Abstract
Background and purpose
To develop a class solution for prostate Stereotactic Ablative Radiotherapy (SABR) using Volumetric Modulated Arc Therapy (VMAT).
Materials and methods
Seven datasets were used to compare plans using one 360° arc (1FA), one 210° arc (1PA), two full arcs and two partial arcs. Subsequently using 1PA, fifteen datasets were compared using (i) 6 mm CTV–PTV margins, (ii) 8 mm CTV–PTV margins and (iii) including the proximal SV within the CTV. Monaco™ 3.2 (Elekta™) was used for planning with the Agility™ MLC system (Elekta™).
Results
Highly conformal plans were produced using all four arc arrangements. Compared to 1FA, 1PA resulted in significantly reduced rectal doses, and monitor units and estimated delivery times were reduced in six of seven cases. Using 6 mm CTV–PTV margins, planning constraints were met for all fifteen datasets. Using 8 mm margins required relaxation of the uppermost bladder constraint in three cases to achieve adequate coverage, and, compared to 6 mm margins, rectal and bladder doses significantly increased. Including the proximal SV required relaxation of the uppermost bladder and rectal constraints in two cases, and rectal and bladder doses significantly increased.
Conclusions
Prostate SABR VMAT is optimal using 1PA. 6 mm CTV–PTV margins, compatible with daily fiducial-based IGRT, are consistently feasible in terms of target objectives and OAR constraints.
Keywords: Image Guided RadioTherapy (IGRT), Prostate cancer, Stereotactic Ablative Body Radiotherapy (SABR), Volumetric Modulated Arc Therapy (VMAT)
Prostate cancer (PCa) is the most common cancer in European men, accounting for over one fifth of male cancer diagnoses [1]. Escalated radiation doses in localized disease result in improved biochemical control [2]. Ultra-hypofractionation within the context of stereotactic ablative radiotherapy (SABR) is an attractive approach to dose escalation. Evidence suggests PCa has a low α/β ratio (∼1.5 Gy), making it theoretically more sensitive to large dose per fraction treatments [3].
Volumetric modulated arc therapy (VMAT) uses a linac to deliver radiotherapy in one or more arcs [4]. Dose rate, gantry rotation speed, and MLC positions are altered to create highly conformal plans [4–6]. In comparison to IMRT, VMAT plans display at least comparable conformity with more efficient monitor unit (MU) use and faster delivery times [4–6].
Delivering prostate SABR with VMAT is an attractive option: it offers dose escalation, the theoretical benefits of hypofractionation, the convenience of a few fraction treatment, together with the high conformity, MU efficiency and rapid delivery achievable with VMAT. While much has been published regarding VMAT in PCa and regarding prostate SABR, very little exists in the literature regarding the PCa SABR planning with VMAT.
This planning study assesses prostate SABR using VMAT as a key preparatory step in facilitating future clinical studies. The impact of different arc arrangements is assessed and CTV-PTV margins consistent with daily online fiducial based image guidance and cone beam CT (CBCT) are compared. The impact of the inclusion of the proximal seminal vesicles (proxSV) within the CTV is also evaluated.
Materials and methods
Patients and volumes
Datasets from 15 early PCa patients were chosen. Patients had full bladders and enemas prior to scanning. The bladder, rectum (anus to recto-sigmoid junction), femoral heads (FH), penile bulb (PB) and bowel were contoured as organs-at-risk. The CTV was the whole prostate gland.
Part I
Seven datasets were used. The CTV was expanded isotropically by 6 mm to create the PTV. Each dataset was planned using four arc arrangements:
-
•
One full 360° arc (1FA).
-
•
One partial 210° arc (255° → 105°; 1PA).
-
•
Two full 360° arcs (2FA).
-
•
Two partial arcs (210° (255° → 105°) and 180° (270° → 90°); 2PA).
Part II
Fifteen datasets were planned using 1PA and 6 mm CTV–PTV margins, reflecting margins used with fiducial marker based daily online image guidance (IGRT) [7–9]. All 15 datasets were re-planned using 8 mm CTV–PTV margins, reflecting margins compatible with daily cone beam CT (CBCT) (without fiducials) [10].
Part III
The fifteen datasets were re-planned including the prostate and proximal 1 cm of SV within the CTV, expanded by 6 mm to PTV.
Dose and PTV coverage
The PTV dose was 42.7 Gy in 7 fractions. Compared to 78 Gy in 39 fractions (standard European prostate fractionation), this delivers a higher biologically effective dose (BED) to the prostate (216.3 Gy vs. 182.0 Gy; α/β 1.5 Gy) but an equivalent dose to late responding tissues (129.5 Gy vs. 130.0 Gy; α/β 3.0 Gy; see Supplementary Material for further explanation).
We specified:
-
(i)
95% of the PTV receives at least 95% of the prescription dose (D95% ⩾ 40.6 Gy).
-
(ii)
Minimum prostate dose: ⩾40.6 Gy, (Dmin ⩾ 40.6 Gy (95%)).
-
(iii)
Dose received by 99% of the PTV: ⩾38.4 Gy (D99% ⩾ 38.4 Gy (90%)).
-
(iv)
Maximum dose: ⩽120% (Dmax 51.2 Gy).
-
(v)
Conformity index (to limit high dose spill; CI; see below) should be less than 1.2.
-
(vi)
Where feasible, dose received by 98% of the PTV: ⩾95% of the PD (D98% ⩾ 95%) and dose received by 2% of the PTV: ⩽107% (D2% ⩽ 107%).
As SABR generally encourages dose escalation, it was acceptable if the median dose exceeded the prescription dose of 42.7 Gy.
Defining organ-at-risk constraints
The appropriate organ-at-risk constraints for prostate SABR are unknown. The Hypo-RT-PC trial, a phase III trial comparing 42.7 Gy in 7 fractions with 78 Gy in 39 fractions delivered using IMRT or 3D-CRT, specifies three rectal constraints for the SABR schedule covering high and intermediate doses [11]. Additional constraints were employed to cover the very high and low dose regions, which were biologically equivalent to those used in the 74 Gy in 37 fractions arm of the UK CHHiP trial (which reported low 2-year grade 2+ bowel and bladder toxicity at 4.3% and 2.2%, respectively [12,13]) (Table 1; See Supplementary Material for further explanation).
Table 1.
Dose volume constraints adopted for planning study.
| Volume | Constraints |
|---|---|
| Rectum | V41.4 Gy (97%) < 3% |
| V38.4 Gy (90%) < 15%⁎ | |
| V32.0 Gy (75%) ⩽ 35%⁎ | |
| V28.0 Gy (65%) ⩽ 45%⁎ | |
| V24.8 Gy (58%) < 70% | |
| V19.6 Gy (46%) < 80% | |
| Bladder | V41.4 Gy (97%) < 5% |
| V34.7 Gy (81%) < 25% | |
| V29.9 Gy (70%) < 50% | |
| Femoral heads | Dmax ⩽ 29.9 Gy (70%)⁎ |
| V29.9 Gy (70%) < 50% | |
| Bowel | V29.9 Gy (70%) < 17 cc |
| Penile bulb (objective only)⁎⁎ | V29.9 Gy (70%) < 50% |
| V34.7 Gy (81%) < 10% |
Dose volume constraints adopted from Hypo-RT-PC phase III trial [11]. Those constraints without an asterisk are biologically equivalent to those used in the CHHiP trial for 74 Gy in 37 fraction treatments [12].
Constraints for the penile bulb were for guidance only and did not have to be achieved.
Biologically equivalent constraints to 74 Gy in 37 fractions for a 7 fraction schedule were also derived for bladder, FH, bowel and PB (Table 1).
Planning and evaluation
Monaco 3.2 (Elekta™) with a Monte Carlo (MC) calculation, Agility 5 mm MLC system (Elekta™), 150 control points per arc, 1% MC variance per plan, 6 MV photons and a 3 mm calculation grid were employed.
The following were recorded:
-
•
CTV: median dose (D50%), D2%, D98% and volume receiving 100% of PD (V100%).
-
•
PTV: D50%, D2%, D98% and D95%.
-
•
Organ-at-risk mean doses and D2%, volume of rectum and bladder receiving at least 95% (V95%), 80% (V80%), 50% (V50%) and 20% (V20%) of PD to reflect very high, high, intermediate and low doses, respectively.
-
•
CI: volume of 95% isodose/PTV volume [14].
-
•
Conformation number (CN): (volume of PTV receiving 95% isodose/PTV volume) × (volume of PTV receiving 95% isodose/volume of 95% isodose) [14].
-
•
Homogeneity index (HI): (D2–D98%)/D50% [15].
-
•
R50: volume of 50% isodose/PTV volume.
-
•
Maximum dose 2 cm from PTV (Dmax 2 cm).
-
•
MU per fraction.
-
•
Estimated delivery time (EDT).
Statistics
The Wilcoxon signed-rank test was used to compare parameters as data were not normally distributed. Median values and ranges are therefore presented. SPSS v19.0 was used for calculations. Tests were two-tailed.
Multiple statistical comparisons are made but a full Bonferroni correction would be over-conservative as several tests are not independent. In part I of the study, the small sample size limits the degree of statistical significance achievable. As a pragmatic approach, p ⩽ 0.02 was considered statistically significant for part I of the study, and p ⩽ 0.005 was considered significant in parts II and III.
Results
Part I: arc arrangements
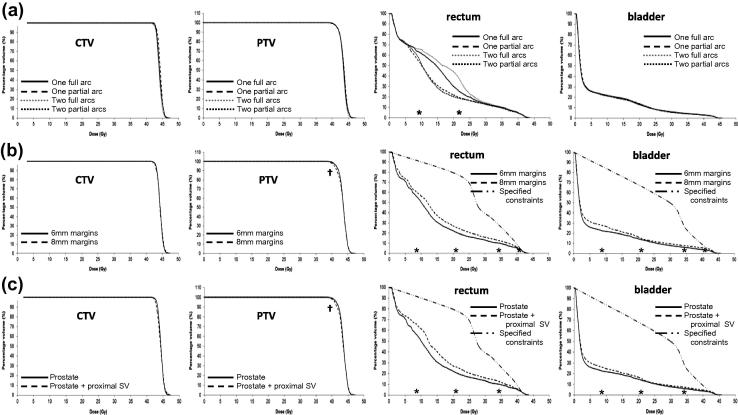
Adequate CTV (prostate only) and PTV coverage was achieved and organ-at-risk constraints were met using all arc arrangements (Fig. 1a–d in Supplementary Material). Plans were highly conformal with CI <1.2 and CN ⩾0.81, and doses were homogeneous (HI: 0.08–0.12). Compared to 1FA, there were no significant differences in CTV and PTV coverage with different arc arrangements, with the exception of 2FA, where there was a significant reduction in CTV V100% (1FA vs. 2FA: 97.9% vs. 95.2%, p = 0.016; median values presented) and a statistically significant, but clinically insignificant, reduction in PTV D50% (43.5 Gy vs. 43.4 Gy, p = 0.016, Table 4 in Supplementary Material).
Compared to 1FA, partial arc arrangements resulted in significant reductions in rectal mean dose (1FA vs. 1PA: 15.1 Gy vs. 13.2 Gy, p = 0.016, 1FA vs. 2PA: 15.1 Gy vs. 13.0 Gy, p = 0.016), V50% and V20%, (Fig. 1a, Table 4 in Supplementary Material). Compared to 1FA, there were no statistically significant differences in bladder doses. Partial arc arrangements resulted in significant increases in FH mean doses and D2% (Table 4 in Supplementary Material), although doses remained well within tolerance. A statistically significant, but clinically insignificant, increase in R50 occurred using 2FA (3.7 vs. 3.9, p = 0.016). Compared to 1FA, 1PA resulted in reduced EDTs in 6 of 7 cases but this result did not reach our selected level for statistical significance (173 s vs. 152 s, p = 0.047). Similarly, compared to 1FA, 1PA resulted in reduced MU requirements in 6 of 7 cases but this also did not reach statistical significance (2049 MU vs. 1785 MU, p = 0.031). There was a significant increase in EDT using 2FA (173 s vs. 206 s, p = 0.016). See Tables 4–7 in Supplementary Material for full planning data.
Given target coverage and conformity equivalence, significant reductions in rectal mean dose, V50% and V20%, together with the MU and EDT advantages, the 210° partial arc was selected for further investigation.
Part II: CTV–PTV margins
Fifteen datasets were planned using 1PA and 6 mm CTV–PTV margins. Adequate CTV and PTV coverage was achieved and organ-at-risk constraints were met, and were generally well within desired limits (Fig. 1b).
Fig. 1.
(a) Rectal DVH using four beam arrangements (median values plotted). ∗Rectal V20% and V50% for one partial arc and two partial arcs significantly less than one full arc (p < 0.02). (b) DVH comparisons for 6 mm and 8 mm CTV to PTV margins (median values plotted). †PTV D95% significantly less using 8 mm margins compared to 6 mm margins (p < 0.005). ∗Rectal/bladder V20%, V50%, V80% and V95% significantly less using 6 mm vs. 8 mm margins (p < 0.005). (c) DVH comparisons for CTV containing prostate alone and CTV containing prostate + proximal seminal vesicles (median values plotted), SV: seminal vesicles. †PTV D95% significantly less with prostate + proximal seminal vesicles in CTV compared to prostate alone (p < 0.005). ∗Rectal/bladder V20%, V50%, and V80% significantly less with prostate alone in CTV compared to prostate plus proximal seminal vesicles (p ⩽ 0.005).
Datasets were re-planned using 1PA and 8 mm CTV–PTV margins (Fig. 1e in Supplementary Material). In 12 cases (80%) it was possible to achieve CTV and PTV coverage and meet organ-at-risk constraints. In three cases it was necessary to relax the uppermost bladder constraint (V41.4 Gy <5%) to up to 8.7% to achieve adequate coverage. Other bladder constraints were achieved. There was a small reduction in homogeneity using 8 mm margins (6 mm vs. 8 mm HI: 0.11 vs. 0.13, p < 0.001).
Of the three patients where the uppermost bladder constraint had to be relaxed, two had median lobes protruding into the bladder and relatively small bladder volumes (208 ml and 249 ml). The third patient had a very large median lobe and the largest volume prostate in the series (60.0 cm3).
Compared to plans using 6 mm CTV–PTV margins, there was no significant difference in CTV and PTV D50% although 8 mm margins resulted in a small but significant reductions in PTV D95% (41.4 Gy vs. 40.8 Gy, p < 0.001, Fig. 1b, Fig. 2a in Supplementary Material), and PTV D98% (40.6 Gy vs. 40.0 Gy, p = 0.001), and significant increases in rectal and bladder mean doses and V95%, V80%, V50% and V20% (Fig. 1b, Table 5 in Supplementary Material). There were statistically significant, but clinically insignificant, increases in mean bowel dose using 8 mm margins. There was also a significant increase in right mean FH dose, but this remained well within tolerance, as well as an increase in PB mean dose and D2% (Table 5 in Supplementary Material).
There was no significant difference in CI, which considers 95% isodose and PTV volumes (but not PTV coverage), nor CN, which reflects PTV coverage as well as high dose spill.
Part III: inclusion of proxSV
Datasets were re-planned using 1PA and 6 mm CTV–PTV margins but including the proxSV within the CTV (Fig. 1f in Supplementary Material). In 13 cases CTV and PTV coverage was achieved and all organ-at-risk constraints were met. In two cases (13%; the same two cases with small bladder volumes and median lobe hypertrophy requiring relaxation of the uppermost bladder constraints using 8 mm CTV–PTV margins) it was necessary to relax the uppermost bladder and rectal constraints up to 6.6% and 3.9%, respectively to achieve coverage. Other constraints were met. It was possible to re-plan both to achieve coverage and meet all constraints by defining two PTVs: prostate plus 6 mm, prescribed 42.7 Gy, and prostate and proximal 1 cm of SV plus 6 mm, prescribed 32.4 Gy (76%).
Adequate CTV and PTV coverage was achieved although, compared to treating prostate alone, there were small but significant reductions in PTV D95% (41.4 Gy vs. 40.9 Gy, p = 0.001, Fig 1c, Fig. 2b in Supplementary Material) and PTV D98% (40.6 Gy vs. 40.2 Gy, p < 0.001) and a small increase in CTV D2% (45.9 Gy vs. 46.4 Gy, p = 0.001). The bladder and rectum received significantly higher mean doses and V80%, V50% and V20% (Fig. 1c, Table 5 in Supplementary Material). There were significant increases in left FH D2%, and bowel mean dose and D2%, although these remained well within tolerance (Table 5 in Supplementary Material). Compared to treating the prostate alone, plans were less homogeneous (prostate alone vs. prostate + SV HI: 0.11 vs. 0.13, p < 0.001) and required increased MU. (1814 vs. 1910 MU, p = 0.002).
Discussion
Much has been published regarding the use of VMAT in PCa, and regarding SABR in PCa. There is, however, very little in the literature, regarding the optimal planning of prostate SABR using VMAT. It is important and relevant to develop linac-based solutions for prostate SABR as this delivery method is more widely available than alternatives such as Cyberknife™ (Accuray™). We found prostate SABR planned with VMAT is optimal using 1PA. Using 6 mm CTV–PTV margins, compatible with daily fiducial based IGRT, is consistently feasible in terms of target objectives and organ-at-risk constraints. All arc arrangements investigated resulted in highly conformal plans but a single 210° partial arc was preferred: conformity was maintained while rectal mean dose, V50% and V20% were reduced and most patients also benefitted in terms of EDT and MU requirements. FH doses increased but remained well within tolerance.
The optimal organ-at-risk constraints for prostate SABR remain unknown. In this study we adopted constraints from the HYPO-RT-PC trial and added additional constraints which were biologically equivalent to those used in the CHHiP trial which reported low 2-year toxicity [11–13].
In the Hypo-RT-PC trial, which employs the same SABR dose, no constraint is specified for very high rectal doses, and there are no bladder constraints [11]. Several of the Cyberknife™ prostate SABR trials stipulate that rectal V100% should not exceed 5% and do not specify very high bladder dose constraints [16–18]. Our study specified a 3% restriction on rectal V97% and a 5% restriction on bladder V97%. Our approach, therefore, may be considered conservative. With a new technique, however, caution is appropriate. Furthermore, when uppermost constraints were met, or minimally exceeded, in our study, all lower constraints were more than adequately achieved. This may translate into low late toxicity rates when these constraints are employed clinically. Caution must be exercised, however, when comparing the constraints adopted between studies as the length of contoured rectum may differ, and this should be specified to aid meaningful comparisons.
The current evidence for SABR as primary treatment for localized PCa is mainly in the form of small trials or series, 13 using Cyberknife™ and 6 using linacs [18–24]. There is variation in dose-fractionation schedules, organ-at-risk constraints, use of androgen deprivation, CTV–PTV margins, IGRT techniques and inclusion of SV within the CTV (most often the SV are not treated, even in non-low risk patients). Overall, toxicity rates and PSA control appear encouraging.
Delivering prostate SABR using VMAT has not been widely practiced. Two groups report production and delivery of SABR VMAT plans [25,26]. Agazaryan et al., using RapidArc VMAT (Varian) for 10 patients, delivering 40 Gy in 5 fractions, found two full arcs resulted in improved homogeneity and conformity compared to one. It is currently uncertain whether homogenous or heterogeneous dose distributions are preferable. Our planning study aimed for a degree of homogeneity where feasible. In contrast to the RapidArc study, our study found no significant improvement in homogeneity or conformity using 2 arcs which may relate to differences in the planning algorithms and linac delivery associated with each technique.
Miften et al. delivered 50 Gy in 5 fractions to 6 patients mainly using 1FA [26]. CI ranged from 1.09 to 1.21, CN from 0.75 to 0.82 and treatment times from 8 to 13 min. Our study demonstrated similar CI, slightly improved CN, and EDT were shorter than those measured by Miften et al. We have also delivered 3 1PA treatments, with measured delivery times of 196, 203 and 213 s per fraction.
Robust IGRT is required for SABR. Several SABR trials employ intra-fraction motion tracking and correction, allowing small CTV–PTV margins (3–5 mm) [16,18,20,24]. We evaluated 6 mm margins which are sufficient to account for residual set-up inaccuracy and uncorrected intra-fraction motion when using fiducial markers for daily online IGRT [7–9]. Larger CTV–PTV margins carry the risk of increased toxicity but with 6 mm margins, planning was successful in terms of coverage and organ-at-risk constraints.
CBCT (without fiducials) is an alternative IGRT technique. Given uncertainties and inter-observer variability, CTV–PTV margins of about 8 mm are required [10]. When planning with 8 mm margins, although PTV coverage was adequate, there was a small but significant reduction in D95%, and significant increases in rectal and bladder mean doses and in volumes receiving very high, high, intermediate and low doses. Furthermore, in 3 patients (20%) it was necessary to relax the uppermost bladder constraint to achieve coverage: two had small bladder volumes and median lobe hyperplasia and one had the largest volume prostate in the series and a very large median lobe (all resulting in a larger proportion of bladder within or close to the PTV). The clinical consequences of such bladder overdoses are unknown [27]. Since 6 mm margins were consistently feasible in terms of organ-at-risk constraints, then implanted fiducial markers, and the accompanying smaller CTV–PTV margins, should be used in preference.
When including the proxSV in the CTV, and using 6 mm CTV–PTV margins, a potential solution for patients with early intermediate risk disease, although PTV coverage was adequate, there was a small but significant reduction in D95% and significant increases in rectal and bladder mean doses and in volumes receiving high, intermediate and low doses. Furthermore, in 13% of cases it was necessary to relax the uppermost bladder and rectal constraints. Ensuring full bladders and using biodegradable spacers to increase prostate to rectal distance could potentially allow proxSV inclusion, without exceeding constraints [28]. The use of neo-adjuvant hormone deprivation could also facilitate planning. Another strategy is to create two PTVs (prostate and prostate plus proxSV) and prescribe a reduced dose to the prostate plus SV PTV. Given that constraints could not be met consistently when including the proximal 1 cm of SV, it is unlikely that prescribing the same dose to greater lengths of SV would be feasible, thus excluding higher risk patients from this linac based treatment option. Treating the prostate alone, as in most of the existing SABR trials, appears likely to be the safest option.
In conclusion, delivering prostate SABR using VMAT offers dose escalation, the theoretical benefits of hypofractionation, the convenience of a few fraction treatment, and the highly conformal plans, MU efficiency and rapid delivery achievable with VMAT. We have demonstrated that prostate SABR planning using VMAT is consistently feasible when treating the prostate alone using 6 mm CTV–PTV margins, compatible with fiducial marker daily online IGRT. A 210° arc was optimal. Clinical trials are required to evaluate this technique in practice.
Conflict of interest statement
St. James’s Institute of Oncology has a research contract with Elekta™.
Acknowledgements
With thanks to: Dr. Mitchell Naisbit, Clinical Scientist, Department of Medical Physics, St. James’s Institute of Oncology, Cancer Research UK Centre, St. James’s University Hospital, Beckett St, Leeds LS9 7TF, UK.
Dr. Helene Thygesen, Statistician, Leeds Cancer Research UK Centre, University of Leeds, Leeds LS2 9JT, UK. (Grant number C37059/A11941).
Dr. Louise Murray is funded as a Cancer Research UK Clinical Research Fellow (Grant number C37059/A11941).
Appendix A. Supplementary data
This document file contains Supplementary Materials.
References
- 1.EUROCARE. Survival of cancer patients in Europe. The EUROCARE-4 study. Survival analysis 1995–1999. 2009 [cited 2012 15.03.2012]; Available from: http://www.eurocare.it/Portals/0/CDEU4/Index.htm.
- 2.D’Ambrosio D.J., Pollack A., Harris E.E. Assessment of external beam radiation technology for dose escalation and normal tissue protection in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:671–677. doi: 10.1016/j.ijrobp.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Miralbell R., Roberts S.A., Zubizarreta E., Hendry J.H. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 4.Palma D.A., Verbakel W.F., Otto K., Senan S. New developments in arc radiation therapy: a review. Cancer Treat Rev. 2010;36:393–399. doi: 10.1016/j.ctrv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Palma D., Vollans E., James K. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Wolff D., Stieler F., Welzel G. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93:226–233. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Quon H., Loblaw D.A., Cheung P.C. Intra-fraction motion during extreme hypofractionated radiotherapy of the prostate using pre- and post-treatment imaging. Clin Oncol (R Coll Radiol) 2012;24:640–645. doi: 10.1016/j.clon.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Adamson J., Wu Q., Yan D. Dosimetric effect of intrafraction motion and residual setup error for hypofractionated prostate intensity-modulated radiotherapy with online cone beam computed tomography image guidance. Int J Radiat Oncol Biol Phys. 2011;80:453–461. doi: 10.1016/j.ijrobp.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltran C., Herman M.G., Davis B.J. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int J Radiat Oncol Biol Phys. 2008;70:289–295. doi: 10.1016/j.ijrobp.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Kupelian P.A., Langen K.M., Willoughby T.R., Zeidan O.A., Meeks S.L. Image-guided radiotherapy for localized prostate cancer: treating a moving target. Semin Radiat Oncol. 2008;18:58–66. doi: 10.1016/j.semradonc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Franzen LWA. Trial protocol: Fase III studie om hypofraktioneret strileterapi til patienter med prostatacancer I intermediaer riskogruppe-Hypo-RT-PC. Version 4; 2009.
- 12.Dearnaley D. CHHIP trial physics plan assessment form. 2006; Available from: http://rttrialsqa.dnsalias.org/chhip/CHHIP%20Physics%20Plan%20Assessment%20Form%20v4%5B1%5D.0.pdf.
- 13.Dearnaley D., Syndikus I., Sumo G. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13:43–54. doi: 10.1016/S1470-2045(11)70293-5. [DOI] [PubMed] [Google Scholar]
- 14.Feuvret L., Noel G., Mazeron J.J., Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 15.ICRU Report 83. 3. Special considerations regarding absorbed-dose and dose–volume prescribing and reporting in IMRT. J ICRU. 2010;10:14. doi: 10.1093/jicru/ndq008. [DOI] [PubMed] [Google Scholar]
- 16.King C.R., Brooks J.D., Gill H., Presti J.C., Jr. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–882. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Freeman D.E., King C.R. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oermann E.K., Suy S., Hanscom H.N. Low incidence of new biochemical and clinical hypogonadism following hypofractionated stereotactic body radiation therapy (SBRT) monotherapy for low- to intermediate-risk prostate cancer. J Hematol Oncol. 2011;4:12. doi: 10.1186/1756-8722-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang C.I., Loblaw D.A., Cheung P. Phase I/II study of a five-fraction hypofractionated accelerated radiotherapy treatment for low-risk localised prostate cancer: early results of pHART3. Clin Oncol (R Coll Radiol) 2008;20:729–737. doi: 10.1016/j.clon.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Townsend N.C., Huth B.J., Ding W. Acute toxicity after cyberknife-delivered hypofractionated radiotherapy for treatment of prostate cancer. Am J Clin Oncol. 2011;34:6–10. doi: 10.1097/COC.0b013e3181c4c7c4. [DOI] [PubMed] [Google Scholar]
- 21.Boike T.P., Lotan Y., Cho L.C. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29:2020–2026. doi: 10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbari S., Weinberg V.K., Kaprealian T. Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: technique, early toxicity, and PSA response. Int J Radiat Oncol Biol Phys. 2012;82:228–234. doi: 10.1016/j.ijrobp.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Action Team. national radiotherapy implementation group report. Stereotactic body radiotherapy clinical review of the evidence for SBRT. 2011 [cited 2012 16.08.2012]; Available from: http://www.ncat.nhs.uk/sites/default/files/clinical%20evidence%20review%20Dec%2010%20-%20Final%20J11.pdf.
- 24.Seisen T., Drouin S.J., Phe V. Current role of image-guided robotic radiosurgery (Cyberknife((R))) for prostate cancer treatment. BJU Int. 2013;111:761–766. doi: 10.1111/bju.12000. [DOI] [PubMed] [Google Scholar]
- 25.Agazaryan NTS, Chow P, et al. Volumetric arc therapy treatment protocol for hypo-fractionated stereotactic body radiotherapy for localised prostate cancer. Poster 3431 presented ASTRO 2010. 2011; Available from: http://astro2010.abstractsnet.com/handouts/011553_Agazaryan_Poster_ASTRO_2010_Final.pdf.
- 26.Miften MKB, Timmerman R, Diot Q, Papiez L. Volumetric modulated arc therapy for the stereotactic body radiation therapy of the prostate. Poster 3453 presented ASTRO 2011. 2011; Available from: http://astro2011.abstractsnet.com/handouts/011567_Prostate_SBRT_ASTRO_2011.pdf.
- 27.Viswanathan A.N., Yorke E.D., Marks L.B., Eifel P.J., Shipley W.U. Radiation dose–volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–122. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatiboglu G., Pinkawa M., Vallee J.P., Hadaschik B., Hohenfellner M. Application technique: placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110:e647–652. doi: 10.1111/j.1464-410X.2012.11373.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document file contains Supplementary Materials.