Abstract
Leishmania braziliensis are intracellular parasites that cause unique clinical forms of cutaneous leishmaniasis. Previous studies with other leishmania species demonstrated that reactive oxygen species (ROS) control promastigotes, the infective stage of the parasite, but not the amastigote form that exists in the mammalian host. Here we show that ROS inhibits growth of L. braziliensis amastigotes in resting monocytes, and that classical monocytes are primarily responsible for this control. ROS, but not nitric oxide, also contributed to killing of L. braziliensis by IFN-γ activated monocytes. Furthermore, by gene expression profiling of human lesions we found greater expression of genes associated with ROS, but not nitric oxide, compared to normal skin. This study shows that ROS are important for control of L. braziliensis both at the initial stages of infection, as well as at later time points, and highlights that monocyte subsets may play different roles during leishmaniasis.
Keywords: Leishmania braziliensis, reactive oxygen species, human monocytes, nitric oxide, amastigotes
Leishmania braziliensis are intracellular parasites that replicate in phagocytic cells. Following the bite of an infected sand fly the infection is initiated in the skin by the promastigote form of the parasite, which rapidly invades phagocytes and transforms into intracellular forms known as amastigotes. Monocytes, as well as other inflammatory cells, infiltrate the infection site and either become infected by promastigotes in the initial hours following infection or at later times by amastigotes that are released from infected cells. In murine studies it was shown that the infiltrating monocytes were among the first cells to be recruited to the site of leishmania infection and that they contribute to control of the parasites [1]. However, we know much less about how human monocytes, and human monocytes subsets, control Leishmania.
The development of a Th1 response leads to interferon γ (IFN-γ) dependent activation of phagocytes and leishmania killing [2, 3]. In mice, parasite killing is dependent on the expression of inducible nitric oxide synthase (iNOS) and the resulting production of nitric oxide (NO) [4]. However, the role of iNOS and NO in mediating control of pathogens by human phagocytes is less clear [5, 6]. In fact, human monocyte-derived macrophages do not produce NO after classical macrophage activation [7] or upon infection with Leishmania parasites [8]. Nevertheless, some reports suggest that although NO cannot be measured in cultures, its inhibition promotes Leishmania growth in phagocytes [8]. In addition, in vitro killing via NO production of other pathogens such as Trypanosoma cruzi [9] and Mycobacterium avium-intracellulare [10] has been demonstrated. Thus, it still remains unclear if NO is required for controlling leishmania parasites.
Although IFN-γ mediated activation of cells is essential for the eventual control of leishmania parasites and resolution of the disease, innate mechanisms also contribute to parasite control. Thus, neutrophils, monocytes, and macrophages can control parasites by reactive oxygen species (ROS) that are produced during phagocytosis in a process called the respiratory burst (RB) [11]. The formation of these ROS requires the assembly of the nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) complex at the cell surface [11]. Several studies indicate that murine macrophages kill promastigotes in vitro through the production of ROS, in some cases with the assistance of neutrophils [8, 12–15]. However, in mice this pathway is not essential, as mice deficient in the NADPH oxidase complex are just slightly more susceptible to leishmania infection [16] (Novais et al, unpublished data). In humans, in vitro studies with macrophages show that ROS can also control leishmania promastigotes, although whether this pathway is essential in humans is more difficult to determine than in the mouse [12, 14, 17, 18]. Moreover, several studies indicate that amastigotes may not induce a RB [13, 19, 20]. Nevertheless, macrophages derived from patients with chronic granulomatous disease, an immunodeficiency due to mutations in the NADPH oxidases required for the RB, are reported to exhibit increased susceptibility to leishmania in vitro [18]. Taken together, these results suggest that the role of both NO and ROS in controlling leishmania in human cells is unclear and may differ between mice and humans.
In this article, we demonstrate that ROS is induced by the interaction of human monocytes and both promastigote and amastigote forms of L. braziliensis, and that ROS production leads to parasite control. As monocytes are a heterogeneous population, we looked into the capacity of the different monocyte subsets to interact with leishmania parasites. We found that L. braziliensis parasites were capable of either binding or infecting the 3 monocytes subsets equally; however, the classical monocyte subset had a greater capacity to induce a RB and were better able to control the parasites. The induction of a RB, but not NO production, led to increased control of the parasites in IFN-γ activated monocytes. Using an unbiased approach, we showed that genes associated with the RB are highly expressed in the skin from L. braziliensis infected patients, whereas we did not see changes in the expression of NOS2 in lesions when compared to naive skin. Overall, we propose that in humans, classical monocytes control L. braziliensis through the production of ROS but not NO, both in an innate fashion, and also following activation of monocytes by IFN-γ.
METHODS
Ethics Statement
This study was conducted according to the principles specified in the Declaration of Helsinki and under local ethical guidelines by the University of Pennsylvania Institutional Review Board (Philadelphia, PA; 813 390).
Parasites
Transgenic mCherry+ L. braziliensis (MHOM/BR/01/BA788) [21] or wild-type strains of cutaneous patients from Bahia (MHOM/BR/01/BA788) and Ceará (MHOM/BR/94/H3227) or mucosal patient (13 330) from Bahia, were grown in Schneider insect medium (GIBCO) supplemented with 20% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin and kept at 26°C. Metacyclic enriched promastigotes were used for infection [22] and the axenic culture of L. braziliensis amastigotes was initiated from stationary-phase promastigotes and maintained in Grace insect cell culture medium (Gibco, Invitrogen Corporation) in acidic pH (5.5) supplemented with 20% FBS and 1% L-glutamine and maintained at 34°C. Limiting dilution was used to quantitate the parasites in some experiments [23].
Human Peripheral Blood Monocytes
Peripheral blood monocytes were obtained from healthy donors from the Human Immunology Core at the University of Pennsylvania. The RosetteSep human monocyte enrichment cocktail kit was used to purify monocytes. Cells were infected with amastigotes (10:1 or 2:1) or promastigotes (10:1) for 2 hours after which extracellular parasites where removed by centrifugation. NAC (10 mM, Sigma), L-NMMA (1 mM, EMD Millipore) and/or human IFN-γ (400 U/mL, R&D Systems) were added to cultures where indicated. Cytospins were performed at 2 and 72 hours, stained with Diff-Quik (Siemens), and counted using light microscopy.
ROS Detection, Flow Cytometry and Cell Sorting
Freshly isolated human monocytes (1 × 106/mL) were loaded with dihydrorhodamine 123 (DHR, Cayman Chemical; 20 ng/mL) for 5 minutes at 37°C in a CO2 incubator. Cells were exposed to metacyclic promastigotes or axenic amastigotes for 20 minutes at 37°C in a CO2 incubator. For nitroblue tetrazolium (NBT) staining, monocytes were incubated for 20 minutes with 500 µg/mL NBT in the presence of L. braziliensis promastigotes or amastigotes and stained with Diff-Quik. Monocytes were stained for surface markers with anti-CD14 (M5E2, Pacific Blue) and anti-CD16 (3G8, APC-Cy7) (BD Pharmingen) and the fluorescence intensity of the cells assessed by flow cytometry using a LSRII Fortessa (BD) and analyzed using FlowJo software (Tree Star). Monocytes were sorted using FACS Aria (BD) based on the expression of CD14 and CD16.
Microarray-based Expression Profiling of Human Lesions
For whole genome expression microarray, lesion biopsies were prepared as described elsewhere [21]. Illumina HumanHT-12 version 4 expression beadchips were hybridized with cRNA from 26 L. braziliensis lesion biopsies and 10 biopses from uninfected donors. Data were quantile normalized and differential expression analysis was carried out using GenomeStudio v1.8 software (Illumina). Genes were considered differentially regulated if expression increased or decreased ≥3-fold with a diffscore of ≥13 or ≤−13 (equivalent to P ≤ .05). Data were deposited on the Gene Expression Omnibus database for public access (GSE GSE43880). Heat map tools available on GenePattern [24] were used to graphically display differentially regulated genes in Figure 5C.
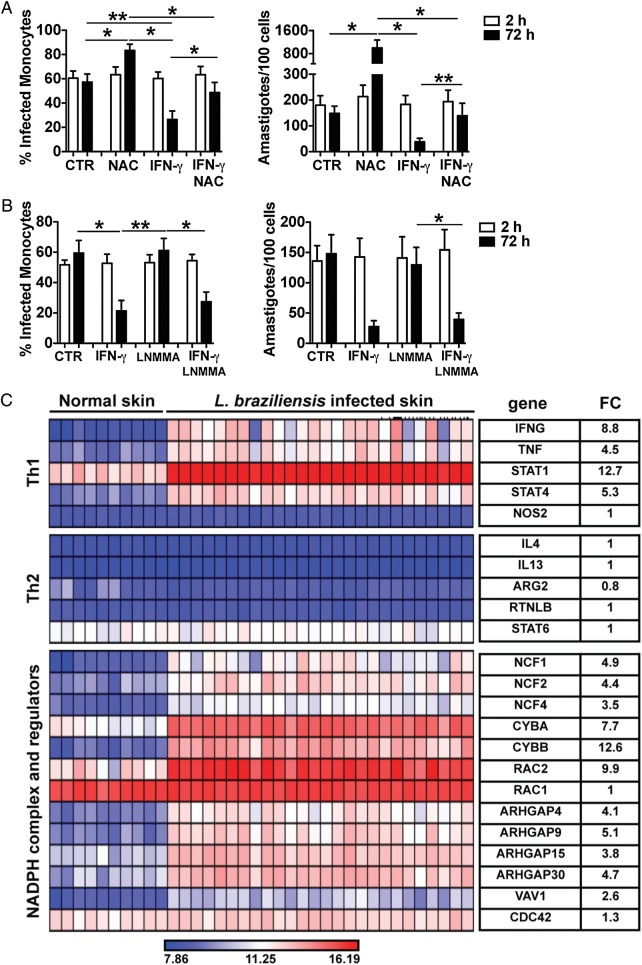
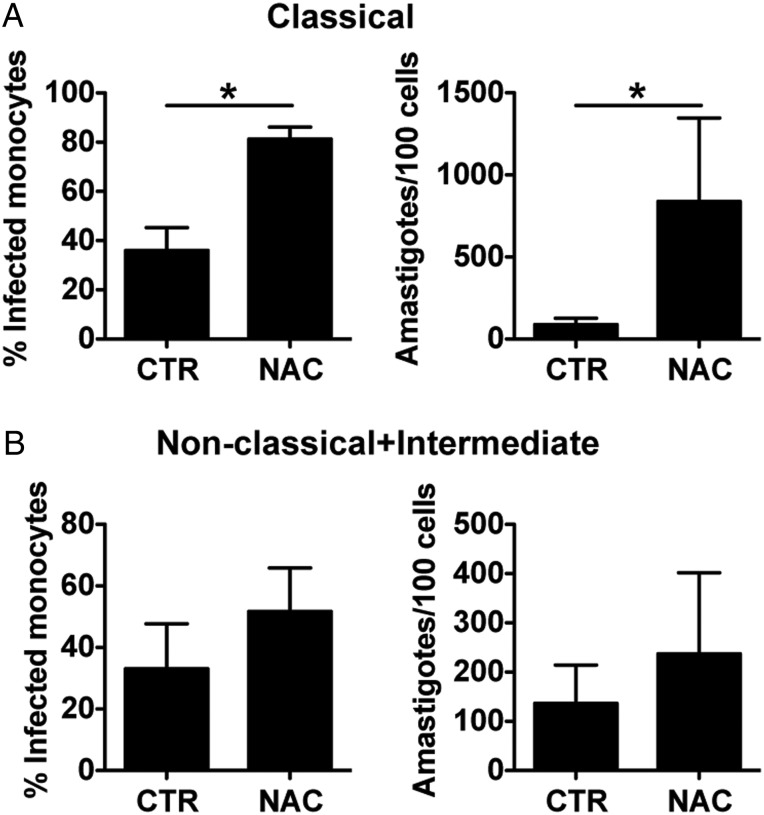
Figure 5.
ROS, but not NO, production is implicated in the control of Leishmania braziliensis in vitro and in the skin of L. braziliensis patients. Human monocytes were infected with amastigotes of L. braziliensis (2:1) and cytospins of cultures were performed at 2 and 72 hours. Cultures were assessed for the percentage of infected cells and the number of amastigotes per 100 cells using light microscopy. Cells were treated with or without (A) NAC, IFN-γ or NAC + IFN-γ; (B) LNMMA, IFN-γ or LNMMA + IFN-γ. Data from 6 donors from experiments performed independently are shown. *P ≤ .05; **P ≤ .01. (C) Heatmap showing induction of genes obtained from a microarray profiling of 26 human lesions and 10 normal skin biopsies. Average fold change (FC) for each gene in lesion samples, relative to normal skin controls, is shown. Abbreviations: IFN-γ, interferon γ; NAC, N-acetylcysteine; NO, nitric oxide; ROS, reactive oxygen species.
Statistical Analysis
The data are presented as the mean ± SD of the mean. The significance of the results was calculated using nonparametric statistical tests: Mann-Whitney (2-sided t test), for comparisons between 2 groups or Kruskal-Wallis, followed by Dunn multiple comparison test, for comparisons between 3 groups.
RESULTS
Promastigotes and Amastigotes of L. braziliensis Induce a RB in Human Monocytes
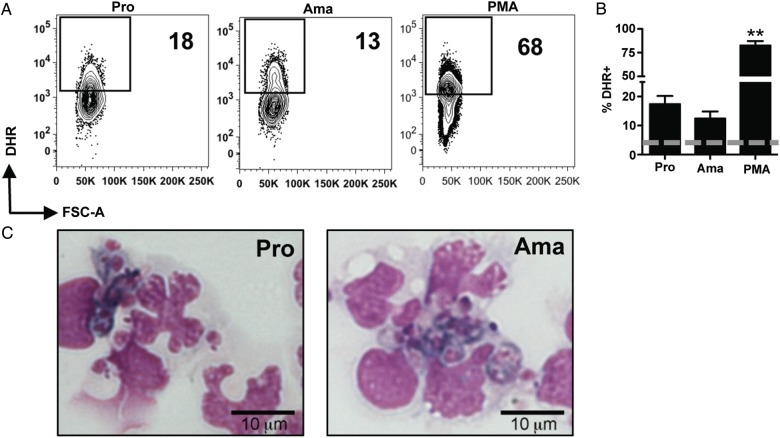
In studies with other leishmania species, promastigotes, but not amastigotes, were able to induce ROS production by phagocytes [13, 19, 20]. However, L. brazilienesis parasites are distinct from other leishmanias and are associated with more severe immunopathology, but often better control of the parasite. Thus, it was possible that they might exhibit differences in their ability to induce ROS. Therefore, we first addressed whether both promastigotes and amastigotes of L. braziliensis could induce ROS in human monocytes. To do so, we obtained purified monocytes from healthy individuals and incubated them with dihydrorhodamine 123 (DHR), a cell permeable probe that once oxidized by ROS becomes fluorescent [25]. DHR-loaded monocytes were then co-cultured with promastigotes or amastigotes of mCherry expressing L. braziliensis or PMA, as a positive control, and analyzed by flow cytometry. Human monocytes in contact with either promastigotes or amastigotes of L. braziliensis were capable of producing ROS as measured by DHR oxidation (Figure 1A and 1B). These results were confirmed by staining infected monocytes with nitroblue tetrazolium (Figure 1C). Overall, the data indicate that human monocytes exhibit a RB after contact with both life cycle stages of leishmania parasites, which raises the question of whether monocytes control L. braziliensis through the production of ROS.
Figure 1.
Amastigotes and promastigotes of Leishmania braziliensis induce ROS production in human monocytes. Human monocytes were loaded with DHR for 5 minutes and exposed to mCherry expressing L. braziliensis (10:1) or PMA for 20 minutes. Depicted are (A) representative contour plots and (B) bar graphs of DHR expression in mCherry+ monocytes for infected monocytes, or total monocytes for PMA. The dashed lines represent background DHR expression in unstimulated cells. C, Monocytes were exposed to L. braziliensis promastigote (10:1) or amastigotes (10:1) and incubated with nitroblue tetrazolium. After 20 minutes the cells were harvested and stained with Diff-Quik. Data from donors [16 (promastigotes), 5 (amastigotes) and 6 (PMA)] from independent experiments are shown. **P ≤ .01. Abbreviations: DHR, dihydrorhodamine; PMA, phorbol myristate acetate.
ROS Control L. braziliensis Growth in Human Monocytes
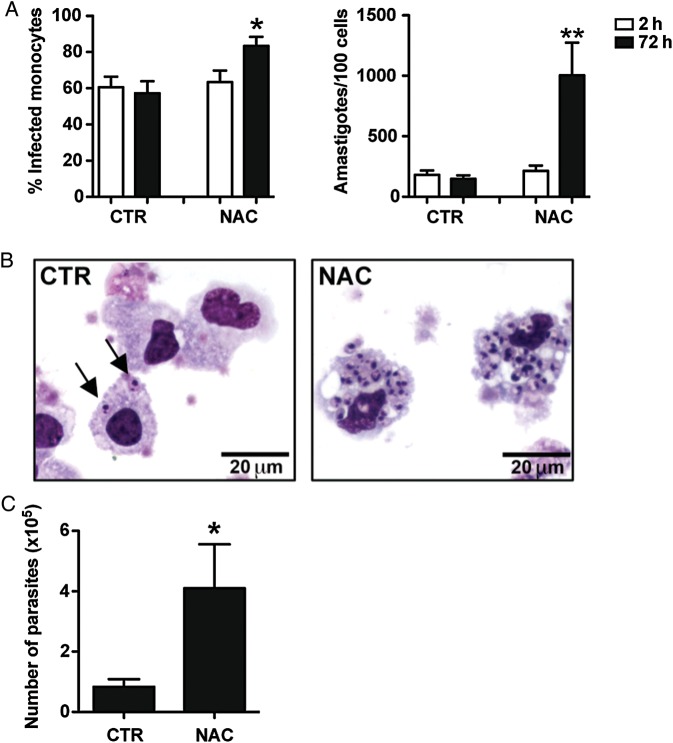
To assess if ROS inhibited amastigote growth in human monocytes, monocytes were infected with amastigotes of L. braziliensis and cultured in vitro. In some cultures we added N-acetylcysteine (NAC), a ROS scavenger. After 2 and 72 hours, the monocytes were harvested, and the percent of infected cells and the number of parasites were quantified microscopically. The percentage of monocytes infected, as well as the overall number of parasites, was significantly greater in the presence of NAC following infection (Figure 2A and 2B). To confirm these results we infected human monocytes with amastigotes in the presence or in the absence of NAC and after overnight incubation, we harvested the cells and performed a limiting dilution assay to quantify the number of parasites. Consistent with the results above, we found that parasites grew better in monocytes that were treated with NAC in comparison to untreated monocytes (Figure 2C). Together, these results suggest that human monocytes prevent L. braziliensis replication by producing ROS.
Figure 2.
ROS production by human monocytes controls Leishmania braziliensis infection. Human monocytes were infected with amastigotes of L. braziliensis (2:1) and treated with NAC or were left untreated. A, Cytospins of cultures were performed at 2 and 72 hours and assessed for the percentage of infected cells and the number of amastigotes per 100 cells using light microscopy. Data from 5 donors from experiments performed independently are shown. B, Light microscopy pictures of representative slides stained with H&E from CTR and NAC treated cultures. C, Parasite titration from cultures of human monocytes treated or not with NAC. Data from 8 donors from independent experiments are shown. *P ≤ .05; **P ≤ .01. Abbreviations: H&E, hematoxylin and eosin; NAC, N-acetylcysteine; ROS, reactive oxygen species.
Classical Monocytes Have a Greater RB Than Intermediate and Nonclassical Monocytes
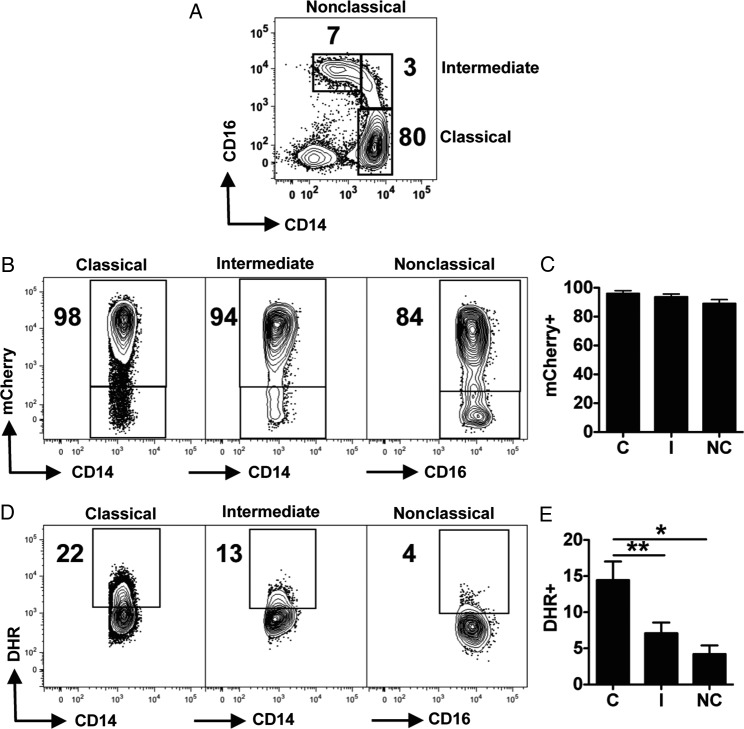
Human monocytes are a heterogeneous population and have been divided into 3 different subsets based on the surface expression of CD14 and CD16 [26]. The 3 subsets have major differences in terms of their capacity to produce cytokines, chemokines and present antigen [27–29]. To investigate if there was preferential production of ROS by a particular monocyte subset, we examined the expression of DHR by the monocyte subsets after exposure to mCherry L. braziliensis amastigotes (Figure 3A). L. braziliensis did not preferentially interact with classical, intermediate or nonclassical monocytes, as a similar percentage of mCherry+ cells were detected for the 3 subsets (Figure 3B and 3C). However, this interaction led to different levels of ROS production by the monocytes subsets (Figure 3D and 3E). Classical monocytes showed a greater capacity to produce ROS when in contact with L. braziliensis, followed by intermediate and nonclassical monocytes (Figure 3D and 3E). The outcome was the same with promastigotes of L. braziliensis (Supplementary Figure 1). Thus, classical monocytes have a greater capacity to induce ROS upon encounter with leishmania parasites.
Figure 3.
Classical monocytes have a greater RB after interaction with amastigotes of Leishmania braziliensis. Human monocytes were loaded with DHR for 5 minutes and stimulated with amastigotes of L. braziliensis expressing mCherry. Cells were stained with CD14 and CD16 antibodies and analyzed by flow cytometry. Depicted are (A) representative coutour plots from one donor showing the monocyte subset gating strategy; (B) representative contour plots and (C) bar graph from interactions with the monocyte subsets and L. braziliensis; (D) counter plots and (E) bar graph from the DHR expression among the distinct monocyte subsets. Data from 6 donors from independent experiments are shown. *P ≤ .05; **P ≤ .01. Abbreviations: DHR, dihydrorhodamine; RB, respiratory burst.
Classical Monocytes Control of L. braziliensis Parasites is Dependent on ROS
We found that scavenging ROS with NAC led to an increase in the growth of parasites within monocytes. Because we found that classical monocytes have a greater RB, we wanted to determine if classical monocytes were preferentially able to control L. braziliensis. In order to do so, monocytes from healthy donors were sorted by flow cytometry into CD14highCD16neg monocytes or CD16pos monocytes and infected with amastigotes of L. braziliensis with or without NAC for 72 hours. Inhibition of the RB using NAC enhanced both the percentage and number of amastigotes found in classical monocyte cultures (Figure 4A). As predicted, nonclassical and intermediate monocytes did not control L. braziliensis by ROS production, as inhibition with NAC did not significantly increase the number of infected cells or parasites present in the cultures (Figure 4B).
Figure 4.
Classical monocytes control Leishmania braziliensis by ROS production. Human monocytes were stained for flow cytometry and sorted as classical or intermediate and nonclassical based on their expression of CD14 and CD16. Sorted monocytes were infected with amastigotes of L. braziliensis (2:1) with or without NAC. Cytospins of cultures were performed at 72 hours and assessed for the percentage of infected cells and the number of amastigotes per 100 cells using light microscopy. Data from 4 donors from experiments performed independently are shown. Abbreviations: NAC, N-acetylcysteine; ROS, reactive oxygen species.
IFN-γ Induced Killing by Human Monocytes Is Dependent on ROS
Our results indicate that without activation monocytes control L. braziliensis by the production of ROS. In an established leishmanial lesion, however, there is an intense pro-inflammatory environment, where IFN-γ, a key cytokine in phagocyte activation, is present [3]. To determine if IFN-γ dependent killing also involved ROS, we infected monocytes with L. braziliensis, and stimulated some of the cells with IFN-γ. Confirming the data shown above, in amastigote-infected monocytes there is more infection and replication of parasites in the absence of ROS (Figure 5A). As expected, addition of IFN-γ increased the capacity of human monocytes to control L. braziliensis as determined by the percentage of infected monocytes, as well as the number of amastigotes present at 72 hours (Figure 5A). Inhibition of ROS in IFN-γ treated monocytes partially restored the parasite growth/survival in cultures treated with NAC alone by both frequency of monocytes infected and number of parasites (Figure 5A). These results suggest that ROS is important for the control of L. braziliensis in both resting and IFN-γ activated monocytes. However, the ability of NAC to reverse the killing of parasites induced by IFN-γ was only partial, which raises the question if NO production might also contribute to killing.
Nitric Oxide Does not Control L. braziliensis in vitro or in Leishmanial Lesions
NO is essential in the control of leishmania in the mouse. Therefore, we next tested if NO could be playing a role in IFN-γ dependent control of L. braziliensis in human monocytes. Monocytes were infected with L. braziliensis and activated with IFN-γ in the presence or absence of L-NMMA, a nitric oxide synthase inhibitor. Treatment of infected monocytes with L-NMMA had no effect on the frequency of infected monocytes or the number of amastigotes found in cultures, for both IFN-γ treated or untreated monocytes (Figure 5B). In addition, we also assayed the culture supernatants and were unable to detect NO (data not shown). We performed similar experiments with L. braziliensis isolated from a cutaneous leishmaniasis patient (13 330) and a mucosal patient (CE3227), with similar results (Supplementary Figure 2). Thus, L. braziliensis parasites are killed by IFN-γ activated monocytes without generating NO.
Although the in vitro experiments clearly demonstrate that NO is not required for IFN-γ dependent killing of L. braziliensis, it was possible that NO was contributing to parasite control within the lesions from patients. To address this we carried out whole genome expression profiling of lesions from patients infected with L. braziliensis. Over 500 genes were more highly expressed (≥3-fold with P ≤ .05) in lesions compared to normal skin from uninfected donors (data not shown). As expected, we found an increase in genes associated with a Th1 response, including IFN-γ, tumor necrosis factor α (TNF-α), STAT-1 and STAT4 (Figure 5C). In contrast, there were minimal changes in Th2 associated genes, as seen by similar expression levels of interleukin 4 (IL-4), interleukin 13 (IL-13), STAT-6, Arginase (ARG2), and Resistin-like beta (RETNLB) between normal skin and lesions (Figure 5C). To determine how parasites might be controlled within these lesions with a strong Th1 environment, we examined the expression of genes associated with the RB and NO production. Despite the Th1 cytokine signature detected in L. braziliensis skin lesions, there was no differential expression of NOS2 (inducible nitric oxide), whereas we found that all the components of the NADPH oxidase complex (NCF1, NCF2, NCF4, CYBA, CYBB) increased in lesions in comparison to normal skin (Figure 5C). In addition, we observed increased gene expression of members of the Rho family of GTPases (ARHGAP4, ARHGAP9, ARHGAP15, ARHGAP30, VAV1) that positively regulate NADPH oxidases. Notably, RAC2, a Rac isoform expressed in hematopoietic cells, is present at higher levels than in normal skin. This is in contrast to RAC1, which is known to be expressed in nonhematopoietic cells, suggesting that the assembly of the NADPH oxidase is happening in phagocytes present in the skin [30]. In contrast, there is no changed expression in CDC42, a negative regulator of RAC2. These results suggest that in humans the production of ROS is an important mechanism in controlling L. braziliensis, although it is likely that NO is not playing a major role in the control of leishmania parasites in the human skin.
DISCUSSION
Leishmania infects and replicates in monocytes, macrophages and dendritic cells, and their control is dependent upon these cells killing the parasites. The overwhelming majority of studies directed at understanding how this occurs have been done with macrophages, in spite of the fact that other myeloid lineage cells play a role in the infection. Many of these studies have been done with L. major or L. donovani, and how other parasite species are controlled is less well defined. Furthermore, most of the focus has been given on how the interactions between macrophages and promastigotes–the infective form transmitted by the sand fly–rather than the amastigote form–which will be interacting with host cells throughout the infection. Here we have focused on how human monocytes control the amastigote form of L. braziliensis, a parasite that causes significant disease in South America. We show that resting human monocytes are able to control L. braziliensis amastigotes by the production of ROS, and that once activated by IFN-γ, it is still ROS, rather than NO, that contributes to parasite control.
The generation of ROS by phagocytes is a major protective mechanism used to control pathogens. Importantly, ROS are produced by phagocytes without prior activation, and thus play a crucial role in innate resistance [11]. Previous studies found that promastigotes induced, and were sensitive to, ROS [8, 12, 14, 17, 19, 20, 31, 32]. In contrast, several studies indicated that amastigotes fail to induce ROS [13, 19, 20] with few exceptions [33]. The consequence of these findings is that the role for ROS is limited to the first few hours of the infection, before the promastigotes have transformed to amastigotes. However, our studies indicate that L. braziliensis amastigotes survive and replicate much better in the absence of ROS, suggesting that in individuals infected with L. braziliensis ROS-producing monocytes recruited to the lesions may help control the parasites long after the initial infection. This indicates that ROS may play a more important role in L. braziliensis infections than with other species of leishmania.
The mechanisms of control of leishmania parasites in mice are very clear and require the activation of Th1 responses, which leads to iNOS upregulation by phagocytic cells [2]. This results in NO production and parasite killing [34]. In humans, however, the capacity of phagocytic cells to upregulate iNOS and produce NO appears to be under much tighter regulation [35]. Indeed, although NOS2 dependent NO may be produced in certain situations by human cells [8, 10, 32, 36, 37], in vitro activated human phagocytes do not produce NO [35]. This has led to a continuing controversy as to the importance of iNOS in human phagocyte-mediated killing [5, 6]. One explanation for this controversy may be that specific tissues can differentially regulate killing mechanisms [16]. Furthermore, Leishmania parasites may regulate the production of effector molecules, for example, by inducing the production of interleukin 10 (IL-10) by immune cells [38]. Our studies indicate that for L. braziliensis infections, NO is unlikely to contribute to parasite control. First, we were unable to measure any NO produced by human monocytes following activation with IFN-γ, in spite of the ability of these cells to kill the parasites. Moreover, to ensure that some low level of NO was not influencing killing, we blocked NO production with the inhibitor L-NMMA, and found no effect. Finally, we performed transcriptional analysis on biopsies from L. braziliensis patients, and saw no change in the expression of NOS2. Taken together, we conclude that in human L. braziliensis infections NO does not play a role in controlling parasites in the skin or in vitro by human monocytes. The ROS scavenger NAC only partially block the killing induced by IFN-γ, and we hypothesize that the amount of NAC added to cultures was not enough to scavenge all the ROS produced by IFN-γ activated monocytes. Alternatively, other as yet to be defined mechanisms might be involved in IFN-γ mediated killing.
Human monocytes are a heterogeneous population, based on their CD14 and CD16 expression [29], although the functional roles for these subsets is still being explored [27, 28]. Notably, the frequency of the least abundant subsets, intermediate and nonclassical, increases during certain diseases, including leishmaniasis [39–42] (Carvalho et al, unpublished data). The significance of these increases remains unclear, but it indicates that it is critical to understand their role not only in healthy individuals but also during disease. We found that only classical monocytes control L. braziliensis by the production of ROS, supporting the idea that human monocyte subsets play different immunological roles during infection.
Overall, this study indicates that ROS is an important mechanism of parasite control, not only during the initial stage of L. braziliensis infection, but potentially throughout the disease. This may contribute to the fact that L. braziliensis parasites are scarce in patients’ lesions. However, the ability of parasite to stimulate ROS production throughout the infection may also contribute to the immunopathology that is characteristic of L. braziliensis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors wish to thank Enaldo Lago, and Drs Adriano Queiroz, Luiz Guimaraes, and Gordon Ruthel for technical assistance.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health [UO1 AI88650].
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Goncalves R, Zhang X, Cohen H, Debrabant A, Mosser D. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J Exp Med. 2011;208:1253–65. doi: 10.1084/jem.20101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 3.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 4.Stenger S, Thüring H, Röllinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–93. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang F, Nathan C. Man is not a mouse: reply. J Leukoc Biol. 2007;81:580. doi: 10.1189/jlb.1206715. [DOI] [PubMed] [Google Scholar]
- 6.Schneemann M, Schoeden G. Macrophage biology and immunology: man is not a mouse. J Leukoc Biol. 2007;81 doi: 10.1189/jlb.1106702. [DOI] [PubMed] [Google Scholar]
- 7.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358–63. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- 8.Gantt K, Goldman T, McCormick M, et al. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz-Fernández M, Fernández M, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 10.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–7. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 11.Brüne B, Dehne N, Grossmann N, et al. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19:595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang H, Thalhofer C, Duerkop B, et al. Oxidant generation by single infected monocytes after short-term fluorescence labeling of a protozoan parasite. Infect Immun. 2007;75:1017–24. doi: 10.1128/IAI.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channon J, Roberts M, Blackwell J. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984;53:345–55. [PMC free article] [PubMed] [Google Scholar]
- 14.Novais F, Santiago R, Báfica A, et al. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol. 2009;183:8088–98. doi: 10.4049/jimmunol.0803720. [DOI] [PubMed] [Google Scholar]
- 15.Zarley J, Britigan B, Wilson M. Hydrogen peroxide-mediated toxicity for Leishmania donovani chagasi promastigotes. Role of hydroxyl radical and protection by heat shock. J Clin Invest. 1991;88:1511–21. doi: 10.1172/JCI115461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha F, Schleicher U, Mattner J, Alber G, Bogdan C. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect Immun. 2007;75:3823–32. doi: 10.1128/IAI.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khouri R, Bafica A, Silva M, et al. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol. 2009;182:2525–31. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 18.Murray H, Cartelli D. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983;72:32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallinson D, Coombs G. Interaction of leishmania metacyclics with macrophages. Int J Parasitol. 1989;19:647–56. doi: 10.1016/0020-7519(89)90043-x. [DOI] [PubMed] [Google Scholar]
- 20.Pham N-K, Mouriz J, Kima P. Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infect Immun. 2005;73:8322–33. doi: 10.1128/IAI.73.12.8322-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novais FO, Carvalho LP, Graff JW, et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013;9:e1003504. doi: 10.1371/journal.ppat.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Späth G, Beverley S. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 23.Uzonna JE, Joyce KL, Scott P. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon gamma-producing CD8+ T cells. J Exp Med. 2004;199:1559–66. doi: 10.1084/jem.20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov J. GenePattern 2.0. Nat Genet. 2006;38:500–1. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 25.Richardson M, Ayliffe M, Helbert M, Davies E. A simple flow cytometry assay using dihydrorhodamine for the measurement of the neutrophil respiratory burst in whole blood: comparison with the quantitative nitrobluetetrazolium test. J Immunol Methods. 1998;219:187–93. doi: 10.1016/s0022-1759(98)00136-7. [DOI] [PubMed] [Google Scholar]
- 26.Passlick B, Flieger D, Ziegler-Heitbrock H. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 27.Wong K, Tai J, Wong W-C, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 28.Zawada A, Rogacev K, Rotter B, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 30.Bokoch G, Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–48. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- 31.Da Silva R, Hall B, Joiner K, Sacks D. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989;143:617–22. [PubMed] [Google Scholar]
- 32.Giudice A, Vendrame C, Bezerra C, et al. Macrophages participate in host protection and the disease pathology associated with Leishmania braziliensis infection. BMC Infect Dis. 2012;12:75. doi: 10.1186/1471-2334-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haidaris C, Bonventre P. A role for oxygen-dependent mechanisms in killing of Leishmania donovani tissue forms by activated macrophages. J Immunol. 1982;129:850–5. [PubMed] [Google Scholar]
- 34.Van Assche T, Deschacht M, da Luz R, Maes L, Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med. 2011;51:337–51. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Kröncke K, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–56. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Annane D, Sanquer S, Sébille V, et al. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet. 2000;355:1143–8. doi: 10.1016/S0140-6736(00)02063-8. [DOI] [PubMed] [Google Scholar]
- 37.Blos M, Schleicher U, Soares Rocha F, Meissner U, Röllinghoff M, Bogdan C. Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur J Immunol. 2003;33:1224–34. doi: 10.1002/eji.200323825. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Lombana C, Gimblet C, Bacellar O, et al. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog. 2013;9:e1003243. doi: 10.1371/journal.ppat.1003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellery P, Tippett E, Chiu Y-L, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 40.Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock H. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 41.Jaworowski A, Kamwendo D, Ellery P, et al. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 42.Soares G, Barral A, Costa J, Barral-Netto M, Van Weyenbergh J. CD16+ monocytes in human cutaneous leishmaniasis: increased ex vivo levels and correlation with clinical data. J Leukoc Biol. 2006;79:36–9. doi: 10.1189/jlb.0105040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.