Abstract
Adipose tissue is a dynamic neuroendocrine organ that is involved in multiple physiological and pathological processes, and when excessive, results in obesity. Clinical and population-based data suggest that migraine and chronic daily headache are associated with obesity, as estimated by anthropometric indices. In addition, translational and basic science research shows multiple areas of overlap between migraine pathophysiology and the central and peripheral pathways regulating feeding. Specifically, neurotransmittors such as serotonin, peptides such as orexin, and adipocytokines such as adiponectin and leptin have been suggested to have roles in both feeding and migraine. In this article, we first review the definition and ascertainment of obesity. This is followed by a review of the clinical and population-based studies evaluating the associations between obesity and chronic daily headache and migraine. We then discuss the central and peripheral pathways involved in the regulation of feeding, where it overlaps with migraine pathophysiology, and where future research may be headed in light of these data.
Keywords: migraine, obesity, BMI, abdominal obesity, adipocytokines, adiponectin
Obesity affects more than a billion adults worldwide.1 In the United States alone it has been estimated that 31% of men and 33% of women fulfill criteria for general/total body obesity, while 42% of men and 61% of women fulfill criteria for abdominal obesity.2 Both general and abdominal obesity have been shown to be associated with an increased risk of morbidity and mortality.3–6 Obesity has also been shown to be associated with a reduced quality of life and pain disorders, such as low-back pain and more recently headache.7,8 While in the past, the pain associated with obesity had been largely attributed to structural changes,9 it is difficult to attribute headache to structural changes. In order to improve our understanding as to how a non-structurally related pain disorder may be associated with obesity, it is necessary to understand the nature of obesity as well as usefulness and limitation of different obesity measurements.
Normal Adipose Tissue Distribution
The human body contains lipids that are stored in the form of triglycerides in adipose tissue cells called adipocytes. Adipose tissue and adipocytes exhibit a sexual dimorphism which first becomes evident after puberty.10–12 During puberty, men begin depositing adipose tissue centrally (in abdominal depots), a pattern which persists throughout adulthood.10 In contrast, during puberty women preferentially deposit adipose tissue in the subcutaneous depots in the gluteo-femoral region, but changes to an abdominal pattern postmenopausally.10–12
Differences in adipocyte function and expression of proteins have been shown to exist based on depot locations, as either gluteo-femoral or abdominal, and based on the depth as either subcutaneous adipose tissue (SAT) or visceral adipose tissue (VAT) (Table 1).10–12 Adult men have less SAT and more VAT than adult women, with VAT representing approximately 20% of total body fat in men as compared with 6% in women.11 Although women demonstrate an increase in VAT deposition peri- and postmenopausally, the total volume never reaches the levels seen in men of similar age.13,14
Table 1.
Major Characteristics of Adipose Cells From Subcutaneous Adipose Tissue (SAT) Depots and Visceral Adipose Tissue (VAT) Depots10–12
| Biochemical factor | Regional difference | Physiological effect |
|---|---|---|
| Adiponectin secretion | SAT > VAT | Increased insulin sensitivity |
| Leptin | SAT > VAT | Decreased insulin sensitivity |
| Secretion | Decreased CNS regulation of VAT | |
| Estrogen receptor-β | SAT > VAT | |
| Insulin anti-lipolytic effect | SAT > VAT | Increased free fatty acids and triglyceride turnover |
| Interleukin-6 | VAT > SAT | Cardiovascular risk |
| Inflammation | ||
| Catecholamine lipolytic response | VAT > SAT | |
| Angiotensinogen | VAT > SAT | Increased blood pressure |
| Plasminogen activator inhibitor-1 | VAT > SAT | Cardiovascular risk |
| Glucocorticoid receptors | VAT > SAT | Increased lipoprotein lipase and triglyceride storage |
SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
Obesity
Excessive adipose tissue in relation to fat-free mass results in obesity. Multiple factors can impact the effect of obesity on various diseases states.15,16 Age is one such factor.16 In several disease states, obesity increases the risk of disease in reproductive aged adults, but is attenuated in older populations.17,18 For example, in adults of reproductive age, obesity has been consistently associated with an increased risk of mortality and cardiovascular disease, regardless of the anthropometric index evaluated. In contrast, in elderly populations this association is less clear, with several studies reporting that obesity is not associated, or even inversely associated, with mortality and cardiovascular disease.17,18 Recently it has been suggested that abdominal obesity in female migraineurs may show a similar age variation in disease risk, with an increased odds of migraine and severe headaches in younger women with abdominal obesity, and a decreased odds of migraine and severe headaches in older women with abdominal obesity.14 Several reasons may account for this finding, including change in the association between risk factors and disease state in aging populations, selective survival, or the lack of change in the definitions used to estimate obesity based on the body mass index (BMI) and waist circumference (WC) in aging populations.19
In addition to gender and age, the distribution of adipose tissue can impact the effect of obesity on disease risk as well.14 Specifically, although both total body obesity (TBO) and abdominal obesity (Ab-O) have been shown to be associated with an increased risk of several disease states, Ab-O has frequently been shown to be a better predictor of disease than TBO as estimated by the BMI (eg, diabetes, cardiovascular disease).1,4,5 Furthermore, how obesity is estimated can also impact obesity-related findings.
Obesity is most accurately estimated by direct demonstration of an increase in adipose tissue to fat-free mass, such as with CT or MRI imaging.10,15,16 However, direct measurements are not practical and are substantially more expensive than indirect measurements. Thus, indirect estimates of general or TBO, based on the BMI, and of regional or abdominal obesity, based on WC measurements, are often used (see Table 2). Consequently, obesity is often “de facto” defined as excess in relative body weight, which includes skin, organs, and muscle mass, in addition to adipose tissue mass, rather than just excessive adipose tissue mass.15,16
Table 2.
WHO Criteria for Total Body Obesity as Estimated Based on Body Mass Index and Abdominal Obesity as Estimated Based on Waist Circumference
| WHO criteria for total body obesity for men and women | ||
| BMI < 18.5 | Underweight | |
| BMI 18.5–24.9 | Normal weight | |
| BMI 25–29.9 | Grade I obesity | Overweight |
| BMI 30–39.9 | Grade II obesity | Severe overweight |
| BMI ≥ 40 | Grade III obesity | Morbid obesity |
|
| ||
| WHO criteria for abdominal obesity | ||
| Men | ||
| WC < 94 | Normal weight | |
| WC 94–102 | Action level 1 | Overweight |
| WC > 102 cm | Action level 2 | Abdominal obesity |
| Women | ||
| WC < 80 | Normal weight | |
| WC 80–88 cm | Action level 1 | Overweight |
| WC > 88 cm | Action level 2 | Abdominal obesity |
BMI = body mass index; WC = waist circumference.
Several methodological issues have been discussed in evaluating TBO with indirect measures such as BMI. While most anthropometric measures of TBO perform reasonably well in predicting future diseases, differences may exist in evaluating short-term effects and effects on specific disease entities.1,4,5 First, individuals tend to overestimate their height and underestimate their weight when self-reporting, including migraineurs.20,21 This can lead to non-differential misclassification in prospectively collected data (ie, TBO is ascertained before disease occurs) but may lead to a differential bias in cross-sectional or case–control studies. Second, the implications of the BMI changes with advancing age, as the ratio of adipose tissue to fat-free mass increases with age – even in individuals who maintain the same BMI.16,22 Thus, using the same definition of obesity (based on a BMI cut-point) in older and younger adults may not be appropriate and may be one explanation for changing associations of obesity on outcomes in the elderly. Finally, BMI does not take into consideration gender-specific differences in adipose tissue distribution.16
Despite their limitations, BMI and WC can be valuable tools to estimate and track gross population changes in obesity in a cost-efficient manner. The World Health Organization criteria and grades for general/TBO and abdominal obesity are noted in Table 2.23,24 In the following section, we review the current research evaluating the relationship between obesity and chronic daily headache or migraine using these indirect estimates of TBO and Ab-O. We will then discuss the central and peripheral pathways involved in the regulation of feeding, where it overlaps with migraine pathophysiology, and then briefly touch on where future research may be headed.
THE EPIDEMIOLOGY OF OBESITY AND HEADACHE
Obesity and Chronic Daily Headache
The first study to identify an association between frequent headache and obesity was a study by Scher and colleagues in 2003 (Table 3).8 A total of 1932 participants between 18 and 65 years of age were evaluated, of whom 1134 participants were chronic daily headache (CDH) sufferers, and 798 participants were episodic headache sufferers. Data, including self-reported height and weight, were collected at 2 time points, 11 months apart. Two important findings were reported from this study. First, the prevalence of CDH was associated with those who self-reported having TBO (OR 1.34; CI: 1.0–1.8) or being overweight (OR 1.26; 1.0–1.7). Second, compared with those of normal weight, individuals with episodic headache who also had TBO at baseline were at increased odds of having CDH at follow-up (OR 5.28; CI: 1.3–21.1). Specifically, 30% (7/23) of newly identified cases of CDH fulfilled criteria for TBO, as compared with only 13% (94/726) of those who remained episodic.
Table 3.
Migraine and Obesity in Chronic Daily Headache
| Reference | Study Design | Sample Size | Gender | Mean age (range) | Obesity diagnosis | HA diagnosis | Findings |
|---|---|---|---|---|---|---|---|
| Scher et al (2003)8 | Long-Gen Pop | Total: 1, 932 EH: 798 CDH: 1,134 |
Both together | EH: 41 years CDH: 40 years (18–65) |
Self-reported TBO | Non-ICHD | No difference in TBO prevalence in those with EH as compared with those with CDH. |
| The prevalence of CDH was higher in those with TBO ≥ grade II (OR 1.34; CI: 1.0–1.8). | |||||||
| Transformation of EM to CDH (or incident CDH) at 11 months was associated with TBO (OR 5.28; CI: 1.3–21.1) | |||||||
| Bigal and Liptoi (2006)25 | CS-Gen Pop | Total: 30,849 CDH: 1,243 – 401 TM – 863 CTTH |
Both together | 39 years (18–89) | Self-reported TBO | Modified Silberstein-Lipton Criteria TM ICHD CTTH | The prevalence of CDH was higher in those with TBO ≥ grade II (OR 1.3; CI: 1.1–1.6). |
| The prevalence of transformed migraine was associated with those with TBO ≥ grade II (BMI ≥ 30), while the prevalence of CTTH was only associated with those with a BMI ≥ 35. | |||||||
| Peterlin et al (2008)26 | Clinic-based Pop (age and BMI matched) | Total: 37 CDH: 12 EM: 12 |
Women only | 34 years | Measured TBO and Ab-OB | ICHD | Despite participants being BMI matched, CDH sufferers had greater frequency of abdominal obesity, as estimated by the WHR, than EM and controls, P = .049. |
Ab-OB = abdominal obesity; BMI = body mass index; CDH = chronic daily headache; CS = cross-sectional; EH = episodic headache; EM = episodic migraine; ICHD = International Classification of Headache Disorders; Long = longitudinal; NL = normal; TBO = total body obesity; WC = waist circumference; WHR = waist to hip ratio.
These results were later confirmed by Bigal and Lipton (Table 3).25 Of the 1243 individuals who fulfilled criteria for CDH, approximately 401 fulfilled criteria for transformed migraine and 863 fulfilled criteria for chronic tension-type headache (CTTH). As in the study by Scher, the prevalence of CDH was higher in those with self-reported TBO as compared with the normal-weight group. Specifically, 6.8% of those with a BMI ≥ 35 (OR 1.8; CI: 1.4–2.2) and 5% of those with a BMI ≥ 30 (OR 1.3; CI: 1.1–1.6) had CDH, as compared with 3.9% of those with a BMI between 18.5 and 24.9. In addition Bigal and Lipton showed that the association between CDH and TBO was stronger in transformed migraine than in CTTH.
Finally, a small clinic-based study of 27 women of reproductive age evaluated abdominal obesity in CDH sufferers and migraineurs (Table 3).26 Although the primary aim of the study was to compare serum levels of adiponectin, a protein secreted from adipocytes, between healthy controls and migraine or CDH sufferers, body mass indices were measured, including height, weight and waist and hip circumference. Headache diagnoses were based on international classification of headache disorders (ICHD)-II criteria. Despite participants having been matched based on BMI, results showed that the women with CDH had a greater frequency of abdominal obesity (based on the waist to hip ratio) as compared with controls and those with episodic migraine.
General population studies evaluating association between CDH and Ab-O are warranted.
The prevalence of CDH is increased in those with TBO.
TBO is associated with an increased risk of transforming from episodic to chronic daily headache.
Ab-O may be associated with CDH.
Obesity and Episodic Headache and Migraine
CDH & obesity conclusions
Migraine and adipose tissue both exhibit a sexual dimorphism; and both have been linked to estrogen and the hormonal life-cycle of women. The prevalence of migraine occurs more commonly in adult women of reproductive age than men, (being 2–3 times greater in women than men) with increases in migraine prevalence first being seen in women during puberty and decreases after menopause.27
Similarly, a sexual dimorphism is found with adipose tissue distribution.11–14 Following puberty, an increase in the total volume of SAT and a decrease in the volume of VAT is seen in girls as compared with boys. This pattern persists throughout the life span of men and throughout the reproductive ages of women.11,13 Postmenopausal women have an increase in both total adipose tissue volume and VAT volume, as compared with premenopausal women. The increase in VAT in older women has been demonstrated to first occur around the ages of 40–50 years and again from 50 to 60 years of age.11,13
Given that adipose tissue distribution varies by age and reproductive status, we distinguish between 2 categories: (1) studies that specifically recruited periand postmenopausal women or whose mean age of participants was over 50 (predominantly peri- & post-reproductive age); and (2) studies whose mean age was under 50 years of age or whose methods did not specifically recruit peri- and postmenopausal women (predominantly reproductive age).
Obesity and Migraine in Reproductive-Age Participants
In 2005, 2 small clinic-based studies reported an increased frequency of migraine attacks in those with TBO (Table 4).28,29 In the first, Peres et al compared 74 patients with TBO (mean age of 39 years) who presented to an obesity surgery clinic to 70 age-matched controls.28 A total of 75% of those with TBO had a life-time headache diagnosis as compared with 42% of the controls, P < .001. Furthermore, ICHD migraine was reported by 66% of those with TBO as compared with 18.5% of the non-obese controls, P < .0001. Similarly, in the second clinic-based study by Horev et al, 63% of 27 patients with TBO reported episodic headache and 48% fulfilled migraine criteria.29
Table 4.
Migraine and Obesity in Reproductive-Aged Participants
| Reference | Study design | Sample size | Gender | Mean age (range) | Obesity diagnosis | HA diagnosis | Findings |
|---|---|---|---|---|---|---|---|
| Peres et al (2005)28 | Clinic-based Pop | Total: 144 (74 pts TBO vs 70 age-matched controls) | Both together | 39 years (14–69) | ? | ICHD | 75% of those w/TBO had life-time HA diagnosis as compared with 42% of controls, P < .001. |
| Migraine reported by 66% those w/TBO as compared with 18.5% of non-obese controls, P < .0001. | |||||||
| Horev et al (2005)29 | Clinic-based Pop | Total: 27 (27 TBO pts) | Women only | 40 years (21–61) | ? | ICHD | 63% of those w/TBO had EH |
| 48% of those w/TBO had migraine | |||||||
| Bigal et al (2006)30 | CS-Gen Pop | Total: 30,215 EM: 3,791 |
Combined and Separate | 39 years (18–89) | Self-reported TBO | ICHD | The crude prevalence of migraine in both men and women together was higher in those underweight (15.8%) vs those w/NL weight (13.1 %) and remained significant in women after adjustments. |
| The crude prevalence of migraine in men w/TBO as estimated by BMI ≥ 35 (8.8%) was greater than in those w/NL weight, (7.2%), P < .01), but did not remain significant after adjustments. | |||||||
| The prevalence of HFEM was increased in participants w/TBO (13.6%) as compared with those w/NL weight (4.4%). | |||||||
| Keith et al (2008)31 | CS-Gen Pop Metaanalysis of 11 databases |
Total: >200,000 EM:? |
Women only | ? (16 to 94) | Mixed self-reported and measured TBO | Non-ICHD | Obese women had increased risk for headache as compared with those with BMI 20. |
| Ford et al (2008)32 | CS-Gen Pop | Total 7,601 EM: 1,649 |
Combined and Separate | 46 years (20–85) | Measured TBO | Non-ICHD | Those who were underweight (OR 2.01; CI: 1.34, 3.02) or with TBO (OR 1.37; CI: 1.09, 172) had increased odds for having migraine or severe headaches compared with those with BMI of 18.5 to 24.9. |
| Peterlin et al (2009)14 | CS-Gen Pop | Total 15,531 (≤55 years) EM: 3,915 |
Genders separate | 38 years (20–55) | Measured TBO and Ab-OB | Non-ICHD | Migraine prevalence was increased in those with TBO, using measured height & weight. (Women: OR 1.39; CI: 1.25,1.56; Men: OR 1.38; CI: 1.20,1.59) |
| Migraine prevalence was increased in those with abdominal obesity (WC). (Women OR 1.39; CI: 1.25,1.56; Men: 1.30; CI: 1.13,1.48) |
Ab-OB = abdominal obesity; BMI = body mass index; CDH = chronic daily headache; CS = cross-sectional; EH = episodic headache; EM = episodic migraine; ICHD = International Classification of Headache Disorders; Long = longitudinal; NL = normal; TBO = total body obesity; WC = waist circumference; WHR = waist to hip ratio.
These 2 studies were subsequently followed by 4 cross-sectional, general population-based studies evaluating obesity in those of reproductive age with varying results.14,30–32 One of these studies found no association between migraine prevalence and TBO;30 another found no association between migraine prevalence and TBO, but did find an association between headache prevalence and obesity.31 The other 2 studies reported a positive association between the prevalence of migraine or severe headaches and obesity.14,32
In the first of the general population studies, Bigal et al evaluated 30,215 participants, of whom 3791 fulfilled ICHD migraine criteria and 25,150 were controls (Table 3).30 The age of participants ranged from 18 to 89 years with a mean of 39 years. TBO was estimated using self-reported height and weight. Several findings from this study are of note. First, the crude and adjusted prevalence of migraine was increased in women who were underweight. In addition the crude prevalence of migraine was increased in men with a BMI ≥ 35 (8.8%) as compared with men of normal weight (7.2%),P < .01;however, this finding did not remain significant after adjusting for demographics. Finally, although migraine prevalence was not found to be associated with self-reported BMI, the prevalence of high-frequency episodic migraine was associated with TBO. Specifically, while only 4.4% of those with a BMI between 18.5 and 24.9 reported migraine attacks occurring 10–14 days per month, 13.6% (OR 2.9; CI: 1.9–4.4) of those with a BMI ≥ 30 reported attacks between 10 and 14 days per month.30
In the second general population study, Keith et al conducted a meta-analysis of data from women in 11 different general population databases (Table 4).31 Demographics, self-report of headache or migraine and TBO was obtained from over 200,000 women, ranging in age from 16 to 94 years. TBO was estimated in all but one of the 11 datasets using self-reported height and weight. Although migraine prevalence was not able to be shown to be associated with TBO, obese women had an increase risk for headache as compared with those with a BMI of 20.
The above studies were subsequently followed by 2 studies which evaluated the prevalence of migraine or severe headaches in those with obesity using measured body mass indices.14,32 In the first of these studies, Ford et al evaluated 7601 participants ranging in age from 20 to 85 years of age.32 Migraine or severe headaches was self-reported by participants. TBO was estimated using measured height and weight to calculate the BMI. Similar to the study by Bigal, migraine prevalence was increased in those who were underweight. In addition those with TBO had a 37% increased odds for having migraine or severe headaches compared with those of normal weight, (OR 1.37; CI: 1.09–1.72).32
In the second general population study, Peterlin et al evaluated the prevalence of migraine in those with TBO using measured body mass indices.14 A total of 15,531 reproductive-age participants, ranging from 20 to 55 years of age, who self-reported migraine or severe headaches were evaluated. TBO was estimated using measured height and weight to calculate the BMI. In addition Ab-O was evaluated based on a WC. As in the Ford study,32 the odds of migraine or severe headache was increased by approximately 39% in woman of reproductive age with TBO (OR 1.39; CI: 1.24–1.56), with a similar result in men of the same age group (OR 1.38; CI: 1.20–1.59). These associations were independent of abdominal obesity for both genders. In addition a very similar association was found between abdominal obesity and migraine or severe headaches in women. In those women of reproductive-age with abdominal obesity, the odds of migraine or severe headache was increased by approximately 39%, (OR 1.39; CI: 1.25–1.56); and this association was independent of TBO. In contrast, while the odds of migraine or severe headache were also increased in men with abdominal obesity, (OR 1.30; CI: 1.13–1.48), it was not independent of TBO.14
Confirmatory studies using ICHD criteria and measured body mass indices are needed.
Migraine prevalence and obesity in those of reproductive age conclusions
The prevalence of migraine may be increased in those who are underweight as estimated by TBO.
The prevalence of migraine may be increased in those with TBO, Confirmatory studies using ICHD criteria and measured body mass indices are needed.
The prevalence of migraine may be increased in those with Ab-O.
Obesity and Migraine in Peri- and Postmenopausal Age
Three general population studies have evaluated the migraine and obesity relationship in periand postmenopausal aged participants14,33,34 All 3 studies reported no association between migraine prevalence and TBO in women, and one of the studies found a decreased prevalence of migraine in women with abdominal obesity.
In the first of these studies, Mattsson evaluated 684 peri- and postmenopausal women between 40 and 74 years of age, with a mean age of 54 (Table 5).33 Importantly, this was the first general population study to use both ICHD criteria for the diagnosis of migraine and to estimate TBO using measured body mass indices. Neither migraine prevalence nor migraine attack frequency was associated with TBO.33
Table 5.
Migraine and Obesity in Peri- and Post menopausal-Aged Participants
| Reference | Study design | Sample size | Gender | Mean age (range) | Obesity diagnosis | HA diagnosis | Findings |
|---|---|---|---|---|---|---|---|
| Mattsson (2007)33 | CS-Gen Pop | Total: 684 EM: 130 |
Women only | 54 years (40–74 years) | Measured TBO | ICHD | Neither migraine prevalence or migraine attack frequency was assoc w/TBO, using measured body mass indices in older women. |
| Winter et al (2009)34 | CS-Gen Pop | Total: 63,467 EM: 9,195 |
Women only | 54 years (≥45 years) | Self-reported TBO | Based on ICHD | There was no association between TBO, by self-reported BMI, and active migraine or prior history of migraine in older women. |
| Peterlin et al (2009)14 | CS-Gen Pop | Total: 6,152 (>55 years) EM: 749 |
Both genders separately | 68 years (≥55 years) | Measured TBO and Ab-OB | Non-ICHD | Migraine prevalence was not associated with measured total body or abdominal obesity in older men. |
| Migraine prevalence was not associated with measured TBO in older women. | |||||||
| Migraine prevalence was decreased in older women with abdominal obesity (WC). |
Ab-OB = abdominal obesity; BMI = body mass index; CDH = chronic daily headache; CS = cross-sectional; EH = episodic headache; EM = episodic migraine; ICHD = International Classification of Headache Disorders; Long = longitudinal; NL= normal; TBO = total body obesity; WC = waist circumference; WHR = waist to hip ratio.
In the second study of peri- and post menopausal women, Winter et al evaluated over 63,000 women 45 years of age or older, with a mean age of 54, (Table 5).34 The diagnosis of migraine was evaluated using self-reported information in a manner previously shown to correlate well with ICHD criteria.35 TBO was evaluated using self-reported height and weight. Two important findings were noted. First, although an age-adjusted increased relative risk for the prevalence of migraine was found in those with a BMI ≥ 35, adjustment for major cardiovascular risk factors and postmenopausal status completely attenuated this association. This finding supports results from Mattsson et al that there is no association between migraine prevalence and obesity in peri- and postmenopausal women. Second, the Winter et al data support the findings from Bigal et al suggesting that the association between BMI and migraine frequency may be J-shaped. Compared with women with a BMI of 27 and 29, the relative odds of having daily migraine attacks was over 3-fold increased for women with active migraine and a BMI of ≥ 35; in addition there was an over 2-fold increase for women with active migraine and a BMI of <23.34
The third general population study evaluating the association between migraine and TBO also evaluated older men and evaluated the prevalence of migraine and severe headaches in those older individuals with abdominal obesity.14 As in the previous studies, Peterlin et al found that TBO was not associated with migraine prevalence in older women and extended this finding to older men (Table 5). However, abdominal obesity was associated with a 26% decreased odds of migraine or severe headache in women (OR 74; CI: 0.58–0.94), a finding independent of TBO. These results may suggest that the sexual dimorphism and aging-related changes in the metabolic function of adipose tissue, such as seen with cardiovascular disease or all cause mortality or a survivorship bias may play a role in the obesity–migraine relationship.14
Further studies using ICHD criteria and measured body mass indices are needed.
Migraine prevalence and obesity in those of peri- and post menopausal age conclusions
Migraine prevalence is not associated with TBO in older women and men.
The odds of migraine or severe headache are not increased in older women and men with abdominal obesity.
THE OVERLAP BETWEEN THE CENTRAL AND PERIPHERAL REGULATION OF FEEDING AND MIGRAINE PATHOPHYSIOLOGY
The reason population-based data suggest an association between obesity with high-frequency episodic migraine and chronic daily headache (and possibly migraine prevalence) is likely multifactorial and may involve more complex mechanisms than just similar peripheral proinflammatory states. Below, we discuss these potential mechanisms that could, at least in part, explain this association. Specifically, the central and peripheral pathways regulating feeding and adipose tissue function share extensive overlap with pathways implicated in migraine pathophysiology. In the following section we will describe a broad overview of the central and peripheral regulation of feeding and then focus on some of the protein and peptides which play a role in both feeding and migraine pathophysiology.
The Hypothalamic Regulation of Feeding
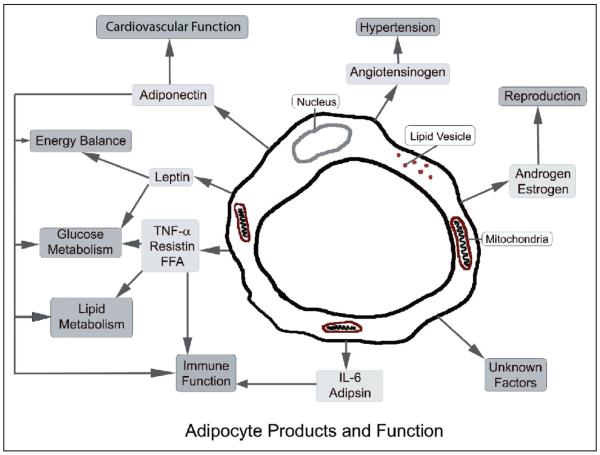
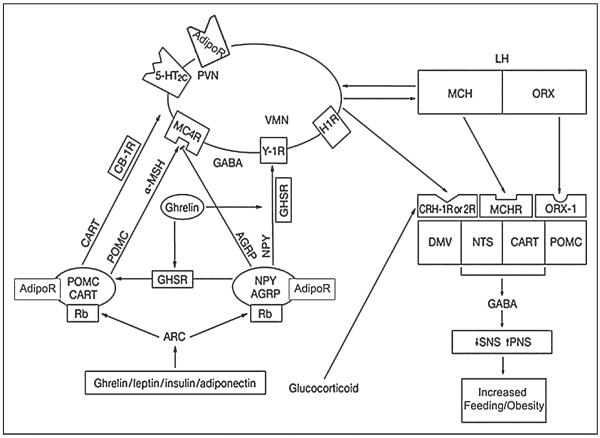
Adipose tissue is an active endocrine organ with roles in energy homeostasis, reproduction, as well as immune and inflammatory process among others (Fig. 2).36 Centrally, the regulation of feeding is controlled by the system involving the arcuate nucleus (ARC) of the hypothalamus and its connections (Fig. 1).37
Fig 2.
Adipocyte Products and Function (Modified with permission from Macmillan Publishers Ltd: Nature Reviews Immunology, Adipocytokines: mediators linking adipose tissue, inflammation and immunity, copyright 2006.)
Fig 1.
The Hypothalamic Regulation of Feeding and Adipose Tissue (Modified with permission from Macmillian Publishers LTD: Nature Clinical Practice Gastroenterology & Hepatology, copyright 2005.) One of the most important hypothalamic nuclei involved in regulating feeding is the arcuate nucleus (ARC). The ARC consists of orexigenic peptides that stimulate feeding (the agouti-gene-related protein [AgRP] and neuropeptide Y [NPY] containing neurons,) and anorexigenic peptides that inhibit feeding (the pro-opiomelanocortin [POMC] and cocaine and amphetamine-regulated transcript [CART] expressing neurons). There is feedback regulation of the central and peripheral signals involved in feeding and energy balance. For example, signals from adiponectin and leptin act on the ARC to produce reciprocal activation or inhibition of the POMC/CART neurons while inhibiting or activating the NPY/AGRP neurons (AdipoR, adiponectin receptor; Rb, leptin receptor). The ARC neurons or “first order neurons” are also defined as “the melanocortin system” for their target, the melanocortin receptor 4. POMC activation from peripheral signals triggers the release of α-melanocyte stimulating hormone (MSH) from axon terminals, which then activates melanocortin-4-receptors (MC4R), resulting in suppression of food intake. Signals from the ARC neurons are then transmitted to “second order neurons” in several other hypothalamic nuclei that also play a role in energy regulation, including the paraventricular (PVN), ventromedial (VM), and lateral hypothalamus (LH). Specifically in the LH are 2 groups of neurons, the orexin neurons, which stimulate feeding, and the melanin-concentrating hormone (MCH) neurons, which inhibit feeding. These second order neurons (the LH, VM, and PVN) modulate the activity of the autonomic nervous system (ANS) and project to the brainstem nuclei, the tractus solitarius (NTS), and the dorsomotor nucleus of the vagus (DMV), where the hypothalamic signals are integrated with peripheral inputs from the gastrointestinal system and liver.
The ARC neurons and its connections are also defined as “the melanocortin system” for their target, the melanocortin receptor. The ARC, or “first order neurons” of the melanocortin system, contains orexigenic and anorexigenic neuropeptides that are the main regulators of energy expenditure and appetite. The primary orexigenic peptides of the ARC consist of the agouti-gene-related protein (AgRP) and neuropeptide Y (NPY) containing neurons which stimulate feeding. The primary anorexigenic peptides consist of the pro-opiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART) expressing neurons, which inhibit feeding (Fig. 1).38,39
There is feedback regulation of the central and peripheral signals involved in feeding and energy balance. For example, signals from adiponectin, leptin, and ghrelin act on the ARC to produce reciprocal activation or inhibition of the POMC/CART neurons while inhibiting or activating the NPY/AGRP neurons.37 Signals from the ARC neurons are then transmitted to “second order neurons” in several other hypothalamic nuclei which also play a role in energy regulation, including the paraventricular (PVN) nucleus, which express adiponectin and leptin receptors, as well as the ventromedial (VM) and lateral hypothalamus (LH) nuclei.36–39
In the LH, there are 2 groups of neurons, the orexin neurons, which stimulate feeding, and the melanin-concentrating hormone (MCH) neurons, which inhibit food intake. In addition the LH, VM, and PVN modulate the activity of the autonomic nervous system. Neurons project from these second order neurons to the brainstem nuclei, the nucleus tractus solitarius (NTS), and the dorsomotor nucleus of the vagus (DMV), where the descending hypothalamic inputs are integrated with the peripheral inputs from the liver and gastrointestinal tract (Fig. 1).37–39
The Hypothalamus and Its Role in Migraine
Hypothalamic involvement has been well described in several headache disorders, including migraine.40,41 Its role in migraine was initially suggested based on the observations of premonitory symptoms in migraineurs, such as changes in thirst, food cravings, mood, and sleep disturbances.40 More recently, functional imaging data support these observations, with hypothalamic activation being demonstrated during acute migraine attacks.41 It has also been demonstrated that several hypothalamic peptides, proteins, and neurotransmittors involved in feeding have been implicated in migraine pathophysiology. Notably, these include serotonin, orexin, adiponectin, and leptin.
Serotonin
Serotonin is a neurotransmitter synthesized from the essential amino acid tryptophan. It is hydroxylated by tryptophan hydroxylase to 5-hydroxytryptamine (5-HT) and then decarboxylated to produce serotonin. After release, synaptic serotonin continues to stimulate pre and post synaptic receptors until it is converted to 5-hydroxyindole acetic acid (5-HIAA) or reabsorbed into the presynaptic neuron.42
The serotonin receptors currently thought to be most directly implicated in feeding control mechanisms are 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2C receptors, with the postsynaptic 5-HT1B and 5-HT2C receptors being directly involved in satiety. Activation of either 5-HT1B or 5-HT2C produces hypophagia.43 Mice with a disruption of the 5-HT2C receptor exhibit increase feeding and develop late-onset obesity and diabetes.44
Serotonin has also been linked to several neuronal cell bodies and neuropeptides involved in feeding, including POMC, NPY, and orexin. Serotonergic compounds directly activate the anorexigenic POMC neurons and cause the release of α-melanocyte-stimulating hormone (MSH) in the hypothalamus.43 In addition, fenfluramine, a 5-HT1B and 5-HT2C agonist, has been shown to block NPY induced hyperphagia. And NPY levels have been shown to decrease after treatment with serotonin receptor agonists and to increase after administration of serotonin receptor antagonists. Finally, serotonergic neurons in the dorsal raphe nucleus express orexin receptors and are excited by orexin A.43
A full review of the connection between serotonin and migraine is beyond the scope of this article; however, we will briefly summarize key points. Specifically low brain serotoninergic activity has been implicated as one of the components in the cascade ultimately resulting in migraine. Inter-ictal levels of plasma serotonin have been shown to be low in migraineurs, along with a 60% increase in 5-HT plasma levels during attacks.42 Thus, it has been hypothesized that migraine may be a syndrome of chronically low serotonin, which would promote an increased drive to feed but with migraine attacks triggered by a sudden raise in 5-HT release, which would coincide with a decreased feeding drive.45 Not surprisingly, several drugs that modulate serotonin and its receptors, including those receptors most directly implicated in satiety, the 5-HT1B and 5-HT2C receptors, are also used in the management of migraine.42,43
Orexin
As with serotonin, orexin has been linked to migraine and feeding. Specifically, orexin (OX) A and OXB are peptides with neuronal cell bodies primarily localized in the LH (Fig. 1). However, orexin containing neurons have been shown to project to the cortex, thalamus, hypothalamus, brainstem (including the locus coeruleus and the raphe nucleus), as well to the gastrointestinal tract.46 Orexin acts on 2 G-protein coupled receptors, OXR1 and OXR2, which have been shown to contribute to the regulation of food intake as well as arousal and pain.47–49
In animal studies, centrally administered orexin increases food intake and has also been shown to reverse the cholecystokinin-induced loss of appetite. In addition in VAT orexin has been shown to decrease the expression of hormone-sensitive lipase, which suggests that orexin might also modulate adipose tissue metabolism by inhibiting lipolysis.49
In addition to their role in feeding, the orexins also participate in inflammatory processes. Several animal studies have demonstrated anti-nociceptive properties of the orexins. In mouse and rat models of nociception and hyperalgesia, intravenous OXA has been shown to be analgesic with an efficacy similar to that of morphine in both the hotplate test and carrageenan-induced thermal hyperalgesia tests.48 Similarly, intrathecally administered OXA in rats has been shown to inhibit heat-evoked hyperalgesia as well as to reduce mechanical allodynia.50 Finally, OXA has also been shown to inhibit neurogenic vasodilation as well as TNC neuronal nociceptive responses to electrical stimulation of the dura mater in rats.51,52
However, the orexins may also have a pro-nociceptive role. The orexins have been shown to excite the histaminergic neurons in the tuberomammillary nucleus (which terminates in the dorsal raphe nucleus and periaqueductal grey region), which can attenuate the antinociceptive effects of OXA. Specifically OXA activates histamine receptors, H1 and H2; and intra-cerebro-ventricular (ICV) injections of a histamine receptor antagonist along with OXA in mice has shown greater antinociceptive effects than ICV OXA alone.47 Furthermore, OXA levels have been shown to be elevated in the cerebrospinal fluid of chronic daily headache sufferers.53 This would suggest that the orexin receptor antagonists, such as ACT-078573 or SB649868, which have been reported to be under development by Actelion and GSK for sleep disorders, could also have a role in migraine therapy.54,55
Thus, although the full role of the orexins and their receptors in migraine is still being determined, the current data suggest that the OXA can modulate neurogenic vasodilation, TNC activation, and pain. In addition, the existing data linking OXA and migraine further support the importance of the regulation of the hypothalamus, in not just feeding, but also pain. Further research evaluating orexin levels during or between migraine attacks is warranted.
Adipocytokines
In addition to peptides and neurotransmitters, adipocytokines participate in energy homeostasis and the regulation of feeding (Fig. 2).56 Adiponectin and leptin are 2 such adipocytokines that have been shown to have central and peripheral roles in the regulation of feeding and have been suggested to be altered in migraineurs.26,57,58
Adiponectin
Adiponectin (ADP) is a protein primarily secreted from adipocytes, with receptors expressed in the brain (notably in POMC and NPY neurons of the hypothalamus), the endothelium of blood vessels, as well as in liver and muscle.56,59 Human plasma ADP can exist as one of several characteristic oligomers or multimers, including high molecular weight (HMW), middle molecular weight (MMW), or low molecular weight (LMW)-ADP.56,60 It has been noted that women have higher ADP levels than men by puberty.60,61
The ADP is most often reported as having anti-inflammatory properties based on the observations that total-ADP (T-ADP) levels are reduced in obesity and type II diabetes. However, elevated levels have been noted in type I diabetes, preeclampsia, and arthritis.26 Furthermore, several lines of evidence now support adiponectin as exerting either pro- or anti-inflammatory properties depending on the form or multimer involved. For example, the globular head of ADP (gADP) can induce self-tolerance to re-exposure of gADP, as well as tolerance to other pro-inflammatory stimuli,62 suggesting that a pattern similar to what is seen with serotonin in migraineurs, could exist with gADP, ie, low levels of gADP inter-ictally and increases during acute attacks.56 In addition the LMW, multimer of ADP has been shown to have anti-inflammatory properties through reduction of interleukin (IL)-6 secretion, while HMW-ADP has been shown to activate nuclear factor kappa-β (NFkβ) pathways and to induce IL-6 secretion in humans.63
The first study to evaluate adiponectin and its multimers in headache sufferers found elevated levels of T-ADP in chronic daily headache sufferers, predominantly due to an elevation in the HMW multimer.26 And although episodic migraineurs showed a similar trend with higher levels of both T-ADP and HMW-ADP as compared with controls, it did not reach significance. Further and larger studies evaluating adiponectin levels both inside and outside an acute migraine attack are needed to evaluate this relationship more fully.
Leptin
Leptin is an adipocytokine with roles in appetite suppression and modulation of inflammatory processes. Like adiponectin, leptin is primarily produced by adipocytes, but also by several other tissues including the brain. In addition, leptin receptors are abundantly expressed in the ARC and DM hypothalamus.64 Leptin is inhibited by testosterone and increased by ovarian sex steroids, with women exhibiting levels that are 2–3 times higher than men even when matched for age and BMI.65,66
Mice with a mutation in the gene encoding leptin (ob/ob mice) or the leptin receptor (db/db mice) express an obese phenotype and have defects in immune function.36 Although elevated leptin levels are associated with an increase in the expression of anorexigenic POMC expression and a decrease in orexigenic NPY and AGRP expression, serum leptin levels are increased in obesity due to leptin resistance. Thus, both leptin deficiency and leptin resistance result in obesity.38
Leptin has also been shown to modulate inflammation. Leptin can induce eicosanoid synthesis and the production of nitric oxide and several cytokines including tumor necrosis factor (TNF)-α and IL-6. Similarly, it has been shown that leptin increases IL-6 production in microglia via several pathways, which include leptin receptors and the pro-inflammatory NFkβ pathways.67,68 In models of acute inflammation circulating leptin levels are promptly and greatly increased. Acute infection, sepsis, and rheumatoid arthritis have all been shown to be associated with increased leptin synthesis.67 Finally, intraperitoneal injections of leptin in mice have also been shown to be associated with an increase in pain sensitivity.69 However, there are some data suggesting an anti-inflammatory role for leptin. In human adipocytes, chronic stimulation with proinflammatory cytokines for 24 hours has been shown to cause a suppression of leptin production.70,71
The first study to evaluate leptin levels in migraineurs evaluated serum leptin levels pre- and post treatment of amitriptyline in 19 patients and of flunarazine in 20 patients.57 BMI and serum leptin levels were found to be increased at both 4 weeks and 12 weeks post treatment as compared with baseline levels. This suggests that serum leptin levels may have been low at baseline in these patients. However, response to therapy was not evaluated and it is unclear if the changes in leptin levels were entirely due to weight gain or a therapeutic response. In addition, disease duration, abdominal obesity, and sex hormones were not evaluated, all of which could affect leptin levels.57
More recently, Guldiken et al evaluated interictal serum leptin levels in migraineurs as compared with age- and gender-matched controls.58 Lower leptin levels and lower fat mass was found in episodic migraineurs. However, after adjusting for fat mass, there was no significant difference in leptin levels between the groups.58 It should also be noted that neither sex hormones nor the phase of the menstrual cycle were controlled for in this study. Thus, no firm conclusion as to the change in level of leptin levels in migraineurs can currently be drawn. However, one could speculate that leptin levels may be low in migraineurs who have had the disease for longer durations since the data suggest that chronic exposure to inflammation may be associated with decreased leptin levels.70,71 Additionally, it is possible that serum leptin levels in migraineurs are elevated, as has been found in other acute inflammatory conditions. Thus, future studies evaluating serum leptin levels in migraineurs with special attention given to disease duration, sex hormones, and the menstrual and life cycle stage in women are warranted.
The Peripheral Regulation
Expansion of adipose tissue during weight gain leads to the recruitment of macrophages and T-cells, as well as changes in the synthesis of cytokines and adipocytokine by adipocytes.36 Specifically, weight gain leads to the induction of adipocytokines and several pro-inflammatory cytokines, including TNF-α, IL-1, and IL-6; all of which can contribute to local and systemic inflammation (Fig. 2).36,72 In the next section we will briefly review the role of cytokines in feeding and their link to migraine.
Cytokines
Pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, are proteins that are predominantly produced by activated immune cells and are involved in amplification of the inflammatory response. Interleukin-6, IL-10, and TNF-α are also expressed or modulated by adipocytes.37 The extent to which adipocytes modulates their activity varies based on body fat. For example TNF-α is mainly produced by macrophages; and with the increase in resident adipose tissue macrophages with obesity, this results in the main source of TNF-α coming from adipose tissue macrophages. TNF-α has also been shown to induce insulin resistance and inhibit adipocyte differentiation.56 Similarly one-third of the IL-6 concentration in the circulation of obese individuals comes from adipocytes.37,60
Several alterations in cytokines have been reported in patients with migraine. Specifically, serum TNF-α and IL-6 have been shown to be increased ictally in episodic migraineurs, while increased cerebrospinal fluid TNF-α has been demonstrated in chronic daily headache sufferers.73,74 In addition, serum levels of the anti-inflammatory cytokine, IL-10 have also been shown to be lower following treatment of acute attacks with sumatriptan, suggesting elevated levels of IL-10 during acute attacks.75 Adiponectin and leptin have been shown to be modulated and to modulate several of these cytokines. Thus, future studies evaluating the effect of cytokines on adipocytokines and of adipocytokines on cytokines in migraineurs would be of interest.
CONCLUSION
Adipose tissue is a dynamic neuroendocrine organ that participates in multiple physiological and pathological processes, including inflammation.48 Clinical, population-based, translational, and basic science research show multiple areas of overlap between the central and peripheral pathways regulating feeding and migraine pathophysiology. The current epidemiological research suggests that chronic daily headache prevalence is increased in adults with obesity and that the prevalence of episodic headaches may be increased in reproductive-aged adults with obesity as well. In order to define this relationship more fully, future studies should use standardized methods to estimate obesity and migraine. Further, the gender- and age-related changes of both obesity and migraine should be taken into account.
In addition to the epidemiological associations, basic and translational research has suggested that several proteins and neurotransmittors, which modulate the pathways regulating feeding and energy homeostasis, may also play a role in migraine pathophysiology, including serotonin, orexin, and adipocytokines. Further research into the role of obesity-related neuroendocrine peptides and neurotransmitters, their receptors and biochemical-signaling pathways may help elucidate migraine disease mechanisms and may initiate new preventive strategies.
Acknowledgments
The authors would like to thank Ann Scher for her helpful comments and suggestions.
Conflict of Interest: Dr. Peterlin has received honoraria and/or grants from GSK, Endo, Merck, OrthoMcNeil, and Pfizer, has a patent for the use of adiponectin modulating agents in migraine, and is an associate editor for Headache. Dr. Rapoport consults for and/or is on the ad boards of Boston Scientific, Nupathe MAP, Pfizer, and Roxro, and is on the speakers bureau of Endo, Merck, and Pfizer. He owns no pharmaceutical stocks and is an editor for Headache. Dr. Kurth has received investigator-initiated research funding as Principal or Co-Investigator from the National Institutes of Health, McNeil Consumer & Specialty Pharmaceuticals, Merck, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety and World Health Information Science consultants, LLC, and has received honoraria from Genzyme, Merck, and Pfizer for educational lectures.
Abbreviations
- 5-HT
5-hydroxytryptamine/serotonin
- Ab-O
abdominal obesity
- ADP
adiponectin
- AgRP
agouti-gene related protein
- ARC
arcuate nucleus of the hypothalamus
- BMI
body mass index
- CART
cocaine and amphetamine-regulated transcript
- CDH
chronic daily headache
- DMV
dorsomotor nucleus of the vagus
- HMW
high molecular weight
- ICHD
international classification of headache disorders
- ICV
intra-cerebro-ventricular
- IL
interleukins
- LH
lateral hypothalamus
- LMW
low molecular weight
- MCH
melanin-concentrating hormone
- MMW
middle molecular weight
- MSH
melanocyte-stimulating hormone
- NFk-β
nuclear factor kappa-β
- NPY
neuropeptide-Y
- NTS
nucleus tractus solitarius
- OX
orexin
- OXR
orexin receptor
- POMC
pro-opiomelanocortin
- SAT
subcutaneous adipose tissue
- TBO
total body obesity
- TNC
trigeminal nucleus caudalis
- VAT
visceral adipose tissue
- VM
ventromedial hypothalamus
- WC
waist circumference
REFERENCES
- 1.Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52:605–615. doi: 10.1016/j.jacc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among U.S. adults. Obesity. 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 3.Kurth T, Gaziano JM, Rexrode KM, et al. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111:1992–1998. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 6.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 7.Lean ME, Han TS, Seidell JC. Impairment of health and quality of life in people with large waist circumference. Lancet. 1998;351:853–856. doi: 10.1016/s0140-6736(97)10004-6. [DOI] [PubMed] [Google Scholar]
- 8.Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106:81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 9.Wendelboe AM, Hegmann KT, Biggs JJ, et al. Relationships between body mass indices and surgical replacements of knee and hip joints. Am J Prev Med. 2003;25:290–295. doi: 10.1016/s0749-3797(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 10.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 11.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 12.Dieudonne MN, Leneveu MC, Giudicelli Y, et al. Evidence for functional estrogen receptors alpha and beta in human adipose cells: Regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 13.Shen W, Punyanitya M, Silva AM, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: A cross-sectional whole-body magnetic resonance imaging study. Nutr Metab. 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterlin BL, Rosso AL, Rapoport AM, Scher AI. Obesity and migraine: The effect of age, gender and adipose tissue distribution. Headache. 2009 doi: 10.1111/j.1526-4610.2009.01459.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boogerd A, Alverdy J, Kumar S, et al. Part II. Obesity. Dis Mon. 2002;48:725–742. doi: 10.1067/mda.2002.130135. [DOI] [PubMed] [Google Scholar]
- 16.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 17.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 18.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: The Iowa Women's Health Study. Arch Intern Med. 2000;24:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Gorber SC, Tremblay M, Moher D, Gorber D. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systemic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 21.Katsnelson MJ, Peterlin BL, Rosso AL, et al. Self-reported versus measured body mass indices in migraineurs. Headache. 2009;49:663–668. doi: 10.1111/j.1526-4610.2009.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen W, St-Onge MP, Wang Z, Heymsfield SB. Study of body composition:An overview. In: Heymsfiel SB, Lohman TG, Wang Z, Going SB, editors. Human Body Composition. Human Kinetics; Champaign, IL: 2005. pp. 3–14. [Google Scholar]
- 23.WHO . Obesity-Preventing and Managing the Global Epidemic. Report of WHO Consultation on Obesity. World Health Organization; Geneva: 1997. [PubMed] [Google Scholar]
- 24.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252–257. doi: 10.1212/01.wnl.0000225052.35019.f9. [DOI] [PubMed] [Google Scholar]
- 26.Peterlin BL, Alexander G, Tabby D, Reichenberger E. Oligomerization state-dependent elevations of adiponectin in chronic daily headache. Neurology. 2008;70:1905–1911. doi: 10.1212/01.wnl.0000312278.40250.6e. [DOI] [PubMed] [Google Scholar]
- 27.Ashkenazi A, Silberstein SD. Hormone-related headache: Pathophysiology and treatment. CNS Drugs. 2006;20:125–141. doi: 10.2165/00023210-200620020-00004. [DOI] [PubMed] [Google Scholar]
- 28.Peres MFP, Lerario DDG, Garrido AB, Zukerman E. Primary headaches in obese patients. Arq Neuropsiquiatr. 2005;63:931–933. doi: 10.1590/s0004-282x2005000600005. [DOI] [PubMed] [Google Scholar]
- 29.Horev A, Wirguin I, Lantsberg L, Ifergane G. A high incidence of migraine with aura among morbidly obese women. Headache. 2005;45:936–938. doi: 10.1111/j.1526-4610.2005.05162.x. [DOI] [PubMed] [Google Scholar]
- 30.Bigal M, Liberman JN, Lipton RB. Obesity and migraine. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- 31.Keith SW, Wang C, Fontaine KR, et al. BMI and headache among women: Results from 11 epidemiologic datasets. Obesity. 2008;16:377–383. doi: 10.1038/oby.2007.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford ES, Li C, Pearson WS, et al. Body mass index and headaches: Findings from a national sample of US adults. Cephalalgia. 2008;28:1270–1276. doi: 10.1111/j.1468-2982.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 33.Mattsson P. Migraine headache and obesity in women aged 40–74 years: A population-based study. Cephalalgia. 2007;27:877–880. doi: 10.1111/j.1468-2982.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 34.Winter AC, Berger K, Buring JE, Kurth T. Body mass index, migraine frequency and migraine features in women. Cephalalgia. 2009;29:269–278. doi: 10.1111/j.1468-2982.2008.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schurks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women's Health Study. Cephalalgia. 2009;29:1086–1090. doi: 10.1111/j.1468-2982.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA. Drug insight: Appetite suppressants. Nat Clin Pract Gastroenterol Hepatol. 2005;2:89–95. doi: 10.1038/ncpgasthep0092. [DOI] [PubMed] [Google Scholar]
- 38.Coppola A, Diano S. Hormonal regulation of the arcuate nucleus melanocortin system. Front Biosci. 2007;12:3519–3530. doi: 10.2741/2331. [DOI] [PubMed] [Google Scholar]
- 39.Crowley VEF, Yeo GSH, O'Rahilly S. Obesity therapy: Alternating the energy intake-and-expenditure balance sheet. Nature. 2002;1:276–286. doi: 10.1038/nrd770. [DOI] [PubMed] [Google Scholar]
- 40.Blau JN. Migraine prodromes separated from the aura: Complete migraine. BMJ. 1980;281:658–660. doi: 10.1136/bmj.281.6241.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47:1418–1426. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 42.Peterlin BL, Rapoport AM. Clinical pharmacology of the serotonin receptor agonist, zolmitriptan. Expert Opin Drug Metab Toxicol. 2007;3:899–911. doi: 10.1517/17425255.3.6.899. [DOI] [PubMed] [Google Scholar]
- 43.Halford JCG, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: Effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 44.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receoptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 45.Hamele E, Saxena PR. 5-Hydroxytryptamine involvement in migraines. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The Headaches. 3rd edn Lippincott, Williams and Wilkins Publishers; Philadelphia, PA: 2006. pp. 275–281. [Google Scholar]
- 46.Budakov D, Alexopoulus H. Metabolic state signaling through central hypocretin/orexin neurons. J Cell Mol Med. 2005;9:795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mobarakeh JI, Takahashi K, Sakurada S, et al. Enhanced antinociception by intracerebroventricularly administered orexin A in histamine H1 or H2 receptor gene knockout mice. Pain. 2005;118:254–262. doi: 10.1016/j.pain.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Bingham S, Davey PT, Babbs AJ, et al. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. doi: 10.1016/s0304-3959(00)00470-x. [DOI] [PubMed] [Google Scholar]
- 49.Heinonen MV, Purhonen AK, Makela KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiol. 2008;192:471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 50.Cheng J, Chou RC, Hwang L, Chiou L. Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain. J Pharmacol Exp Ther. 2003;303:1065–1071. doi: 10.1124/jpet.103.056663. [DOI] [PubMed] [Google Scholar]
- 51.Holland PR, Akerman S, Goadsby PJ. Orexin 1 receptor activation attenuates neurogenic dural vasodilation in an animal model of trigeminovascular nociception. J Pharmacol Exp Ther. 2005;315:1380–1385. doi: 10.1124/jpet.105.090951. [DOI] [PubMed] [Google Scholar]
- 52.Holland PR, Akerman S, Goadsby PJ. Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat. Eur J Neurosci. 2006;24:2825–2833. doi: 10.1111/j.1460-9568.2006.05168.x. [DOI] [PubMed] [Google Scholar]
- 53.Sarchielli P, Rainero I, Coppola F, et al. Involvement of corticotrophin-releasing factor and orexin-A in chronic migraine and medication-overuse headache: Findings form cerebrospinal fluid. Cephalalgia. 2008;28:714–722. doi: 10.1111/j.1468-2982.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- 54.Cormac S. Actelion, GSK ink $3.2B deal with Almacexant as centerpiece. http://www.bioworld.com/servlet/com.accumedia.web.Dispatcher?next=bioWorldHeadlines_article&forceid=48183.
- 55. http://en.wikipedia.org/wiki/Orexin.
- 56.Peterlin BL, Bigl M, Tepper SJ, Urakaze M, Sheftell FD, Rapoport AM. Migraine and adiponectin: Is there a connection? Cephalalgia. 2007;27:435–446. doi: 10.1111/j.1468-2982.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- 57.Berilgen MS, Bulut S, Gonen M, et al. Comparison of the effects of amitriptyline and flunarizine on weight gain and serum leptin, C peptide and insulin levels when used as migraine preventive treatment. Cephalalgia. 2005;25:1048–1053. doi: 10.1111/j.1468-2982.2005.00956.x. [DOI] [PubMed] [Google Scholar]
- 58.Guldiken B, Guldiken S, Demir M, Turgut N, Tugrul A. Low leptin levels in migraine: A case control study. Headache. 2008;40:1103–1107. doi: 10.1111/j.1526-4610.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 59.Kos K, Harte AL, da Silva NF, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in human hypothalamus. J Clin Endocrinol Metab. 2007;92:1139–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 60.Tsao TS, Tomas E, Murrey HE, et al. Role of disulfide bonds in acrp30/adiponectin structure and signaling specificity. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 61.Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein aidponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 62.Tsatsanis C, Zacharioudaki V, Androulidaki A, et al. Adiponectin induces TNF-α and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254–1263. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 63.Haugen F, Drevon CA. Activation of nuclear factor-kB by high molecular weight and globular adiponectin. Endocrinology. 2007;148:5478–5486. doi: 10.1210/en.2007-0370. [DOI] [PubMed] [Google Scholar]
- 64.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Tena-Sempere M, Manna PR, Zhang FP, et al. Molecular mechanisms of leptin action in adult rat testis: Potential targets for leptin-induced inhibition of steroidogenesis and pattern of leptin receptor messenger ribonucleic acid expression. J Endocrinol. 2001;170:413–423. doi: 10.1677/joe.0.1700413. [DOI] [PubMed] [Google Scholar]
- 66.Lin KC, Sagawa N, Yura S, Itoh H, Fujii S. Simulataneous increases of leptin and gondadotropin-releasing hormone following exogenous estrogen administration in women with normally menstrual cycle. Endocr J. 2005;52:449–454. doi: 10.1507/endocrj.52.449. [DOI] [PubMed] [Google Scholar]
- 67.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates pro-inflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 68.Tang CH, Lu DY, Yang RS, et al. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kb, and p300 pathway in migroglia. Immunology. 2007;179:1292–1302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- 69.Kutlu S, Canpolat S, Sandal S, Ozcan M, Sarsilmaz M, Kelestimur H. Effects of central and peripheral administration of leptin on pain threshold in rats and mice. Neuro Endocrinol Lett. 2003;24:193–196. [PubMed] [Google Scholar]
- 70.Otero M, Lago R, Gomez R, et al. Towards a proinflammoatory and immunomodulatory emerging role of leptin. Rheumatology. 2006;45:944–950. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, Trayhurn P. Acute and prolonged effects of TNF-α on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflugers Arch. 2006;452:418–427. doi: 10.1007/s00424-006-0055-8. [DOI] [PubMed] [Google Scholar]
- 72.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarchielli P, Alberti A, Baldi A, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 74.Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47:1050–1055. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 75.Munno I, Marinaro M, Bassi A, et al. Immunological aspects in migraine: Increase of IL-10 plasma levels during attack. Headache. 2001;41:764–767. doi: 10.1046/j.1526-4610.2001.01140.x. [DOI] [PubMed] [Google Scholar]