Abstract
Background. Semen is the main vector for human immunodeficiency virus (HIV) transmission from men to women. We investigated the influence of cytokines in semen on local HIV burden and activated T cells.
Methods. Blood and semen were collected from 42 HIV-negative and 38 HIV-positive men. Concentrations of 20 cytokines were measured by Luminex, and frequencies of activated T cells were measured by flow cytometry.
Results. Semen contained higher concentrations of proinflammatory (monocyte chemotactic protein-1, interleukin [IL]-8, IL-6, Fractalkine, macrophage inflammatory protein (MIP)-1β, granulocyte macrophage colony-stimulating factor) and adaptive cytokines (IL-7 and IL-15) and higher frequencies of activated T cells compared to blood. Plasma IL-2, eotaxin, MIP-1β, and IL-15 and semen eotaxin and granulocyte colony-stimulating factor (G-CSF) concentrations were associated with T-cell activation. Cytokines in semen were highly coregulated in HIV-negative men; however, this network was disrupted during HIV infection. Several cytokines in semen correlated with HIV shedding (G-CSF, tumor necrosis factor-alpha [TNF-α], interferon-gamma [IFN-γ], IL-10).
Conclusion. Higher levels of inflammation and T-cell activation were observed in semen compared with blood. Seminal G-CSF, which influences neutrophil survival, T-cell function, and dendritic cell activation, was associated with T-cell activation and HIV shedding and may be an important target for reducing HIV shedding or risk.
Keywords: semen, HIV, activation, T cells, inflammation, cytokines
Sexual transmission of human immunodeficiency virus (HIV)-1 is the major route of new infections. Semen serves as the main vehicle for HIV transmission from men to their partners during sexual transmission, with risk of transmission being influenced by concentrations of HIV in semen [1, 2]. Other infectious and noninfectious cofactors may enhance the risk of HIV transmission to a partner by increasing local HIV replication in the male genital tract (MGT), thereby increasing the number of HIV-infected cells and the local HIV shedding in semen. The immunological milieu and the highly regulated cytokine network in the MGT can be altered during HIV infection and in the presence of sexually transmitted infections including cytomegalovirus (CMV), Chlamydia trachomatis, herpes simplex virus (HSV)-2, and other herpes viruses [3–5]. In fact, CMV reactivation in the seminal compartment promotes HIV shedding [6].
Although little is known about the extent of local HIV target-cell activation in semen during HIV infection, significantly increased numbers of highly activated memory T cells are found in blood [7, 8]. Because the major coreceptor for HIV entry into target cells is the chemokine receptor CCR5, the distribution of T cells that express CCR5 in semen during HIV infection may be of specific importance for the potential to transmit HIV through sexual contact. In HIV-uninfected men, high concentrations of inflammatory cytokines and chemokines in semen may recruit and activate potential CCR5+ HIV target cells, thereby increasing susceptibility to HIV infection. While CD4+CCR5+ T cells have been found in the foreskin and other epithelial layers of the penis [9], the frequency of these cells in semen and factors that drive their recruitment are less well understood.
Here, we investigate the role of cytokines and their networks in semen in order to determine the extent of local HIV shedding and T-cell activation. This may provide insight into the role of local immune activation and inflammatory markers in semen when determining the risk for HIV acquisition (in uninfected individuals) and transmission potential (in infected men).
MATERIALS AND METHODS
Study Participants
Thirty-eight HIV-infected (HIV+) and 42 HIV-uninfected (HIV−) men were enrolled from the Empilisweni Clinic in Athlone, Cape Town, South Africa. All men gave written informed consent, and the Research Ethics Committee of the University of Cape Town approved all aspects of the study.
Sample Collection and Processing
Ejaculates were collected in sterile specimen jars following voluntary self-masturbation and processed as described previously [10]. Whole blood was collected by venipuncture and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Histopaque (Sigma-Aldrich, Egham, Runnymede, UK) and LeucoSep tubes (Greiner Bio-one, Frickenhausen, Germany). Blood and seminal plasma samples were stored at −80°C for viral load determination and cytokine measurements. Blood CD4 counts were performed using Flow CARE PLG kits (Beckman Coulter, Inc., Brea, CA) according to the manufacturer's protocol.
Quantification of HIV and CMV in Semen and Blood
Plasma and seminal HIV-1 RNA concentrations (copies/milliliter) were quantified using NucliSENS EasyQ HIV-1 (version 2.0, bioMérieux SA, Lyon, France) according to the manufacturer's protocol. The assay had a lower limit of detection of 70 copies of HIV-1 RNA/mL and a linear range of detection up to 10 × 106 copies of HIV-1 RNA/mL. Seminal CMV DNA (copies/milliliter) was quantified using CMV R-gene polymerase chain reaction (PCR; Argene, Verniolle, France) according to the manufacturer's protocol. The assay had a linear range of detection up to 10 × 107, with a lower limit of detection of 150 copies of CMV DNA/mL.
Detection of Sexually Transmitted Infection
A real-time multiplex PCR (M-PCR) assay (CDC, Atlanta, GA) was used to detect Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, and Mycoplasma genitalium in DNA extracted from seminal fluid. DNA extraction was performed using the X-tractor gene platform (Qiagen, Germany) and M-PCRs were performed using Rotor Gene 3000 platform (Corbett Research, Australia) as described previously [11].
Flow-Cytometric Analysis
Flow cytometry was used to investigate CD38 and CCR5 expression on CD4+ and CD8+ T cells in blood and semen of all participants. Seminal mononuclear cells and PBMCs were stained with anti-CD3 phycoerythrin (PE), anti-CD4 fluorescein isothiocyanate (FITC), anti-CD8 PerCP-Cy5.5, and anti-CCR5 allophycocyanin (APC) or with anti-CD3 PE, anti-CD4 FITC, anti-CD8 PerCP-Cy5.5 and anti-CD38 APC (all BD Biosciences, San Diego, CA). All antibodies were pretitered to determine optimal staining dilutions. Surface staining of cells was performed for 20 minutes at room temperature. Cells were fixed in Cell Fix (BD Biosciences, San Jose, CA) and samples acquired on a FACSCalibur (BD Biosciences, San Jose, CA). All flow cytometric events were collected for semen, whereas 500 000 events were collected for PBMCs. The median CD3+ T-cell yields in semen were 13 490 (interquartile range [IQR], 6205–37191). Data were analyzed using FlowJo software, version 8.5.3 (Tree Star Inc, Ashland, OR). Gates for CD38 and CCR5 were set using fluorescence minus one (FMO) controls. The gating strategy used is included in Supplementary Figure 1.
Measurement of Cytokines and Chemokine Concentrations
Twenty cytokines were measured in semen and blood plasma of all HIV+ men and 28 HIV− men using high-sensitivity human and human cytokine milliplex MAP kits (Millipore Corporation, St. Charles, MO). The following analytes were measured with the high-sensitivity kit: interleukin-1 beta (IL-1β), IL-2, IL-6, IL-7, IL-12p70, granulocyte macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α). The sensitivity of the kit ranged from 0.05 pg/mL to 0.46 pg/mL for each cytokine measured. The following analytes were measured with the human cytokine kit: IL-1α, IL-8, IL-12p40, IL-15, Eotaxin (CCL11), Fractalkine (CX3CL1), granulocyte colony-stimulating factor (G-CSF), monocyte chemotactic protein (MCP-1; CCL2), macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), and regulated upon activation normal T cell expressed and secreted (RANTES; CCL5). The sensitivity of this kit ranged from 0.2 to 10.5 pg/mL for each cytokine measured. Plasma samples were thawed and filtered by centrifugation using 0.2-µm cellulose acetate filters (Sigma) prior to cytokine/chemokine measurements. Data were collected using a Bio-Plex Suspension Array Reader (Bio-Rad Laboratories Inc., Hercules, CA) and BIO-plex manager software (version 4). Cytokine concentrations below the lower limits of detection were reported as the midpoint between the lowest concentration and zero for each cytokine measured, as described previously [12].
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego) and STATA version 10 (StataCorp., College Station, TX). The Mann–Whitney U test was applied for independent nonparametric sample comparisons, the Wilcoxon signed rank test was used for matched nonparametric comparisons, and Spearman Rank correlation was applied for assessing nonparametric associations. The Kruskall–Wallis test with Dunn's multiple comparison test was used to test for differences among more than 2 independent groups. For linear regression, all variables were log10-transformed and used to evaluate the relationships between cytokine levels and activated T-cell subsets in blood and semen. All tests were 2-tailed and P values ≤ .05 were considered significant. P values were adjusted using the false discovery rate step-down procedure in order to reduce false-positive results when multiple comparisons were made [13]. For heat maps, unsupervised hierarchical clustering was used to visualize the variation in cytokine concentrations in individual men and to cluster men according to the relatedness of their cytokine expression profiles (QlucoreOmics Explorer, Sweden).
RESULTS
To define the relationship between cytokine composition and the extent of T-cell activation (CD38 or CCR5) present in semen, 80 men were recruited, of whom 38 were HIV+ and 42 were HIV− (Table 1). Of the HIV+ men, 12/38 (31.6%) were on highly active antiretroviral therapy (HAART+). The median age was 42 years (range, 23–58) and did not differ significantly between groups. The median absolute CD4 count for HIV+HAART− men was 391 cells/mL (IQR, 278–507 cells/mL) compared with 340 cells/mL (IQR, 234–532 cells/mL) in HIV+HAART+ men. The median plasma viral load in HIV+ HAART− men was 11 000 RNA copies/mL (IQR, undetectable−300 000 RNA copies/mL), which was significantly higher than the matching median seminal viral load (1389 RNA copies/mL; IQR, undetectable−135 000 RNA copies/mL; P = .04). Furthermore, the majority of HIV+HAART− men in our study were shedding HIV in semen, showing that they have the potential to put their partners at risk of HIV infection (73.1%, 19/26; Supplementary Figure 2A). Viral loads in blood significantly predicted HIV shedding in semen (P = .04, r = 0.4; Supplementary Figure 2B). In HIV+ men, lower CD4 counts were associated with higher plasma viral loads (P = .005, r = −0.75). Almost half of HIV+ men (47%, 17/36) were shedding CMV in their semen compared with less than a third of HIV− men (30%, 6/20). However, there was no difference in seminal HIV viral load between men who were shedding CMV and those who were not, nor was there a correlation between CMV and HIV shedding (data not shown). Twelve of 64 (19%) men were positive for 1 of the following sexually transmitted infection (STIs) assayed: N. gonorrhoeae, C. trachomatis, T. vaginalis, or M. genitalium. Four of the men were HIV− and 8 were HIV+, of which 3 were HAART+.
Table 1.
Participant Characteristics
| Characteristic | HIV- | HIV+ (untreated) | HIV+ HAART+ |
|---|---|---|---|
| N | 42 | 26 | 12 |
| Age, y (median [IQR]) | 44 (37–51) | 39 (34–44) | 43 (39–46) |
| CD4 count, cell/mm3 (median [IQR]) | - | 391 (278–507) | 340 (234–532) |
| Plasma viral load, RNA copies/mL (median [IQR]) | - | 10 200 (2250–40 000) | LDLa (LDL-LDL) |
| Number of men with detectable HIV RNA in plasma, (N/total (%) | - | 25/26 (96.2) | 2/12 (16.6)b |
| Genital tract viral load, RNA copies/mL (median [IQR]) | - | 1389 (LDLa-20 060) | LDLa (LDL-LDL) |
| Number of men with detectable HIV RNA in semen, N/total (%) | - | 19/26 (73.1) | 2/12 (16.6)b |
| Number of men with detectable CMV in semen, N/total (%) | 6/20 | 13/25 | 4/11 |
| Genital tract CMV viral load, DNA copies/mL (median [IQR]) | LDLa (LDLa-800) | 374 (LDLa-174 025) | LDLa (LDLa-43 375) |
Abbreviations: CMV,cytomegalovirus; HAART,highly active antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; LDL, lower than detection level.
a LDL, 70 HIV-1 RNA copies/mL.
b Two participants had plasma viral loads of 740 and 880 copies/mL; an additional 2 participants had seminal viral loads of 414 and 60 200 copies/mL.
Distinct Composition of Cytokines in Semen vs Blood
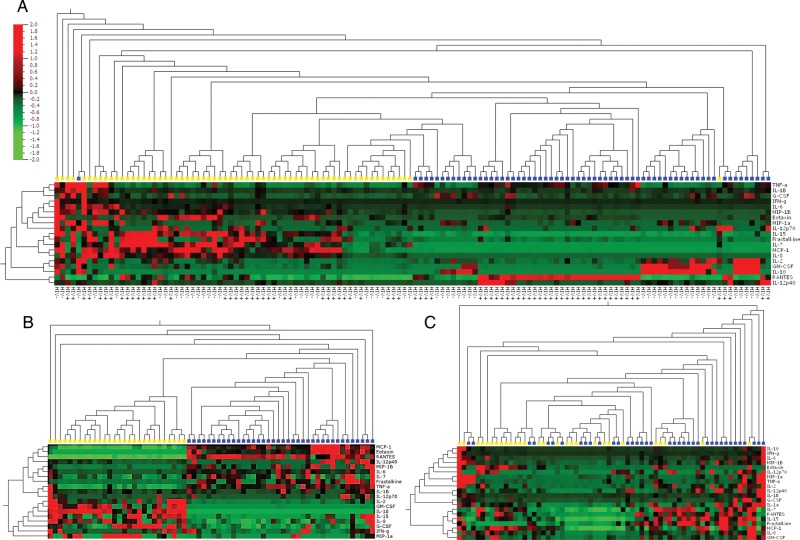
Generally, all classes of cytokines were present in noticeably higher concentrations in semen than in matching blood samples, with MCP-1, IL-8, IL-6, Fractalkine, MIP-1β, GM-CSF, IL-7, and IL-15 being detected at significantly higher concentrations, whereas TNF-α, Eotaxin, and RANTES were present at higher concentrations in blood (Table 2). Unsupervised hierachical clustering showed that semen cytokine signatures were distinct from those detected in blood, irrespective of HIV status (Figure 1A). Interestingly, while unsupervised clustering clearly distinguished HIV+ from HIV− men according to their plasma cytokine concentrations (Figure 1B), concentrations of cytokines in semen from HIV+ vs HIV− men were generally similar (Figure 1C), with only IL-10 significantly decreased in semen from HIV+ compared with HIV− men in both blood (P = .021; Table 2) and semen (P = .035; Table 2). In semen, cytokine concentrations in HIV+HAART+ men did not differ from their HAART− counterparts (data not shown).
Table 2.
Cytokine and Chemokine Concentrations in Blood and Semen of Human Immunodeficiency Virus (HIV)-Uninfected, HIV-Infected (Antiretroviral [ARV]-naive), and HIV-infected (ARV-treated) Men
| Cytokines/Chemokines | Median Concentration (IQR)a |
P Valueb |
||||||
|---|---|---|---|---|---|---|---|---|
| HIV- |
Untreated |
HAART+ |
||||||
| Proinflammatory | Blood | Semen | Blood | Semen | Blood | Semen | Blood | Semen |
| IL-1α | - | 16.3 (5.5–32.9) | - | 17.2 (5.3–79.3) | - | 17.4 (6.5–82) | - | 0.48 |
| IL-1β | 0.15 (0.04–0.33) | 0.25 (0.08–0.4) | 0.26 (0.012–0.59) | 0.17 (0.05–0.69) | 0.09 (0.012–0.35) | 0.32 (0.044–1) | 0.14 | 0.82 |
| IL-6 | 6.5 (4–12.3) | 32.5 (6.8–118.3) | 6.6 (4–14.1) | 22 (8.7–122) | 5.5 (3.2–9.2) | 33.4 (7.3–147.2) | 0.67 | 0.98 |
| IL-8 | 2.3 (0.27–4.7) | 1486 (241–2771) | 2.5 (1.07–3.7) | 1110 (643–2115) | 2.8 (0.04–9.2) | 1141 (540.5–2947) | 0.8 | 0.86 |
| IL-12p40 | 5.4 (0.47–44.7) | 2.8 (1.3–3.5) | 0.47 (0.47–46.1) | 1.9 (1.4–3.5) | 0.47 (0.47–3.07) | 3.1 (1.78–9.85) | 0.21 | 0.2 |
| IL-12p70 | 0.01 (0.01–0.17) | 0.05 (0.03–1.9) | 0.01 (0.01–1.16) | 0.036 (0.02–0.99) | 0.01 (0.01–0.01) | 1.2 (0.036–2.57) | 0.39 | 0.21 |
| TNF-α | 5.9 (4.6–7) | 1.82 (0.79–4.5) | 10.1 (7.02–12.5) | 1.2 (0.57–3.95) | 7.6 (6.4–9.8) | 1.88 (0.27–5.9) | 0.0003 | 0.88 |
| Eotaxin | 89.1 (37.8–182.8) | 23 (4.4–38.9) | 89.2 (59–195.1) | 16.3 (8.6–37.9) | 49.8 (44.2–68.3) | 28.2 (23.3–41.3) | 0.083 | 0.28 |
| Fractalkine | 42.6 (11.1–102.3) | 802.9 (333–1636) | 42.6 (18.8–82.4) | 655.8 (251–1489) | 0.59 (0.59–42.6) | 875.6 (250–1019) | 0.021 | 0.66 |
| MCP-1 | 216.7 (174.1–301.1) | 14 110 (3908–31 028) | 221.3 (168.4–364.7) | 9805 (3211–18 029) | 220.3 (155.2–303.8) | 17 720 (7486–27 430) | 0.72 | 0.18 |
| MIP-1α | 1.95 (1.95–9.52) | 3.15 (1.3–4.9) | 1.95 (1.95–7.24) | 7.5 (2.3–36) | 1.95 (1.95–5.9) | 15.7 (5.5–86.4) | 0.67 | 0.013 |
| MIP-1β | 8.7 (1.5–12.7) | 78.1 (31.9–112.2) | 3.01 (1.5–12.7) | 53 (35.4–171.6) | 5.1 (1.5–16.1) | 83.4 (30.2–195.8) | 0.85 | 0.92 |
| RANTES | 2809 (1997–3156) | 370.9 (189.2–649.3) | 2663 (2080–3207) | 266.5 (98.6–818.3) | 2637 (2483–3332) | 419.5 (178.6–1239) | 0.97 | 0.56 |
| G-CSF | 25.8 (15.5–47.8) | 29.5 (12.2–60.5) | 40.02 (24.6–53.2) | 21.2 (7.5–84.3) | 23.3 (15.5–30.7) | 20 (12.9–58.1) | 0.034 | 0.48 |
| GM-CSF | 0.01 (0.01–0.86) | 2.4 (0.65–7.35) | 0.26 (0.01–0.69) | 2.9 (1.6–6.2) | 0.02 (0.01–0.69) | 5.05 (2.1–14.7) | 0.76 | 0.38 |
| Regulatory | ||||||||
| IL-10 | 1.26 (0.88–2.02) | 27.1 (7.1–52.4) | 2.02 (1.26–2.8) | 10.9 (2.02–17.5) | 1.07 (0.88–1.5) | 7.7 (0.8–24.5) | 0.021 | 0.035 |
| Adaptive | ||||||||
| IFN-γ | 0.86 (0.37–2.9) | 6.3 (2.7–21.9) | 2.4 (0.86–7.8) | 5.6 (1.9–10.2) | 1.21 (0.37–1.33) | 9.8 (0.78–40.5) | 0.039 | 0.75 |
| IL-2 | 0.1 (0.01–0.98) | 0.4 (0.03–2.56) | 0.47 (0.05–1.39) | 0.2 (0.026–1.22) | 0.08 (0.01–0.86) | 1.6 (0.022–3.52) | 0.24 | 0.71 |
| IL-7 | 3.12 (1.27–7.44) | 786.4 (395.9–1930) | 3.13 (1.5–5.26) | 715.8 (347–1865) | 1.91 (0.54–4.9) | 524.4 (414–2011) | 0.43 | 0.8 |
| IL-15 | 0.37 (0.25–1.25) | 27.8 (8.8–45.4) | 0.25 (0.25–1.25) | 27.6 (10.1–64.8) | 0.25 (0.25–0.25) | 30.7 (27.6–39.5) | 0.065 | 0.74 |
Abbreviations: ARV, antiretroviral; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HIV, human immunodeficiency virus; IFN, interferon; IL, interleukin; IQR, interquartile range; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted; TNF, tumor necrosis factor.
a IQR, Interquartile range.
b P-value: Kruskal-Wallis significance between 3 groups.
Figure 1.
Two-way unsupervised hierarchical clustering of cytokine concentrations present in semen and blood. A, Heat map showing a cluster analysis of cytokines in all men (n = 66); yellow blocks represent semen and blue blocks represent blood. B, Heat map showing a cluster analysis of cytokines in blood and semen (C); yellow blocks represent HIV− men and blue blocks represent HIV+ men. The analysis was performed using Qlucore Omics Explorer software. Cytokine levels are expressed as color scales using normalized values for each cytokine and depicted according to a color scale, where red represents high concentrations and green represents low concentrations. Dendograms above classify the samples according to human immunodeficiency virus status and dendrograms on the left side reflect proximities of cytokines to each other. Abbreviations: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HIV, human immunodeficiency virus; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted; TNF, tumor necrosis factor.
Since CMV is a common chronic viral infection that can reactivate and be shed from mucosal surfaces, we examined the impact of CMV DNA (indicating reactivation) on cytokine concentrations in semen (Supplementary Table 1). After adjusting for multiple comparisons, none of the 20 cytokines differed between men who were CMV DNA+ vs DNA− in semen.
Levels of inflammation between STI− and STI+ groups showed that G-CSF, TNF-α, IL-10, IFN-γ, and IL-6 were all significantly higher in the STI+ group after controlling for multiple comparisons (data not shown).
Compartmental Cytokine Concentrations Predict HIV Plasma Viral Load or Shedding
TNF-α concentrations in plasma and semen positively predicted viral loads in plasma (r = 0.53, P = .005) and semen (r = 0.55, P = .01; Table 3). Furthermore, concentrations of G-CSF, IFN-γ, and IL-10 in semen were also significantly positively correlated with semen viral loads (G-CSF: r = 0.61, P = .006; IL-10: r = 0.61, P = .006; IFN-γ: r = 0.57, P = .01; Table 3).
Table 3.
Relationship Between Human Immunodeficiency Virus Viral Loads and Cytokine Concentrations
| Class | Cytokine | Plasma Spearman Rho | Semen Spearman Rho |
|---|---|---|---|
| Proinflammatory | IL-1αa | - | −0.012 |
| IL-1β | 0.26 | 0.38 | |
| IL-6 | 0.14 | 0.27 | |
| IL-8 | −0.32 | 0.01 | |
| IL-12p40 | −0.068 | 0.11 | |
| IL-12p70 | 0.17 | 0.28 | |
| TNF-α | 0.53b | 0.55b | |
| Eotaxin | 0.21 | 0.38 | |
| Fractalkine | 0.11 | 0.17 | |
| MCP-1 | −0.035 | −0.41 | |
| MIP-1α | −0.071 | 0.32 | |
| MIP-1β | −0.21 | 0.25 | |
| RANTES | −0.15 | 0.037 | |
| G-CSF | −0.19 | 0.61b | |
| GM-CSF | −0.054 | −0.04 | |
| Regulatory | IL-10 | 0.12 | 0.61b |
| Adaptive | IFN-γ | 0.31 | 0.57b |
| IL-2 | 0.22 | 0.065 | |
| IL-7 | 0.046 | −0.0035 | |
| IL-15 | −0.079 | 0.068 |
Abbreviations: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted; TNF, tumor necrosis factor.
a IL-1α was undetectable in blood plasma and was excluded.
b In bold: Spearman P ≤ .01.
Altered Cytokine Networks in Semen During HIV Infection
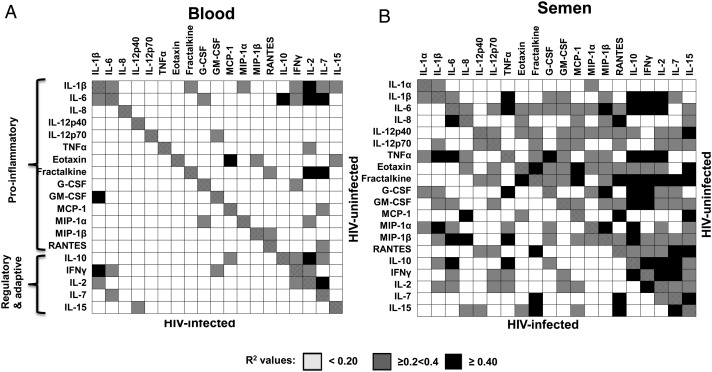
During an inflammatory response, a broad panel of cytokines (all act via the Nuclear Factor Kappa Beta (NF-kB) pathway) generally correlate with one another as they form part of one cascade [14]. In support of this, we found that more than half of the potential correlations (120/190, 63%) we could infer from the 20 cytokines measured in this study were statistically significantly correlated in semen of HIV− men (Figure 2). In particular, the concentration of the regulatory cytokine IL-10 in semen was strongly predictive of IFN-γ, IL-2, and TNF-α concentrations (R2 = 0.79, 0.74, and 0.72, respectively; Figure 2B) and had the highest number of correlations with R2 values > 0.2. In contrast, significantly fewer (62/190, 32.6%) of these relationships between cytokines remained significantly correlated in semen during HIV infection (P < .0001), and this was independent of HAART status. In blood, far fewer cytokines correlated significantly with each other (17.5% in HIV− and 6.4% in HIV+ plasma; Figure 2A). These analyses suggest that cytokines in the MGT are highly coregulated in the absence of HIV infection, more so than in blood. Furthermore, changes in the concentrations of particular cytokines and other factors during HIV infection, such as IL-10, an important regulatory cytokine, may disrupt these tightly regulated networks.
Figure 2.
Human immunodeficiency virus (HIV) infection interrupts coregulation of cytokine networks in (A) blood and (B) semen. Depicted is a table divided diagonally between HIV-uninfected (top right) and HIV-infected (bottom left) with shading representing the R2 valuesof correlations between all cytokines and chemokines assayed. Each rectangle represents an R2 value indicative of the level of correlation between each cytokine or chemokine assayed. R2 values < 0.20 = white rectangles; ≥0.2 to <0.4 = gray rectangles; ≥0.4 = black rectangles. Abbreviations: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HIV, human immunodeficiency virus; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted; TNF, tumor necrosis factor.
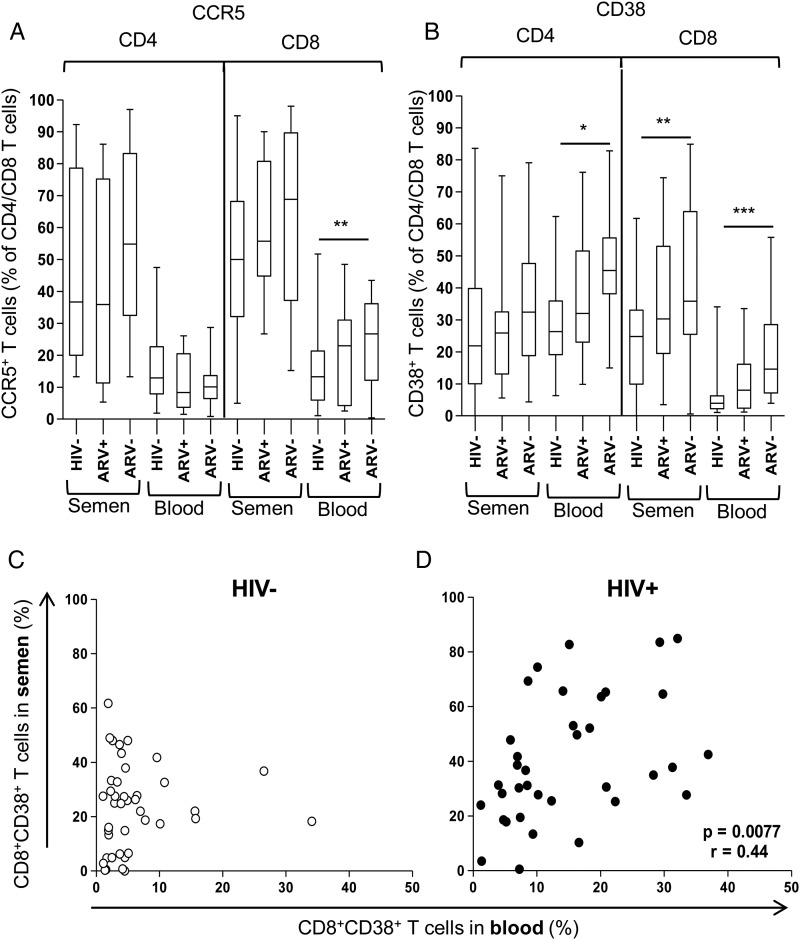
T cells in Semen Are More Activated and Express More CCR5 Compared With Those in Blood
Semen from HIV− and HIV+ men (irrespective of HAART status) had significantly higher frequencies of CD4+CCR5+, CD8+CCR5+, and CD8+CD38+ T cells than that found in matched blood (P < .001; Figure 3A CCR5+ T cells; Figure 3B CD38+ T cells), indicating that levels of activated T cells were markedly higher in semen than circulating in blood. In blood, the frequencies of CD8+CCR5+ and CD8+CD38+ T cells in HIV+ men were significantly correlated (P = .044, r = 0.4), indicative of HIV-induced expansion of these subsets. However, the frequencies of CD4 and CD8 T-cell activation between semen and blood did not correlate (CD4+CCR5+, CD4+CD38+, or CD8+CCR5+), suggesting that the level of activation between compartments was not linked. However, we did observe a significant relationship between frequencies of CD8+CD38+ T cells between blood and semen in HIV+ men (P = .0077, r = 0.44; Figure 3D) that was clearly absent in HIV− men (Figure 3C).
Figure 3.
Elevated activation marker expression (CD38 and CCR5) on T cells in semen compared with blood, irrespective of human immunodeficiency virus (HIV) or highly active antiretroviral therapy status. A, Frequency of CCR5+CD4+ and CD8+ T cells. B, Frequency of CD38+ CD4+ and CD8+ T cells. Each box-and-whisker plot indicates the median, interquartile range, and 10%–90% range (error bars). *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .0001, using the Mann–Whitney U test for the comparison of groups, with P values ≤ .05 considered significant. C and D, Correlation of CD8+CD38+ T cells between compartments in HIV− and HIV+ men, respectively. Spearman rank test was used for correlations and P values ≤ .05 were considered significant.
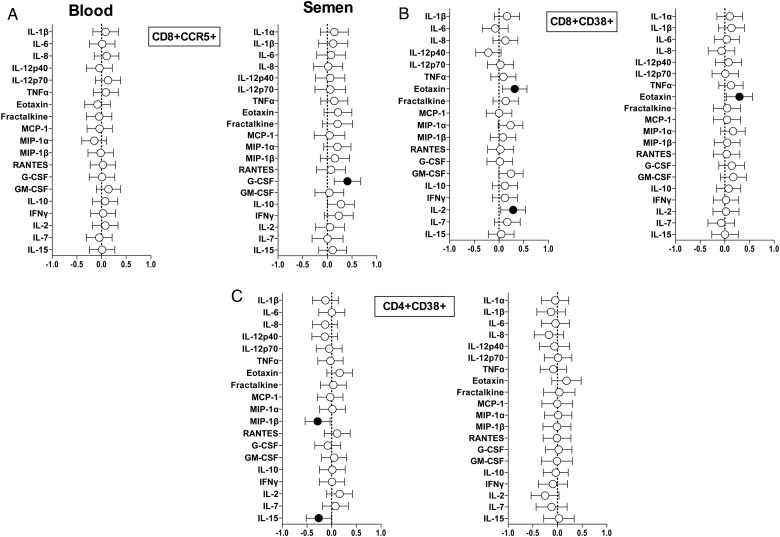
Restricted Influence of Cytokine Concentrations on Compartmental T-Cell Activation
Linear regression was performed in order to assess the relationships between cellular activation and cytokine concentrations in semen and blood. Resulting regression coefficients were adjusted for the potential influence of HIV, antiretroviral (ARV), and STI status in a multivariate analysis (Figure 4). High concentrations of G-CSF in semen were significantly associated with high frequencies of CD8+CCR5+ T cells (a 1 log10 CD8+CCR5+ T-cell frequency increase for every 0.4 log10pg/mL G-CSF increase; Figure 4A) but not frequencies of CD8+CD38+ T cells. Eotaxin concentrations in both blood and semen were significantly associated with high frequencies of CD8+CD38+ T cells and remained significant after adjustment (Figure 4B). Both plasma IL-15 (P = .03) and MIP-1β (P = .013) concentrations showed a significant inverse relationship with blood CD4+CD38+ T-cell frequencies after adjustment (Figure 4C).
Figure 4.
Relationship between cytokine concentrations and frequency of activated T-cell subsets in blood and semen. A, The CD8+CCR5+, (B) CD8+CD38+, and (C) CD4+CD38+ T-cell relationship with cytokines in blood and semen of human immunodeficiency virus (HIV)-infected men. β coefficients were calculated using linear regression for each of the 20 cytokines, adjusting for HIV, antiretroviral therapy, and sexually transmitted infection status in each compartment. A positive β coefficient indicates that higher concentrations of the cytokine are associated with elevated frequencies of activated T cells, while a negative β coefficient indicates an inverse association. Associations that were significant (P ≤ .05) after adjusting for multiple comparisons are shown in black. Abbreviations: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted; TNF, tumor necrosis factor.
DISCUSSION
In this study, we investigated the central role of seminal inflammatory, adaptive, and regulatory cytokines in association with both local HIV shedding and mucosal target-cell activation. We show that semen has a cytokine profile that is distinct from blood, with semen having elevated concentrations of several proinflammatory (MCP-1, IL-8, IL-6, Fractalkine, MIP-1β, and GM-CSF) and adaptive cytokines (IL-7 and IL-15), while blood had higher concentrations of RANTES, Eotaxin, and G-CSF. Many of these cytokines, as part of the inflammatory cascade during infection, are known to be highly coregulated, and we showed this to be true in semen from HIV− men. HIV infection, however, disrupted these networks. Importantly, we showed that frequencies of activated T cells were generally higher in semen than in blood (regardless of HIV status) and that concentrations of G-CSF in semen significantly predicted both HIV shedding and T-cell activation.
The MGT is a mucosal effector site to which immune cells from the systemic circulation migrate to in order to provide defense against pathogens. This generally happens in response to chemotactic cytokine gradients [15], and our finding that many proinflammatory cytokines were present at higher concentrations in semen than in blood supports this. The concentration of MCP-1, in particular, was 60 times higher in semen than in blood. This observation is important because MCP-1 regulates the infiltration of monocytes, memory T cells, dendritic cells, and NK cells [16]. Because the cognate receptor for MCP-1 is CCR2, which is expressed at high levels by memory CD4+ T cells, MCP-1 in semen may also recruit these HIV target cells to the MGT [17]. Sharkey et al. showed that endogenous MCP-1 in the female genital tract (FGT) was elevated following exposure to seminal plasma in a dose-dependent manner, indicating that exogenous MCP-1 may also play a role in recruiting immune cells to FGT following ejaculation, which may influence HIV transmission [18].
Aside from MCP-1, 5-fold higher concentrations of IL-8, Fractalkine, GM-CSF, IL-7, and IL-15 were observed in semen compared with blood. This finding confirms and extends previous reports from Politch et al. and Anderson et al. that showed higher concentrations of IL-7 and IL-8 in semen than in blood, regardless of HIV status [19, 20]. These cytokines and chemokines are involved in recruitment, maturation, and proliferation of monocytes, T and B cells, dendritic cells, and NK cells at potential sites of inflammation [21–26]. Thus, semen passing through the MGT contains high concentrations of immune mediators that are involved in the recruitment, maturation, and survival of immune cells at this site.
Coregulation between cytokines in semen of HIV− but not HIV+ men highlights the complexity and fragility of these regulatory networks. Dysregulation of inflammatory cytokine networks during HIV infection [5, 27, 28] may have serious implications for local seminal viral replication and the associated increased risk of HIV transmission. An association between seminal viral loads and levels of IL-1β, IL-6, IL-12, IFNγ, and RANTES has been shown [29–31]. Seminal levels of TNF-α, IFN-γ, G-CSF, and IL-10 were positively associated with seminal viral load, whereas only plasma levels of TNF-α were positively associated with plasma viral loads.
In the present study, higher seminal levels of CCR5+ CD4+ and CD38+CD8+ T cells of both HIV+ and HIV− men were found compared with levels in blood. There were also significantly higher frequencies of CD8+CD38+ T cells in the semen of HIV+ men compared with HIV− men. HIV+ individuals have significantly elevated levels of CD38 expression on both CD4+ and CD8+ T cells in blood [32–36], and there are elevated levels in the genital tract of HIV+ women [36]. CD4+CCR5+ T cells are the main targets for HIV infection [37–39], and immune activation exacerbates the depletion of these cells in HIV infection by promoting surface expression of CCR5 [40, 41]. Because the genital tract is an effector site, higher levels of CCR5-expressing T cells are to be expected, and CD38 upregulation on CD8+ T cells in the semen of HIV+ men is known to occur [7]. Furthermore, in the present study, CD8+CD38+ T-cell frequencies correlated between blood and semen of HIV+ men, suggesting that systemic immune activation may be used to predict levels in the genital tract. While frequencies of activated CD8+ T cells in semen were significantly elevated in HIV infection, frequencies of these cells, as well as CCR5-expressing CD4+ and CD8+ T cells, were also significantly higher in semen of HIV− men compared with frequencies in blood. This further emphasizes that the MGT, as an effector site where naturally high levels of HIV target cells occur, may place men in danger of HIV acquisition.
In HAART+ men, a rapid reduction in T-cell activation in blood has been demonstrated [42]. However, T-cell activation may persist in some individuals at levels higher than in healthy individuals despite years of ARV treatment [32]. In the present study, despite effective suppression of viral replication in most men, a reduction in activation levels to those found in HIV- men was not achieved in either semen or blood, supporting previous findings. We did not have data available on the duration of ARV treatment or the duration of viral suppression. Hunt et al. speculated that elevated immune activation, despite suppressive ARV treatment, may be due to ongoing antigenic stimulation from low-level HIV replication, other infections, or persistence of immune damage, such as gut damage that leads to microbial translocation and immune activation [32]. Although that study was performed using blood, the same findings may be relevant to the seminal compartment evaluated in the present study.
HIV-induced immune activation can lead to enhanced HIV replication, promoting further cellular activation. This cycle is further exacerbated by proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, working synergistically to elevate levels of activation [27, 43]. In semen, higher concentrations of G-CSF were associated with higher frequencies of CD8+CCR5+ T cells in HIV-infected men. G-CSF is a proinflammatory cytokine produced by macrophages and endothelial cells at sites of infection and it attracts and promotes the survival of neutrophils [22]. Interestingly, G-CSF levels correlated positively with concentrations of the β-chemokines MIP-1α and MIP-1β, which may lead to migration of the CD8+CCR5+ subset into the seminal compartment. G-CSF has also been implicated in influencing T-cell function and in activating dendritic cells (reviewed in [44]). G-CSF may function in the same way in the MGT, where high levels of neutrophils protect against pathogens. This restricted relationship between local inflammation and local activation of T cells suggests that HIV target cells are not activated locally but rather recruited in greater numbers in an already activated state to the MGT. We found an STI prevalence of 19% in our cohort, which is consistent with previous studies in South African men [45], and there was a relationship between the presence of STIs and elevated G-CSF levels. Thus, the role of genital tract inflammation (irrespective of the cause) in driving local HIV target-cell recruitment to this mucosal compartment has implications for placing HIV− men at higher risk for HIV acquisition.
In conclusion, we provide evidence of preexisting high levels of activated and CCR5-expressing T cells in the MGT, which are further elevated upon HIV infection. We show that even though HIV infection disrupts normal cytokine/chemokine networks, certain proinflammatory mediators, such as G-CSF, are upregulated in association with CD8+CCR5+ T cells. Taken together, our study highlights the complex interplay between immune activation and inflammation, particularly at mucosal surfaces, and the role it might play in increasing both risk for HIV acquisition (in HIV− men) and transmission (from HIV+ men). Seminal G-CSF, which is a key player in neutrophil survival, T-cell function, and dendritic cell activation, may be an important target for reducing HIV shedding or risk.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the participants and the staff at the Empilisweni Clinic, Athlone. We thank Andreia Soares, Gerald Chege, and Marcel Tongo for critical review of the manuscript and Kathy Norman for administrative assistance.
Financial support. This work was supported by the South African AIDS Vaccine Initiative and the National Health Laboratory Service Trust. The research on this cohort was also supported by the Poliomyelitis Research Foundation, Medical Research Council, Swedish International Development Cooperation Agency, Cancer Association of South Africa, National Research Foundation, and South African Research Chairs Initiative of the Department of Science and Technology. A. J. O. received postdoctoral funding from the Clinical Infectious Diseases Research Initiative at the University of Cape Town and the National Research Foundation of South Africa. W. A. B. is a Wellcome Trust Intermediate Fellow in Public Health and Tropical Medicine.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Krieger JN, Coombs RW, Collier AC, et al. Intermittent shedding of human immunodeficiency virus in semen: implications for sexual transmission. J Urol. 1995;154:1035–40. [PubMed] [Google Scholar]
- 2.Vernazza PL. HIV in semen: still more to be learned. AIDS Res Ther. 2005;2:11. doi: 10.1186/1742-6405-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kokab A, Akondi MM, Sadeghi MR, et al. Raised inflammatory markers in semen from men with asymptomatic chlamydial infection. J Androl. 2010;31:114–20. doi: 10.2164/jandrol.109.008300. [DOI] [PubMed] [Google Scholar]
- 4.Gianella S, Morris SR, Anderson C, et al. Herpes viruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS. 2013;27:39–47. doi: 10.1097/QAD.0b013e3283573305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisco A, Munawwar A, Introini A, et al. Semen of HIV-1-infected individuals: local shedding of herpes viruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheth PM, Danesh A, Sheung A, et al. Disproportionately high semen shedding of HIV is associated with compartmentalized Cytomegalovirus reactivation. J Inf Dis. 2006;193:45–8. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 7.Lo Caputo S, Trabattoni D, Vichi F, et al. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS. 2003;17:531–9. doi: 10.1097/00002030-200303070-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gianella S, Strain MC, Rought SE, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol. 2012;86:1307–15. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–5. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 10.Olivier AJ, Liebenberg LJ, Coetzee D, Williamson A-L, Passmore J-AS, Burgers WA. Isolation and characterization of T cells from semen. J Immunol Methods. 2012;375:223–31. doi: 10.1016/j.jim.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Mhlongo S, Magooa P, Muller EE, et al. Etiology and STI/HIV coinfections among patients with urethral and vaginal discharge syndromes in South Africa. Sex Transm Dis. 2010;37:566–70. doi: 10.1097/OLQ.0b013e3181d877b7. [DOI] [PubMed] [Google Scholar]
- 12.Bebell LM, Passmore JA, Williamson C, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–4. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 13.Columb MO, Sagadai S. Multiple comparisons. Curr Anaes Crit Care. 2006;17:233–6. [Google Scholar]
- 14.Blackwell TS, Christman BW. The role of nuclear factor kappa beta in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 15.Kutteh WH, Mestecky J, Wira CR. Mucosal immunity in the human female reproductive tract. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal immunology. 3rd ed. Vol 2. Oxford: Elsevier Academic Press; 2005. pp. 1631–46. [Google Scholar]
- 16.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cyt. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 19.Politch JA, Mayer KH, Anderson DJ. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50:283–9. doi: 10.1097/QAI.0b013e3181989870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JA, Ping LH, Dibben O, et al. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathogens. 2010;6:e1001053. doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong AM, Robinson LA, Steeber DA, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–9. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas AK, Lichtman AH. Cytokines. In: Abbas AK, Lichtman AH, editors. Cellular and molecular immunology. 6th ed. Philadelphia: Elsevier & Saunders; 2007. pp. 243–74. [Google Scholar]
- 23.Mueller YM, Katsikis PD. IL-15 in HIV infection: pathogenic or therapeutic potential? Eur Cyt Net. 2010;21:219–21. doi: 10.1684/ecn.2010.0198. [DOI] [PubMed] [Google Scholar]
- 24.Chahroudi A, Silvestri G. Interleukin-7 in HIV pathogenesis and therapy. Eur Cyt Net. 2010;21:202–7. doi: 10.1684/ecn.2010.0205. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan WP, Hunt P, Simonet S, Ulrich TR. Hematological effects of cytokines. In: Remick DG, Friedland JS, editors. Cytokines in health and disease. 2nd ed. New York: Marcel Dekker; 1997. pp. 487–505. [Google Scholar]
- 26.Male D. Mechanisms of innate immunity. In: Male D, Brostoff J, Roth DB, Roitt I, editors. Immunology. 7th ed. Philadelphia: Mosby Elsevier; 2006. pp. 127–44. [Google Scholar]
- 27.Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–59. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- 28.Lisco A, Introini A, Munawwar A, et al. HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol. 2012;68:515–21. doi: 10.1111/aji.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheth PM, Danesh A, Shahabi K, et al. HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J Immunol. 2005;175:4789–96. doi: 10.4049/jimmunol.175.7.4789. [DOI] [PubMed] [Google Scholar]
- 30.Storey DF, Dolan MJ, Anderson SA, Meier PA, Walter EA. Seminal plasma RANTES levels positively correlate with seminal plasma HIV-1 RNA levels. AIDS. 1999;13:2169–71. doi: 10.1097/00002030-199910220-00023. [DOI] [PubMed] [Google Scholar]
- 31.Berlier W, Bourlet T, Levy R, Lucht F, Pozzetto B, Delezay O. Amount of seminal IL-1beta positively correlates to HIV-1 load in the semen of infected patients. J Clin Virol. 2006a;36:204–7. doi: 10.1016/j.jcv.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 33.Eggena MP, Barugahare B, Okello M, et al. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 34.Benito JM, Lopez M, Lozano S, Martinez P, Gonzalez-Lahoz J, Soriano V. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res Hum Retro. 2004;20:227–33. doi: 10.1089/088922204773004950. [DOI] [PubMed] [Google Scholar]
- 35.Almeida M, Cordero M, Almeida J, Orfao A. Relationship between CD38 expression on peripheral blood T-cells and monocytes, and response to antiretroviral therapy: a one-year longitudinal study of a cohort of chronically infected ART-naive HIV-1+ patients. Cyt Part B Clin Cyt. 2007;72:22–33. doi: 10.1002/cyto.b.20144. [DOI] [PubMed] [Google Scholar]
- 36.Restrepo C, Rallon NI, del Romero J, et al. Low-level exposure to HIV induces virus-specific T cell responses and immune activation in exposed HIV-seronegative individuals. J Immunol. 2010;185:982–9. doi: 10.4049/jimmunol.1000221. [DOI] [PubMed] [Google Scholar]
- 37.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;6:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Paxton WA, Kassam N, Ruffing N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Nat Ac Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgi JV, Majchrowicz MA, Johnson TD, Hultin P, Matud J, Detels R. Immunologic effects of combined protease inhibitor and reverse transcriptase inhibitor therapy in previously treated chronic HIV-1 infection. AIDS. 1998;12:1833–44. doi: 10.1097/00002030-199814000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 44.Roberts AW. G-CSF: a key regulator of neutrophil production, but that's not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 45.Johnson LF, Coetzee DJ, Dorrington RE. Sentinel surveillance of sexually transmitted infections in South Africa: a review. Sex Trans Infect. 2005;81:287–93. doi: 10.1136/sti.2004.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.