Abstract
The age-related reduction in muscle force cannot be fully explained by the loss of muscle fiber mass or degeneration of myofibers. Our previous study showed that changes in lateral transmission of force could affect the total force transmitted to the tendon. The extracellular matrix (ECM) of skeletal muscle plays an important role in lateral transmission of force. The objective of this study was to define the effects of aging on lateral transmission of force in skeletal muscles, and explore possible underlying mechanisms. In vitro contractile tests were performed on extensor digitorum longus (EDL) muscle of young and old rats with series of tenotomy and myotomy. We concluded that lateral transmission of force was impaired in the old rats, and this deficit could be partly due to increased thickness of the ECM induced by aging.
Keywords: Aging, Lateral Transmission, Extracellular Matrix of Skeletal Muscle, Tenotomy, Myotomy
1. Introduction
By the year 2030, 20% of the population of the United States will be over 65 years old (U.S. Census Bureau, 2008). Disabilities associated with muscle weakness, known as sarcopenia, are common, and significantly influence the quality of their daily life (Morley et. al., 2001; Roubenoff, 2001; Janssen et al., 2002). The age-related reduction in muscle force has been commonly attributed to the loss of muscle mass. However, muscle force is lost to a greater extent than the loss of muscle mass, suggesting that other factors are involved (Nair, 2005).
The loss of muscle force in aged muscles is not simply caused by degeneration of myofibers. Changes in ATPase activity, metabolite levels, or myosin isoforms have been proposed to be the possible mechanisms; however, none of them can fully explain the loss of force in aged muscles (Philips et al., 1993; Lowe et al., 2001; Lowe et al., 2002). Previous studies showed that although the specific force (force per area) of the whole muscles decreases with aging (~13% – 20%), there is no significant deficit in specific force of single skinned muscle fibers between the young and old groups (Eddinger et al., 1986; Brooks and Faulkner, 1988, 1994; Philips et al., 1991). The deficit in specific force of whole muscles cannot be explained by unimpaired intrinsic force-generating capacity of cross bridges in aged muscles, suggesting that the force transmission from muscle fibers to the tendon in aged muscles could be impaired, leading to the loss of skeletal muscle strength.
Two pathways are involved in transmitting force from muscle fibers to tendon: 1) longitudinal transmission, i.e., transmission along the muscle fibers via the myotendinous junctions (MTJ) to the tendon; and 2) lateral transmission, i.e., transmission laterally across one muscle fiber to the adjacent connective tissue network, the extracellular matrix (ECM), and finally to the tendon (Huijing, 1999). The myotendinous junction has been thought to be the main site of force transmission. However, muscle fibers frequently terminate within the fascicles without reaching the MTJ for muscles across species (Gaunt and Gans, 1992; Trotter, 1993; Trotter and Purslow, 1992; Huijing, 1999). This anatomic structure suggests that the force generated in these muscle fibers has to be transmitted laterally via the ECM, and then to the tendon. Therefore, the force transmitted to the end of muscle could be significantly affected by the ECM (Zhang and Gao, 2012; Gao et al., 2009). As the stiffness and the thickness of ECM in skeletal muscles increase with aging (Nishimura et al., 1997; Kjaer, 2004; Gao et al., 2008), we believe that lateral transmission of force could be affected due to these changes. However, previous studies by Ramaswamy et al. (2011) found that significant difference on lateral transmission only exists between the young (3 months) and very old rats (36–38 months), but not between the young and old (30–33 months). The conflicting findings motivated us to re-investigate the effects of aging on lateral transmission.
The objective of this study was to determine the effects of aging on the lateral transmission of force in skeletal muscle, and explore the potential underlying mechanisms. We hypothesized that lateral transmission pathway is impaired in aged skeletal muscle, and the impaired lateral transmission could be partly due to increased thickness of the ECM. To test our hypothesis, in vitro isometric contractile tests were performed on the extensor digitorum longus (EDL) muscle of young and old rats with series of tenotomy and myotomy between adjacent heads. Proportions of forces transmitted laterally and longitudinally were then calculated and compared between young and old rats.
2. Methods
Two groups of male Brown Norway rats were used in our experiments: young (3–4 months old, n = 6) from Harlan Laboratories (Indianapolis, IN), and old (32 months old, n = 5) from National Institutes of Aging (Baltimore, MD). All procedures used in this study were approved by Cornell University's Institutional Animal Care and Use Committee (IACUC).
2.1 Experimental procedures
After anesthesia, the left extensor digitorum longus (EDL) muscle of each rat was isolated. The EDL is a multi-tendon muscle with distal insertions on digits II–V of the foot, a well-established model for characterizing the force transmission through ECM between the four muscle heads (Huijing et al., 1998; Maas et al., 2003). Silk suture was tied to the distal tendon of the muscle as proximally as possible without damaging the muscle. The suture stayed intact throughout the experiments.
After dissected free from the body, the EDL was fixed to an in vitro contractile testing system (1205A, Aurora Scientific, Toronto, ON) with the proximal end fixed by a clamp, and the suture on the distal end attached to force transducer (resolution 1.0mN). The EDL was placed in mammalian ringer’s solution, and stimulated by a 100Hz, 30V stimulation signal for maximum force generation for 600ms (Brooks & Faulkner, 1988). Three minutes rest was applied between each contraction. The optimal length was then determined, and maximum isometric tetanic force generated was measured and recorded as F0.
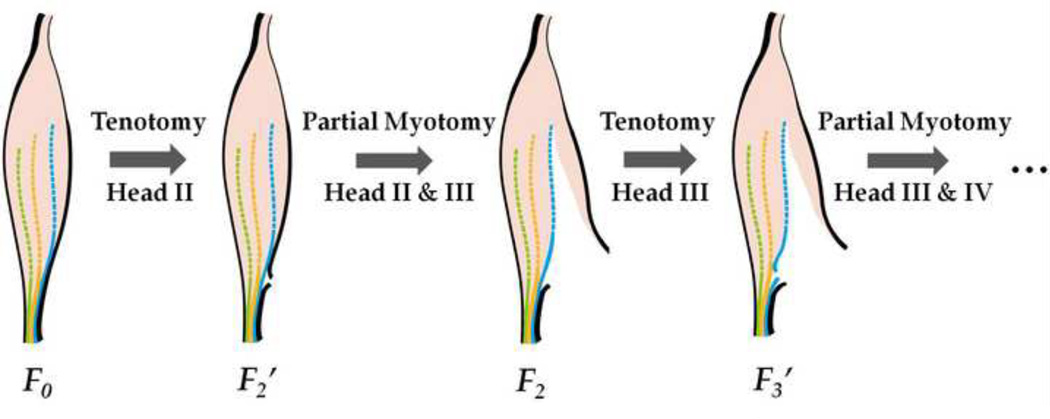
The distal end of the EDL was then detached from the force transducer. The tendon of head II was cut (tenotomy) without touching the suture on the tendon, and the distal end was reattached to force transducer. The same electrical simulation was then applied to the whole muscle at the optimal length. The force measured was recorded as F2', which is the force measured after longitudinal transmission pathway is cut off. After measuring force generated with tenotomy to head II, the distal end was detached again, and the ECM between heads II and III was cut to separate heads II and III. Force generated by the EDL with this process was measured as F2, which is the force measured after both longitudinal and lateral transmissions were cut off for head II. We assume the difference between F0 and F2, i.e., F0 − F2, was the force generated by head II; the difference between F0 and F2', i.e., F0 − F2' was the force generated by head II and transmitted through tendon II; and the difference between F2' and F2, i.e., F2'− F2, was the force generated by head II and transmitted laterally through the ECM between head II and head III. Repeated tenotomy and myotomy were then applied to heads III, IV and the interface between corresponding heads to measure F3', F3, F4', and F4, respectively.
A schematic illustration of the procedure is shown in Fig. 1. For each force measurement, three contractile tests were taken and an average value was calculated. To reduce the geometric variances between EDLs, we normalized each measurement to the maximum force generated by the whole EDL muscle, F0, i.e., (i = 2, 3, and 4). The contribution of each head to the total force of the whole muscle was calculated as (i = 2, 3, and 4, F1 = F0). The force generated in each head Fi−1 − Fi (i = 2, 3, and 4, F1 = F0) was divided into two parts, the force transmitted laterally (Fi'− Fi) and the force transmitted longitudinally (Fi−1 − Fi'), and two respective ratios and (i = 2, 3, and 4, F1 = F0) were calculated.
Fig. 1.
A schematic representation of contractile tests with sequential tenotomy and myotomy between different heads in EDL. First of all, maximum isometric tetanic force of the intact muscle, F0, was measured at the optimal length. F2' is the force measured after tenotomy of tendon of head II, and F2 is the force measured after a partial myotomy, cutting of the ECM, between head II and head III. Same procedure was then repeated for heads III and IV.
The right EDL of each rat was dissected from rats and fixed in 10% neutral buffered formalin solution. The tissue was then fixed and cut into 3µm sections for hematoxylin and eosin (H&E) staining. ImageJ (National Institutes of Health, Bethesda, MD) was used to measure the thickness of the perimysium.
2.2 Statistics analysis
The variance equality and the normality of measurements were checked, and student’s t-test between two groups of small samples was conducted to compare forces transmitted through the ECM between young and old groups. Differences in proportion of force transmitted laterally between young and old groups were assessed using analysis of variance (ANOVA). Difference was considered statistically significant at p < 0.05.
3. Results
The average maximum isometric force, represented by F0, generated in young group is 2.04 ± 0.22 N, and that of the aged group is 1.60 ± 0.20 N (values are presented as mean ± s.d.). Significant difference of F0 was found between young and old groups (p<0.01). There was no significant difference on force contribution from each head () between the young and old groups (p-values for heads II, III, and IV are 0.64, 0.15, and 0.58, respectively).
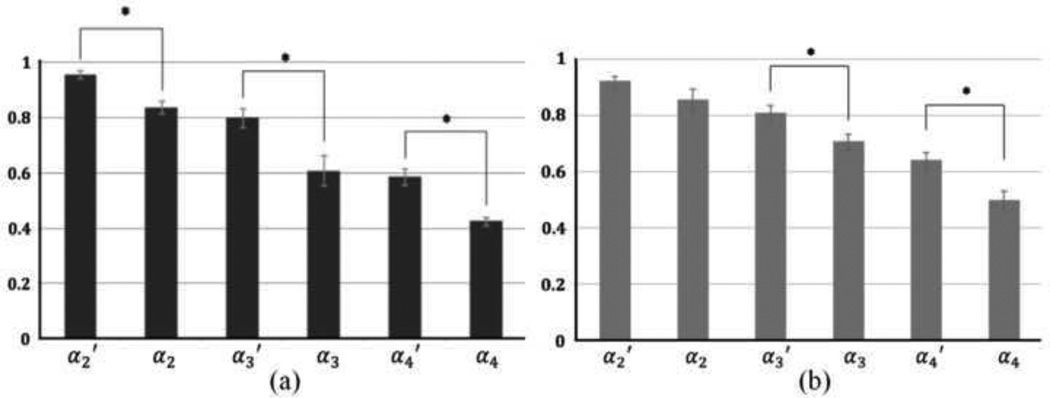
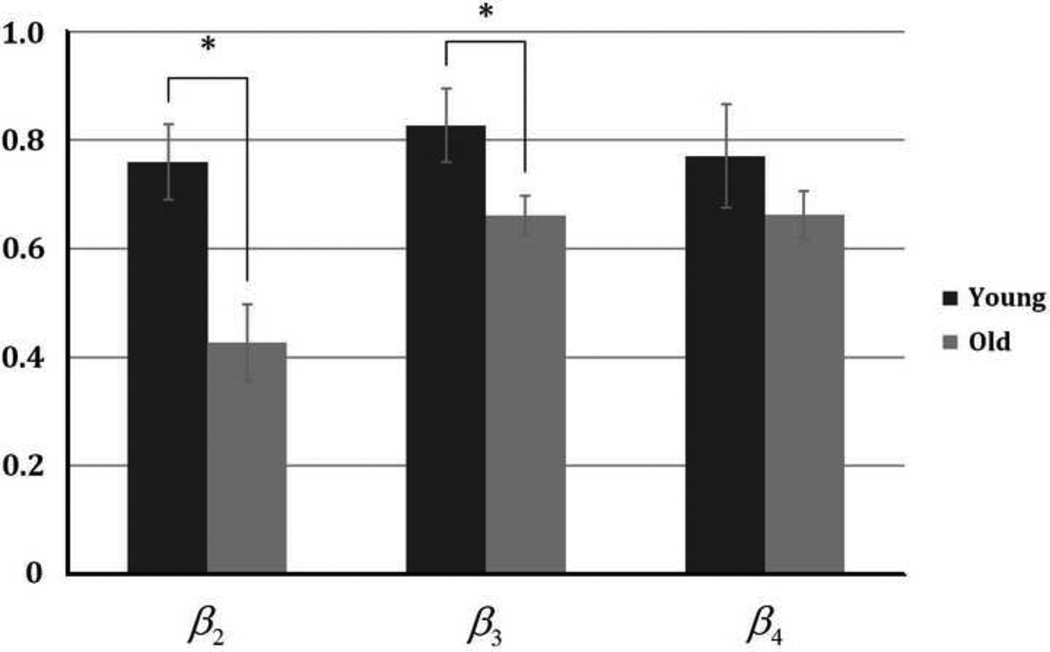
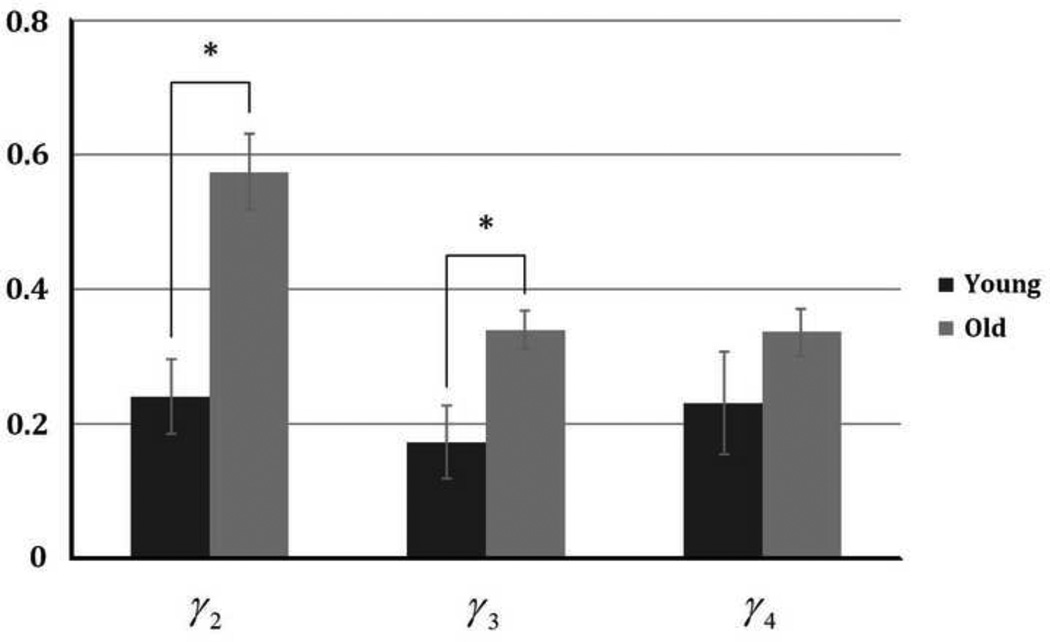
Total forces transmitted to the end of the EDL in the young group during series of tenotomy and myotomy were shown in Fig. 2a. Significant differences between α2' and α2, α3' and α3, and α4' and α4 were observed. There is no significant difference between α2 and α3', and α3 and α4'. Similar results were found for the aged group, which is shown in Fig. 2b, except that α2' and α2 are not significantly different for EDLs in aged group. As shown in Fig. 3, there are significant differences in β2 and β3 between young and old groups, suggesting larger fractions of force generated in heads II and III were transmitted laterally in young muscle. This result indicates that the efficiency of lateral transmission of force in the EDL muscle is impaired in old rats. Significant differences in γ2 and γ3 between young and old groups were also seen (Fig. 4), suggesting that in heads II and III, the force that needed to be transmitted longitudinally increased in the old group. No significant difference was found in β4 or γ4 between two groups.
Fig. 2.
(a) Forces transmitted during series of tenotomy and partial myotomy of the young group (b) Forces transmitted during series of tenotomy and partial myotomy of the old group. ( (i = 2, 3, and 4))
Fig. 3.
Comparison in the proportion of force transmitted laterally in each head ( (i = 2, 3, and 4, F1 = F0)) between young and old groups.
Fig. 4.
Comparison in the proportion of force transmitted longitudinally in each head ( (i = 2, 3, and 4, F1 = F0)) between young and old groups.
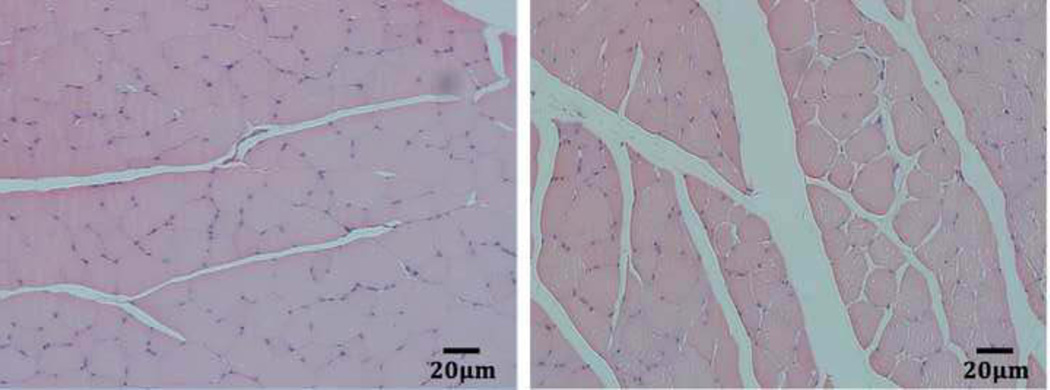
ECM thickness of the old EDL is significantly larger than that of young rats as shown in Fig. 5. The average thickness of perimysium in the young group was 16.87 ± 5.5µm, and that measured in the old group was 44.14 ± 16.55µm. The average thickness of perimysium in the old group was significantly larger than that of the young group (p < 0.0001). In Fig. 5, the cross section of the EDL in young rats demonstrated a more integrated structure and compact myofibers. In contrast, small segments of myofibers isolated by much thicker perimysium were seen in the EDL of aged rats.
Fig. 5.
HE staining of cross-sections of right EDL of young (left) and old (right) rats.
4. Discussion
We demonstrated lateral transmission of force is significantly impaired in old muscle. Parametric analysis by a previous single muscle fiber model suggests that the increased thickness of the ECM induced by aging could be one of the potential compelling mechanisms that cause impaired lateral transmission in old muscles.
The role of lateral transmission through the ECM is well demonstrated in this study. This is supported by two observations in both young and old groups. First of all, no significant differences in contractile force measurement after tenotomy only (α2 vs. α3', and α3 vs. α4'). Secondly, significant reduction in force is seen after the myotomy processed at the interface, i.e., αi' vs. αi. This suggests that cutting the myotendinous junction only does not affect force transmission as the force can still be transmitted laterally through the ECM to the fascicles that are still connected to the tendon. However, cutting the ECM between muscle heads after tenotomy removes the lateral pathway of force transmission, therefore, the force generated by that head of the EDL cannot be transmitted. This result is consistent with a previous study, in which lateral transmission in young rats was determined (Huijing et al., 1998). They found that cutting the longitudinal pathway for approximate 55% of the total EDL mass only caused about 15% drop on the total force transmitted to the end. However, the following myotomy at the interface between head III and head IV caused the force transmitted dropped to approximate 50% of the entire muscle.
Our experimental results suggest that the lateral transmission of force is impaired in old EDL muscle. Although cutting ECM after tenotomy caused decreased muscle force measured in both young and old groups, significant higher force reduction was observed in the young group, suggesting that more force is transferred to the tendon through lateral transmission in the young group. In addition, we measured that the total force transmitted laterally in the entire EDL decreased by about 16% (approximated as ) due to aging, which is consistent with previous experimental observations of about 13% – 20% deficit in aged muscles (Brooks and Faulkner, 1988; Philips et al., 1991). This finding further suggested that the impairment in lateral transmission with aging could be one of the main reasons for the decreased specific force of entire muscle.
In current study, alternately cutting the tendon and the ECM between different heads is performed. This allows us to perform parametric analysis experimentally so that we can quantitatively determine the role of each removed tendon head on longitudinal transmission and the role of the cut ECM between each head on lateral transmission. For example, in addition to aforementioned impaired lateral transmission in old muscle, longitudinal transmission needs to play a more important role with aging, indicated by the difference on βi and γi between young and old groups. For the force generated by head II and III, the proportion of force transmitted laterally (β2 and β3) is significantly smaller in old EDL, and as a result, the proportion of force transmitted longitudinally (γ2 and γ3) in each head significantly increased due to aging.
Different from the conclusion that Ramaswamy et al. (2011) made, we showed impaired lateral transmission of force in old rats of 32 months old. We believe that these two different observations are due to the following two reasons. Firstly, the yoke (Ramaswamy et al., 2011) sutured to the middle of EDL could cause damage to epimysium, which is one of the key components for lateral transmission. Secondly, in the lateral transmission measurement, the yoke instead of the distal tendon was connected to the force transducer, resulting nearly one half of the fibers, i.e., the segment between the yoke and the distal end of the muscle, tend to change from the optimal length to slack length, which would significantly affect the force measured.
Increased thickness of the ECM induced by aging could be an important reason of the impaired lateral transmission of force in old muscle. Similar to other previous studies (Alnaqeeb et al., 1984; Purslow, 2002; Fang et al., 1999), significant increase in thickness of ECM in old rats is observed in present study. From a previously developed FE model (Zhang & Gao, 2012), we believe that increasing the thickness of ECM leads to decreased force transmitted to the end of muscle. This is consistent with a different model in which increased thickness of the ECM can result in drop of force transmitted laterally (Sharafi & Blemker, 2011). In lateral transmission, force is transferred between muscle fibers by shear stresses in the ECM. Considering skeletal muscle as a fiber-reinforced composite (Huijing, 1999; Yucesoy et al., 2002; Blemker et al., 2005; Zhang & Gao, 2012), the reduction of lateral transmission of force with thicker ECM could also be explained by shear lag model of short fiber-reinforced composite (Cox, 1952), in which states that the thicker the matrix is, the less efficient of lateral transmission is.
However, increased thickness of the ECM does not fully explain the impaired lateral transmission of force in old muscle. We only found an approximate 5% drop of force transmitted when doubling the thickness of ECM, which is much lower than 13–20% drop of force in aged muscle (Eddinger et al., 1986; Brooks & Faulkner, 1988), indicating other mechanisms are involved. The stiffness of the ECM of old muscle could increase to 1.5 – 2 times of that of the adult (Alnaqeeb et al., 1984; Gao et al., 2008), which could affect the transmission of force. In addition, according to mechanics of fiber-reinforced composite, changes in interface between the fiber and matrix could affect the force transmission between them. In muscle, dystrophin–glycoprotein complex (DGC) is one of the structures that connect the ECM and myofibers laterally. Therefore, any change in the number or type of DGC could affect the lateral transmission. How the number and type of DGC are affected by aging is controversial and unclear. For example, Ramaswamy et al. (2011) found that number of dystrophin decreases in EDL in very old rats, and therefore, believed that aging caused significant reduction in the expression of dystrophin is correlated to the impaired lateral transmission of force. Rice et al. (2006), however, reported that the amount of both dystrophin and β-dystroglycan increased in aged EDL. They also stated that the age-related DGC change also depended on the type of skeletal muscle. Therefore, further studies on the effects of aging on changes in the molecular linkages between the ECM and the fiber, such as dystrophin, vinculin, talin, etc., are needed.
The lateral transmission of force involves very complicated mechanical interactions between muscle fiber and the ECM. Aging is associated with changes in geometrical, structural and mechanical properties of ECM, muscle fibers and the molecules in between. Experimental results can only show the overall effects of all changes in structural and mechanical properties, and the effect of each individual factor is difficult to be determined. Mathematical model has the advantage of analyzing effects of individual factor by parametric analysis. In the future, a computational model that incorporates the physiological structures and appropriate material properties will be developed. Comparing the parametric analysis of the computational model to our experimental results will allow us to identify the underlying mechanisms of impaired lateral transmission associated with aging.
Acknowledgement
This study was supported by NIH grant 5R03AR59225-2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
We would like to declare that we do not have any conflict of interest to report in this research.
References
- Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. Journal of Anatomy. 1984;139(4):677–689. [PMC free article] [PubMed] [Google Scholar]
- Blemker SS, Pinsky PM, Delp SL. A 3D model of muscle reveals the causes of nonuniform strains in the biceps brachii. Journal of Biomechanics. 2005;38:657–665. doi: 10.1016/j.jbiomech.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. Journal of Physiology. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Isometric, shortening, and lengthening contractions of muscle fiber segments from adult and old mice. The American Journal of Physiology. 1994;207:C507–C513. doi: 10.1152/ajpcell.1994.267.2.C507. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. Journal of Morphology. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Cox HL. The elasticity and strength of paper and other fibrous materials. British Journal of Applied Physics. 1952;3:72–79. [Google Scholar]
- Eddinger TJ, Cassens RG, Moss RL. Mechanical and histochemical characterization of skeletal muscles from senescent rats. American Journal of Physiology-Cell Physiology. 1986;251:C421–C430. doi: 10.1152/ajpcell.1986.251.3.C421. [DOI] [PubMed] [Google Scholar]
- Fang SH, Nishimura T, Takahashi K. Relationship between development of intramusclular connective tissue and toughness of pork during growth of pigs. Journal of Animal Science. 1999;77:120–130. doi: 10.2527/1999.771120x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Faulkner JA, Kostrominova TY, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. Journal of Biomechanics. 2008;41(2):465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Waas AM, Wineman AS. Time dependent lateral transmission of force in skeletal muscle. Proceedings of the Royal Society of London, Series A. 2009;12(2009):1–20. [Google Scholar]
- Gaunt AS, Gans C. Serially arranged myofibers: an unappreciated variant in muscle architecture. Experientia. 1992;48:864–868. [Google Scholar]
- Huijing PA, Baan G, Rebel G. Non-myotendinous force transmission in rat extensor digitorum longus muscle. Journal of Experimental Biology. 1998;201:683–691. [PubMed] [Google Scholar]
- Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. Journal of Biomechanics. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- Hull D, Clyne TW. An Introduction to Composite Materials. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (Sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological Reviews. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. American Journal of Physiology-Cell Physiology. 2001;280(3):C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. American Journal of Physiology-Cell Physiology. 2002;283:C187–C192. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- Maas H, Jaspers RT, Baan GC, Huijing PA. Myofascial force transmission between a single muscle head and adjacent tissues: length effects of head III of rat EDL. Journal of Applied Physiology. 2003;95(5):2004–2013. doi: 10.1152/japplphysiol.00220.2003. [DOI] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. Journal of Laboratory and Clinical Medicine. 2001;137(4):231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- Nair KS. Aging muscles. The American Journal of Clinical Nutrition. 2005;81(5):953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Ojima K, Hattori A, Takahashi K. Developmental expression of extracellular matrix components in intramuscular connective tissue of bovine semitendinosus muscle. Histochemistry and Cell Biology. 1997;107:215–221. doi: 10.1007/s004180050106. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Bruce SA, Woledge RC. In mice, the muscle weakness due to age is absent during stretching. Journal of Physiology. 1991;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SK, Wiseman RW, Woledge RC, Kushmerick MJ. Neither changes in phosphorus metabolite levels nor myosin isoforms can explain the weakness in aged mouse muscle. Journal of Physiology. 1993;463:157–167. doi: 10.1113/jphysiol.1993.sp019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comparative Biochemistry and Physiology Part A. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. The Journal of Physiology. 2011;589(5):1195–1208. doi: 10.1113/jphysiol.2010.201921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KM, Preston DL, Neff D, Norton M, Blough ER. Age-related dystrophin–glycoprotein complex structure and function in the rat extensor digitorum longus and soleus muscle. Journal of Gerontology: Biological Sciences. 2006;61A(11):1119–1129. doi: 10.1093/gerona/61.11.1119. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Origins and clinical relevance of sarcopenia. Canadian Journal of Applied Physiology. 2001;26(1):78–89. doi: 10.1139/h01-006. [DOI] [PubMed] [Google Scholar]
- Sharafi B, Blemker SS. A mathematical model of force transmission from intrafascicularly terminating muscle fibers. Journal of Biomechanics. 2011;44:2031–2039. doi: 10.1016/j.jbiomech.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter JA. Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel) 1993;146(4):205–222. doi: 10.1159/000147459. [DOI] [PubMed] [Google Scholar]
- Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. Journal of Morphology. 1992;212(2):109–122. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- Yucesoy CA, Koopman BH, Huijing PA, Grootenboer HJ. Three-dimensional finite element modeling of skeletal muscle using a two-domain approach: linked fiber-matrix mesh model. Journal of Biomechanics. 2002;35:1253–1262. doi: 10.1016/s0021-9290(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Zhang C, Gao Y. Finite element analysis of mechanics of lateral transmission of force in single muscle fiber. Journal of Biomechanics. 2012;45:2001–2006. doi: 10.1016/j.jbiomech.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]