Abstract
IMPORTANCE
Pediatric anxiety disorders are highly prevalent and impairing and are considered gateway disorders in that they predict adult psychiatric problems. Although they can be effectively treated in the short term, data are limited on the long-term outcomes in treated children and adolescents, particularly those treated with medication.
OBJECTIVE
To determine whether acute clinical improvement and treatment type (ie, cognitive behavioral therapy, medication, or their combination) predicted remission of anxiety and improvement in global functioning at a mean of 6 years after randomization and to examine predictors of outcomes at follow-up.
DESIGN, SETTING, AND PARTICIPANTS
This naturalistic follow-up study, as part of the Child/Adolescent Anxiety Multimodal Extended Long-term Study (CAMELS), was conducted at 6 academic sites in the United States and included 288 youths (age range, 11–26 years; mean age, 17 years). Youths were randomized to 1 of 4 interventions (cognitive behavioral therapy, medication, combination, or pill placebo) in the Child/Adolescent Anxiety Multimodal Study (CAMS) and were evaluated a mean of 6 years after randomization. Participants in this study constituted 59.0% of the original CAMS sample.
EXPOSURES
Participants were assessed by independent evaluators using a semistructured diagnostic interview to determine the presence of anxiety disorders, the severity of anxiety, and global functioning. Participants and their parents completed questionnaires about mental health symptoms, family functioning, life events, and mental health service use.
MAIN OUTCOMES AND MEASURES
Remission, defined as the absence of all study entry anxiety disorders.
RESULTS
Almost half of the sample (46.5%) were in remission a mean of 6 years after randomization. Responders to acute treatment were significantly more likely to be in remission (odds ratio, 1.83; 95% CI, 1.08–3.09) and had less severe anxiety symptoms and higher functioning; the assigned treatment arm was unrelated to outcomes. Several predictors of remission and functioning were identified.
CONCLUSIONS AND RELEVANCE
Youths rated as responders during the acute treatment phase of CAMS were more likely to be in remission a mean of 6 years after randomization, although the effect size was small. Relapse occurred in almost half (48%) of acute responders, suggesting the need for more intensive or continued treatment for a sizable proportion of youths with anxiety disorders.
Anxiety disorders are highly prevalent in childhood and severely disrupt the developmental trajectories of affected children and adolescents. Considered gateway disorders, they predict adult mental health problems, including anxiety, depression, and substance use.1–3 These disorders can be treated in the short term with cognitive behavioral therapy (CBT), medication, and/or their combination.4 However, long-term treatment outcome data (ie, ≥5 years after treatment) exist only for CBT.5 To our knowledge, no study has compared the long-term outcomes of CBT with those of medication or combined treatment (CBT plus medication).
Findings from the CBT studies indicate that initial treatment response is largely maintained over time. The largest CBT follow-up study to date reassessed 86 youths (91% of the original sample) a mean of 7 years after treatment and found that 81% of the sample no longer met criteria for their original primary anxiety diagnosis5 and that treatment responders, compared with nonresponders, were less likely to report using alcohol and cannabis. Despite these encouraging findings, the existing CBT follow-up literature is limited by the fact that most studies report only the loss of the youth’s primary anxiety disorder rather than remission (ie, loss of all study entry anxiety disorders). Remission is a more stringent criterion, particularly because anxiety disorders are highly comorbid.6
The current study, part of the Child/Adolescent Anxiety Multimodal Extended Long-term Study (CAMELS), examined the long-term outcomes in youths diagnosed with an anxiety disorder who had been randomized to 1 of 4 treatment conditions (ie, CBT, sertraline, combination, or pill placebo) as part of the Child/Adolescent Anxiety Multimodal Study (CAMS).4 The primary aims of this study were to assess whether children and adolescents who responded favorably to short-term treatments for anxiety (those assigned a score of 1 or 2 on the Clinical Global Impression–Improvement Scale) were more likely to be in remission (ie, free of all of the study entry anxiety disorders), had lower anxiety symptom severity, and had higher functioning than those who did not respond favorably to these treatments (ie, nonresponders). Treatment type was examined to determine whether it was associated with remission, anxiety severity, or global functioning. Several predictors of these outcomes were examined in an exploratory manner (ie, age, sex, race/ethnicity, socioeconomic status [SES], baseline severity of anxiety symptoms, baseline comorbid disorders, parental symptoms of psychopathology, family functioning, negative life events, and mental health service use since CAMS). We hypothesized that CAMS treatment responders (compared with nonresponders) and those receiving combination treatment (compared with other treatment arms) would more likely be in remission, have lower anxiety severity, and have higher functioning at this first CAMELS visit.
Method
Participants
Eligible participants were recruited from 465 youths who participated in CAMS (23 of the original 488 CAMS participants declined to be contacted for future studies). Recruitment for CAMS occurred between 2002 and 2007 at 6 sites.4 All participants met Diagnostic and Statistical Manual of Mental Disorders (4th edition, text revision) diagnostic criteria for social, separation, or generalized anxiety disorder. Participants were randomized in CAMS to 12 weeks of CBT (the Coping Cat treatment manual),7 medication (sertraline), a combination of both, or pill placebo at a 2:2:2:1 ratio. Detailed descriptions of the sample, methods, and acute outcomes have been published elsewhere.4,8–10Recruitment for CAMELS began in January2011 and will end in 2015. Recruited participants are assessed every 6 months. Each year there is a “long visit” that includes a semistructured diagnostic interview by an independent evaluator and numerous questionnaires completed by youths, parents, and study staff (approximately 4–6 hours). The second annual assessment is a “short visit,” in which participants are sent questionnaires to complete at home and contacted by telephone to assess service use. Data for the current study were based on participants’ first CAMELS long visit.
Outcome Assessments
Primary outcomes were assessed by independent evaluators (ie, efforts were made to keep evaluators blind to initial CAMS treatment assignment and responder status) who interviewed participants and their parents. Additional outcomes were determined from youth participant and parent reports and were selected to maintain continuity of measures from CAMS. Specifically, the age-appropriate versions of the Anxiety Disorders Interview Schedule11 were used to assess a broad range of psychiatric disorders, including all 3 of the primary CAMS entry anxiety disorders. The interrater agreement for diagnostic status was high (intraclass correlation coefficient, 0.82–0.88) and was based on a random review of 10% of CAMS baseline and week 12 videotaped independent evaluator assessments. The Clinical Global Impression–Severity scale,12 a single-item measure of anxiety symptom severity, and the Children’s Global Assessment Scale,13 used to rate global functioning, were also rated by an independent evaluator.
The following predictor variables were collected at the CAMS baseline: the Brief Family Assessment Measure,14 completed by parents to assess overall family functioning, with higher scores indicating more family dysfunction (Cronbach α = 0.84); the Brief Symptom Inventory,15 a 53-item parent-report measure of global psychiatric symptoms (Cronbach α = 0.95); the State-Trait Anxiety Inventory–Trait,16 a 20-item parent-report measure that assesses trait anxiety in parents, with higher scores indicating higher anxiety symptoms (Cronbach α = 0.91); and demographic information collected through parent report. The following measures were collected at the first CAMELS visit and examined as predictors: the Anxiety Disorders Interview Schedule Supplemental Services Form,11 administered by a research assistant and used to assess whether psychiatric medication and therapy services were received any time after the acute phase of treatment, with a dichotomized score (ie, yes or no) in each category (psychotherapy, medication). The Life Events Scale,17 a self-report 35-item measure of exposure to a range of stressful events, with a total score reflecting the number of endorsed negative life events, was also obtained.
Procedure
The 465 CAMS participants who agreed to be contacted for future studies were called by telephone and sent a letter of invitation to participate in CAMELS. Families expressing interest completed a written informed consent and then an in-person or telephone evaluation during which all of the described measures were collected. Each family was reimbursed $130 for completion of this assessment. During this evaluation, an independent evaluator administered the age-appropriate diagnostic interview and other key measures. A research assistant supervised administration of youth-report and parent-report questionnaires to ensure that forms were understood and fully completed. All sites had institutional review board approval for this study.
Statistical Analysis
Preliminary Analyses and Handling of Missing Data
Initial analyses compared baseline demographic and clinical characteristics between CAMS participants who enrolled in CAMELS (n = 288) and those who did not (n = 200), using t tests for continuous measures and χ2 tests for categorical variables. Before analyses, missing data were handled using the multiple imputation feature of SPSS software, version 21 (SPSS Institute Inc). Following published guidelines,18,19 we created 20 imputed data sets using the fully conditional specification algorithm, which uses a regression-based approach to generate multiple data sets with different estimates of missing values. The imputation model included all baseline demographic and clinical variables, treatment responder status, and the examined covariates. Binary variables were imputed from a logistic model; all other variables were imputed using linear regression. Overall rates of missing data on examined variables for CAMELS participants were low (<5%), and no variable was missing more than 7% of its values (range, 0%–7%).
Primary Analysis
Logistic regression analyses using multiple imputation were used to examine whether acute treatment responders, compared with nonresponders, had higher rates of remission (ie, were free of the 3 study entry anxiety disorders) at the first CAMELS study visit. Multiple regression analyses examined the effect of response status on anxiety symptom severity and global functioning. Treatment conditions were dummy-coded with the placebo condition as the reference group, and planned contrasts between the 3 active treatment conditions were conducted to examine the effect of treatment condition on outcome variables. Finally, additional predictors of remission, severity of anxiety symptoms, and functioning at this first follow-up visit were examined by using similar procedures. All analyses controlled for study site, baseline CAMS anxiety severity determined with the Clinical Global Impression–Severity Scale, time since CAMS randomization (in years), and mental health service use after the acute phase of CAMS (to account for interim service use). Analyses examined predictors individually along with the designated control variables. To determine the unique contributions of each predictor, all variables were included together in a final multiple regression model. Rubin’s formulas20 were used to combine parameter estimates and standard errors to create pooled estimates. Magnitude of effects for analyses were assessed using Cohen’s effect size guidelines,21 such that odds ratios in logistic regression analyses of 1.44, 2.47, and 4.25 and R2 values in hierarchical regression analyses of 0.01, 0.09, and 0.25 indicate small, medium, and large effects, respectively.
Results
Study Participants
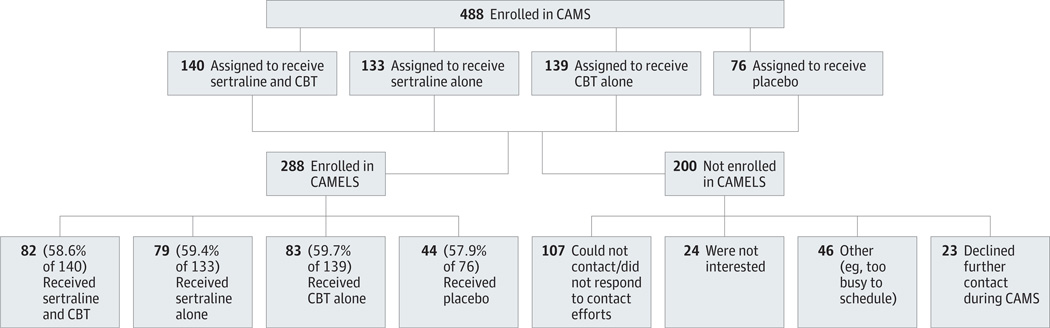
This report is based on the first assessment of 288 CAMELS participants representing 59.0% of youths randomized in CAMS from all CAMS sites (Figure). The CAMELS sample included 160 female participants (55.6%), the mean (SD) age was 16.8 (3.2) years (range, 11–26years), and the racial/ethnic composition was 81.6% white, 8.7% African American, 3.1% Asian, and 6.6% other (eg, American Indian); 8.0% of the sample identified as Hispanic or Latino. The mean time since CAMS randomization was 6.2 years (range, 3.7–9.9 years).
Figure. Flowchart for Child/Adolescent Anxiety Multimodal Study (CAMS) and Child/Adolescent Anxiety Multimodal Extended Long-term Study (CAMELS).
Flowchart shows treatment assignments and breakdown between CAMELS participants and nonparticipants among the original 488 CAMS participants. CBT indicates cognitive behavioral therapy.
Results comparing CAMELS participants and nonparticipants (ie, those who participated only in CAMS) revealed no differences in percentage of treatment responders, baseline anxiety severity, number of baseline comorbid disorders, or assigned treatment condition (Table 1). With respect to demographic variables, the CAMELS sample included more female participants (P = .002) and fewer Hispanics (P = .001) and was of higher SES, as measured by the Hollingshead SES Index (P < .001). On average, CAMELS participants were randomized 3 months later than nonparticipants (t[486] = −2.19; P = .03). Across the CAMELS sample, 46.5% (n = 134) were in remission (ie, did not meet criteria for any study entry anxiety disorder). Among the 53.5% (n = 154) who did meet criteria for a current anxiety disorder, 46.8% (n = 72) had a comorbid internalizing disorder (eg, non–study entry anxiety or mood disorder), and 27.3% (n = 42) had a comorbid externalizing disorder (eg, oppositional defiant disorder or attention deficit hyperactivity disorder); these participants had a mean (SD) of 2.7 (1.3) diagnoses. Among youths who did not meet criteria for a current study entry anxiety disorder (n = 134), 10.4% (n = 14) had an internalizing disorder and 9.7% (n = 13) had an externalizing disorder; they had a mean (SD) of 0.28 (0.51) diagnoses. Table 2 summarizes the diagnostic status of all CAMELS participants.
Table 1.
Comparison of CAMELS Participants and Nonparticipants
| Variable | CAMELS Year 1 Participants (n = 288) |
Nonparticipants (n = 200) |
χ2 or t Statistica |
|---|---|---|---|
| Female sex, % | 55.6 | 41.0 | 10.00b |
| Baseline age, mean (SD), y | 10.5 (2.8) | 11.0 (2.8) | 1.65 |
| Hispanic ethnicity, % | 8.0 | 18.0 | 11.14b |
| Nonwhite race, % | 18.4 | 25.0 | 3.09 |
| Hollingshead SES Index, mean (SD) | 49.34 (10.40) | 43.72 (13.01) | 5.20c |
| Baseline CAMS CGI-S score, mean (SD) | 5.02 (.70) | 5.04 (.77) | 0.34 |
| Baseline CAMS CGAS score, mean (SD) | 50.36 (7.05) | 51.22 (7.18) | 1.32 |
| CAMS treatment responder, % | 62.2 | 54.0 | 3.24 |
| Baseline CAMS diagnoses, mean (SD), No. | 2.99 (1.18) | 2.93 (1.29) | 0.50 |
| Comorbid disorder, % | |||
| Any | 56.3 | 54.0 | 0.24 |
| Internalizing | 44.4 | 43.5 | 0.04 |
| Externalizing | 18.8 | 18.0 | 0.04 |
| Baseline parent BFAM score, mean (SD) | 10.87 (5.12) | 11.21 (5.64) | 0.67 |
| Baseline STAI total score, mean (SD) | 38.74 (9.74) | 38.56 (9.21) | 0.21 |
| Baseline parent BSI GSI score, mean (SD) | .47 (.41) | .48 (.44) | 0.21 |
| Treatment condition, % | |||
| Combination treatment | 28.5 | 29.0 | 0.09 |
| Sertraline | 27.4 | 27.0 | |
| CBT | 28.8 | 28.0 | |
| Placebo | 15.3 | 16.0 |
Abbreviations: BFAM, Brief Family Assessment Measure; BSI GSI, Brief Symptom Inventory Global Severity Index; CAMELS, Child/Adolescent Anxiety Multimodal Extended Long-term Study; CAMS, Child/Adolescent Anxiety Multimodal Study; CBT, cognitive behavioral therapy; CGAS, Children’s Global Assessment Scale; CGI-S, Clinical Global Impression–Severity Scale; SES, socioeconomic status; STAI, State-Trait Anxiety Inventory–Trait.
χ2 Test statistics are presented for categorical variables, t test statistics for continuous variables.
P < .01.
P < .001.
Table 2.
Diagnoses in 288 CAMELS Participants
| Diagnosis | Participants, No. (%) | |
|---|---|---|
| Principal Diagnosis |
Present in Diagnostic Profile |
|
| No diagnosis | 95 (33.0) | 95 (33.0) |
| Separation anxiety disorder | 13 (4.5) | 22 (7.6) |
| Social phobia | 70 (24.3) | 115 (39.9) |
| Generalized anxiety disorder | 48 (16.7) | 98 (34.0) |
| Obsessive compulsive disorder | 3 (1.0) | 13 (4.5) |
| Specific phobia | 12 (4.2) | 52 (18.1) |
| Panic without agoraphobia | 0 | 2 (0.7) |
| Panic with agoraphobia | 2 (0.7) | 3 (1.0) |
| Agoraphobia without panic | 1 (0.3) | 6 (2.1) |
| PTSD | 5 (1.7) | 8 (2.8) |
| Anxiety, not otherwise specified | 0 | 1 (0.3) |
| MDD | 5 (1.7) | 19 (6.6) |
| Dysthymia | 0 | 6 (2.1) |
| Bipolar disorder | 0 | 0 |
| ADHD | 16 (5.6) | 48 (16.7) |
| Conduct | 0 | 0 |
| ODD | 4 (1.4) | 12 (4.2) |
| Tic disorder | 2 (0.7) | 5 (1.7) |
| Substance use or abuse | 3 (1.0) | 10 (3.5) |
| Other | 9 (3.1) | 26 (9.0) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CAMELS, Child/Adolescent Anxiety Multimodal Extended Long-term Study; MDD, major depressive disorder; ODD, oppositional defiant disorder; PTSD, posttraumatic stress disorder.
Interim Service Use
Information on interim therapy services received between CAMS and CAMELS is presented in Table 3. Among CAMELS participants, 46.9% received both medication and therapy at some point between CAMS and CAMELS; 14.9% received only medication, 9.0% received only psychological therapy, and 28.1% received no interim mental health services (6 participants did not complete the service use form). The χ2 tests indicated no differences in supplemental service use by active CAMS treatment condition (ie, CBT, medication, or combination).
Table 3.
Interim Mental Health Service Use Between CAMS and CAMELS Participation by Treatment Conditiona
| Treatment Condition | Mental Health Service Use, No. (%) | |||
|---|---|---|---|---|
| Any Medication or Therapy | Medication and Therapy | Medication Only | Therapy Only | |
| Total (N = 288) | 201 (69.8) | 135 (46.9) | 43 (14.9) | 26 (9.0) |
| Combination (n = 82) | 55 (67.1) | 36 (43.9) | 16 (19.5) | 3 (3.7) |
| Sertraline (n = 79) | 56 (70.9) | 39 (49.4) | 12 (15.2) | 5 (6.3) |
| CBT (n = 83) | 52 (62.7) | 36 (43.4) | 9 (10.8) | 7 (8.4) |
| Placebo (n = 44) | 38 (86.4) | 24 (54.5) | 6 (13.6) | 11 (25.0) |
Abbreviations: CAMELS, Child/Adolescent Anxiety Multimodal Extended Long-term Study; CAMS, Child/Adolescent Anxiety Multimodal Study; CBT, cognitive behavioral therapy.
Eighteen of the 44 youths receiving placebo received a CAMS treatment of their choice (CBT, sertraline, or combination) after completing 12 weeks of treatment with placebo. The “Any Medication or Therapy” variable includes both open-label CAMS treatments and post-CAMS treatments; the other 3 service variables were obtained from the Anxiety Disorders Interview Schedule Supplemental Services Form. Results of χ2 tests indicated no differences in service use variables by active treatment condition (all P >.05).
Primary Outcomes
Treatment Responders vs Nonresponders
Treatment responders were significantly more likely to be in remission (ie, free of all CAMS entry anxiety disorders) (odds ratio, 1.83; 95% CI, 1.08–3.09; P = .03), to have lower anxiety severity scores (R2 = 0.02; P = .02), and to have higher global functioning scores (R2 = 0.01; P = .02) at this follow-up; these were all small effects. The percentages of CAMS responders and nonresponders in remission at the first CAMELS assessment were 52.0% and 37.6%, respectively; as noted, the remission rate for the total sample was 46.5%. When analyses were performed without participants assigned to placebo treatment, responder status predicted only lower anxiety severity scores (R2 = 0.02; P = .04[small effect]); differences in remission (odds ratio, 1.76; 95% CI, 0.97–3.18) and global functioning (R2 = 0.01) were reduced to trends (P = .06 and .08, respectively).
Treatment Condition
The CAMS treatment condition was not associated with remission, anxiety severity scores, or global functioning at follow-up (P > .05 for all). Post hoc comparisons between the active treatment conditions appear in Table 4. The proportion of CAMELS participants in remission in each active treatment condition were 48.8%, 51.9%, and 45.8% for combination treatment, medication, and CBT, respectively.
Table 4.
Predictors of Remission, Symptom Severity, and Functioning at CAMELS Visit 1a
| Predictor Variable | Remission Statusb | CGI-S at CAMELS | CGAS at CAMELS | |||
|---|---|---|---|---|---|---|
| B (SE) |
Odds Ratio (95% CI) | β | ΔR2c | β | ΔR2c | |
| Responder status | .60 (.27) | 1.83 (1.08–3.09)d | −.14d | 0.02 | .12d | 0.01 |
| Treatment condition | ||||||
| Combination | .77 (.41) | 2.16 (0.96–4.85) | −.09 | <0.01 | .05 | <0.01 |
| Sertraline | .76 (.41) | 2.15 (0.96–4.82) | −.04 | .06 | ||
| CBT | .62 (.41) | 1.85 (0.83–4.13) | −.04 | .04 | ||
| Active treatment condition contrasts | ||||||
| Combination vs CBT | .26 (.36) | 1.29 (0.65–2.59) | −.06 | <0.01 | .02 | <0.01 |
| Combination vs sertraline | < −.01 (.34) | 1.00 (0.51–1.95) | −.06 | <0.01 | .01 | <0.01 |
| Sertraline vs CBT | .26 (.35) | 1.29 (0.66–2.54) | −.01 | <0.01 | .03 | <0.01 |
| Demographic variables | ||||||
| Sex | .53 (.26) | 1.69 (1.01–2.84)d | −.15d | 0.02 | .08 | 0.01 |
| Age | −.08 (.05) | 0.93 (0.84–1.02) | .10 | 0.01 | −.10 | 0.01 |
| SES | .02 (.01) | 1.02 (1.00–1.05) | −.14d | 0.02 | .12d | 0.01 |
| Racial minority status | −.24 (.35) | 0.79 (0.40–1.57) | .07 | <0.01 | −.10 | 0.01 |
| Hispanic ethnicity | −.24 (.55) | 0.79 (0.27–2.30) | .06 | <0.01 | −.10 | 0.01 |
| Parent educational levele | ||||||
| Some or standard college | .07 (.36) | 1.07 (0.53–2.18) | −.11 | 0.01 | .05 | 0.01 |
| Advanced graduate degree | .13 (.38) | 1.14 (0.54–2.40) | −.14 | .11 | ||
| Parent/family predictors at baseline | ||||||
| BSI GSI | −.25 (.32) | 0.78 (0.41–1.47) | .07 | <0.01 | −.09 | 0.01 |
| STAI total | <.01 (.01) | 1.00 (0.98–1.03) | .02 | <0.01 | −.05 | <0.01 |
| BFAM | −.03 (.01) | 0.97 (0.94–1.00)d | .12d | 0.02 | −.10 | 0.01 |
| Youth clinical variables at baseline | ||||||
| CGI-Sf | −.43 (.19) | 0.65 (0.45–0.94)d | .08 | 0.01 | −.07 | <0.01 |
| Internalizing comorbid disorder | −.01 (.28) | 0.98 (0.58–1.71) | .05 | <0.01 | −.07 | <0.01 |
| Externalizing comorbid disorder | −.22 (.34) | 0.80 (0.41–1.55) | .07 | <0.01 | −.13d | 0.02 |
| Life Events Scale total score | −.05 (.03) | 0.96 (0.90–1.02) | .15d | 0.02 | −.17g | 0.03 |
| Interim service use | ||||||
| Medication or therapyf | −.71 (.29) | 0.49 (0.28–0.86)d | .13 | 0.02 | −.18g | 0.03 |
| Medication and therapyf | −.46 (.26) | 0.63 (0.38–1.06) | .14g | 0.02 | −.16g | 0.02 |
| Medication onlyf | −.52 (.38) | 0.59 (0.28–1.24) | .01 | <0.01 | −.03 | <0.01 |
| Therapy onlyf | .41 (.44) | 1.51 (0.63–3.58) | −.03 | <0.01 | .01 | <0.01 |
Abbreviations: BFAM, Brief Family Assessment Measure; BSI GSI, Brief Symptom Inventory Global Severity Index; CAMELS, Child/Adolescent Anxiety Multimodal Extended Long-term Study; CBT, cognitive behavioral therapy; CGAS, Children’s Global Assessment Scale; CGI-S, Clinical Global Impression–Severity Scale; SE, standard error; SES, socioeconomic status; STAI, State-Trait Anxiety Inventory–Trait.
For all logistic regressions, df = 9 except for treatment condition (df = 11), parent education (df = 10), and baseline CGI-S and interim service use (df = 8). All hierarchical regressions had degrees of freedom of F9,277 except for treatment condition (F11,275), combination vs CBT (F9,154), combination vs sertraline (F9,151), sertraline vs CBT (F9,151), parent education (F10,276), and baseline CGI-S and interim service use (F8,278).
Remission was defined as absence of all study entry diagnoses (ie, separation anxiety disorder, social phobia, and generalized anxiety disorder).
Denotes change in R2 value after controlling for baseline severity, years since CAMS randomization, treatment site, and supplemental service use (use of medication or therapy).
P < .05.
Dummy coded with high school educational level as the reference group.
Not included as a control variable in this analysis to assess independent contribution of the variable.
P < .01.
Secondary Outcomes
Predictors of Remission at Follow-Up
Fourteen variables (ie, 6 demographic, 3 parent/family, and 5 clinical) were examined as predictors of remission, anxiety severity, and functioning (Table 4). With respect to demographic variables, male sex significantly predicted remission and lower anxiety severity scores at follow-up; higher SES predicted lower anxiety severity and better functioning at follow-up. Better family functioning predicted remission and lower anxiety severity at follow-up. With respect to youth clinical variables, lower baseline anxiety severity predicted remission, and the absence of baseline externalizing comorbid disorders predicted better functioning at follow-up. Fewer negative life events during the follow-up interval predicted lower anxiety severity and higher functioning. In general, youths who did not receive interim services (psychological therapy and/or medication) between CAMS and CAMELS were more likely to be in remission and had higher global functioning.
To determine the unique contribution of each predictor, all variables (including responder status and treatment condition) were combined into a final model for each outcome. With respect to remission, only better family functioning (P = .02) and male sex (P = .04) were significant predictors; responder status (P = .08), baseline anxiety severity (P = .11), and interim mental health service use (P = .10) were no longer significant predictors of remission. With respect to anxiety severity, only responder status (P = .04) and male sex (P = .007) remained significant predictors; SES (P = .16), family functioning (P = .11), life events (P = .05), and interim mental health service use (P = .14) were no longer significant predictors. In terms of global functioning, responder status (P = .03), absence of baseline externalizing comorbid disorders (P = .02), fewer negative life events (P = .03), and absence of interim medication and/or therapy use (P = .003) remained significant predictors of higher functioning; SES (P = .20) was no longer significant.
Post Hoc Analyses
Four post hoc analyses were conducted to clarify findings. First, because male sex was a significant predictor of remission and male CAMS participants were less likely than female CAMS participants to participate in CAMELS, rates of CAMELS participation were compared between male CAMS treatment responders and male nonresponders. No significant differences were found (; P = .23).
Second, because published long-term CBT follow-up studies often use “loss of primary anxiety disorder” as the primary outcome (rather than remission or loss of all study entry anxiety disorders) we examined this outcome to facilitate comparisons with published CBT studies. The percentage of youths no longer meeting diagnostic criteria for their primary anxiety disorder in the overall sample was 64.9% (n = 187), slightly higher than remission rates. Responders were more likely to be free of their primary anxiety disorder (odds ratio, 2.58; 95% CI, 1.52–4.40; P < .001); 72.6% of responders and 52.3% of nonresponders lost their primary anxiety diagnosis, and these proportions did not vary by active CAMS treatment condition (ie, 65.9%, 64.6%, and 69.9% in the combination, medication [ie, sertraline], and CBT [ie, Coping Cat treatment] groups, respectively).
Third, because interpretation of the primary analyses may be confounded by interim mental health service use (which was controlled for in all statistical analyses), we examined the primary research aims for those youths who did not receive any interim mental health services (n = 81; overall remission rate, 60.5%). The remission rate for CAMS responders (n = 55) was 63.6% compared with 53.8% for nonresponders (n = 26). The remission rate for each active treatment condition (among youths who had no interim service use) was 65.2% for combination treatment (n = 23), 64.5% for CBT (n = 31), 47.6% for sertraline (n = 21), and (although the sample was small) 66.7% for placebo (n = 6). Results of χ2 tests indicated that these differences were not statistically significant (remission, [P = .40]; treatment condition, [P = .58]).
Finally, to check whether nonsignificant results for the primary analyses were due to a lack of statistical power, post hoc power analyses using G*Power22 were carried out with power (1 − β) set at .80 and α at .05. Results revealed that, overall, the sample size was sufficient to detect small to medium effect sizes for all primary analyses; thus, it is unlikely that negative findings can be attributed to a limited sample size.
Discussion
This study examined the outcomes in youths with anxiety disorders a mean of 6 years after randomization in the CAMS study.4 Although previous studies have examined the long-term (ie, ≥5-year) outcomes in youths receiving CBT, ours is the first to determine the long-term (ie, >1-year) outcomes in anxious youths treated with medication and combination treatment. Our findings revealed that 46.5% of youths with anxiety disorders randomized in the CAMS treatment trial were in remission (ie, did not meet diagnostic criteria for any of the 3 study entry anxiety disorders) at a mean of 6 years after randomization (some may have experienced relapse during the interim but been in remission by the first CAMELS assessment), highlighting both the gains made by some participants and the chronic nature of these disorders for others. Youths showing clinically meaningful improvement after 12 weeks of treatment were more likely to be in remission, had less severe anxiety, and had better functioning than those who showed minimal or no initial clinical improvement. Although the effect size was small, these findings suggest that early clinical improvement provides protection against reoccurrence of anxiety disorders and associated disability. The fact that youths who received any of the 3 active CAMS treatments showed similar outcomes at the present study’s naturalistic cross-sectional follow-up suggests that practitioners have multiple treatment options with regard to attaining clinical remission.
It is worth noting that the remission rate for CBT observed in this study was slightly lower than rates reported in the existing literature concerning pediatric CBT for anxiety.5 This finding is in part due to variations in the definitions of remission used across studies (ie, absence of all 3 study entry anxiety disorders vs absence of the primary anxiety disorder), and it may be related to other differences in the treatment-seeking preferences of study samples (eg, more severe anxiety, openness to medication, and treatment seeking at a medical institution). Relative to published data on the 1-year outcomes of medication use, the current rates are also slightly lower.23,24 For instance, in 1 study, the responder rate was 67% for children and adolescents (aged 6–18 years) openly treated with sertraline for 1 year23; however, the definition of responder in that study was less stringent than our criteria for remission. Although adult anxiety treatment studies also vary in methods and in assessment and definition of outcomes, a small 5-year CBT follow-up study of adults with social phobia by Mörtberg et al25 found a 65% remission rate (with remission defined as no longer meeting diagnostic criteria for social phobia based on an interview).
The current study also examined a number of predictors of remission, anxiety severity, and functioning. The most consistent predictors of remission were family functioning (based on parent reports) at baseline and male sex. Specifically, youths whose parents reported that their family had clear rules, more trust, and higher-quality interactions when they entered CAMS were more likely to be in remission at this 6-year follow-up. This finding underscores the importance of families in the emotional adjustment of youth and suggests that a greater focus on enhancing family relations in treatment may enhance the durability of existing treatment approaches for pediatric anxiety. Future studies, however, should include youths’ perspectives on family functioning.
Results also revealed that male participants were consistently more likely than female participants to be in remission and have lower anxiety severity scores at 6-year follow-up. The greater risk for female participants parallels sex differences in the rates of anxiety and depressive disorders among older adolescents and adults.2,7 This pattern of heightened risk for female participants was also found in a study examining the long-term outcomes in youths treated for depression.26 Although several reasons for these sex differences have been proposed (eg, gender role orientation, pubertal onset, and related hormones), additional research is needed, and tailored relapse prevention strategies are warranted.
Another demographic predictor of outcomes (ie, lower anxiety severity and better functioning) was higher socioeconomic background. Higher SES is probably a proxy for access to high-quality services that may have helped improve outcomes. A large literature has documented that lower income is a risk factor for psychopathological conditions in general,27,28 and the current data suggest that this pattern is true for anxiety.
Some outcomes were also predicted by certain clinical characteristics of youths at initial presentation for treatment. Youths with lower baseline severity were more likely to be in remission, and those without comorbid externalizing disorders (eg, attention-deficit/hyperactivity and oppositional defiant disorders) had higher functioning, findings that are consistent with other studies.11 Finally, several factors that occurred after acute treatment seem to play a key role in determining outcomes at this 6-year follow-up and are worthy of assessment. Youths who had fewer negative life events (eg, did not report fights with friends, breakups with significant others, or change in parental income status) and participants who did not use mental health services during the follow-up period had better outcomes (eg, lower anxiety severity and higher global functioning). Finally, when all variables were examined together, youths experiencing initial clinical improvement and male participants had the best outcomes at this follow-up assessment.
Findings from this study should be interpreted in the context of several limitations. The most problematic is the use of a naturalistic design. Consequently, although treatments received during the follow-up period were measured and statistically controlled, concomitant treatment use during the follow-up was not restricted and may have affected outcomes. However, the parallel findings based on the exploratory analyses examining youths who received no interim services suggest that interim service use had minimal effect on the pattern of findings (although service use in general was associated with lower remission rates and poorer functioning). Future studies will be needed to examine the effect of specific interim treatments and other events on long-term outcomes. Moreover, the current measure of interim service use was rather crude (use was dichotomized as yes or no) and does not account for duration of treatment, dose, and/or other important aspects of service use. In addition, given the naturalistic design, unmeasured and/or unexamined confounding variables could provide alternative explanations for some of the results. The naturalistic design prohibits claims of causality between CAMS treatments and later outcomes. As in the CAMS trial, there was no formal assessment to determine whether the independent evaluators remained blind, limiting conclusions that can be claimed when comparing blinded and un-blinded treatment arms. Finally, the current assessment was cross-sectional, providing only a snapshot of current functioning, and does not provide data on remission or recurrences of illness during the follow-up period.
The CAMELS sample represented only 59.0% of the CAMS sample, and findings may thus be biased (eg, remission rates may be higher or lower). In addition, the CAMELS sample varied from the CAMS sample on several variables, limiting the generalizability of the findings, particularly to male youths and youths from nonwhite and lower-SES backgrounds. The current study also restricted its examination of outcomes to anxiety and global functioning; future reports will examine the onset of substance use, depression, and other disorders that are expected to emerge as the sample ages and enters a high-risk period for these illnesses. Finally, despite the relatively large size of the current sample, effect sizes were generally modest, suggesting that additional variables need to be explored to elucidate predictors of remission and recurrence of anxiety disorders.
Conclusions
In summary, findings from this naturalistic follow-up study indicated that those youths who experienced a positive response to acute treatment for their anxiety disorder, regardless of treatment type, had better outcomes after a mean of 6 years since randomization. However, almost half of these responders relapsed, suggesting the need for more intensive or continued treatment for a sizable proportion of anxious youth. Predictors of remission (eg, male sex and better family functioning) suggest potential targets for intervention and identify risk factors for poorer outcomes related to anxiety disorders.
Acknowledgments
Dr Ginsburg reports receiving funding from the National Institute of Mental Health (NIMH) and the Department of Education. Dr Sakolsky reports receiving research funding from the NIMH and the Brain & Behavior Research Foundation (formerly NARSAD), receiving an honorarium from the American Academy of Child and Adolescent Psychiatry, and serving as an editorial board member of Child and Adolescent Psychopharmacology News; her spouse is a computer programmer for Thermo Fisher Scientific. Dr Piacentini reports receiving research support from Pfizer Pharmaceuticals, consultant fees from Bayer Schering Pharma, and book royalties from Oxford University Press. Dr Albano reports receiving grant support from the NIMH and Duke University, royalties from Oxford Press and Lynn Sonberg Books, and honoraria from the American Psychological Association and Brackett Global. Dr Compton reports receiving research support from the NIMH and Shire Pharmaceuticals and being the associate editor of Journal of Consulting and Clinical Psychology and Journal of Child and Adolescent Psychopharmacology. Dr Peris reports receiving grants/funding from the NIMH and the International OCD Foundation. Dr Birmaher reports receiving research funding from the NIMH and royalties for publications from Random House and Lippincott Williams & Wilkins. Dr Rynn reports receiving grants/funding from the NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Eli Lilly, Pfizer, Merck, and Shire and royalties from APPI press. Dr Kendall reports receiving author royalties from the sales of treatment materials (Guilford, Oxford University Press, and Workbook Publishing); his spouse has a financial interest in and is affiliated with Workbook Publishing.
Funding/Support: This research was supported by the NIMH (grants MH064089 to Dr Ginsburg, MH064003 to Dr Sakolsky, MH64088 to Dr Piacentini, MH64092 to Dr Albano, MH64107 to Dr March, and MH063747 to Dr Kendall).
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Ginsburg and Ms Becker had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ginsburg, Keeton, Sakolsky, Piacentini, Albano, Compton, Iyengar, Birmaher, Rynn, March, Kendall.
Acquisition of data: Ginsburg, Keeton, Sakolsky, Piacentini, Albano, Compton, Sullivan, Caporino, Peris, Birmaher, Rynn, March, Kendall.
Analysis and interpretation of data: Ginsburg, Becker, Keeton, Sakolsky, Piacentini, Albano, Compton, Iyengar, Sullivan, Birmaher, Rynn, Kendall.
Drafting of the manuscript: Ginsburg, Becker, Keeton, Sakolsky, Sullivan, Rynn, Kendall.
Critical revision of the manuscript for important intellectual content: Ginsburg, Sakolsky, Piacentini, Albano, Compton, Iyengar, Sullivan, Caporino, Peris, Birmaher, Rynn, March, Kendall.
Statistical analysis: Becker, Sakolsky, Compton, Iyengar, Sullivan, Rynn.
Obtained funding: Ginsburg, Sakolsky, Piacentini, Albano, Rynn, March, Kendall.
Administrative, technical, or material support: Ginsburg, Keeton, Sakolsky, Piacentini, Albano, Sullivan, Peris, Rynn, March.
Study supervision: Ginsburg, Piacentini, Albano, Caporino, Peris, Rynn, Kendall.
Conflict of Interest Disclosures: No other disclosures were reported.
Additional Contributions: We thank the following individuals who worked so diligently to complete this study: Columbia University Medical Center: A. Sarubbi, J. Guerry, O. Jablonka, K. Camacho, A. Rapp, C. Heleniak, and M. Goldfine; Duke University: J. Curry, D. Hood, B. Glass-Thomas, C. Mauro, N. Heilbron, J. Sapyta, R. Dingfelder, J. Compton, and P. Cuper; The Johns Hopkins University School of Medicine: J. Walkup, N. Affrunti, M. Crosby Budinger, K. Drake, T. Drazdowski, A. Gordon-Hollingsworth, J. Christofferson, S. Makhzoumi, R. Teetsel, E. Santana, and R. Bloom; Temple University: C. Settipani, C. Wei, D. Brodman, J. Peterman, H. Makover, and J. Edmunds; UCLA Semel Institute for Neuroscience and Human Behavior: L. Bergman, J. McCracken, S. Chang, J. Podell, R. Vivrette, D. Solis, M. Wu, and N. Abrahami; Western Psychiatric Institute and Clinic: N. Curcio, J. Bender, H. Kumar, and K. Monk.
REFERENCES
- 1.Buckner JD, Schmidt NB. Marijuana effect expectancies: relations to social anxiety and marijuana use problems. Addict Behav. 2008;33(11):1477–1483. doi: 10.1016/j.addbeh.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Green JG, Gruber M, et al. The National Comorbidity Survey Adolescent Supplement (NCS-A), III: concordance of DSMIV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry. 2009;48:386–399. doi: 10.1097/CHI.0b013e31819a1cbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendall PC, Safford S, Flannery-Schroeder E, Webb A. Child anxiety treatment: outcomes in adolescence and impact on substance use and depression at 7.4-year follow-up. J Consult Clin Psychol. 2004;72(2):276–287. doi: 10.1037/0022-006X.72.2.276. [DOI] [PubMed] [Google Scholar]
- 6.Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631–648. doi: 10.1016/j.chc.2005.06.003. vii. [DOI] [PubMed] [Google Scholar]
- 7.Kendall PC, Hedtke K. Cognitive-Behavioral Therapy for Anxious Children: Therapist Manual. 3rd ed. Ardmore, PA: Workbook Publishing; 2006. [Google Scholar]
- 8.Compton SN, Walkup JT, Albano AM, et al. Rationale, design, and methods of the Child/Adolescent Anxiety Multimodal Study (CAMS) Child Adolesc Psychiatry Ment Health. 2010;4:1–15. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsburg GS, Kendall PC, Sakolsky D, et al. Remission after acute treatment in children and adolescents with anxiety disorders: findings from the CAMS. J Consult Clin Psychol. 2011;79(6):806–813. doi: 10.1037/a0025933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–365. doi: 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albano AM, Silverman WK. Clinician’s Guide to the Anxiety Disorders Interview Schedule for DSM-IV, Child and Parent Versions. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 12.Guy W. ECDEU Assessment Manual for Psychopharmacology—Revised. Rockville, MD: US Dept of Health, Education, and Welfare; 1976. pp. 218–222. DHEW publication ADM 76–338. [Google Scholar]
- 13.Shaffer D, Gould MS, Brasic J, et al. A Children’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 14.Skinner H, Steinhauer P, Santa-Barbara J. Family Assessment Measure-III (FAM-III) North Tonawanda, NY: Multi-Health Systems; 1995. [Google Scholar]
- 15.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 16.Spielberger CD. State-Trait Anxiety Inventory: Bibliography. 2nd ed. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- 17.Coddington RD. The significance of life events as etiologic factors in the diseases of children, II: a study of a normal population. J Psychosom Res. 1972;16(3):205–213. doi: 10.1016/0022-3999(72)90045-1. [DOI] [PubMed] [Google Scholar]
- 18.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 19.Enders CK. Applied Missing Data Analysis. New York, NY: Guilford Press; 2010. [Google Scholar]
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- 21.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 22.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 23.Cook EH, Wagner KD, March JS, et al. Long-term sertraline treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2001;40(10):1175–1181. doi: 10.1097/00004583-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Clark DB, Birmaher B, Axelson D, et al. Fluoxetine for the treatment of childhood anxiety disorders: open-label, long-term extension to a controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(12):1263–1270. doi: 10.1097/01.chi.0000183464.41777.c1. [DOI] [PubMed] [Google Scholar]
- 25.Mörtberg E, Clark DM, Bejerot S. Intensive group cognitive therapy and individual cognitive therapy for social phobia: sustained improvement at 5-year follow-up. J Anxiety Disord. 2011;25(8):994–1000. doi: 10.1016/j.janxdis.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Curry J, Silva S, Rohde P, et al. Recovery and recurrence following treatment for adolescent major depression. Arch Gen Psychiatry. 2011;68(3):263–269. doi: 10.1001/archgenpsychiatry.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley RH, Convyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States, II: relations with behavioral development through age thirteen. Child Dev. 2001;72(6):1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- 28.Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental origins of child and adolescent behavior problems: from age three to age fifteen. Child Dev. 1995;66(1):55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]