Abstract
Background. Human immunodeficiency virus (HIV)–infected (HIV+) men are more susceptible to sexually transmitted infections, and may be superinfected by HIV. We hypothesized that HIV induces immune alterations in the foreskin that may impact the subsequent acquisition/clearance of genital coinfections.
Methods. Foreskin tissue and blood were obtained from 70 HIV-uninfected and 20 HIV+ men undergoing circumcision. T cells were characterized by flow cytometry, immunohistochemistry, and polymerase chain reaction.
Results. There was substantial influx of CD8 T-cells into the foreskins of HIV+ men (108.8 vs 23.1 cells/mm2; P < .001); but foreskin CD4 T-cell density was unchanged (43.0 vs 33.7/mm2; P = .67), despite substantial blood depletion (409.0 vs 877.8 cells/µL; P < .001). While frequencies of foreskin C-C chemokine receptor type 5+ (CCR5+) T cells, T regulatory cells, and T-helper 17 cells were unaltered in HIV+ men, CD8 T-cell production of tumor necrosis factor α (TNFα) was decreased. HIV-specific CD8 T cells were present in the foreskins of HIV+ men, although their frequency and function was reduced compared to the blood.
Conclusions. Foreskin CD4 T-cell density and CCR5 expression were not reduced during HIV infection, perhaps explaining susceptibility to HIV superinfection. Foreskin CD8 T-cell density was increased, but decreased production of TNFα may enhance susceptibility to genital coinfections in HIV+ men.
Keywords: circumcision, cytokines, HIV, sexually transmitted infections, T cells
Infection with human immunodeficiency virus type 1 (HIV) is associated with gradual CD4 T-cell loss in the peripheral blood, systemic immune activation, and an increased risk of opportunistic infections and cancers. There is more rapid and profound CD4 T-cell loss within the gut mucosa, which impairs the gut mucosal barrier, causing microbial translocation and systemic immune activation [1, 2]. CD4 depletion is also apparent within the female genital tract mucosa [3, 4], which may increase susceptibility to genital infections such as herpes, candidiasis, and human papillomavirus (HPV).
Male circumcision reduces HIV incidence by 50%–60% [5–7] and HPV and herpes simplex virus 2 (HSV-2) to a lesser degree [8], suggesting that the foreskin is a major site of acquisition for these infections in heterosexual, uncircumcised men. While the immune defenses of the foreskin are poorly understood, normal adult foreskin contains numerous T cells and dendritic cells [9], with enrichment of T-cell subsets such as T-helper 17 (Th17) cells [10] that have important antibacterial and antifungal functions. The effect of HIV on these functional T-cell subsets is not known.
HIV-infected men are more susceptible to HSV-2 infection and reactivation [11–13]. In addition, HIV-infected women have an increased incidence of HPV, gonorrhea, chancroid, candidiasis, and trichomoniasis [11, 14–17], and demonstrate delayed HPV clearance [18]. This increased rate of genital coinfections has important implications for the sexual health of HIV-infected individuals, and the resulting local inflammation may also increase HIV shedding and the likelihood of HIV transmission to sexual partner(s) [19–27] .
We hypothesized that the increased susceptibility to foreskin-acquired infections in HIV-infected men might be caused by HIV-induced immune changes in the foreskin, particularly the loss of CD4 T cell subsets such as Th17 cells. In order to investigate this hypothesis, we collected blood and foreskin tissue from adult men undergoing elective, male circumcision at the Rakai Health Science Program (Uganda), and used flow cytometry and immunohistochemistry to determine whether HIV infection was associated with alterations in foreskin T-cell populations.
METHODS
Study Participants
Participants consisted of 90 men recruited from an established community cohort in Rakai, Uganda [28] who elected to undergo adult circumcision at the Rakai Health Sciences Program in Kalisizo, Uganda. If urethral discharge or genital ulceration was present, surgery was deferred until treatment was completed and symptoms resolved. Participants were offered voluntary HIV counseling and testing, and HIV-infected men were referred to the Rakai Health Sciences HIV care and treatment program. HIV-exposed seronegative men who reported regular unprotected sex with an HIV-infected woman were excluded from this analysis to avoid any potential bias due to mucosal immune differences in this population [29]. All participants provided written informed consent, and ethical approval was obtained through the Institutional Review Boards at the University of Toronto, Uganda Virus Research Institute, Karolinska Institute, and from Western Institutional Review Board. All assays were performed by lab personnel blinded to participant HIV status, and all assays were performed on each foreskin sample collected (n = 90 throughout all analyses).
Sample Collection and Diagnostic Testing
Prior to surgery, a clinical officer administered a behavioral questionnaire, performed a physical examination, and collected blood (ethylenediaminetetraacetic acid anticoagulant) and a subpreputial swab. Swabs were collected using a FLOQswab (COPAN Diagnostics, Murrieta, CA) by the same medical officers throughout the study in a consistent manner, then resuspended in 1 mL AMPLICOR STD Specimen Transport Kit medium (Roche Diagnostic Systems, Branchburg, NJ) and cryopreserved at −80°C.
One HIV-uninfected participant was found to have genital ulceration, and surgery was deferred until symptoms were resolved (3 weeks). Foreskins were processed immediately upon surgical removal: 2 sections snap-frozen in Optimal Cutting Temperature (OCT) compound (Fisher Scientific, Toronto, Canada) for immunohistochemistry, 2 sections placed in RNAlater (Applied Biosystems, Carlsbad, CA) at 4°C overnight before storage at −80°C for polymerase chain reaction (PCR) analysis, and 1 large section collected for T-cell isolation [30].
All diagnostics were performed at the Rakai Health Sciences Program by members of the Genital Immunology Research Group. HIV serology was performed on the day of surgery using 2 enzyme-linked immunosorbent assays (Murex HIV-1.2.O, Abbott, Abbott Park, IL; and Vironistika HIV Uni-Form II plus O Mircoelisa System, bioMerieux; Marcy l'Etoile, France) and discordant results confirmed by Western blot (GS HIV-1 Western Blot, BioRad; Hercules, CA). All participants were also screened for plasma HIV-1 RNA by PCR. RNA was extracted using the Abbott Sample Preparation System, amplified using the Real Time HIV-1 Amplification Reagent Kit (Abbott), and run on the M2000rt RealTime System (Abbott). CD4 counts were performed using Tritest Reagent in combination with Trucount Tubes and analyzed on a FACSCalibur (BD Biosciences, San Jose, CA). HSV-2 serology (Herpes Simplex Type 2 IgG ELISA, Kalon Biological Ltd., Guildford, UK) used cutoffs previously validated in Rakai [30].
T-cell Isolation From the Foreskin and Blood
Foreskins and blood were processed for flow cytometry within 15 minutes of surgery. T cells were isolated by tissue disruption with a combination of mechanical and enzymatic digestion as described, the resulting cell suspension filtered to remove undigested tissue, and cells rested (37°C, 5% CO2) for 3–7 hours. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Ficoll-Paque Plus; Amersham Biosciences; Uppsala, Sweden).
Characterization of T-cell Subsets
Flow cytometry characterization was performed immediately using fresh cells (without cryopreservation) at the Rakai Health Sciences Program. PBMC and foreskin mononuclear cell counts were determined by trypan blue exclusion. Amounts of 1 × 106 PBMCs and 10–20 × 106 foreskin mononuclear cells (depending on yield) were plated in 500-µL culture medium with 5 µg/mL Brefeldin A (GolgiPlug, BD Biosciences) and stimulated with either: 1 ng/mL phorbol-12-myristate-13-acetate (PMA) and 1 µg/mL ionomycin (Sigma; St. Louis, MO), 102 µg/mL HIV peptides, or vehicle (0.1% dimethylsulfoxide). HIV peptides consisted of a pool of 51 predefined epitopes (9–11 amino acids; JPT Peptide Technologies, Berlin, Germany) from a variety of HIV genes, often conserved across clades A–D and shown to be highly antigenic in an East African population [31]. After stimulation, samples were washed with cold 2% fetal bovine serum (FBS) in PBS and stained for CD3 (UCHT1), CD4 (RPA-T4), CD8 (SK1), C-C chemokine receptor type 5 (CCR5) (2D7/CCR5), and CD25 (M-A251; all BD Biosciences). Samples for intracellular staining were permeabilized using either the eBioscience fixation/permeabilization solution for forkhead box P3 (FoxP3) staining (eBiosciences; San Diego, CA) or the BD Cytofix/Cytoperm solution (BD Biosciences). Cells were then stained for tumor necrosis factor α (TNFα) (MAb11; BD Biosciences), interferon (INγ) (B27; BD Biosciences), interleukin (IL) 17a (eBio64DEC17; eBioscience), IL22 (22URTI; eBioscience), and FoxP3 (PCH101; eBioscience). Samples were using the FACSCalibur platform (BD Biosciences) as described [30].
CD3 Density Quantification by Immunohistochemistry
To express flow cytometry–derived T-cell proportions as absolute numbers (T cells per mm2 of tissue), 2 sections of foreskin were stained for CD3 by immunohistochemistry at the University of Toronto, after OCT-cryopreservation, sectioning, and blocking as previously described [29]. Sections were stained with anti-CD3 antibody (Vector Labs), followed by biotin-labeled secondary and Alkaline Phosphatase Streptavidin Labeling Reagent (all Vector Labs). Color development was performed with Alkaline Phosphatase Substrate Kit Vector Red (Vector Labs) and slides were counterstained with Mayer's Hematoxylin (Fisher Scientific). The number of CD3+ T cells per mm2 of tissue was derived from an average of 2 tissue sections from separate foreskin locations. A median of 6.10 mm2 of foreskin tissue was analyzed per patient. Whole sections were scanned using the TissueScope 4000 (Huron Technologies, Waterloo, Canada), and image analysis software (Definiens, München, Germany) was used to delineate the apical edge of the epidermis to a depth of 300 µm (excluding artifacts or folds). CD3 cells in this area were manually counted by an investigator blinded to HIV status. A CD3-positive cell was defined as hematoxylin staining overlapping with, or directly adjacent to, Vector Red staining.
Detection and Quantification of mRNA
Detection and quantification of mRNA was performed at the Karolinska Institute [9]. Foreskin samples were disrupted in lysis buffer using a mechanical rotor; RNA was extracted using an RNeasy kit (QIAgen; Hilden, Germany) and converted to cDNA in a single reverse-transcriptase reaction using superscript reverse transcriptase (Invitrogen, Carlsbad, CA) and random hexanucleotide primers (Roche, Basel, Switzerland). Amplification of ubiquitin C, CD3, CD4, and CD8 cDNA was performed using the ABI PRISM 7700 sequence detection system and FAM-labeled TaqMan MGB probes and primers (Applied Biosystems, Foster City, CA). Each sample was run in triplicate. Relative quantity of target cDNA was computed using the comparative threshold (Ct) method [32]. Ct values for target cDNA were normalized to ubiquitin C using the normalized expression ratio 2−dCT, so that amounts are described as a relative quantity to ubiquitin C.
Cytokine and Chemokine Analysis of Subpreputial Swabs
Subpreputial swabs were assayed for cytokine levels using an electrochemiluminescent detection system at the University of Toronto. A custom Human Ultra-Sensitive 7-spot kit from Meso Scale Discovery (Rockville, MD) was designed to assess the following analytes: IL-1α (interleukin-1α); IL-8, monocyte chemotactic protein-1 (MCP-1); macrophage-derived chemokine (MDC); monokine induced by γ-interferon (MIG); macrophage inflammatory protein-3α (MIP-3α), and regulated on activation, normal T cell expressed and secreted (RANTES). Due to low chemokine concentrations in subpreputial swabs, only levels of IL-8 were quantifiable in >2/3 of men, so cytokines other than IL-8 were treated as a binary outcome.
Statistical Analysis
T-cell populations and levels of mRNA and IL-8 were compared between HIV-infected and negative men by Mann–Whitney U test. Variables found to be associated (P < .1) with HSV-2 status [34] or condom use were controlled for by multivariate general linear regression and adjusted P values are reported. Proportions of men with HIV-specific CD8 T cell responses and detectable MCP-1, MIG, and RANTES were compared by Fisher's exact test; differences in the magnitude of HIV-specific response between blood and foreskin CD8 T cells were determined using paired Wilcoxon signed rank test. Statistical tests were run using SPSS v.20.0 for Mac (IBM; New York, NY). Flow cytometry data was analyzed in FlowJo v.9.5.2 (Treestar; Ashland, OR) and Excel v.12.3.5 (Microsoft; Redmond, WA) prior to statistical testing.
RESULTS
Study Population
Participants consisted of men with prevalent HIV infection (n = 20) and HIV-uninfected men (n = 70) from Rakai, Uganda, who were undergoing elective male circumcision. HIV-infected men had a mean viral load of 30 690 copies/mL (range 0–335 900 copies/mL) and a mean peripheral blood CD4 T cell count of 409 cells/cm3 (range 6–935 cells/cm3). HIV-infected men were more likely to be coinfected with HSV-2 than HIV-uninfected men (90% vs 35%; P < .001; Table 1), and all subsequent immune analyses were controlled for HSV-2 status.
Table 1.
Patient Demographics
| HIV+ (n = 20) | HIV− (n = 70) | sig. | |
|---|---|---|---|
| Age (y) | 38 (28–50) | 35 (22–51) | ns |
| HSV-2 serology (%) | 90.0 | 35.7 | <0.001 |
| Viral load (copies/mL) | 30 693 (0–335 903) | 0 | <0.001 |
| CD4 count (cells/µL) | 409 (6–935) | 877.8 (143.8–2171.7) | <0.001 |
| Antiretroviral therapy (%) | |||
| Treatment-naive | 85.0 | n/a | n/a |
| Recent initiationa | 15.0 | n/a | n/a |
| On TMP/SMX (%) | 25.0 | n/a | n/a |
| Condom use (%) | |||
| Always | 10.0 | 17.1 | <0.001 |
| Sometimes | 40.0 | 2.9 | |
| Not using | 50.0 | 80.0 | |
| Sexual partners in past year (%) | |||
| Single partner | 75.0 | 86.0 | ns |
| Multiple partners | 25.0 | 14.0 | |
| Extramarital relationship (%) | 10.0 | 14.3 | ns |
Abbreviations: HIV, human immunodeficiency virus; HSV-2, herpes simplex virus 2; n/a, not applicable; ns, not significant; TMP/SMX, trimethoprim and sulfamethoxazole.
aDefined as initiation within 6 months, with detectable viral load at the time of circumcision; exclusion of these 3 individuals did not alter analysis outcome.
Foreskin CD4 and CD8 T-cell Proportions
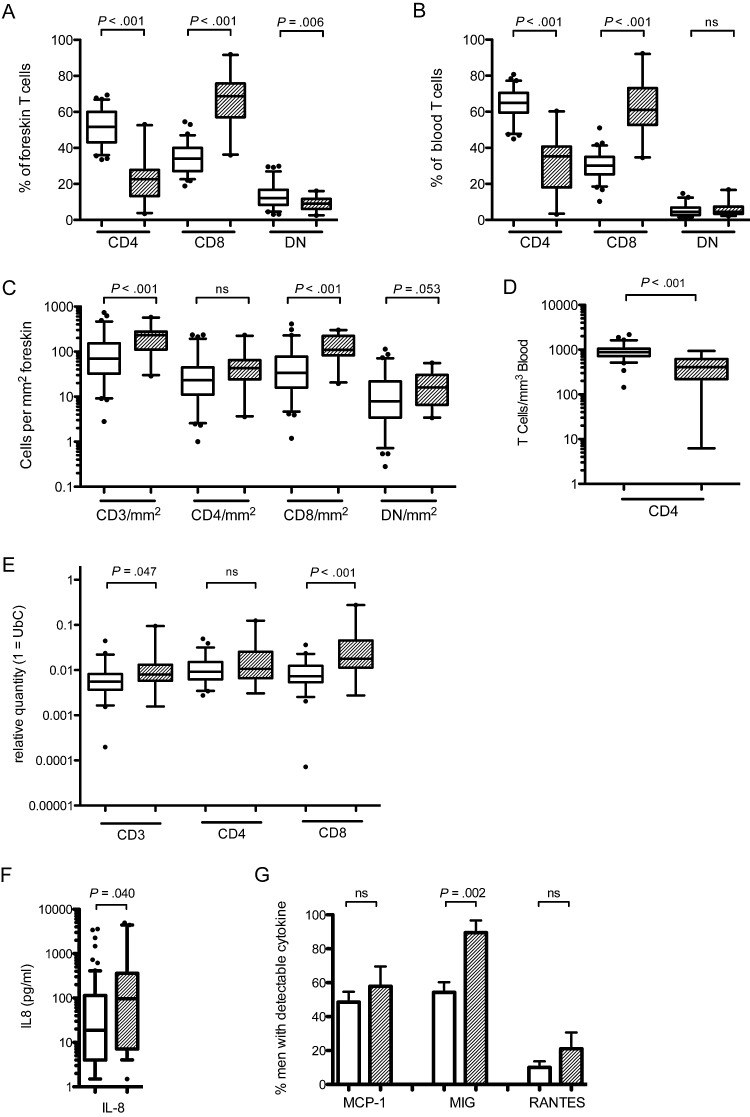
In HIV-uninfected participants, the blood T-cell CD4/CD8 ratio was 2.2 (64.9% CD4 vs 30.1% CD8), while the foreskin had a higher proportion of CD8 T cells, resulting in a ratio of 1.5 (51.7% CD4 vs 34.0% CD8; P < .001 vs blood; Figure 1A and B). In HIV-infected individuals, the CD4:CD8 T-cell ratio was significantly lower in both the blood (0.6 in HIV-infected men vs 2.2 in HIV-uninfected men; P < .001) and the foreskin (0.3 vs 1.5; P < .001). While the decreased CD4:CD8 ratio in the blood of HIV-infected individuals was due to both an increase in CD8 T-cell numbers and a loss of CD4 T cells (absolute count 409.0 vs 877.8 CD4 T cells/µL blood, in HIV+ vs HIV−; P < .001, Figure 1D), in the foreskin the reduced ratio was driven solely by an increase in the absolute number of CD8 T cells. HIV-infected men had over 4-fold more CD8 T cells/mm2 of foreskin tissue (108.8 vs 23.1/mm2; P < .001; Figure 1C), with no reduction in the absolute number of CD4 T cells (43.0 vs 33.7/mm2; P = .67). This was observed in conjunction with increased levels of chemokines in the subpreputial space; HIV-infected men had increased level of IL-8 (97.1 vs 18.8 pg/mL; P = .04; Figure 1F) and were more likely to have detectable MIG (85.0% vs 54.3% of men; P = .002; Figure 1G) than HIV-uninfected men. Frequency of detection of MCP-1 and RANTES did not differ between HIV-infected and uninfected men. IL-1α, MDC, and MIP-3α were not detected in subpreputial swabs.
Figure 1.
T-cell subsets in the blood and foreskin of HIV-infected ( ) and uninfected (
) and uninfected ( ) men. Relative proportions of CD4, CD8, and double-negative (DN) CD3+ T cells were measured in the foreskin (A) and blood (B) using flow cytometry. Number of CD3 T cells per mm2 (C) was obtained through immunohistochemistry and used to calculate the absolute number of foreskin CD4, CD8, and DN T cells from flow cytometry proportions. The absolute numbers of blood CD4 T cells was obtained from clinical CD4 counts (D). Foreskin tissue quantities of T-cell markers were confirmed with PCR (E). Chemokine levels in the subpreputial space were assayed using a multiplex immunoassay system: IL-8 (F) was quantifiable in all swabs and was treated as a continuous outcome; detection of MIG, MCP-1, and RANTES was variable, and therefore presence of the chemokine was compared between HIV-infected and uninfected men (G). Statistical comparisons made by Mann–Whitney U test; CD3, CD4, and CD8 T-cell densities controlled for HSV-2 status by multivariate general linear regression and adjusted P values are reported.
) men. Relative proportions of CD4, CD8, and double-negative (DN) CD3+ T cells were measured in the foreskin (A) and blood (B) using flow cytometry. Number of CD3 T cells per mm2 (C) was obtained through immunohistochemistry and used to calculate the absolute number of foreskin CD4, CD8, and DN T cells from flow cytometry proportions. The absolute numbers of blood CD4 T cells was obtained from clinical CD4 counts (D). Foreskin tissue quantities of T-cell markers were confirmed with PCR (E). Chemokine levels in the subpreputial space were assayed using a multiplex immunoassay system: IL-8 (F) was quantifiable in all swabs and was treated as a continuous outcome; detection of MIG, MCP-1, and RANTES was variable, and therefore presence of the chemokine was compared between HIV-infected and uninfected men (G). Statistical comparisons made by Mann–Whitney U test; CD3, CD4, and CD8 T-cell densities controlled for HSV-2 status by multivariate general linear regression and adjusted P values are reported.
Abbreviations: HIV, human immunodeficiency virus; HSV-2, herpes simplex virus 2; IL-8, interleukin 8; MCP-1, monocyte chemotactic protein-1; MIG, monokine induced by γ-interferon, PCR, polymerase chain reaction; RANTES, regulated on activation, normal T cell expressed and secreted.
Tissue levels of CD3, CD4, and CD8 mRNA were then quantified by PCR in corresponding foreskin biopsies (Figure 1E). Confirming our flow cytometry and immunohistochemistry results, we found no change in expression of CD4 mRNA in the foreskins of HIV-infected men, but instead an increase in the expression of both CD3 (8.0 × 10−3 vs 5.6 × 10−3 relative quantity; P = .047) and CD8 (17.8 × 10−3 vs 7.3 × 10−3 relative quantity; P < .001) mRNA.
CD4 T-cell Subsets in the Foreskin
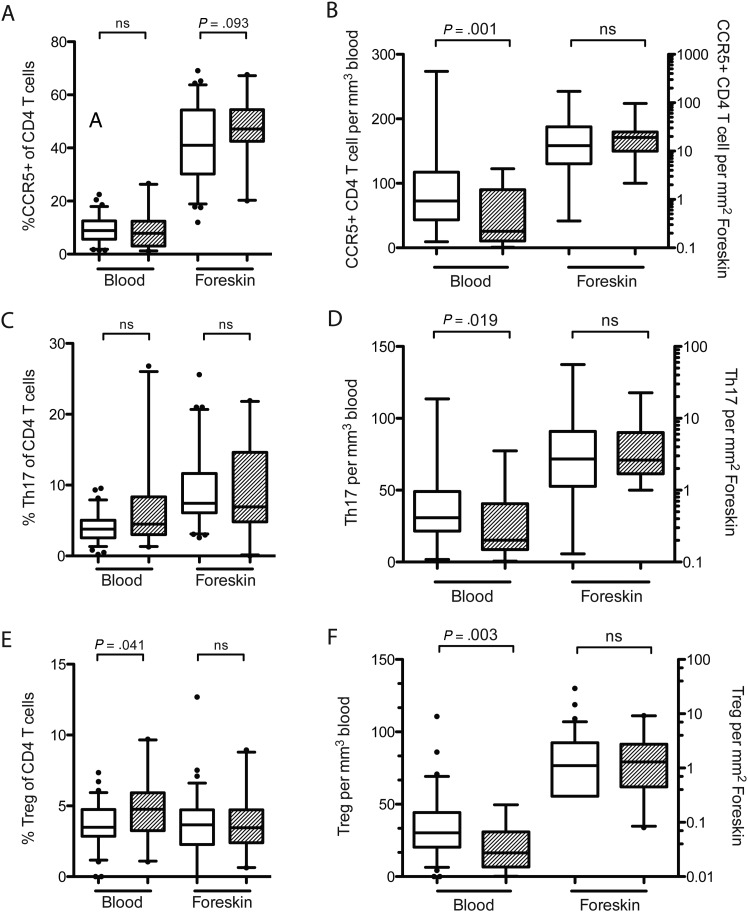
We next assessed the impact of HIV infection on the frequency of CCR5 expression, Th17 cells, and T regulatory cells (Tregs) in the blood and foreskin. The relative proportion of each CD4 T-cell type was determined using flow cytometry, and these proportions were converted to absolute numbers using either the blood CD4 T-cell count or the foreskin CD3+ cell density measured by immunohistochemistry.
After controlling for HSV-2 status, there were no differences in the proportion (Figure 2A) or absolute number (Figure 2B) of foreskin CD4 T cells expressing CCR5. There were also no changes in the proportion of blood CD4 T cells expressing CCR5 in HIV-infected men (Figure 2A). However, due to the overall depletion of CD4 T cells from the blood, the absolute number of CCR5+ CD4 T cells in the blood of HIV-infected men was decreased (25.7 vs 72.6 cells/µL; P = .001; Figure 2B).
Figure 2.
CD4 T-cell subsets in the blood and foreskin of HIV-infected ( ) and uninfected (
) and uninfected ( ) men. Tregs were defined as CD4 T cells coexpressing CD25 and FoxP3; Th17 cells were defined as CD4 T cells producing IL17a in response to PMA and ionomycin stimulation; CCR5+ cells were CD4 T cells expressing the HIV-coreceptor CCR5. The proportion of CD4 T cells that were Tregs (A), Th17 cells (B), or expressed CCR5 (C) were measured using flow cytometry. Proportions were converted into absolute numbers of each cell type (B, D, E) using either the CD3 T-cell density obtained through immunohistochemistry (for foreskin tissue) or clinical CD4 counts (for blood). Statistical comparisons made by Mann–Whitney U test; CCR5+ T-cell proportions and densities controlled for HSV-2 using multivariate general linear regression and adjusted P values are reported.
) men. Tregs were defined as CD4 T cells coexpressing CD25 and FoxP3; Th17 cells were defined as CD4 T cells producing IL17a in response to PMA and ionomycin stimulation; CCR5+ cells were CD4 T cells expressing the HIV-coreceptor CCR5. The proportion of CD4 T cells that were Tregs (A), Th17 cells (B), or expressed CCR5 (C) were measured using flow cytometry. Proportions were converted into absolute numbers of each cell type (B, D, E) using either the CD3 T-cell density obtained through immunohistochemistry (for foreskin tissue) or clinical CD4 counts (for blood). Statistical comparisons made by Mann–Whitney U test; CCR5+ T-cell proportions and densities controlled for HSV-2 using multivariate general linear regression and adjusted P values are reported.
Abbreviations: CCR5, C-C chemokine receptor type 5; FoxP3, forkhead box P3; HIV, human immunodeficiency virus; HSV-2, herpes simplex virus 2; IL-17a, interleukin 17a; PMA, phorbol-12-myristate-13-acetate; Th17, T-helper 17; Tregs, T regulatory cells.
Th17 cells were defined as CD4 T cells producing the cytokine IL17a in response to mitogen stimulation. HIV infection was not associated with any alteration in the proportion of Th17 cells in either the foreskin or the blood (Figure 2C); the absolute number of Th17 cells was significantly decreased in the blood (15.2 vs 30.9 cells/µL; P = .019) but not the foreskin (2.61 vs 2.71 cells/mm2; P = .694) of HIV-infected men (Figure 2D).
Tregs were defined as CD4 T cells coexpressing CD25 and the transcription factor FoxP3. While the proportion of Tregs was increased in the blood of HIV-infected men (4.7% vs 3.5%; P = .041; Figure 2E), the absolute number of Tregs/µL was decreased in HIV-infected men (16.4 vs 30.3 cells/µL; P = .003; Figure 2F). There were no HIV-associated alterations in either the proportion or absolute number of Tregs in the foreskin (Figure 2E and 2F).
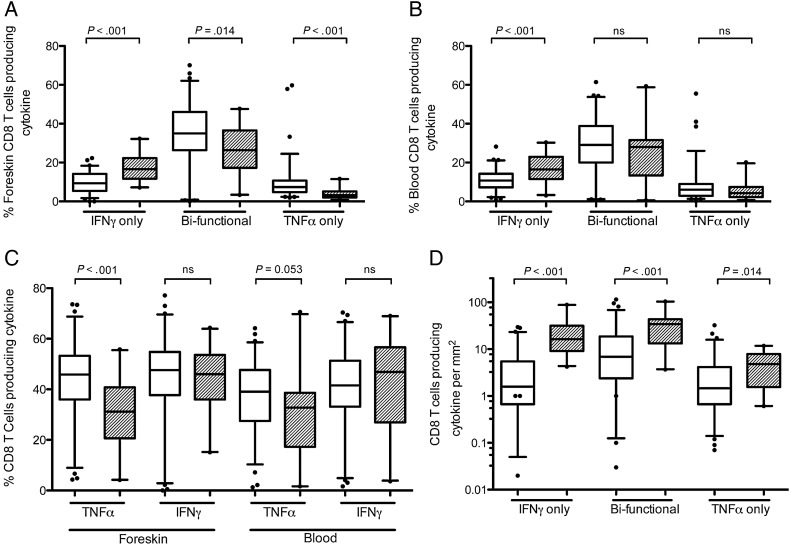
HIV Status and CD8 T-cell Cytokine Production in the Foreskin
We next measured the proportion and absolute number of CD8 T cells producing the cytokines TNFα and interferon γ (IFNγ) in the blood and foreskin after mitogen (PMA-ionomycin) stimulation (Figure 3). Compared to HIV-uninfected men, the foreskins of HIV-infected men had a greater proportion of CD8 T cells that produced only IFNγ (16.6% vs 9.4%; P < .001), while the proportions of CD8 T cells producing both TNFα and IFNγ (bifunctional cells, 26.5% vs 35.0%; P = .014), or only TNFα (3.3% vs 7.3%; P < .001; Figure 3A), were reduced. Therefore, this increase in foreskin CD8 T cell IFNγ monoproduction was driven by a decrease in the proportion of cells able to produce TNFα, as opposed to a gain in IFNγ production: when bifunctional cells were included, there was no overall increase in the proportion of cells producing IFNγ, but instead a decrease in the proportion of cells producing TNFα (31.2% vs 47.6%; P < .001; Figure 3C). However, despite this proportionate reduction in TNFα production, the increased CD8 T-cell infiltration in the foreskin of HIV-infected men meant that there was actually an increase in the absolute number of foreskin CD8 T cells producing TNFα/µL (4.8 vs 1.5 cells/µL; P = .014; Figure 3D).
Figure 3.
Production of inflammatory cytokines by CD8 T cells in the foreskin and blood of HIV-infected ( ) and uninfected (
) and uninfected ( ) men. T cells isolated from foreskin tissue (A) and blood (B) were stimulated with PMA and ionomycin, and subsequent TNFα and IFNγ production by CD8 T cells was measured by flow cytometry, allowing for identification of cells producing only IFNγ, both IFNγ and TNFα (bifunctional cells), or cells that produce TNFα only. Panel (C) shows the overall proportion of cells producing each cytokine (values include bifunctional cells). Proportions of foreskin cell populations were converted into absolute numbers (D) using immunohistochemistry (Figure 1). Statistical comparisons made by Mann–Whitney U test.
) men. T cells isolated from foreskin tissue (A) and blood (B) were stimulated with PMA and ionomycin, and subsequent TNFα and IFNγ production by CD8 T cells was measured by flow cytometry, allowing for identification of cells producing only IFNγ, both IFNγ and TNFα (bifunctional cells), or cells that produce TNFα only. Panel (C) shows the overall proportion of cells producing each cytokine (values include bifunctional cells). Proportions of foreskin cell populations were converted into absolute numbers (D) using immunohistochemistry (Figure 1). Statistical comparisons made by Mann–Whitney U test.
Abbreviations: HIV, human immunodeficiency virus; IFNγ, interferon γ; PMA, phorbol-12-myristate-13-acetate; TNFα, tumor necrosis factor α.
HIV-Specific CD8+ T-cell Responses in the Foreskin
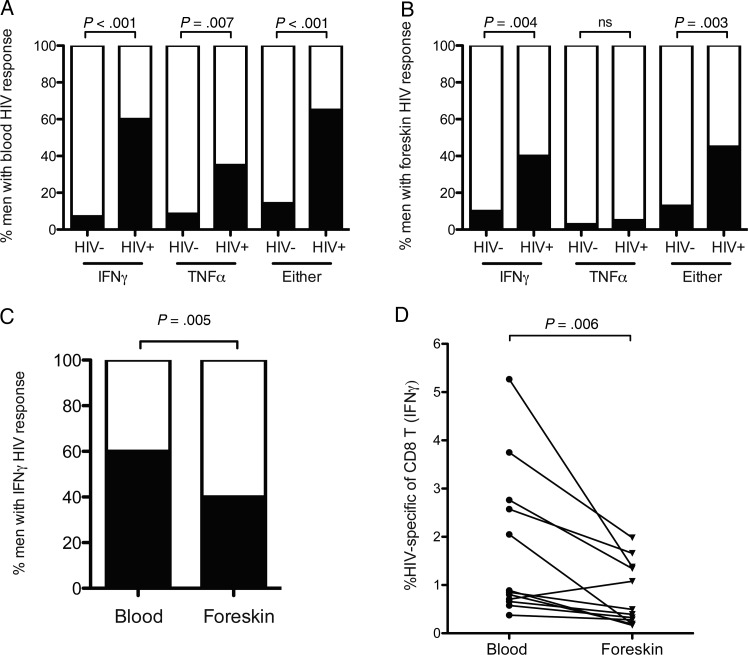
Blood and foreskin cell suspensions were stimulated with a pool of optimized HIV class I epitope peptides previously shown to be highly antigenic in a cohort of East African women [31], and TNFα and IFNγ production were measured by flow cytometry. A positive HIV-specific response was defined as: (1) ≥0.3% of CD8 T cells producing either TNFα or IFNγ in response to HIV peptides, and (2) a percentage of CD8 T cells producing cytokine after peptide stimulation that exceeded the vehicle-only control by at least 3-fold.
HIV-specific CD8 T-cell responses were present in both the blood and foreskin of a large proportion of HIV-infected men. However, while HIV-specific CD8 T cells in the blood produced both IFNγ (60% HIV+ men vs 7.1% HIV− men; P < .001) and TNFα (34% HIV+ men vs 8.6% HIV− men; P = .007; Figure 4A), HIV-specific CD8 T cells in the foreskin produced IFNγ almost exclusively (40.0% HIV+ men vs 10.0% HIV− men; P = .004; Figure 4B). HIV-specific TNFα responses in the foreskin were very infrequent, and their frequency did not differ from HIV-uninfected men (5.0% vs 2.9%; Figure 4B).
Figure 4.
HIV-specific CD8 T-cell responses in the blood and foreskin. CD8 T cells isolated from foreskin tissue or blood were challenged with a pool of cross-clade HIV peptides previously shown to be highly antigenic in an East African population. TNFα and IFNγ production by CD8 T cells was measured by flow cytometry; an HIV-specific response was defined as cytokine production 3 times greater than that observed in unstimulated cells, and a minimum of 0.3% of cells responding (shown in black). The proportion of men with HIV-specific cytokine production in the blood (A) and foreskin (B) is shown (Fisher exact test). Panel (C) compares the proportion of men with a blood-versus-foreskin IFNγ response (Fisher exact test). Among HIV-infected men who had an HIV-specific response, panel (D) compares the percentage of CD8 T cells that were HIV-specific in the blood and foreskin (Wilcoxon signed rank test).
Abbreviations: HIV, human immunodeficiency virus; IFNγ, interferon γ; TNFα, tumor necrosis factor α.
Foreskin HIV-specific CD8 T-cell responses were both less frequent and of a smaller magnitude than blood responses. The proportion of HIV-infected men with a response detected in the foreskin was lower than that with a response in the blood (40.0% vs 60.0%; P = .005; Figure 4C). Furthermore, of those men who did have an HIV-specific CD8 T-cell response in the foreskin, the strength of the response (% of total CD8 T cells) was lower than that in the blood (median difference between foreskin and blood, −0.7%; P = .006; Figure 4D).
DISCUSSION
We demonstrate that HIV infection was associated with a 4-fold increase in the tissue density of CD8 T cells in the foreskin, with no reduction in the density of CD4 T cells. The proportion of CD8 T cells in the foreskin that were able to produce TNFα was decreased during HIV infection, leading to a functional skewing from bifunctional cells able to produce both TNFα and IFNγ, toward IFNγ monoproduction. While HIV-specific CD8 T cells were readily detected in the foreskin tissue of HIV-infected men, the frequency, functional diversity, and magnitude of foreskin HIV-specific responses were lower than in blood.
Maintenance of CD4 T-cell numbers in foreskin tissue during HIV infection despite a substantial reduction in absolute blood CD4 counts was in contrast to mucosal pathogenesis in the cervix of HIV-infected women, where significant CD4 depletion was observed in some [3, 33] but not all [35] studies. Previous studies in the foreskin showed that monoinfection by HIV (ie, without HSV-2) was associated with a loss of CD4+ cells from the foreskin, but that no CD4+ loss was observed in men coinfected with both HIV and HSV-2 [36]. This may be because the host immune response against HSV-2 is characterized by long-lasting mucosal infiltration with antigen-specific CD4+ T cells [37]. Because the great majority of HIV-infected men in our study were coinfected by HSV-2, this limited our ability to assess the impact of HIV monoinfection on foreskin CD4 T-cell numbers. However, we found no HSV-2–associated increase in CD4 T-cell density among HIV-uninfected controls, and point estimates of outcomes were similar after stratification for HSV-2 infection status (significance unaltered by stratification, data not shown).
In contrast to HIV mucosal pathogenesis in the gastrointestinal tract [38], we did not observe any HIV-associated loss of CCR5+ CD4 T cells or Th17 cells from the foreskin. Sexual transmission of HIV occurs almost exclusively through R5-tropic viral strains [39], and Th17 cells are highly susceptible to HIV infection in vitro [40, 41]. This raises the intriguing possibility that the maintenance of highly HIV-susceptible CCR5+ CD4 T cells and Th17 cells in the foreskin of HIV-infected men may permit HIV superinfection, something observed with relatively high frequency in this population-based cohort [42]. Superinfection might also be aided by the fact that virus-specific CD8 T-cell responses were reduced in frequency in the foreskin of HIV-infected men, as has been observed in the female genital mucosa [33, 43], and had a functional profile that was skewed toward IFNγ monoproduction.
HIV infection was both associated with an increased tissue density of CD8 T cells in the foreskin, and with changes in the quality of their cytokine production. Specifically, the proportion of CD8 T cells producing TNFα was significantly decreased, as was the proportion of CD8 T cells producing only TNFα, and of bifunctional cells producing both TNFα and IFNγ. Because TNFα production is a key component of the host immune response to several genital infections (including HSV-2 [44], syphilis [45], and gonorrhea [46, 47]), this may impair immune responses to these pathogens and contribute to their increased incidence (syphilis, gonorrhea, HPV), duration (HPV), and/or recurrence (HSV-2) in HIV-infected men.
Whether the T-cell alterations that we observed in the foreskin of HIV-infected men are causally related to a subsequent increase in the risk of genital infections or HIV superinfection cannot be determined in observational studies. Animal models and/or ex vivo foreskin explant models may be useful ways to explore the direction of causality in the future. In addition, our onsite FACSCaliber flow cytometer only permits the measurement of 4 immune parameters, which limited our capacity to assess T-cell polyfunction (such as production of additional cytokines, including IL-2, MIP-1β, or perforin). Based on the importance of T-cell polyfunctionality in HIV disease progression [48], future studies are warranted to explore these T-cell functions in the foreskin.
In conclusion, we found that foreskin CD4 T-cell numbers, including Th17 cells and CCR5+ CD4 T-cell subsets, were maintained during HIV infection, while overall foreskin CD8 T-cell numbers were increased. However, a reduced functional capacity in both bulk and HIV-specific CD8+ T cells in the foreskin may impair local immune defenses against genital coinfections and HIV superinfection.
Notes
Acknowledgments. Rakai Genital Immunology Group Members: Kighoma Nehemiah, Tumuramye Denis, Mbagiira Emma, Kubaawo John-Bosco, Isabirye Yahaya, Mulema Patrick, Teba James, Atukunda Boru, Mayengo Herbert, Nakafeero Mary, Mugamba Stephen, Nakyeyune Mary, Anyokorit Margaret, Male Deo, Kayiwa Dan, Kalibbala Sarah, Lubyayi Lawrence, Otobi Ouma Joseph, Kakanga Moses, Okech John Baptist, Okello Grace, Aluma Gerald, Ssebugenyi Ivan, and Balikudembe Ambrose.
Financial support. This work was supported by grants from the Bill and Melinda Gates Foundation (22006.03) the National Institutes of Health (R01AI087409-01A1, UO1AI51171, and 1UO1AI075115-O1A1), and the Canadian Institutes of Health Research (HBF-126787). Partial support was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaspan HB, Liebenberg L, Hanekom W, et al. Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis. 2011;204:1550–6. doi: 10.1093/infdis/jir591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quayle AJ, Kourtis AP, Cu-Uvin S, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007;44:292–8. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 6.Bailey R, Moses S, Parker C, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 7.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLOS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobian AAR, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirbod T, Bailey RC, Agot K, et al. Abundant expression of HIV target cells and C-type lectin receptors in the foreskin tissue of young Kenyan men. Am J Pathol. 2010;176:2798–805. doi: 10.2353/ajpath.2010.090926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prodger JL, Gray R, Kigozi G, et al. Foreskin T-cell subsets differ substantially from blood with respect to HIV co-receptor expression, inflammatory profile, and memory status. Mucosal Immunol. 2012;5:121–8. doi: 10.1038/mi.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamali A, Nunn AJ, Mulder DW, Van Dyck E, Dobbins JG, Whitworth JA. Seroprevalence and incidence of genital ulcer infections in a rural Ugandan population. Sex Transm Infect. 1999;75:98–102. doi: 10.1136/sti.75.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland W, Gwanzura L, Bassett MT, et al. Prevalence and incidence of herpes simplex virus type 2 infection among male Zimbabwean factory workers. J Infect Dis. 1999;180:1459–65. doi: 10.1086/315076. [DOI] [PubMed] [Google Scholar]
- 13.Nasio JM, Nagelkerke NJ, Mwatha A, Moses S, Ndinya-Achola JO, Plummer FA. Genital ulcer disease among STD clinic attenders in Nairobi: association with HIV-1 and circumcision status. Int J STD AIDS. 1996;7:410–4. doi: 10.1258/0956462961918374. [DOI] [PubMed] [Google Scholar]
- 14.Kaul R, Kimani J, Nagelkerke NJ, et al. Risk factors for genital ulcerations in Kenyan sex workers. The role of human immunodeficiency virus type 1 infection. Sex Transm Dis. 1997;24:387–92. doi: 10.1097/00007435-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 15.McClelland RS, Lavreys L, Katingima C, et al. Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study. J Infect Dis. 2005;191:333–8. doi: 10.1086/427262. [DOI] [PubMed] [Google Scholar]
- 16.Ohmit SE, Sobel JD, Schuman P, et al. Longitudinal study of mucosal Candida species colonization and candidiasis among human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188:118–27. doi: 10.1086/375746. [DOI] [PubMed] [Google Scholar]
- 17.Plummer FA, Simonsen JN, Chubb H, et al. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989;83:1472–6. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feola TD, Albert MB, Shahabi K, Endy T. Prevalence of HPV in HIV-infected women in the designated AIDS center at Upstate Medical University and the potential benefit of vaccination regardless of age. J Assoc Nurses AIDS Care. 2013;24:176–9. doi: 10.1016/j.jana.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 19.McClelland RS, Baeten JM. Reducing HIV-1 transmission through prevention strategies targeting HIV-1-seropositive individuals. J Antimicrob Chemother. 2006;57:163–6. doi: 10.1093/jac/dki433. [DOI] [PubMed] [Google Scholar]
- 20.Paz-Bailey G, Sternberg M, Puren AJ, Steele L, Lewis DA. Determinants of HIV type 1 shedding from genital ulcers among men in South Africa. Clin Infect Dis. 2010;50:1060–7. doi: 10.1086/651115. [DOI] [PubMed] [Google Scholar]
- 21.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. Aids. 2001;15:105–10. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 22.Baeten JM, McClelland RS, Overbaugh J, et al. Vitamin A supplementation and human immunodeficiency virus type 1 shedding in women: results of a randomized clinical trial. J Infect Dis. 2002;185:1187–91. doi: 10.1086/339823. [DOI] [PubMed] [Google Scholar]
- 23.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. Aids. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 24.McClelland RS, Wang CC, Richardson BA, et al. A prospective study of hormonal contraceptive use and cervical shedding of herpes simplex virus in human immunodeficiency virus type 1-seropositive women. J Infect Dis. 2002;185:1822–5. doi: 10.1086/340639. [DOI] [PubMed] [Google Scholar]
- 25.Baeten JM, McClelland RS, Corey L, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004;189:1466–71. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- 26.Martin HL, Jr., Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–9. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 27.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 28.Gray RH, Niwanuka N, Quinn TC, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. AIDS. 2000;14:2371–81. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 29.Prodger JL, Hirbod T, Kigozi G, et al. Immune correlates of HIV exposure without infection in foreskins of men from Rakai, Uganda. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.83. doi:10.1038/mi.2013.83. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamiel JL, Tobian AAR, Laeyendecker OB, et al. Improved performance of enzyme-linked immunosorbent assays and the effect of human immunodeficiency virus coinfection on the serologic detection of herpes simplex virus type 2 in Rakai, Uganda. Clin Vaccine Immunol. 2008;15:888–90. doi: 10.1128/CVI.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaul R, Rowland-Jones SL, Kimani J, et al. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol Lett. 2001;79:3–13. doi: 10.1016/s0165-2478(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Gumbi PP, Jaumdally SZ, Salkinder AL, et al. CD4 T cell depletion at the cervix during HIV infection is associated with accumulation of terminally differentiated T cells. J Virol. 2011;85:13333–41. doi: 10.1128/JVI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prodger JL, Gray R, Kigozi G, et al. Impact of asymptomatic Herpes simplex virus-2 infection on T cell phenotype and function in the foreskin. AIDS. 2012;26:1319–22. doi: 10.1097/QAD.0b013e328354675c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirbod T, Kimani J, Tjernlund A, et al. Stable CD4 expression and local immune activation in the ectocervical mucosa of HIV-infected women. J Immunol. 2013;191:3948–54. doi: 10.4049/jimmunol.1301220. [DOI] [PubMed] [Google Scholar]
- 36.Johnson KE, Redd AD, Quinn TC, et al. Effects of HIV-1 and herpes simplex virus type 2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis. 2011;203:602–9. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posavad C, Zhao L, Mueller D, Huang ML, Herold B, Wald A, Corey L. Persistence of Mucosal T Cell Responses to HSV-2 in the Female Genital Tract; 2013. International Conference of Mucosal Immunology. Vancouver, British Columbia, Canada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chege D, Sheth PM, Kain T, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. Aids. 2011;25:741–9. doi: 10.1097/QAD.0b013e328344cefb. [DOI] [PubMed] [Google Scholar]
- 39.Grivel J-C, Shattock RJ, Margolis LB. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? J Transl Med. 2010;9:S6. doi: 10.1186/1479-5876-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prendergast A, Prado JG, Kang Y-H, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro P, Gosselin A, Wacleche VS, et al. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J Immunol. 2011;186:4618–30. doi: 10.4049/jimmunol.1004151. [DOI] [PubMed] [Google Scholar]
- 42.Redd AD, Mullis CE, Serwadda D, et al. The rates of HIV superinfection and primary HIV incidence in a general population in Rakai, Uganda. J Infect Dis. 2012;206:267–74. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knudsen A, Benfield T, Kofoed K. Cytokine expression during syphilis infection in HIV-1-infected individuals. Sex Transm Dis. 2009;36:300–4. doi: 10.1097/OLQ.0b013e318193ca26. [DOI] [PubMed] [Google Scholar]
- 46.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–81. doi: 10.1128/CMR.17.4.965-981.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Jong MA, de Witte L, Oudhoff MJ, Gringhuis SI, Gallay P, Geijtenbeek TB. TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. J Clin Invest. 2008;118:3440–52. doi: 10.1172/JCI34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]