Abstract
Background. Human immunodeficiency virus (HIV)–infected individuals are at increased risk of cardiovascular disease (CVD) due in part to inflammation. Statins decrease inflammation in the general population, but their effect during HIV infection is largely unknown.
Methods. This is an ongoing randomized, double-blinded, placebo-controlled trial to evaluate the effect of statin therapy on inflammatory markers during HIV infection. Subjects received rosuvastatin 10 mg daily or placebo for 24 weeks. Subjects were receiving stable (>12 weeks) antiretroviral therapy and had a low-density lipoprotein (LDL) cholesterol level of ≤130 mg/dL and evidence of heightened immune activation or inflammation. This was a prespecified interim analysis.
Results. A total of 147 subjects were enrolled (78% were male, 70% were black, and the median age was 47 years). By 24 weeks, LDL cholesterol levels had decreased in the statin group, compared with an increase in the placebo group (−28% vs +3.8%; P < .01). A 10% reduction in the lipoprotein-associated phospholipase A2 (Lp-PLA2) level was seen in the statin group, compared with a 2% reduction in the placebo group (P < .01). In multivariable regression, receipt of statin treatment and having a nadir CD4+ T-cell count of ≤100 cell/µL were the only statistically significant predictors of a decrease in Lp-PLA2 level. Markers of systemic inflammation did not change significantly between groups.
Conclusions. Twenty-four weeks of rosuvastatin therapy significantly decreased the level of Lp-PLA2, a vascular-specific, inflammatory enzyme that predicts cardiovascular events in the general population. Statins may hold promise as a means of attenuating CVD risk in HIV-infected individuals by decreasing Lp-PLA2 levels.
Keywords: HIV, statin, cardiovascular disease, inflammation, lipoprotein-associated phospholipase A2, coagulation
(See the editorial commentary by Dubé on pages 1149–50.)
Human immunodeficiency virus (HIV)–infected patients are at increased risk of cardiovascular disease (CVD) compared with the general population [1]. This increased risk is due in part to a high prevalence of traditional risk factors [2]. However, some patients may have few or no traditional risk factors yet are still at increased risk because of independent effects of HIV infection, despite virological suppression with antiretroviral therapy (ART) [3, 4].
There is growing evidence that heightened inflammation, immune activation, and altered coagulation play key roles in the increased CVD risk during HIV infection. For example, levels of the proinflammatory cytokines high-sensitivity C-reactive protein (hsCRP) and interleukin 6 (IL-6) predict CVD events and mortality [5–7], as well as subclinical atherosclerosis in HIV infection [4, 8, 9]. Increased IL-6 and D-dimer levels are associated with all-cause mortality [5].
Interventions are urgently needed to decrease HIV-associated CVD risk. Pharmacologic approaches aimed at decreasing inflammation may represent a promising strategy. In the general population, statins have been shown to possess antiinflammatory properties, thereby significantly reducing levels of plasma markers of inflammation, T-cell activation, and monocyte/macrophage infiltration in vessels [10, 11]. Several studies have shown that statins given to people with high levels of hsCRP but normal or relatively low levels of low-density lipoprotein (LDL) cholesterol reduce the risk of cardiovascular events and all-cause mortality [12–15]. To date, no randomized studies have investigated changes in inflammation with statins during HIV infection, but a retrospective study showed a reduction in hsCRP, IL-6, and tumor necrosis factor α (TNF-α) levels in HIV-infected subjects with hypercholesterolemia [16].
The purpose of this study was to prospectively investigate the effects of statins on levels of systemic and vascular inflammation and coagulation associated with CVD in HIV-infected individuals receiving ART who had few traditional risk factors for CVD but heightened inflammation and/or immune activation. We hypothesized that statins would decrease cardiovascular biomarkers in these subjects, lending credence to its use as an adjuvant therapy to attenuate HIV-related CVD risk. The primary objectives of the study were to compare differences in changes in inflammatory cytokines, cellular adhesion molecules, and coagulation markers between HIV-infected subjects treated with 24 weeks of statin therapy, compared with those who received placebo.
METHODS
Study Design and Population
The SATURN-HIV (Stopping Atherosclerosis and Treating Unhealthy Bone With Rosuvastatin in HIV) study is a randomized, double-blinded, placebo-controlled trial designed to measure the effect of rosuvastatin on the progression of subclinical CVD in subjects with heightened inflammation or T-cell activation but a normal LDL cholesterol level. The intervention consisted of rosuvastatin 10 mg daily or matching placebo, which were provided free of charge to study participants and donated by Astra Zeneca. Randomization was done by an investigational pharmacist and stratified by protease inhibitor (PI) use at entry. Here, we present the preplanned, interim analysis that assessed changes from baseline to week 24 in markers of systemic and vascular inflammation and coagulation.
Enrollment occurred between March 2011 and August 2012 and included HIV-infected adults ≥18 years old who had a fasting LDL cholesterol level of ≤130 mg/dL and either a hsCRP level of ≥2 mg/L and/or expression of CD38 and HLA-DR antigens on ≥19% of CD8+ T cells at screening, which occurred ≤30 days before enrollment. Additional inclusion criteria included a cumulative ART duration of ≥6 months; receipt of a stable, unchanged ART regimen for ≥12 weeks before entry; no plans to change the current ART, diet, or exercise regimen; an HIV-1 RNA plasma level of <1000 copies/mL; a fasting triglycerides level of ≤500 mg/dL; a Karnofsky performance score of ≥80, and 1 CVD risk factor (ie, male sex, smoking, hypertension, or family history). Main exclusion criteria included a clinically important illness or an abnormal laboratory finding within 14 days before study entry that might place the subject at risk by being exposed to statins or that might confound the interpretation of this investigation, pregnancy/lactation, known CVD, uncontrolled diabetes (ie, a hemoglobin A1C level of ≥8.5%), an active or chronic uncontrolled inflammatory condition or opportunistic infection, known underlying myositis/muscle disease, a change in lipid or cholesterol modification pharmacotherapy, or statin use in the 6 months before entry.
The study was reviewed and approved by the Institutional Review Board of University Hospitals Case Medical Center, Cleveland, Ohio. Written informed consent was provided by all participants. The study was registered on clinicaltrials.gov (NCT01218802).
Clinical Assessments
Physical examination and weight and height measurements were obtained for all subjects by use of the same scale, and blood pressure was obtained with an automated, calibrated blood pressure machine. Extensive chart reviews were conducted, and subject-reported data were collected for relevant variables. HIV-1 RNA plasma level and CD4+ T-cell count were measured concomitantly as part of clinical care.
Laboratory Assessments
For both screening and entry visits, blood specimens were collected after a 12-hour fast. Whole blood was collected in ethylenediaminetetraacetic acid–coated tubes and underwent processing for flow cytometry in real time. An additional blood specimen was collected for plasma and serum isolation and frozen at −70°C without prior thawing until analysis. For all laboratory assessments, laboratory personnel were blinded to clinical information of patients.
To determine eligibility, plasma levels of hsCRP were determined by particle-enhanced immunonephelometric assays on a BNII nephelometer (Siemens, San Diego, CA). Interassay coefficients of variance were <12%. CD8+ T-cell activation was determined by flow cytometry.
Plasma levels of inflammation, cellular adhesion molecules, and coagulation markers that are associated with CVD during HIV infection and/or in the general population and/or elevated during HIV infection were selected [4–6, 17–26], including the proinflammatory cytokines IL-6 and soluble receptors of tumor necrosis factor α (sTNFR-I and sTNFR-II), interferon γ–inducible protein 10 (IP-10), the cellular adhesion molecules soluble vascular cell adhesion molecule 1 (sVCAM-1) and soluble intercellular adhesion molecule 1 (sICAM-1), the coagulation markers D-dimer and fibrinogen, and the inflammatory enzyme lipoprotein-associated phospholipase A2 (Lp-PLA2). IL-6, sVCAM-1, sICAM-1, IP-10, sTNFR-I, and sTNFR-II levels were determined by quantitative sandwich enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Minneapolis, MN). The D-dimer level was determined by immunoturbidimetric assay on a STA-R Coagulation Analyzer (DiagnosticaStago, Parsippany, NJ). The fibrinogen level was determined by particle-enhanced immunonephelometric assay on a BNII nephelometer (Siemens). All interassay coefficients of variance were <12%, except for very low D-dimer values (<0.20 µg/mL), for which the interassay coefficient of variance was 21%. Intraassay coefficients of variance were not calculated for all but one marker because of the small number of samples in each run; for IP-10, the interassay coefficient of variance was 3.3%. Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2) concentrations were measured with an (ELISA; PLAC Test, diaDexus, South San Francisco, CA). Interassay and intraassay coefficients of variance were <5% and <9%, respectively. Calculated medians of the measurements were used in the analysis.
Statistical Analysis
All analyses were performed using intent-to-treat principles based on randomized treatment assignment and used all available data, and modifications to randomized treatment and missing values were ignored. Results of as-treated analyses did not differ from results of intent-to-treat analyses; therefore, only the former data are presented.
Variables are described by study group. Continuous measures are described by medians and interquartile ranges, and nominal variables are described with frequencies and percentages.
Nominal variables were compared using χ2 analysis or the Fisher exact test. Continuous measures were tested for normality. For between-group comparisons (baseline and changes from baseline to 24 weeks), normally distributed variables were compared using the t test, and nonnormally distributed variables were compared using the Wilcoxon rank sum test. For within-group changes from baseline to 24 weeks, normally distributed variables were compared using the paired t test, and nonnormally distributed variables were compared using the Wilcoxon signed rank test.
After the primary results were known, we conducted a post-hoc exploratory analysis using multivariable linear regression to assess predictors of changes in Lp-PLA2 level. This model was performed with both groups combined. Then, a similar model was performed in only the statin group. Variables were chosen for inclusion in the model on the basis of clinical significance and/or univariate results.
All statistical tests were 2-sided with a .05 significance level. Analyses were performed with SAS, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Subject Characteristics
Twenty-nine, 80, and 65 subjects qualified with a hsCRP level of ≥2 μg/mL, expression of CD38+HLA-DR+ on ≥19% of CD8+ T cells, or both, respectively, and were randomly assigned to receive either rosuvastatin (72 subjects) or placebo (75 subjects; Figure 1). Baseline characteristics are summarized in Table 1. Overall, the median age was 47 years, with 78% of subjects male and 70% black. The median CD4+ T-cell count was 613 cells/μL; 76% of subjects had an HIV-1 RNA level of <50 copies/mL. The median duration of HIV infection was 139 months, with 49% receiving a PI.
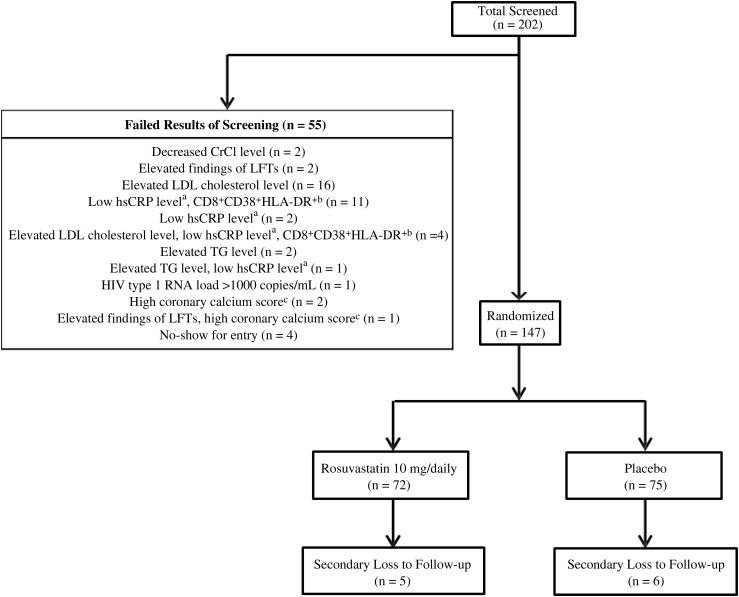
Figure 1.
Patient enrollment flow chart. Two hundred and two patients were screened for enrollment; screening of 55 patients failed, resulting in enrollment of 147 patients (72 in the rosuvastatin arm, and 75 in the placebo arm). ahigh-sensitivity C-reactive protein level of <2 mg/L; bCD8+ T-cell expression of CD38 and HLA-DR antigens <19%; cthe patient was found to have very high coronary calcium score by computed tomography on the same day as screening, and it was deemed unethical for the patient to be potentially randomly assigned to the placebo group. Abbreviations: CrCl, creatinine clearance; LDL, low-density lipoprotein; LFT, liver function test; TG, triglycerides.
Table 1.
Baseline Characteristics of Subjects, by Study Group
| Characteristic | Statin (n = 72) | Placebo (n = 75) |
|---|---|---|
| Age, y | 45.6 (41.1–51.4) | 46.9 (39.2–53.6) |
| Male sex | 58 (81) | 57 (76) |
| African American race | 51 (71) | 52 (69) |
| Body mass indexa | 26.6 (23.4–30.0) | 27.2 (23.5–30.5) |
| Systolic blood pressure, mmHg | 122 (112–136) | 120 (110–132) |
| Diastolic blood pressure, mm Hg | 79 (73–85) | 80 (72–83) |
| LDL cholesterol level, mg/dL | 96 (76–107) | 97 (77–121) |
| HDL cholesterol level, mg/dL | 47 (38–58) | 46 (37–57) |
| Triglycerides level, mg/dL | 105 (77–184) | 121 (87–184) |
| Current smoking | 43 (60) | 54 (72) |
| CD4+ T-cell count, cells/μL | 608 (440–848) | 627 (398–853) |
| Nadir CD4+ T-cell count, cells/μL | 173 (84–312) | 190 (89–281) |
| HIV type 1 RNA plasma level <50 copies/mL | 56 (78) | 58 (77) |
| HIV infection duration, mo | 133 (75–199) | 145 (73–232) |
| ART, duration mo | 63 (37–119) | 71 (39–116) |
| PI treatment at entry | 36 (50) | 36 (48) |
| PI treatment duration, mo | 47 (13–106) | 39 (2–80) |
| CD8+CD38+HLA-DR+ cellsb | 13.3 (9.0–19.1) | 11.5 (8.0–16.5) |
| hsCRP level, μg/mL | 1.6 (0.8–4.9) | 2.0 (0.7–5.2) |
| IL-6 level, pg/mL | 2.9 (1.9–4.1) | 2.7 (2.0–5.3) |
| sTNFR-I level, ng/mL | 1.59 (1.32–2.15) | 1.50 (1.23–2.44) |
| sTNFR-II level, ng/mL | 2.48 (1.78–3.01) | 2.16 (1.61–2.62) |
| sVCAM-1 level, ng/mL | 697 (574–828) | 640 (533–727) |
| sICAM-1 level, ng/mL | 223 (170–318) | 245 (172–305) |
| IP-10 level, pg/mL | 225 (169–353) | 219 (144–344) |
| D-dimer level, μg/mL | 0.19 (0.13–0.33) | 0.18 (0.09–0.29) |
| Fibrinogen level, mg/dL | 380 (338–512) | 391 (331–477) |
| Lp-PLA2 level, ng/mL | 165 (134–199) | 169 (142–206) |
| High-risk Lp-PLA2c | 17 (24) | 20 (27) |
Data are median (interquartile range) or no. (%) of individuals. There were no significant differences between groups for any of the variables listed in this table.
Abbreviations: ART, antiretroviral therapy; HDL, high-density lipoprotein; HLA, human leukocyte antigen; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; IP-10, interferon γ–inducible protein 10; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; PI, protease inhibitor; sICAM-1, soluble intercellular adhesion molecule I; sTNFR-I, soluble tumor necrosis factor receptor I; sTNFR-II, soluble tumor necrosis factor receptor II; sVCAM-1, soluble vascular cellular adhesion molecule I.
a Defined as the weight in kilograms divided by the height in meters squared.
b Percentage of CD8+ T cells expressing CD38 and HLA-DR antigens.
c Percentage of subjects with an Lp-PLA2 level in the high-risk cardiovascular disease category (>200 ng/mL).
Groups were well balanced across arms. There was no difference in the number of subjects from each group who were receiving antihypertensive therapy (20 in the statin group vs 18 in the placebo group; P = .60), and only 1 subject had diabetes, which was well controlled with metformin therapy. Seven subjects had active hepatitis B (3 in the statin group vs 4 in the placebo group; P = .74); 12 subjects had active hepatitis C (5 in the statin group vs 7 in the placebo group; P = .60). Sixteen subjects in each group had metabolic syndrome, as defined by the American Heart Association, with no difference in the prevalence between groups (P = .90) [27].
Eleven subjects (5 in the statin group and 6 in the placebo group) withdrew before 24 weeks: 10 withdrawals were secondary to loss to follow-up, transportation-related issues, and/or schedule-related issues, and 1 subject withdrew because of a potential adverse event (on day 4 of study, grade 2 myalgias caused the subject to refuse to continue in the study). One additional subject in the statin group stopped treatment at week 5 because of hospitalization for hydration secondary to grade 3 myalgias without rhabdomyolysis or renal compromise but continued to be followed during the study but without receiving the study drug.
Two subjects changed ART regimens between baseline and 24 weeks: 1 initiated abacavir therapy instead of didanosine therapy, and 1 stopped treatment with lamivudine/zidovudine and started treatment with emtricitabine/tenofovir and maraviroc. One subject stopped receiving ART and had an HIV-1 RNA level of >12 000 copies/mL at 24 weeks, but there was no statistically significant difference in the percentage of subjects with an undetectable HIV-1 RNA level at baseline, compared with the percentage at 24 weeks, in either group (at 24 weeks, 83% and 84% in the statin and placebo groups, respectively).
Changes in Lipoprotein Profiles
By 24 weeks, the LDL cholesterol level decreased by 28% in the statin group, compared with a 3.8% increase in the placebo group (P< .01 for between-group differences). This decrease within the statin group was statistically significant (P< .01), as was the increase within the placebo group (P = .04). There were no significant differences in percentage changes between groups at 24 weeks in high-density lipoprotein (HDL) cholesterol or triglycerides levels; however, within-group changes in the statin group were significant for the HDL cholesterol level (7% increase; P < .01).
Changes in Markers of Inflammation, Cellular Adhesion, and Coagulation
Percentage changes in levels of inflammatory and coagulation markers are shown in Table 2. By 24 weeks, the Lp-PLA2 level decreased 10% in the rosuvastatin group, compared with a decrease of 2% in the placebo group (P < .01). Within-group percentage changes in Lp-PLA2 levels were also significant within the statin group (P < .01) but not within the placebo group (P = .33). There was no statistically significant difference in percentage changes between groups for the other measured biomarkers.
Table 2.
Percentage Change in Markers of Inflammation, Cellular Adhesion, Coagulation, and Low-Density Lipoprotein (LDL) Cholesterol, by Study Group
| Marker | Statin, Percentage Change (n = 72a) | Placebo, Percentage Change (n = 75a) | P |
|---|---|---|---|
| hsCRP level | −12.5 (−54.6 to 22.2) | 0.0 (−42.4 to 54.7) | .14 |
| IL-6 level | −24.2 (−42.4 to 29.4) | −2.1 (−39.5 to 32.9) | .24 |
| sTNFR-I level | −7.1 (−41.7 to 6.3)b | −13.5 (−38.3 to 7.2)b | .77 |
| sTNFR-II level | 5.6 (−23.3 to 78.7)b | 22.5 (−9.9 to 63.4)b | .27 |
| sVCAM-1 level | 3.8 (−11.5 to 15.3) | 1.9 (−7.4 to 11.4) | .72 |
| sICAM-1 level | 6.2 (−8.2 to 19.0)b | 4.6 (8.1–20.7) | .56 |
| IP-10 level | −22 (−34.3 to −4.2)b | −12.1 (34.7–4.0) | .34 |
| D-dimer level | 6.9 (43.8 to −35.0) | 21.9 (−9.1 to 73.3)b | .23 |
| Fibrinogen level | 0.2 (−20.1 to 22.2) | 9.8 (−6.4 to 23.5)b | .16 |
| Lp-PLA2 level | −9.9 (−20.1 to −1.0)b | −1.9 (−8.6 to 13.3) | <.01 |
| LDL cholesterol level | −28 (−43 to −16)b | 3.8 (−0.7% to 17.2)b | <.01 |
Abbreviations: hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; IP-10, interferon γ–inducible protein 10; Lp-PLA2, lipoprotein-associated phospholipase A2; sTNFR-I, soluble tumor necrosis factor receptor I; sTNFR-II, soluble tumor necrosis factor receptor II; sICAM-1, soluble intercellular adhesion molecule I; sVCAM-1, soluble vascular cellular adhesion molecule I.
a Baseline numbers. At 24 weeks, 5 subjects in the statin group and 6 in the placebo group had withdrawn or were lost to follow-up.
b P < .05 for within-group changes.
Within both groups, sTNFR-I levels decreased, whereas sTNFR-II levels increased significantly (P < .01 for both comparisons). The level of soluble ICAM-1 increased in the statin group (P < .01). The level of IP-10 decreased within the statin group (P = .04), whereas the D-dimer level increased within the placebo group (P = .02).
When looking at absolute changes, the Lp-PLA2 level decreased a median of 15 ng/mL in the rosuvastatin group, compared with a decrease of 4 ng/mL in the placebo group (P < .01). Of the subjects who had both entry and 24-week data on Lp-PLA2 levels (excluding those who were lost to follow-up), 15 subjects in the statin group and 19 in the placebo group had baseline values in the high-risk CVD category (>200 ng/mL, according to general population guidelines) [28]. Lp-PLA2 levels decreased to ≤200 ng/mL by 24 weeks in 8 subjects (53%) and 5 subjects (26%), respectively (P = .23 for the between-group difference).
To evaluate the role of medications that could interfere with results, sensitivity analyses were conducted by excluding subjects who were receiving either aspirin (4 in the statin group and 6 in the placebo group), steroid therapy (4 in the statin group and 7 in the placebo group), or nonsteroidal antiinflammatory agents (3 in the statin group and 3 in the placebo group). Of the subjects receiving steroid therapy, 1 was receiving 10 mg of oral prednisone daily, and the rest were receiving inhaled steroids; there were no changes to regimens between entry and 24 weeks. The analyses were repeated with exclusion of all subjects who were receiving any of these medications (11 in the statin group and 15 in the placebo group). There was no significant difference in results (data not shown).
Analyses were repeated within the rosuvastatin group for subjects >40 versus ≤40 years old, as well as among subjects with a CD4+ T-cell count of >500 versus ≤500 cells/µL, a nadir CD4+ T-cell count of >200 versus ≤200 cells/µL, and a nadir CD4+ T-cell count of >100 versus ≤100 cells/µL. The triglyceride level decreased by 20% among subjects ≤40 years old but remained unchanged among those >40 years old (P = .03). The sTNFR-I level decreased by 18% in subjects with CD4+ T-cell counts of >500 cells/µL but increased by 4% in subjects with CD4+ T-cell counts of ≤500 cells/µL (P = .02). The difference in the percentage decrease in the Lp-PLA2 level was important but nonsignificant between subjects with a nadir CD4+ T-cell count of ≤100 cells/µL, compared with those with a nadir CD4+ T-cell count of >100 cells/µL (14% vs 9%; P = .08); this trend was not seen when 200 cells/µL was used to stratify the nadir CD4+ T-cell count (P = .33).
There was no difference in percentage changes in Lp-PLA2 levels in the statin group among those with and those without metabolic syndrome (P = .38). There was also no difference in percentage changes for any of the biomarkers within the statin group between subjects receiving and those not receiving a PI-based regimen.
In univariate analysis, decreases in Lp-PLA2 levels were positively correlated with decrease in LDL cholesterol levels (R = 0.25; P < .01). In multivariable regression analysis, however, receipt of statin treatment and having a nadir CD4+ T-cell count of ≤100 cell/µL were the only statistically significant predictors of a decrease in Lp-PLA2 level (Table 3). The regression model was then repeated within the statin group only and included age of >40 years, male sex, nadir CD4+ T-cell count of < 100 cells/µL, HIV-1 RNA plasma level of <50 copies/mL, and changes in LDL cholesterol level. No variables remained significant in this model, including changes in LDL cholesterol level.
Table 3.
Multivariable Regression Analysis for Changes in the Lipoprotein-Associated Phospholipase A2 Level
| Characteristic | β ± SE | P |
|---|---|---|
| Statin treatment groupa | −0.0829 ± 0.0317 | <.001 |
| Change in LDL cholesterol level | 0.0781 ± 0.0526 | .1375 |
| Current PI use | −0.0312 ± 0.0274 | .2552 |
| CD8+CD38+HLA-DR+ ≥19%b | −0.0382 ± 0.0342 | .2644 |
| Age >40 yc | −0.0004 ± 0.0322 | .9912 |
| Male sex | −0.0037 ± 0.0333 | .9105 |
| Nadir CD4+ T-cell count <100 cells/µLd | 0.0619 ± 0.0302 | .04 |
| HIV type 1 RNA load <50 copies/mLe | 0.0123 ± 0.0344 | .7201 |
R2 = 0.1660.
Abbreviations: HLA, human leukocyte antigen; LDL, low-density lipoprotein; PI, protease inhibitor; SE, standard error.
a Statin treatment group vs placebo group.
b Percentage of CD8+ T cells expressing CD38 and HLA-DR antigens ≥19% vs <19%.
c Age >40 vs ≤40 years.
d Nadir CD4+ T-cell count <100 vs ≥100 cells/µL.
e HIV type 1 RNA load <50 vs >50 copies/mL.
DISCUSSION
We investigated the effects of 24 weeks of statin therapy on systemic and vascular inflammation and coagulation in HIV-infected individuals with ART-suppressed HIV-1 RNA plasma levels, normal LDL cholesterol levels, but heightened inflammation and/or immune activation. Despite LDL cholesterol levels of ≤130 mg/dL at baseline, LDL cholesterol levels decreased significantly over the study period in the statin group, compared with the placebo group. There were also several inflammatory markers that decreased within the statin group. Perhaps the most important finding was a significant decrease in LP-PLA2 levels in the statin group, which was independent of the changes in LDL cholesterol levels. Both statin use and a nadir CD4+ T-cell count of ≤100 cell/µL were predictive of greater decreases in LP-PLA2 levels when the groups were considered together.
LP-PLA2 is an inflammatory enzyme produced by monocytes, macrophages, T lymphocytes, and mast and liver cells and is upregulated in atherosclerotic plaques and rupture-prone fibrous caps. It has high specificity for vascular inflammation, as opposed to systemic inflammation [29, 30], making it potentially valuable during HIV infection because most patients have a high level of systemic inflammation, compared with healthy controls [4]. Several prospective studies have shown that increases in Lp-PLA2 concentration or activity predict primary and recurrent cardiovascular events in the general population [22–26]. Like hsCRP, an elevated Lp-PLA2 level doubles the risk for first and recurrent CVD events. In fact, general population guidelines include testing of Lp-PLA2 levels for patients with intermediate and moderate CVD risk and for some high-risk patients [31].
LP-PLA2 circulates primarily bound to LDL particles and is the enzyme responsible for hydrolysis of oxidized phospholipids on LDL particles within the arterial intima, thereby producing 2 highly inflammatory mediators (lysophosphatidylcholine and oxidized fatty acid). These initiate a cascade of events causing upregulation of cellular adhesion molecules, expression of inflammatory cytokines, monocyte recruitment into the intimal space, and differentiation of monocytes into macrophages that engulf oxidized LDL, producing foam cells, which aggregate to form a fatty streak covered by a fibrous cap. The atherosclerotic plaque produces proteases and cytokines, which destroy collagen within the fibrous cap, making it unstable and prone to rupture. This leads to an acute coronary event [32].
In the general population, statins decrease Lp-PLA2 levels [33, 34]. Some studies showed that changes in Lp-PLA2 levels were correlated with changes in LDL cholesterol levels; however, LDL concentration changes could not always explain the extent of the reduction in the Lp-PLA2 level [35, 36]. This suggests that decreases in Lp-PLA2 levels may result in antiinflammatory effects beyond merely decreasing the level of LDL cholesterol. While the mechanisms remain elusive, data suggest that statins inhibit lipopolysaccharide-induced increases in Lp-PLA2 expression and secreted activity in human monocyte–derived macrophages [37].
In this current study, statins significantly decreased Lp-PLA2 levels, compared with no change in the placebo group. In fact, statin use was an independent predictor of decreases in Lp-PLA2 levels. There was a univariate relationship between changes in Lp-PLA2 levels and changes in LDL cholesterol levels, suggesting that some of the decrease may have been related to the effect of statin therapy on LDL cholesterol. However, the relationship between changes in LDL cholesterol and Lp-PLA2 levels did not hold up in multivariable regression analysis with both groups considered together, as well as within the statin group alone. This indicates that changes in Lp-PLA2 levels were not solely due to decreases in the LDL cholesterol concentration and may represent an antiinflammatory effect of statins. This novel finding deserves further attention, and the SATURN-HIV study will be able to assess whether favorable changes in the Lp-PLA2 level will ultimately decrease the subclinical atherosclerotic burden within this population.
In studies involving the general population, there are conflicting findings on whether a decrease in the Lp-PLA2 level translates into a reduction in the incidence of coronary events. In an analysis of the JUPITER trial, Lp-PLA2 levels were no longer associated with CVD events when the LDL cholesterol level decreased to approximately 50 mg/dL [38]. However, studies have shown that a reduced level of circulating Lp-PLA2 is an independent risk factor for coronary plaque regression in patients with acute coronary syndrome [39], and animal studies have shown that reductions in the Lp-PLA2 concentration in atherosclerotic plaques attenuate local inflammatory response and improve plaque stability [40]. Thus, it is likely that decreases in Lp-PLA2 levels result in reductions in the incidences of clinical events. Studies are underway to evaluate this relationship.
In this current study, there was a greater decrease in sTNFR-I level within the statin group in subjects with a CD4+ T-cell count of >500 cells/µL and a trend toward a greater decrease in Lp-PLA2 levels in subjects whose nadir CD4+ T-cell count was ≤100 cells/µL. This latter relationship was also significant in the multivariable regression analysis when groups were evaluated together but not within the statin group alone. These 2 findings appear to be contradictory. Previous studies have shown that HIV-infected individuals with lower CD4+ T-cell counts and nadirs have an increased risk of CVD and myocardial infarction, compared with those with higher CD4+ T-cell counts and nadirs [41–45]. While the decrease in sTNFR-I levels among subjects with higher CD4+ T-cell counts is in line with these studies, the decrease in Lp-PLA2 level among subjects with a lower nadir CD4+ T-cell count is not. In this latter case, the Lp-PLA2 level decreased more in subjects with a lower nadir CD4+ T-cell count, despite no difference in baseline levels between subjects with high versus those with low nadirs. However, this is consistent with other studies that have looked at measures of subclinical atherosclerosis and endothelial function, in which the nadir CD4+ T-cell count is an independent predictor of carotid intima-media thickness progression and brachial artery flow-mediated vasodilation [42, 43]. There were only 11 and 12 subjects who had a current CD4+ T-cell count of >500 cells/µL and a nadir CD4+ T-cell count of <100 cells/µL in the statin and placebo groups, respectively; thus, this is likely not merely a reflection of subjects with higher CD4+ T-cell counts. This suggests that patients with low nadirs may benefit from statin therapy to attenuate CVD risk, but longer follow-up is needed to further assess this relationship.
We saw significant increases and decreases in levels of other inflammatory markers within the statin group. The significance of these findings is unclear and likely attributable to the relatively short follow-up period. Continued follow-up is ongoing to determine whether further changes can be detected and whether they result in measurable changes in subclinical atherosclerosis and/or arterial stiffness. Interestingly, IL-6 levels decreased by 24% and hsCRP levels decreased by 12% in the statin group, compared with decreases of 2% and 0%, respectively, in the placebo group; these differences were not statistically significant, however. Higher levels of hsCRP and IL-6 have been associated with cardiovascular events during HIV infection [6, 7]. Again, further follow-up is needed to determine whether these early changes will become statistically significant and/or whether these decreases will improve CVD risk.
We demonstrated a significant decrease in LDL cholesterol levels in the statin group, even though all subjects started with a level ≤130 mg/dL. This finding alone is important, because studies in the general population have shown a linear relationship between reductions in LDL cholesterol levels and coronary and other major vascular events even when the LDL cholesterol level was lowered substantially below recommended levels [46, 47].
There are limitations to this study. We investigated a specific HIV-infected population: those receiving stable ART with a low or undetectable HIV-1 RNA level and a normal LDL cholesterol level. Thus, generalizability of our findings to the HIV-infected population as a whole should be determined. In addition, we evaluated changes in Lp-PLA2 plasma concentrations. Some studies have suggested that using Lp-PLA2 activity rather than mass concentration may be more accurate [48]. We also investigated a number of inflammatory markers, increasing the risk of type I errors. As stated previously, this analysis focused on the first 24 weeks of the ongoing SATURN-HIV study and, as such, involved data collected during a relatively short period and could not yet assess changes in measures of subclinical atherosclerosis. Finally, we used T-cell activation as one of the inclusion criteria. Although there are studies that show T-cell activation is independently associated with increased carotid intima-media thickness, arterial stiffness, and carotid plaques [8, 49], we did not use monocyte activation for screening, which is also implicated in CVD risk [50].
In conclusion, this study offers novel data that support statin use during HIV infection as a means of decreasing inflammation. It remains to be seen whether altering inflammation, especially Lp-PLA2 concentrations, will affect CVD risk and plaque stability. However, further follow-up and assessment of changes in atherosclerotic burden and arterial stiffness are warranted. Increased CVD risk during HIV infection is a growing concern, and measures to minimize this risk are urgently needed. Statin therapy in HIV-infected individuals, even in the absence of traditional risk factors, may hold promise.
Notes
Financial support. The work was supported by the National Institute of Nursing Research, National Institutes of Health (NIH; R01 NR012642 to G. A. M.); the National Institute of Child Health and Development, NIH (K23 HD069199 to A. R. E.); and the Center for AIDS Research, Case Western Reserve University (P30 AI36219). Study drugs and matching placebo were donated by Astra Zeneca.
Potential conflicts of interest. A. R. E. has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor for Gilead. G. A. M. serves as a consultant and speaker and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Tibotec. G. A. M. currently chairs a data safety monitoring board for a Pfizer-funded study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 3.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross AC, Rizk N, O'Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–27. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–73. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PloS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longenecker C, Funderburg N, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–90. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsue PY, Scherzer R, Hunt PW, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1:jah3–e000422. doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325–30. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33F. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 13.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 16.Calza L, Trapani F, Bartoletti M, et al. Statin therapy decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-alpha in HIV-infected patients treated with ritonavir-boosted protease inhibitors. HIV Clin Trials. 2012;13:153–61. doi: 10.1310/hct1303-153. [DOI] [PubMed] [Google Scholar]
- 17.Amar J, Fauvel J, Drouet L, et al. Interleukin 6 is associated with subclinical atherosclerosis: a link with soluble intercellular adhesion molecule 1. J Hypertens. 2006;24:1083–8. doi: 10.1097/01.hjh.0000226198.44181.0c. [DOI] [PubMed] [Google Scholar]
- 18.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 20.Hileman CO, Longenecker CT, Carman TL, et al. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. 2012;17:1345–9. doi: 10.3851/IMP2297. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 22.Vittos O, Toana B, Vittos A, Moldoveanu E. Lipoprotein-associated phospholipase A2 (Lp-PLA2): a review of its role and significance as a cardiovascular biomarker. Biomarkers. 2012;17:289–302. doi: 10.3109/1354750X.2012.664170. [DOI] [PubMed] [Google Scholar]
- 23.Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–44. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137–44. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 25.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Int Med. 2006;166:2073–80. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 26.Sabatine MS, Morrow DA, O'Donoghue M, et al. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2007;27:2463–9. doi: 10.1161/ATVBAHA.107.151670. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 28.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–31. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 30.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–8. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 31.Davidson MH, Corson MA, Alberts MJ, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol. 2008;101:51F–7F. doi: 10.1016/j.amjcard.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Braun LT, Davidson MH. Lp-PLA2: A new target for statin therapy. Curr Atheroscler Rep. 2010;12:29–33. doi: 10.1007/s11883-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer EJ, McNamara JR, Asztalos BF, et al. Effects of atorvastatin versus other statins on fasting and postprandial C-reactive protein and lipoprotein-associated phospholipase A2 in patients with coronary heart disease versus control subjects. Am J Cardiol. 2005;95:1025–32. doi: 10.1016/j.amjcard.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Saougos VG, Tambaki AP, Kalogirou M, et al. Differential effect of hypolipidemic drugs on lipoprotein-associated phospholipase A2. Arterioscler Thromb Vasc Biol. 2007;27:2236–43. doi: 10.1161/ATVBAHA.107.147280. [DOI] [PubMed] [Google Scholar]
- 35.Reddy KJ, Singh M, Batsell RR, Bangit JR, Miraskar RA, Zaheer MS. Lipoprotein-associated phospholipase A2 mass is significantly reduced in dyslipidemic patients treated with lifestyle modification and combination lipid-modifying drug therapy. Prev Cardiol. 2010;13:130–4. doi: 10.1111/j.1751-7141.2009.00060.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Kang SM, Park S, Jang Y, Chung N, Choi D. The effects of statin monotherapy and low-dose statin/ezetimibe on lipoprotein-associated phospholipase A(2) Clin Cardiol. 2011;34:108–12. doi: 10.1002/clc.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song JX, Ren JY, Chen H. Simvastatin reduces lipoprotein-associated phospholipase A2 in lipopolysaccharide-stimulated human monocyte-derived macrophages through inhibition of the mevalonate-geranylgeranyl pyrophosphate-RhoA-p38 mitogen-activated protein kinase pathway. J Cardiovasc Pharmacol. 2011;57:213–22. doi: 10.1097/FJC.0b013e31820376ac. [DOI] [PubMed] [Google Scholar]
- 38.Mora S, Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97:33A–41A. doi: 10.1016/j.amjcard.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Dohi T, Miyauchi K, Okazaki S, et al. Decreased circulating lipoprotein-associated phospholipase A2 levels are associated with coronary plaque regression in patients with acute coronary syndrome. Atherosclerosis. 2011;219:907–12. doi: 10.1016/j.atherosclerosis.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Qiao Z, Ren J, Chen H. Simvastatin reduces expression and activity of lipoprotein-associated phospholipase A(2) in the aorta of hypercholesterolaemic atherosclerotic rabbits. J Internat Med Res. 2009;37:1029–37. doi: 10.1177/147323000903700407. [DOI] [PubMed] [Google Scholar]
- 41.Ho JE, Deeks SG, Hecht FM, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS. 2010;24:1897–905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho JE, Scherzer R, Hecht FM, et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS. 2012;26:1115–20. doi: 10.1097/QAD.0b013e328352ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 45.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–9. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 47.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A(2) mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin chem. 2012;58:877–86. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–45. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]