T and CD19+ B cells in LNs 9 d p.i. and (C) of IFN-γ producing CD4+ and CD8+ T cells restimulated for 24 hours by C. psittaci-infected BMDCs were determined by FACS analyses. There was almost no IFN-γ detectable in controls with non-infected APCs (data not shown). The following antibodies were used to stain surface markers of the lymphocytes and intracellular IFN-γ: anti-mouse CD3-APC; CD4-FITC; CD8-PE; (130-092-977; 130-091-608; 130-091-603; Miltenyi Biotec) and anti-mouse CD8-PerCP/Cy5.5; CD19-PerCP; IFN-γ-PE (100 733; 115 531; 505 807; BioLegend). Data were statistically analyzed with 2-way ANOVA and Bonferroni Posttest after logarithmic transformation. Group size for WT mice was n = 9 and for C3aR−/− mice n = 13 or 14. Means ± SD are depicted. Asterisks indicate significant differences between absolute cell numbers or cellular distributions of naïve and/or infected WT and C3aR−/− mice (*P ≤ .05; **P ≤ .01; ***P ≤ .001; n.s. = not significant). Abbreviations: ANOVA, analysis of variance; APC, antigen-presenting cell; C3aR, C3a receptor; d p.i., days postinfection; IFN, interferon; SD, standard deviation; WT, wild type.
C3aR−/− Mice Lack Chlamydia-specific Antibodies and Can Be Partially Protected Only by Early Administration of Hyperimmune Serum Before Infection
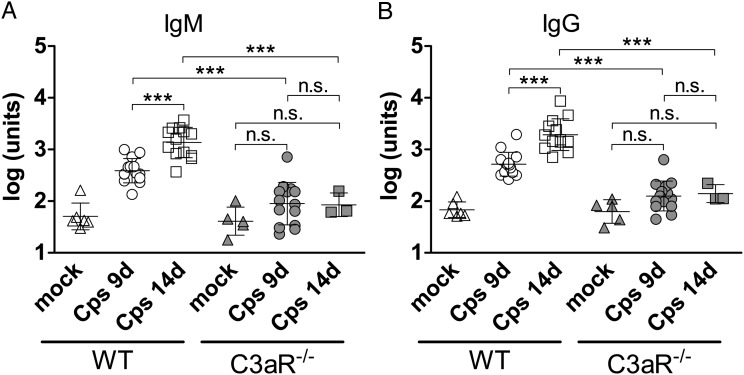
The drastically smaller number of B cells in ldLNs might suggest impaired antibody production in C3aR−/− mice. Indeed, whereas WT animals produced high levels of C. psittaci-specific IgM and IgG 9 days postinfection, further rising during the following days, there was no significant increase in C. psittaci-specific antibodies of C3aR−/− mice upon chlamydial infection (Figure 5A and 5B) despite the even higher bacterial load (Figure 2).
Figure 5.
C3aR−/− mice are not able to produce Chlamydia-specific antibodies. C. psittaci-specific IgM (A) and IgG levels (B) in C. psittaci-infected WT and C3aR−/− mice on day 9 and 14 p.i. and mock-treated mice. Data were statistically analyzed with 1-way ANOVA and Tukey multiple comparison test. Asterisks indicate significant differences between infected WT and C3aR−/− as well as between the different time points (***P ≤ .001; n.s. = not significant). No significant differences could be found between mock-treated WT and C3aR−/− mice as well as between mock-treated and infected C3aR−/− mice. The group size for data on WT mice was: mock n = 6; 9 d p.i. n = 14; 14 d p.i. n = 13, and on C3aR−/− mice: mock n = 5; 9 d p.i. n = 14; 14 d p.i. n = 3. Abbreviations: ANOVA, analysis of variance; C3aR, C3a receptor; d p.i., days postinfection; IgG, immunglobulin G; IgM, immunoglobulin M; SD, standard deviation; WT, wild type.
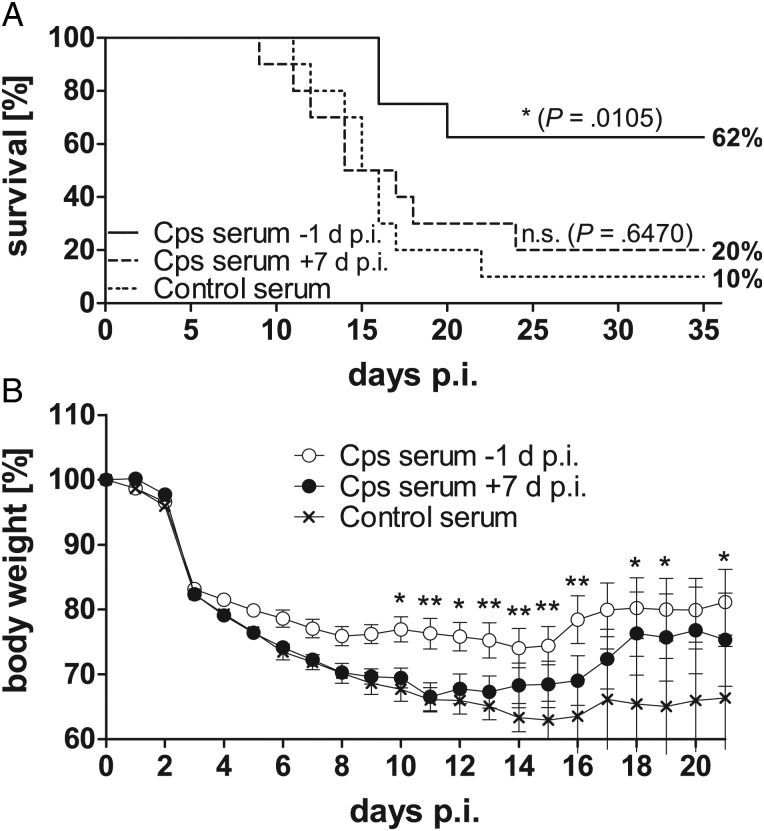
To determine the contribution of C. psittaci-specific antibodies to protection of WT mice, serum transfer experiments were performed. WT mice were repeatedly infected with Chlamydia. Hyperimmune serum from these mice was transferred to C3aR−/− mice after their recovery. C. psittaci-specific antibody titers in the recipients were comparable or even slightly higher to the titers present in WT mice 9 days postinfection (data not shown). To clarify whether the lack of antibodies in C3aR−/− mice can explain their high lethality in our model, hyperimmune serum was administered on day 7 of ongoing primary infection. Infected C3aR−/− mice of this group gained slightly (but not significantly) more weight several days after transfer compared to infected mice receiving control serum. However, C. psittaci-specific IgG present from day 7 postinfection on had no effect on survival. This suggests that serum transfer at a late stage of infection does not protect C3aR−/− mice against lethal chlamydial infection. In contrast, transfer of C. psittaci-specific serum 1 day before infection protected C3aR−/− mice increasing survival rates from 10% to 62% and limited early weight loss (Figure 6A and 6B). This indicates that immune serum transfer partially restored the WT phenotype in receptor knockouts suggesting a protective role of antibodies (and C3a/C3aR) in C. psittaci reinfection.
Figure 6.
Only early transfer of C. psittaci-specific antibodies partially protects C3aR−/− mice from severe pneumonia and increases their survival rate. Survival of mice (A) was statistically analyzed with the Log-rank (Mantel-Cox) test. Body weight (B) was analyzed with 2-way ANOVA and Bonferroni Posttest. Ten C3aR−/− mice received C. psittaci-specific hyperimmune serum one day before infection, 8 mice received this serum 7 d p.i., and n = 8 mice received only control serum. Hyperimmune serum was positive in the anti-C. psittaci ELISA for IgG, in contrast to pooled serum of not-infected mice providing control serum (data not shown). After the transfer of hyperimmune serum, C. psittaci-specific IgG reached levels similar to those observed 2 or 3 weeks after primary infection of WT mice (data not shown). Mice were monitored for up to 35 days to determine survival rates. Means ± standard errors of the means are depicted. Asterisks indicate significant differences between infected C3aR−/− recipient mice receiving C. psittaci-specific serum 1 day before or 7 days after chlamydial infection, or control serum one day before infection (*P ≤ .05; **P ≤ .01; ***P ≤ .001). Abbreviations: ANOVA, analysis of variance; C3aR, C3a receptor; d p.i., days postinfection; ELISA, enzyme-linked immunosorbent assay; IgG, immunglobulin G; WT, wild type.
DISCUSSION
Here we show that C3aR is a key factor determining susceptibility to Chlamydia-caused death in mice. Although complement is generally considered as a first line of defense, we observed that C3aR was essential only after more than one week, a time point which coincides with the onset of Chlamydia-directed adaptive immune defense mechanisms. Older studies suggested that C3a suppresses polyclonal and antigen-specific antibody responses [42, 43]. However, in our hands C. psittaci-infected C3aR−/− mice exhibited fewer B cells in ldLNs and were incapable of effective C. psittaci-specific IgM or IgG production. We therefore propose that C3a/C3aR play a central role in adaptive immunity and that current concepts limiting antibody response-modulating effects of the complement system solely to complement receptors (CRs) 1 and 2 must be reconsidered [24, 44, 45].
Transfer of specific hyperimmune serum before infection improved disease outcome in C3aR−/− mice indicating that antibody responses are protective against secondary C. psittaci infection. In line with this, further reports indicated antibody-mediated protection in C. muridarum and C. trachomatis reinfections, suggesting a general function of antibodies in protection against secondary chlamydial infections [12, 13, 46].
Yet, transfer of C. psittaci-specific IgG to C3aR−/− mice on day 7 postinfection, that is, during active infection, had only a very limited effect on disease progression and did not protect from lethality. This indicates that in addition to humoral responses other C3a-dependent functions must be critical for recovery and decreased lethality in primary infection. Indeed, absence of C3a/C3aR signaling resulted in poor T cell responses, including reduced accumulation of CD4+ T cells in ldLNs and impaired generation of antigen-specific CD4+ and CD8+ T cells during infection.
Our data are in agreement with observations in influenza virus infection, which, like C. psittaci infections, are characterized by activation of the complement system. Interestingly, C3 is also essential for survival during this viral lung infection [20]. Moreover, virus-specific IgG responses as well as priming and recruitment of T cells in mice are C3-dependent. Remarkably, they are independent of CR1 and 2 [18]. The IgG response to infection with live vesicular stomatitis virus is also largely independent of CR2-mediated stimulation of B cells. However, for an antibody response against non-replicating virus CR2 is needed [45]. One might speculate that antibody responses and recovery from viral infections might depend on C3aR signaling.
Thus, observations reported here and further published evidence question the common believe that the anaphylatoxin C3a primarily serves as proinflammatory mediator. Instead, C3a and its receptor might rather act as important immune modulators, here fine-tuning various aspects of C. psittaci-directed immunity. During influenza infection, C3a (and C5a) provide migratory signals for lung DCs in mice [20]. Moreover, C3a can influence antigen-specific T cell immunity and Th1 cytokine production via modulation of DCs and macrophages (reviewed by Zhou) [31]; and the anaphylatoxins provide costimulatory signals to naive CD4+ T cells, which are essential for their sustained viability [47]. These examples illustrate the complexity of complement-mediated effects, and we propose that the complement system might similarly modulate various aspects of the immune response directed against intracellular bacteria.
We found similar levels of IFN-γ in lungs of WT and C3aR−/− mice. Consequently, besides C. psittaci-specific IFN-γ producing T cells, an additional C3aR-independent source of IFN-γ in the lung must exist - potentially, activated NK cells. Furthermore, there must be other C3aR-dependent but IFN-γ-independent functions causing bacterial clearance. This might be apoptosis of C. psittaci-infected cells induced by C. psittaci-specific cytotoxic T cells, or stimulation of activated lung macrophages by their C3aR.
Noteworthy, the newly identified C3aR functions alone do not explain all data obtained on C3−/− mice: These animals are even more susceptible than C3aR−/− to C. psittaci and die with an even lower infectious dose ([19], data for C3aR−/− not shown). Moreover, the results from C3−/− mice demonstrate that C. psittaci-activated complement can be harmful to the host during the first week postinfection: Only mice lacking all effector functions downstream of C3 lose less weight than WT mice (Supplementary Figure 2) and show a better clinical score [19]. An improved uptake of C3b-tagged elementary bodies via CR(s) might cause this C3aR-independent effect. Thus, other complement components besides C3a/C3aR additionally modify the course of disease in Chlamydia infection.
The precise function of C3aR in immune modulation and protection needs to be further investigated. The lower numbers of B and T cells in ldLNs might be explained with their reduced proliferation or shortened survival through enhanced apoptosis. This might be caused by hampered C3aR-mediated costimulation of specific cytotoxic T and Th1 cells (and Th2 cells as helpers of B cells). These effects could be alternatively caused by inefficient C3aR-dependent migration of APCs and antigen presentation, or by decreased inhibition of regulatory T cells, as recently described in another context [32, 33]. Further experiments, including adoptive T cell transfer from WT to C3aR−/− mice will help to clarify the role of these mechanisms in C. psittaci infection, to identify the involved target cells of C3a, and to dissect the contribution of the C3aR on CD4+ or CD8+ T cells to the control of primary C. psittaci lung infection.
In summary, the present study indicates an important function of C3aR in the defense against intracellular C. psittaci during mouse lung infection. Moreover, we have identified two new functions of C3a/C3aR in T and B cell-mediated immune protection that might well extend to other infections besides those caused by Chlamydia.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank G. Bartling and E. Wiebe for excellent help as technical assistants, and Prof S. Suerbaum for his support.
Financial support. This work was supported by the DFG-funded CRC587 “Immunoreactions of the lung in allergy and infection” N A16, additionally by the Federal Ministry of Education and Research (BMBF) of Germany under Grant No. 01Kl10011F “Zoonotic Chlamydiae” IX (A. K.), and partially by the DFG-funded European Research Training Group 1273 “Strategies of human pathogens to establish acute and chronic infections” (P. D.) as well as by NIH RO1 AI025011 (R.A.W.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gaede W, Reckling KF, Dresenkamp B, et al. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health. 2008;55:184–8. doi: 10.1111/j.1863-2378.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 2.Rohde G, Straube E, Essig A, Reinhold P, Sachse K. Chlamydial zoonoses. Dtsch Arztebl Int. 2010;107:174–80. doi: 10.3238/arztebl.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longbottom D, Coulter LJ. Animal chlamydioses and zoonotic implications. J Comp Pathol. 2003;128:217–44. doi: 10.1053/jcpa.2002.0629. [DOI] [PubMed] [Google Scholar]
- 4.Theegarten D, Sachse K, Mentrup B, Fey K, Hotzel H, Anhenn O. Chlamydophila spp. infection in horses with recurrent airway obstruction: similarities to human chronic obstructive disease. Respir Res. 2008;9:14. doi: 10.1186/1465-9921-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhold P, Sachse K, Kaltenboeck B. Chlamydiaceae in cattle: Commensals, trigger organisms, or pathogens? Vet J. 2010;189:257–67. doi: 10.1016/j.tvjl.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Vanrompay D, Harkinezhad T, van de WM, et al. Chlamydophila psittaci transmission from pet birds to humans. Emerg Infect Dis. 2007;13:1108–10. doi: 10.3201/eid1307.070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–59. doi: 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Bastidas RJ, Elwell CA, Engel JN, Valdivia RH. Chlamydial intracellular survival strategies. Cold Spring Harb Perspect Med. 2013;3:a010256. doi: 10.1101/cshperspect.a010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 10.Buchholz KR, Stephens RS. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect Immun. 2008;76:3150–5. doi: 10.1128/IAI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rusconi B, Greub G. Chlamydiales and the innate immune response: friend or foe? FEMS Immunol Med Microbiol. 2011;61:231–44. doi: 10.1111/j.1574-695X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 12.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–9. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roan NR, Starnbach MN. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008;10:9–19. doi: 10.1111/j.1462-5822.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore T, Ekworomadu CO, Eko FO, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–24. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 15.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothfuchs AG, Kreuger MR, Wigzell H, Rottenberg ME. Macrophages, CD4+ or CD8+ cells are each sufficient for protection against Chlamydia pneumoniae infection through their ability to secrete IFN-gamma. J Immunol. 2004;172:2407–15. doi: 10.4049/jimmunol.172.4.2407. [DOI] [PubMed] [Google Scholar]
- 17.Klos A, Wende E, Wareham KJ, Monk PN. International union of pharmacology. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol Rev. 2013;65:500–43. doi: 10.1124/pr.111.005223. [DOI] [PubMed] [Google Scholar]
- 18.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–8. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 19.Bode J, Dutow P, Sommer K, et al. A new role of the complement system: C3 provides protection in a mouse model of lung infection with intracellular Chlamydia psittaci. PLoS ONE. 2012;7:e50327. doi: 10.1371/journal.pone.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandasamy M, Ying PC, Ho AW, et al. Complement mediated signaling on pulmonary CD103(+) dendritic cells is critical for their migratory function in response to influenza infection. PLoS Pathog. 2013;9:e1003115. doi: 10.1371/journal.ppat.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wende E, Laudeley R, Bleich A, et al. The complement anaphylatoxin C3a receptor (C3aR) contributes to the inflammatory response in dextran sulfate sodium (DSS)-induced colitis in mice. PLoS ONE. 2013;8:e62257. doi: 10.1371/journal.pone.0062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–8. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MC, Brennan FH, Lynch JP, et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci U S A. 2013;110:9439–44. doi: 10.1073/pnas.1218815110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 26.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–35. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011;17:317–29. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemper C, Atkinson JP. T cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 29.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–24. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Kemper C, Kohl J. Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol. 2013;56:181–90. doi: 10.1016/j.molimm.2013.05.223. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W. The new face of anaphylatoxins in immune regulation. Immunobiology. 2012;217:225–34. doi: 10.1016/j.imbio.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:162–71. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting edge: receptors for c3a and c5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol. 2013;190:5921–5. doi: 10.4049/jimmunol.1300847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Megran DW, Stiver HG, Bowie WR. Complement activation and stimulation of chemotaxis by Chlamydia trachomatis. Infect Immun. 1985;49:670–3. doi: 10.1128/iai.49.3.670-673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JS, Yan LL, Ho Y, Rice PA. Early complement components enhance neutralization of Chlamydia trachomatis infectivity by human sera. Infect Immun. 1992;60:2547–50. doi: 10.1128/iai.60.6.2547-2550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes C, Ferreira VP, Pangburn MK. Native properdin binds to Chlamydia pneumoniae and promotes complement activation. Infect Immun. 2011;79:724–31. doi: 10.1128/IAI.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goellner S, Schubert E, Liebler-Tenorio E, Hotzel H, Saluz HP, Sachse K. Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect Immun. 2006;74:4801–8. doi: 10.1128/IAI.01487-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sommer K, Njau F, Wittkop U, et al. Identification of high- and low-virulent strains of Chlamydia pneumoniae by their characterization in a mouse pneumonia model. FEMS Immunol Med Microbiol. 2009;55:206–14. doi: 10.1111/j.1574-695X.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- 39.Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406–9. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- 40.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A. 1995;92:11490–4. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutow P, Lingner S, Laudeley R, et al. Severity of allergic airway disease due to house dust mite allergen is not increased after clinical recovery of lung infection with Chlamydia pneumoniae in mice. Infect Immun. 2013;81:3366–74. doi: 10.1128/IAI.00334-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan EL, Weigle WO, Hugli TE. Anaphylatoxin-mediated regulation of human and murine immune responses. Fed Proc. 1984;43:2543–7. [PubMed] [Google Scholar]
- 43.Morgan EL. The role of prostaglandins in C3a-mediated suppression of human in vitro polyclonal antibody responses. Clin Immunol Immunopathol. 1987;44:1–11. doi: 10.1016/0090-1229(87)90046-8. [DOI] [PubMed] [Google Scholar]
- 44.Molina H, Holers VM, Li B, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93:3357–61. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochsenbein AF, Pinschewer DD, Odermatt B, Carroll MC, Hengartner H, Zinkernagel RM. Protective T cell-independent antiviral antibody responses are dependent on complement. J Exp Med. 1999;190:1165–74. doi: 10.1084/jem.190.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams DM, Grubbs BG, Pack E, Kelly K, Rank RG. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–82. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.