To the Editor:
The beneficial role of pet exposure and the development of allergen-specific IgG antibodies induced by natural exposure is a controversial issue. Increased levels of IgG antibodies to Fel d 1 were found to be associated with decreased sensitization in children,1 and higher levels of IgG/IgG4 to mouse allergens were found to be associated with decreased symptoms in laboratory workers.2 However, another study reported that exposure and high IgG levels to cat were not associated with a lower risk of allergic respiratory symptoms.3 One possibility for this discrepancy may be that allergen-specific IgE and IgG responses are not synchronized and directed to the same allergens/epitopes. To address this question and to define the most frequently recognized animal allergens, we investigated allergen-specific IgE and IgG responses by using microarrayed allergens.
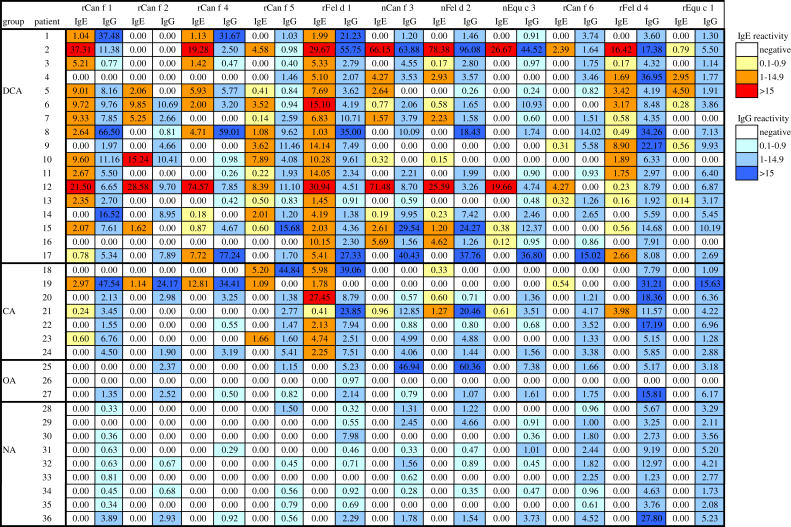
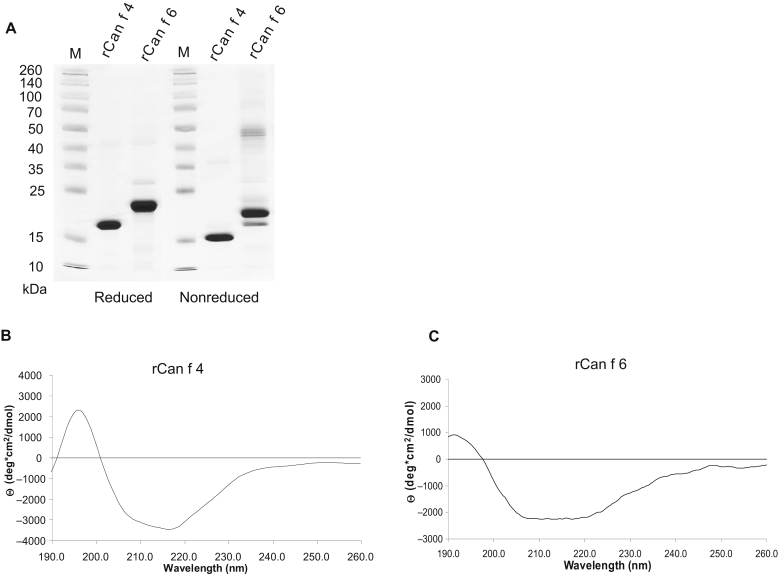
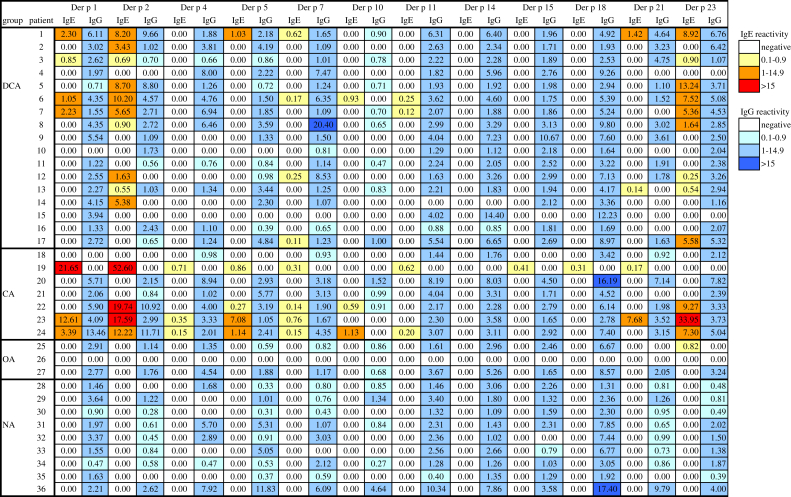
Sera from patients with allergic symptoms clearly attributable to cat exposure with and without concomitant allergy to dogs and horses, from allergic patients without allergy to animals, and from subjects without allergy were studied (see Table E1 in this article's Online Repository at www.jacionline.org). By using the ImmunoCAP ISAC technology (Thermo Fisher, Uppsala, Sweden, and Vienna, Austria), customized allergen microarrays containing in addition to Can f 1, Can f 2, Can f 3, and Can f 5, 2 recently described dog allergens, that is, Can f 4 and Can f 6, were prepared.4 Fig E1 in this article's Online Repository at www.jacionline.org shows that recombinant Can f 4 (rCan f 4) and rCan f 6 exhibit correct molecular weight, are pure, and are folded. In addition, the chip also contained the cat allergens rFel d 1, natural Fel d 2 (nFel d 2), and rFel d 4 and the horse allergens rEqu c 1 and nEqu c 3. The simultaneous analysis of IgE and IgG responses toward 11 animal allergens showed that allergen-specific IgE and IgG responses were only poorly correlated (Fig 1 and Table I). High correlation between IgE and IgG antibodies was found only for Can f 4 (r = 0.728; P < .001), moderate correlations were observed for Can f 1 (r = 0.581; P < .05), Can f 2 (r = 0.504; P < .05), and Equ c 3 (r = 0.550; P < .05), and no correlations were observed for the other animal-derived allergens (Table I). Often, animal-allergic patients without IgE reactivity to certain allergen components mounted pronounced IgG responses toward these allergens (Fig 1 and Table I). Furthermore, allergic patients without animal allergy and nonallergic individuals exhibited specific IgG antibody responses toward animal allergens similar to those of animal-allergic patients (Fig 1). Almost for each tested allergen (ie, Can f 2, Can f 3, Can f 4, Can f 5, Can f 6, Fel d 1, and Fel d 2), we found allergic patients who showed selective IgE reactivity without detectable IgG antibodies (Fig 1 and Table I). Similar finding were made for house dust mite allergens (see Fig E2 in this article's Online Repository at www.jacionline.org). Moderate correlation between IgE and IgG was observed for only 2 of the 12 allergens, namely, Der p 2 and Der p 23 (r = 0.560, P < .05, and r = 0.545, P < .05, respectively; Fig E2).
Table E1.
Demographic, clinical, and serological characteristics of patients and control subjects
| Allergy | Subject | Age (y) | Sex | Symptoms on contact with the following animals | Symptoms to animals | Other allergies | Symptoms to other allergen sources | Specific IgE level (kUA/L) |
Total IgE level (kU/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cat (e1) | Dog (e5) | |||||||||
| 1 | 23 | F | Dog, cat, horse, rabbit | AS, RH, | tp, gp, hdm, nu | AS, RH, CO, OAS | 4.31 | 4.96 | 140 | |
| 2 | 42 | F | Dog, cat, horse | AS, RH, CO | tp, gp, hdm, ve, he, cm | AS, RH, CO, AD | >100 | 66.70 | >2000 | |
| 3 | 26 | F | Dog, cat, horse, rodents | AS, RH, CO, U | tp, gp, hdm, fi, nu | AS, RH, CO | >100 | 14.60 | 570 | |
| 4 | 36 | F | Dog, cat, horse | AS, U | gp, hdm | RH | 15.70 | 6.27 | 218 | |
| DCA | 5 | 29 | M | Dog, cat | AS, RH, CO | tp, gp, hdm | AS, RH, CO, AD | 19.70 | 63.00 | >2000 |
| 6 | 34 | F | Dog, cat | AS, CO, AD | tp, gp, hdm | AS, RH, CO, AD | 75.60 | 78.10 | >2000 | |
| 7 | 26 | M | Dog, cat, guinea pig, sheep | CO, AD | tp, gp, hdm, he, nu | AS, RH, CO, AD, OAS | 19.30 | 4.47 | >2000 | |
| 8 | 23 | M | Dog, cat | AS, RH, CO | tp, gp, hdm, fr | AS, RH, CO, OAS | 10.00 | 16.40 | 316 | |
| 9 | 27 | M | Dog, cat, horse, rabbit, guinea pig | RH, CO | tp, me | RH | 31.30 | 4.44 | 129 | |
| 10 | 26 | M | Dog, cat, horse, rabbit, guinea pig | AS, AD | tp, fr, nu | RH, CO, AD, OAS | 52.70 | 12.70 | >5000 | |
| 11 | 37 | M | Dog, cat, horse | AS, RH, CO, AD | tp, gp | RH, CO | 3.60 | 1.46 | 141 | |
| 12 | 47 | F | Dog, cat | AS, RH, AD | tp, gp, hdm, fi | AS, RH, AD, U | >100 | >100 | 1224 | |
| 13 | 41 | M | Dog, cat | RH | tp, gp, ve | RH, CO, OAS | 0.55 | 1.81 | 44 | |
| 14 | 30 | F | Dog, cat, guinea pig | AS, RH, CO | tp, gp, hdm, mo, me, fi, nu | AS, RH, CO, OAS | 3.55 | 5.20 | 997 | |
| 15 | 47 | M | Dog, cat, horse | AS, RH, CO, U | tp, gp | AS, RH, CO | 13.20 | 20.10 | 420 | |
| 16 | 26 | M | Dog, cat, horse, guinea pig | RH, CO | tp, gp | RH, CO | 62.20 | 6.00 | 798 | |
| 17 | 27 | M | Dog, cat, guinea pig | AS, RH | tp, gp, hdm, fr | AS, RH, OAS | 61.00 | 20.80 | 938 | |
| 18 | 27 | M | Cat | AS | gp, me | AS | >100 | 73.20 | 2706 | |
| 19 | 19 | M | Cat | AS | gp, hdm | AS | 4.97 | 9.97 | 1771 | |
| 20 | 36 | F | Cat | AS, RH, CO | wf | RH | >100 | 17.10 | 1035 | |
| CA | 21 | 29 | F | Cat, horse | AS, RH, CO | me | U | 11.30 | 2.46 | 101 |
| 22 | 29 | F | Cat | RH, CO | tp, gp, hdm, fr | AS, RH, CO, OAS | 3.51 | 0.75 | 490 | |
| 23 | 27 | F | Cat, horse | RH, CO | tp, gp, hdm, fr | RH, CO, OAS | 3.47 | 2.89 | >5000 | |
| 24 | 26 | M | Cat | RH, CO | gp, hdm | AS, RH, CO | 3.20 | 0.48 | 296 | |
| 25 | 23 | M | 0 | 0 | tp, gp, fr, ve | RH, CO, OAS | 0 | 0.78 | 410 | |
| OA | 26 | 25 | F | 0 | 0 | tp, gp, hdm, fr, nu | RH, CO, OAS | 0.41 | 0.68 | 130 |
| 27 | 34 | M | 0 | 0 | gp | RH, CO | 0 | 0 | 45 | |
| 28 | 29 | F | 0 | 0 | 0 | 0 | 0 | 0 | 31 | |
| 29 | 29 | M | 0 | 0 | 0 | 0 | 0 | 0 | 44 | |
| 30 | 27 | F | 0 | 0 | 0 | 0 | 0 | 0 | 8 | |
| NA | 31 | 49 | M | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| 32 | 44 | F | 0 | 0 | 0 | 0 | 0 | 0 | 52 | |
| 33 | 22 | F | 0 | 0 | 0 | 0 | 0 | 0 | 19 | |
| 34 | 36 | M | 0 | 0 | 0 | 0 | 0 | 0 | 19 | |
| 35 | 39 | M | 0 | 0 | 0 | 0 | 0 | 0 | 20 | |
| 36 | 54 | F | 0 | 0 | 0 | 0 | 0 | 0 | 8 | |
Demographic data and symptoms and sensitization to animal allergens are displayed for 24 dog/cat-allergic patients, 3 controls with allergy to pollen and/or house dust mite, and 9 nonallergic individuals. Total and allergen-specific IgE levels were measured by using ImmunoCAP and are displayed in kilo units/liter (kU/L) and kilo units of antigen/liter (kUA/L), respectively. The cutoff value is 0.35 kUA/L.
AD, Atopic dermatitis; AS, asthma; CA, cat allergy; cm, cow's milk; CO, conjunctivitis; DCA, dog or cat allergic; e1, cat dander extract; e5, dog dander extract; F, female; fi, fish; fr, fruits; gp, grass pollen; hdm, house dust mite; he, hens egg; M, male; me, medicaments; mo, moulds; NA, not allergic; nu, nuts; OA, other allergies than to animal dander; OAS, oral allergy syndrome; RH, rhinitis; tp, tree pollen; U, urticaria; ve, vegetables; wf, wheat flour.
Fig 1.
Heat map of patients' IgE and IgG reactivity. IgE and IgG levels (inserts show color codes for the levels) specific for microarrayed dog (Can f 1-6), cat (Fel d 1, Fel d 2, and Fel d 4), and horse (Equ c 1 and Equ c 3) allergens are displayed for 4 groups of subjects (DCA, patients with symptoms to dog and/or cat; CA, cat-allergic patients; OA, allergic patients without allergy to animals; NA, nonallergic subjects). n, Natural; r, recombinant.
Table I.
Correlations between allergen-specific IgE and IgG levels of animal-derived allergens
| IgG | IgE | Can f 1 | Can f 2 | Can f 3 | Can f 4 | Can f 5 | Can f 6 | Fel d 1 | Fel d 2 | Fel d 4 | Equ c 1 | Equ c 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Can f 1 |
r = 0.581∗ P = .003 |
|||||||||||
| Can f 2 |
r = 0.504† P = .01 |
|||||||||||
| Can f 3 |
r = 0.281 P > .05 |
|||||||||||
| Can f 4 |
r = 0.728∗ P < .001 |
|||||||||||
| Can f 5 |
r = 0.304 P > .05 |
|||||||||||
| Can f 6 |
r = −0.168 P > .05 |
|||||||||||
| Fel d 1 |
r = 0.100 P > .05 |
|||||||||||
| Fel d 2 |
r = 0.337 P > .05 |
|||||||||||
| Fel d 4 |
r = 0.154 P > .05 |
|||||||||||
| Equ c 1 |
r = 0.048 P > .05 |
|||||||||||
| Equ c 3 |
r = 0.550∗ P < .005 |
|||||||||||
P values less than .005 were considered highly significant.
P values less than .05 were considered significant.
In the case of a strictly sequential class-switch from allergen-specific IgG to IgE production, one would expect a good correlation between IgE and IgG responses but our results provide evidence for a direct switch from IgM to allergen-specific IgE without intermediate IgG response. Our results therefore may explain why natural allergen exposure does not always induce protective IgG responses leading to immunological tolerance as has been suggested for cat allergy because IgG is directed to other allergens/epitopes than is IgE.
In the group of dog-allergic patients (Table E1: patients 1-17), the frequencies of IgE reactivity to the individual dog allergens were as follows: Can f 1, 13 of 17 (76%); Can f 3, 10 of 17 (59%); Can f 5, 12 of 17 (71%); Can f 4, 10 of 17 (59%); Can f 2, 6 of 17 (35%), and Can f 6, 4 of 17 (23%) (Fig 1). In the group of cat-allergic patients, the frequencies of IgE reactivity to cat allergens (Table E1: patients 1-24) were as follows: Fel d 1, 24 of 24 (100%); Fel d 4, 15 of 24 (63%); and Fel d 2, 13 of 24 (54%). Using Equ c 1 and Equ c 3, only 6 of the 11 patients (Table E1: patients 1-4, 9-11, 15, 16, 21, and 23) reporting symptoms on contact with horses were identified, indicating that additional horse allergen components were needed. Each of the patients who had reported allergic symptoms on contact with dogs showed IgE reactivity to at least 1 of the microarrayed dog allergens and each of the patients who had reported symptoms on contact with cats reacted with at least 1 of the cat allergens present on the chip, indicating high sensitivity of the microarray for diagnosing cat and dog allergy. No IgE binding to microarrayed animal allergen components was detected in sera from nonallergic subjects or allergic patients with house dust mite and/or pollen allergy without animal allergy, indicating specificity of the microarray. Interestingly, IgE reactivity to dog and cat allergen extracts was found by ImmunoCAP measurements in allergic patients without clinical animal allergy (Table E1, patients 25 and 26). Our findings need to be confirmed in a larger population of patients to identify the most relevant animal allergens. Nevertheless, the chip should be useful for studying dog and cat allergen-specific IgE responses to follow the evolution of IgE responses in birth cohorts5 and in populations from various countries.
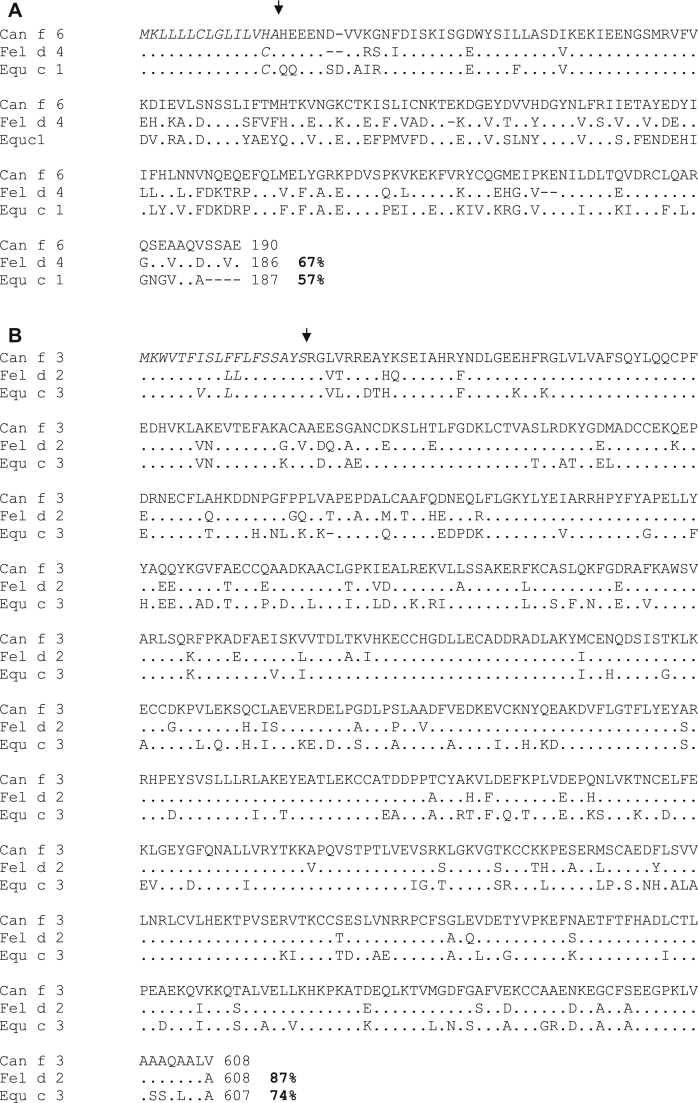
Fig E3 in this article's Online Repository at www.jacionline.org shows that there is a sequence identity of 67% and 57% between the lipocalins Fel d 4 and Equ c 1 with the dog allergen Can f 6, respectively, and a very high sequence identity of more than 74% between the albumins from dog, cat, and horse (ie, Can f 3, Fel d 2, and Equ c 3). Dog, cat, and horse lipocalin allergens Can f 6, Fel d 4, and Equ c 1, which had previously been reported to be cross-reactive at the IgE level,6 showed a significant correlation of IgE reactivities between Fel d 4 and Equ c 1 (r = 0.557, P < .005; see Table E2 in this article's Online Repository at www.jacionline.org) but not between the 2 other pairs of lipocalins (Can f 6: Fel d 4, r = 0.163, P > .05; Can f 6: Equ c 1, r = 0.325, P > .05). The weak IgE cross-reactivity among the lipocalin allergens was also evident by the fact that many patients displayed selective IgE reactivity to members of this allergen family. For example, 9 patients showed selective IgE reactivity to Fel d 4 but not to Equ c 1 and 11 patients showed IgE reactivity to Fel d 4 but not to Can f 6 (Fig 1; see Table E2).
Table E2.
Correlations between specific IgE levels to homologous allergens for the group of patients with dog and/or cat allergy (n = 24)
| Can f 6 | Fel d 4 | Equ c 1 | Can f 3 | Fel d 2 | Equ c 3 | |
|---|---|---|---|---|---|---|
| Can f 6 | 1 |
r = 0.163 P > .05 |
r = 0.325 P > .05 |
|||
| Fel d 4 | 1 |
r = 0.557∗ P < .003 |
||||
| Equ c 1 | 1 | |||||
| Can f 3 | 1 |
r = 0.788∗ P < .001 |
r = 0.690∗ P < .001 |
|||
| Fel d 2 | 1 |
r = 0.684∗ P < .001 |
||||
| Equ c 3 | 1 |
P values less than .005 were considered highly significant.
Significant correlations in IgE reactivity were found between serum albumins from dog, cat, and horse (Can f 3 vs Fel d 2, r = 0.788, P < .001; Can f 3 vs Equ c 3, r = 0.690, P < .001; Equ c 3 vs Fel d 2, r = 0.684, P < .001; Table E2). However, for the albumins also, several patients were found with selective IgE reactivities toward certain albumins (Fig 1 and Fig E2). The poor associations of IgE reactivities between the lipocalins and albumins may also be due to IgG competing with IgE for chip-bound allergens.
In summary, we report a microarray containing 11 purified recombinant and natural allergens from dog, cat, and horse and its usefulness for the diagnosis of IgE sensitization to dogs and cats and the parallel analysis of allergen-specific IgG responses. The microarray not only allowed sensitive and specific detection of dog and cat allergen-specific IgE but also identified allergens that may be relevant components of vaccines and allowed to reveal species-specific sensitizations due to limited cross-reactivity of the allergen components. Furthermore, we discovered an interesting dissociation of allergen-specific IgE and IgG responses that indicates that nonsequential class-switch mechanisms are operative in animal allergy and may explain why naturally occurring allergen-specific IgG is not always protective.
Acknowledgments
We thank Prof Oswald Wagner for his support and Nadja Balic for ImmunoCAP testing, both from the Department of Laboratory Medicine, Medical University of Vienna.
Footnotes
This study was supported by the SFB program F46 of the Austrian Science Fund (FWF) project F4605 and by research grants from Biomay AG, Vienna, Austria; the Christian Doppler Research Association, Austria; the FP7-funded EU project MeDALL; the Swedish Research Council; and the Stockholm County Council.
Disclosure of potential conflict of interest: C. Lupinek has received lecture fees from Thermo Fisher. M. van Hage has received lecture fees from Thermo Fisher Scientific, Novartis, and ALK-Abelló. R. Valenta has received research support from FWF Austrian Science Fund, Phadia, Thermo Fisher, and Biomay; and has received consulting fees from Phadia, Thermo Fisher, and Biomay. The rest of the authors declare that they have no relevant conflicts of interest.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Methods
Patients' sera
Sera from 24 adult animal-allergic patients suffering from rhinitis, conjunctivitis, asthma, urticaria, and/or atopic dermatitis were analyzed. All patients had reported allergic symptoms attributable to contact with cat (patients 1-24, Table E1), and 1 subset exhibited allergic symptoms to cat and additionally to dog and/or horse (patients 1-17, Table E1). As controls, sera from 3 subjects with allergy to pollen or/and house dust mite but without allergy to animal dander and from 9 nonallergic subjects were analyzed. Control subjects were exposed to cats and dogs. All subjects were from Austria. The anonymized analysis of sera was approved by the ethics committee of the Medical University of Vienna, and subjects had signed approved consent forms. For each patient, a detailed case history summarizing allergic symptoms to animals and other allergen sources was established. In particular, patients were asked in detail about the occurrence, type, and intensity of allergic symptoms on contact with animals (ie, dog, cat, horse, rabbit, mice, guinea pig, cow, and sheep) (Table E1). None of the animal-allergic patients had received allergen-specific immunotherapy for animal allergies. Symptoms on contact with animals were recorded, and symptoms on contact with other allergen sources were also noted (Table E1). Animal-allergic patients were frequently cosensitized to house dust mites and grass/tree pollen. Each serum was tested regarding total IgE levels and for IgE reactivity to natural dog dander (e5) and cat dander (e1) allergen extracts as determined by the ImmunoCAP System (Thermo Fisher Scientific/Phadia, Uppsala, Sweden). Table E1 summarizes the demographic, clinical, and serologic characteristics of the patients.
Expression and purification of recombinant dog allergens
Genes coding for the mature forms of Can f 4 (ACY38525.1) and Can f 6 (E2QYS2) was synthesized by using PCR-based assembly of oligonucleotides (GenScript, Piscataway, NJ) and inserted into the NdeI/EcoRI sites of pET-27b (Novagen, Darmstadt, Germany). Both genes contained sequences coding for a hexahistidine tag at the C terminus of the protein, and the gene sequences were optimized for Escherichia coli expression. The DNA sequences were confirmed by means of restriction enzyme analysis with corresponding restriction enzymes (Roche, Mannheim, Germany) and by automated sequencing of both DNA strands. E coli BL 21 DE3 (Stratagene, La Jolla, Calif) transformed with pET 27b-Can f 4 and pET 27b-Can f 6 were grown at 37°C for approximately 10 hours in a GFL 3033 incubator (GFL, Burgwedel, Germany) in Luria Bertani medium containing kanamycin (30 μg/mL) until a cell density (OD600nm) of 0.5 for Can f 4 and 0.2 for Can f 6 was reached. Protein expression was induced by adding 0.5 mmol/L isopropyl-β-thiogalactopyranoside (Calbiochem, Merck, Darmstadt, Germany). Cells were harvested by centrifugation at 6000g for 10 minutes. rCan f 4 was purified under native conditions. For this purpose, cell pellets were lysed with an Ultra-Turrax (Janke & Kunkel-IKA Labortechnik, Staufen, Germany) in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8). rCan f 6 was purified under denaturing conditions. In this case, cell pellets were lysed in lysis buffer A (100 mM NaH2PO4, 10 mM Tris-HCl, 8 mol/L Urea, pH 8). Both recombinant proteins were purified by Ni2+ metal-ion affinity chromatography according to the manufacturer's protocol (Qiagen, Hilden, Germany). Finally, purified Can f 4 was dialysed against PBS (pH 9) and rCan 6 was refolded by extensive dialysis against 10 mM NaH2PO4 (pH 7.5). The purity of the recombinant proteins was analyzed by using SDS-PAGEE1 and Coomassie blue staining under reducing and nonreducing conditions. The molecular masses were determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker, Billerica, Mass), and the protein concentration was determined by Micro-BCA Protein Assay Kit (Pierce, Rockford, Ill).
Circular dichroism analysis
The circular dichroism spectra of purified recombinant rCan f 4 and rCan f 6 were measured on a JASCO J-810 spectropolarimeter (Tokyo, Japan). Measurements were performed at protein concentrations of 0.1 mg/mL in a rectangular quartz cuvette with a path length of 0.2 cm. Spectra were recorded from 190 to 260 nm with a resolution of 0.5 nm at a scan speed of 50 nm/min and were derived from 3 scans. Final spectra were corrected by subtracting the baseline spectra obtained with the buffers alone. Results are displayed as the mean residue ellipticities (Θ) at given wavelengths. The secondary structure contents of the proteins were calculated with the secondary structure estimation programs CDSSTR and CONTIN.E2
Extended ISAC microarray
The animal allergen spectrum of ImmunoCAP ISAC (Thermo Fisher, Uppsala, Sweden) was expanded regarding rCan f 4 and rCan f 6. Allergens were spotted in triplicates, and IgE standards were added as for ImmunoCAP ISAC (Thermofisher). The animal allergen repertoire of the chip comprised dog allergens (rCan f 1, rCan f 2, nCan f 3, rCan f 4, rCan f 5, and rCan f 6), cat allergens (rFel d 1, nFel d 2, and rFel d 4), and horse allergens (rEqu c 1 and nEqu c 3).E3 Quality controls were performed with IgE and IgG standards with established IgE and IgG reactivity to allergen components on the chip. For IgE detection, 30 μL of serum, or for IgG detection 30 μL of a 1:50 dilution of serum, was added to the microarray and incubated for 120 minutes, followed by washing and incubation with fluorescence-labeled anti-IgE or anti-IgG antibodies (Thermo Fisher), respectively, for 30 minutes. Then, chips were washed, dried, and analyzed with a Laser Scan Confocal microarray reader (LuxScan 10K/A; Capital-Bio, Beijing, China) and evaluated with Phadia Microarray Image Analysis software. Antibody levels are given as semiquantitative ISAC Standardized Units (ISUs) with a cutoff of 0.1 ISU. The allergen-specific antibody levels were classified as low (0.1-1 ISUs), moderate to high (>1-15 ISUs), or very high (>15 ISUs).
Statistical analysis
For all statistical analyses, the statistical Program SPSS (2008, version 16.0; SPSS, Inc, Chicago, Ill) was used. P values of less than .05 were considered significant. Correlations between variables were analyzed by using the Spearman-Rho test.
Fig E1.
Biochemical and structural characterization of rCan f 4 and rCan f 6. A, Coomassie brilliant blue–stained SDS-PAGE containing rCan f 4 and rCan f 6 under reducing and nonreducing conditions. Molecular weights (M) are indicated at the left margins. B, Circular dichroism spectra of rCan f 4 and rCan f 6. The mean residue ellipticities (Θ) (y-axes) are shown at given wavelengths (x-axes).
Fig E2.
Heat map of patients' IgE and IgG reactivity. IgE and IgG levels (inserts show color codes for the levels) specific for microarrayed house dust mite allergens (nDer p 1, rDer p 2, rDer p 4, rDer p 5, rDer p 7, rDer p 10, rDer p 11, rDer p 14, rDer p 15, rDer p 18, rDer p 21, and rDer p 23) are displayed for 4 groups of subjects (DCA, patients with symptoms to dog and/or cat; CA, cat-allergic patients; OA, allergic patients without allergy to animals; NA, nonallergic subjects). n, Natural; r, recombinant.
Fig E3.
Alignment of protein sequences of Can f 6, Fel d 4, and Equ c 1 (A) and Can f 3, Fel d 2, and Equ c 3 (B). Predicted signal sequences are marked in italics, and arrows mark the first amino acid of the mature form of protein. Points indicate identical residues, and dashes indicate gaps. Sequence identities of allergens to the mature Can f 6 and Can f 3 allergens are shown on the right side of the last lines of the alignments.
References
- 1.Platts-Mills T., Vaughan J., Squillace S., Woodfolk J., Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 2.Matsui E.C., Diette G.B., Krop E.J., Aalberse R.C., Smith A.L., Curtin-Brosnan J. Mouse allergen-specific immunoglobulin G and immunoglobulin G4 and allergic symptoms in immunoglobulin E-sensitized laboratory animal workers. Clin Exp Allergy. 2005;35:1347–1353. doi: 10.1111/j.1365-2222.2005.02331.x. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis D., Zock J.P., Heinrich J., Svanes C., Verlato G., Olivieri M. Cat and dust mite allergen levels, specific IgG and IgG4, and respiratory symptoms in adults. J Allergy Clin Immunol. 2007;119:697–704. doi: 10.1016/j.jaci.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Bublin M, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2013 Oct 22. 10.1016/j.ymeth.2013.10.008. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 5.Bousquet J., Anto J., Sunyer J., Nieuwenhuijsen M., Vrijheid M., Keil T., MeDALL Study Group. CHICOS Study Group. ENRIECO Study Group. GA²LEN Study Group Pooling birth cohorts in allergy and asthma: European Union-funded initiatives - a MeDALL, CHICOS, ENRIECO, and GA²LEN joint paper. Int Arch Allergy Immunol. 2013;161:1–10. doi: 10.1159/000343018. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson O.B., Binnmyr J., Zoltowska A., Saarne T., van Hage M., Grönlund H. Characterization of the dog lipocalin allergen Can f 6: the role in cross-reactivity with cat and horse. Allergy. 2012;67:751–757. doi: 10.1111/j.1398-9995.2012.02826.x. [DOI] [PubMed] [Google Scholar]
References
- Lämmli U. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sreerama N., Woody R.W. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Bublin M, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2013 Oct 22. 10.1016/j.ymeth.2013.10.008. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]